Melatonin Attenuates H2O2-Induced Oxidative Injury by Upregulating LncRNA NEAT1 in HT22 Hippocampal Cells
Abstract
1. Introduction
2. Results
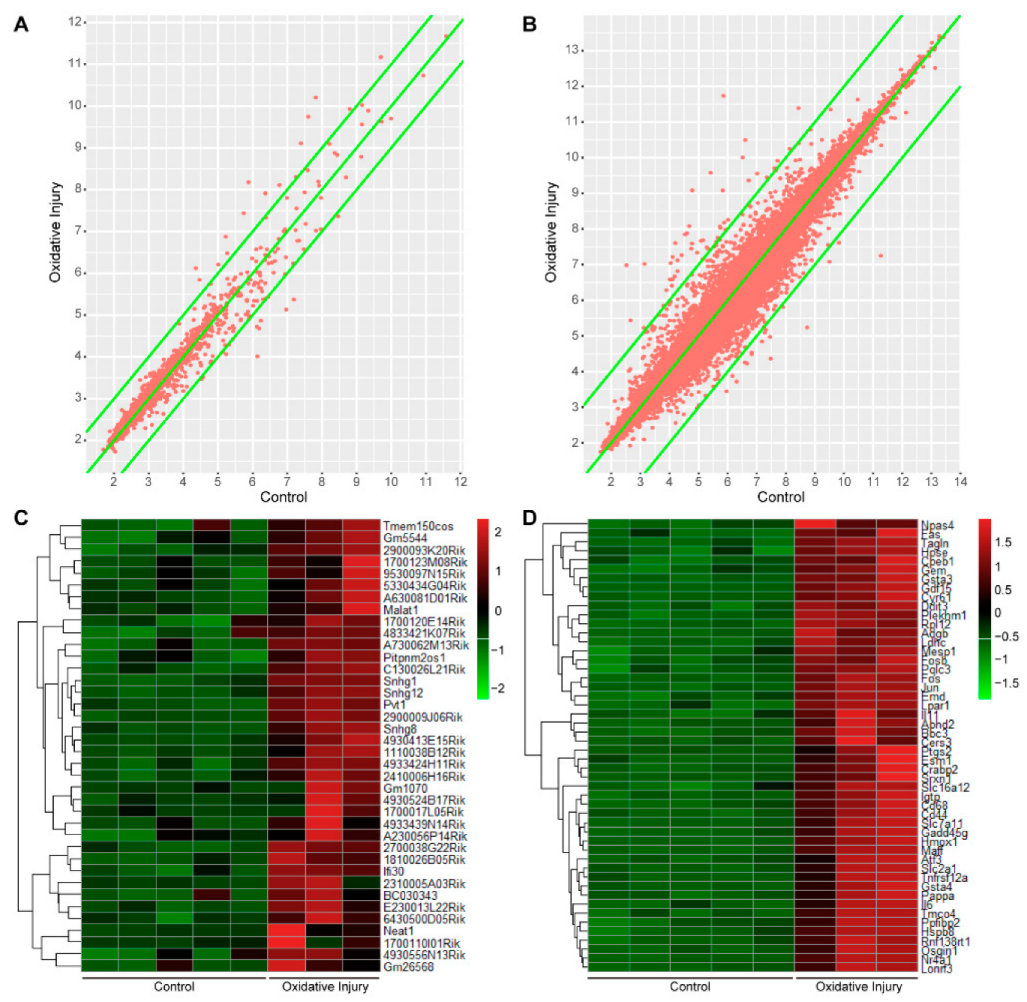
2.1. LncRNA and mRNA Expression Profile of Oxidative Injury in Mouse Neurons
2.2. Differential Expression Analysis of lncRNA and mRNA
2.3. Homology Analysis of Differentially Expressed lncRNA
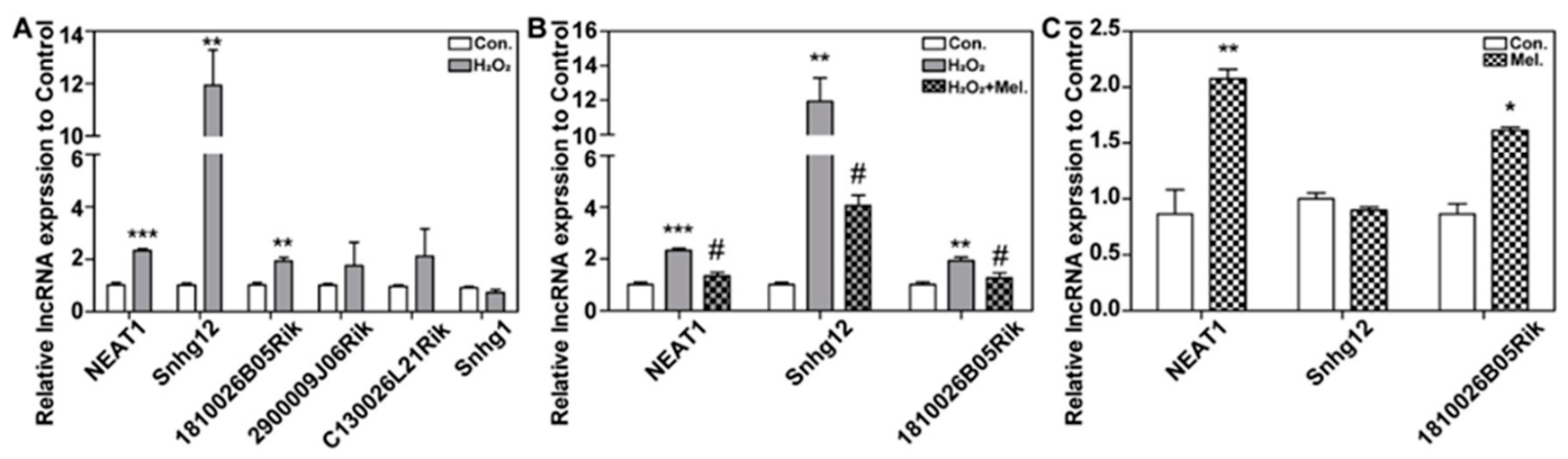
2.4. LncRNA NEAT1 and 1810026B05Rik Were Involved in Melatonin Protecting against H2O2-Induced Oxidative Injury in HT22 Cells
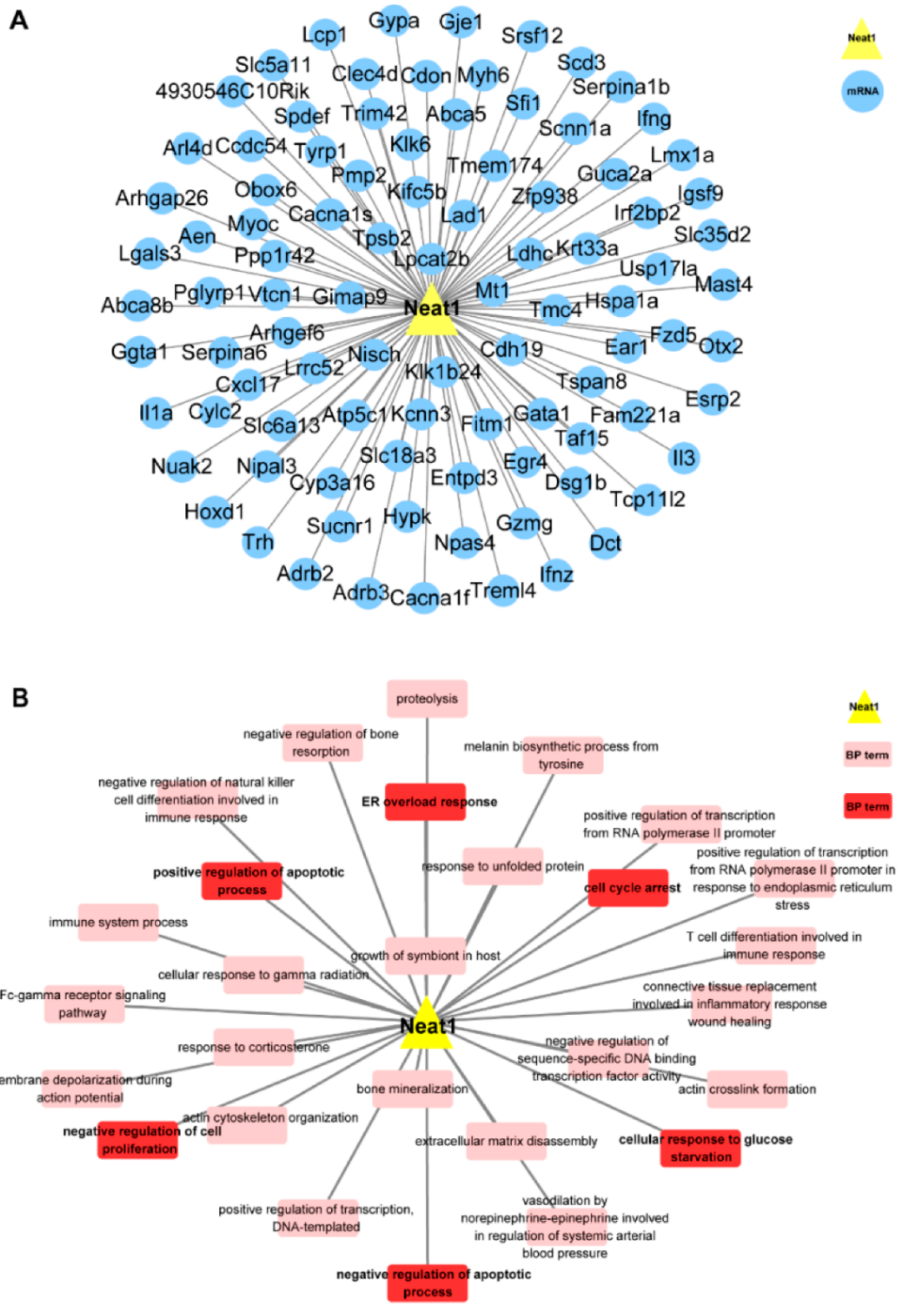
2.5. Gene Ontology Analysis for the Potential Functionalities of NEAT1
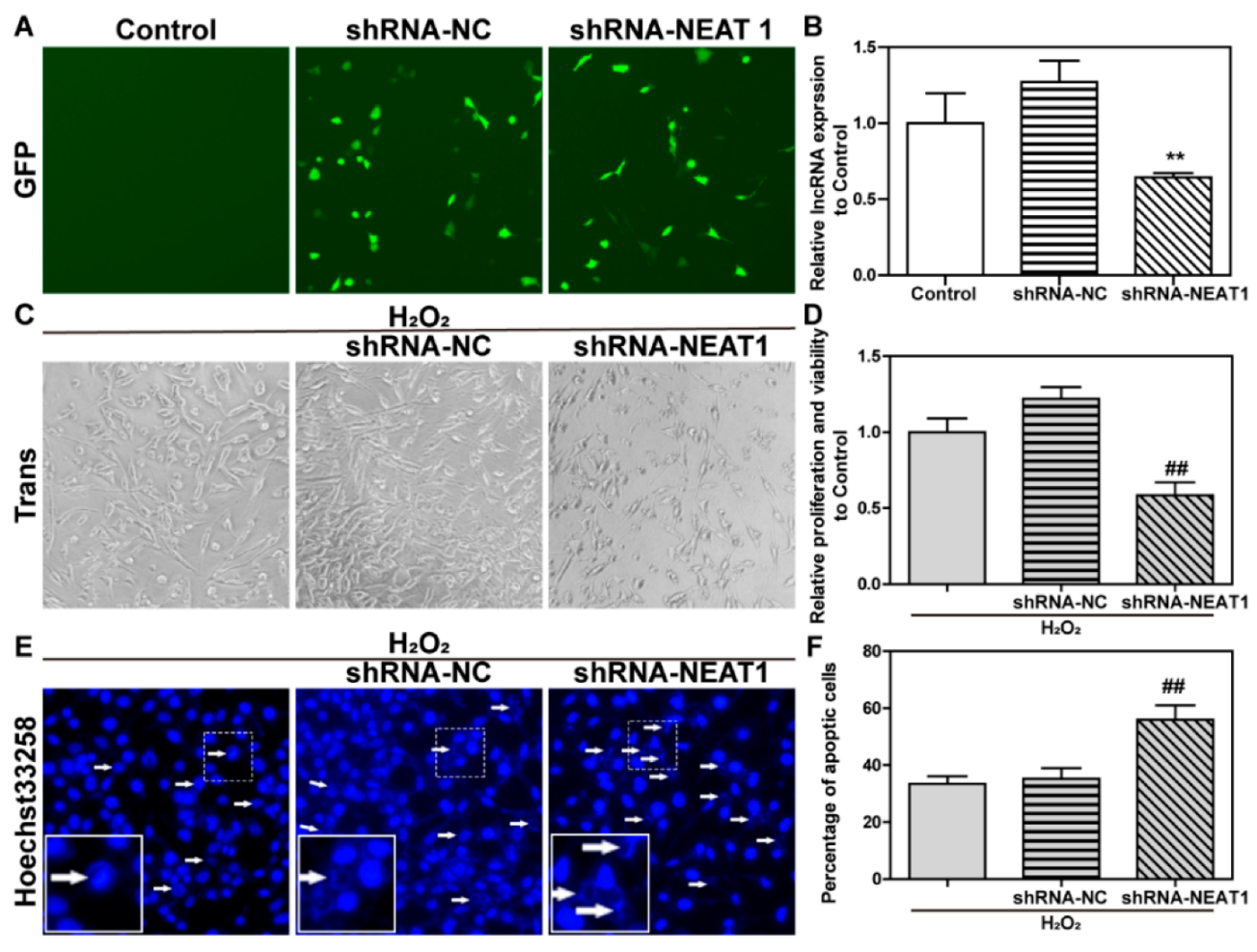
2.6. The Knockdown of lncRNA NEAT1 Aggravated H2O2-Induced Oxidative Injury in HT22 Cells
2.7. The Knockdown of lncRNA NEAT1 Inhibited the Protective Effect of Melatonin on H2O2-Induced Oxidative Injury
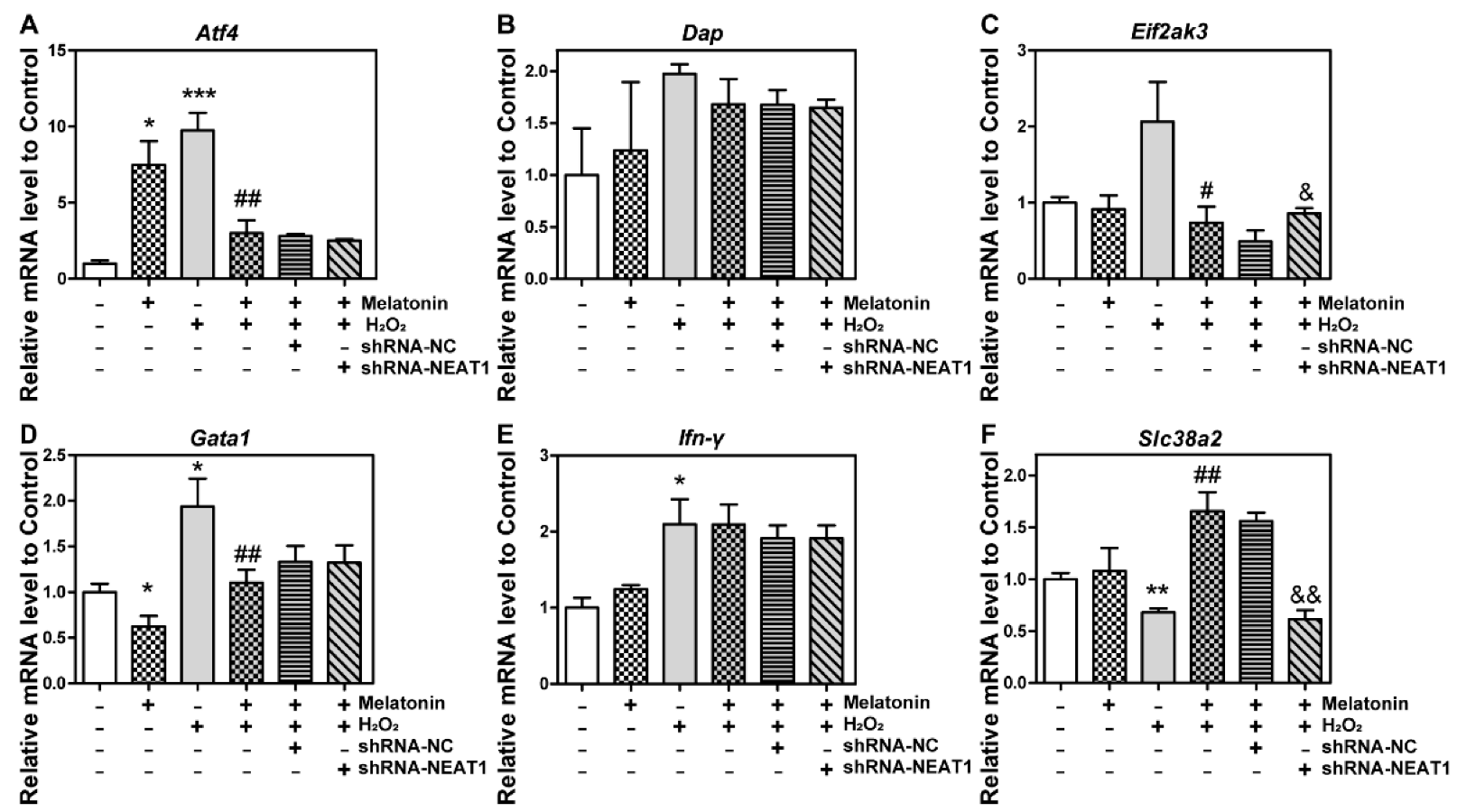
2.8. The Knockdown of NEAT1 Disturbed mRNA Level of Eif2ak3 and Slc38a2
2.9. The Knockdown of NEAT1 Inhibited Protein Level of Slc38a2
3. Discussion
4. Materials and Methods
4.1. RNA Microarray Data of Oxidative Injury in Mouse Neurons
4.2. Construction of lncRNA and mRNA Expression Profiles
4.3. Cluster Analysis of Differential Expression
4.4. Homology Analysis
4.5. Pearson Correlation Analysis
4.6. Functional Enrichment Analysis
4.7. Cell Culture
4.8. Construction of shRNA Expression Vector
4.9. Transfection of shRNA Expression Vector for RNA Interference
4.10. CCK-8 Assay
4.11. Apoptosis-Associated Hoechst Staining
4.12. Western Blot Analysis
4.13. Immunofluorescence Staining
4.14. qPCR
4.15. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bettio, L.E.B.; Rajendran, L.; Gil-Mohapel, J. The effects of aging in the hippocampus and cognitive decline. Neurosci. Biobehav. Rev. 2017, 79, 66–86. [Google Scholar] [CrossRef]
- Martinez, E.; Navarro, A.; Ordonez, C.; Del Valle, E.; Tolivia, J. Oxidative stress induces apolipoprotein D overexpression in hippocampus during aging and Alzheimer’s disease. J. Alzheimer’s Dis. 2013, 36, 129–144. [Google Scholar] [CrossRef] [PubMed]
- Giorgio, M.; Trinei, M.; Migliaccio, E.; Pelicci, P.G. Hydrogen peroxide: A metabolic by-product or a common mediator of ageing signals? Nat. Rev. Mol. Cell Biol. 2007, 8, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Fang, J.; Li, S.; Gaur, U.; Xing, X.; Wang, H.; Zheng, W. Artemisinin Attenuated Hydrogen Peroxide (H2O2)-Induced Oxidative Injury in SH-SY5Y and Hippocampal Neurons via the Activation of AMPK Pathway. Int. J. Mol. Sci. 2019, 20, 2680. [Google Scholar] [CrossRef]
- Sunwoo, J.S.; Lee, S.T.; Im, W.; Lee, M.; Byun, J.I.; Jung, K.H.; Park, K.I.; Jung, K.Y.; Lee, S.K.; Chu, K.; et al. Altered Expression of the Long Noncoding RNA NEAT1 in Huntington’s Disease. Mol. Neurobiol. 2017, 54, 1577–1586. [Google Scholar] [CrossRef]
- Li, R.; Yin, F.; Guo, Y.Y.; Zhao, K.C.; Ruan, Q.; Qi, Y.M. Knockdown of ANRIL aggravates H2O2-induced injury in PC-12 cells by targeting microRNA-125a. Biomed. Pharmacother. = Biomed. Pharmacother. 2017, 92, 952–961. [Google Scholar] [CrossRef] [PubMed]
- El Bassit, G.; Patel, R.S.; Carter, G.; Shibu, V.; Patel, A.A.; Song, S.; Murr, M.; Cooper, D.R.; Bickford, P.C.; Patel, N.A. MALAT1 in Human Adipose Stem Cells Modulates Survival and Alternative Splicing of PKCdeltaII in HT22 Cells. Endocrinology 2017, 158, 183–195. [Google Scholar]
- Shu, T.; Fan, L.; Wu, T.; Liu, C.; He, L.; Pang, M.; Bu, Y.; Wang, X.; Wang, J.; Rong, L.; et al. Melatonin promotes neuroprotection of induced pluripotent stem cells-derived neural stem cells subjected to H2O2-induced injury in vitro. Eur. J. Pharmacol. 2018, 825, 143–150. [Google Scholar] [CrossRef]
- Jeong, J.K.; Moon, M.H.; Lee, Y.J.; Seol, J.W.; Park, S.Y. Melatonin-induced autophagy protects against human prion protein-mediated neurotoxicity. J. Pineal Res. 2012, 53, 138–146. [Google Scholar] [CrossRef]
- Shukla, M.; Govitrapong, P.; Boontem, P.; Reiter, R.J.; Satayavivad, J. Mechanisms of Melatonin in Alleviating Alzheimer’s Disease. Curr. Neuropharmacol. 2017, 15, 1010–1031. [Google Scholar] [CrossRef]
- Gao, Q.; Guo, X.; Cao, Y.; Jia, X.; Xu, S.; Lu, C.; Zhu, H. Melatonin Protects HT22 Hippocampal Cells from H2O2-induced Injury by Increasing Beclin1 and Atg Protein Levels to Activate Autophagy. Curr. Pharm. Des. 2021, 27, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Cai, B.; Ma, W.; Bi, C.; Yang, F.; Zhang, L.; Han, Z.; Huang, Q.; Ding, F.; Li, Y.; Yan, G.; et al. Long noncoding RNA H19 mediates melatonin inhibition of premature senescence of c-kit+ cardiac progenitor cells by promoting miR-675. J. Pineal Res. 2016, 61, 82–95. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Tang, W.; He, Y.; Wen, L.; Sun, B.; Li, S. Long non-coding RNA and microRNA-675/let-7a mediates the protective effect of melatonin against early brain injury after subarachnoid hemorrhage via targeting TP53 and neural growth factor. Cell Death Dis. 2018, 9, 99. [Google Scholar] [CrossRef]
- Ma, L.; Bajic, V.B.; Zhang, Z. On the classification of long non-coding RNAs. RNA Biol. 2013, 10, 925–933. [Google Scholar] [CrossRef] [PubMed]
- Noviello, T.M.R.; Di Liddo, A.; Ventola, G.M.; Spagnuolo, A.; D’Aniello, S.; Ceccarelli, M.; Cerulo, L. Detection of long non-coding RNA homology, a comparative study on alignment and alignment-free metrics. BMC Bioinform. 2018, 19, 407. [Google Scholar] [CrossRef] [PubMed]
- Cortijo, S.; Bhattarai, M.; Locke, J.C.W.; Ahnert, S.E. Co-expression Networks From Gene Expression Variability Between Genetically Identical Seedlings Can Reveal Novel Regulatory Relationships. Front. Plant Sci. 2020, 11, 599464. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhang, N.; Lv, C.; Li, N.; Li, X.; Li, W. lncRNA SNHG1 Knockdown Alleviates Amyloid-beta-Induced Neuronal Injury by Regulating ZNF217 via Sponging miR-361-3p in Alzheimer’s Disease. J. Alzheimer’s Dis. 2020, 77, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Huang, Y.; Cai, J.; Zhang, D.; Liu, S.; Pang, B. LncRNA SNHG12 Improves Cerebral Ischemic-reperfusion Injury by Activating SIRT1/FOXO3a Pathway through I nhibition of Autophagy and Oxidative Stress. Curr. Neurovasc. Res. 2020, 17, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zheng, J.; Li, Y.; Yan, M.; Li, P.; Ma, L. LncRNA EPIC1 downregulation mediates hydrogen peroxide-induced neuronal cell injury. Aging 2019, 11, 11463–11473. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; He, L.; Pang, M.; Ke, Z.; Zheng, X.; Feng, F.; Yang, B.; Wang, N.; Liu, B.; Wu, T.; et al. Melatonin Promotes Neuroprotection of H2O2-induced Neural Stem Cells via lncRNA MEG3/miRNA-27a-3p/MAP2K4 axis. Neuroscience 2020, 446, 69–79. [Google Scholar] [CrossRef]
- Shaath, H.; Vishnubalaji, R.; Elkord, E.; Alajez, N.M. Single-Cell Transcriptome Analysis Highlights a Role for Neutrophils and Inflammatory Macrophages in the Pathogenesis of Severe COVID-19. Cells 2020, 9, 2374. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Zhang, D.; Guo, J.; Chen, Z.; Chen, Y.; Zhang, J. Deficiency of NEAT1 prevented MPP+-induced inflammatory response, oxidative stress and apoptosis in dopaminergic SK-N-SH neuroblastoma cells via miR-1277-5p/ARHGAP26 axis. Brain Res. 2021, 1750, 147156. [Google Scholar] [CrossRef] [PubMed]
- Ernst, E.H.; Nielsen, J.; Ipsen, M.B.; Villesen, P.; Lykke-Hartmann, K. Transcriptome Analysis of Long Non-coding RNAs and Genes Encoding Paraspeckle Proteins During Human Ovarian Follicle Development. Front. Cell Dev. Biol. 2018, 6, 78. [Google Scholar] [CrossRef] [PubMed]
- Bluthgen, N.; van Bentum, M.; Merz, B.; Kuhl, D.; Hermey, G. Profiling the MAPK/ERK dependent and independent activity regulated transcriptional programs in the murine hippocampus in vivo. Sci. Rep. 2017, 7, 45101. [Google Scholar] [CrossRef]
- Kim, Y.S.; Hwan, J.D.; Bae, S.; Bae, D.H.; Shick, W.A. Identification of differentially expressed genes using an annealing control primer system in stage III serous ovarian carcinoma. BMC Cancer 2010, 10, 576. [Google Scholar] [CrossRef]
- Zhong, J.; Jiang, L.; Huang, Z.; Zhang, H.; Cheng, C.; Liu, H.; He, J.; Wu, J.; Darwazeh, R.; Wu, Y.; et al. The long non-coding RNA Neat1 is an important mediator of the therapeutic effect of bexarotene on traumatic brain injury in mice. Brain Behav. Immun. 2017, 65, 183–194. [Google Scholar] [CrossRef]
- Liu, X.; Yu, X.; He, Y.; Wang, L. Long noncoding RNA nuclear enriched abundant transcript 1 promotes the proliferation and migration of Schwann cells by regulating the miR-34a/Satb1 axis. J. Cell Physiol. 2019, 234, 16357–16366. [Google Scholar] [CrossRef]
- Hetz, C.; Mollereau, B. Disturbance of endoplasmic reticulum proteostasis in neurodegenerative diseases. Nat. Rev. Neurosci. 2014, 15, 233–249. [Google Scholar] [CrossRef]
- Costa-Mattioli, M.; Gobert, D.; Stern, E.; Gamache, K.; Colina, R.; Cuello, C.; Sossin, W.; Kaufman, R.; Pelletier, J.; Rosenblum, K.; et al. eIF2alpha phosphorylation bidirectionally regulates the switch from short- to long-term synaptic plasticity and memory. Cell 2007, 129, 195–206. [Google Scholar] [CrossRef]
- Hughes, D.; Mallucci, G.R. The unfolded protein response in neurodegenerative disorders—Therapeutic modulation of the PERK pathway. FEBS J. 2019, 286, 342–355. [Google Scholar] [CrossRef]
- Ounallah-Saad, H.; Sharma, V.; Edry, E.; Rosenblum, K. Genetic or pharmacological reduction of PERK enhances cortical-dependent taste learning. J. Neurosci. 2014, 34, 14624–14632. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Ounallah-Saad, H.; Chakraborty, D.; Hleihil, M.; Sood, R.; Barrera, I.; Edry, E.; Kolatt Chandran, S.; Ben Tabou de Leon, S.; Kaphzan, H.; et al. Local Inhibition of PERK Enhances Memory and Reverses Age-Related Deterioration of Cognitive and Neuronal Properties. J. Neurosci. 2018, 38, 648–658. [Google Scholar] [CrossRef] [PubMed]
- Stockwell, S.R.; Platt, G.; Barrie, S.E.; Zoumpoulidou, G.; Te Poele, R.H.; Aherne, G.W.; Wilson, S.C.; Sheldrake, P.; McDonald, E.; Venet, M.; et al. Mechanism-based screen for G1/S checkpoint activators identifies a selective activator of EIF2AK3/PERK signalling. PLoS ONE 2012, 7, e28568. [Google Scholar] [CrossRef] [PubMed]
- Grewal, S.; Defamie, N.; Zhang, X.; De Gois, S.; Shawki, A.; Mackenzie, B.; Chen, C.; Varoqui, H.; Erickson, J.D. SNAT2 amino acid transporter is regulated by amino acids of the SLC6 gamma-aminobutyric acid transporter subfamily in neocortical neurons and may play no role in delivering glutamine for glutamatergic transmission. J. Biol. Chem. 2009, 284, 11224–11236. [Google Scholar] [CrossRef] [PubMed]
- Gjymishka, A.; Palii, S.S.; Shan, J.; Kilberg, M.S. Despite increased ATF4 binding at the C/EBP-ATF composite site following activation of the unfolded protein response, system A transporter 2 (SNAT2) transcription activity is repressed in HepG2 cells. J. Biol. Chem. 2008, 283, 27736–27747. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.J.; Khelifa, S.; Ratnikov, B.; Scott, D.A.; Feng, Y.; Parisi, F.; Ruller, C.; Lau, E.; Kim, H.; Brill, L.M.; et al. Regulation of glutamine carrier proteins by RNF5 determines breast cancer response to ER stress-inducing chemotherapies. Cancer Cell 2015, 27, 354–369. [Google Scholar] [CrossRef] [PubMed]
- Duval, F.; Santos, E.D.; Poidatz, D.; Serazin, V.; Gronier, H.; Vialard, F.; Dieudonne, M.N. Adiponectin Inhibits Nutrient Transporters and Promotes Apoptosis in Human Villous Cytotrophoblasts: Involvement in the Control of Fetal Growth. Biol. Reprod. 2016, 94, 111. [Google Scholar] [CrossRef]
- Malabanan, K.P.; Khachigian, L.M. Activation transcription factor-4 and the acute vascular response to injury. J. Mol. Med. 2010, 88, 545–552. [Google Scholar] [CrossRef]
- Luhr, M.; Torgersen, M.L.; Szalai, P.; Hashim, A.; Brech, A.; Staerk, J.; Engedal, N. The kinase PERK and the transcription factor ATF4 play distinct and essential roles in autophagy resulting from tunicamycin-induced ER stress. J. Biol. Chem. 2019, 294, 8197–8217. [Google Scholar] [CrossRef]
- Gully, J.C.; Sergeyev, V.G.; Bhootada, Y.; Mendez-Gomez, H.; Meyers, C.A.; Zolotukhin, S.; Gorbatyuk, M.S.; Gorbatyuk, O.S. Up-regulation of activating transcription factor 4 induces severe loss of dopamine nigral neurons in a rat model of Parkinson’s disease. Neurosci. Lett. 2016, 627, 36–41. [Google Scholar] [CrossRef]
- Galehdar, Z.; Swan, P.; Fuerth, B.; Callaghan, S.M.; Park, D.S.; Cregan, S.P. Neuronal apoptosis induced by endoplasmic reticulum stress is regulated by ATF4-CHOP-mediated induction of the Bcl-2 homology 3-only member PUMA. J. Neurosci. 2010, 30, 16938–16948. [Google Scholar] [CrossRef] [PubMed]
- B’Chir, W.; Maurin, A.C.; Carraro, V.; Averous, J.; Jousse, C.; Muranishi, Y.; Parry, L.; Stepien, G.; Fafournoux, P.; Bruhat, A. The eIF2alpha/ATF4 pathway is essential for stress-induced autophagy gene expression. Nucleic Acids Res. 2013, 41, 7683–7699. [Google Scholar] [CrossRef] [PubMed]
- Rouschop, K.M.; van den Beucken, T.; Dubois, L.; Niessen, H.; Bussink, J.; Savelkouls, K.; Keulers, T.; Mujcic, H.; Landuyt, W.; Voncken, J.W.; et al. The unfolded protein response protects human tumor cells during hypoxia through regulation of the autophagy genes MAP1LC3B and ATG5. J. Clin. Investig. 2010, 120, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, A.; Shimizu, R. GATA1 Activity Governed by Configurations of cis-Acting Elements. Front. Oncol. 2016, 6, 269. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, R.; Huang, Z.; Gurley, E.C.; Wang, X.; Wang, J.; He, H.; Yang, H.; Lai, G.; Zhang, L.; et al. Cholangiocyte-derived exosomal long noncoding RNA H19 promotes cholestatic liver injury in mouse and humans. Hepatology 2018, 68, 599–615. [Google Scholar] [CrossRef]
- Huang, Y.S.; Hsieh, H.Y.; Shih, H.M.; Sytwu, H.K.; Wu, C.C. Urinary Xist is a potential biomarker for membranous nephropathy. Biochem. Biophys. Res. Commun. 2014, 452, 415–421. [Google Scholar] [CrossRef]


| Data Categories | Data Quantity |
|---|---|
| Sample | 8 (control group 5, oxidative injury group 3) |
| Original probe | 45,057 |
| Specific probe | 38,300 |
| lncRNA related probe | 1800 |
| mRNA related probe | 31,929 |
| Detected lncRNA | 1439 |
| Detected mRNA | 15,965 |
| Gene Symbol | Seq. Name | Fold Change | p-Value | Reg. | RNA Length (b) | Model (Normalized) | Control (Normalized) |
|---|---|---|---|---|---|---|---|
| C130026L21Rik | BB_632665 | 7.9 | <0.0001 | up | 2472 | 7.899769333 | 0.999999926 |
| SNHG1 | BQ_177137 | 4.783 | <0.0001 | up | 605 | 4.325219667 | 0.958542270 |
| SNHG12 | BI_180630 | 5.053 | <0.0001 | up | 935 | 5.052909467 | 1.000000068 |
| 4930413E15Rik | AK_015127 | 10.694 | <0.0001 | up | 1290 | 10.69391797 | 1.000000032 |
| E230013L22Rik | BB_549061 | 7.115 | <0.0001 | up | 1856 | 7.115391 | 0.999999986 |
| A730062M13Rik | BB_254594 | 4.611 | <0.0001 | up | 1910 | 4.611339767 | 1.000000040 |
| 1110038B12Rik | BE_133150 | 4.261 | <0.0001 | up | 785 | 4.261468833 | 1.000000048 |
| 1810026B05Rik | BB_327381 | 3.974 | <0.0001 | up | 5140 | 3.413172133 | 0.999999991 |
| 2310005A03Rik | AK_009160 | 8.082 | <0.0001 | up | 1398 | 8.0818338 | 0.999999980 |
| 1700123M08Rik | BB_408149 | 5.446 | <0.0001 | up | 770 | 5.445621267 | 0.999999948 |
| Gene Symbol | Seq. Name | Fold Change | p-Value | Reg. | RNA Length (b) | Model (Normalized) | Control (Normalized) |
|---|---|---|---|---|---|---|---|
| Ptgs2 | M94967 | 62.36897551 | 0.049713300 | up | 4453 | 62.368978 | 1.000000040 |
| Gsta3 | AI_172943 | 15.11052768 | 0.001807316 | up | 1457 | 15.11052724 | 0.999999971 |
| Cers3 | AI_429073 | 6.985921284 | 0.026982869 | up | 3250 | 6.9859212 | 0.999999988 |
| Hmox1 | NM_010442 | 73.17679532 | 0.005781206 | up | 1569 | 73.1768 | 1.000000064 |
| Gdf15 | NM_011819 | 20.12441644 | 0.006973339 | up | 1545 | 20.12441467 | 0.999999912 |
| Npas4 | AV_348246 | 11.1304136 | 0.027445049 | up | 3277 | 11.13041367 | 1.000000006 |
| Ptgr1 | BC_014865 | 15.36713818 | 0.040184910 | up | 2296 | 15.36713833 | 1.000000010 |
| Gem | U10551 | 5.740063778 | 0.004308775 | up | 1994 | 5.740063767 | 0.999999998 |
| Cpeb1 | NM_007755 | 5.963823015 | 0.003356431 | up | 3111 | 5.9638228 | 0.999999964 |
| Tlk2 | NM_011903 | 2.990434232 | 0.002009714 | up | 5494 | 2.990434167 | 0.999999978 |
| Dclre1b | BB_763339 | 0.258067778 | 0.002308249 | down | 4113 | 0.259593163 | 1.005910792 |
| Olig2 | AB_038697 | 0.076296681 | 0.021070358 | down | 2437 | 0.076296676 | 0.999999928 |
| Pdgfra | AW_537708 | 0.115138148 | 0.010354445 | down | 2876 | 0.114080087 | 0.990810510 |
| Hes5 | AV_337579 | 0.118642876 | 0.041823638 | down | 1273 | 0.118642871 | 0.999999957 |
| Sox8 | AV_345303 | 0.151596202 | 0.006506986 | down | 3000 | 0.151596204 | 1.000000018 |
| Ascl1 | BB_425719 | 0.137524736 | 0.009259114 | down | 2481 | 0.137524738 | 1.000000016 |
| Nim1k | BB_359887 | 0.285656255 | 0.007794788 | down | 4103 | 0.285656243 | 0.999999960 |
| Mki67 | X82786 | 0.229441042 | 0.008072360 | down | 100,061 | 0.22944107 | 1.000000120 |
| Homez | AV_298304 | 0.20154121 | 0.031646867 | down | 5609 | 0.20154121 | 1.000000000 |
| Bbs12 | AI_449447 | 0.188051542 | 0.023960328 | down | 2433 | 0.188051538 | 0.999999980 |
| Gene Symbol | Homology Score | Homology Degree |
|---|---|---|
| 2900009J06Rik | 222 | High |
| 1810026B05Rik | 286 | High |
| NEAT1 | 273 | High |
| SNHG12 | 163 | Medium |
| SNHG1 | 165 | Medium |
| C130026L21Rik | 102 | Medium |
| Gene Symbol | Seq. Name | Biotype | Fold Change | p-Value | Reg. | RNA Length (b) | Chrom | Strand | txStart | txEnd |
|---|---|---|---|---|---|---|---|---|---|---|
| 2900009J06Rik | BB_663189 | Antisense | 2.544 | <0.0001 | up | 465 | 1 | − | 127,756,757 | 127,767,978 |
| 1810026B05Rik | BB_327381 | lincRNA | 3.974 | <0.0001 | up | 5140 | 7 | − | 73,539,801 | 73,558,395 |
| NEAT1 | AK_018202 | lincRNA | 7.38 | <0.0001 | up | 20,771 | 19 | − | 5,824,708 | 5,845,478 |
| SNHG12 | BI_180630 | lincRNA | 5.053 | <0.0001 | up | 935 | 4 | + | 132,308,678 | 132,311,024 |
| SNHG1 | BQ_177137 | lincRNA | 4.783 | <0.0001 | up | 605 | 19 | + | 8,723,475 | 8,726,443 |
| C130026L21Rik | BB_632665 | lincRNA | 7.9 | <0.0001 | up | 2472 | 5 | + | 111,581,422 | 111,587,945 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Q.; Zhang, C.; Li, J.; Xu, H.; Guo, X.; Guo, Q.; Zhao, C.; Yao, H.; Jia, Y.; Zhu, H. Melatonin Attenuates H2O2-Induced Oxidative Injury by Upregulating LncRNA NEAT1 in HT22 Hippocampal Cells. Int. J. Mol. Sci. 2022, 23, 12891. https://doi.org/10.3390/ijms232112891
Gao Q, Zhang C, Li J, Xu H, Guo X, Guo Q, Zhao C, Yao H, Jia Y, Zhu H. Melatonin Attenuates H2O2-Induced Oxidative Injury by Upregulating LncRNA NEAT1 in HT22 Hippocampal Cells. International Journal of Molecular Sciences. 2022; 23(21):12891. https://doi.org/10.3390/ijms232112891
Chicago/Turabian StyleGao, Qiang, Chi Zhang, Jiaxin Li, Han Xu, Xiaocheng Guo, Qi Guo, Chen Zhao, Haixu Yao, Yuhan Jia, and Hui Zhu. 2022. "Melatonin Attenuates H2O2-Induced Oxidative Injury by Upregulating LncRNA NEAT1 in HT22 Hippocampal Cells" International Journal of Molecular Sciences 23, no. 21: 12891. https://doi.org/10.3390/ijms232112891
APA StyleGao, Q., Zhang, C., Li, J., Xu, H., Guo, X., Guo, Q., Zhao, C., Yao, H., Jia, Y., & Zhu, H. (2022). Melatonin Attenuates H2O2-Induced Oxidative Injury by Upregulating LncRNA NEAT1 in HT22 Hippocampal Cells. International Journal of Molecular Sciences, 23(21), 12891. https://doi.org/10.3390/ijms232112891






