Red and Blue Light Differently Influence Actinidia chinensis Performance and Its Interaction with Pseudomonas syringae pv. Actinidiae
Abstract
1. Introduction
2. Results
2.1. Effect of Light Treatment on the Growth of Uninfected or Infected Plants
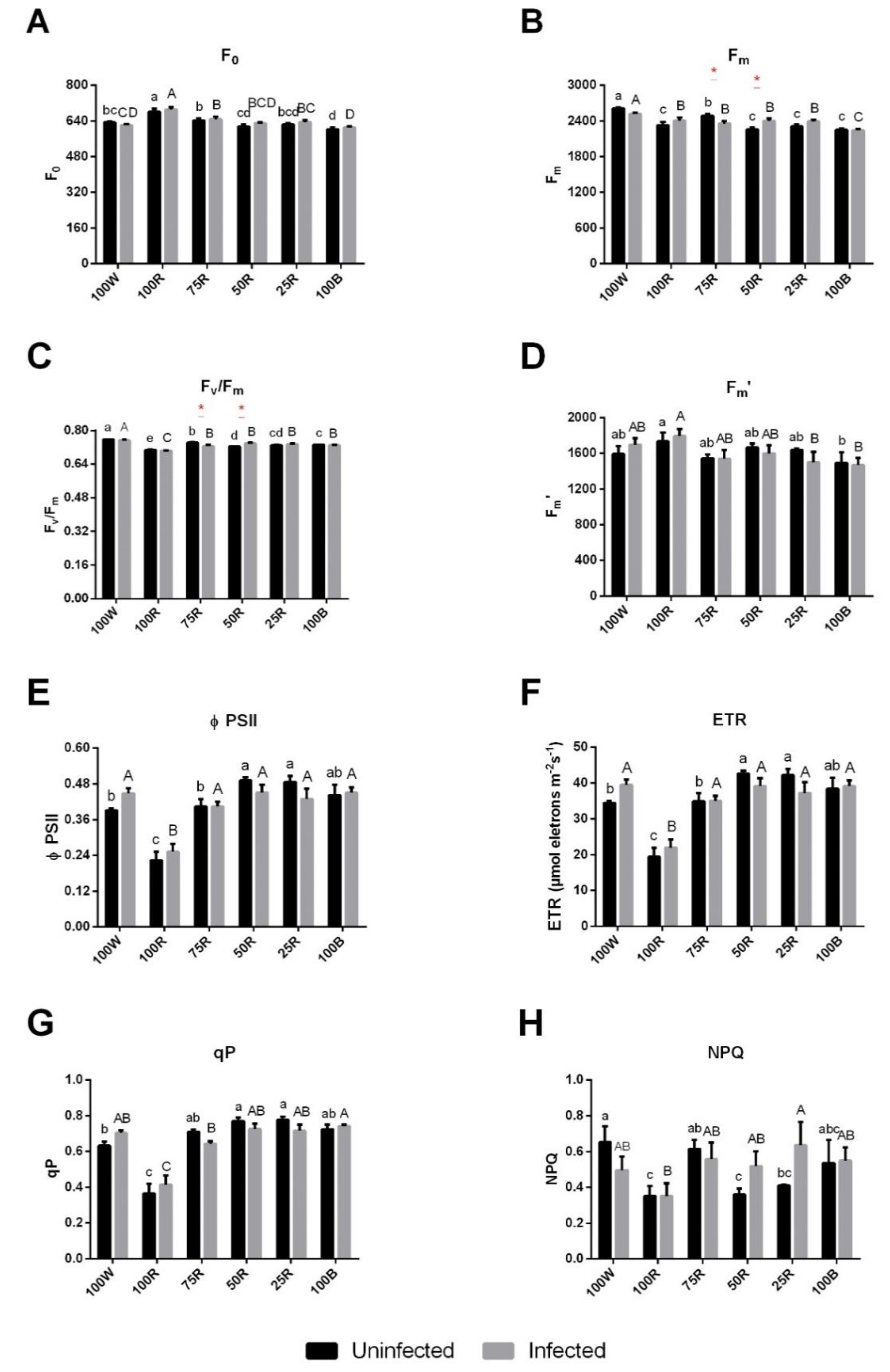
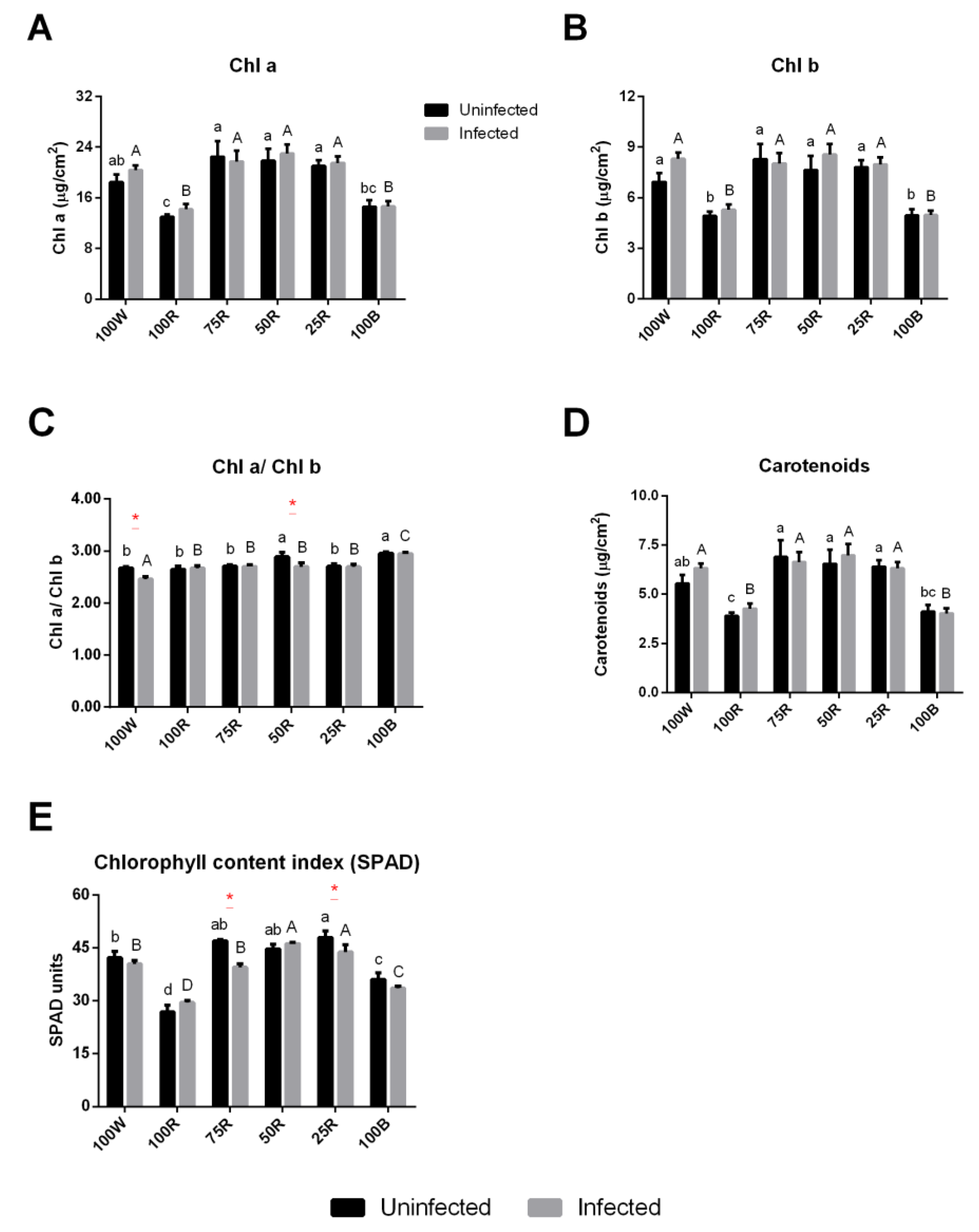
2.2. Effect of Light Treatment on Chl a Fluorescence and Pigment Content of Uninfected or Infected Plants
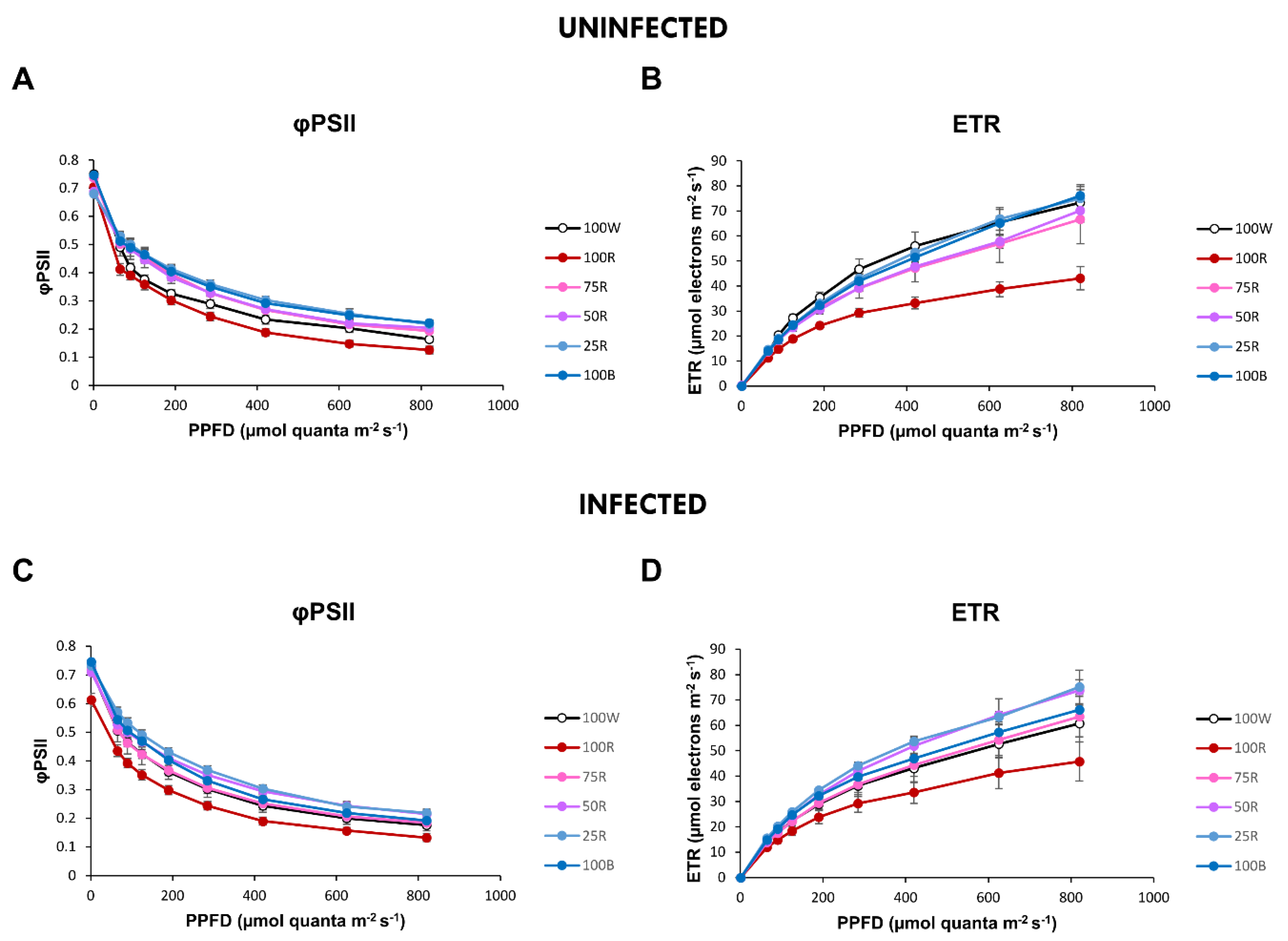
2.3. Rapid Light Curves (RLC)
2.4. Gas Exchange
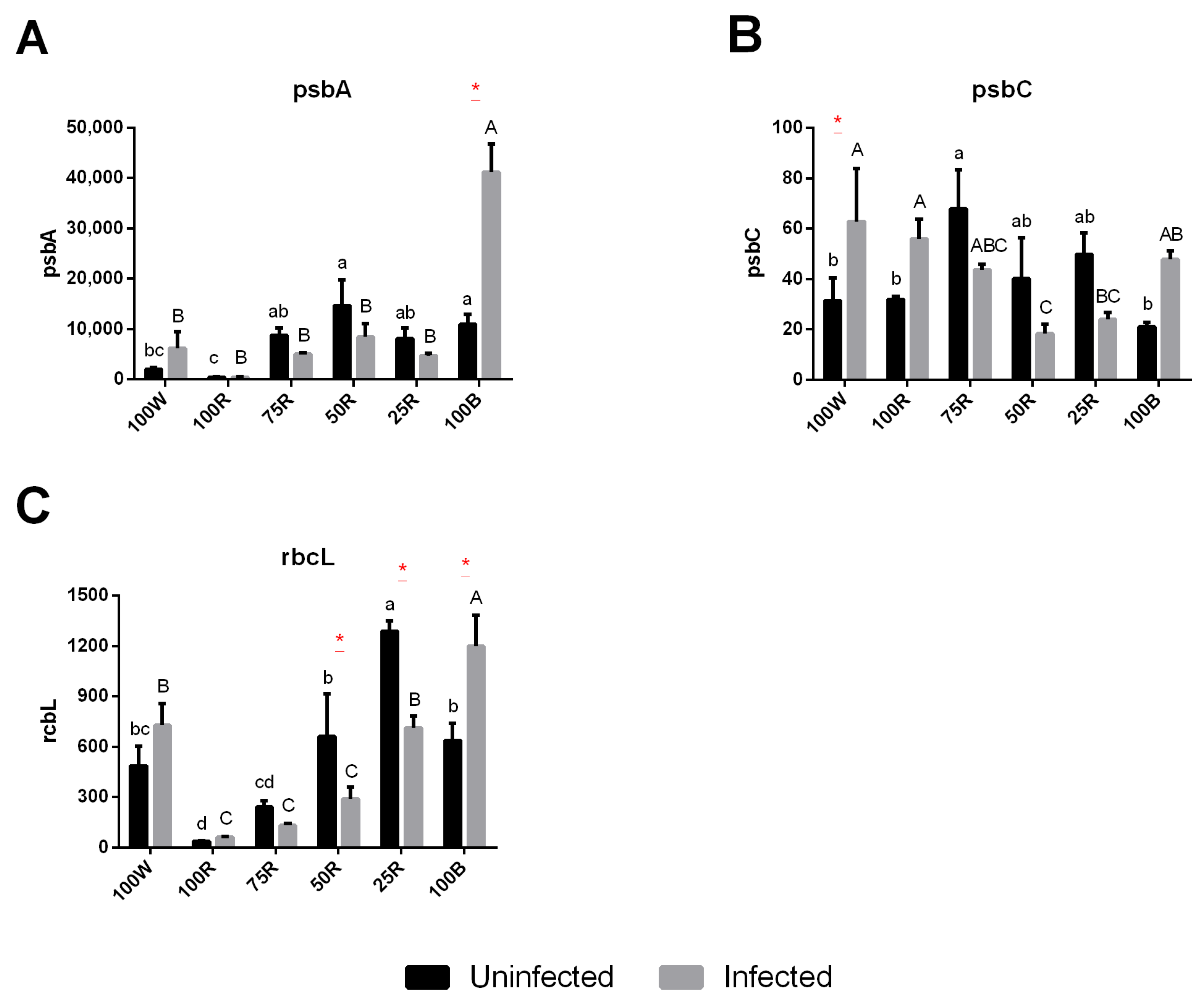
2.5. Gene Expression
2.6. Hierarchical Clustering Analysis
2.7. Effect of Light Treatments on Disease Development
3. Discussion
3.1. Light and Photosynthesis in Kiwifruit Plants
3.2. Light Quality, Psa and the Pathosystem
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Plant Inoculation
4.3. Measurements of Plant Biometric Parameters
4.4. Chlorophyll a Fluorescence and Pigments Quantification
4.5. Rapid Light Curves (RLC)
4.6. Gas-Exchange Measurements and Soluble Sugars
4.7. Photosynthesis-Related Gene Expression
4.8. Psa Quantification by Real-Time PCR (qPCR)
4.9. Hierarchical Clustering
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Wit, M.; Galvao, V.C.; Fankhauser, C. Light-mediated hormonal regulation of plant growth and development. Annu. Rev. Plant Biol. 2016, 67, 513–537. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Shi, H. Direct regulation of phytohormone actions by photoreceptors. Trends Plant Sci. 2019, 24, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.; Munné-Bosch, S. Hormonal impact on photosynthesis and photoprotection in plants. Plant Physiol. 2021, 185, 1500–1522. [Google Scholar] [CrossRef] [PubMed]
- Wobbe, L.; Bassi, R.; Kruse, O. Multi-level light capture control in plants and green algae. Trends Plant Sci. 2016, 21, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Kong, S.G.; Okajima, K. Diverse photoreceptors and light responses in plants. J. Plant Res. 2016, 129, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Paik, I.; Huq, E. Plant photoreceptors: Multi-functional sensory proteins and their signaling networks. In Seminars in Cell & Developmental Biology, August, 2019; Academic Press: Cambridge, MA, USA, 2019; Volume 92, pp. 114–121. [Google Scholar]
- Paradiso, R.; Proietti, S. Light-Quality Manipulation to Control Plant Growth and Photomorphogenesis in Greenhouse Horticulture: The State of the Art and the Opportunities of Modern LED Systems. J. Plant Growth Regul. 2022, 41, 742–780. [Google Scholar] [CrossRef]
- Zheng, L.; Van Labeke, M.C. Long-term effects of red-and blue-light emitting diodes on leaf anatomy and photosynthetic efficiency of three ornamental pot plants. Front. Plant Sci. 2017, 8, 917. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; He, H.; Song, W. Application of light-emitting diodes and the effect of light quality on horticultural crops: A review. HortScience 2019, 54, 1656–1661. [Google Scholar] [CrossRef]
- Carotti, L.; Potente, G.; Pennisi, G.; Ruiz, K.B.; Biondi, S.; Crepaldi, A.; Orsini, F.; Gianquinto, G.; Antognoni, F. Pulsed LED Light: Exploring the Balance between Energy Use and Nutraceutical Properties in Indoor-Grown Lettuce. Agronomy 2021, 11, 1106. [Google Scholar] [CrossRef]
- Kataoka, I.; Sugiyama, A.; Beppu, K. Role of ultraviolet radiation in accumulation of anthocyanin in berries of ‘Gros Colman’ grapes (Vitis vinifera L.). J. Jpn. Soc. Hortic. Sci. 2003, 72, 1–6. [Google Scholar] [CrossRef]
- Li, Q.; Kubota, C. Effects of supplemental light quality on growth and phytochemicals of baby leaf lettuce. Environ. Exp. Bot. 2009, 67, 59–64. [Google Scholar] [CrossRef]
- Pennisi, G.; Sanyé-Mengual, E.; Orsini, F.; Crepaldi, A.; Nicola, S.; Ochoa, J.; Fernandez, J.A.; Gianquinto, G. Modelling environmental burdens of indoor-grown vegetables and herbs as affected by red and blue LED lighting. Sustainability 2019, 11, 4063. [Google Scholar] [CrossRef]
- Pennisi, G.; Pistillo, A.; Orsini, F.; Cellini, A.; Spinelli, F.; Nicola, S.; Fernandez, J.A.; Crepaldi, A.; Gianquinto, G.; Marcelis LF, M. Optimal light intensity for sustainable water and energy use in indoor cultivation of lettuce and basil under red and blue LEDs. Sci. Hortic. 2020, 272, 109508. [Google Scholar] [CrossRef]
- Terashima, I.; Hanba, Y.T.; Tazoe, Y.; Vyas, P.; Yano, S. Irradiance and phenotype: Comparative eco-development of sun and shade leaves in relation to photosynthetic CO2 diffusion. J. Exp. Bot. 2006, 57, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Athanasiou, K.; Dyson, B.C.; Webster, R.E.; Johnson, G.N. Dynamic acclimation of photosynthesis increases plant fitness in changing environments. Plant Physiol. 2010, 152, 366–373. [Google Scholar] [CrossRef]
- Kono, M.; Terashima, I. Long-term and short-term responses of the photosynthetic electron transport to fluctuating light. J. Photochem. Photobiol. B Biol. 2014, 137, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Vialet-Chabrand, S.; Matthews, J.S.; Simkin, A.J.; Raines, C.A.; Lawson, T. Importance of fluctuations in light on plant photosynthetic acclimation. Plant Physiol. 2017, 173, 2163–2179. [Google Scholar] [CrossRef] [PubMed]
- Savvides, A.; Fanourakis, D.; van Ieperen, W. Co-ordination of hydraulic and stomatal conductances across light qualities in cucumber leaves. J. Exp. Bot. 2012, 63, 1135–1143. [Google Scholar] [CrossRef]
- Miao, Y.X.; Wang, X.Z.; Gao, L.H.; Chen, Q.Y.; Mei, Q.U. Blue light is more essential than red light for maintaining the activities of photosystem II and I and photosynthetic electron transport capacity in cucumber leaves. J. Integr. Agric. 2016, 15, 87–100. [Google Scholar] [CrossRef]
- Fan, X.; Zang, J.; Xu, Z.; Guo, S.; Jiao, X.; Liu, X.; Gao, Y. Effects of different light quality on growth, chlorophyll concentration and chlorophyll biosynthesis precursors of non-heading Chinese cabbage (Brassica campestris L.). Acta Physiol. Plant. 2013, 35, 2721–2726. [Google Scholar] [CrossRef]
- Tiansawat, P.; Dalling, J.W. Differential seed germination responses to the ratio of red to far-red light in temperate and tropical species. Plant Ecol. 2013, 214, 751–764. [Google Scholar] [CrossRef]
- OuYang, F.; Ou, Y.; Zhu, T.; Ma, J.; An, S.; Zhao, J.; Wang, J.; Kong, L.; Zhang, H.; Tigabu, M. Growth and Physiological Responses of Norway Spruce (Picea abies (L.) H. Karst) Supplemented with Monochromatic Red, Blue and Far-Red Light. Forests 2021, 12, 164. [Google Scholar] [CrossRef]
- Ohashi-Kaneko, K.; Matsuda, R.; Goto, E.; Fujiwara, K.; Kurata, K. Growth of rice plants under red light with or without supplemental blue light. Soil Sci. Plant Nutr. 2006, 52, 444–452. [Google Scholar] [CrossRef]
- Matsuda, R.; Ohashi-Kaneko, K.; Fujiwara, K.; Kurata, K. Analysis of the relationship between blue-light photon flux density and the photosynthetic properties of spinach (Spinacia oleracea L.) leaves with regard to the acclimation of photosynthesis to growth irradiance. Soil Sci. Plant Nutr. 2007, 53, 459–465. [Google Scholar] [CrossRef]
- Hogewoning, S.W.; Trouwborst, G.; Maljaars, H.; Poorter, H.; van Ieperen, W.; Harbinson, J. Blue light dose-responses of leaf photosynthesis, morphology, and chemical composition of Cucumis sativus grown under different combinations of red and blue light. J. Exp. Bot. 2010, 61, 3107–3117. [Google Scholar] [CrossRef]
- Pennisi, G.; Blasioli, S.; Cellini, A.; Maia, L.; Crepaldi, A.; Braschi, I.; Spinelli, F.; Nicola, S.; Fernandez, J.A.; Stanghellini, C.; et al. Unraveling the role of red: Blue LED lights on resource use efficiency and nutritional properties of indoor grown sweet basil. Front. Plant Sci. 2019, 10, 305. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Jiang, Y.P.; Yu, H.J.; Xia, X.J.; Shi, K.; Zhou, Y.H.; Yu, J.Q. Light quality affects incidence of powdery mildew, expression of defenses-related genes and associated metabolism in cucumber plants. Eur. J. Plant Pathol. 2010, 127, 125–135. [Google Scholar] [CrossRef]
- Hui, X.U.; FUY, N.; LIT, L.; Rui WA, N.G. Effects of different LED light wavelengths on the resistance of tomato against Botrytis cinerea and the corresponding physiological mechanisms. J. Integr. Agric. 2017, 16, 106–114. [Google Scholar]
- Moriconi, V.; Sellaro, R.; Ayub, N.; Soto, G.; Rugnone, M.; Shah, R.; Pathak, G.P.; Gärtner, W.; Casal, J.J. LOV-domain photoreceptor, encoded in a genomic island, attenuates the virulence of Pseudomonas syringae in light-exposed Arabidopsis leaves. Plant J. 2013, 76, 322–331. [Google Scholar] [PubMed]
- Chandra-Shekara, A.C.; Gupte, M.; Navarre, D.; Raina, S.; Raina, R.; Klessig, D.; Kachroo, P. Light-dependent hypersensitive response and resistance signaling against Turnip Crinkle Virus in Arabidopsis. Plant J. 2006, 45, 320–334. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Yang, H.Q. CRYPTOCHROME 1 is implicated in promoting R protein-mediated plant resistance to Pseudomonas syringae in Arabidopsis. Mol. Plant 2010, 3, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.X.; Wang, M.M.; Yin, Y.L.; Onac, E.; Zhou, G.F.; Peng, S.; Xia, X.J.; Shi, K.; Yu, J.Q.; Zhou, Y.H. RNA-seq analysis reveals the role of red light in resistance against Pseudomonas syringae pv. tomato DC3000 in tomato plants. BMC Genom. 2015, 16, 120. [Google Scholar] [CrossRef] [PubMed]
- Cellini, A.; Donati, I.; Farneti, B.; Khomenko, I.; Buriani, G.; Biasioli, F.; Cristescu, S.M.; Spinelli, F. A Breach in Plant Defences: Pseudomonas syringae pv. actinidiae Targets Ethylene Signalling to Overcome Actinidia chinensis Pathogen Responses. Int. J. Mol. Sci. 2021, 22, 4375. [Google Scholar] [CrossRef]
- Kim, K.; Kook, H.; Jang, Y.; Lee, W.; Kamala-Kannan, S.; Chae, J.; Lee, K. The effect of blue-light-emitting diodes on antioxidant properties and resistance to Botrytis cinerea in tomato. J. Plant Pathol. Microbiol. 2013, 4, 10–4172. [Google Scholar]
- Islam, S.Z.; Honda, Y.; Arase, S. Light-induced resistance of broad bean against Botrytis cinerea. J. Phytopathol. 1998, 146, 479–485. [Google Scholar] [CrossRef]
- Oberpichler, I.; Rosen, R.; Rasouly, A.; Vugman, M.; Ron, E.Z.; Lamparter, T. Light affects motility and infectivity of Agrobacterium tumefaciens. Environ. Microbiol. 2008, 10, 2020–2029. [Google Scholar] [CrossRef] [PubMed]
- Gomelsky, M.; Hoff, W.D. Light helps bacteria make important lifestyle decisions. Trends Microbiol. 2011, 19, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Río-Álvarez, I.; Rodríguez-Herva, J.J.; Martínez, P.M.; Gonzalez-Melendi, P.; García-Casado, G.; Rodriguez-Palenzuela, P.; Lopez-Solanilla, E. Light regulates motility, attachment and virulence in the plant pathogen Pseudomonas syringae pv tomato DC3000. Environ. Microbiol. 2013, 16, 2072–2085. [Google Scholar] [CrossRef]
- Santamaría-Hernando, S.; Rodríguez-Herva, J.J.; Martínez-García, P.M.; Río-Álvarez, I.; González-Melendi, P.; Zamorano, J.; Tapia, C.; Rodríguez-Palenzuela, P.; López-Solanilla, E. Pseudomonas syringae pv. tomato exploits light signals to optimize virulence and colonization of leaves. Environ. Microbiol. 2018, 20, 4261–4280. [Google Scholar] [CrossRef]
- Cheng, D.D.; Liu, M.J.; Sun, X.B.; Zhao, M.; Chow, W.S.; Sun, G.Y.; Zhang, Z.S.; Hu, Y.B. Light Suppresses Bacterial Population through the Accumulation of Hydrogen Peroxide in Tobacco Leaves Infected with Pseudomonas syringae pv. tabaci. Front. Plant Sci. 2016, 7, 512. [Google Scholar] [CrossRef]
- Donati, I.; Cellini, A.; Sangiorgio, D.; Vanneste, J.L.; Scortichini, M.; Balestra, G.M.; Spinelli, F. Pseudomonas syringae pv. actinidiae: Ecology, infection dynamics and disease epidemiology. Microb. Ecol. 2020, 80, 81–102. [Google Scholar] [CrossRef] [PubMed]
- Colombi, E.; Straub, C.; Künzel, S.; Templeton, M.D.; McCann, H.C.; Rainey, P.B. Evolution of copper resistance in the kiwifruit pathogen Pseudomonas syringae pv. actinidiae through acquisition of integrative conjugative elements and plasmids. Environ. Microbiol. 2017, 19, 819–832. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Kim, G.H.; Song, Y.R.; Oh, C.S.; Koh, Y.J.; Jung, J.S. Streptomycin Resistant Isolates of Pseudomonas syringae pv. actinidiae in Korea. Res. Plant Dis. 2020, 26, 44–47. [Google Scholar] [CrossRef]
- Huot, B.; Yao, J.; Montgomery, B.L.; He, S.Y. Growth-defense tradeoffs in plants: A balancing act to optimize fitness. Mol. Plant 2014, 7, 1267–1287. [Google Scholar] [CrossRef]
- Berger, S.; Sinha, A.K.; Roitsch, T. Plant physiology meets phytopathology: Plant primary metabolism and plant–pathogen interactions. J. Exp. Bot. 2007, 58, 4019–4026. [Google Scholar] [CrossRef]
- Pérez-Bueno, M.L.; Pineda, M.; Díaz-Casado, E.; Barón, M. Spatial and temporal dynamics of primary and secondary metabolism in Phaseolus vulgaris challenged by Pseudomonas syringae. Physiol. Plant. 2015, 153, 161–174. [Google Scholar] [CrossRef]
- Petriccione, M.; Di Cecco, I.; Salzano, A.M.; Arena, S.; Scaloni, A.; Scortichini, M. A proteomic approach to investigate the Actinidia spp./Pseudomonas syringae pv. actinidiae pathosystem. In I International Symposium on Bacterial Canker of Kiwifruit. Mt Maunganui, New Zealand, 19–22 November 2013. Acta Hortic. 2013, 1095, 95–101. [Google Scholar]
- Michelotti, V.; Lamontanara, A.; Buriani, G.; Orrù, L.; Cellini, A.; Donati, I.; Vanneste, J.L.; Cattivelli, L.; Tacconi, G.; Spinelli, F. Comparative transcriptome analysis of the interaction between Actinidia chinensis var. chinensis and Pseudomonas syringae pv. actinidiae in absence and presence of acibenzolar-S-methyl. BMC Genom. 2018, 19, 585. [Google Scholar] [CrossRef]
- Poorter, H. Interspecific variation in relative growth rate: On ecological causes and physiological consequences. Causes Conseq. Var. Growth Rate Product. High. Plants 1989, 24, 45–68. [Google Scholar]
- Miao, Y.; Chen, Q.; Qu, M.; Gao, L.; Hou, L. Blue light alleviates ‘red light syndrome’by regulating chloroplast ultrastructure, photosynthetic traits and nutrient accumulation in cucumber plants. Sci. Hortic. 2019, 257, 108680. [Google Scholar] [CrossRef]
- Gurrea-Ysasi, G.; Blanca-Gimenez, V.; Fita, I.C.; Fita, A.; Prohens, J.; Rodriguez-Burruezo, A. Spectral comparison of diffuse PAR irradiance under different tree and shrub shading conditions and in cloudy days. J. Photochem. Photobiol. B Biol. 2018, 189, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Pennisi, G.; Orsini, F.; Blasioli, S.; Cellini, A.; Crepaldi, A.; Braschi, I.; Spinelli, F.; Nicola, S.; Fernandez, J.A.; Stanghellini, C.; et al. Resource use efficiency of indoor lettuce (Lactuca sativa L.) cultivation as affected by red: Blue ratio provided by LED lighting. Sci. Rep. 2019, 9, 14127. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Kang, C.; Kaiser, E.; Kuang, Y.; Yang, Q.; Li, T. Red/blue light ratios induce morphology and physiology alterations differently in cucumber and tomato. Sci. Hortic. 2021, 281, 109995. [Google Scholar] [CrossRef]
- Johnson, R.E.; Kong, Y.; Zheng, Y. Elongation growth mediated by blue light varies with light intensities and plant species: A comparison with red light in arugula and mustard seedlings. Environ. Exp. Bot. 2020, 169, 103898. [Google Scholar] [CrossRef]
- Sager, J.C.; Smith, W.O.; Edwards, J.L.; Cyr, K.L. Photosynthetic efficiency and phytochrome photoequilibria determination using spectral data. Trans. ASAE 1988, 31, 1882–1889. [Google Scholar] [CrossRef]
- Whitelam, G.; Halliday, K. Annual Plant Reviews, Light and Plant Development; Blackwell Publishing: Oxford, UK, 2007. [Google Scholar]
- Boardman, N.T. Comparative photosynthesis of sun and shade plants. Annu. Rev. Plant Physiol. 1977, 28, 355–377. [Google Scholar] [CrossRef]
- Middleton, L. Shade-Tolerant flowering plants: Adaptations and horticultural implications. In XX International Eucarpia Symposium, Section Ornamentals, Strategies for New Ornamentals-Part I. Melle, Belgium, 1 July 2001. ISHS Acta Hortic. 2001, 552, 95–102. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence-a practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Hou, H.; Najafpour, M.M.; Moore, G.F.; Allakhverdiev, S.I. Photosynthesis: Structures, Mechanisms and Applications; Springer International Publishing: Cham, Switzerland, 2017. [Google Scholar]
- Tian, Y.; Wang, H.; Zhang, Z.; Zhao, X.; Wang, Y.; Zhang, L. An RNA-seq Analysis Reveals Differential Transcriptional Responses to Different Light Qualities in Leaf Color of Camellia sinensis cv. Huangjinya. J. Plant Growth Regul. 2022, 41, 612–627. [Google Scholar] [CrossRef]
- Kiss, A.Z.; Ruban, A.V.; Horton, P. The PsbS protein controls the organization of the photosystem II antenna in higher plant thylakoid membranes. J. Biol. Chem. 2008, 283, 3972–3978. [Google Scholar] [CrossRef]
- Ruban, A.V. Nonphotochemical chlorophyll fluorescence quenching: Mechanism and effectiveness in protecting plants from photodamage. Plant Physiol. 2016, 170, 1903–1916. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Xu, X.M.; Cui, J. The importance of blue light for leaf area expansion, development of photosynthetic apparatus, and chloroplast ultrastructure of Cucumis sativus grown under weak light. Photosynthetica 2015, 53, 213–222. [Google Scholar] [CrossRef]
- Takemiya, A.; Inoue, S.I.; Doi, M.; Kinoshita, T.; Shimazaki, K.I. Phototropins promote plant growth in response to blue light in low light environments. Plant Cell 2005, 17, 1120–1127. [Google Scholar] [CrossRef] [PubMed]
- Shimazaki, K.I.; Doi, M.; Assmann, S.M.; Kinoshita, T. Light regulation of stomatal movement. Annu. Rev. Plant Biol. 2007, 58, 219–247. [Google Scholar] [CrossRef]
- Roden, L.C.; Ingle, R.A. Lights, rhythms, infection: The role of light and the circadian clock in determining the outcome of plant–pathogen interactions. Plant Cell 2009, 21, 2546–2552. [Google Scholar] [CrossRef]
- Heil, M.; Bostock, R.M. Induced systemic resistance (ISR) against pathogens in the context of induced plant defences. Ann. Bot. 2002, 89, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Berger, S.; Papadopoulos, M.; Schreiber, U.; Kaiser, W.; Roitsch, T. Complex regulation of gene expression, photosynthesis and sugar levels by pathogen infection in tomato. Physiol. Plant. 2004, 122, 419–428. [Google Scholar] [CrossRef]
- Scharte, J.; Schön, H.; Weis, E. Photosynthesis and carbohydrate metabolism in tobacco leaves during an incompatible interaction with Phytophthora nicotianae. Plant Cell Environ. 2005, 28, 1421–1435. [Google Scholar] [CrossRef]
- Swarbrick, P.J.; Schulze-Lefert, P.; Scholes, J.D. Metabolic consequences of susceptibility and resistance in barley leaves challenged with powdery mildew. Plant Cell Environ. 2006, 29, 1061–1076. [Google Scholar] [CrossRef]
- Kopczewski, T.; Kuźniak, E.; Kornaś, A.; Rut, G.; Nosek, M.; Ciereszko, I.; Szczepaniak, L. Local and Systemic Changes in Photosynthetic Parameters and Antioxidant Activity in Cucumber Challenged with Pseudomonas syringae pv lachrymans. Int. J. Mol. Sci. 2020, 21, 6378. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, H.; Xu, Z.; Wang, Y.; Liu, X.; Li, Y.; Tian, B.; Sun, G.; Zhang, H. TMT-based quantitative proteomic analysis of the effects of Pseudomonas syringae pv. tabaci (Pst) infection on photosynthetic function and the response of the MAPK signaling pathway in tobacco leaves. Plant Physiol. Biochem. 2021, 166, 657–667. [Google Scholar] [CrossRef] [PubMed]
- Kook, H.S.; Park, S.H.; Jang, Y.J.; Lee, G.W.; Kim, J.S.; Kim, H.M.; Oh, B.T.; Chae, J.C.; Lee, K.J. Blue LED (light-emitting diodes)-mediated growth promotion and control of Botrytis disease in lettuce. Acta Agric. Scand. Sect. B Soil Plant Sci. 2013, 63, 271–277. [Google Scholar]
- Ahn, S.Y.; Kim, S.A.; Yun, H.K. Inhibition of Botrytis cinerea and accumulation of stilbene compounds by light-emitting diodes of grapevine leaves and differential expression of defense-related genes. Eur. J. Plant Pathol. 2015, 143, 753–765. [Google Scholar] [CrossRef]
- Chen, L.J.; Zhao, F.F.; Zhang, M.; Lin, H.H.; Xi, D.H. Effects of light quality on the interaction between Cucumber Mosaic Virus and Nicotiana tabacum. J. Phytopathol. 2015, 163, 1002–1013. [Google Scholar] [CrossRef]
- Carvalho, S.D.; Castillo, J.A. Influence of light on plant–phyllosphere interaction. Front. Plant Sci. 2018, 9, 1482. [Google Scholar] [CrossRef] [PubMed]
- Nunes da Silva, M.; Carvalho, S.M.; Rodrigues, A.M.; Gómez-Cadenas, A.; António, C.; Vasconcelos, M.W. Defence-related pathways, phytohormones and primary metabolism are key players in kiwifruit plant tolerance to Pseudomonas syringae pv. actinidiae. Plant Cell Environ. 2022, 45, 528–541. [Google Scholar] [CrossRef]
- Melotto, M.; Underwood, W.; Koczan, J.; Nomura, K.; He, S.Y. Plant stomata function in innate immunity against bacterial invasion. Cell 2006, 126, 969–980. [Google Scholar] [CrossRef]
- Tanaka, Y.; Sano, T.; Tamaoki, M.; Nakajima, N.; Kondo, N.; Hasezawa, S. Ethylene inhibits abscisic acid-induced stomatal closure in Arabidopsis. Plant Physiol. 2005, 138, 2337–2343. [Google Scholar] [CrossRef]
- Bonomi, H.R.; Toum, L.; Sycz, G.; Sieira, R.; Toscani, A.M.; Gudesblat, G.E.; Leskow, F.C.; Goldbaum, F.A.; Vojnov, A.A.; Malamud, F. Xanthomonas campestris attenuates virulence by sensing light through a bacteriophytochrome photoreceptor. EMBO Rep. 2016, 17, 1565–1577. [Google Scholar] [CrossRef]
- Wu, L.; McGrane, R.S.; Beattie, G.A. Light regulation of swarming motility in Pseudomonas syringae integrates signaling pathways mediated by a bacteriophytochrome and a LOV protein. MBio 2013, 4, e00334-13. [Google Scholar] [CrossRef]
- Kraiselburd, I.; Alet, A.I.; Tondo, M.L.; Petrocelli, S.; Daurelio, L.D.; Monzon, J.; Ruiz, O.A.; Losi, A.; Orellano, E.G. A LOV protein modulates the physiological attributes of Xanthomonas axonopodis pv. citri relevant for host plant colonization. PLoS ONE 2012, 7, e38226. [Google Scholar]
- Santamaría-Hernando, S.; Cerna-Vargas, J.P.; Martínez-García, P.M.; de Francisco-de Polanco, S.; Nebreda, S.; Rodríguez-Palenzuela, P.; Rodríguez-Herva, J.J.; López-Solanilla, E. Blue-light perception by epiphytic Pseudomonas syringae drives chemoreceptor expression, enabling efficient plant infection. Mol. Plant Pathol. 2020, 21, 1606–1619. [Google Scholar] [CrossRef]
- Verma, R.K.; Biswas, A.; Kakkar, A.; Lomada, S.K.; Pradhan, B.B.; Chatterjee, S. A bacteriophytochrome mediates Interplay between light sensing and the second messenger cyclic Di-GMP to control social behavior and virulence. Cell Rep. 2020, 32, 108202. [Google Scholar] [CrossRef] [PubMed]
- Vandelle, E.; Colombo, T.; Regaiolo, A.; Maurizio, V.; Libardi, T.; Puttilli, M.R.; Danzi, D.; Polverari, A. Transcriptional profiling of three Pseudomonas syringae pv. actinidiae biovars reveals different responses to apoplast-like conditions related to strain virulence on the host. Mol. Plant-Microbe Interact. 2021, 34, 376–396. [Google Scholar] [CrossRef] [PubMed]
- Basile, B.; Giaccone, M.; Cirillo, C.; Ritieni, A.; Graziani, G.; Shahak, Y.; Forlani, M. Photo-selective hail nets affect fruit size and quality in Hayward kiwifruit. Sci. Hortic. 2012, 141, 91–97. [Google Scholar] [CrossRef]
- Cellini, A.; Fiorentini, L.; Buriani, G.; Yu, J.; Donati, I.; Cornish, D.A.; Novak, B.; Costa, G.; Vanneste, J.L.; Spinelli, F. Elicitors of the salicylic acid pathway reduce incidence of bacterial canker of kiwifruit caused by Pseudomonas syringae pv. actinidae. Ann. Appl. Biol. 2014, 165, 441–453. [Google Scholar] [CrossRef]
- Murchie, E.H.; Lawson, T. Chlorophyll fluorescence analysis: A guide to good practice and understanding some new applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef]
- Sims, D.A.; Gamon, J.A. Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. Remote Sens. Environ. 2002, 81, 337–354. [Google Scholar] [CrossRef]
- White, A.J.; Critchley, C. Rapid light curves: A new fluorescence method to assess the state of the photosynthetic apparatus. Photosynth. Res. 1999, 59, 63–72. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Schansker, G.; Ladle, R.J.; Goltsev, V.; Bosa, K.; Allakhverdiev, S.I.; Brestic, M.; Bussotti, F.; Calatayud, A.; Dąbrowski, P.; et al. Frequently asked questions about in vivo chlorophyll fluorescence: Practical issues. Photosynth. Res. 2014, 122, 121–158. [Google Scholar] [CrossRef]
- Machado, F.; Dias, C.; Pinho, P.; Araújo, A.; Pinto, D.; Correia, C.; Santos, C. Photosynthetic performance and volatile organic compounds profile in Eucalyptus globulus after UVB radiation. Environ. Exp. Bot. 2017, 140, 141–149. [Google Scholar] [CrossRef]
- Dias, M.C.; Pinto, M.D.; Correia, C.; Silva AM, S.; Santos, C. UV-B radiation modulates physiology and lipophilic metabolite profile in Olea europaea. J. Plant Physiol. 2018, 222, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Petriccione, M.; Mastrobuoni, F.; Zampella, L.; Scortichini, M. Reference gene selection for normalization of RT-qPCR gene expression data from Actinidia deliciosa leaves infected with Pseudomonas syringae pv. actinidiae. Sci. Rep. 2015, 5, 16961. [Google Scholar] [PubMed]
- Ramakers, C.; Ruijter, J.M.; Deprez RH, L.; Moorman AF, M. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci. Lett. 2003, 339, 62–66. [Google Scholar] [CrossRef]
- Simon, P. Q-Gene: Processing quantitative real-time RT–PCR data. Bioinformatics 2003, 19, 1439–1440. [Google Scholar] [CrossRef]
- Pfaffl, M.W.; Horgan, G.W.; Dempfle, L. Relative expression software tool (REST©) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002, 30, e36. [Google Scholar] [CrossRef]
- Gallelli, A.; Talocci, S.; Pilotti, M.; Loreti, S. Real-time and qualitative PCR for detecting Pseudomonas syringae pv. actinidiae isolates causing recent outbreaks of kiwifruit bacterial canker. Plant Pathol. 2014, 63, 264–276. [Google Scholar] [CrossRef]
- Mercado, J.A.; El Mansouri, I.; Jiménez-Bermúdez, S.; Pliego-Alfaro, F.; Quesada, M.A. A convenient protocol for extraction and purification of DNA from Fragaria. Vitr. Cell. Dev. Biol. -Plant 1999, 35, 152–153. [Google Scholar] [CrossRef]
- Huvenne, H.; Debode, J.; Maes, M.; Heungens, K. Real-time PCR mediated monitoring of Fusarium foetens in symptomatic and non-symptomatic hosts. Eur. J. Plant Pathol. 2011, 131, 705–717. [Google Scholar] [CrossRef]
- Ares, A.; Tacão, M.; Figueira, D.; Garcia, E.; Costa, J. Draft genome resources sequences of six Pseudomonas syringae pv. actinidiae strains isolated from Actinidia chinensis var. deliciosa leaves in Portugal. Phytopathol. 2021, 111, 237–239. [Google Scholar] [CrossRef]
- Howe, E.A.; Sinha, R.; Schlauch, D.; Quackenbush, J. RNA-Seq analysis in MeV. Bioinformatics 2011, 27, 3209–3210. [Google Scholar] [CrossRef] [PubMed]




| Gene | Primer Sequence | |
|---|---|---|
| rubisco | F | ACATGGACAACTGTGTGGAC |
| R | AGTTTCTTCTCCAGCAACGG | |
| psbA | F | ATTCGTGAGCCTGTTTCTGG |
| R | TCATCAACAGATGCCGCTTC | |
| psbC | F | TGGTGGGGAAGTTATAGACACC |
| R | ATACCGCCAAAGCCCAATAC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Correia, C.; Magnani, F.; Pastore, C.; Cellini, A.; Donati, I.; Pennisi, G.; Paucek, I.; Orsini, F.; Vandelle, E.; Santos, C.; et al. Red and Blue Light Differently Influence Actinidia chinensis Performance and Its Interaction with Pseudomonas syringae pv. Actinidiae. Int. J. Mol. Sci. 2022, 23, 13145. https://doi.org/10.3390/ijms232113145
Correia C, Magnani F, Pastore C, Cellini A, Donati I, Pennisi G, Paucek I, Orsini F, Vandelle E, Santos C, et al. Red and Blue Light Differently Influence Actinidia chinensis Performance and Its Interaction with Pseudomonas syringae pv. Actinidiae. International Journal of Molecular Sciences. 2022; 23(21):13145. https://doi.org/10.3390/ijms232113145
Chicago/Turabian StyleCorreia, Cristiana, Federico Magnani, Chiara Pastore, Antonio Cellini, Irene Donati, Giuseppina Pennisi, Ivan Paucek, Francesco Orsini, Elodie Vandelle, Conceição Santos, and et al. 2022. "Red and Blue Light Differently Influence Actinidia chinensis Performance and Its Interaction with Pseudomonas syringae pv. Actinidiae" International Journal of Molecular Sciences 23, no. 21: 13145. https://doi.org/10.3390/ijms232113145
APA StyleCorreia, C., Magnani, F., Pastore, C., Cellini, A., Donati, I., Pennisi, G., Paucek, I., Orsini, F., Vandelle, E., Santos, C., & Spinelli, F. (2022). Red and Blue Light Differently Influence Actinidia chinensis Performance and Its Interaction with Pseudomonas syringae pv. Actinidiae. International Journal of Molecular Sciences, 23(21), 13145. https://doi.org/10.3390/ijms232113145









