Capability of Human Dendritic Cells Pulsed with Autologous Induced Pluripotent Stem Cell Lysate to Induce Cytotoxic T Lymphocytes against HLA-A33-Matched Cancer Cells
Abstract
1. Introduction
2. Results
2.1. Human iPSCs Expressed Tumor-Specific and Tumor-Associated Antigens
2.2. The Established iPSCs-Expressed TAAs
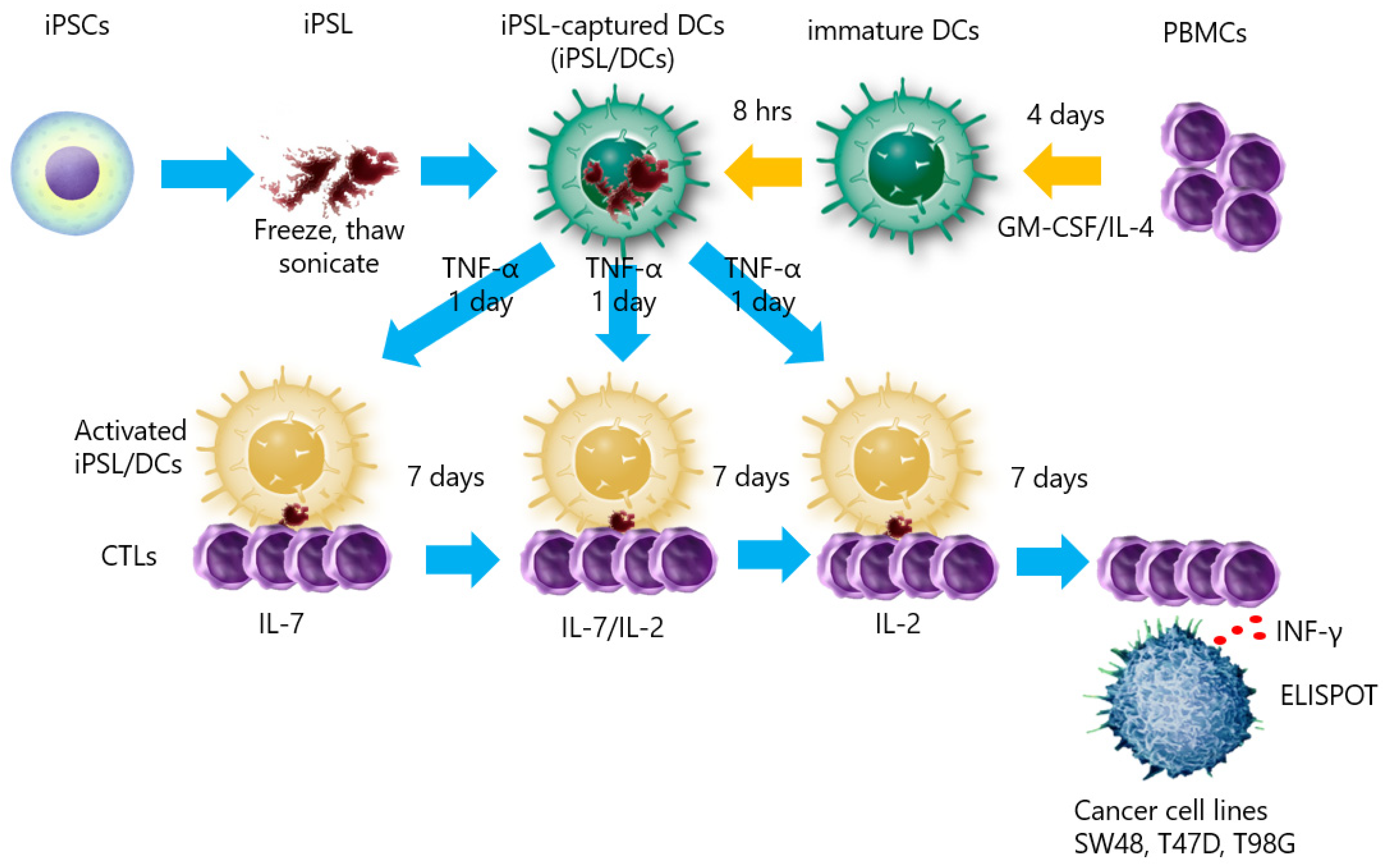
2.3. Autologous iPSL/DCs-Induced Tumor-Cell-responsive CTLs Derived from a HLA-A33 Donor
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Ethics
5.2. Peripheral Blood Mononuclear Cells
5.3. Human Cell Lines
5.4. Generation of iPSCs and Lysate Production
5.4.1. iPSC Generation and Passage
5.4.2. Cellular Characterization of iPSCs
5.4.3. Preparation of iPS Lysate
5.5. Comprehensive Gene Expression Analysis
5.5.1. RNA Extraction and Microarray Gene Expression Assay
5.5.2. Differentiation Ability of iPSCs into Ebs
5.5.3. Comparison of the Expression Patterns of Tumor-associated Genes in iPSCs
5.6. Preparation of iPSL-Loaded DCs and CTL Induction
5.7. ELISPOT Assays
5.8. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, R.F.; Wang, H.Y. Immune targets and neoantigens for cancer immunotherapy and precision medicine. Cell Res. 2017, 27, 11–37. [Google Scholar] [CrossRef]
- Richard, G.; Princiotta, M.F.; Bridon, D.; Martin, W.D.; Steinberg, G.D.; De Groot, A.S. Neoantigen-based personalized cancer vaccines: The emergence of precision cancer immunotherapy. Expert Rev. Vaccines 2022, 21, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, T.N.; Schreiber, R.D. Neoantigens in cancer immunotherapy. Science 2015, 348, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Bobisse, S.; Foukas, P.G.; Coukos, G.; Harari, A. Neoantigen-based cancer immunotherapy. Ann. Transl. Med. 2016, 4, 262. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.G.; Li, F.; Roszik, J.; Lizee, G. Exploiting Tumor Neoantigens to Target Cancer Evolution: Current Challenges and Promising Therapeutic Approaches. Cancer Discov. 2021, 11, 1024–1039. [Google Scholar] [CrossRef]
- Zacharakis, N.; Chinnasamy, H.; Black, M.; Xu, H.; Lu, Y.C.; Zheng, Z.; Pasetto, A.; Langhan, M.; Shelton, T.; Prickett, T.; et al. Immune recognition of somatic mutations leading to complete durable regression in metastatic breast cancer. Nat. Med. 2018, 24, 724–730. [Google Scholar] [CrossRef]
- Chiang, C.L.; Benencia, F.; Coukos, G. Whole tumor antigen vaccines. Semin. Immunol. 2010, 22, 132–143. [Google Scholar] [CrossRef]
- Chiang, C.L.; Coukos, G.; Kandalaft, L.E. Whole Tumor Antigen Vaccines: Where Are We? Vaccines 2015, 3, 344–372. [Google Scholar] [CrossRef]
- Fong, L.; Engleman, E.G. Dendritic cells in cancer immunotherapy. Annu. Rev. Immunol. 2000, 18, 245–273. [Google Scholar] [CrossRef]
- Anguille, S.; Smits, E.L.; Lion, E.; van Tendeloo, V.F.; Berneman, Z.N. Clinical use of dendritic cells for cancer therapy. Lancet Oncol. 2014, 15, e257–e267. [Google Scholar] [CrossRef]
- Ouyang, X.; Telli, M.L.; Wu, J.C. Induced Pluripotent Stem Cell-Based Cancer Vaccines. Front. Immunol. 2019, 10, 1510. [Google Scholar] [CrossRef]
- Ben-David, U.; Benvenisty, N. The tumorigenicity of human embryonic and induced pluripotent stem cells. Nat. Rev. Cancer 2011, 11, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Hiyama, E.; Hiyama, K. Telomere and telomerase in stem cells. Br. J. Cancer 2007, 96, 1020–1024. [Google Scholar] [CrossRef] [PubMed]
- Heisig, J.; Weber, D.; Englberger, E.; Winkler, A.; Kneitz, S.; Sung, W.K.; Wolf, E.; Eilers, M.; Wei, C.L.; Gessler, M. Target gene analysis by microarrays and chromatin immunoprecipitation identifies HEY proteins as highly redundant bHLH repressors. PLoS Genet. 2012, 8, e1002728. [Google Scholar] [CrossRef] [PubMed]
- Evans, P.M.; Liu, C. Roles of Krupel-like factor 4 in normal homeostasis, cancer and stem cells. Acta Biochim. Biophys. Sin. 2008, 40, 554–564. [Google Scholar] [CrossRef]
- Sperger, J.M.; Chen, X.; Draper, J.S.; Antosiewicz, J.E.; Chon, C.H.; Jones, S.B.; Brooks, J.D.; Andrews, P.W.; Brown, P.O.; Thomson, J.A. Gene expression patterns in human embryonic stem cells and human pluripotent germ cell tumors. Proc. Natl. Acad. Sci. USA 2003, 100, 13350–13355. [Google Scholar] [CrossRef]
- Ben-Porath, I.; Thomson, M.W.; Carey, V.J.; Ge, R.; Bell, G.W.; Regev, A.; Weinberg, R.A. An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nat. Genet. 2008, 40, 499–507. [Google Scholar] [CrossRef]
- Neveu, P.; Kye, M.J.; Qi, S.; Buchholz, D.E.; Clegg, D.O.; Sahin, M.; Park, I.H.; Kim, K.S.; Daley, G.Q.; Kornblum, H.I.; et al. MicroRNA profiling reveals two distinct p53-related human pluripotent stem cell states. Cell Stem. Cell 2010, 7, 671–681. [Google Scholar] [CrossRef]
- Calvanese, V.; Horrillo, A.; Hmadcha, A.; Suarez-Alvarez, B.; Fernandez, A.F.; Lara, E.; Casado, S.; Menendez, P.; Bueno, C.; Garcia-Castro, J.; et al. Cancer genes hypermethylated in human embryonic stem cells. PLoS ONE 2008, 3, e3294. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef]
- Bock, C.; Kiskinis, E.; Verstappen, G.; Gu, H.; Boulting, G.; Smith, Z.D.; Ziller, M.; Croft, G.F.; Amoroso, M.W.; Oakley, D.H.; et al. Reference Maps of human ES and iPS cell variation enable high-throughput characterization of pluripotent cell lines. Cell 2011, 144, 439–452. [Google Scholar] [CrossRef]
- Mallon, B.S.; Chenoweth, J.G.; Johnson, K.R.; Hamilton, R.S.; Tesar, P.J.; Yavatkar, A.S.; Tyson, L.J.; Park, K.; Chen, K.G.; Fann, Y.C.; et al. StemCellDB: The human pluripotent stem cell database at the National Institutes of Health. Stem Cell Res. 2013, 10, 57–66. [Google Scholar] [CrossRef]
- Mallon, B.S.; Hamilton, R.S.; Kozhich, O.A.; Johnson, K.R.; Fann, Y.C.; Rao, M.S.; Robey, P.G. Comparison of the molecular profiles of human embryonic and induced pluripotent stem cells of isogenic origin. Stem Cell Res. 2014, 12, 376–386. [Google Scholar] [CrossRef]
- Soldner, F.; Hockemeyer, D.; Beard, C.; Gao, Q.; Bell, G.W.; Cook, E.G.; Hargus, G.; Blak, A.; Cooper, O.; Mitalipova, M.; et al. Parkinson’s disease patient-derived induced pluripotent stem cells free of viral reprogramming factors. Cell 2009, 136, 964–977. [Google Scholar] [CrossRef]
- Kooreman, N.G.; Kim, Y.; de Almeida, P.E.; Termglinchan, V.; Diecke, S.; Shao, N.Y.; Wei, T.T.; Yi, H.; Dey, D.; Nelakanti, R.; et al. Autologous iPSC-Based Vaccines Elicit Anti-tumor Responses In Vivo. Cell Stem Cell 2018, 22, 501–513.e507. [Google Scholar] [CrossRef]
- Albihn, A.; Johnsen, J.I.; Henriksson, M.A. MYC in oncogenesis and as a target for cancer therapies. Adv. Cancer Res. 2010, 107, 163–224. [Google Scholar] [CrossRef]
- Tian, Y.; Luo, A.; Cai, Y.; Su, Q.; Ding, F.; Chen, H.; Liu, Z. MicroRNA-10b promotes migration and invasion through KLF4 in human esophageal cancer cell lines. J. Biol. Chem. 2010, 285, 7986–7994. [Google Scholar] [CrossRef]
- Lambertini, C.; Pantano, S.; Dotto, G.P. Differential control of Notch1 gene transcription by Klf4 and Sp3 transcription factors in normal versus cancer-derived keratinocytes. PLoS ONE 2010, 5, e10369. [Google Scholar] [CrossRef]
- Rageul, J.; Mottier, S.; Jarry, A.; Shah, Y.; Theoleyre, S.; Masson, D.; Gonzalez, F.J.; Laboisse, C.L.; Denis, M.G. KLF4-dependent, PPARgamma-induced expression of GPA33 in colon cancer cell lines. Int. J. Cancer 2009, 125, 2802–2809. [Google Scholar] [CrossRef] [PubMed]
- Asadi, M.H.; Mowla, S.J.; Fathi, F.; Aleyasin, A.; Asadzadeh, J.; Atlasi, Y. OCT4B1, a novel spliced variant of OCT4, is highly expressed in gastric cancer and acts as an antiapoptotic factor. Int. J. Cancer 2011, 128, 2645–2652. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Zheng, P.S. Expression of Sox2 in human cervical carcinogenesis. Hum. Pathol. 2010, 41, 1438–1447. [Google Scholar] [CrossRef] [PubMed]
- Sholl, L.M.; Barletta, J.A.; Yeap, B.Y.; Chirieac, L.R.; Hornick, J.L. Sox2 protein expression is an independent poor prognostic indicator in stage I lung adenocarcinoma. Am. J. Surg. Pathol. 2010, 34, 1193–1198. [Google Scholar] [CrossRef] [PubMed]
- Schoenhals, M.; Kassambara, A.; De Vos, J.; Hose, D.; Moreaux, J.; Klein, B. Embryonic stem cell markers expression in cancers. Biochem. Biophys. Res. Commun. 2009, 383, 157–162. [Google Scholar] [CrossRef]
- Ouyang, X.; Liu, Y.; Zhou, Y.; Guo, J.; Wei, T.T.; Liu, C.; Lee, B.; Chen, B.; Zhang, A.; Casey, K.M.; et al. Antitumor effects of iPSC-based cancer vaccine in pancreatic cancer. Stem. Cell Rep. 2021, 16, 1468–1477. [Google Scholar] [CrossRef]
- International Stem Cell, I.; Adewumi, O.; Aflatoonian, B.; Ahrlund-Richter, L.; Amit, M.; Andrews, P.W.; Beighton, G.; Bello, P.A.; Benvenisty, N.; Berry, L.S.; et al. Characterization of human embryonic stem cell lines by the International Stem Cell Initiative. Nat. Biotechnol. 2007, 25, 803–816. [Google Scholar] [CrossRef]
- Itskovitz-Eldor, J.; Schuldiner, M.; Karsenti, D.; Eden, A.; Yanuka, O.; Amit, M.; Soreq, H.; Benvenisty, N. Differentiation of human embryonic stem cells into embryoid bodies compromising the three embryonic germ layers. Mol. Med. 2000, 6, 88–95. [Google Scholar] [CrossRef]
- Cheever, M.A.; Allison, J.P.; Ferris, A.S.; Finn, O.J.; Hastings, B.M.; Hecht, T.T.; Mellman, I.; Prindiville, S.A.; Viner, J.L.; Weiner, L.M.; et al. The prioritization of cancer antigens: A national cancer institute pilot project for the acceleration of translational research. Clin. Cancer Res. 2009, 15, 5323–5337. [Google Scholar] [CrossRef]
- Andree, H.A.; Reutelingsperger, C.P.; Hauptmann, R.; Hemker, H.C.; Hermens, W.T.; Willems, G.M. Binding of vascular anticoagulant alpha (VAC alpha) to planar phospholipid bilayers. J. Biol. Chem. 1990, 265, 4923–4928. [Google Scholar] [CrossRef]
- Wang, J.; Shao, L.; Wu, L.; Ma, W.; Zheng, Y.; Hu, C.; Li, F. Expression levels of a gene signature in hiPSC associated with lung adenocarcinoma stem cells and its capability in eliciting specific antitumor immune-response in a humanized mice model. Thorac. Cancer 2020, 11, 1603–1612. [Google Scholar] [CrossRef]
- Yaddanapudi, K.; Mitchell, R.A.; Putty, K.; Willer, S.; Sharma, R.K.; Yan, J.; Bodduluri, H.; Eaton, J.W. Vaccination with embryonic stem cells protects against lung cancer: Is a broad-spectrum prophylactic vaccine against cancer possible? PLoS ONE 2012, 7, e42289. [Google Scholar] [CrossRef]
- Gabka-Buszek, A.; Kwiatkowska-Borowczyk, E.; Jankowski, J.; Kozlowska, A.K.; Mackiewicz, A. Novel Genetic Melanoma Vaccines Based on Induced Pluripotent Stem Cells or Melanosphere-Derived Stem-Like Cells Display High Efficacy in a Murine Tumor Rejection Model. Vaccines 2020, 8, 147. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Chen, X.H.; Chang, X.H.; Ye, X.; Li, Y.; Cui, H. Human embryonic stem cells—A potential vaccine for ovarian cancer. Asian Pac. J. Cancer Prev. 2012, 13, 4295–4300. [Google Scholar] [CrossRef]
- Dong, W.; Du, J.; Shen, H.; Gao, D.; Li, Z.; Wang, G.; Mu, X.; Liu, Q. Administration of embryonic stem cells generates effective antitumor immunity in mice with minor and heavy tumor load. Cancer Immunol. Immunother. 2010, 59, 1697–1705. [Google Scholar] [CrossRef]
- Hu, J.L.; Omofoye, O.A.; Rudnick, J.D.; Kim, S.; Tighiouart, M.; Phuphanich, S.; Wang, H.; Mazer, M.; Ganaway, T.; Chu, R.M.; et al. A Phase I Study of Autologous Dendritic Cell Vaccine Pulsed with Allogeneic Stem-like Cell Line Lysate in Patients with Newly Diagnosed or Recurrent Glioblastoma. Clin. Cancer Res. 2022, 28, 689–696. [Google Scholar] [CrossRef]
- Aerts, J.; de Goeje, P.L.; Cornelissen, R.; Kaijen-Lambers, M.E.H.; Bezemer, K.; van der Leest, C.H.; Mahaweni, N.M.; Kunert, A.; Eskens, F.; Waasdorp, C.; et al. Autologous Dendritic Cells Pulsed with Allogeneic Tumor Cell Lysate in Mesothelioma: From Mouse to Human. Clin. Cancer Res. 2018, 24, 766–776. [Google Scholar] [CrossRef]
- Lowenfels, A.B.; Maisonneuve, P.; DiMagno, E.P.; Elitsur, Y.; Gates, L.K., Jr.; Perrault, J.; Whitcomb, D.C. Hereditary pancreatitis and the risk of pancreatic cancer. International Hereditary Pancreatitis Study Group. J. Natl. Cancer Inst. 1997, 89, 442–446. [Google Scholar] [CrossRef]
- Weiss, F.U. Pancreatic cancer risk in hereditary pancreatitis. Front. Physiol. 2014, 5, 70. [Google Scholar] [CrossRef]
- Zhao, S.; Chen, L.; Zang, Y.; Liu, W.; Liu, S.; Teng, F.; Xue, F.; Wang, Y. Endometrial cancer in Lynch syndrome. Int. J. Cancer 2022, 150, 7–17. [Google Scholar] [CrossRef]
- Pantziarka, P.; Blagden, S. Inhibiting the Priming for Cancer in Li-Fraumeni Syndrome. Cancers 2022, 14, 1621. [Google Scholar] [CrossRef] [PubMed]
- Kisielow, P. How does the immune system learn to distinguish between good and evil? The first definitive studies of T cell central tolerance and positive selection. Immunogenetics 2019, 71, 513–518. [Google Scholar] [CrossRef] [PubMed]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakazawa, T.; Maeoka, R.; Morimoto, T.; Matsuda, R.; Nakamura, M.; Nishimura, F.; Yamada, S.; Nakagawa, I.; Park, Y.-S.; Nakase, H.; et al. Capability of Human Dendritic Cells Pulsed with Autologous Induced Pluripotent Stem Cell Lysate to Induce Cytotoxic T Lymphocytes against HLA-A33-Matched Cancer Cells. Int. J. Mol. Sci. 2022, 23, 12992. https://doi.org/10.3390/ijms232112992
Nakazawa T, Maeoka R, Morimoto T, Matsuda R, Nakamura M, Nishimura F, Yamada S, Nakagawa I, Park Y-S, Nakase H, et al. Capability of Human Dendritic Cells Pulsed with Autologous Induced Pluripotent Stem Cell Lysate to Induce Cytotoxic T Lymphocytes against HLA-A33-Matched Cancer Cells. International Journal of Molecular Sciences. 2022; 23(21):12992. https://doi.org/10.3390/ijms232112992
Chicago/Turabian StyleNakazawa, Tsutomu, Ryosuke Maeoka, Takayuki Morimoto, Ryosuke Matsuda, Mitsutoshi Nakamura, Fumihiko Nishimura, Shuichi Yamada, Ichiro Nakagawa, Young-Soo Park, Hiroyuki Nakase, and et al. 2022. "Capability of Human Dendritic Cells Pulsed with Autologous Induced Pluripotent Stem Cell Lysate to Induce Cytotoxic T Lymphocytes against HLA-A33-Matched Cancer Cells" International Journal of Molecular Sciences 23, no. 21: 12992. https://doi.org/10.3390/ijms232112992
APA StyleNakazawa, T., Maeoka, R., Morimoto, T., Matsuda, R., Nakamura, M., Nishimura, F., Yamada, S., Nakagawa, I., Park, Y.-S., Nakase, H., & Tsujimura, T. (2022). Capability of Human Dendritic Cells Pulsed with Autologous Induced Pluripotent Stem Cell Lysate to Induce Cytotoxic T Lymphocytes against HLA-A33-Matched Cancer Cells. International Journal of Molecular Sciences, 23(21), 12992. https://doi.org/10.3390/ijms232112992







