Absence of CCR2 Promotes Proliferation of Alveolar Macrophages That Control Lung Inflammation in Acute Respiratory Distress Syndrome in Mice
Abstract
1. Introduction
2. Results
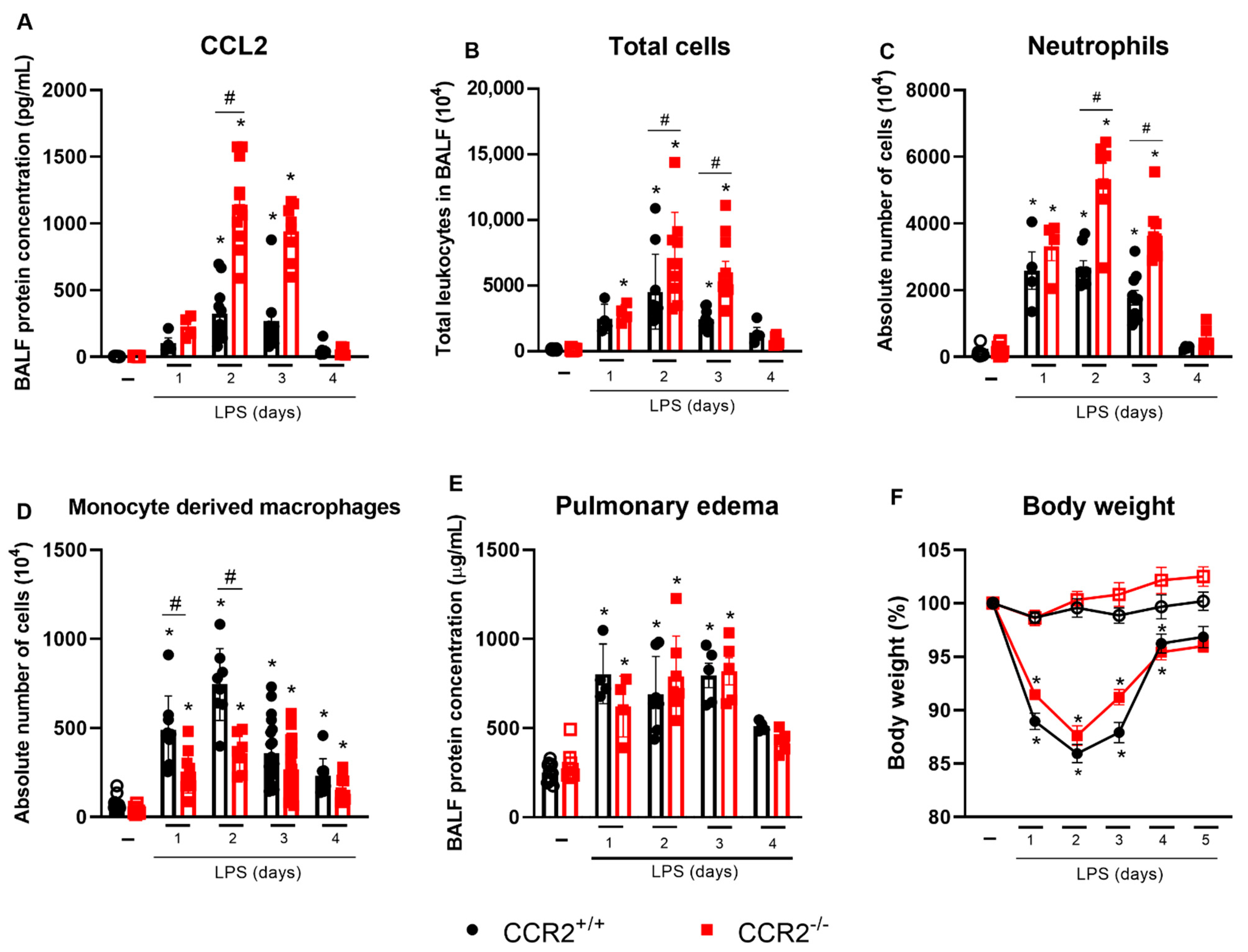
2.1. Lack of CCR2 Modifies the Recruitment Profile of Monocytes and Neutrophils in Early Time Points after LPS Instillation
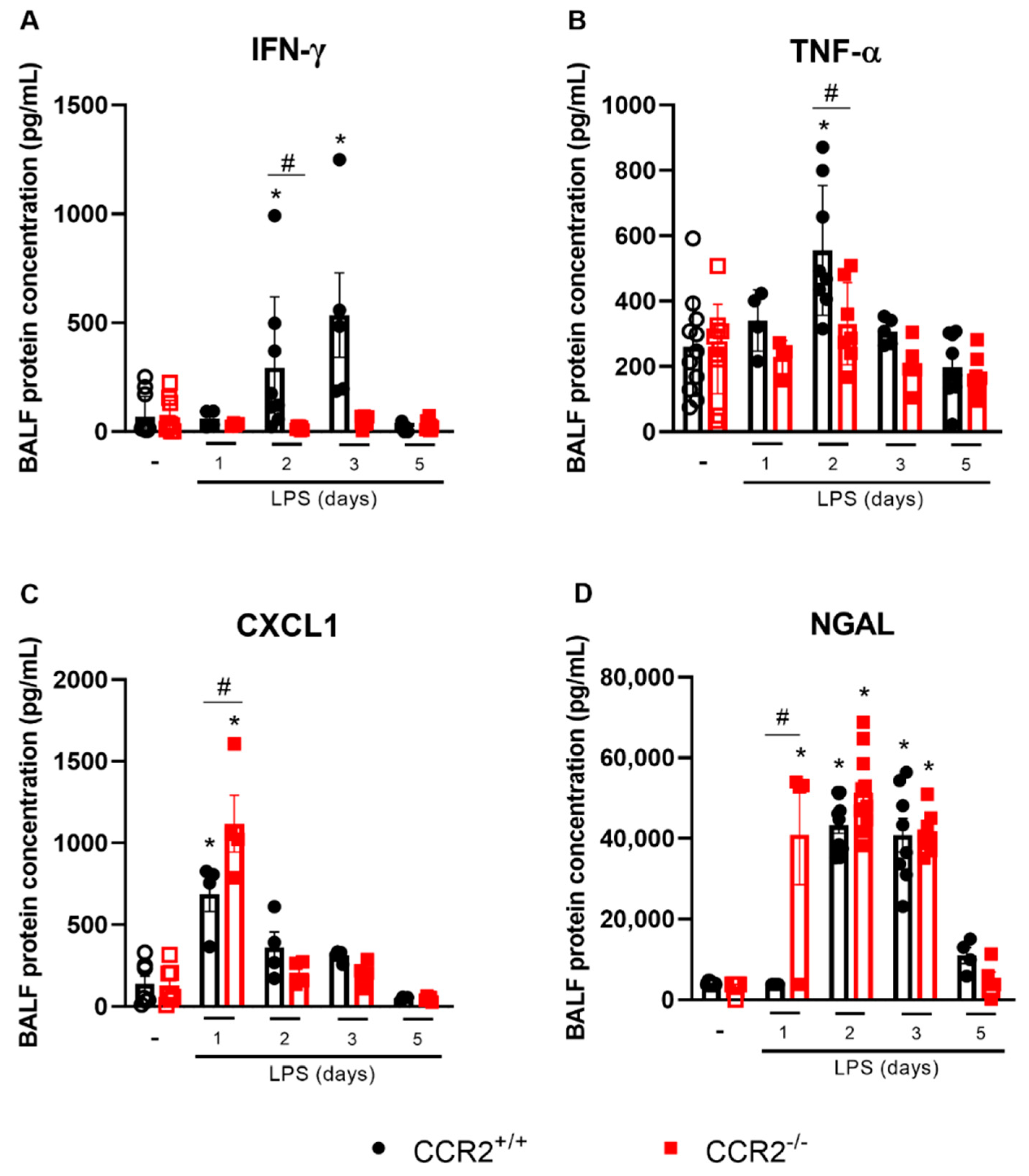
2.2. Cytokine Production in the Initial Phases of Inflammation Is Altered in the Absence of CCR2, but Does Not Impact the Tissue Damage
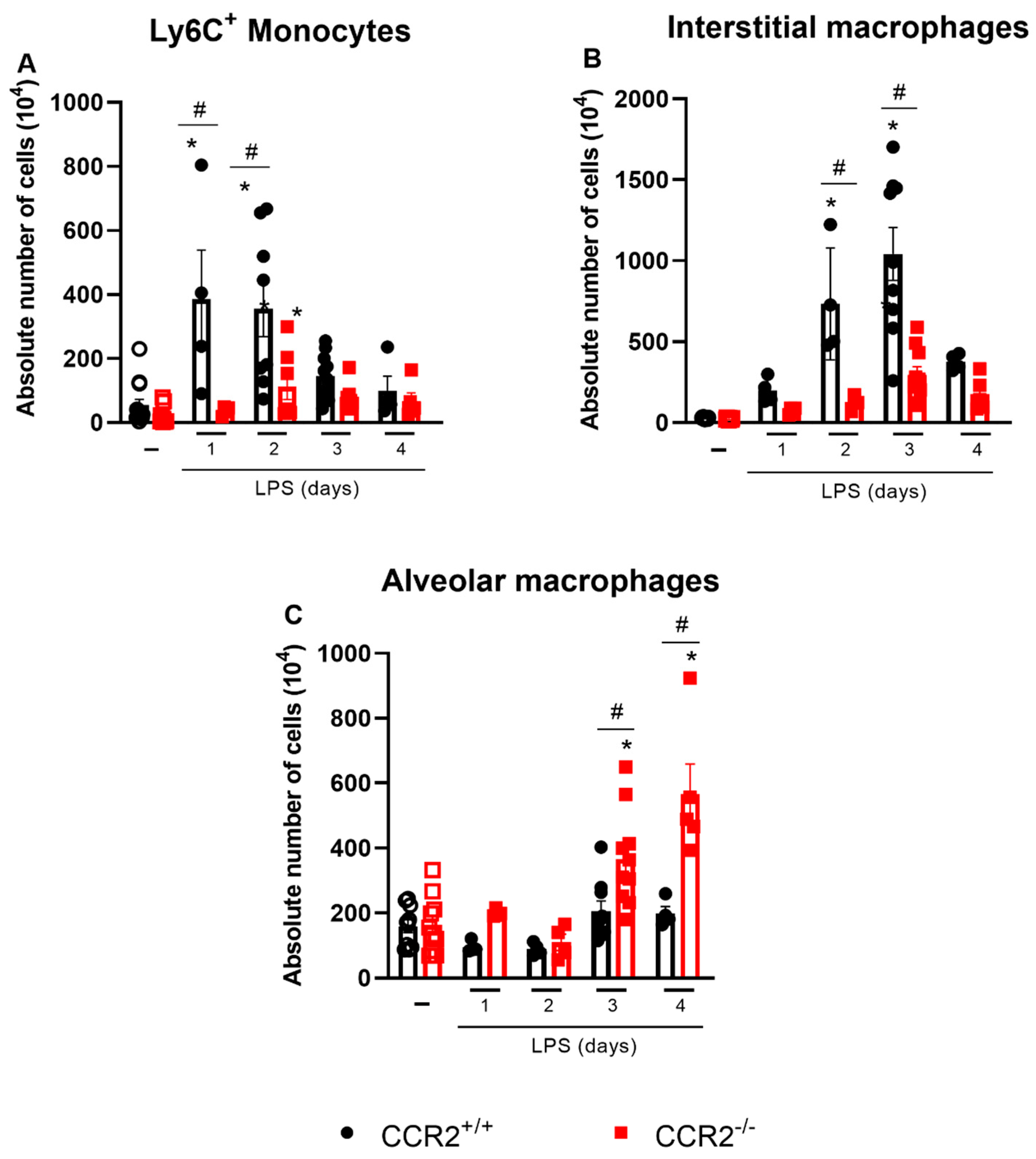
2.3. The Profile of Monocytes/Macrophages Varies between CCR2+/+ and CCR2−/− Mice
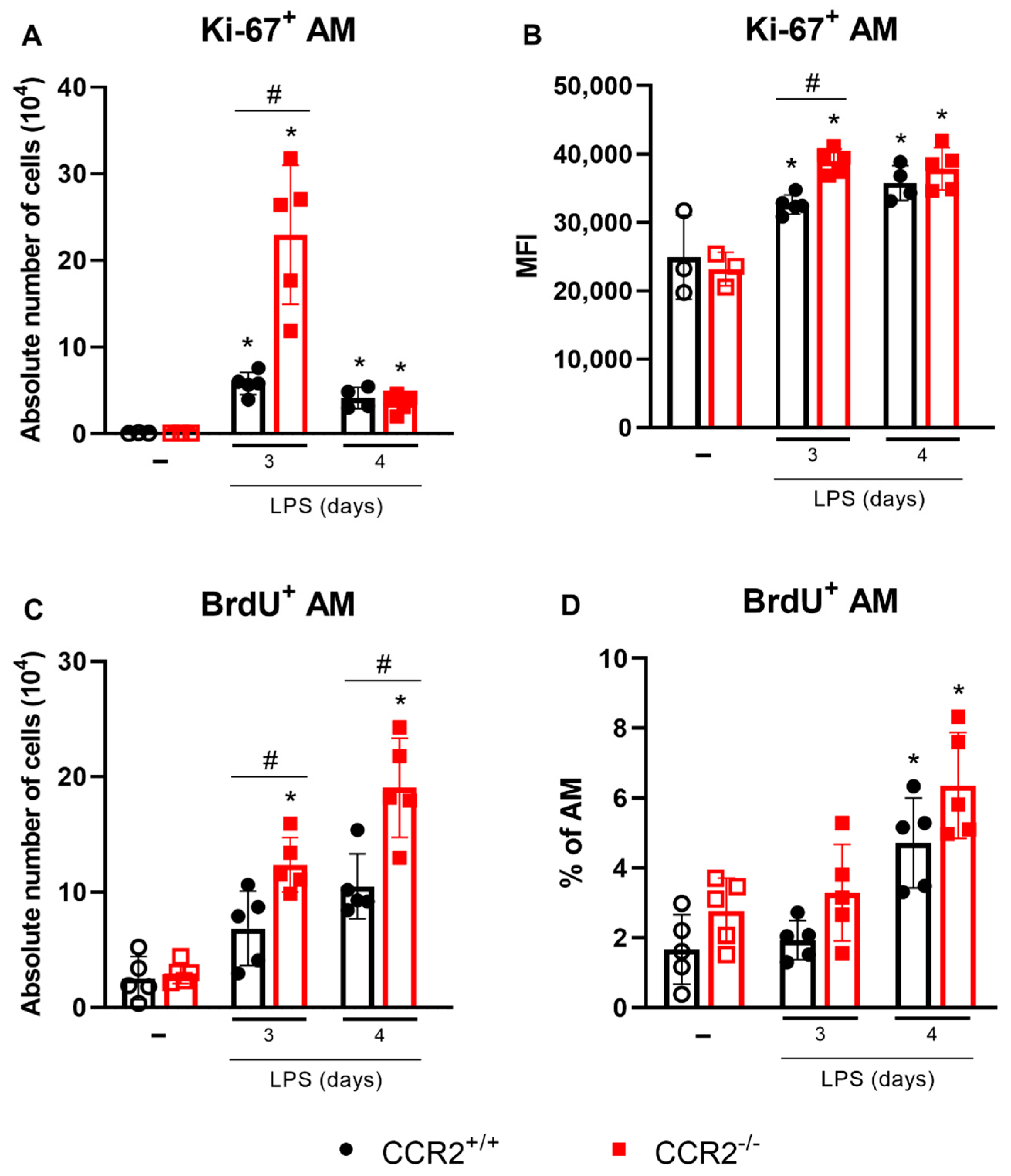
2.4. Alveolar Macrophages Can Be Associated with the Final Events of Tissue Inflammation and Its Resolution in the Absence of CCR2
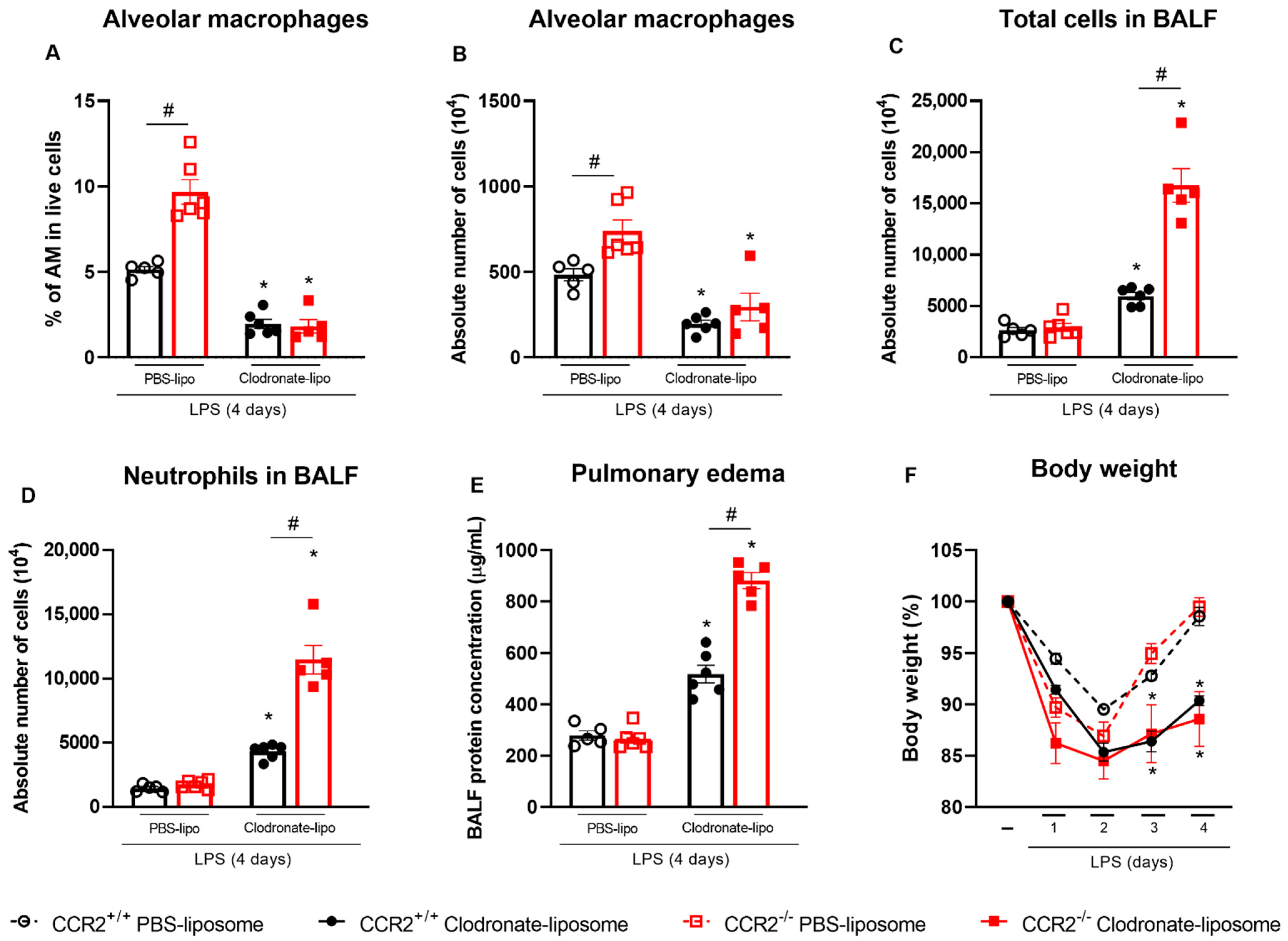
2.5. Depletion of Alveolar Macrophages before the LPS Challenge Leads to Uncontrolled Inflammation Which Is Worsened in the Absence of CCR2
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. ARDS Model
4.3. BALF Protein Concentration
4.4. Isolation of Single Cells from the Lungs
4.5. Staining and Flow Cytometry
4.6. Proliferation Assays
4.6.1. Ki-67 Staining
4.6.2. BrdU Staining
4.7. Quantitation of Neutrophil Products, Growth Factors and Cytokines in BALF by ELISA
4.8. Histology
4.9. qPCR Analysis
4.10. Depletion of Alveolar Macrophages Using Clodronate Loaded Liposomes
4.11. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ashbaugh, D.; Boyd Bigelow, D.; Petty, T.; Levine, B. ACUTE RESPIRATORY DISTRESS IN ADULTS. Lancet 1967, 290, 319–323. [Google Scholar] [CrossRef]
- Johnson, E.R.; Matthay, M.A. Acute Lung Injury: Epidemiology, Pathogenesis, and Treatment. J. Aerosol. Med. Pulm. Drug Deliv. 2010, 23, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Zemans, R.L.; Colgan, S.P.; Downey, G.P. Transepithelial Migration of Neutrophils. Am. J. Respir. Cell Mol. Biol. 2009, 40, 519–535. [Google Scholar] [CrossRef]
- Calfee, C.S.; Eisner, M.D.; Parsons, P.E.; Thompson, B.T.; Conner, E.R.; Matthay, M.A.; Ware, L.B. Soluble Intercellular Adhesion Molecule-1 and Clinical Outcomes in Patients with Acute Lung Injury. Intensive Care Med. 2009, 35, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Matthay, M.A.; Zemans, R.L.; Zimmerman, G.A.; Arabi, Y.M.; Beitler, J.R.; Mercat, A.; Herridge, M.; Randolph, A.G.; Calfee, C.S. Acute Respiratory Distress Syndrome. Nat. Rev. Dis. Prim. 2019, 5, 18. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, R.M.; McAuley, D.F. Acute Respiratory Distress Syndrome. Lancet 2016, 388, 2416–2430. [Google Scholar] [CrossRef]
- Sadikot, R.T.; Rubinstein, I. Long-Acting, Multi-Targeted Nanomedicine: Addressing Unmet Medical Need in Acute Lung Injury. J. Biomed. Nanotechnol. 2009, 5, 614–619. [Google Scholar] [CrossRef]
- Zoulikha, M.; Xiao, Q.; Boafo, G.F.; Sallam, M.A.; Chen, Z.; He, W. Pulmonary Delivery of SiRNA against Acute Lung Injury/Acute Respiratory Distress Syndrome. Acta Pharm. Sin. B 2022, 12, 600–620. [Google Scholar] [CrossRef]
- Dowdy, D.W.; Eid, M.P.; Dennison, C.R.; Mendez-Tellez, P.A.; Herridge, M.S.; Guallar, E.; Pronovost, P.J.; Needham, D.M. Quality of Life after Acute Respiratory Distress Syndrome: A Meta-Analysis. Intensive Care Med. 2006, 32, 1115–1124. [Google Scholar] [CrossRef]
- Choi, H.; Song, I.-A.; Oh, T.K. Quality of Life and Mortality among Survivors of Acute Respiratory Distress Syndrome in South Korea: A Nationwide Cohort Study. J. Anesth. 2022, 36, 230–238. [Google Scholar] [CrossRef]
- Fullerton, J.N.; Gilroy, D.W. Resolution of Inflammation: A New Therapeutic Frontier. Nat. Rev. Drug Discov. 2016, 15, 551–567. [Google Scholar] [CrossRef] [PubMed]
- Holt, P.G.; Strickland, D.H.; Wikström, M.E.; Jahnsen, F.L. Regulation of Immunological Homeostasis in the Respiratory Tract. Nat. Rev. Immunol. 2008, 8, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Melo, E.M.; Oliveira, V.L.S.; Boff, D.; Galvão, I. Pulmonary Macrophages and Their Different Roles in Health and Disease. Int. J. Biochem. Cell Biol. 2021, 141, 1060–1095. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Xiu, H.; Zhang, S.; Zhang, G. The Role of Macrophages in the Pathogenesis of ALI/ARDS. Mediat. Inflamm. 2018, 2018, 1264913. [Google Scholar] [CrossRef]
- Machado-Aranda, D.; Suresh, M.V.; Yu, B.; Dolgachev, V.; Hemmila, M.R.; Raghavendran, K. Alveolar Macrophage Depletion Increases the Severity of Acute Inflammation Following Nonlethal Unilateral Lung Contusion in Mice. J. Trauma Acute Care Surg. 2014, 76, 982–990. [Google Scholar] [CrossRef]
- Petermann, M.; Orfanos, Z.; Sellau, J.; Gharaibeh, M.; Lotter, H.; Fleischer, B.; Keller, C. CCR2 Deficiency Impairs Ly6Clo and Ly6Chi Monocyte Responses in Orientia Tsutsugamushi Infection. Front. Immunol. 2021, 12, 670219. [Google Scholar] [CrossRef]
- Rose, C.E.; Sung, S.S.J.; Fu, S.M. Significant Involvement of CCL2 (MCP-1) in Inflammatory Disorders of the Lung. Microcirculation 2003, 10, 273–288. [Google Scholar] [CrossRef]
- Levy, B.D.; Vachier, I.; Serhan, C.N. Resolution of Inflammation in Asthma. Clin. Chest Med. 2012, 33, 559–570. [Google Scholar] [CrossRef]
- Buckley, C.D.; Gilroy, D.W.; Serhan, C.N.; Stockinger, B.; Tak, P.P. The Resolution of Inflammation. Nat. Rev. Immunol. 2013, 13, 59–66. [Google Scholar] [CrossRef]
- Basil, M.C.; Levy, B.D. Specialized Pro-Resolving Mediators: Endogenous Regulators of Infection and Inflammation. Nat. Rev. Immunol. 2016, 16, 51–67. [Google Scholar] [CrossRef]
- Levy, B.D.; Serhan, C.N. Resolution of Acute Inflammation in the Lung. Annu. Rev. Physiol. 2014, 76, 467–492. [Google Scholar] [CrossRef] [PubMed]
- Rittirsch, D.; Flierl, M.A.; Day, D.E.; Nadeau, B.A.; McGuire, S.R.; Hoesel, L.M.; Ipaktchi, K.; Zetoune, F.S.; Sarma, J.V.; Leng, L.; et al. Acute Lung Injury Induced by Lipopolysaccharide Is Independent of Complement Activation. J. Immunol. 2008, 180, 7664–7672. [Google Scholar] [CrossRef] [PubMed]
- Butt, Y.; Kurdowska, A.; Allen, T.C. Acute Lung Injury: A Clinical and Molecular Review. Arch. Pathol. Lab. Med. 2016, 140, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Bell, T.; Salek-Ardakani, S.; Hussell, T. Macrophage Adaptation in Airway Inflammatory Resolution. Eur. Respir. Rev. 2015, 24, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Bakhshayesh, M.; Zaker, F.; Hashemi, M.; Katebi, M.; Solaimani, M. TGF- Β1-Mediated Apoptosis Associated with SMAD-Dependent Mitochondrial Bcl-2 Expression. Clin. Lymphoma Myeloma Leuk. 2012, 12, 138–143. [Google Scholar] [CrossRef]
- Atri, C.; Guerfali, F.Z.; Laouini, D. Role of Human Macrophage Polarization in Inflammation during Infectious Diseases. Int. J. Mol. Sci. 2018, 19, 1801. [Google Scholar] [CrossRef]
- Arabpour, M.; Saghazadeh, A.; Rezaei, N. Anti-Inflammatory and M2 Macrophage Polarization-Promoting Effect of Mesenchymal Stem Cell-Derived Exosomes. Int. Immunopharmacol. 2021, 97, 107823. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.; Tong, L.; Wang, J.; Dou, M.; Ji, S.; Bi, J.; Chen, C.; Yang, D.; He, H.; et al. Recovery from Acute Lung Injury Can Be Regulated via Modulation of Regulatory T Cells and Th17 Cells. Scand. J. Immunol. 2018, 88, e12715. [Google Scholar] [CrossRef]
- Feehan, K.T.; Gilroy, D.W. Is Resolution the End of Inflammation? Trends Mol. Med. 2019, 25, 198–214. [Google Scholar] [CrossRef]
- Tsou, C.L.; Peters, W.; Si, Y.; Slaymaker, S.; Aslanian, A.M.; Weisberg, S.P.; Mack, M.; Charo, I.F. Critical Roles for CCR2 and MCP-3 in Monocyte Mobilization from Bone Marrow and Recruitment to Inflammatory Sites. J. Clin. Invest. 2007, 117, 902–909. [Google Scholar] [CrossRef]
- Ip, W.K.; Wong, C.K.; Lam, C.W.K. Interleukin (IL)-4 and IL-13 up-Regulate Monocyte Chemoattractant Protein-1 Expression in Human Bronchial Epithelial Cells: Involvement of P38 Mitogen-Activated Protein Kinase, Extracellular Signal-Regulated Kinase 1/2 and Janus Kinase-2 but Not c-Jun NH2. Clin. Exp. Immunol. 2006, 145, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Flores-Villanueva, P.O.; Ruiz-Morales, J.A.; Song, C.H.; Flores, L.M.; Jo, E.K.; Montaño, M.; Barnes, P.F.; Selman, M.; Granados, J. A Functional Promoter Polymorphism in Monocyte Chemoattractant Protein-1 Is Associated with Increased Susceptibility to Pulmonary Tuberculosis. J. Exp. Med. 2005, 202, 1649–1658. [Google Scholar] [CrossRef] [PubMed]
- Venosa, A.; Cowman, S.; Katzen, J.; Tomer, Y.; Armstrong, B.S.; Mulugeta, S.; Beers, M.F. Role of CCR2+ Myeloid Cells in Inflammation Responses Driven by Expression of a Surfactant Protein-C Mutant in the Alveolar Epithelium. Front. Immunol. 2021, 12, 665818. [Google Scholar] [CrossRef] [PubMed]
- Dal-Secco, D.; Wang, J.; Zeng, Z.; Kolaczkowska, E.; Wong, C.H.Y.; Petri, B.; Ransohoff, R.M.; Charo, I.F.; Jenne, C.N.; Kubes, P. A Dynamic Spectrum of Monocytes Arising from the in Situ Reprogramming of CCR2+ Monocytes at a Site of Sterile Injury. J. Exp. Med. 2015, 212, 447–456. [Google Scholar] [CrossRef]
- Lavine, K.J.; Epelman, S.; Uchida, K.; Weber, K.J.; Nichols, C.G.; Schilling, J.D.; Ornitz, D.M.; Randolph, G.J.; Mann, D.L. Distinct Macrophage Lineages Contribute to Disparate Patterns of Cardiac Recovery and Remodeling in the Neonatal and Adult Heart. Proc. Natl. Acad. Sci. USA 2014, 111, 16029–16034. [Google Scholar] [CrossRef]
- Epelman, S.; Lavine, K.J.; Beaudin, A.E.; Sojka, D.K.; Carrero, J.A.; Calderon, B.; Brija, T.; Gautier, E.L.; Ivanov, S.; Satpathy, A.T.; et al. Embryonic and Adult-Derived Resident Cardiac Macrophages Are Maintained through Distinct Mechanisms at Steady State and during Inflammation. Immunity 2014, 40, 91–104. [Google Scholar] [CrossRef]
- Deshmane, S.L.; Kremlev, S.; Amini, S.; Sawaya, B.E. Monocyte Chemoattractant Protein-1 (MCP-1): An Overview. J. Interf. Cytokine Res. 2009, 29, 313–325. [Google Scholar] [CrossRef]
- Juskewitch, J.E.; Knudsen, B.E.; Platt, J.L.; Nath, K.A.; Knutson, K.L.; Brunn, G.J.; Grande, J.P. LPS-Induced Murine Systemic Inflammation Is Driven by Parenchymal Cell Activation and Exclusively Predicted by Early MCP-1 Plasma Levels. Am. J. Pathol. 2012, 180, 32–40. [Google Scholar] [CrossRef]
- Cui, T.X.; Brady, A.E.; Fulton, C.T.; Zhang, Y.J.; Rosenbloom, L.M.; Goldsmith, A.M.; Moore, B.B.; Popova, A.P. CCR2 Mediates Chronic LPS-Induced Pulmonary Inflammation and Hypoalveolarization in a Murine Model of Bronchopulmonary Dysplasia. Front. Immunol. 2020, 11, 579628. [Google Scholar] [CrossRef]
- Maus, U.; von Grote, K.; Kuziel, W.A.; Mack, M.; Miller, E.J.; Cihak, J.; Stangassinger, M.; Maus, R.; Schlöndorff, D.; Seeger, W.; et al. The Role of CC Chemokine Receptor 2 in Alveolar Monocyte and Neutrophil Immigration in Intact Mice. Am. J. Respir. Crit. Care Med. 2002, 166, 268–273. [Google Scholar] [CrossRef]
- Maus, U.A.; Waelsch, K.; Kuziel, W.A.; Delbeck, T.; Mack, M.; Blackwell, T.S.; Christman, J.W.; Schlöndorff, D.; Seeger, W.; Lohmeyer, J. Monocytes Are Potent Facilitators of Alveolar Neutrophil Emigration During Lung Inflammation: Role of the CCL2-CCR2 Axis. J. Immunol. 2003, 170, 3273–3278. [Google Scholar] [CrossRef] [PubMed]
- Francis, M.; Groves, A.M.; Sun, R.; Cervelli, J.A.; Choi, H.; Laskin, J.D.; Laskin, D.L. CCR2 Regulates Inflammatory Cell Accumulation in the Lung and Tissue Injury Following Ozone Exposure. Toxicol. Sci. 2017, 155, 474–484. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Zhou, Q.; Gu, C.; Li, D.; Zhu, L. Depletion of Circulating Monocytes Suppresses IL-17 and HMGB1 Expression in Mice with LPS-Induced Acute Lung Injury. Am. J. Physiol.—Lung Cell. Mol. Physiol. 2017, 312, L231–L242. [Google Scholar] [CrossRef]
- Trinchieri, G. Interleukin-12 and the Regulation of Innate Resistance and Adaptive Immunity. Nat. Rev. Immunol. 2003, 3, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Tötemeyer, S.; Sheppard, M.; Lloyd, A.; Roper, D.; Dowson, C.; Underhill, D.; Murray, P.; Maskell, D.; Bryant, C. IFN-γ Enhances Production of Nitric Oxide from Macrophages via a Mechanism That Depends on Nucleotide Oligomerization Domain-2. J. Immunol. 2006, 176, 4804–4810. [Google Scholar] [CrossRef]
- Sugimoto, M.A.; Sousa, L.P.; Pinho, V.; Perretti, M.; Teixeira, M.M. Resolution of Inflammation: What Controls Its Onset? Front. Immunol. 2016, 7, 160. [Google Scholar] [CrossRef]
- Shechter, R.; London, A.; Varol, C.; Raposo, C.; Cusimano, M.; Yovel, G.; Rolls, A.; Mack, M.; Pluchino, S.; Martino, G.; et al. Infiltrating Blood-Derived Macrophages Are Vital Cells Playing an Anti-inflammatory Role in Recovery from Spinal Cord Injury in Mice. PLoS Med. 2009, 28, 17. [Google Scholar]
- Pollenus, E.; Pham, T.-T.; Vandermosten, L.; Possemiers, H.; Knoops, S.; Opdenakker, G.; Van den Steen, P.E. CCR2 Is Dispensable for Disease Resolution but Required for the Restoration of Leukocyte Homeostasis Upon Experimental Malaria-Associated Acute Respiratory Distress Syndrome. Front. Immunol. 2021, 11, 628643. [Google Scholar] [CrossRef]
- Ruytinx, P.; Proost, P.; Van Damme, J.; Struyf, S. Chemokine-Induced Macrophage Polarization in Inflammatory Conditions. Front. Immunol. 2018, 9, 1930. [Google Scholar] [CrossRef]
- Ishida, Y.; Kimura, A.; Nosaka, M.; Kuninaka, Y.; Hemmi, H.; Sasaki, I.; Kaisho, T.; Mukaida, N.; Kondo, T. Essential Involvement of the CX3CL1-CX3CR1 Axis in Bleomycin-Induced Pulmonary Fibrosis via Regulation of Fibrocyte and M2 Macrophage Migration. Sci. Rep. 2017, 7, 16833. [Google Scholar] [CrossRef]
- Mantovani, A.; Sica, A.; Sozzani, S.; Allavena, P.; Vecchi, A.; Locati, M. The Chemokine System in Diverse Forms of Macrophage Activation and Polarization. Trends Immunol. 2004, 25, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Lee, Y.; Song, J.; Lee, J.; Chang, S.-Y. Tissue-Specific Role of CX3CR1 Expressing Immune Cells and Their Relationships with Human Disease. Immune Netw. 2018, 18, e5. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Huang, L.; Sung, S.S.J.; Vergis, A.L.; Rosin, D.L.; Rose, C.E.; Lobo, P.I.; Okusa, M.D. The Chemokine Receptors CCR2 and CX3CR1 Mediate Monocyte/Macrophage Trafficking in Kidney Ischemia-Reperfusion Injury. Kidney Int. 2008, 74, 1526–1537. [Google Scholar] [CrossRef] [PubMed]
- Evren, E.; Ringqvist, E.; Willinger, T. Origin and Ontogeny of Lung Macrophages: From Mice to Humans. Immunology 2020, 160, 126–138. [Google Scholar] [CrossRef]
- Guilliams, M.; De Kleer, I.; Henri, S.; Post, S.; Vanhoutte, L.; De Prijck, S.; Deswarte, K.; Malissen, B.; Hammad, H.; Lambrecht, B.N. Alveolar Macrophages Develop from Fetal Monocytes That Differentiate into Long-Lived Cells in the First Week of Life via GM-CSF. J. Exp. Med. 2013, 210, 1977–1992. [Google Scholar] [CrossRef]
- Opalek, J.M.; Ali, N.A.; Lobb, J.M.; Hunter, M.G.; Marsh, C.B. Alveolar Macrophages Lack CCR2 Expression and Do Not Migrate to CCL2. J. Inflamm. 2007, 4, 1–10. [Google Scholar] [CrossRef]
- Liegeois, M.; Legrand, C.; Desmet, C.J.; Marichal, T.; Bureau, F. The Interstitial Macrophage: A Long-Neglected Piece in the Puzzle of Lung Immunity. Cell. Immunol. 2018, 330, 91–96. [Google Scholar] [CrossRef]
- Schyns, J.; Bureau, F.; Marichal, T. Lung Interstitial Macrophages: Past, Present, and Future. J. Immunol. Res. 2018, 2018, 1–10. [Google Scholar] [CrossRef]
- Herold, S.; Mayer, K.; Lohmeyer, J. Acute Lung Injury: How Macrophages Orchestrate Resolution of Inflammation and Tissue Repair. Front. Immunol. 2011, 2, 65. [Google Scholar] [CrossRef]
- D’Alessio, F.R.; Craig, J.M.; Singer, B.D.; Files, D.C.; Mock, J.R.; Garibaldi, B.T.; Fallica, J.; Tripathi, A.; Mandke, P.; Gans, J.H.; et al. Enhanced Resolution of Experimental ARDS through IL-4-Mediated Lung Macrophage Reprogramming. Am. J. Physiol. Cell. Mol. Physiol. 2016, 310, L733–L746. [Google Scholar] [CrossRef]
- Beck-Schimmer, B.; Schwendener, R.; Pasch, T.; Reyes, L.; Booy, C.; Schimmer, R.C. Alveolar Macrophages Regulate Neutrophil Recruitment in Endotoxin-Induced Lung Injury. Respir. Res. 2005, 6, 61. [Google Scholar] [CrossRef] [PubMed]
- Mahida, R.Y.; Scott, A.; Parekh, D.; Lugg, S.T.; Hardy, R.S.; Lavery, G.G.; Matthay, M.A.; Naidu, B.; Perkins, G.D.; Thickett, D.R. Acute Respiratory Distress Syndrome Is Associated with Impaired Alveolar Macrophage Efferocytosis. Eur. Respir. J. 2021, 58, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Ushach, I.; Zlotnik, A. Biological Role of Granulocyte Macrophage Colony-Stimulating Factor (GM-CSF) and Macrophage Colony-Stimulating Factor (M-CSF) on Cells of the Myeloid Lineage. J. Leukoc. Biol. 2016, 100, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Lukic, A.; Larssen, P.; Fauland, A.; Samuelsson, B.; Wheelock, C.E.; Gabrielsson, S.; Radmark, O. GM-CSF– and M-CSF–Primed Macrophages Present Similar Resolving but Distinct Inflammatory Lipid Mediator Signatures. FASEB J. 2017, 31, 4370–4381. [Google Scholar] [CrossRef]
- Hamilton, T.A.; Zhao, C.; Pavicic, P.G.; Datta, S. Myeloid Colony-Stimulating Factors as Regulators of Macrophage Polarization. Front. Immunol. 2014, 5, 554. [Google Scholar] [CrossRef]
- Horvat, J.C.; Beagley, K.W.; Wade, M.A.; Preston, J.A.; Hansbro, N.G.; Hickey, D.K.; Kaiko, G.E.; Gibson, P.G.; Foster, P.S.; Hansbro, P.M. Neonatal Chlamydial Infection Induces Mixed T-Cell Responses That Drive Allergic Airway Disease. Am. J. Respir. Crit. Care Med. 2007, 176, 556–564. [Google Scholar] [CrossRef]
- Sencio, V.; Barthelemy, A.; Tavares, L.P.; Machado, M.G.; Soulard, D.; Cuinat, C.; Queiroz-Junior, C.M.; Noordine, M.-L.; Salomé-Desnoulez, S.; Deryuter, L.; et al. Gut Dysbiosis during Influenza Contributes to Pulmonary Pneumococcal Superinfection through Altered Short-Chain Fatty Acid Production. Cell Rep. 2020, 30, 2934–2947.e6. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, V.L.S.d.; Pollenus, E.; Berghmans, N.; Queiroz-Junior, C.M.; Blanter, M.; Mattos, M.S.; Teixeira, M.M.; Proost, P.; Van den Steen, P.E.; Amaral, F.A.; et al. Absence of CCR2 Promotes Proliferation of Alveolar Macrophages That Control Lung Inflammation in Acute Respiratory Distress Syndrome in Mice. Int. J. Mol. Sci. 2022, 23, 12920. https://doi.org/10.3390/ijms232112920
Oliveira VLSd, Pollenus E, Berghmans N, Queiroz-Junior CM, Blanter M, Mattos MS, Teixeira MM, Proost P, Van den Steen PE, Amaral FA, et al. Absence of CCR2 Promotes Proliferation of Alveolar Macrophages That Control Lung Inflammation in Acute Respiratory Distress Syndrome in Mice. International Journal of Molecular Sciences. 2022; 23(21):12920. https://doi.org/10.3390/ijms232112920
Chicago/Turabian StyleOliveira, Vivian Louise Soares de, Emilie Pollenus, Nele Berghmans, Celso Martins Queiroz-Junior, Marfa Blanter, Matheus Silvério Mattos, Mauro Martins Teixeira, Paul Proost, Philippe E. Van den Steen, Flávio Almeida Amaral, and et al. 2022. "Absence of CCR2 Promotes Proliferation of Alveolar Macrophages That Control Lung Inflammation in Acute Respiratory Distress Syndrome in Mice" International Journal of Molecular Sciences 23, no. 21: 12920. https://doi.org/10.3390/ijms232112920
APA StyleOliveira, V. L. S. d., Pollenus, E., Berghmans, N., Queiroz-Junior, C. M., Blanter, M., Mattos, M. S., Teixeira, M. M., Proost, P., Van den Steen, P. E., Amaral, F. A., & Struyf, S. (2022). Absence of CCR2 Promotes Proliferation of Alveolar Macrophages That Control Lung Inflammation in Acute Respiratory Distress Syndrome in Mice. International Journal of Molecular Sciences, 23(21), 12920. https://doi.org/10.3390/ijms232112920





