Cannabidiol Exerts a Neuroprotective and Glia-Balancing Effect in the Subacute Phase of Stroke
Abstract
:1. Introduction
2. Results
2.1. Ischemia-Induced Neurodegeneration and Neurological Deficit Are Reduced after Cannabidiol Treatment
2.2. Less Activated Microglia Cells in the Ischemic Brain after Cannabidiol Treatment
2.3. In Vivo 2P-LSM Revealed a Reduced Number of Microglia Cells with Smaller Somata in the Somatosensory Cortex of Ischemic Animals after Cannabidiol Treatment
2.4. Cannabidiol Balances Ischemia-Induced Alterations of Astroglial Ca2+ Signaling after MCAO In Vivo
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Ethics Statement
5.2. Animals
5.3. Tamoxifen Induced Recombination
5.4. Cannabidiol Treatment
5.5. Middle Cerebral Artery Occlusion
5.6. Cortical Craniotomy
5.7. Neurological Score
5.8. Immunohistochemistry
5.9. Fluoro-Jade C Staining
5.10. Microscopic Analysis and Quantification on Fixed Brain Slices
5.11. Two-Photon Laser-Scanning Microscopy
5.12. Analyses of Ca2+ Imaging Data
5.13. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feigin, V.L.; Stark, B.; Johnson, C.O.; Roth, G.; Bisignano, C.; Abady, G.G.; Abbasifard, M.; Abbasi-Kangevari, M.; Abd-Allah, F.; Abedi, V.; et al. Global, regional, and national burden of stroke and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021, 20, 795–820. [Google Scholar] [CrossRef]
- Kumar, S.; Selim, M.H.; Caplan, L.R. Medical complications after stroke. Lancet Neurol. 2010, 9, 105–118. [Google Scholar] [CrossRef]
- Paul, S.; Candelario-Jalil, E. Emerging neuroprotective strategies for the treatment of ischemic stroke: An overview of clinical and preclinical studies. Exp. Neurol. 2021, 335, 113518. [Google Scholar] [CrossRef] [PubMed]
- Shi, K.; Tian, D.-C.; Li, Z.-G.; Ducruet, A.F.; Lawton, M.T.; Shi, F.-D. Global brain inflammation in stroke. Lancet Neurol. 2019, 18, 1058–1066. [Google Scholar] [CrossRef]
- Lambertsen, K.L.; Finsen, B.; Clausen, B.H. Post-stroke inflammation—Target or tool for therapy? Acta Neuropathol. 2019, 137, 693–714. [Google Scholar] [CrossRef] [Green Version]
- Verkhratsky, A. Glial calcium signaling in physiology and pathophysiology. Acta Pharmacol. Sin. 2006, 27, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Verkhratsky, A.; Orkand, R.K.; Kettenmann, H. Glial Calcium: Homeostasis and Signaling Function. Physiol. Rev. 1998, 78, 99–141. [Google Scholar] [CrossRef]
- Choudhury, G.R.; Ding, S. Reactive astrocytes and therapeutic potential in focal ischemic stroke. Neurobiol. Dis. 2016, 85, 234–244. [Google Scholar] [CrossRef] [Green Version]
- Hayakawa, K.; Irie, K.; Sano, K.; Watanabe, T.; Higuchi, S.; Enoki, M.; Nakano, T.; Harada, K.; Ishikane, S.; Ikeda, T.; et al. Therapeutic Time Window of Cannabidiol Treatment on Delayed Ischemic Damage via High-Mobility Group Box1-Inhibiting Mechanism. Biol. Pharm. Bull. 2009, 32, 1538–1544. [Google Scholar] [CrossRef] [Green Version]
- Qin, C.; Zhou, L.-Q.; Ma, X.-T.; Hu, Z.-W.; Yang, S.; Chen, M.; Bosco, D.B.; Wu, L.-J.; Tian, D.-S. Dual Functions of Microglia in Ischemic Stroke. Neurosci. Bull. 2019, 35, 921–933. [Google Scholar] [CrossRef]
- Shen, X.-Y.; Gao, Z.-K.; Han, Y.; Yuan, M.; Guo, Y.-S.; Bi, X. Activation and Role of Astrocytes in Ischemic Stroke. Front. Cell. Neurosci. 2021, 15, 755955. [Google Scholar] [CrossRef] [PubMed]
- De Meyer, S.F.; Denorme, F.; Langhauser, F.; Geuss, E.; Fluri, F.; Kleinschnitz, C. Thromboinflammation in Stroke Brain Damage. Stroke 2016, 47, 1165–1172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crippa, J.A.; Guimarães, F.S.; Campos, A.C.; Zuardi, A.W. Translational Investigation of the Therapeutic Potential of Cannabidiol (CBD): Toward a New Age. Front. Immunol. 2018, 9, 2009. [Google Scholar] [CrossRef] [Green Version]
- Laux, L.C.; Bebin, E.M.; Checketts, D.; Chez, M.; Flamini, R.; Marsh, E.D.; Miller, I.; Nichol, K.; Park, Y.; Segal, E.; et al. Long-term safety and efficacy of cannabidiol in children and adults with treatment resistant Lennox-Gastaut syndrome or Dravet syndrome: Expanded access program results. Epilepsy Res. 2019, 154, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.D.; Mazurkiewicz-Bełdzińska, M.; Chin, R.F.; Gil-Nagel, A.; Gunning, B.; Halford, J.J.; Mitchell, W.; Perry, M.S.; Thiele, E.A.; Weinstock, A.; et al. Long-term safety and efficacy of add-on cannabidiol in patients with Lennox–Gastaut syndrome: Results of a long-term open-label extension trial. Epilepsia 2021, 62, 2228–2239. [Google Scholar] [CrossRef]
- Syed, Y.Y.; McKeage, K.; Scott, L.J. Delta-9-Tetrahydrocannabinol/Cannabidiol (Sativex®): A Review of Its Use in Patients with Moderate to Severe Spasticity Due to Multiple Sclerosis. Drugs 2014, 74, 563–578. [Google Scholar] [CrossRef]
- Devinsky, O.; Patel, A.D.; Cross, J.H.; Villanueva, V.; Wirrell, E.C.; Privitera, M.; Greenwood, S.M.; Roberts, C.; Checketts, D.; VanLandingham, K.E.; et al. Effect of Cannabidiol on Drop Seizures in the Lennox–Gastaut Syndrome. N. Engl. J. Med. 2018, 378, 1888–1897. [Google Scholar] [CrossRef] [Green Version]
- Dos-Santos-Pereira, M.; Da-Silva, C.A.; Guimarães, F.S.; Del-Bel, E. Co-administration of cannabidiol and capsazepine reduces L-DOPA-induced dyskinesia in mice: Possible mechanism of action. Neurobiol. Dis. 2016, 94, 179–195. [Google Scholar] [CrossRef]
- Junior, N.C.F.; dos-Santos-Pereira, M.; Guimarães, F.S.; Del Bel, E. Cannabidiol and Cannabinoid Compounds as Potential Strategies for Treating Parkinson’s Disease and l-DOPA-Induced Dyskinesia. Neurotox. Res. 2020, 37, 12–29. [Google Scholar] [CrossRef]
- Martín-Moreno, A.M.; Reigada, D.; Ramírez, B.G.; Mechoulam, R.; Innamorato, N.; Cuadrado, A.; de Ceballos, M.L. Cannabidiol and Other Cannabinoids Reduce Microglial Activation In Vitro and In Vivo: Relevance to Alzheimer’s Disease. Mol. Pharmacol. 2011, 79, 964–973. [Google Scholar] [CrossRef]
- Cheng, D.; Spiro, A.S.; Jenner, A.M.; Garner, B.; Karl, T. Long-Term Cannabidiol Treatment Prevents the Development of Social Recognition Memory Deficits in Alzheimer’s Disease Transgenic Mice. J. Alzheimer Dis. 2014, 42, 1383–1396. [Google Scholar] [CrossRef] [PubMed]
- Carlini, E.; Cunha, J.M. Hypnotic and Antiepileptic Effects of Cannabidiol. J. Clin. Pharmacol. 1981, 21, 417S–427S. [Google Scholar] [CrossRef] [PubMed]
- Patra, P.H.; Barker-Haliski, M.; White, H.S.; Whalley, B.J.; Glyn, S.; Sandhu, H.; Jones, N.; Bazelot, M.; Williams, C.M.; McNeish, A.J. Cannabidiol reduces seizures and associated behavioral comorbidities in a range of animal seizure and epilepsy models. Epilepsia 2019, 60, 303–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozela, E.; Lev, N.; Kaushansky, N.; Eilam, R.; Rimmerman, N.; Levy, R.; Ben-Nun, A.; Juknat, A.; Vogel, Z. Cannabidiol inhibits pathogenic T cells, decreases spinal microglial activation and ameliorates multiple sclerosis-like disease in C57BL/6 mice. Br. J. Pharmacol. 2011, 163, 1507–1519. [Google Scholar] [CrossRef] [Green Version]
- Mecha, M.; Feliú, A.; Iñigo, P.; Mestre, L.; Carrillo-Salinas, F.; Guaza, C. Cannabidiol provides long-lasting protection against the deleterious effects of inflammation in a viral model of multiple sclerosis: A role for A2A receptors. Neurobiol. Dis. 2013, 59, 141–150. [Google Scholar] [CrossRef]
- Giacoppo, S.; Rajan, T.S.; Galuppo, M.; Pollastro, F.; Grassi, G.; Bramanti, P.; Mazzon, E. Purified Cannabidiol, the main non-psychotropic component of Cannabis sativa, alone, counteracts neuronal apoptosis in experimental multiple sclerosis. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 4906–4919. [Google Scholar]
- da Silva, N.R.; Gomes, F.V.; Sonego, A.B.; da Silva, N.R.; Guimarães, F.S. Cannabidiol attenuates behavioral changes in a rodent model of schizophrenia through 5-HT1A, but not CB1 and CB2 receptors. Pharmacol. Res. 2020, 156, 104749. [Google Scholar] [CrossRef]
- Pedrazzi, J.F.; Sales, A.J.; Guimarães, F.S.; Joca, S.R.; Crippa, J.A.; Del Bel, E. Cannabidiol prevents disruptions in sensorimotor gating induced by psychotomimetic drugs that last for 24-h with probable involvement of epigenetic changes in the ventral striatum. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 111, 110352. [Google Scholar] [CrossRef]
- Meyer, E.; Bonato, J.M.; Mori, M.A.; Mattos, B.A.; Guimarães, F.S.; Milani, H.; de Campos, A.C.; de Oliveira, R.M.W. Cannabidiol Confers Neuroprotection in Rats in a Model of Transient Global Cerebral Ischemia: Impact of Hippocampal Synaptic Neuroplasticity. Mol. Neurobiol. 2021, 58, 5338–5355. [Google Scholar] [CrossRef]
- Mishima, K.; Hayakawa, K.; Abe, K.; Ikeda, T.; Egashira, N.; Iwasaki, K.; Fujiwara, M. Cannabidiol Prevents Cerebral Infarction Via a Serotonergic 5-Hydroxytryptamine 1A Receptor–Dependent Mechanism. Stroke 2005, 36, 1071–1076. [Google Scholar] [CrossRef] [Green Version]
- Kwiatkoski, M.; Guimarães, F.S.; Del-Bel, E. Cannabidiol-treated Rats Exhibited Higher Motor Score After Cryogenic Spinal Cord Injury. Neurotox. Res. 2012, 21, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Lin, Z.; Meng, Q.; Wang, K.; Wu, J.; Yan, H. Cannabidiol administration reduces sublesional cancellous bone loss in rats with severe spinal cord injury. Eur. J. Pharmacol. 2017, 809, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Schiavon, A.P.; Soares, L.M.; Bonato, J.M.; Milani, H.; Guimarães, F.S.; De Oliveira, R.M.W. Protective Effects of Cannabidiol Against Hippocampal Cell Death and Cognitive Impairment Induced by Bilateral Common Carotid Artery Occlusion in Mice. Neurotox. Res. 2014, 26, 307–316. [Google Scholar] [CrossRef]
- Mori, M.A.; Meyer, E.; Soares, L.M.; Milani, H.; Guimarães, F.S.; de Oliveira, R.M.W. Cannabidiol reduces neuroinflammation and promotes neuroplasticity and functional recovery after brain ischemia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2017, 75, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Ceprián, M.; Jiménez-Sánchez, L.; Vargas, C.; Barata, L.; Hind, W.; Martínez-Orgado, J. Cannabidiol reduces brain damage and improves functional recovery in a neonatal rat model of arterial ischemic stroke. Neuropharmacology 2017, 116, 151–159. [Google Scholar] [CrossRef]
- Khaksar, S.; Bigdeli, M.R. Anti-excitotoxic effects of cannabidiol are partly mediated by enhancement of NCX2 and NCX3 expression in animal model of cerebral ischemia. Eur. J. Pharmacol. 2017, 794, 270–279. [Google Scholar] [CrossRef]
- Hayakawa, K.; Mishima, K.; Nozako, M.; Hazekawa, M.; Irie, K.; Fujioka, M.; Orito, K.; Abe, K.; Hasebe, N.; Egashira, N.; et al. Delayed treatment with cannabidiol has a cerebroprotective action via a cannabinoid receptor-independent myeloperoxidase-inhibiting mechanism. J. Neurochem. 2007, 102, 1488–1496. [Google Scholar] [CrossRef]
- Hayakawa, K.; Mishima, K.; Irie, K.; Hazekawa, M.; Mishima, S.; Fujioka, M.; Orito, K.; Egashira, N.; Katsurabayashi, S.; Takasaki, K.; et al. Cannabidiol prevents a post-ischemic injury progressively induced by cerebral ischemia via a high-mobility group box1-inhibiting mechanism. Neuropharmacology 2008, 55, 1280–1286. [Google Scholar] [CrossRef]
- Khaksar, S.; Bigdeli, M.R. Intra-cerebral cannabidiol infusion-induced neuroprotection is partly associated with the TNF-α/TNFR1/NF-κB pathway in transient focal cerebral ischaemia. Brain Inj. 2017, 31, 1932–1943. [Google Scholar] [CrossRef]
- Jung, S.; Aliberti, J.; Graemmel, P.; Sunshine, M.J.; Kreutzberg, G.W.; Sher, A.; Littman, D.R. Analysis of Fractalkine Receptor CX 3 CR1 Function by Targeted Deletion and Green Fluorescent Protein Reporter Gene Insertion. Mol. Cell. Biol. 2000, 20, 4106–4114. [Google Scholar] [CrossRef] [Green Version]
- Mori, T.; Tanaka, K.; Buffo, A.; Wurst, W.; Kühn, R.; Götz, M. Inducible gene deletion in astroglia and radial glia-A valuable tool for functional and lineage analysis. Glia 2006, 54, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Paukert, M.; Agarwal, A.; Cha, J.; Doze, V.A.; Kang, J.U.; Bergles, D.E. Norepinephrine Controls Astroglial Responsiveness to Local Circuit Activity. Neuron 2014, 82, 1263–1270. [Google Scholar] [CrossRef] [Green Version]
- Kuriakose, D.; Xiao, Z. Pathophysiology and Treatment of Stroke: Present Status and Future Perspectives. Int. J. Mol. Sci. 2020, 21, 7609. [Google Scholar] [CrossRef] [PubMed]
- Hughes, B.; Herron, C.E. Cannabidiol Reverses Deficits in Hippocampal LTP in a Model of Alzheimer’s Disease. Neurochem. Res. 2019, 44, 703–713. [Google Scholar] [CrossRef] [PubMed]
- Huestis, M.A.; Solimini, R.; Pichini, S.; Pacifici, R.; Carlier, J.; Busardò, F.P. Cannabidiol Adverse Effects and Toxicity. Curr. Neuropharmacol. 2019, 17, 974–989. [Google Scholar] [CrossRef]
- Dos Santos, R.G.; Guimarães, F.S.; Crippa, J.A.S.; Hallak, J.E.C.; Rossi, G.; Rocha, J.M.; Zuardi, A.W. Serious adverse effects of cannabidiol (CBD): A review of randomized controlled trials. Expert Opin. Drug Metab. Toxicol. 2020, 16, 517–526. [Google Scholar] [CrossRef]
- McGuire, P.; Robson, P.; Cubała, W.; Vasile, D.; Morrison, P.D.; Barron, R.; Taylor, A.; Wright, S. Cannabidiol (CBD) as an Adjunctive Therapy in Schizophrenia: A Multicenter Randomized Controlled Trial. Am. J. Psychiatry 2018, 175, 225–231. [Google Scholar] [CrossRef] [Green Version]
- Masataka, N. Anxiolytic Effects of Repeated Cannabidiol Treatment in Teenagers With Social Anxiety Disorders. Front. Psychol. 2019, 10, 2466. [Google Scholar] [CrossRef] [Green Version]
- Geffrey, A.L.; Pollack, S.F.; Bruno, P.L.; Thiele, E.A. Drug-drug interaction between clobazam and cannabidiol in children with refractory epilepsy. Epilepsia 2015, 56, 1246–1251. [Google Scholar] [CrossRef]
- Devinsky, O.; Patel, A.D.; Thiele, E.A.; Wong, M.H.; Appleton, R.; Harden, C.L.; Greenwood, S.; Morrison, G.; Sommerville, K. On behalf of the GWPCARE1 Part A Study Group Randomized, dose-ranging safety trial of cannabidiol in Dravet syndrome. Neurology 2018, 90, e1204–e1211. [Google Scholar] [CrossRef] [Green Version]
- Tang, R.; Fang, F. Trial of Cannabidiol for Drug-Resistant Seizures in the Dravet Syndrome. N. Engl. J. Med. 2017, 377, 699–700. [Google Scholar] [CrossRef] [PubMed]
- Gaston, T.E.; Bebin, E.M.; Cutter, G.R.; Liu, Y.; Szaflarski, J.P.; The Uab Cbd Program. Interactions between cannabidiol and commonly used antiepileptic drugs. Epilepsia 2017, 58, 1586–1592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thiele, E.; Marsh, E.D.; French, J.; Mazurkiewicz-Beldzinska, M.; Benbadis, S.R.; Joshi, C.; Lyons, P.D.; Taylor, A.; Roberts, C.; Sommerville, K.; et al. Cannabidiol in patients with seizures associated with Lennox-Gastaut syndrome (GWPCARE4): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2018, 391, 1085–1096. [Google Scholar] [CrossRef]
- Hattori, K.; Lee, H.; Hurn, P.D.; Crain, B.J.; Traystman, R.J.; Devries, A.C. Cognitive Deficits After Focal Cerebral Ischemia in Mice. Stroke 2000, 31, 1939–1944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Truong, D.T.; Venna, V.R.; McCullough, L.D.; Fitch, R.H. Deficits in auditory, cognitive, and motor processing following reversible middle cerebral artery occlusion in mice. Exp. Neurol. 2012, 238, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Linden, J.; Fassotte, L.; Tirelli, E.; Plumier, J.-C.; Ferrara, A. Assessment of behavioral flexibility after middle cerebral artery occlusion in mice. Behav. Brain Res. 2014, 258, 127–137. [Google Scholar] [CrossRef]
- Hayakawa, K.; Mishima, K.; Abe, K.; Hasebe, N.; Takamatsu, F.; Yasuda, H.; Ikeda, T.; Inui, K.; Egashira, N.; Iwasaki, K.; et al. Cannabidiol prevents infarction via the non-CB1 cannabinoid receptor mechanism. NeuroReport 2004, 15, 2381–2385. [Google Scholar] [CrossRef]
- Braida, D.; Pegorini, S.; Arcidiacono, M.V.; Consalez, G.G.; Croci, L.; Sala, M. Post-ischemic treatment with cannabidiol prevents electroencephalographic flattening, hyperlocomotion and neuronal injury in gerbils. Neurosci. Lett. 2003, 346, 61–64. [Google Scholar] [CrossRef]
- Pazos, M.R.; Mohammed, N.; Lafuente, H.; Santos, M.; Martínez-Pinilla, E.; Moreno, E.; Valdizan, E.; Romero, J.; Pazos, A.; Franco, R.; et al. Mechanisms of cannabidiol neuroprotection in hypoxic–ischemic newborn pigs: Role of 5HT1A and CB2 receptors. Neuropharmacology 2013, 71, 282–291. [Google Scholar] [CrossRef]
- Iadecola, C.; Anrather, J. The immunology of stroke: From mechanisms to translation. Nat. Med. 2011, 17, 796–808. [Google Scholar] [CrossRef]
- Kim, J.Y.; Park, J.; Chang, J.Y.; Kim, S.-H.; Lee, S.-H.K.A.J.E. Inflammation after Ischemic Stroke: The Role of Leukocytes and Glial Cells. Exp. Neurobiol. 2016, 25, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Jayaraj, R.L.; Azimullah, S.; Beiram, R.; Jalal, F.Y.; Rosenberg, G.A. Neuroinflammation: Friend and foe for ischemic stroke. J. Neuroinflamm. 2019, 16, 142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yasuda, Y.; Shimoda, T.; Uno, K.; Tateishi, N.; Furuya, S.; Tsuchihashi, Y.; Kawai, Y.; Naruse, S.; Fujita, S. Temporal and sequential changes of glial cells and cytokine expression during neuronal degeneration after transient global ischemia in rats. J. Neuroinflamm. 2011, 8, 70. [Google Scholar] [CrossRef] [Green Version]
- Benakis, C.; Garcia-Bonilla, L.; Iadecola, C.; Anrather, J. The role of microglia and myeloid immune cells in acute cerebral ischemia. Front. Cell. Neurosci. 2014, 8, 461. [Google Scholar] [CrossRef]
- Fumagalli, S.; Perego, C.; Pischiutta, F.; Zanier, E.; de Simoni, M.G. The Ischemic Environment Drives Microglia and Macrophage Function. Front. Neurol. 2015, 6, 81. [Google Scholar] [CrossRef] [Green Version]
- Morioka, T.; Kalehua, A.N.; Streit, W.J. Characterization of microglial reaction after middle cerebral artery occlusion in rat brain. J. Comp. Neurol. 1993, 327, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Baron, J.-C.; Yamauchi, H.; Fujioka, M.; Endres, M. Selective Neuronal Loss in Ischemic Stroke and Cerebrovascular Disease. J. Cereb. Blood Flow Metab. 2014, 34, 2–18. [Google Scholar] [CrossRef] [Green Version]
- Emmrich, J.V.; Ejaz, S.; Neher, J.J.; Williamson, D.J.; Baron, J.-C. Regional Distribution of Selective Neuronal Loss and Microglial Activation across the MCA Territory after Transient Focal Ischemia: Quantitative versus Semiquantitative Systematic Immunohistochemical Assessment. J. Cereb. Blood Flow Metab. 2015, 35, 20–27. [Google Scholar] [CrossRef] [Green Version]
- Park, J.H.; Cho, J.H.; Ahn, J.H.; Choi, S.Y.; Lee, T.-K.; Lee, J.-C.; Na Shin, B.; Hong, S.; Jeon, Y.H.; Kim, Y.-M.; et al. Neuronal loss and gliosis in the rat striatum subjected to 15 and 30 minutes of middle cerebral artery occlusion. Metab. Brain Dis. 2018, 33, 775–784. [Google Scholar] [CrossRef]
- Mohammed, N.; Ceprian, M.; Jiménez-Sánchez, L.; Pazos, M.R.; Martinez-Orgado, J. Neuroprotective Effects of Cannabidiol in Hypoxic Ischemic Insult. The Therapeutic Window in Newborn Mice. CNS Neurol. Disord. Drug Targets 2017, 16, 102–108. [Google Scholar] [CrossRef]
- Dos-Santos-Pereira, M.; Guimarães, F.S.; Del-Bel, E.; Raisman-Vozari, R.; Michel, P.P. Cannabidiol prevents LPS-induced microglial inflammation by inhibiting ROS/NF-κB-dependent signaling and glucose consumption. Glia 2020, 68, 561–573. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Chen, N.; Liu, Y.; Godlewski, G.; Kaplan, H.J.; Shrader, S.H.; Song, Z.-H.; Shao, H. Studies of involvement of G-protein coupled receptor-3 in cannabidiol effects on inflammatory responses of mouse primary astrocytes and microglia. PLoS ONE 2021, 16, e0251677. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.J.; Anrather, J.; Nishimura, N.; Schaffer, C.B. Diverse Inflammatory Response After Cerebral Microbleeds Includes Coordinated Microglial Migration and Proliferation. Stroke 2018, 49, 1719–1726. [Google Scholar] [CrossRef]
- Tanaka, R.; Komine-Kobayashi, M.; Mochizuki, H.; Yamada, M.; Furuya, T.; Migita, M.; Shimada, T.; Mizuno, Y.; Urabe, T. Migration of enhanced green fluorescent protein expressing bone marrow-derived microglia/macrophage into the mouse brain following permanent focal ischemia. Neuroscience 2003, 117, 531–539. [Google Scholar] [CrossRef]
- Jolivel, V.; Bicker, F.; Binamé, F.; Ploen, R.; Keller, S.; Gollan, R.; Jurek, B.; Birkenstock, J.; Poisa-Beiro, L.; Bruttger, J.; et al. Perivascular microglia promote blood vessel disintegration in the ischemic penumbra. Acta Neuropathol. 2015, 129, 279–295. [Google Scholar] [CrossRef]
- Liu, Z.; Chopp, M. Astrocytes, therapeutic targets for neuroprotection and neurorestoration in ischemic stroke. Prog. Neurobiol. 2016, 144, 103–120. [Google Scholar] [CrossRef] [Green Version]
- Caudal, L.C.; Gobbo, D.; Scheller, A.; Kirchhoff, F. The Paradox of Astroglial Ca2 + Signals at the Interface of Excitation and Inhibition. Front. Cell. Neurosci. 2020, 14, 609947. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Wang, T.; Cui, W.; Haydon, P.G. Photothrombosis ischemia stimulates a sustained astrocytic Ca2+ signaling in vivo. Glia 2009, 57, 767–776. [Google Scholar] [CrossRef] [Green Version]
- Fordsmann, J.C.; Murmu, R.P.; Cai, C.; Brazhe, A.; Thomsen, K.J.; Zambach, S.A.; Lønstrup, M.; Lind, B.L.; Lauritzen, M. Spontaneous astrocytic Ca2+ activity abounds in electrically suppressed ischemic penumbra of aged mice. Glia 2019, 67, 37–52. [Google Scholar] [CrossRef] [Green Version]
- Rakers, C.; Petzold, G.C. Astrocytic calcium release mediates peri-infarct depolarizations in a rodent stroke model. J. Clin. Investig. 2017, 127, 511–516. [Google Scholar] [CrossRef] [Green Version]
- Lafuente, H.; Alvarez, F.J.; Pazos, M.R.; Alvarez, A.; Rey-Santano, M.C.; Mielgo, V.; Murgia-Esteve, X.; Hilario, E.; Martinez-Orgado, J. Cannabidiol Reduces Brain Damage and Improves Functional Recovery After Acute Hypoxia-Ischemia in Newborn Pigs. Pediatr. Res. 2011, 70, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Esposito, G.; Scuderi, C.; Valenza, M.; Togna, G.I.; Latina, V.; De Filippis, D.; Cipriano, M.; Carratù, M.R.; Iuvone, T.; Steardo, L. Cannabidiol Reduces Aβ-Induced Neuroinflammation and Promotes Hippocampal Neurogenesis through PPARγ Involvement. PLoS ONE 2011, 6, e28668. [Google Scholar] [CrossRef] [PubMed]
- Jahn, H.M.; Kasakow, C.V.; Helfer, A.; Michely, J.; Verkhratsky, A.; Maurer, H.H.; Scheller, A.; Kirchhoff, F. Refined protocols of tamoxifen injection for inducible DNA recombination in mouse astroglia. Sci. Rep. 2018, 8, 5913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deiana, S.; Watanabe, A.; Yamasaki, Y.; Amada, N.; Arthur, M.; Fleming, S.; Woodcock, H.; Dorward, P.; Pigliacampo, B.; Close, S.; et al. Plasma and brain pharmacokinetic profile of cannabidiol (CBD), cannabidivarine (CBDV), Δ9-tetrahydrocannabivarin (THCV) and cannabigerol (CBG) in rats and mice following oral and intraperitoneal administration and CBD action on obsessive–compulsive behaviour. Psychopharmacology 2012, 219, 859–873. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Bai, X.; Meyer, E.; Scheller, A. Acute brain injuries trigger microglia as an additional source of the proteoglycan NG. Acta Neuropathol. Commun. 2020, 8, 146. [Google Scholar] [CrossRef]
- Koizumi, J.-I.; Yoshida, Y.; Nakazawa, T.; Ooneda, G. Experimental studies of ischemic brain edema. Jpn. J. Stroke 1986, 8, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Cupido, A.; Catalin, B.; Steffens, H.; Kirchhoff, F. Surgical Procedures to Study Microglial Motility in the Brain and in the Spinal Cord by In Vivo Two-Photon Laser-Scanning Microcopy. In Confocal and Multiphoton Laser-Scanning Microscopy of Neuronal Tissue: Applications and Quantitative Image Analysis; Bakota, L., Brandt, R., Eds.; Springer: Berlin, Germany, 2014; pp. 37–50. [Google Scholar]
- Bieber, M.; Gronewold, J.; Scharf, A.-C.; Schuhmann, M.K.; Langhauser, F.; Hopp, S.; Mencl, S.; Geuss, E.; Leinweber, J.; Guthmann, J.; et al. Validity and Reliability of Neurological Scores in Mice Exposed to Middle Cerebral Artery Occlusion. Stroke 2019, 50, 2875–2882. [Google Scholar] [CrossRef]
- Bederson, J.B.; Pitts, L.H.; Tsuji, M.; Nishimura, M.C.; Davis, R.L.; Bartkowski, H. Rat middle cerebral artery occlusion: Evaluation of the model and development of a neurologic examination. Stroke 1986, 17, 472–476. [Google Scholar] [CrossRef] [Green Version]
- Schmued, L.C.; Hopkins, K.J. Fluoro-Jade: Novel Fluorochromes for Detecting Toxicant-Induced Neuronal Degeneration. Toxicol. Pathol. 2000, 28, 91–99. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Pologruto, T.; Sabatini, B.L.; Svoboda, K. ScanImage: Flexible software for operating laser scanning microscopes. Biomed. Eng. Online 2003, 2, 13. [Google Scholar] [CrossRef] [PubMed]
- Stopper, L.C.; Caudal, P.; Rieder, D.; Gobbo, L.; Felix, K.; Everaerts, X.; Bai, L.; Stopper, C.R.; Scheller, A.R.; Kirchhoff, F. Novel algorithms for improved detection and analysis of fluorescent signal fluctuations. bioRxiv 2022. [Google Scholar] [CrossRef]
- Rieder, P.; Gobbo, D.; Stopper, G.; Welle, A.; Damo, E.; Kirchhoff, F.; Scheller, A. Astrocytes and Microglia Exhibit Cell-Specific Ca2+ Signaling Dynamics in the Murine Spinal Cord. Front. Mol. Neurosci. 2022, 15, 840948. [Google Scholar] [CrossRef] [PubMed]
- Luisier, F.; Blu, T.; Unser, M. Image Denoising in Mixed Poisson–Gaussian Noise. IEEE Trans. Image Process. 2011, 20, 696–708. [Google Scholar] [CrossRef]
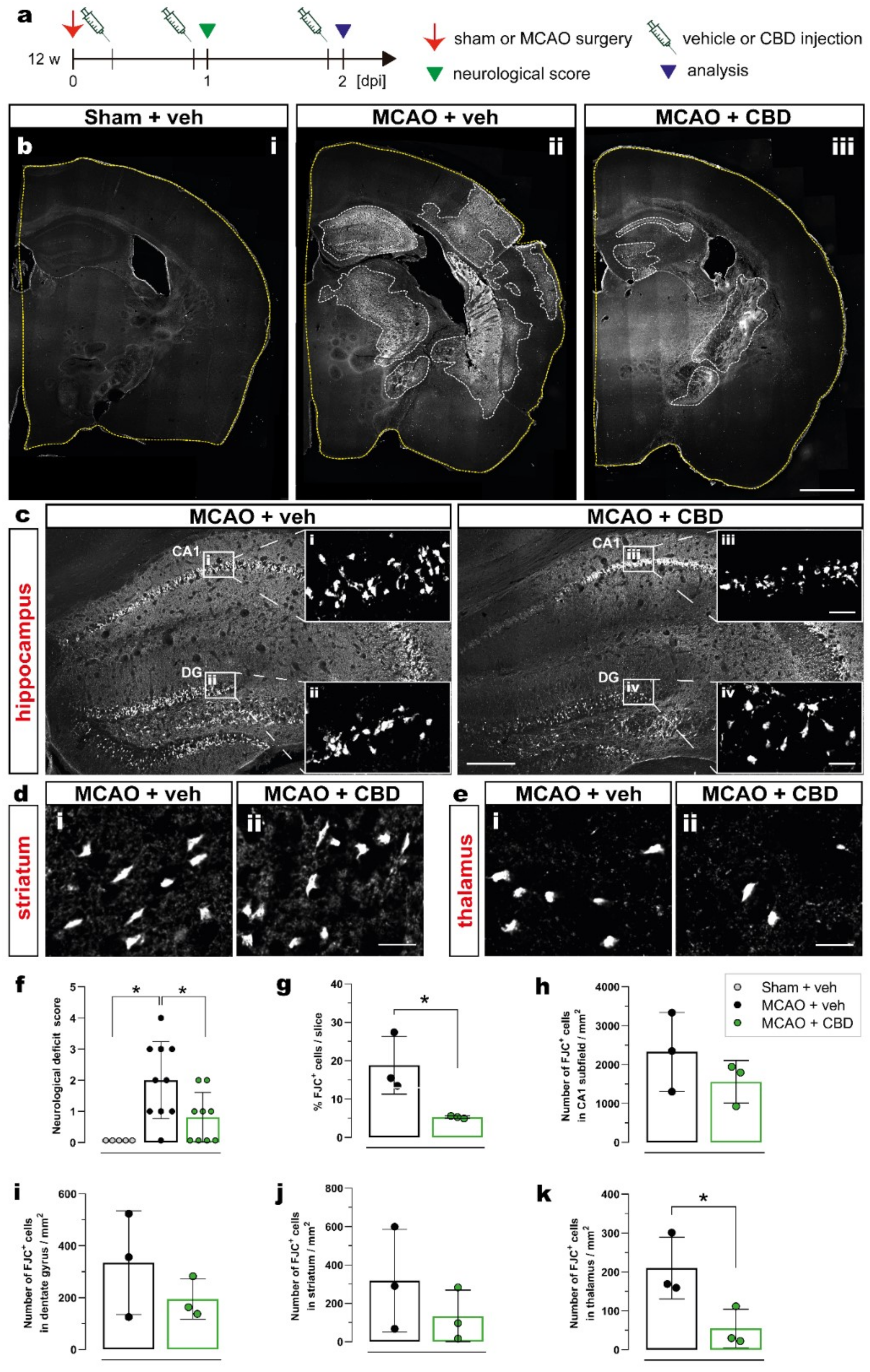
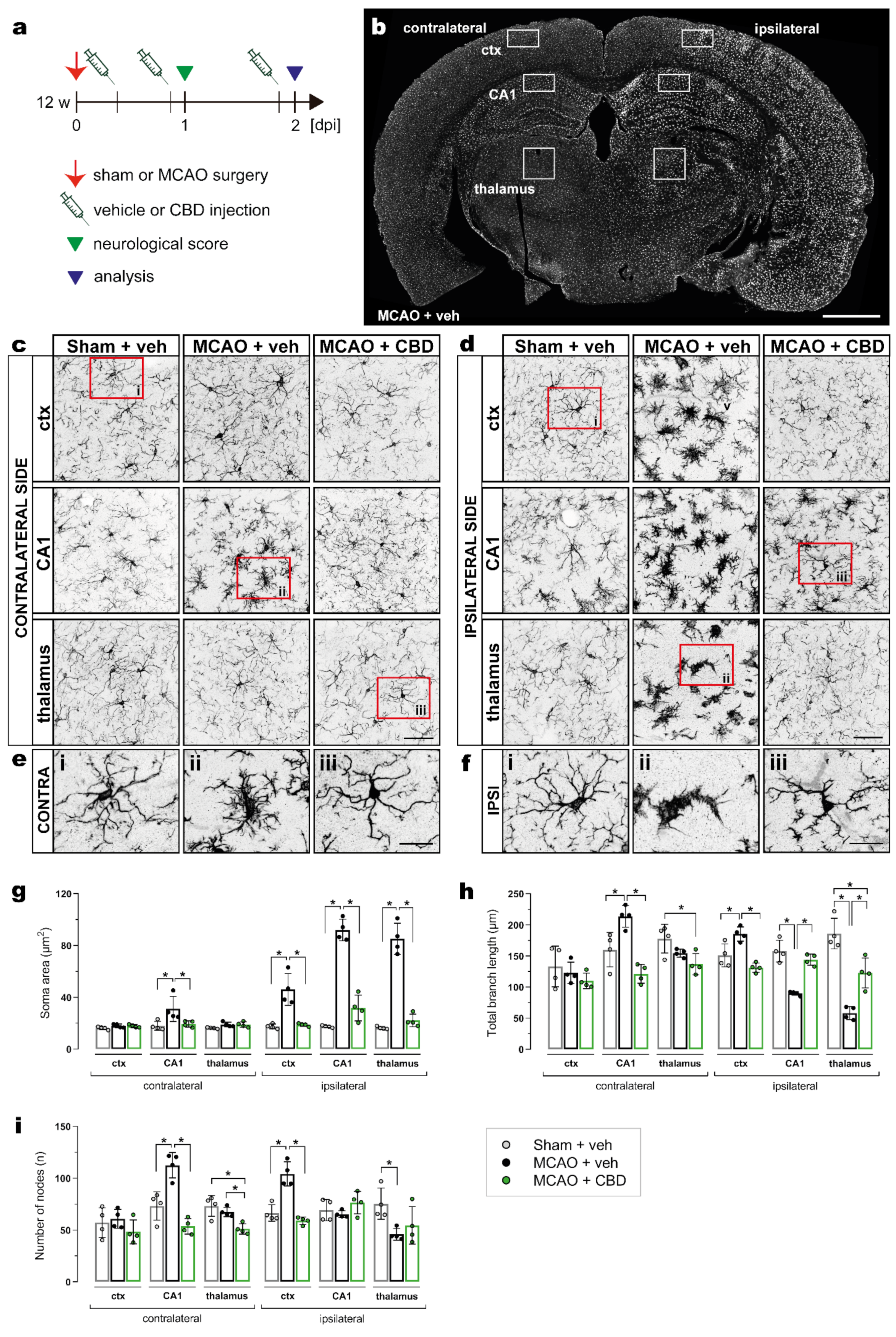
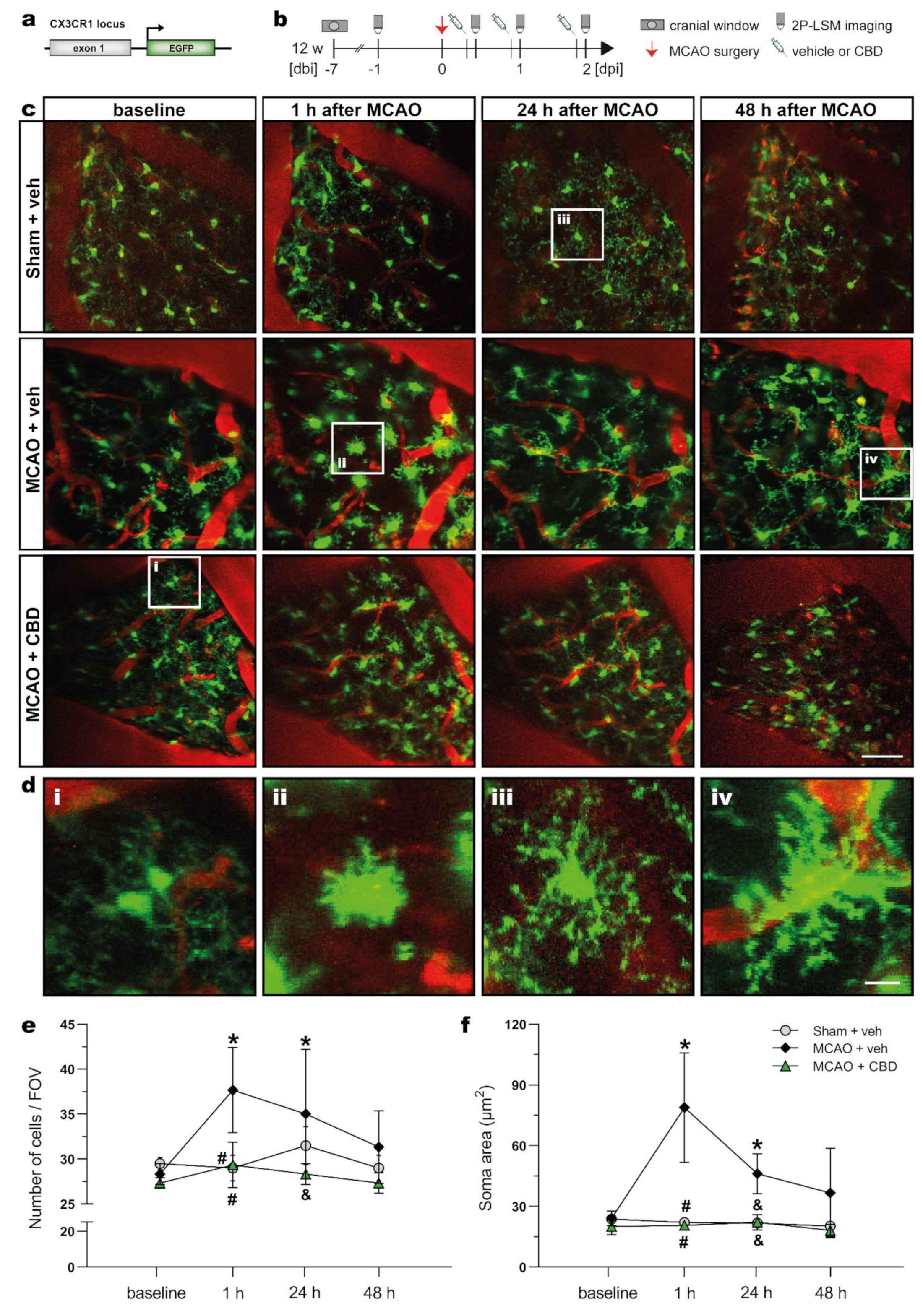
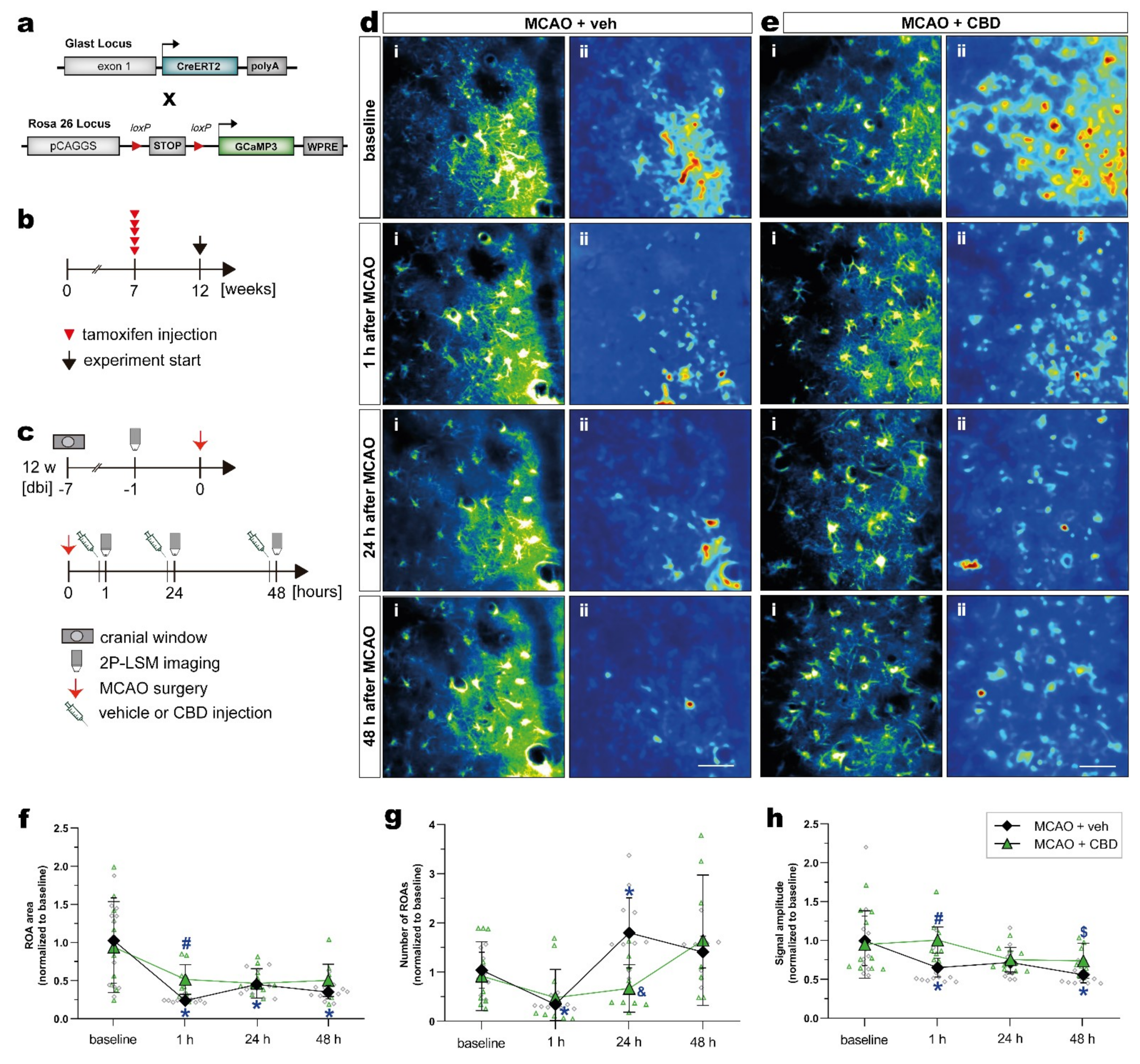
| Analyses | Experimental Groups and Respective Sample Size | ||
|---|---|---|---|
| Sham + veh | MCAO + veh | MCAO + CBD | |
| Neurological score | 5 | 10 | 9 |
| Fluoro-Jade C staining | - | 3 | 3 |
| Immunohistochemistry | 4 | 4 | 4 |
| Time-lapse imaging of microglia | - | 3 | 3 |
| Time-lapse imaging of astroglial Ca2+ signals | - | 3 | 3 |
| Score | Bederson Neurological Score (0–5) |
|---|---|
| 0 | No observable deficit in motor behavior |
| 1 | Forelimb flexion |
| 2 | Forelimb flexion and decreased resistance to lateral push |
| 3 | Circling |
| 4 | Circling and spinning around the cranial–caudal axis |
| 5 | No spontaneous movement |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meyer, E.; Rieder, P.; Gobbo, D.; Candido, G.; Scheller, A.; de Oliveira, R.M.W.; Kirchhoff, F. Cannabidiol Exerts a Neuroprotective and Glia-Balancing Effect in the Subacute Phase of Stroke. Int. J. Mol. Sci. 2022, 23, 12886. https://doi.org/10.3390/ijms232112886
Meyer E, Rieder P, Gobbo D, Candido G, Scheller A, de Oliveira RMW, Kirchhoff F. Cannabidiol Exerts a Neuroprotective and Glia-Balancing Effect in the Subacute Phase of Stroke. International Journal of Molecular Sciences. 2022; 23(21):12886. https://doi.org/10.3390/ijms232112886
Chicago/Turabian StyleMeyer, Erika, Phillip Rieder, Davide Gobbo, Gabriella Candido, Anja Scheller, Rúbia Maria Weffort de Oliveira, and Frank Kirchhoff. 2022. "Cannabidiol Exerts a Neuroprotective and Glia-Balancing Effect in the Subacute Phase of Stroke" International Journal of Molecular Sciences 23, no. 21: 12886. https://doi.org/10.3390/ijms232112886






