Molecular Docking and Intracellular Translocation of Extracellular Vesicles for Efficient Drug Delivery
Abstract
1. Introduction
2. EVs in Cancer Metastasis and Malignant Transformation
3. EV-Mediated Immune Escape of Cancer Cells
4. Intracellular Translocation of Nucleic Acids and Proteins via EVs
5. EV Uptake in Target Cells via Macropinocytosis
6. Techniques for Functional Peptide Modifications on the Exosomal Membrane
7. Storages of EVs as a Long-Term Strategy
8. Clinical Application
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Matsuzaka, Y.; Yashiro, R. Extracellular Vesicles as Novel Drug-Delivery Systems through Intracellular Communications. Membranes 2022, 12, 550. [Google Scholar] [CrossRef] [PubMed]
- Giovannelli, P.; Di Donato, M.; Galasso, G.; Monaco, A.; Licitra, F.; Perillo, B.; Migliaccio, A.; Castoria, G. Communication between cells: Exosomes as a delivery system in prostate cancer. Cell Commun. Signal. 2021, 19, 110. [Google Scholar] [CrossRef]
- Choi, H.; Choi, Y.; Yim, H.Y.; Mirzaaghasi, A.; Yoo, J.K.; Choi, C. Biodistribution of Exosomes and Engineering Strategies for Targeted Delivery of Therapeutic Exosomes. Tissue Eng. Regen. Med. 2021, 18, 499–511. [Google Scholar] [CrossRef]
- Gaurav, I.; Thakur, A.; Iyaswamy, A.; Wang, X.; Chen, X.; Yang, Z. Factors Affecting Extracellular Vesicles Based Drug Delivery Systems. Molecules 2021, 26, 1544. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Zhou, Y.; Jia, C.; Chao, Z.; Qin, H.; Liang, J.; Liu, X.; Liu, Z.; Sun, T.; Yuan, Y.; et al. Caspase-1-mediated extracellular vesicles derived from pyroptotic alveolar macrophages promote inflammation in acute lung injury. Int. J. Biol. Sci. 2022, 18, 1521–1538. [Google Scholar] [CrossRef] [PubMed]
- Tayebi, M.; Yang, D.; Collins, D.J.; Ai, Y. Deterministic Sorting of Submicrometer Particles and Extracellular Vesicles Using a Combined Electric and Acoustic Field. Nano Lett. 2021, 21, 6835–6842. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, T.; Oyama, T.; Hu, H.T.; Fujioka, T.; Hanawa-Suetsugu, K.; Ikeda, K.; Yamada, S.; Kawana, H.; Saigusa, D.; Ikeda, H.K.; et al. Filopodium-derived vesicles produced by MIM enhance the migration of recipient cells. Dev. Cell 2021, 56, 842–859.e8. [Google Scholar] [CrossRef]
- Liu, T.; Hooda, J.; Atkinson, J.M.; Whiteside, T.L.; Oesterreich, S.; Lee, A.V. Exosomes in Breast Cancer—Mechanisms of Action and Clinical Potential. Mol. Cancer Res. 2021, 19, 935–945. [Google Scholar] [CrossRef] [PubMed]
- Lazar, S.; Goldfinger, L.E. Platelets and extracellular vesicles and their cross talk with cancer. Blood 2021, 137, 3192–3200. [Google Scholar] [CrossRef]
- Menck, K.; Sivaloganathan, S.; Bleckmann, A.; Binder, C. Microvesicles in Cancer: Small Size, Large Potential. Int. J. Mol Sci. 2020, 21, 5373. [Google Scholar] [CrossRef]
- Wang, C.; Liu, J.; Yan, Y.; Tan, Y. Role of Exosomes in Chronic Liver Disease Development and Their Potential Clinical Applications. J. Immunol. Res. 2022, 2022, 1695802. [Google Scholar] [CrossRef] [PubMed]
- Shang, X.; Fang, Y.; Xin, W.; You, H. The Application of Extracellular Vesicles Mediated miRNAs in Osteoarthritis: Current Knowledge and Perspective. J. Inflamm. Res. 2022, 15, 2583–2599. [Google Scholar] [CrossRef]
- Fu, Y.; Sui, B.; Xiang, L.; Yan, X.; Wu, D.; Shi, S.; Hu, X. Emerging understanding of apoptosis in mediating mesenchymal stem cell therapy. Cell Death Dis. 2021, 12, 596. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Song, J.; Huang, X.; Pan, Z.; Goldbrunner, R.; Stavrinou, L.; Lin, S.; Hu, W.; Zheng, F.; Stavrinou, P. Exosomes Derived from Mesenchymal Stem Cells: Novel Effects in the Treatment of Ischemic Stroke. Front. Neurosci. 2022, 16, 899887. [Google Scholar] [CrossRef]
- Xu, Y.; Hu, Y.; Xu, S.; Liu, F.; Gao, Y. Exosomal microRNAs as Potential Biomarkers and Therapeutic Agents for Acute Ischemic Stroke: New Expectations. Front. Neurol. 2022, 12, 747380. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, J.P.; Schulz, A.; Morrison, H. The role of exosomes in intercellular and inter-organ communication of the peripheral nervous system. FEBS Lett. 2022, 596, 655–664. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, Y.; Yin, Y.; Jia, X.; Mao, L. Mechanism of cargo sorting into small extracellular vesicles. Bioengineered 2021, 12, 8186–8201. [Google Scholar] [CrossRef] [PubMed]
- Waldenmaier, M.; Seibold, T.; Seufferlein, T.; Eiseler, T. Pancreatic Cancer Small Extracellular Vesicles (Exosomes): A Tale of Short- and Long-Distance Communication. Cancers 2021, 13, 4844. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Verma, H.; Dhiman, M.; Tell, G.; Gigli, G.L.; Janes, F.; Mantha, A.K. Brain Exosomes: Friend or Foe in Alzheimer’s Disease? Mol. Neurobiol. 2021, 58, 6610–6624. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ren, L.; Li, S.; Li, W.; Zheng, X.; Yang, Y.; Fu, W.; Yi, J.; Wang, J.; Du, G. The biology, function, and applications of exosomes in cancer. Acta Pharm. Sin. B 2021, 11, 2783–2797. [Google Scholar] [CrossRef]
- Yang, Y.; Yuan, L.; Du, X.; Zhou, K.; Qin, L.; Wang, L.; Yang, M.; Wu, M.; Zheng, Z.; Xiang, Y.; et al. Involvement of epithelia-derived exosomes in chronic respiratory diseases. Biomed. Pharmacother 2021, 143, 112189. [Google Scholar] [CrossRef]
- U Stotz, H.; Brotherton, D.; Inal, J. Communication is key: Extracellular vesicles as mediators of infection and defence during host-microbe interactions in animals and plants. FEMS Microbiol. Rev. 2022, 46, fuab044. [Google Scholar] [CrossRef]
- Hade, M.D.; Suire, C.N.; Suo, Z. Mesenchymal Stem Cell-Derived Exosomes: Applications in Regenerative Medicine. Cells 2021, 10, 1959. [Google Scholar] [CrossRef] [PubMed]
- Thej, C.; Kishore, R. Unfathomed Nanomessages to the Heart: Translational Implications of Stem Cell-Derived, Progenitor Cell Exosomes in Cardiac Repair and Regeneration. Cells 2021, 10, 1811. [Google Scholar] [CrossRef]
- Whiteside, T.L.; Diergaarde, B.; Hong, C.S. Tumor-Derived Exosomes (TEX) and Their Role in Immuno-Oncology. Int. J. Mol. Sci. 2021, 22, 6234. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Kang, M.H.; Kim, J.H. A Comprehensive Review on Factors Influences Biogenesis, Functions, Therapeutic and Clinical Implications of Exosomes. Int. J. Nanomed. 2021, 16, 1281–1312. [Google Scholar] [CrossRef]
- Rezaie, J.; Aslan, C.; Ahmadi, M.; Zolbanin, N.M.; Kashanchi, F.; Jafari, R. The versatile role of exosomes in human retroviral infections: From immunopathogenesis to clinical application. Cell Biosci. 2021, 11, 19. [Google Scholar] [CrossRef] [PubMed]
- Koohsarian, P.; Talebi, A.; Rahnama, M.A.; Zomorrod, M.S.; Kaviani, S.; Jalili, A. Reviewing the role of cardiac exosomes in myocardial repair at a glance. Cell Biol. Int. 2021, 45, 1352–1363. [Google Scholar] [CrossRef] [PubMed]
- Murugesan, S.; Saravanakumar, L.; Powell, M.F.; Rajasekaran, N.S.; Kannappan, R.; Berkowitz, D.E. Role of exosomal microRNA signatures: An emerging factor in preeclampsia-mediated cardiovascular disease. Placenta 2021, 103, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Kita, S.; Shimomura, I. Stimulation of exosome biogenesis by adiponectin, a circulating factor secreted from adipocytes. J. Biochem. 2021, 169, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Jayaseelan, V.P. Emerging role of exosomes as promising diagnostic tool for cancer. Cancer Gene Ther. 2020, 27, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Hesari, A.; Golrokh Moghadam, S.A.; Siasi, A.; Rahmani, M.; Behboodi, N.; Rastgar-Moghadam, A.; Ferns, G.A.; Ghasemi, F.; Avan, A. Tumor-derived exosomes: Potential biomarker or therapeutic target in breast cancer? J. Cell Biochem. 2018, 119, 4236–4240. [Google Scholar] [CrossRef] [PubMed]
- Li, S.R.; Man, Q.W.; Gao, X.; Lin, H.; Wang, J.; Su, F.C.; Wang, H.Q.; Bu, L.L.; Liu, B.; Chen, G. Tissue-derived extracellular vesicles in cancers and non-cancer diseases: Present and future. J. Extracell. Vesicles 2021, 10, e12175. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Wu, P.; Li, L.; Xu, W.; Qian, H. Exosomes: Emerging Therapy Delivery Tools and Biomarkers for Kidney Diseases. Stem Cells Int. 2021, 2021, 7844455. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, J.; Li, X.; Shu, K. Cell-Derived Exosomes as Therapeutic Strategies and Exosome-Derived microRNAs as Biomarkers for Traumatic Brain Injury. J. Clin. Med. 2022, 11, 3223. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.M.; Amin Mahdian, S.M.; Ebrahimi, M.S.; Taghizadieh, M.; Vosough, M.; Sadri Nahand, J.; Hosseindoost, S.; Vousooghi, N.; Javar, H.A.; Larijani, B.; et al. Microfluidics for detection of exosomes and microRNAs in cancer: State of the art. Mol. Ther. Nucleic Acids 2022, 28, 758–791. [Google Scholar] [CrossRef]
- Sun, H.; Sun, R.; Song, X.; Gu, W.; Shao, Y. Mechanism and clinical value of exosomes and exosomal contents in regulating solid tumor radiosensitivity. J. Transl. Med. 2022, 20, 189. [Google Scholar] [CrossRef]
- Suire, C.N.; Hade, M.D. Extracellular Vesicles in Type 1 Diabetes: A Versatile Tool. Bioengineering 2022, 9, 105. [Google Scholar] [CrossRef]
- Nikdoust, F.; Pazoki, M.; Mohammadtaghizadeh, M.; Aghaali, M.K.; Amrovani, M. Exosomes: Potential Player in Endothelial Dysfunction in Cardiovascular Disease. Cardiovasc. Toxicol. 2022, 22, 225–235. [Google Scholar] [CrossRef]
- Feng, Z.Y.; Zhang, Q.Y.; Tan, J.; Xie, H.Q. Techniques for increasing the yield of stem cell-derived exosomes: What factors may be involved? Sci. China Life Sci. 2022, 65, 1325–1341. [Google Scholar] [CrossRef] [PubMed]
- Rajool Dezfuly, A.; Safaee, A.; Salehi, H. Therapeutic effects of mesenchymal stem cells-derived extracellular vesicles’ miRNAs on retinal regeneration: A review. Stem Cell Res. Ther. 2021, 12, 530. [Google Scholar] [CrossRef] [PubMed]
- Aoi, W.; Tanimura, Y. Roles of Skeletal Muscle-Derived Exosomes in Organ Metabolic and Immunological Communication. Front. Endocrinol 2021, 12, 697204. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, M.G.; Lee, S.H.; Abdelazim, A.M.; Saadeldin, I.M.; Abomughaid, M.M. Role of Extracellular Vesicles in Compromising Cellular Resilience to Environmental Stressors. Biomed. Res. Int. 2021, 2021, 9912281. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, R.; Zamani, F.; Hajibaba, M.; Rasouli-Saravani, A.; Noroozbeygi, M.; Gorgani, M.; Hosseini-Fard, S.R.; Jalalifar, S.; Ajdarkosh, H.; Abedi, S.H.; et al. The pathogenic, therapeutic and diagnostic role of exosomal microRNA in the autoimmune diseases. J. Neuroimmunol. 2021, 358, 577640. [Google Scholar] [CrossRef]
- Bahrami, A.; Binabajm, M.M.; Ferns, A.G. Exosomes: Emerging modulators of signal transduction in colorectal cancer from molecular understanding to clinical application. Biomed. Pharmacother. 2021, 141, 111882. [Google Scholar] [CrossRef] [PubMed]
- Liew, F.F.; Chew, B.C.; Ooi, J. Wound Haling Properties of Exosomes—A Review and Modelling of Combinatorial Analysis Strategies. Curr. Mol. Med. 2022, 22, 165–191. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M. Extracellular vesicles: Their emerging roles in the pathogenesis of respiratory diseases. Respir. Investig. 2021, 59, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Kanada, M.; Ye, J.; Deng, Y.; He, Q.; Lei, Z.; Chen, Y.; Li, Y.; Qin, P.; Zhang, J.; et al. Exosome-mediated remodeling of the tumor microenvironment: From local to distant intercellular communication. Cancer Lett. 2022, 543, 215796. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Chen, H.; Guo, Z.; Shen, J.; Luo, W.; Xie, F.; Wan, Y.; Wang, S.; Li, J.; He, J. Circular RNAs and Their Role in Exosomes. Front. Oncol. 2022, 12, 848341. [Google Scholar] [CrossRef]
- Thakur, A.; Johnson, A.; Jacobs, E.; Zhang, K.; Chen, J.; Wei, Z.; Lian, Q.; Chen, H.J. Energy Sources for Exosome Communication in a Cancer Microenvironment. Cancers 2022, 14, 1698. [Google Scholar] [CrossRef] [PubMed]
- Hsu, M.T.; Wang, Y.K.; Tseng, Y.J. Exosomal Proteins and Lipids as Potential Biomarkers for Lung Cancer Diagnosis, Prognosis, and Treatment. Cancers 2022, 14, 732. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, A.; Butler, A.E.; Jamialahmadi, T.; Sahebkar, A. The role of exosomal miRNA in nonalcoholic fatty liver disease. J. Cell Physiol. 2022, 237, 2078–2094. [Google Scholar] [CrossRef] [PubMed]
- Yun, B.D.; Choi, Y.J.; Son, S.W.; Cipolla, G.A.; Berti, F.C.B.; Malheiros, D.; Oh, T.J.; Kuh, H.J.; Choi, S.Y.; Park, J.K. Oncogenic Role of Exosomal Circular and Long Noncoding RNAs in Gastrointestinal Cancers. Int. J. Mol. Sci. 2022, 23, 930. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xing, J.; Dai, Z.; Wang, D.; Tang, D. Exosomes: The key of sophisticated cell-cell communication and targeted metastasis in pancreatic cancer. Cell Commun. Signal 2022, 20, 9. [Google Scholar] [CrossRef] [PubMed]
- Rizk, N.I.; Abulsoud, A.I.; Kamal, M.M.; Kassem, D.H.; Hamdy, N.M. Exosomal-long non-coding RNAs journey in colorectal cancer: Evil and goodness faces of key players. Life Sci. 2022, 292, 120325. [Google Scholar] [CrossRef]
- Cione, E.; Cannataro, R.; Gallelli, L.; De Sarro, G.; Caroleo, M.C. Exosome microRNAs in Metabolic Syndrome as Tools for the Early Monitoring of Diabetes and Possible Therapeutic Options. Pharmaceuticals 2021, 14, 1257. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, N. Anti-Cancer Role and Therapeutic Potential of Extracellular Vesicles. Cancers 2021, 13, 6303. [Google Scholar] [CrossRef]
- Teles, R.H.G.; Yano, R.S.; Villarinho, N.J.; Yamagata, A.S.; Jaeger, R.G.; Meybohm, P.; Burek, M.; Freitas, V.M. Advances in Breast Cancer Management and Extracellular Vesicle Research, a Bibliometric Analysis. Curr. Oncol. 2021, 28, 4504–4520. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Peng, H.; Liu, Y. The Roles of Exosomes in Immunoregulation and Autoimmune Thyroid Diseases. Front. Immunol. 2021, 12, 757674. [Google Scholar] [CrossRef]
- Khodamoradi, K.; Golan, R.; Dullea, A.; Ramasamy, R. Exosomes as Potential Biomarkers for Erectile Dysfunction, Varicocele, and Testicular Injury. Sex Med. Rev. 2022, 10, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, Q.; Yang, Y.; Zhou, S.; Zhang, P.; Feng, T. The role of exosomal lncRNAs in cancer biology and clinical management. Exp. Mol. Med. 2021, 53, 1669–1673. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; He, R.; He, B.; Xu, L.; Zhang, S. Potential Roles of Exosomal lncRNAs in the Intestinal Mucosal Immune Barrier. J. Immunol. Res. 2021, 2021, 7183136. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Xie, X. Association of Exosomal miR-210 with Signaling Pathways Implicated in Lung Cancer. Genes 2021, 12, 1248. [Google Scholar] [CrossRef] [PubMed]
- Heydari, R.; Abdollahpour-Alitappeh, M.; Shekari, F.; Meyfour, A. Emerging Role of Extracellular Vesicles in Biomarking the Gastrointestinal Diseases. Expert. Rev. Mol. Diagn. 2021, 21, 939–962. [Google Scholar] [CrossRef] [PubMed]
- Ghafouri-Fard, S.; Niazi, V.; Hussen, B.M.; Omrani, M.D.; Taheri, M.; Basiri, A. The Emerging Role of Exosomes in the Treatment of Human Disorders with a Special Focus on Mesenchymal Stem Cells-Derived Exosomes. Front. Cell Dev. Biol. 2021, 9, 653296. [Google Scholar] [CrossRef]
- Cricrì, G.; Bellucci, L.; Montini, G.; Collino, F. Urinary Extracellular Vesicles: Uncovering the Basis of the Pathological Processes in Kidney-Related Diseases. Int. J. Mol. Sci. 2021, 22, 6507. [Google Scholar] [CrossRef]
- Alhamwe, A.B.; Potaczek, D.P.; Miethe, S.; Alhamdan, F.; Hintz, L.; Magomedov, A.; Garn, H. Extracellular Vesicles and Asthma-More Than Just a Co-Existence. Int. J. Mol. Sci. 2021, 22, 4984. [Google Scholar] [CrossRef]
- Gysler, S.M.; Drapkin, R. Tumor innervation: Peripheral nerves take control of the tumor microenvironment. J. Clin. Investig. 2021, 131, e147276. [Google Scholar] [CrossRef]
- Qiu, Y.; Li, P.; Zhang, Z.; Wu, M. Insights into Exosomal Non-Coding RNAs Sorting Mechanism and Clinical Application. Front. Oncol. 2021, 11, 664904. [Google Scholar] [CrossRef]
- Albacete-Albacete, L.; Sánchez-Álvarez, M.; Del Pozo, M.A. Extracellular Vesicles: An Emerging Mechanism Governing the Secretion and Biological Roles of Tenascin-C. Front. Immunol. 2021, 12, 671485. [Google Scholar] [CrossRef]
- Wu, H.; Fu, M.; Liu, J.; Chong, W.; Fang, Z.; Du, F.; Liu, Y.; Shang, L.; Li, L. The role and application of small extracellular vesicles in gastric cancer. Mol. Cancer 2021, 20, 71. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.F.; Pi, J.; Xu, J.F. Emerging Role of Exosomes in Tuberculosis: From Immunity Regulations to Vaccine and Immunotherapy. Front. Immunol. 2021, 12, 628973. [Google Scholar] [CrossRef] [PubMed]
- Perocheau, D.; Touramanidou, L.; Gurung, S.; Gissen, P.; Baruteau, J. Clinical applications for exosomes: Are we there yet? Br. J. Pharmacol. 2021, 178, 2375–2392. [Google Scholar] [CrossRef] [PubMed]
- Jafari, A.; Babajani, A.; Abdollahpour-Alitappeh, M.; Ahmadi, N.; Rezaei-Tavirani, M. Exosomes and cancer: From molecular mechanisms to clinical applications. Med. Oncol. 2021, 38, 45. [Google Scholar] [CrossRef]
- Casari, I.; Howard, J.A.; Robless, E.E.; Falasca, M. Exosomal integrins and their influence on pancreatic cancer progression and metastasis. Cancer Lett. 2021, 507, 124–134. [Google Scholar] [CrossRef]
- Lizarraga-Valderrama, L.R.; Sheridan, G.K. Extracellular vesicles and intercellular communication in the central nervous system. FEBS Lett. 2021, 595, 1391–1410. [Google Scholar] [CrossRef]
- Esfandyari, S.; Elkafas, H.; Chugh, R.M.; Park, H.S.; Navarro, A.; Al-Hendy, A. Exosomes as Biomarkers for Female Reproductive Diseases Diagnosis and Therapy. Int. J. Mol. Sci. 2021, 22, 2165. [Google Scholar] [CrossRef]
- Saludas, L.; Oliveira, C.C.; Roncal, C.; Ruiz-Villalba, A.; Prósper, F.; Garbayo, E.; Blanco-Prieto, M.J. Extracellular Vesicle-Based Therapeutics for Heart Repair. Nanomaterials 2021, 11, 570. [Google Scholar] [CrossRef]
- Wei, X.; Shi, Y.; Dai, Z.; Wang, P.; Meng, X.; Yin, B. Underlying metastasis mechanism and clinical application of exosomal circular RNA in tumors (Review). Int. J. Oncol. 2021, 58, 289–297. [Google Scholar] [CrossRef]
- Makarova, J.; Turchinovich, A.; Shkurnikov, M.; Tonevitsky, A. Extracellular miRNAs and Cell-Cell Communication: Problems and Prospects. Trends Biochem. Sci. 2021, 46, 640–651. [Google Scholar] [CrossRef]
- Amintas, S.; Vendrely, V.; Dupin, C.; Buscail, L.; Laurent, C.; Bournet, B.; Merlio, J.P.; Bedel, A.; Moreau-Gaudry, F.; Boutin, J.; et al. Next-Generation Cancer Biomarkers: Extracellular Vesicle DNA as a Circulating Surrogate of Tumor DNA. Front. Cell Dev. Biol. 2021, 8, 622048. [Google Scholar] [CrossRef]
- Røsand, Ø.; Høydal, M.A. Cardiac Exosomes in Ischemic Heart Disease—A Narrative Review. Diagnostics 2021, 11, 269. [Google Scholar] [CrossRef] [PubMed]
- Fareez, I.M.; Seng, W.Y.; Zaki, R.M.; Shafiq, A.; Izwan, I.M. Molecular and Epigenetic Basis of Extracellular Vesicles Cell Repair Phenotypes in Targeted Organ-specific Regeneration. Curr. Mol. Med. 2022, 22, 132–150. [Google Scholar] [CrossRef]
- Marostica, G.; Gelibter, S.; Gironi, M.; Nigro, A.; Furlan, R. Extracellular Vesicles in Neuroinflammation. Front. Cell Dev. Biol. 2021, 8, 623039. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.L.; Chia, W.C.; How, C.W.; Tor, Y.S.; Show, P.L.; Looi, Q.H.D.; Foo, J.B. Benchtop Isolation and Characterisation of Small Extracellular Vesicles from Human Mesenchymal Stem Cells. Mol. Biotechnol. 2021, 63, 780–791. [Google Scholar] [CrossRef]
- Poon, I.K.H.; Parkes, M.A.F.; Jiang, L.; Atkin-Smith, G.K.; Tixeira, R.; Gregory, C.D.; Ozkocak, D.C.; Rutter, S.F.; Caruso, S.; Santavanond, J.P.; et al. Moving beyond size and phosphatidylserine exposure: Evidence for a diversity of apoptotic cell-derived extracellular vesicles in vitro. J. Extracell. Vesicles 2019, 8, 1608786. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, S.; Minamisawa, T.; Suga, K.; Kishita, H.; Akagi, T.; Ichiki, T.; Ichikawa, Y.; Shiba, K. Subtypes of tumour cell-derived small extracellular vesicles having differently externalized phosphatidylserine. J. Extracell. Vesicles 2019, 8, 1579541. [Google Scholar] [CrossRef]
- Oyarce, K.; Cepeda, M.Y.; Lagos, R.; Garrido, C.; Vega-Letter, A.M.; Garcia-Robles, M.; Luz-Crawford, P.; Elizondo-Vega, R. Neuroprotective and Neurotoxic Effects of Glial-Derived Exosomes. Front. Cell Neurosci. 2022, 16, 920686. [Google Scholar] [CrossRef]
- Hu, C.; Jiang, W.; Lv, M.; Fan, S.; Lu, Y.; Wu, Q.; Pi, J. Potentiality of Exosomal Proteins as Novel Cancer Biomarkers for Liquid Biopsy. Front. Immunol. 2022, 13, 792046. [Google Scholar] [CrossRef]
- Bano, R.; Ahmad, F.; Mohsin, M. A perspective on the isolation and characterization of extracellular vesicles from different biofluids. RSC Adv. 2021, 11, 19598–19615. [Google Scholar] [CrossRef]
- Gurunathan, S.; Kang, M.H.; Song, H.; Kim, N.H.; Kim, J.H. The role of extracellular vesicles in animal reproduction and diseases. J. Anim. Sci. Biotechnol. 2022, 13, 62. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, N.; Nakanishi, T. Stem Cell Studies in Cardiovascular Biology and Medicine: A Possible Key Role of Macrophages. Biology 2022, 11, 122. [Google Scholar] [CrossRef] [PubMed]
- Pulliam, L.; Sun, B.; Mustapic, M.; Chawla, S.; Kapogiannis, D. Plasma neuronal exosomes serve as biomarkers of cognitive impairment in HIV infection and Alzheimer’s disease. J. Neurovirol. 2019, 25, 702–709. [Google Scholar] [CrossRef]
- Salomon, C.; Nuzhat, Z.; Dixon, C.L.; Menon, R. Placental Exosomes During Gestation: Liquid Biopsies Carrying Signals for the Regulation of Human Parturition. Curr. Pharm. Des. 2018, 24, 974–982. [Google Scholar] [CrossRef]
- Carnino, J.M.; Lee, H. Extracellular vesicles in respiratory disease. Adv. Clin. Chem. 2022, 108, 105–127. [Google Scholar] [CrossRef] [PubMed]
- Pathania, A.S.; Prathipati, P.; Challagundla, K.B. New insights into exosome mediated tumor-immune escape: Clinical perspectives and therapeutic strategies. Biochim. Biophys. Acta Rev. Cancer 2021, 1876, 188624. [Google Scholar] [CrossRef]
- Wu, J.; Xie, Q.; Liu, Y.; Gao, Y.; Qu, Z.; Mo, L.; Xu, Y.; Chen, R.; Shi, L. A Small Vimentin-Binding Molecule Blocks Cancer Exosome Release and Reduces Cancer Cell Mobility. Front. Pharmacol. 2021, 12, 627394. [Google Scholar] [CrossRef]
- Grieco, G.E.; Fignani, D.; Formichi, C.; Nigi, L.; Licata, G.; Maccora, C.; Brusco, N.; Sebastiani, G.; Dotta, F. Extracellular Vesicles in Immune System Regulation and Type 1 Diabetes: Cell-to-Cell Communication Mediators, Disease Biomarkers, and Promising Therapeutic Tools. Front. Immunol. 2021, 12, 682948. [Google Scholar] [CrossRef]
- Burillo, J.; Fernández-Rhodes, M.; Piquero, M.; López-Alvarado, P.; Menéndez, J.C.; Jiménez, B.; González-Blanco, C.; Marqués, P.; Guillén, C.; Benito, M. Human amylin aggregates release within exosomes as a protective mechanism in pancreatic β cells: Pancreatic β-hippocampal cell communication. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 118971. [Google Scholar] [CrossRef]
- Wang, C.A.; Tsai, S.J. Regulation of lymphangiogenesis by extracellular vesicles in cancer metastasis. Exp. Biol. Med. 2021, 246, 2048–2056. [Google Scholar] [CrossRef]
- Kim, K.S.; Park, J.I.; Oh, N.; Cho, H.J.; Park, J.H.; Park, K.S. ELK3 expressed in lymphatic endothelial cells promotes breast cancer progression and metastasis through exosomal miRNAs. Sci. Rep. 2019, 9, 8418. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, W.; Zhang, C.; Wang, L.; Chen, H.; Xu, J. Exosomal non-coding RNAs have a significant effect on tumor metastasis. Mol. Ther. Nucleic Acids 2022, 29, 16–35. [Google Scholar] [CrossRef] [PubMed]
- Monti, M.; Lunardini, S.; Magli, I.A.; Campi, R.; Primiceri, G.; Berardinelli, F.; Amparore, D.; Terracciano, D.; Lucarelli, G.; Schips, L.; et al. Micro-RNAs Predict Response to Systemic Treatments in Metastatic Renal Cell Carcinoma Patients: Results from a Systematic Review of the Literature. Biomedicines 2022, 10, 1287. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Sun, M.; Zhang, H. The Interaction Between Epigenetic Changes, EMT, and Exosomes in Predicting Metastasis of Colorectal Cancers (CRC). Front. Oncol. 2022, 12, 879848. [Google Scholar] [CrossRef]
- Khera, A.; Alajangi, H.K.; Khajuria, A.; Barnwal, R.P.; Kumar, S.; Singh, G. Highlighting the potential role of Exosomes as the targeted nano-therapeutic carrier in metastatic breast cancer. Curr. Drug Deliv. 2022; in press. [Google Scholar] [CrossRef]
- Tămaș, F.; Bălașa, R.; Manu, D.; Gyorki, G.; Chinezu, R.; Tămaș, C.; Bălașa, A. The Importance of Small Extracellular Vesicles in the Cerebral Metastatic Process. Int. J. Mol. Sci. 2022, 23, 1449. [Google Scholar] [CrossRef] [PubMed]
- Sunami, Y.; Häußler, J.; Zourelidis, A.; Kleeff, J. Cancer-Associated Fibroblasts and Tumor Cells in Pancreatic Cancer Microenvironment and Metastasis: Paracrine Regulators, Reciprocation and Exosomes. Cancers 2022, 14, 744. [Google Scholar] [CrossRef]
- Bai, S.; Wei, Y.; Liu, R.; Xu, R.; Xiang, L.; Du, J. Role of tumour-derived exosomes in metastasis. Biomed. Pharmacother. 2022, 147, 112657. [Google Scholar] [CrossRef]
- Zhou, H.; He, X.; He, Y.; Ou, C.; Cao, P. Exosomal circRNAs: Emerging Players in Tumor Metastasis. Front. Cell Dev. Biol. 2021, 9, 786224. [Google Scholar] [CrossRef]
- Pascual-Antón, L.; Cardeñes, B.; Sainz de la Cuesta, R.; González-Cortijo, L.; López-Cabrera, M.; Cabañas, C.; Sandoval, P. Mesothelial-to-Mesenchymal Transition and Exosomes in Peritoneal Metastasis of Ovarian Cancer. Int. J. Mol. Sci. 2021, 22, 11496. [Google Scholar] [CrossRef]
- Shen, B.; Sun, K. Exosomal circular RNAs: A new frontier in the metastasis of digestive system tumors. Oncol. Lett. 2021, 22, 826. [Google Scholar] [CrossRef]
- Singh, M.; Agarwal, S.; Agarwal, V.; Mall, S.; Pancham, P.; Mani, S. Current theranostic approaches for metastatic cancers through hypoxia-induced exosomal packaged cargo. Life Sci. 2021, 286, 120017. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, Y.; Zhang, Y.; Zhang, S.; Qiu, L.; Zhuang, Z.; Wei, M.; Deng, X.; Wang, Z.; Han, J. The Key Role of Exosomes on the Pre-metastatic Niche Formation in Tumors. Front. Mol. Biosci. 2021, 8, 703640. [Google Scholar] [CrossRef] [PubMed]
- Mkhobongo, B.; Chandran, R.; Abrahamse, H. The Role of Melanoma Cell-Derived Exosomes (MTEX) and Photodynamic Therapy (PDT) within a Tumor Microenvironment. Int. J. Mol. Sci. 2021, 22, 9726. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chengalvala, V.; Hu, H.; Sun, D. Tumor-derived exosomes: Nanovesicles made by cancer cells to promote cancer metastasis. Acta Pharm. Sin. B 2021, 11, 2136–2149. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Zhang, N.; Hu, X.; Wang, H. Tumor-associated exosomes promote lung cancer metastasis through multiple mechanisms. Mol. Cancer 2021, 20, 117. [Google Scholar] [CrossRef]
- Seibold, T.; Waldenmaier, M.; Seufferlein, T.; Eiseler, T. Small Extracellular Vesicles and Metastasis-Blame the Messenger. Cancers 2021, 13, 4380. [Google Scholar] [CrossRef]
- Zhao, L.; Ma, X.; Yu, J. Exosomes and organ-specific metastasis. Mol. Ther. Methods Clin. Dev. 2021, 22, 133–147. [Google Scholar] [CrossRef]
- Storti, G.; Scioli, M.G.; Kim, B.S.; Terriaca, S.; Fiorelli, E.; Orlandi, A.; Cervelli, V. Mesenchymal Stem Cells in Adipose Tissue and Extracellular Vesicles in Ovarian Cancer Patients: A Bridge toward Metastatic Diffusion or a New Therapeutic Opportunity? Cells 2021, 10, 2117. [Google Scholar] [CrossRef]
- Zarin, B.; Rafiee, L.; Daneshpajouhnejad, P.; Haghjooy Javanmard, S. A review on the role of CAFs and CAF-derived exosomes in progression and metastasis of digestive system cancers. Tumour Biol. 2021, 43, 141–157. [Google Scholar] [CrossRef]
- Yin, L.; Liu, X.; Shao, X.; Feng, T.; Xu, J.; Wang, Q.; Hua, S. The role of exosomes in lung cancer metastasis and clinical applications: An updated review. J. Transl. Med. 2021, 19, 312. [Google Scholar] [CrossRef]
- Chen, X.; Wang, H.; Huang, Y.; Chen, Y.; Chen, C.; Zhuo, W.; Teng, L. Comprehensive Roles and Future Perspectives of Exosomes in Peritoneal Metastasis of Gastric Cancer. Front. Oncol. 2021, 11, 684871. [Google Scholar] [CrossRef] [PubMed]
- Balaji, S.; Kim, U.; Muthukkaruppan, V.; Vanniarajan, A. Emerging role of tumor microenvironment derived exosomes in therapeutic resistance and metastasis through epithelial-to-mesenchymal transition. Life Sci. 2021, 280, 119750. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Li, S.; Xu, Q.; Zhang, X.; Huang, M.; Dai, X.; Liu, L. Exosomes Promote Pre-Metastatic Niche Formation in Gastric Cancer. Front. Oncol. 2021, 11, 652378. [Google Scholar] [CrossRef] [PubMed]
- Al-Humaidi, R.B.; Fayed, B.; Sharif, S.I.; Noreddin, A.; Soliman, S.S.M. Role of Exosomes in Breast Cancer Management: Evidence-Based Review. Curr. Cancer Drug Targets 2021, 21, 666–675. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Luo, X.; Lv, W.; Hu, W.; Zhao, C.; Xiong, M.; Yi, Y.; Wang, D.; Wang, Y.; Wang, H.; et al. Tumor-derived exosomal components: The multifaceted roles and mechanisms in breast cancer metastasis. Cell Death Dis. 2021, 12, 547. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Pang, B.; Li, J.; Gao, N.; Fan, T.; Li, Y. Emerging Role of Exosomes in Liquid Biopsy for Monitoring Prostate Cancer Invasion and Metastasis. Front. Cell Dev. Biol. 2021, 9, 679527. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, Y.; Ding, K. Roles of exosomes in cancer chemotherapy resistance, progression, metastasis and immunity, and their clinical applications (Review). Int. J. Oncol. 2021, 59, 44. [Google Scholar] [CrossRef]
- Danac, J.M.C.; Uy, A.G.G.; Garcia, R.L. Exosomal microRNAs in colorectal cancer: Overcoming barriers of the metastatic cascade (Review). Int. J. Mol. Med. 2021, 47, 112. [Google Scholar] [CrossRef]
- Akoto, T.; Saini, S. Role of Exosomes in Prostate Cancer Metastasis. Int. J. Mol. Sci. 2021, 22, 3528. [Google Scholar] [CrossRef]
- Fu, C.; Zhang, Q.; Wang, A.; Yang, S.; Jiang, Y.; Bai, L.; Wei, Q. EWI-2 controls nucleocytoplasmic shuttling of EGFR signaling molecules and miRNA sorting in exosomes to inhibit prostate cancer cell metastasis. Mol. Oncol. 2021, 15, 1543–1565. [Google Scholar] [CrossRef]
- Peng, L.; Wang, D.; Han, Y.; Huang, T.; He, X.; Wang, J.; Ou, C. Emerging Role of Cancer-Associated Fibroblasts-Derived Exosomes in Tumorigenesis. Front. Immunol. 2022, 12, 795372. [Google Scholar] [CrossRef]
- Tang, L.B.; Ma, S.X.; Chen, Z.H.; Huang, Q.Y.; Wu, L.Y.; Wang, Y.; Zhao, R.C.; Xiong, L.X. Exosomal microRNAs: Pleiotropic Impacts on Breast Cancer Metastasis and Their Clinical Perspectives. Biology 2021, 10, 307. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Ji, X.; Liu, J.; Fan, D.; Zhou, Q.; Chen, C.; Wang, W.; Wang, G.; Wang, H.; Yuan, W.; et al. Effects of exosomes on pre-metastatic niche formation in tumors. Mol. Cancer 2019, 18, 39. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Jia, J.; Yao, L.; Li, Z. Crosstalk of Exosomal Non-Coding RNAs in The Tumor Microenvironment: Novel Frontiers. Front. Immunol. 2022, 13, 900155. [Google Scholar] [CrossRef]
- Su, M.T.; Kumata, S.; Endo, S.; Okada, Y.; Takai, T. LILRB4 promotes tumor metastasis by regulating MDSCs and inhibiting miR-1 family miRNAs. Oncoimmunology 2022, 11, 2060907. [Google Scholar] [CrossRef] [PubMed]
- Sendi, H.; Yazdimamaghani, M.; Hu, M.; Sultanpuram, N.; Wang, J.; Moody, A.S.; McCabe, E.; Zhang, J.; Graboski, A.; Li, L.; et al. Nanoparticle Delivery of miR-122 Inhibits Colorectal Cancer Liver Metastasis. Cancer Res. 2022, 82, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.J.; Wang, D.D.; Zhong, S.L.; Chen, W.Q.; Wang, F.L.; Zhang, J.; Xu, W.X.; Xu, D.; Zhang, Q.; Li, J.; et al. Tumor-derived exosomal circPSMA1 facilitates the tumorigenesis, metastasis, and migration in triple-negative breast cancer (TNBC) through miR-637/Akt1/β-catenin (cyclin D1) axis. Cell Death Dis. 2021, 12, 420. [Google Scholar] [CrossRef]
- Chen, S.; Chen, X.; Qiu, J.; Chen, P.; Han, X.; Wu, Y.; Zhuang, J.; Yang, M.; Wu, C.; Wu, N.; et al. Exosomes derived from retinoblastoma cells enhance tumour deterioration by infiltrating the microenvironment. Oncol. Rep. 2021, 45, 278–290. [Google Scholar] [CrossRef]
- Moradi-Chaleshtori, M.; Bandehpour, M.; Heidari, N.; Mohammadi-Yeganeh, S.; Mahmoud Hashemi, S. Exosome-mediated miR-33 transfer induces M1 polarization in mouse macrophages and exerts antitumor effect in 4T1 breast cancer cell line. Int. Immunopharmacol. 2021, 90, 107198. [Google Scholar] [CrossRef]
- Zhao, M.; Zhuang, A.; Fang, Y. Cancer-Associated Fibroblast-Derived Exosomal miRNA-320a Promotes Macrophage M2 Polarization In Vitro by Regulating PTEN/PI3Kγ Signaling in Pancreatic Cancer. J. Oncol. 2022, 2022, 9514697. [Google Scholar] [CrossRef]
- Qi, M.; Xia, Y.; Wu, Y.; Zhang, Z.; Wang, X.; Lu, L.; Dai, C.; Song, Y.; Xu, K.; Ji, W.; et al. Lin28B-high breast cancer cells promote immune suppression in the lung pre-metastatic niche via exosomes and support cancer progression. Nat. Commun. 2022, 13, 897. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Yokoi, A.; Kato, T.; Ochiya, T.; Yamamoto, Y. The clinical impact of intra- and extracellular miRNAs in ovarian cancer. Cancer Sci. 2020, 111, 3435–3444. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Kim, H.S.; Song, G.; Lim, W. The potential role of exosomes derived from ovarian cancer cells for diagnostic and therapeutic approaches. J. Cell Physiol. 2019, 234, 21493–21503. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.G.; Zhou, C.F.; Zhang, Y.M.; Yan, R.M.; Wei, W.F.; Chen, X.J.; Yi, H.Y.; Liang, L.J.; Fan, L.S.; Liang, L.; et al. Cancer-derived exosomal miR-221-3p promotes angiogenesis by targeting THBS2 in cervical squamous cell carcinoma. Angiogenesis 2019, 22, 397–410. [Google Scholar] [CrossRef]
- Chen, K.; Wang, Q.; Liu, X.; Wang, F.; Yang, Y.; Tian, X. Hypoxic pancreatic cancer derived exosomal miR-30b-5p promotes tumor angiogenesis by inhibiting GJA1 expression. Int. J. Biol. Sci. 2022, 18, 1220–1237. [Google Scholar] [CrossRef]
- Duréndez-Sáez, E.; Torres-Martinez, S.; Calabuig-Fariñas, S.; Meri-Abad, M.; Ferrero-Gimeno, M.; Camps, C. Exosomal microRNAs in non-small cell lung cancer. Transl. Cancer Res. 2021, 10, 3128–3139. [Google Scholar] [CrossRef]
- Li, C.; Zhou, T.; Chen, J.; Li, R.; Chen, H.; Luo, S.; Chen, D.; Cai, C.; Li, W. The role of Exosomal miRNAs in cancer. J. Transl. Med. 2022, 20, 6. [Google Scholar] [CrossRef]
- Xu, J.L.; Xu, W.X.; Tang, J.H. Exosomal circRNAs: A new communication method in cancer. Am. J. Transl. Res. 2021, 13, 12913–12928. [Google Scholar]
- Tominaga, N.; Kosaka, N.; Ono, M.; Katsuda, T.; Yoshioka, Y.; Tamura, K.; Lötvall, J.; Nakagama, H.; Ochiya, T. Brain metastatic cancer cells release microRNA-181c-containing extracellular vesicles capable of destructing blood-brain barrier. Nat. Commun. 2015, 6, 6716. [Google Scholar] [CrossRef]
- Geng, S.; Tu, S.; Bai, Z.; Geng, Y. Exosomal lncRNA LINC01356 Derived from Brain Metastatic Nonsmall-Cell Lung Cancer Cells Remodels the Blood-Brain Barrier. Front. Oncol. 2022, 12, 825899. [Google Scholar] [CrossRef]
- Curtaz, C.J.; Reifschläger, L.; Strähle, L.; Feldheim, J.; Feldheim, J.J.; Schmitt, C.; Kiesel, M.; Herbert, S.L.; Wöckel, A.; Meybohm, P.; et al. Analysis of microRNAs in Exosomes of Breast Cancer Patients in Search of Molecular Prognostic Factors in Brain Metastases. Int. J. Mol. Sci. 2022, 23, 3683. [Google Scholar] [CrossRef] [PubMed]
- Busatto, S.; Morad, G.; Guo, P.; Moses, M.A. The role of extracellular vesicles in the physiological and pathological regulation of the blood-brain barrier. FASEB Bioadv. 2021, 3, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Deng, S.; Li, L.; Liu, T.; Zhang, T.; Li, J.; Yu, Y.; Xu, Y. TGF-β1-mediated exosomal lnc-MMP2-2 increases blood-brain barrier permeability via the miRNA-1207-5p/EPB41L5 axis to promote non-small cell lung cancer brain metastasis. Cell Death Dis. 2021, 12, 721. [Google Scholar] [CrossRef]
- Yokoi, A.; Yoshioka, Y.; Yamamoto, Y.; Ishikawa, M.; Ikeda, S.I.; Kato, T.; Kiyono, T.; Takeshita, F.; Kajiyama, H.; Kikkawa, F.; et al. Malignant extracellular vesicles carrying MMP1 mRNA facilitate peritoneal dissemination in ovarian cancer. Nat. Commun. 2017, 8, 14470. [Google Scholar] [CrossRef]
- Wang, G.; Xie, L.; Li, B.; Sang, W.; Yan, J.; Li, J.; Tian, H.; Li, W.; Zhang, Z.; Tian, Y.; et al. A nanounit strategy reverses immune suppression of exosomal PD-L1 and is associated with enhanced ferroptosis. Nat. Commun. 2021, 12, 5733. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Tsai, H.I.; Xiao, Y.; Wu, Y.; Su, D.; Yang, M.; Zha, H.; Yan, F.; Liu, X.; Cheng, F.; et al. Engineering Programmed Death Ligand-1/Cytotoxic T-Lymphocyte-Associated Antigen-4 Dual-Targeting Nanovesicles for Immunosuppressive Therapy in Transplantation. ACS Nano 2020, 14, 7959–7969. [Google Scholar] [CrossRef]
- Xie, L.; Li, J.; Wang, G.; Sang, W.; Xu, M.; Li, W.; Yan, J.; Li, B.; Zhang, Z.; Zhao, Q.; et al. Phototheranostic Metal-Phenolic Networks with Antiexosomal PD-L1 Enhanced Ferroptosis for Synergistic Immunotherapy. J. Am. Chem. Soc. 2022, 144, 787–797. [Google Scholar] [CrossRef]
- Chen, J.; Song, Y.; Miao, F.; Chen, G.; Zhu, Y.; Wu, N.; Pang, L.; Chen, Z.; Chen, X. PDL1-positive exosomes suppress antitumor immunity by inducing tumor-specific CD8+ T cell exhaustion during metastasis. Cancer Sci. 2021, 112, 3437–3454. [Google Scholar] [CrossRef]
- Liu, N.; Zhang, J.; Yin, M.; Liu, H.; Zhang, X.; Li, J.; Yan, B.; Guo, Y.; Zhou, J.; Tao, J.; et al. Inhibition of xCT suppresses the efficacy of anti-PD-1/L1 melanoma treatment through exosomal PD-L1-induced macrophage M2 polarization. Mol. Ther. 2021, 29, 2321–2334. [Google Scholar] [CrossRef]
- Shu, S.; Matsuzaki, J.; Want, M.Y.; Conway, A.; Benjamin-Davalos, S.; Allen, C.L.; Koroleva, M.; Battaglia, S.; Odunsi, A.; Minderman, H.; et al. An Immunosuppressive Effect of Melanoma-derived Exosomes on NY-ESO-1 Antigen-specific Human CD8+ T Cells is Dependent on IL-10 and Independent of BRAFV600E Mutation in Melanoma Cell Lines. Immunol. Investig. 2020, 49, 744–757. [Google Scholar] [CrossRef]
- Chen, G.; Huang, A.C.; Zhang, W.; Zhang, G.; Wu, M.; Xu, W.; Yu, Z.; Yang, J.; Wang, B.; Sun, H.; et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature 2018, 560, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Rupareliya, C.; Naqvi, S.; Jani, V.B. Acute Inflammatory Demyelinating Polyneuroradiculopathy with Ipilimumab in Metastatic Melanoma: A Case Report and Review of Literature. Cureus 2017, 9, e1310. [Google Scholar] [CrossRef] [PubMed]
- Mastoraki, A.; Gkiala, A.; Theodoroleas, G.; Mouchtouri, E.; Strimpakos, A.; Papagiannopoulou, D.; Schizas, D. Metastatic malignant melanoma of the breast: Report of a case and review of the literature. Folia. Med. 2022, 64, 354–358. [Google Scholar] [CrossRef] [PubMed]
- Gracia-Hernandez, M.; Munoz, Z.; Villagra, A. Enhancing Therapeutic Approaches for Melanoma Patients Targeting Epigenetic Modifiers. Cancers 2021, 13, 6180. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Anandasabapathy, N. Immune Checkpoint Blockade and Skin Toxicity Pathogenesis. J. Investig. Dermatol. 2022, 142, 951–959. [Google Scholar] [CrossRef] [PubMed]
- Dietz, H.; Weinmann, S.C.; Salama, A.K. Checkpoint Inhibitors in Melanoma Patients with Underlying Autoimmune Disease. Cancer Manag. Res. 2021, 13, 8199–8208. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.M.; Li, W.; Chen, Z.Y.; Wang, Y. Risk of Pneumonitis Associated with Immune Checkpoint Inhibitors in Melanoma: A Systematic Review and Network Meta-Analysis. Front. Oncol. 2021, 11, 651553. [Google Scholar] [CrossRef]
- Zawit, M.; Swami, U.; Awada, H.; Arnouk, J.; Milhem, M.; Zakharia, Y. Current status of intralesional agents in treatment of malignant melanoma. Ann. Transl. Med. 2021, 9, 1038. [Google Scholar] [CrossRef]
- Sakellariou, S.; Zouki, D.N.; Ziogas, D.C.; Pouloudi, D.; Gogas, H.; Delladetsima, I. Granulomatous colitis in a patient with metastatic melanoma under immunotherapy: A case report and literature review. BMC Gastroenterol. 2021, 21, 227. [Google Scholar] [CrossRef]
- Li, J.H.; Huang, L.J.; Zhou, H.L.; Shan, Y.M.; Chen, F.M.; Lehto, V.P.; Xu, W.J.; Luo, L.Q.; Yu, H.J. Engineered nanomedicines block the PD-1/PD-L1 axis for potentiated cancer immunotherapy. Acta Pharmacol. Sin. 2022, 43, 2749–2758. [Google Scholar] [CrossRef]
- Archilla-Ortega, A.; Domuro, C.; Martin-Liberal, J.; Muñoz, P. Blockade of novel immune checkpoints and new therapeutic combinations to boost antitumor immunity. J. Exp. Clin. Cancer Res. 2022, 41, 62. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Zhu, Z.; Chen, X.; Zhang, H.; Huang, J.; Gu, S.; Zhao, X. The Importance of Exosomal PD-L1 in Cancer Progression and Its Potential as a Therapeutic Target. Cells 2021, 10, 3247. [Google Scholar] [CrossRef] [PubMed]
- Palicelli, A.; Croci, S.; Bisagni, A.; Zanetti, E.; De Biase, D.; Melli, B.; Sanguedolce, F.; Ragazzi, M.; Zanelli, M.; Chaux, A.; et al. What Do We Have to Know about PD-L1 Expression in Prostate Cancer? A Systematic Literature Review. Part 3: PD-L1, Intracellular Signaling Pathways and Tumor Microenvironment. Int. J. Mol. Sci. 2021, 22, 12330. [Google Scholar] [CrossRef] [PubMed]
- Awadasseid, A.; Wu, Y.; Zhang, W. Advance investigation on synthetic small-molecule inhibitors targeting PD-1/PD-L1 signaling pathway. Life Sci. 2021, 282, 119813. [Google Scholar] [CrossRef]
- Xing, K.; Zhou, P.; Li, J.; Liu, M.; Zhang, W.E. Inhibitory Effect of PD-1/PD-L1 and Blockade Immunotherapy in Leukemia. Comb. Chem. High Throughput Screen 2021, 25, 1399–1410. [Google Scholar] [CrossRef]
- Huang, H.W.; Chang, C.C.; Wang, C.S.; Lin, K.H. Association between Inflammation and Function of Cell Adhesion Molecules Influence on Gastrointestinal Cancer Development. Cells 2021, 10, 67. [Google Scholar] [CrossRef]
- Han, J.; Xu, X.; Liu, Z.; Li, Z.; Wu, Y.; Zuo, D. Recent advances of molecular mechanisms of regulating PD-L1 expression in melanoma. Int. Immunopharmacol. 2020, 88, 106971. [Google Scholar] [CrossRef]
- Guan, L.; Wu, B.; Li, T.; Beer, L.A.; Sharma, G.; Li, M.; Lee, C.N.; Liu, S.; Yang, C.; Huang, L.; et al. HRS phosphorylation drives immunosuppressive exosome secretion and restricts CD8+ T-cell infiltration into tumors. Nat. Commun. 2022, 13, 4078. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, Y.; Guan, M.; Liu, Y.; Lv, M.; Zhang, C.; Zhang, H.; Zhang, Z. Isolation of circulating exosomes and identification of exosomal PD-L1 for predicting immunotherapy response. Nanoscale 2022, 14, 8995–9003. [Google Scholar] [CrossRef]
- Shen, D.D.; Pang, J.R.; Bi, Y.P.; Zhao, L.F.; Li, Y.R.; Zhao, L.J.; Gao, Y.; Wang, B.; Wang, N.; Wei, L.; et al. LSD1 deletion decreases exosomal PD-L1 and restores T-cell response in gastric cancer. Mol. Cancer 2022, 21, 75. [Google Scholar] [CrossRef]
- Turiello, R.; Capone, M.; Morretta, E.; Monti, M.C.; Madonna, G.; Azzaro, R.; Del Gaudio, P.; Simeone, E.; Sorrentino, A.; Ascierto, P.A.; et al. Exosomal CD73 from serum of patients with melanoma suppresses lymphocyte functions and is associated with therapy resistance to anti-PD-1 agents. J. Immunother. Cancer 2022, 10, e004043. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhong, W.; Wang, B.; Yang, J.; Yang, J.; Yu, Z.; Qin, Z.; Shi, A.; Xu, W.; Zheng, C.; et al. ICAM-1-mediated adhesion is a prerequisite for exosome-induced T cell suppression. Dev. Cell 2022, 57, 329–343.e7. [Google Scholar] [CrossRef] [PubMed]
- Zanella, A.; Vautrot, V.; Aubin, F.; Avoscan, L.; Samimi, M.; Garrido, C.; Gobbo, J.; Nardin, C. PD-L1 in circulating exosomes of Merkel cell carcinoma. Exp. Dermatol. 2022, 31, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Chen, W.; Zhou, S.; Zhu, G. ExoHCR: A sensitive assay to profile PD-L1 level on tumor exosomes for immunotherapeutic prognosis. Biophys. Rep. 2020, 6, 290–298. [Google Scholar] [CrossRef]
- Chen, X.; Du, Z.; Huang, M.; Wang, D.; Fong, W.P.; Liang, J.; Fan, L.; Wang, Y.; Yang, H.; Chen, Z.; et al. Circulating PD-L1 is associated with T cell infiltration and predicts prognosis in patients with CRLM following hepatic resection. Cancer Immunol. Immunother. 2022, 71, 661–674. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Yang, Y.; Yang, R.; Liu, C.; Hsu, J.M.; Jiang, Z.; Sun, L.; Wei, Y.; Li, C.W.; Yu, D.; et al. Activated T cell-derived exosomal PD-1 attenuates PD-L1-induced immune dysfunction in triple-negative breast cancer. Oncogene 2021, 40, 4992–5001. [Google Scholar] [CrossRef]
- Wang, R.; Xu, A.; Zhang, X.; Wu, J.; Freywald, A.; Xu, J.; Xiang, J. Novel exosome-targeted T-cell-based vaccine counteracts T-cell anergy and converts CTL exhaustion in chronic infection via CD40L signaling through the mTORC1 pathway. Cell Mol. Immunol. 2017, 14, 529–545. [Google Scholar] [CrossRef]
- Morrissey, S.M.; Yan, J. Exosomal PD-L1: Roles in Tumor Progression and Immunotherapy. Trends Cancer 2020, 6, 550–558. [Google Scholar] [CrossRef]
- Zhou, K.; Guo, S.; Li, F.; Sun, Q.; Liang, G. Exosomal PD-L1: New Insights into Tumor Immune Escape Mechanisms and Therapeutic Strategies. Front. Cell Dev. Biol. 2020, 8, 569219. [Google Scholar] [CrossRef]
- Xie, F.; Xu, M.; Lu, J.; Mao, L.; Wang, S. The role of exosomal PD-L1 in tumor progression and immunotherapy. Mol. Cancer 2019, 18, 146. [Google Scholar] [CrossRef]
- Liu, J.; Peng, X.; Yang, S.; Li, X.; Huang, M.; Wei, S.; Zhang, S.; He, G.; Zheng, H.; Fan, Q.; et al. Extracellular vesicle PD-L1 in reshaping tumor immune microenvironment: Biological function and potential therapy strategies. Cell Commun. Signal. 2022, 20, 14. [Google Scholar] [CrossRef] [PubMed]
- Yoh, K.E.; Lowe, C.J.; Mahajan, S.; Suttmann, R.; Nguy, T.; Reichelt, M.; Yang, J.; Melendez, R.; Li, Y.; Molinero, L.; et al. Enrichment of circulating tumor-derived extracellular vesicles from human plasma. J. Immunol. Methods 2021, 490, 112936. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Gu, Y.; Kang, B.; Heskia, F.; Pachot, A.; Bonneville, M.; Wei, P.; Liang, J. PD-L1 detection on circulating tumor-derived extracellular vesicles (T-EVs) from patients with lung cancer. Transl. Lung Cancer Res. 2021, 10, 2441–2451. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Kim, H.; Choi, Y.J.; Kim, S.Y.; Lee, J.E.; Sung, K.J.; Sung, Y.H.; Pack, C.G.; Jung, M.K.; Han, B.; et al. Exosomal PD-L1 promotes tumor growth through immune escape in non-small cell lung cancer. Exp. Mol. Med. 2019, 51, 1–13. [Google Scholar] [CrossRef]
- Wang, Y.; Niu, X.; Cheng, Y.; Zhang, Y.; Xia, L.; Xia, W.; Lu, S. Exosomal PD-L1 predicts response with immunotherapy in NSCLC patients. Clin. Exp. Immunol. 2022, 208, 316–322. [Google Scholar] [CrossRef]
- Shin, J.M.; Lee, C.H.; Son, S.; Kim, C.H.; Lee, J.A.; Ko, H.; Shin, S.; Song, S.H.; Park, S.S.; Bae, J.H.; et al. Sulfisoxazole Elicits Robust Antitumour Immune Response Along with Immune Checkpoint Therapy by Inhibiting Exosomal PD-L1. Adv. Sci. 2022, 9, e2103245. [Google Scholar] [CrossRef]
- Gong, Y.; Li, K.; Qin, Y.; Zeng, K.; Liu, J.; Huang, S.; Chen, Y.; Yu, H.; Liu, W.; Ye, L.; et al. Norcholic Acid Promotes Tumor Progression and Immune Escape by Regulating Farnesoid X Receptor in Hepatocellular Carcinoma. Front. Oncol. 2021, 11, 711448. [Google Scholar] [CrossRef]
- Shimada, Y.; Matsubayashi, J.; Kudo, Y.; Maehara, S.; Takeuchi, S.; Hagiwara, M.; Kakihana, M.; Ohira, T.; Nagao, T.; Ikeda, N. Serum-derived exosomal PD-L1 expression to predict anti-PD-1 response and in patients with non-small cell lung cancer. Sci. Rep. 2021, 11, 7830. [Google Scholar] [CrossRef]
- Yin, Z.; Yu, M.; Ma, T.; Zhang, C.; Huang, S.; Karimzadeh, M.R.; Momtazi-Borojeni, A.A.; Chen, S. Mechanisms underlying low-clinical responses to PD-1/PD-L1 blocking antibodies in immunotherapy of cancer: A key role of exosomal PD-L1. J. Immunother. Cancer 2021, 9, e001698. [Google Scholar] [CrossRef]
- Pico de Coaña, Y.; Wolodarski, M.; van der Haar Àvila, I.; Nakajima, T.; Rentouli, S.; Lundqvist, A.; Masucci, G.; Hansson, J.; Kiessling, R. PD-1 checkpoint blockade in advanced melanoma patients: NK cells, monocytic subsets and host PD-L1 expression as predictive biomarker candidates. Oncoimmunology 2020, 9, 1786888. [Google Scholar] [CrossRef]
- Ando, K.; Hamada, K.; Shida, M.; Ohkuma, R.; Kubota, Y.; Horiike, A.; Matsui, H.; Ishiguro, T.; Hirasawa, Y.; Ariizumi, H.; et al. A high number of PD-L1+ CD14+ monocytes in peripheral blood is correlated with shorter survival in patients receiving immune checkpoint inhibitors. Cancer Immunol. Immunother. 2021, 70, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Bae, J.H.; Choe, E.J.; Park, J.M.; Park, S.S.; Cho, H.J.; Song, B.J.; Baek, M.C. Macitentan improves antitumor immune responses by inhibiting the secretion of tumor-derived extracellular vesicle PD-L1. Theranostics 2022, 12, 1971–1987. [Google Scholar] [CrossRef] [PubMed]
- Del Re, M.; van Schaik, R.H.N.; Fogli, S.; Mathijssen, R.H.J.; Cucchiara, F.; Capuano, A.; Scavone, C.; Jenster, G.W.; Danesi, R. Blood-based PD-L1 analysis in tumor-derived extracellular vesicles: Applications for optimal use of anti-PD-1/PD-L1 axis inhibitors. Biochim. Biophys. Acta Rev. Cancer 2021, 1875, 188463. [Google Scholar] [CrossRef] [PubMed]
- Theodoraki, M.N.; Yerneni, S.S.; Hoffmann, T.K.; Gooding, W.E.; Whiteside, T.L. Clinical Significance of PD-L1+ Exosomes in Plasma of Head and Neck Cancer Patients. Clin. Cancer Res. 2018, 24, 896–905. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.L.; Luo, Y.P.; Lin, M.W.; Peng, X.X.; Liu, M.L.; Wang, Y.C.; Li, S.J.; Yang, D.H.; Yang, Z.X. Serum exosomal miR-16-5p functions as a tumor inhibitor and a new biomarker for PD-L1 inhibitor-dependent immunotherapy in lung adenocarcinoma by regulating PD-L1 expression. Cancer Med. 2022, 11, 2627–2643. [Google Scholar] [CrossRef]
- Whiteside, T.L. The emerging role of plasma exosomes in diagnosis, prognosis and therapies of patients with cancer. Contemp. Oncol. 2018, 22, 38–40. [Google Scholar] [CrossRef]
- Muller, L.; Muller-Haegele, S.; Mitsuhashi, M.; Gooding, W.; Okada, H.; Whiteside, T.L. Exosomes isolated from plasma of glioma patients enrolled in a vaccination trial reflect antitumor immune activity and might predict survival. Oncoimmunology 2015, 4, e1008347. [Google Scholar] [CrossRef]
- Del Re, M.; Cucchiara, F.; Rofi, E.; Fontanelli, L.; Petrini, I.; Gri, N.; Pasquini, G.; Rizzo, M.; Gabelloni, M.; Belluomini, L.; et al. A multiparametric approach to improve the prediction of response to immunotherapy in patients with metastatic NSCLC. Cancer Immunol. Immunother. 2021, 70, 1667–1678. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, P.; Wang, Y.; Wang, J.; Su, M.; Wang, Y.; Zhou, L.; Zhou, J.; Xiong, W.; Zeng, Z.; et al. The Biogenesis, Biology, and Clinical Significance of Exosomal PD-L1 in Cancer. Front. Immunol. 2020, 11, 604. [Google Scholar] [CrossRef]
- Wang, M.; Zhai, X.; Li, J.; Guan, J.; Xu, S.; Li, Y.; Zhu, H. The Role of Cytokines in Predicting the Response and Adverse Events Related to Immune Checkpoint Inhibitors. Front. Immunol. 2021, 12, 670391. [Google Scholar] [CrossRef]
- Cha, J.H.; Chan, L.C.; Li, C.W.; Hsu, J.L.; Hung, M.C. Mechanisms Controlling PD-L1 Expression in Cancer. Mol. Cell 2019, 76, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Yi, M.; Niu, M.; Xu, L.; Luo, S.; Wu, K. Regulation of PD-L1 expression in the tumor microenvironment. J. Hematol. Oncol. 2021, 14, 10. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Che, X.; Qu, J.; Hou, K.; Wen, T.; Li, Z.; Li, C.; Wang, S.; Xu, L.; Liu, Y.; et al. Exosomal PD-L1 Retains Immunosuppressive Activity and is Associated with Gastric Cancer Prognosis. Ann. Surg. Oncol. 2019, 26, 3745–3755. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, Y.; Li, L.; He, D.; Chi, J.; Li, Q.; Wu, Y.; Zhao, Y.; Zhang, S.; Wang, L.; et al. Engineered extracellular vesicles and their mimetics for cancer immunotherapy. J. Control. Release 2022, 349, 679–698. [Google Scholar] [CrossRef]
- Yong, T.; Wei, Z.; Gan, L.; Yang, X. Extracellular Vesicle-Based Drug Delivery Systems for Enhanced Anti-Tumor Therapies through Modulating Cancer-Immunity Cycle. Adv. Mater. 2022, 20, e2201054. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, L.; Li, H.; Miao, F.; Zhang, Z.; Hu, C.; Yu, W.; Tang, Q.; Shao, G. Application of lipid nanovesicle drug delivery system in cancer immunotherapy. J. Nanobiotechnol. 2022, 20, 214. [Google Scholar] [CrossRef]
- Calvo, V.; Izquierdo, M. T Lymphocyte and CAR-T Cell-Derived Extracellular Vesicles and Their Applications in Cancer Therapy. Cells 2022, 11, 790. [Google Scholar] [CrossRef]
- Hao, Q.; Wu, Y.; Wu, Y.; Wang, P.; Vadgama, J.V. Tumor-Derived Exosomes in Tumor-Induced Immune Suppression. Int. J. Mol. Sci. 2022, 23, 1461. [Google Scholar] [CrossRef]
- Hosseini, R.; Sarvnaz, H.; Arabpour, M.; Ramshe, S.M.; Asef-Kabiri, L.; Yousefi, H.; Akbari, M.E.; Eskandari, N. Cancer exosomes and natural killer cells dysfunction: Biological roles, clinical significance and implications for immunotherapy. Mol. Cancer 2022, 21, 15. [Google Scholar] [CrossRef]
- Abu, N.; Rus Bakarurraini, N.A.A. The interweaving relationship between extracellular vesicles and T cells in cancer. Cancer Lett. 2022, 530, 1–7. [Google Scholar] [CrossRef]
- Shenoy, G.N.; Bhatta, M.; Bankert, R.B. Tumor-Associated Exosomes: A Potential Therapeutic Target for Restoring Anti-Tumor T Cell Responses in Human Tumor Microenvironments. Cells 2021, 10, 3155. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Vayalil, J.; Lee, G.; Wang, Y.; Peng, G. Emerging role of tumor-derived extracellular vesicles in T cell suppression and dysfunction in the tumor microenvironment. J. Immunother. Cancer 2021, 9, e003217. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, B. The Immunomodulation Potential of Exosomes in Tumor Microenvironment. J. Immunol. Res. 2021, 2021, 3710372. [Google Scholar] [CrossRef] [PubMed]
- Linder, M.; Pogge von Strandmann, E. The Role of Extracellular HSP70 in the Function of Tumor-Associated Immune Cells. Cancers 2021, 13, 4721. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Cai, S.; Li, M.; Salma, K.I.; Zhou, X.; Han, F.; Chen, J.; Huyan, T. Tumor-Derived Extracellular Vesicles: Their Role in Immune Cells and Immunotherapy. Int. J. Nanomed. 2021, 16, 5395–5409. [Google Scholar] [CrossRef]
- Wu, F.; Xie, M.; Hun, M.; She, Z.; Li, C.; Luo, S.; Chen, X.; Wan, W.; Wen, C.; Tian, J. Natural Killer Cell-Derived Extracellular Vesicles: Novel Players in Cancer Immunotherapy. Front. Immunol. 2021, 12, 658698. [Google Scholar] [CrossRef]
- Hou, P.P.; Chen, H.Z. Extracellular vesicles in the tumor immune microenvironment. Cancer Lett. 2021, 516, 48–56. [Google Scholar] [CrossRef]
- Lipinski, S.; Tiemann, K. Extracellular Vesicles and Their Role in the Spatial and Temporal Expansion of Tumor-Immune Interactions. Int. J. Mol. Sci. 2021, 22, 3374. [Google Scholar] [CrossRef]
- Ayala-Mar, S.; Donoso-Quezada, J.; González-Valdez, J. Clinical Implications of Exosomal PD-L1 in Cancer Immunotherapy. J. Immunol. Res. 2021, 2021, 8839978. [Google Scholar] [CrossRef]
- Wang, L.; Sun, Z.; Wang, H. Extracellular vesicles and the regulation of tumor immunity: Current progress and future directions. J. Cell Biochem. 2021, 122, 760–769. [Google Scholar] [CrossRef]
- Srivastava, A.; Rathore, S.; Munshi, A.; Ramesh, R. Extracellular Vesicles in Oncology: From Immune Suppression to Immunotherapy. AAPS J. 2021, 23, 30. [Google Scholar] [CrossRef] [PubMed]
- Lucafò, M.; De Biasi, S.; Curci, D.; Norbedo, A.; Stocco, G.; Decorti, G. Extracellular Vesicles as Innovative Tools for Assessing Adverse Effects of Immunosuppressant Drugs. Curr. Med. Chem. 2022, 29, 3586–3600. [Google Scholar] [CrossRef] [PubMed]
- Negahdaripour, M.; Owji, H.; Eskandari, S.; Zamani, M.; Vakili, B.; Nezafat, N. Small extracellular vesicles (sEVs): Discovery, functions, applications, detection methods and various engineered forms. Expert Opin. Biol. Ther. 2021, 21, 371–394. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.C.; Zhang, T.; Gao, J.Q. The in vivo fate and targeting engineering of crossover vesicle-based gene delivery system. Adv. Drug Deliv. Rev. 2022, 187, 114324. [Google Scholar] [CrossRef]
- Raghav, A.; Jeong, G.B. A systematic review on the modifications of extracellular vesicles: A revolutionized tool of nano-biotechnology. J. Nanobiotechnol. 2021, 19, 459. [Google Scholar] [CrossRef]
- Kučuk, N.; Primožič, M.; Knez, Ž.; Leitgeb, M. Exosomes Engineering and Their Roles as Therapy Delivery Tools, Therapeutic Targets, and Biomarkers. Int. J. Mol. Sci. 2021, 22, 9543. [Google Scholar] [CrossRef]
- Liang, Y.; Duan, L.; Lu, J.; Xia, J. Engineering exosomes for targeted drug delivery. Theranostics 2021, 11, 3183–3195. [Google Scholar] [CrossRef]
- Geng, T.; Pan, P.; Leung, E.; Chen, Q.; Chamley, L.; Wu, Z. Recent Advancement and Technical Challenges in Developing Small Extracellular Vesicles for Cancer Drug Delivery. Pharm. Res. 2021, 38, 179–197. [Google Scholar] [CrossRef]
- Xue, V.W.; Wong, S.C.C.; Song, G.; Cho, W.C.S. Promising RNA-based cancer gene therapy using extracellular vesicles for drug delivery. Expert Opin. Biol. Ther. 2020, 20, 767–777. [Google Scholar] [CrossRef]
- Jabłkowski, M.; Szemraj, M.; Oszajca, K.; Janiszewska, G.; Bartkowiak, J.; Szemraj, J. New type of BACE1 siRNA delivery to cells. Med. Sci. Monit. 2014, 20, 2598–2606. [Google Scholar] [CrossRef]
- Nawrot, B. Targeting BACE with small inhibitory nucleic acids—A future for Alzheimer’s disease therapy? Acta Biochim. Pol. 2004, 51, 431–444. [Google Scholar] [CrossRef]
- Alvarez-Erviti, L.; Seow, Y.; Yin, H.; Betts, C.; Lakhal, S.; Wood, M.J. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011, 29, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Meldolesi, J. News about Therapies of Alzheimer’s Disease: Extracellular Vesicles from Stem Cells Exhibit Advantages Compared to Other Treatments. Biomedicines 2022, 10, 105. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Q.; Zhang, X.; Huang, H.; Tang, S.; Chai, Y.; Xu, Z.; Li, M.; Chen, X.; Liu, J.; et al. Recent advances in exosome-mediated nucleic acid delivery for cancer therapy. J. Nanobiotechnol. 2022, 20, 279. [Google Scholar] [CrossRef] [PubMed]
- Entezari, M.; Ghanbarirad, M.; Taheriazam, A.; Sadrkhanloo, M.; Zabolian, A.; Goharrizi, M.A.S.B.; Hushmandi, K.; Aref, A.R.; Ashrafizadeh, M.; Zarrabi, A.; et al. Long non-coding RNAs and exosomal lncRNAs: Potential functions in lung cancer progression, drug resistance and tumor microenvironment remodeling. Biomed. Pharmacother. 2022, 150, 112963. [Google Scholar] [CrossRef] [PubMed]
- Taghvimi, S.; Vakili, O.; Soltani Fard, E.; Khatami, S.H.; Karami, N.; Taheri-Anganeh, M.; Salehi, M.; Negahdari, B.; Ghasemi, H.; Movahedpour, A. Exosomal microRNAs and long noncoding RNAs: Novel mediators of drug resistance in lung cancer. J. Cell Physiol. 2022, 237, 2095–2106. [Google Scholar] [CrossRef]
- Sohrabi, B.; Dayeri, B.; Zahedi, E.; Khoshbakht, S.; Nezamabadi Pour, N.; Ranjbar, H.; Davari Nejad, A.; Noureddini, M.; Alani, B. Mesenchymal stem cell (MSC)-derived exosomes as novel vehicles for delivery of miRNAs in cancer therapy. Cancer Gene Ther. 2022, 29, 1105–1116. [Google Scholar] [CrossRef]
- Sorop, A.; Constantinescu, D.; Cojocaru, F.; Dinischiotu, A.; Cucu, D.; Dima, S.O. Exosomal microRNAs as Biomarkers and Therapeutic Targets for Hepatocellular Carcinoma. Int. J. Mol. Sci. 2021, 22, 4997. [Google Scholar] [CrossRef]
- Mohammadi, R.; Hosseini, S.A.; Noruzi, S.; Ebrahimzadeh, A.; Sahebkar, A. Diagnostic and Therapeutic Applications of Exosome Nanovesicles in Lung Cancer: State-of-The-Art. Anticancer Agents Med. Chem. 2022, 22, 83–100. [Google Scholar] [CrossRef]
- Li, X.; Jiang, W.; Gan, Y.; Zhou, W. The Application of Exosomal MicroRNAs in the Treatment of Pancreatic Cancer and Its Research Progress. Pancreas 2021, 50, 12–16. [Google Scholar] [CrossRef]
- Mowla, M.; Hashemi, A. Functional roles of exosomal miRNAs in multi-drug resistance in cancer chemotherapeutics. Exp. Mol. Pathol. 2021, 118, 104592. [Google Scholar] [CrossRef] [PubMed]
- Nakase, I.; Kobayashi, N.B.; Takatani-Nakase, T.; Yoshida, T. Active macropinocytosis induction by stimulation of epidermal growth factor receptor and oncogenic Ras expression potentiates cellular uptake efficacy of exosomes. Sci. Rep. 2015, 5, 10300. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, Y.; Arafiles, J.V.V.; Kawaguchi, Y.; Nakase, I.; Hirose, H.; Futaki, S. Stearylated Macropinocytosis-Inducing Peptides Facilitating the Cellular Uptake of Small Extracellular Vesicles. Bioconjug. Chem. 2022, 33, 869–880. [Google Scholar] [CrossRef]
- Noguchi, K.; Obuki, M.; Sumi, H.; Klußmann, M.; Morimoto, K.; Nakai, S.; Hashimoto, T.; Fujiwara, D.; Fujii, I.; Yuba, E.; et al. Macropinocytosis-Inducible Extracellular Vesicles Modified with Antimicrobial Protein CAP18-Derived Cell-Penetrating Peptides for Efficient Intracellular Delivery. Mol. Pharm. 2021, 18, 3290–3301. [Google Scholar] [CrossRef]
- Takenaka, T.; Nakai, S.; Katayama, M.; Hirano, M.; Ueno, N.; Noguchi, K.; Takatani-Nakase, T.; Fujii, I.; Kobayashi, S.S.; Nakase, I. Effects of gefitinib treatment on cellular uptake of extracellular vesicles in EGFR-mutant non-small cell lung cancer cells. Int. J. Pharm. 2019, 572, 118762. [Google Scholar] [CrossRef]
- Nakase, I.; Noguchi, K.; Aoki, A.; Takatani-Nakase, T.; Fujii, I.; Futaki, S. Arginine-rich cell-penetrating peptide-modified extracellular vesicles for active macropinocytosis induction and efficient intracellular delivery. Sci. Rep. 2017, 7, 1991. [Google Scholar] [CrossRef] [PubMed]
- Nakase, I.; Noguchi, K.; Fujii, I.; Futaki, S. Vectorization of biomacromolecules into cells using extracellular vesicles with enhanced internalization induced by macropinocytosis. Sci. Rep. 2016, 6, 34937. [Google Scholar] [CrossRef]
- Nakase, I.; Takatani-Nakase, T. Exosomes: Breast cancer-derived extracellular vesicles; recent key findings and technologies in disease progression, diagnostics, and cancer targeting. Drug Metab. Pharmacokinet. 2022, 42, 100435. [Google Scholar] [CrossRef]
- Noguchi, K.; Hirano, M.; Hashimoto, T.; Yuba, E.; Takatani-Nakase, T.; Nakase, I. Effects of Lyophilization of Arginine-rich Cell-penetrating Peptide-modified Extracellular Vesicles on Intracellular Delivery. Anticancer Res. 2019, 39, 6701–6709. [Google Scholar] [CrossRef]
- Siddiqui, H.; Yevstigneyev, N.; Madani, G.; McCormick, S. Approaches to Visualising Endocytosis of LDL-Related Lipoproteins. Biomolecules 2022, 12, 158. [Google Scholar] [CrossRef]
- Varma, S.; Dey, S.; Palanisamy, D. Cellular Uptake Pathways of Nanoparticles: Process of Endocytosis and Factors Affecting their Fate. Curr. Pharm. Biotechnol. 2022, 23, 679–706. [Google Scholar] [CrossRef]
- Cooke, L.D.F.; Tumbarello, D.A.; Harvey, N.C.; Sethi, J.K.; Lewis, R.M.; Cleal, J.K. Endocytosis in the placenta: An undervalued mediator of placental transfer. Placenta 2021, 113, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Vieira, N.; Rito, T.; Correia-Neves, M.; Sousa, N. Sorting Out Sorting Nexins Functions in the Nervous System in Health and Disease. Mol. Neurobiol. 2021, 58, 4070–4106. [Google Scholar] [CrossRef] [PubMed]
- Renard, H.F.; Boucrot, E. Unconventional endocytic mechanisms. Curr. Opin. Cell Biol. 2021, 71, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, A.; Li, L.A.A.; Grøndahl, L. Chitosan Nanomedicine in Cancer Therapy: Targeted Delivery and Cellular Uptake. Macromol. Biosci. 2021, 21, e2100005. [Google Scholar] [CrossRef] [PubMed]
- Kahlhofer, J.; Leon, S.; Teis, D.; Schmidt, O. The α-arrestin family of ubiquitin ligase adaptors links metabolism with selective endocytosis. Biol. Cell 2021, 113, 183–219. [Google Scholar] [CrossRef] [PubMed]
- Luk, B.T.; Zhang, L. Cell membrane-camouflaged nanoparticles for drug delivery. J. Control. Release 2015, 220, 600–607. [Google Scholar] [CrossRef]
- Lee, N.H.; You, S.; Taghizadeh, A.; Taghizadeh, M.; Kim, H.S. Cell Membrane-Cloaked Nanotherapeutics for Targeted Drug Delivery. Int. J. Mol. Sci. 2022, 23, 2223. [Google Scholar] [CrossRef]
- Smith, S.M.; Smith, C.J. Capturing the mechanics of clathrin-mediated endocytosis. Curr. Opin. Struct. Biol. 2022, 75, 102427. [Google Scholar] [CrossRef]
- Shi, R.; Hou, L.; Wei, L.; Liu, J. Involvement of adaptor proteins in clathrin-mediated endocytosis of virus entry. Microb. Pathog. 2021, 161, 105278. [Google Scholar] [CrossRef]
- Redlingshöfer, L.; Brodsky, F.M. Antagonistic regulation controls clathrin-mediated endocytosis: AP2 adaptor facilitation vs restraint from clathrin light chains. Cells Dev. 2021, 168, 203714. [Google Scholar] [CrossRef] [PubMed]
- Moo, E.V.; van Senten, J.R.; Bräuner-Osborne, H.; Møller, T.C. Arrestin-Dependent and -Independent Internalization of G Protein-Coupled Receptors: Methods, Mechanisms, and Implications on Cell Signaling. Mol. Pharmacol. 2021, 99, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Hu, J.; Li, X.; Lu, Z.; Li, X.; Wang, C.; Yu, S. Receptor-Dependent Endocytosis Mediates α-Synuclein Oligomer Transport into Red Blood Cells. Front. Aging Neurosci. 2022, 14, 899892. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Jadhav, H.R.; Bhatt, T. Dynamin Functions and Ligands: Classical Mechanisms Behind. Mol. Pharmacol. 2017, 91, 123–134. [Google Scholar] [CrossRef]
- Wolfe, B.L.; Trejo, J. Clathrin-dependent mechanisms of G protein-coupled receptor endocytosis. Traffic 2007, 8, 462–470. [Google Scholar] [CrossRef]
- Rejman, J.; Oberle, V.; Zuhorn, I.S.; Hoekstra, D. Size-dependent internalization of particles via the pathways of clathrin- and caveolae-mediated endocytosis. Biochem. J. 2004, 377, 159–169. [Google Scholar] [CrossRef]
- Langston Suen, W.L.; Chau, Y. Size-dependent internalisation of folate-decorated nanoparticles via the pathways of clathrin and caveolae-mediated endocytosis in ARPE-19 cells. J. Pharm. Pharmacol. 2014, 66, 564–573. [Google Scholar] [CrossRef]
- Hackett, B.A.; Cherry, S. Flavivirus internalization is regulated by a size-dependent endocytic pathway. Proc. Natl. Acad. Sci. USA 2018, 115, 4246–4251. [Google Scholar] [CrossRef]
- Ueda, Y.; Sato, M. Cell membrane dynamics induction using optogenetic tools. Biochem. Biophys. Res. Commun. 2018, 506, 387–393. [Google Scholar] [CrossRef]
- Gozzelino, L.; De Santis, M.C.; Gulluni, F.; Hirsch, E.; Martini, M. PI(3, 4)P2 Signaling in Cancer and Metabolism. Front. Oncol. 2020, 10, 360. [Google Scholar] [CrossRef]
- Liu, H.; Qian, F. Exploiting macropinocytosis for drug delivery into KRAS mutant cancer. Theranostics 2022, 12, 1321–1332. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ghosh, D. Intracellular nanoparticle delivery by oncogenic KRAS-mediated macropinocytosis. Int. J. Nanomed. 2019, 14, 6589–6600. [Google Scholar] [CrossRef] [PubMed]
- Michalopoulou, E.; Auciello, F.R.; Bulusu, V.; Strachan, D.; Campbell, A.D.; Tait-Mulder, J.; Karim, S.A.; Morton, J.P.; Sansom, O.J.; Kamphorst, J.J. Macropinocytosis Renders a Subset of Pancreatic Tumor Cells Resistant to mTOR Inhibition. Cell Rep. 2020, 30, 2729–2742.e4. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.S.; Cui, J.D.; Lee, D.; Yuen, V.W.; Chiu, D.K.; Goh, C.C.; Cheu, J.W.; Tse, A.P.; Bao, M.H.; Wong, B.P.Y.; et al. Hypoxia-induced macropinocytosis represents a metabolic route for liver cancer. Nat. Commun. 2022, 13, 954. [Google Scholar] [CrossRef]
- Recouvreux, M.V.; Commisso, C. Macropinocytosis: A Metabolic Adaptation to Nutrient Stress in Cancer. Front. Endocrinol. 2017, 8, 261. [Google Scholar] [CrossRef] [PubMed]
- Commisso, C. The pervasiveness of macropinocytosis in oncological malignancies. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2019, 374, 20180153. [Google Scholar] [CrossRef]
- Sutton, M.N.; Gammon, S.T.; Muzzioli, R.; Pisaneschi, F.; Radaram, B.; Yang, P.; Piwnica-Worms, D. RAS-Driven Macropinocytosis of Albumin or Dextran Reveals Mutation-Specific Target Engagement of RAS p.G12C Inhibitor ARS-1620 by NIR-Fluorescence Imaging. Mol. Imaging Biol. 2022, 24, 498–509. [Google Scholar] [CrossRef]
- Sheng, W.; Geng, J.; Li, L.; Shang, Y.; Jiang, M.; Zhen, Y. An albumin-binding domain and targeting peptide-based recombinant protein and its enediyne-integrated analogue exhibit directional delivery and potent inhibitory activity on pancreatic cancer with K-ras mutation. Oncol. Rep. 2020, 43, 851–863. [Google Scholar] [CrossRef]
- Thu, P.M.; Zheng, Z.G.; Zhou, Y.P.; Wang, Y.Y.; Zhang, X.; Jing, D.; Cheng, H.M.; Li, J.; Li, P.; Xu, X. Phellodendrine chloride suppresses proliferation of KRAS mutated pancreatic cancer cells through inhibition of nutrients uptake via macropinocytosis. Eur. J. Pharmacol. 2019, 850, 23–34. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, Y.; Liu, P.; Zhang, X.; Xu, Z.; Tan, X.; Chen, M.; Wang, J. ICP-MS and Photothermal Dual-Readout Assay for Ultrasensitive and Point-of-Care Detection of Pancreatic Cancer Exosomes. Anal. Chem. 2021, 93, 11540–11546. [Google Scholar] [CrossRef]
- Buscail, E.; Alix-Panabières, C.; Quincy, P.; Cauvin, T.; Chauvet, A.; Degrandi, O.; Caumont, C.; Verdon, S.; Lamrissi, I.; Moranvillier, I.; et al. High Clinical Value of Liquid Biopsy to Detect Circulating Tumor Cells and Tumor Exosomes in Pancreatic Ductal Adenocarcinoma Patients Eligible for Up-Front Surgery. Cancers 2019, 11, 1656. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Dong, Z.; Zhen, L.; Xia, G.; Huang, X.; Wang, T.; Guo, H.; Yang, B.; Xu, C.; Wu, W.; et al. Combined Exosomal GPC1, CD82, and Serum CA19-9 as Multiplex Targets: A Specific, Sensitive, and Reproducible Detection Panel for the Diagnosis of Pancreatic Cancer. Mol. Cancer Res. 2020, 18, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Buscail, E.; Chauvet, A.; Quincy, P.; Degrandi, O.; Buscail, C.; Lamrissi, I.; Moranvillier, I.; Caumont, C.; Verdon, S.; Brisson, A.; et al. CD63-GPC1-Positive Exosomes Coupled with CA19-9 Offer Good Diagnostic Potential for Resectable Pancreatic Ductal Adenocarcinoma. Transl. Oncol. 2019, 12, 1395–1403. [Google Scholar] [CrossRef] [PubMed]
- Zanetti-Domingues, L.C.; Bonner, S.E.; Iyer, R.S.; Martin-Fernandez, M.L.; Huber, V. Cooperation and Interplay between EGFR Signalling and Extracellular Vesicle Biogenesis in Cancer. Cells 2020, 9, 2639. [Google Scholar] [CrossRef] [PubMed]
- Gonda, A.; Kabagwira, J.; Senthil, G.N.; Wall, N.R. Internalization of Exosomes through Receptor-Mediated Endocytosis. Mol. Cancer Res. 2019, 17, 337–347. [Google Scholar] [CrossRef]
- Ramos-Zaldívar, H.M.; Polakovicova, I.; Salas-Huenuleo, E.; Corvalán, A.H.; Kogan, M.J.; Yefi, C.P.; Andia, M.E. Extracellular vesicles through the blood-brain barrier: A review. Fluids Barriers CNS 2022, 19, 60. [Google Scholar] [CrossRef]
- Nakase, I. Biofunctional Peptide-Modified Extracellular Vesicles Enable Effective Intracellular Delivery via the Induction of Macropinocytosis. Processes 2021, 9, 224. [Google Scholar] [CrossRef]
- Sun, W.; Ren, Y.; Lu, Z.; Zhao, X. The potential roles of exosomes in pancreatic cancer initiation and metastasis. Mol. Cancer 2020, 19, 135. [Google Scholar] [CrossRef]
- Beit-Yannai, E.; Tabak, S.; Stamer, W.D. Physical exosome:exosome interactions. J. Cell Mol. Med. 2018, 22, 2001–2006. [Google Scholar] [CrossRef]
- Midekessa, G.; Godakumara, K.; Ord, J.; Viil, J.; Lättekivi, F.; Dissanayake, K.; Kopanchuk, S.; Rinken, A.; Andronowska, A.; Bhattacharjee, S.; et al. Zeta Potential of Extracellular Vesicles: Toward Understanding the Attributes that Determine Colloidal Stability. ACS Omega 2020, 5, 16701–16710. [Google Scholar] [CrossRef]
- Kesimer, M.; Gupta, R. Physical characterization and profiling of airway epithelial derived exosomes using light scattering. Methods 2015, 87, 59–63. [Google Scholar] [CrossRef]
- Yang, Y.; Shen, G.; Wang, H.; Li, H.; Zhang, T.; Tao, N.; Ding, X.; Yu, H. Interferometric plasmonic imaging and detection of single exosomes. Proc. Natl. Acad. Sci. USA 2018, 115, 10275–10280. [Google Scholar] [CrossRef] [PubMed]
- Pedrioli, G.; Paganetti, P. Hijacking Endocytosis and Autophagy in Extracellular Vesicle Communication: Where the Inside Meets the Outside. Front. Cell Dev. Biol. 2021, 8, 595515. [Google Scholar] [CrossRef] [PubMed]
- Kamerkar, S.; LeBleu, V.S.; Sugimoto, H.; Yang, S.; Ruivo, C.F.; Melo, S.A.; Lee, J.J.; Kalluri, R. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature 2017, 546, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Sun, M.; Liu, Z.; Liu, H.; Kong, W.; Wang, R.; Qian, F. Macropinocytic dextran facilitates KRAS-targeted delivery while reducing drug-induced tumor immunity depletion in pancreatic cancer. Theranostics 2022, 12, 1061–1073. [Google Scholar] [CrossRef]
- Mendt, M.; Kamerkar, S.; Sugimoto, H.; McAndrews, K.M.; Wu, C.C.; Gagea, M.; Yang, S.; Blanko, E.V.R.; Peng, Q.; Ma, X.; et al. Generation and testing of clinical-grade exosomes for pancreatic cancer. JCI Insight 2018, 3, e99263. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhao, G.; Yang, S.; Zhu, S.; Zhang, S.; Li, P. The significance of exosomal RNAs in the development, diagnosis, and treatment of pancreatic cancer. Cancer Cell Int. 2021, 21, 364. [Google Scholar] [CrossRef]
- Jing, C.; Cao, H.; Qin, X.; Yu, S.; Wu, J.; Wang, Z.; Ma, R.; Feng, J. Exosome-mediated gefitinib resistance in lung cancer HCC827 cells via delivery of miR-21. Oncol. Lett. 2018, 15, 9811–9817. [Google Scholar] [CrossRef]
- Srivastava, A.; Amreddy, N.; Babu, A.; Panneerselvam, J.; Mehta, M.; Muralidharan, R.; Chen, A.; Zhao, Y.D.; Razaq, M.; Riedinger, N.; et al. Nanosomes carrying doxorubicin exhibit potent anticancer activity against human lung cancer cells. Sci. Rep. 2016, 6, 38541. [Google Scholar] [CrossRef]
- Rizwan, M.N.; Ma, Y.; Nenkov, M.; Jin, L.; Schröder, D.C.; Westermann, M.; Gaßler, N.; Chen, Y. Tumor-derived exosomes: Key players in non-small cell lung cancer metastasis and their implication for targeted therapy. Mol. Carcinog. 2022, 61, 269–280. [Google Scholar] [CrossRef]
- Nakase, I. Development of Intracellular Delivery System Based on Biofunctional Peptide–modified Exosome. Membrane 2016, 41, 209–214. [Google Scholar] [CrossRef]
- Sancho-Albero, M.; Sebastián, V.; Sesé, J.; Pazo-Cid, R.; Mendoza, G.; Arruebo, M.; Martín-Duque, P.; Santamaría, J. Isolation of exosomes from whole blood by a new microfluidic device: Proof of concept application in the diagnosis and monitoring of pancreatic cancer. J. Nanobiotechnol. 2020, 18, 150. [Google Scholar] [CrossRef] [PubMed]
- Nakase, I.; Akita, H.; Kogure, K.; Gräslund, A.; Langel, U.; Harashima, H.; Futaki, S. Efficient intracellular delivery of nucleic acid pharmaceuticals using cell-penetrating peptides. Acc. Chem. Res. 2012, 45, 1132–1139. [Google Scholar] [CrossRef]
- Nakase, I.; Ueno, N.; Katayama, M.; Noguchi, K.; Takatani-Nakase, T.; Kobayashi, N.B.; Yoshida, T.; Fujii, I.; Futaki, S. Receptor clustering and activation by multivalent interaction through recognition peptides presented on exosomes. Chem. Commun. 2016, 53, 317–320. [Google Scholar] [CrossRef] [PubMed]
- Futaki, S.; Nakase, I. Cell-Surface Interactions on Arginine-Rich Cell-Penetrating Peptides Allow for Multiplex Modes of Internalization. Acc. Chem. Res. 2017, 50, 2449–2456. [Google Scholar] [CrossRef]
- Nakase, I.; Osaki, K.; Tanaka, G.; Utani, A.; Futaki, S. Molecular interplays involved in the cellular uptake of octaarginine on cell surfaces and the importance of syndecan-4 cytoplasmic V domain for the activation of protein kinase Cα. Biochem. Biophys. Res. Commun. 2014, 446, 857–862. [Google Scholar] [CrossRef]
- Albrecht, L.V.; Tejeda-Muñoz, N.; Bui, M.H.; Cicchetto, A.C.; Di Biagio, D.; Colozza, G.; Schmid, E.; Piccolo, S.; Christofk, H.R.; De Robertis, E.M. GSK3 Inhibits Macropinocytosis and Lysosomal Activity through the Wnt Destruction Complex Machinery. Cell Rep. 2020, 32, 107973. [Google Scholar] [CrossRef]
- Reggiori, F.; Gabius, H.J.; Aureli, M.; Römer, W.; Sonnino, S.; Eskelinen, E.L. Glycans in autophagy, endocytosis and lysosomal functions. Glycoconj. J. 2021, 38, 625–647. [Google Scholar] [CrossRef]
- Kobayashi, S.; Nakase, I.; Kawabata, N.; Yu, H.H.; Pujals, S.; Imanishi, M.; Giralt, E.; Futaki, S. Cytosolic targeting of macromolecules using a pH-dependent fusogenic peptide in combination with cationic liposomes. Bioconjug. Chem. 2009, 20, 953–959. [Google Scholar] [CrossRef]
- Nakase, I.; Kogure, K.; Harashima, H.; Futaki, S. Application of a fusiogenic peptide GALA for intracellular delivery. Methods Mol. Biol. 2011, 683, 525–533. [Google Scholar] [CrossRef]
- Nakase, I.; Futaki, S. Combined treatment with a pH-sensitive fusogenic peptide and cationic lipids achieves enhanced cytosolic delivery of exosomes. Sci. Rep. 2015, 5, 10112. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaka, Y.; Tanihata, J.; Komaki, H.; Ishiyama, A.; Oya, Y.; Rüegg, U.; Takeda, S.I.; Hashido, K. Characterization and Functional Analysis of Extracellular Vesicles and Muscle-Abundant miRNAs (miR-1, miR-133a, and miR-206) in C2C12 Myocytes and mdx Mice. PLoS ONE 2016, 11, e0167811. [Google Scholar] [CrossRef] [PubMed]
- Sivanantham, A.; Jin, Y. Impact of Storage Conditions on EV Integrity/Surface Markers and Cargos. Life 2022, 12, 697. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Y.; Li, Y.J.; Hu, X.B.; Huang, S.; Xiang, D.X. Preservation of small extracellular vesicles for functional analysis and therapeutic applications: A comparative evaluation of storage conditions. Drug Deliv. 2021, 28, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bi, J.; Huang, J.; Tang, Y.; Du, S.; Li, P. Exosome: A Review of Its Classification, Isolation Techniques, Storage, Diagnostic and Targeted Therapy Applications. Int. J. Nanomed. 2020, 15, 6917–6934. [Google Scholar] [CrossRef] [PubMed]
- Jeyaram, A.; Jay, S.M. Preservation and Storage Stability of Extracellular Vesicles for Therapeutic Applications. AAPS J. 2017, 20, 1. [Google Scholar] [CrossRef]
- Görgens, A.; Corso, G.; Hagey, D.W.; Jawad Wiklander, R.; Gustafsson, M.O.; Felldin, U.; Lee, Y.; Bostancioglu, R.B.; Sork, H.; Liang, X.; et al. Identification of storage conditions stabilizing extracellular vesicles preparations. J. Extracell. Vesicles 2022, 11, e12238. [Google Scholar] [CrossRef]
- Deville, S.; Berckmans, P.; Van Hoof, R.; Lambrichts, I.; Salvati, A.; Nelissen, I. Comparison of extracellular vesicle isolation and storage methods using high-sensitivity flow cytometry. PLoS ONE 2021, 16, e0245835. [Google Scholar] [CrossRef]
- Tsuchiya, A.; Terai, S.; Horiguchi, I.; Homma, Y.; Saito, A.; Nakamura, N.; Sato, Y.; Ochiya, T.; Kino-Oka, M.; Working Group of Attitudes for Preparation and Treatment of Exosomes of Japanese Society of Regenerative Medicine. Basic points to consider regarding the preparation of extracellular vesicles and their clinical applications in Japan. Regen. Ther. 2022, 21, 19–24. [Google Scholar] [CrossRef]
- Gimona, M.; Brizzi, M.F.; Choo, A.B.H.; Dominici, M.; Davidson, S.M.; Grillari, J.; Hermann, D.M.; Hill, A.F.; de Kleijn, D.; Lai, R.C.; et al. Critical considerations for the development of potency tests for therapeutic applications of mesenchymal stromal cell-derived small extracellular vesicles. Cytotherapy 2021, 23, 373–380. [Google Scholar] [CrossRef]
- Umezu, T.; Takanashi, M.; Murakami, Y.; Ohno, S.I.; Kanekura, K.; Sudo, K.; Nagamine, K.; Takeuchi, S.; Ochiya, T.; Kuroda, M. Acerola exosome-like nanovesicles to systemically deliver nucleic acid medicine via oral administration. Mol. Ther. Methods Clin. Dev. 2021, 21, 199–208. [Google Scholar] [CrossRef]
- Fujita, Y.; Kadota, T.; Araya, J.; Ochiya, T.; Kuwano, K. Clinical Application of Mesenchymal Stem Cell-Derived Extracellular Vesicle-Based Therapeutics for Inflammatory Lung Diseases. J. Clin. Med. 2018, 7, 355. [Google Scholar] [CrossRef] [PubMed]
- Urabe, F.; Kosaka, N.; Kimura, T.; Egawa, S.; Ochiya, T. Extracellular vesicles: Toward a clinical application in urological cancer treatment. Int. J. Urol. 2018, 25, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Liew, L.C.; Katsuda, T.; Gailhouste, L.; Nakagama, H.; Ochiya, T. Mesenchymal stem cell-derived extracellular vesicles: A glimmer of hope in treating Alzheimer’s disease. Int. Immunol. 2017, 29, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, G.; Yu, T.; He, J.; Liu, J.; Chai, X.; Zhao, G.; Yin, D.; Zhang, C. Exosomes deliver lncRNA DARS-AS1 siRNA to inhibit chronic unpredictable mild stress-induced TNBC metastasis. Cancer Lett. 2022, 543, 215781. [Google Scholar] [CrossRef] [PubMed]
- Shan, S.; Chen, J.; Sun, Y.; Wang, Y.; Xia, B.; Tan, H.; Pan, C.; Gu, G.; Zhong, J.; Qing, G.; et al. Functionalized Macrophage Exosomes with Panobinostat and PPM1D-siRNA for Diffuse Intrinsic Pontine Gliomas Therapy. Adv. Sci. 2022, 9, e2200353. [Google Scholar] [CrossRef] [PubMed]
- Subhan, M.A.; Torchilin, V.P. siRNA based drug design, quality, delivery and clinical translation. Nanomedicine 2020, 29, 102239. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, H.; Ning, T.; Liu, D.; Deng, T.; Liu, R.; Bai, M.; Zhu, K.; Li, J.; Fan, Q.; et al. Exosome-Delivered c-Met siRNA Could Reverse Chemoresistance to Cisplatin in Gastric Cancer. Int. J. Nanomed. 2020, 15, 2323–2335. [Google Scholar] [CrossRef]
- Zhupanyn, P.; Ewe, A.; Büch, T.; Malek, A.; Rademacher, P.; Müller, C.; Reinert, A.; Jaimes, Y.; Aigner, A. Extracellular vesicle (ECV)-modified polyethylenimine (PEI) complexes for enhanced siRNA delivery in vitro and in vivo. J. Control. Release 2020, 319, 63–76. [Google Scholar] [CrossRef]
- Nishida-Aoki, N.; Tominaga, N.; Takeshita, F.; Sonoda, H.; Yoshioka, Y.; Ochiya, T. Disruption of Circulating Extracellular Vesicles as a Novel Therapeutic Strategy against Cancer Metastasis. Mol. Ther. 2017, 25, 181–191. [Google Scholar] [CrossRef]
- Zafarani, A.; Taghavi-Farahabadi, M.; Razizadeh, M.H.; Amirzargar, M.R.; Mansouri, M.; Mahmoudi, M. The Role of NK Cells and Their Exosomes in Graft Versus Host Disease and Graft Versus Leukemia. Stem Cell Rev. Rep. 2022, in press. [Google Scholar] [CrossRef]
- Fujii, S.; Miura, Y. Immunomodulatory and regenerative effects of MSC-derived extracellular vesicles to treat acute GVHD. Stem Cells 2022, in press. [Google Scholar] [CrossRef]
- Fujii, S.; Miura, Y.; Fujishiro, A.; Shindo, T.; Shimazu, Y.; Hirai, H.; Tahara, H.; Takaori-Kondo, A.; Ichinohe, T.; Maekawa, T. Graft-Versus-Host Disease Amelioration by Human Bone Marrow Mesenchymal Stromal/Stem Cell-Derived Extracellular Vesicles Is Associated with Peripheral Preservation of Naive T Cell Populations. Stem Cells 2018, 36, 434–445. [Google Scholar] [CrossRef] [PubMed]
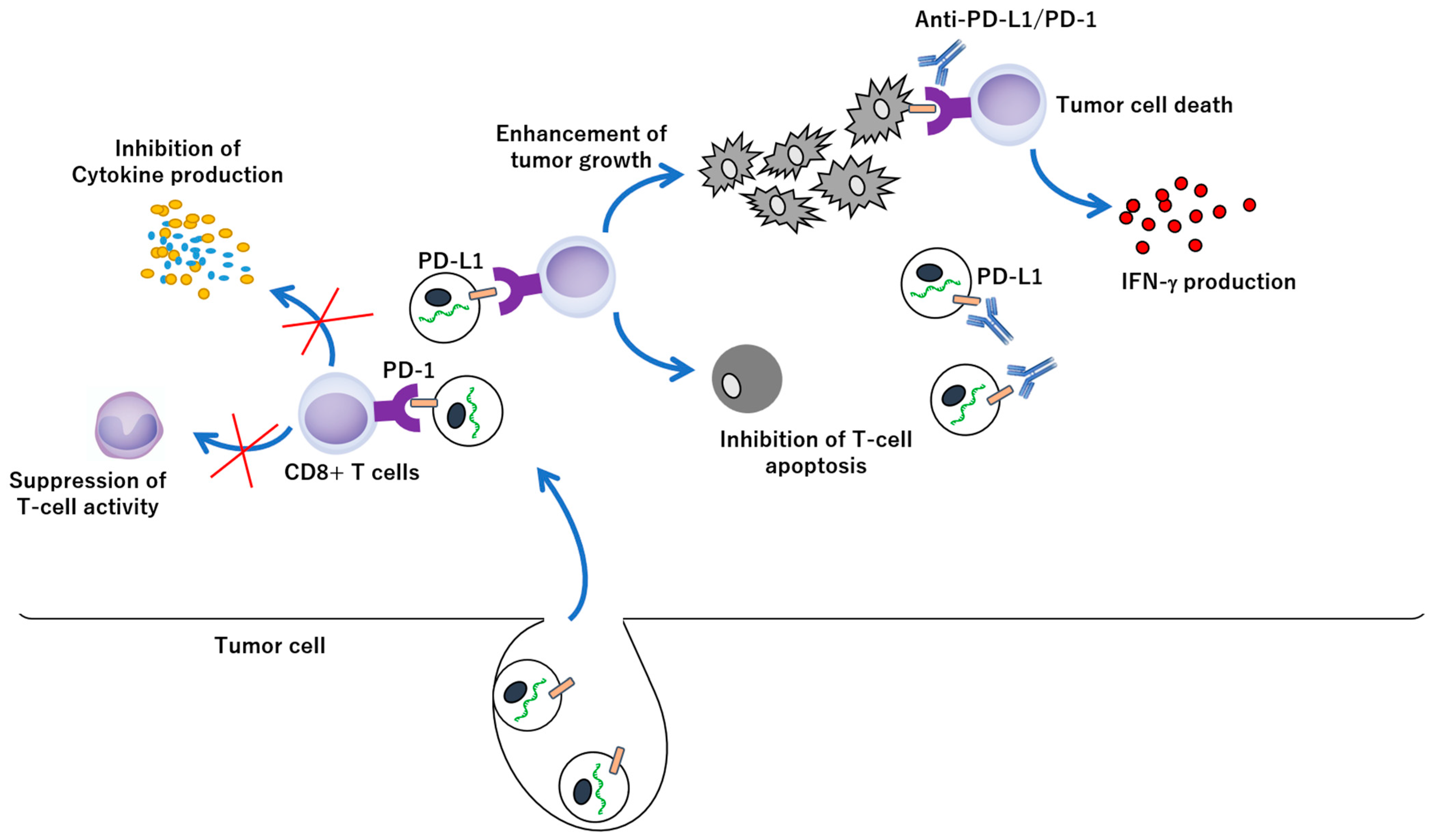
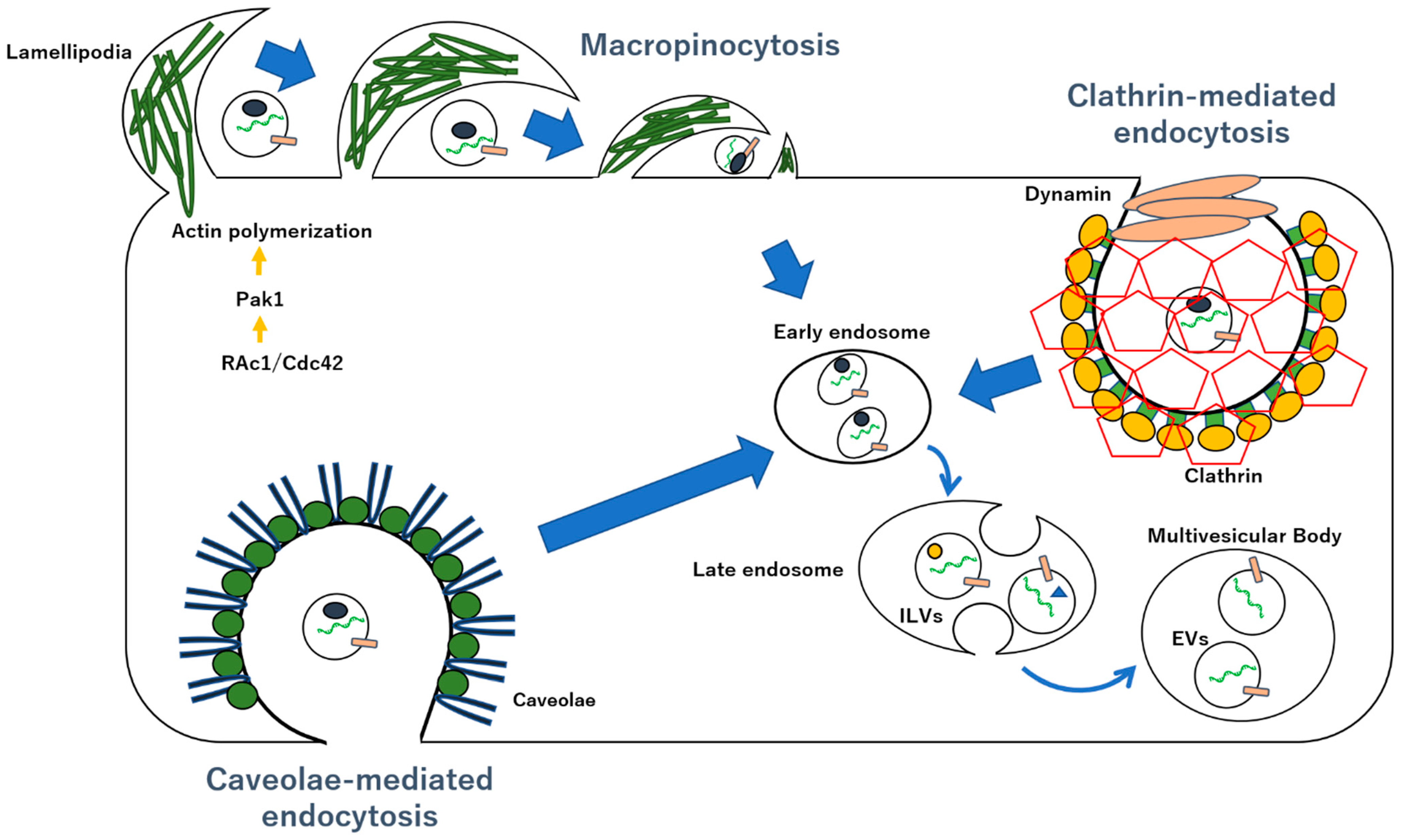
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsuzaka, Y.; Yashiro, R. Molecular Docking and Intracellular Translocation of Extracellular Vesicles for Efficient Drug Delivery. Int. J. Mol. Sci. 2022, 23, 12971. https://doi.org/10.3390/ijms232112971
Matsuzaka Y, Yashiro R. Molecular Docking and Intracellular Translocation of Extracellular Vesicles for Efficient Drug Delivery. International Journal of Molecular Sciences. 2022; 23(21):12971. https://doi.org/10.3390/ijms232112971
Chicago/Turabian StyleMatsuzaka, Yasunari, and Ryu Yashiro. 2022. "Molecular Docking and Intracellular Translocation of Extracellular Vesicles for Efficient Drug Delivery" International Journal of Molecular Sciences 23, no. 21: 12971. https://doi.org/10.3390/ijms232112971
APA StyleMatsuzaka, Y., & Yashiro, R. (2022). Molecular Docking and Intracellular Translocation of Extracellular Vesicles for Efficient Drug Delivery. International Journal of Molecular Sciences, 23(21), 12971. https://doi.org/10.3390/ijms232112971





