Vitamin C Maintenance against Cell Growth Arrest and Reactive Oxygen Species Accumulation in the Presence of Redox Molecular Chaperone hslO Gene
Abstract
1. Introduction
2. Results
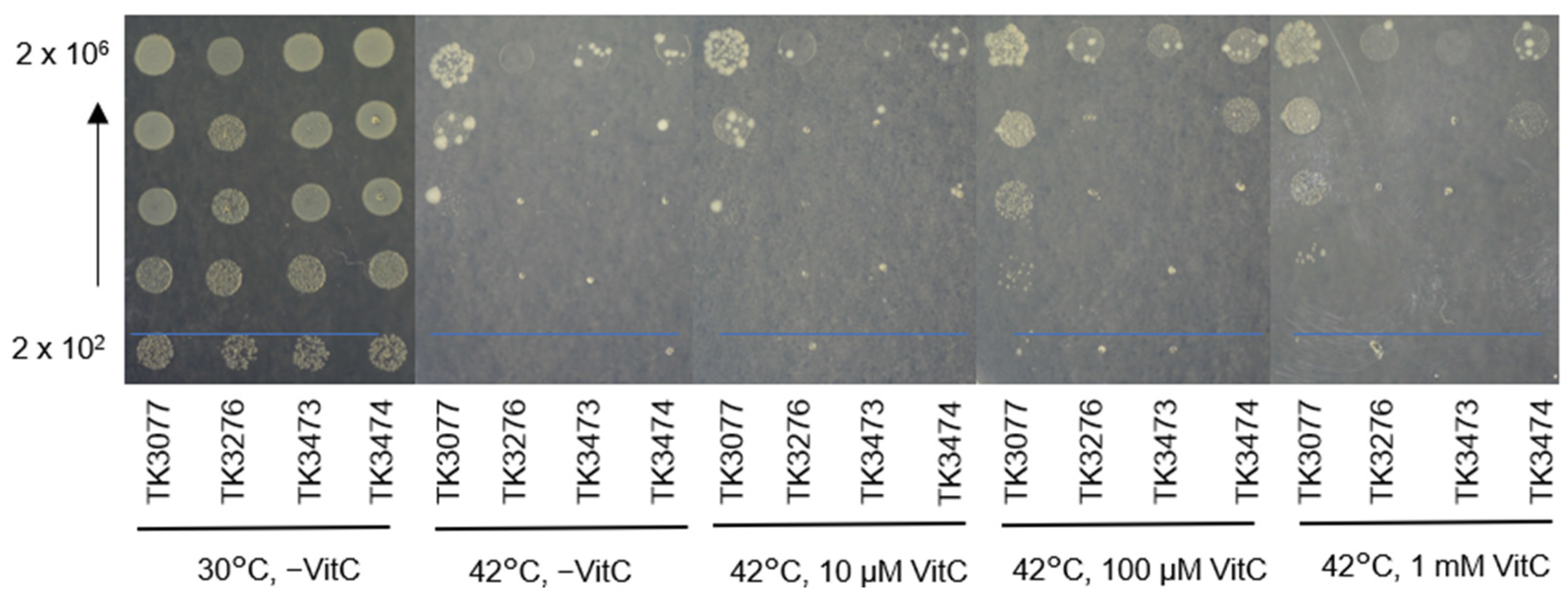
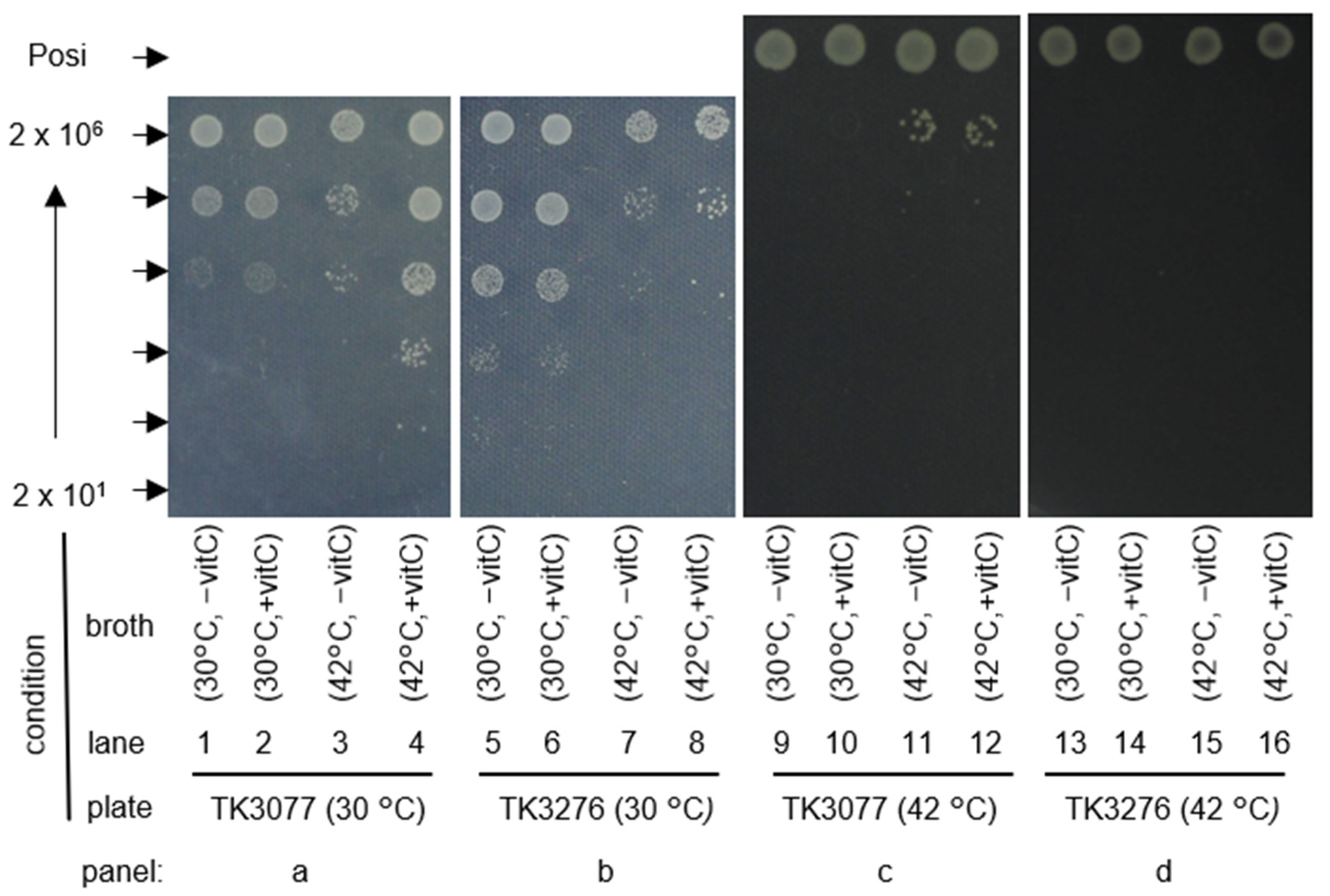
2.1. recAts polA Cells Form Colonies in Restrictive Temperatures in the Presence of Vitamin C
2.2. Vitamin C Is Involved in Maintaining the Colony-Forming Ability of recAts polA Cells at Restrictive Temperatures
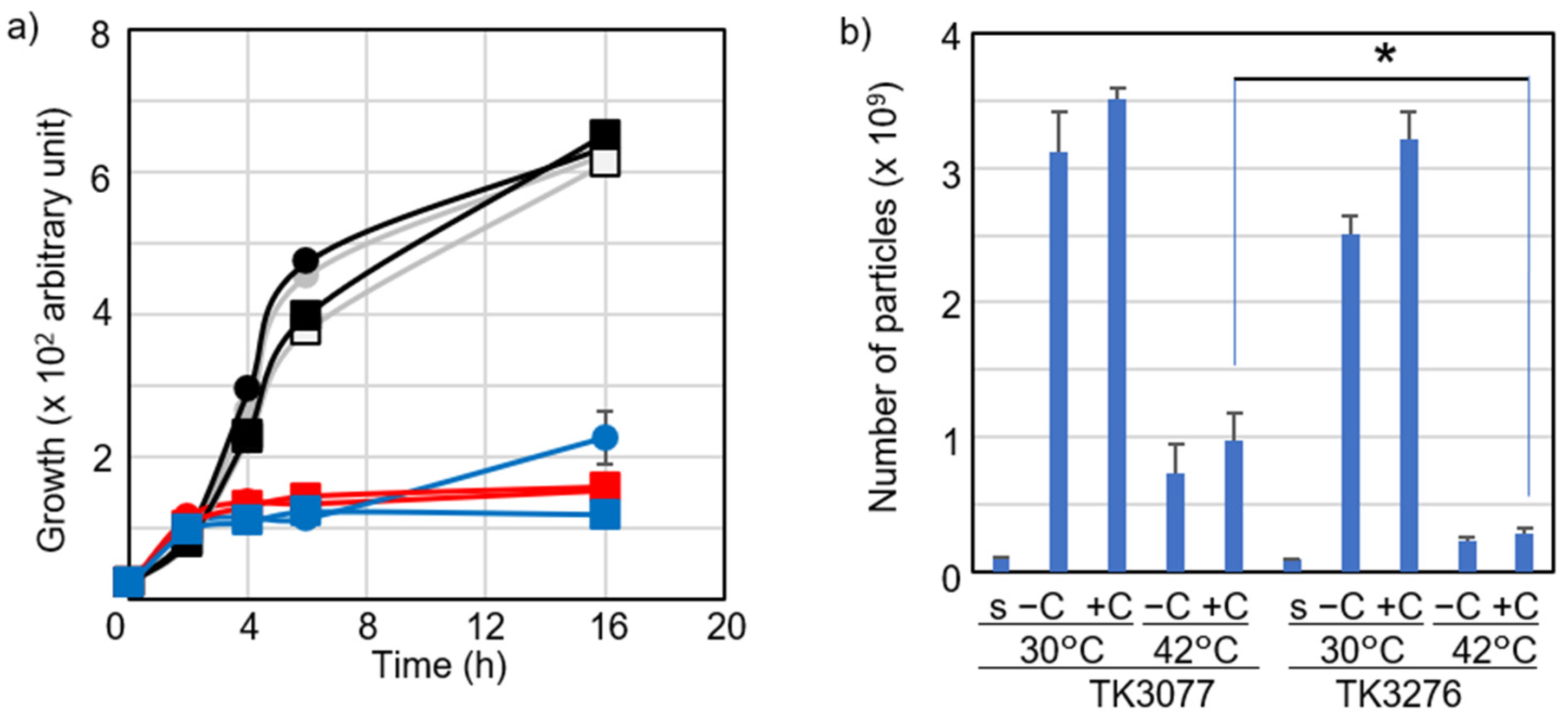
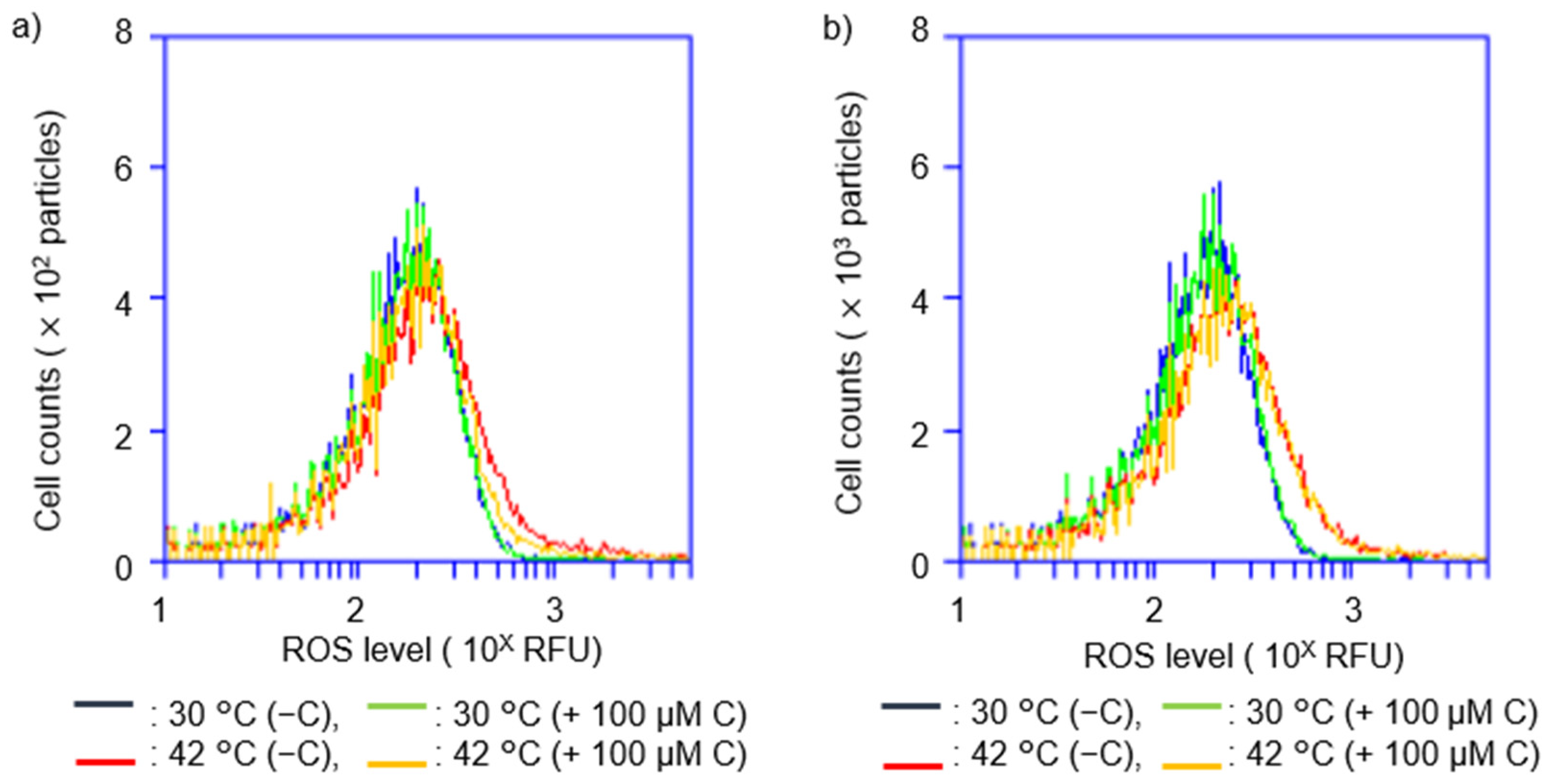
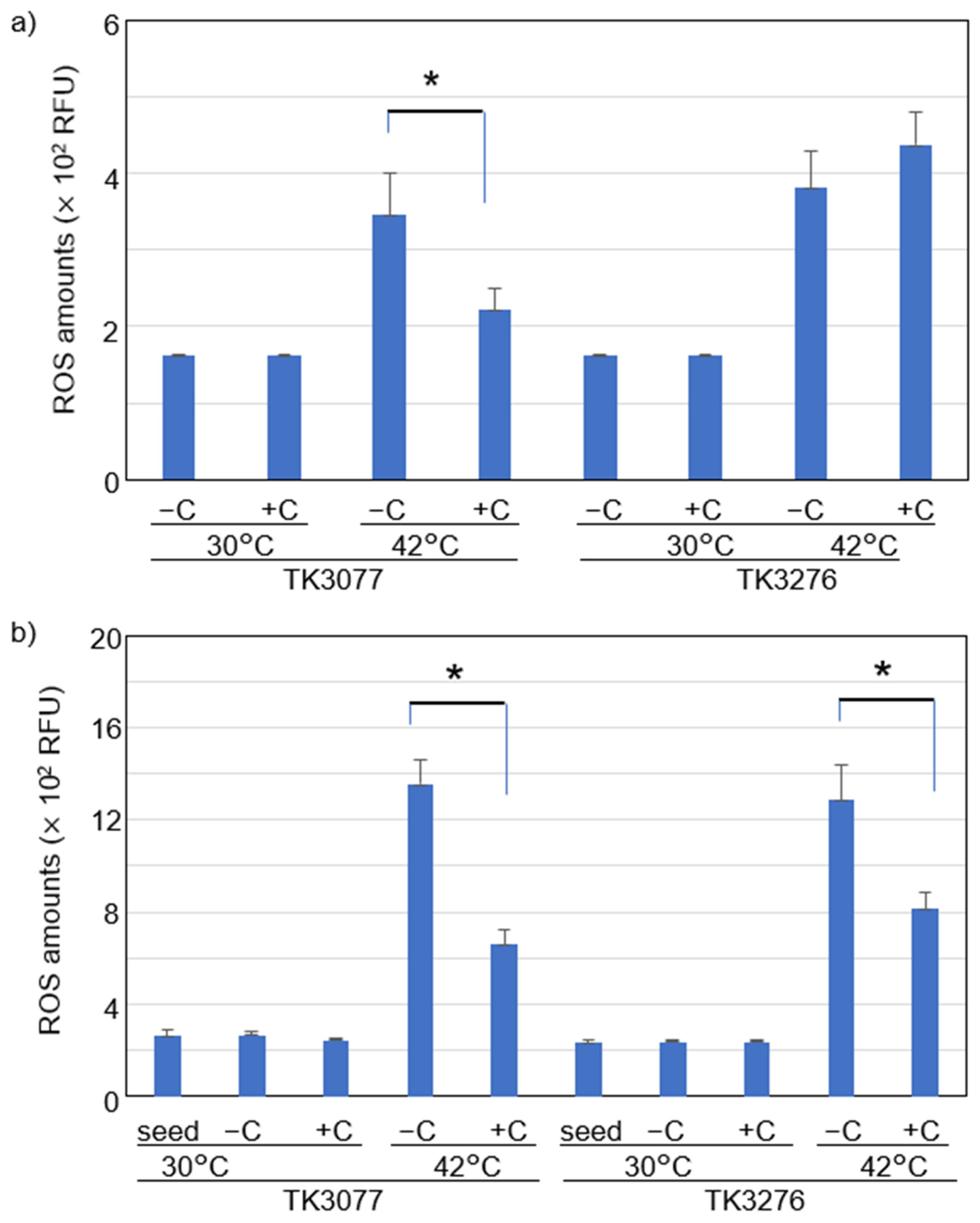
2.3. Vitamin C Reduced Accumulation of Intracellular ROS Levels at Restrictive Temperatures in hslO+ recAts polA Cells
2.4. Vitamin C Affected Intracellular ROS Levels in recAts polA Cells by Synchronizing ROS Levels with Growth
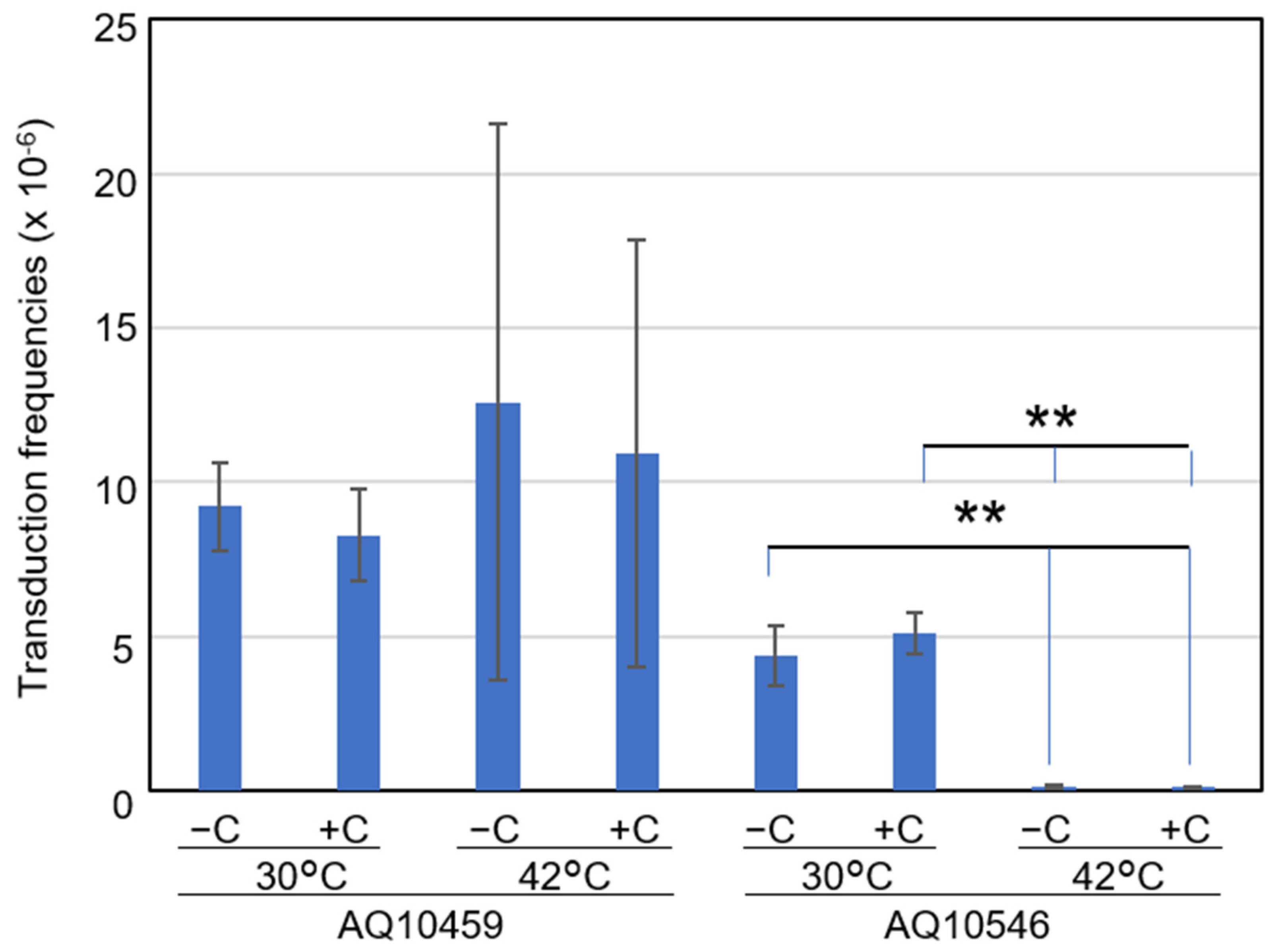
2.5. Vitamin C Influences a Convergence of Cell Population with Chromosomal DNA Level Rather Than the Temperature-Sensitive Recombination of the recAts Mutation
3. Discussion
4. Materials and Methods
4.1. E. coli Strains and Media
4.2. Cultivation and Sampling Methods
4.3. Plasmid Construction
4.4. Determination of Survival Fraction and Cell Recovery
4.5. Flow Cytometry Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cao, Y.; Kogoma, T. The mechanism of recA polA Lethality: Suppression by RecA-independent recombination repair activated by the lexA(Def) mutation in Escherichia coli. Genetics 1995, 139, 1483–1494. [Google Scholar] [CrossRef]
- Kaidow, A.; Ishii, N.; Suzuki, S.; Shiina, T.; Kasahara, H. Reactive oxygen species accumulation is synchronised with growth inhibition of temperature-sensitive recAts polA Escherichia coli. Arch. Microbiol. 2022, 204, 396. [Google Scholar] [CrossRef]
- Kaidow, A.; Ishii, N.; Suzuki, S.; Shiina, T.; Endoh, K.; Murakami, Y.; Kasahara, H. HslO Ameliorates Arrested ΔrecA polA Cell Growth and Reduces DNA Damage and Oxidative Stress Responses. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Hong, Y.; Zeng, J.; Wang, X.; Drlica, K.; Zhao, X. Post-stress bacterial cell death mediated by reactive oxygen species. Proc. Natl. Acad. Sci. USA 2019, 116, 10064–10071. [Google Scholar] [CrossRef] [PubMed]
- Imlay, J.A. Diagnosing oxidative stress in bacteria: Not as easy as you might think. Curr. Opin. Microbiol. 2015, 24, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Li, L.; Luan, G.; Drlica, K.; Zhao, X. Contribution of reactive oxygen species to thymineless death in Escherichia coli. Nat. Microbiol. 2017, 2, 1667–1675. [Google Scholar] [CrossRef] [PubMed]
- Li, R. Vitamin C, a multi-tasking molecule, finds a molecular target in killing cancer cells. React. Oxyg Species 2016, 1, 141–156. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J. Redox signaling: An evolution from free radicals to aging. Free Radic. Biol. Med. 2016, 97, 398–407. [Google Scholar] [CrossRef]
- Butturini, E.; Carcereri de Prati, A.; Mariotto, S. Redox regulation of STAT1 and STAT3 signaling. Int. J. Mol. Sci. 2020, 21, 7034. [Google Scholar] [CrossRef] [PubMed]
- Hübner, C.; Haase, H. Interactions of zinc- and redox-signaling pathways. Redox Biol. 2021, 41, 101916. [Google Scholar] [CrossRef] [PubMed]
- Jakob, U.; Muse, W.; Eser, M.; Bardwell, J.C. Chaperone activity with a redox switch. Cell 1999, 96, 341–352. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Ritz, D.; Planson, A.G.; Jönsson, T.J.; Faulkner, M.J.; Boyd, D.; Beckwith, J.; Poole, L.B. Mutant AhpC peroxiredoxins suppress thiol-disulfide redox deficiencies and acquire deglutathionylating activity. Mol. Cell 2008, 29, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Campos, E.M.C.; Garces, F.; Baldoma, L.; Aguilar, J.; Badia, J. Aerobic L-ascorbate metabolism and associated oxidative stress in Escherichia coli. Microbiology 2007, 153, 3399–3408. [Google Scholar] [CrossRef] [PubMed]
- Pedre, B.; Young, D.; Charlier, D.; Mourenza, Á.; Rosado, L.A.; Marcos-Pascual, L.; Wahni, K.; Martens, E.; de la Rubia, A.G.; Belousov, V.V.; et al. Structural snapshots of OxyR reveal the peroxidatic mechanism of H2O2 sensing. Proc. Natl. Acad. Sci. USA 2018, 115, E11623–E11632. [Google Scholar] [CrossRef]
- Nunoshiba, T. Two-stage gene regulation of the superoxide stress response soxRS system in Escherichia coli. Crit. Rev. Eukaryot. Gene Expr. 1996, 6, 377–389. [Google Scholar] [CrossRef]
- Gu, M.; Imlay, J.A. The SoxRS response of escherichia coli is directly activated by redox-cycling drugs rather than by superoxide. Mol. Microbiol. 2011, 79, 1136–1150. [Google Scholar] [CrossRef]
- Ilbert, M.; Horst, J.; Ahrens, S.; Winter, J.; Graf, P.C.; Lilie, H.; Jakob, U. The redox-switch domain of Hsp33 functions as dual stress sensor. Nat. Struct. Mol. Biol. 2007, 14, 556–563. [Google Scholar] [CrossRef]
- Reichmann, D.; Xu, Y.; Cremers, C.M.; Ilbert, M.; Mittelman, R.; Fitzgerald, M.C.; Jakob, U. Order out of disorder: Working cycle of an intrinsically unfolded chaperone. Cell 2012, 148, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Reichmann, D.; Jakob, U. The roles of conditional disorder in redox proteins. Curr. Opin. Struct. Biol. 2013, 23, 436–442. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Segal, N.A.; Shapira, M. HSP33 in Eukaryotes—An evolutionary tale of a chaperone adapted to photosynthetic organisms. Plant J. 2015, 82, 850–860. [Google Scholar] [CrossRef] [PubMed]
- Groitl, B.; Horowitz, S.; Makepeace, K.A.T.; Petrotchenko, E.V.; Borchers, C.H.; Reichmann, D.; Bardwell, J.C.A.; Jakob, U. Protein unfolding as a switch from self-recognition to high-affinity client binding. Nat. Commun. 2016, 7, 10357. [Google Scholar] [CrossRef] [PubMed]
- Smirnoff, N. Ascorbic acid metabolism and functions: A comparison of plants and mammals. Free Radic. Biol. Med. 2018, 122, 116–129. [Google Scholar] [CrossRef]
- Yokoo, S.; Furumoto, K.; Hiyama, E.; Miwa, N. Slow-down of age-dependent telomere shortening is executed in human skin keratinocytes by hormesis-like-effects of trace hydrogen peroxide or by anti-oxidative effects of pro-vitamin c in common concurrently with reduction of intracellular oxidative stress. J. Cell. Biochem. 2004, 93, 588–597. [Google Scholar] [CrossRef]
- Messer, W.; Weigel, C.; Messer, W.; Weigel, C. Escherichia coli and Salmonella typhimurium: Cellular and Molecular Biology, 2nd ed.; American Society of Microbiology: Washington, DC, USA, 1996; pp. 1579–1601. [Google Scholar]
- Mendoza-Chamizo, B.; Løbner-Olesen, A.; Charbon, G. Coping with reactive oxygen species to ensure genome stability in Escherichia coli. Genes 2018, 9, 565. [Google Scholar] [CrossRef]
- Kontek, R.B.K.; Kontek, B.; Grzegorczyk, K. Vitamin C modulates DNA damage induced by hydrogen peroxide in human Colorectal adenocarcinoma cell lines (HT29) estimated by comet assay in vitro. Arch. Med. Sci. 2013, 9, 1006–1012. [Google Scholar] [CrossRef]
- Deutsch, J.C. Oxygen-accepting antioxidants which arise during ascorbate oxidation. Anal. Biochem. 1998, 265, 238–245. [Google Scholar] [CrossRef]
- Shin, S.-Y.; Kim, I.-S.; Kim, Y.-H.; Park, H.M.; Lee, J.-Y.; Kang, H.-G.; Yoon, H.S. Scavenging reactive oxygen species by rice dehydroascorbate reductase alleviates oxidative stresses in Escherichia coli. Mol. Cells 2008, 26, 616–620. [Google Scholar] [PubMed]
- Fu, L.H.; Wei, Z.Z.; Hu, K.D.; Hu, L.Y.; Li, Y.H.; Chen, X.Y.; Han, Z.; Yao, G.F.; Zhang, H. Hydrogen sulfide inhibits the growth of Escherichia coli through oxidative damage. J. Microbiol. 2018, 56, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Kamariah, N.; Eisenhaber, B.; Eisenhaber, F.; Grüber, G. Molecular mechanism of the Escherichia coli AhpC in the function of a chaperone under heat-shock conditions. Sci. Rep. 2018, 8, 14151. [Google Scholar] [CrossRef]
- Lanzov, V.A.; Bakhlanova, I.V.; Clark, A.J. Conjugational hyperrecombination achieved by derepressing the LexA regulon, altering the properties of RecA protein and inactivating mismatch repair in Escherichia coli K-12. Genetics 2003, 163, 1243–1254. [Google Scholar] [CrossRef] [PubMed]
- Kogoma, T. Stable DNA replication: Interplay between DNA replication, homologous recombination, and transcription. Microbiol. Mol. Biol. Rev. 1997, 61, 212–238. [Google Scholar] [CrossRef] [PubMed]
- Lennox, E.S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology 1955, 1, 190–206. [Google Scholar] [CrossRef]
- Miller, J.H. A Short Course in Bacterial Genetics: A Laboratory Manual and Handbook for Escherichia coli and Related Bacteria; Plainview NY Cold Spring Harbor Laboratory Press: New York, NY, USA, 1992. [Google Scholar] [CrossRef]
- Ferullo, D.J.; Cooper, D.L.; Moore, H.R.; Lovett, S.T. Cell cycle synchronization of Escherichia coli using the stringent response, with fluorescence labeling assays for DNA content and replication. Methods 2009, 48, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Kogoma, T. Requirement for the Polymerization and 5′-->3′ exonuclease activities of DNA polymerase I in initiation of DNA replication at oriK sites in the absence of RecA in Escherichia coli rnhA mutants. J. Bacteriol. 1993, 175, 7254–7259. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaidow, A.; Ishii, N.; Suzuki, S.; Shiina, T.; Kasahara, H. Vitamin C Maintenance against Cell Growth Arrest and Reactive Oxygen Species Accumulation in the Presence of Redox Molecular Chaperone hslO Gene. Int. J. Mol. Sci. 2022, 23, 12786. https://doi.org/10.3390/ijms232112786
Kaidow A, Ishii N, Suzuki S, Shiina T, Kasahara H. Vitamin C Maintenance against Cell Growth Arrest and Reactive Oxygen Species Accumulation in the Presence of Redox Molecular Chaperone hslO Gene. International Journal of Molecular Sciences. 2022; 23(21):12786. https://doi.org/10.3390/ijms232112786
Chicago/Turabian StyleKaidow, Akihiro, Noriko Ishii, Shingo Suzuki, Takashi Shiina, and Hirokazu Kasahara. 2022. "Vitamin C Maintenance against Cell Growth Arrest and Reactive Oxygen Species Accumulation in the Presence of Redox Molecular Chaperone hslO Gene" International Journal of Molecular Sciences 23, no. 21: 12786. https://doi.org/10.3390/ijms232112786
APA StyleKaidow, A., Ishii, N., Suzuki, S., Shiina, T., & Kasahara, H. (2022). Vitamin C Maintenance against Cell Growth Arrest and Reactive Oxygen Species Accumulation in the Presence of Redox Molecular Chaperone hslO Gene. International Journal of Molecular Sciences, 23(21), 12786. https://doi.org/10.3390/ijms232112786






