Abstract
Systemic juvenile idiopathic arthritis (sJIA) and its complication, macrophage activation syndrome (sJIA-MAS), are rare but sometimes very serious or even critical diseases of childhood that can occasionally be characterized by nonspecific clinical signs and symptoms at onset—such as non-remitting high fever, headache, rash, or arthralgia—and are biologically accompanied by an increase in acute-phase reactants. For a correct positive diagnosis, it is necessary to rule out bacterial or viral infections, neoplasia, and other immune-mediated inflammatory diseases. Delays in diagnosis will result in late initiation of targeted therapy. A set of biomarkers is useful to distinguish sJIA or sJIA-MAS from similar clinical entities, especially when arthritis is absent. Biomarkers should be accessible to many patients, with convenient production and acquisition prices for pediatric medical laboratories, as well as being easy to determine, having high sensitivity and specificity, and correlating with pathophysiological disease pathways. The aim of this review was to identify the newest and most powerful biomarkers and their synergistic interaction for easy and accurate recognition of sJIA and sJIA-MAS, so as to immediately guide clinicians in correct diagnosis and in predicting disease outcomes, the response to treatment, and the risk of relapses. Biomarkers constitute an exciting field of research, especially due to the heterogeneous nature of cytokine storm syndromes (CSSs) in the COVID era. They must be selected with utmost care—a fact supported by the increasingly improved genetic and pathophysiological comprehension of sJIA, but also of CSS—so that new classification systems may soon be developed to define homogeneous groups of patients, although each with a distinct disease.
1. Introduction
Juvenile idiopathic arthritis (JIA) is a set of chronic childhood disorders that usually cause inflammation and pain, swelling, and stiffness in the joints of children under the age of 16 years, which may last from months to the entire life of the patient, with a risk of locomotor and ocular disabilities. The unparalleled therapeutic advances since the beginning of this century have turned the remission or minimization of disease activity from an ideal goal to an achievable one for most JIA patients, with fewer joint and extra-articular injuries and reduced incidence of long-term physical disabilities. However, with all current therapeutic means of conventional synthetic disease-modifying anti-rheumatic drugs (csDMARDs) and/or biological DMARDs (bDMARDs), long-term remission is difficult to achieve for some patients [1,2,3].
Nevertheless, there are scientific reports indicating that after 5 years from diagnosis, less than half of JIA patients who received contemporary medication went into remission. At the same time, prolonged anti-inflammatory treatment is associated with high financial costs, high stress for the patient and their family, and potential side effects [4,5].
Current diagnostic guidelines for patients with JIA are not currently accompanied by recommendations for the use of predictive molecular biomarkers for relapses, complications, disease progression, and prognosis.
Action plans based on predictive biomarkers are promising tools in decision-making as to whether to withdraw or discontinue medication if the inflammation is no longer severe enough to show clear clinical symptoms, and could be very beneficial to patients [6].
Systemic juvenile idiopathic arthritis (sJIA) is a subtype manifested by arthritis or arthralgia, in addition to extra-articular symptoms, which include the following: high daily and prolonged fever of unknown origin, persisting for at least 3 consecutive days and recurring for at least 2 weeks; transient rash; and at least one of the following clinical characteristics: enlarged lymph nodes; hepatosplenomegaly; or pleural, pericardial, or peritoneal effusion, sometimes associated with pneumonitis and/or signs of central nervous system damage. sJIA, previously thought to be an autoimmune disease, is now more commonly classified as an autoinflammatory disease, because the genetic abnormalities of the major histocompatibility complex (MHC) and the innate immune system (such as natural killer (NK) cells, polymorphonuclear neutrophils (PMNs), and macrophages (MP))—with the participation of interleukins 1 (IL-1), 6 (IL-6), and 18 (IL-18)—have been shown to be involved. The prognosis and evolution of the disease are unpredictable, ranging from a mild single-phase evolution to a severe chronic cyclic polyarticular disease complicated by extra-articular manifestations that can induce significant morbidity and mortality [7,8,9,10,11].
sJIA has many characteristics of an autoinflammatory pathology, associated with an increased synthesis of pro-inflammatory interleukins such as IL-1, IL-6, and IL-18, as well as S100 proteins; at the same time, in its evolution, sJIA can be complicated by macrophage activation syndrome (MAS). This term is used by rheumatologists to describe the aggressive, life-threatening complications in patients with sJIA or AOSD, and is phenotypically associated with secondary hemophagocytic lymphohistiocytosis (sec-HLH), in which IFN-γ and CXCL9 play essential roles as biomarkers. MAS is manifested by high fever, cytopenia, cytokine storm (CS), severe liver dysfunction, consumptive coagulopathy, seizures, coma, and even death. During the evolution of sJIA as active disease, MAS may occur (sJIA-MAS) in 7–17% of cases, while subclinical cases are even more frequent, found in 30–40% of patients. The criteria for the classification of sJIA-MAS were advanced in 2016 [12,13,14,15,16].
Biomarkers have a high potential as indispensable tools for pediatric rheumatologists in the early differentiation of cytokine storm syndromes (CSSs).
Currently, due to the diversity of cases and the increasingly complicated pathologies that appear after infection with SARS-CoV-2—such as multisystem inflammatory syndrome in children (MIS-C)—the need to refine and maximize these biomarkers has become more and more important, in order to move from the laboratory to the patient’s bedside as soon as possible, to apply the results of research in as many clinical laboratories as possible for sampling the analyses. Based on this approach, a successful positive diagnosis and the early initiation of the appropriate individualized treatment for the respective patient could save their life in the event of CSS-aggravating circumstances.
The aim of this review was to identify the newest and most powerful biomarkers and their synergistic interactions for easy and accurate recognition of sJIA, as well as its complication sJIA-MAS, to immediately guide clinicians in the correct diagnosis and in predicting the outcomes of the disease, the response to treatment, and the risk of relapses.
Biomarkers constitute an exciting field of research, especially due to the heterogeneous nature of CSS in the COVID-19 era. They must be selected with the utmost care—a fact supported by the increasingly improved genetic and pathophysiological comprehension of sJIA, but also of CSS—so that new classification systems can be developed to define homogeneous groups of patients, each with a distinct disease.
The discovery of new biomarkers with the best possible diagnostic/prognostic value could increase the speed of reaching the final goal, i.e., the introduction of new targeted therapies and personalized interventions, improving disease remission percentages while minimizing all undesirable pathophysiological effects of the disease.
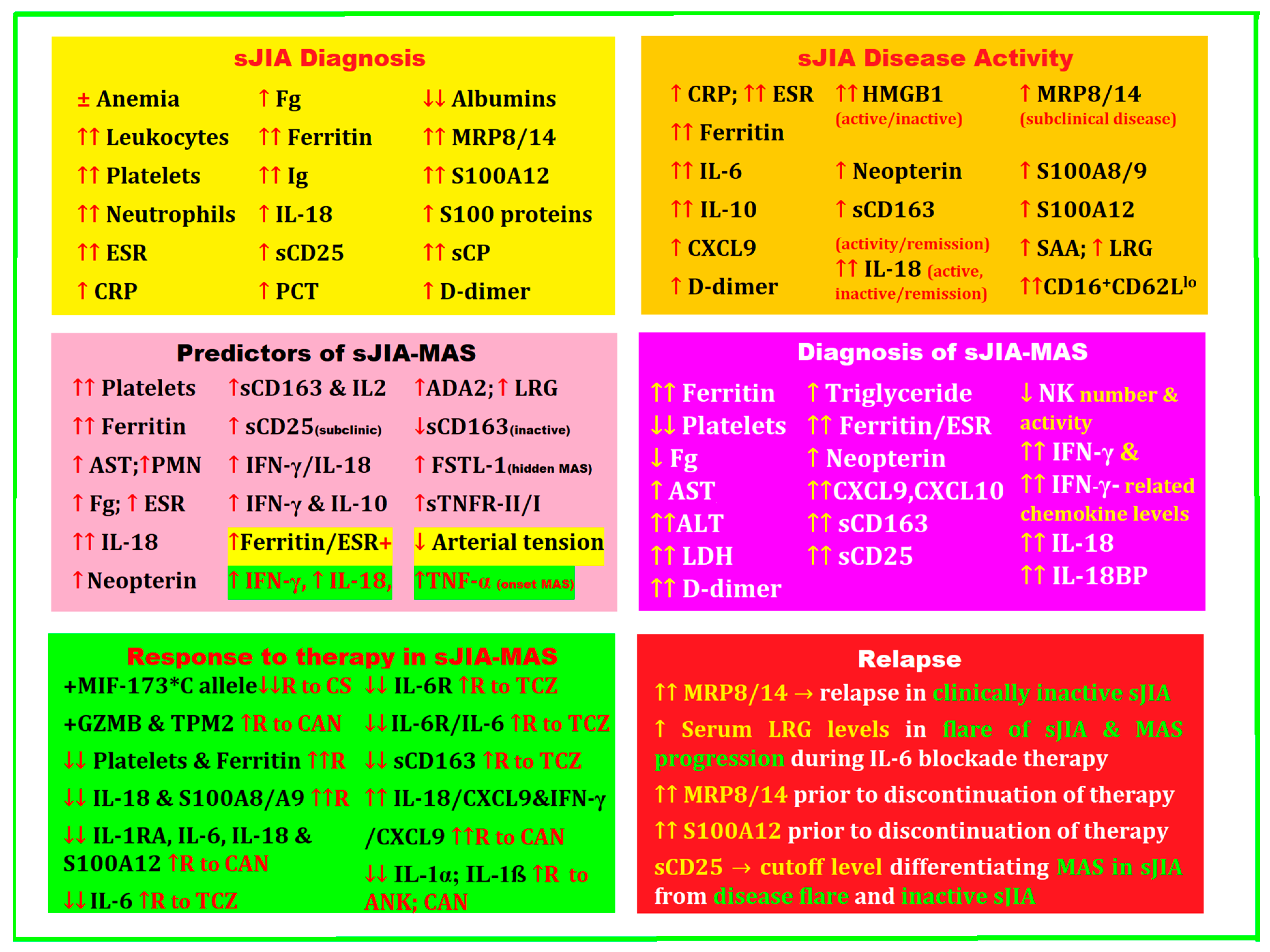
2. Biomarkers for Diagnosis, Treatment, and Disease Activity in sJIA Complicated by MAS
A positive diagnosis of sJIA is initially very difficult, especially when arthritis is absent as a defining symptom. In this case, it is necessary to extend the investigations by making a differential diagnosis with different entities that are caused by viral or bacterial infections, neoplasms, and other inflammatory diseases. It is precisely these issues that generate the urgent need to find biomarkers to help exclude the various other entities for the early diagnosis of sJIA. These biomarkers must have high sensitivity and specificity, be easy to determine at low cost, and be found in the pathogenic mechanisms of the disease.
The absence of arthritis at the beginning of the disease is an impediment for clinicians, as there are currently no reliable diagnostic criteria, and this could lead to a delay in early positive diagnosis, which may have consequences for the initiation of targeted treatment, as well as patients’ outcomes and prognosis [17,18].
The discovery of the roles of IL-1 and IL-6 in the pathogenesis of sJIA had the effect of introducing an extremely effective therapy—especially if administered at the onset of the disease—by targeting these cytokines. However, 20–30% of cases do not respond to initial therapy with anti-IL-1 or anti-IL-6 biological agents, leading to the urgent need to discover new biomarkers for diagnosis, develop a more effective management plan, and accurately predict the course and prognosis of the disease [7,19].
2.1. Genetic Biomarkers
Perception of the genetic hazard posed by sJIA—which, albeit less frequent than the other subtypes of JIA and with an unusual clinical expression at the first visit, can evolve in a severe manner that is sometimes complicated by MAS—has initiated important research to determine the genetic susceptibility to sJIA, as a challenge in a neglected area of investigation, so as to be able to more easily identify genetic biomarkers for early diagnosis, establishing a targeted treatment from the beginning and accurately predicting the long-term response to therapy for these patients.
For 136 sJIA patients at onset, De Benedetti et al. assessed the association of the MIF-173 polymorphism with patients’ long-term response, observed for more than 5 years. The levels of MIF-173 single-nucleotide G-to-C polymorphism of the macrophage migration inhibitory factor (MIF) gene in the serum and synovial fluid of patients with sJIA were studied in correlation with the required amount of glucocorticoids and the outcomes in sJIA patients. Subjects carrying the MIF-173*C allele had significantly higher serum and synovial concentrations of MIF than those with the GG genotype; therefore, the former needed a much longer treatment with glucocorticoids than in MIF-173 GG homozygous patients and had a shorter duration of clinical response to intraarticular injections. Finally, the number of joints with active arthritis, the questionnaires scores, and the number of joints with limited range of motion were significantly higher in patients carrying the MIF-173*C allele, so this could predict poor outcomes of sJIA at onset [20].
Starting from the fact that the polymorphisms in the interferon regulatory factor 5 (IRF5) gene have been linked with susceptibility to autoimmune diseases (ADs), Yanagimachi et al. intended to evaluate the associations of IRF5 gene polymorphisms with the vulnerability to sJIA and MAS. Using TaqMan assays, the authors genotyped three IRF5 single-nucleotide polymorphisms (rs729302, rs2004640, and rs2280714) in 81 patients with sJIA (33 with MAS, 48 without) and 190 controls. A significant association of the rs2004640 T allele with the vulnerability to MAS was highlighted. The IRF5 haplotype (rs729302 A, rs2004640 T, and rs2280714 T), known to be an increased risk factor in systemic lupus erythematosus (SLE), was also significantly associated with susceptibility to MAS in sJIA. IRF5 gene polymorphism influences susceptibility to MAS in sJIA, so IRF5 could trigger MAS in sJIA [21].
At the level of the innate immune system, inflammasomes receive and respond to the signals of the pathological factors, as well to the injuries produced through the activation of caspase-1, the secretion of IL-1β and IL-18, and pyroptosis of macrophages; as a result, Canna et al. found a de novo missense mutation, c.1009A > T, p.Thr337Ser, in the nucleotide-binding domain of the NLRC4 inflammasome that causes early-onset relapsing fever and MAS. Operational analyses shed light on the spontaneous generation of the inflammasome, along with the production of IL-1β- and IL-18-dependent cytokines, revealing a new monoallelic inflammasome defect that broadens the field of monogenic ADs—including MAS—and opens the possibility of new targets in therapy [22].
Ombrello et al. investigated whether genetic variation in the MHC locus on chromosome 6 influenced the susceptibility to the onset of sJIA in a group of 982 children with sJIA and 8010 healthy control subjects from nine countries. The authors used a meta-analysis of directly observed and imputed single-nucleotide polymorphism (SNP) genotypes and imputed classical HLA types, finding that the strongest SNP associated with sJIA was rs151043342 from the HLA class II region. Meta-analysis of the imputed classical HLA-type associations showed that HLA-DRB1*11 (conferring at least a twofold increase in disease risk in each population studied) and its defining amino acid residue—glutamate 58—were strongly associated with sJIA, as were the HLA-DRB1*11 haplotypes, i.e., HLA-DQA1*05–HLA-DQB1*03. This research demonstrated the relationship between the HLA class II region and sJIA, as well as the participation of adaptive immune molecules in the etiopathogenesis of sJIA [23].
In the etiopathogenesis of JIA, the genetic predisposition mainly concerns HLA class II molecules (e.g., HLA-DRB1, HLA-DPB1), although HLA class I molecules and non-HLA genes have also been found to be involved. A meta-analysis of selected papers for the evaluation of the HLA-DRB1 genetic background of patients with JIA compared to healthy controls, conducted by De Silvestri et al., confirmed that HLA-DRB1*04 has a predisposing role in sJIA [24].
Arthur et al., with the largest cohort at the time, investigated whether susceptibility loci previously determined through studies on candidate genes in JIA had a provable association with sJIA. The authors studied SNPs for risk loci in sJIA by association in nine populations, which incorporated 770 patients with sJIA and 6947 controls. The results demonstrated that among the 26 previously reported SNPs there was only one notable association: the promoter region of IL1RN. The IL1RN gene encodes the interleukin-1 receptor antagonist (IL-1Ra). sJIA-associated SNPs are well-correlated with IL1RN expression in lymphoblastoid cell lines (LCLs), with an inverse correlation between sJIA risk and IL1RN expression. The high expression of homozygous IL1RN alleles is strongly correlated with the lack of response to anakinra therapy, paving the way for personalized treatments in sJIA guided by homozygous high-expression alleles with the attributes of ideal biomarkers [25].
Recent advances in “omics” technologies (e.g., genomics, transcriptomics, proteomics, and metabolomics) have made innovative contributions to human genome sequencing, bioinformatics, and analytical tools, significantly facilitating knowledge of the molecular mechanisms of JIA and providing the potential to discover new therapeutic agents. In a review on the multi-omics architecture of JIA, Hou et al. identified several human leukocyte antigen (HLA) alleles (including both HLA class I and class II genes) and 23 non-HLA genetic loci in association with different JIA subtypes. HLA class II alleles are remarkably associated with sJIA. HLA-DRB1*11 has a strong association, while the SNP rs151043342 has the strongest association with sJIA [26].
Zhou et al. attempted to identify key modules and hub genes in the pathophysiology of sJIA, using two datasets on GSE7753 and GSE13501, to accomplish a weighted gene co-expression network analysis (WGCNA). The authors identified a total of 5414 genes for WGCNA, of which the highly correlated ones were divided into 17 modules, where the red module had the highest correlation with sJIA, while the green–yellow one was not related to sJIA. The red module was enriched in the activation of immune responses, infection, nucleosomes, and erythrocyte differentiation, including the ALAS2, AHSP, TRIM10, TRIM58, and KLF1 genes, while the green–yellow module was enriched in inflammation and immune responses, such as the KLRB1, KLRF1, CD160, and KIR genes. These results may deepen the comprehension of the pathophysiology of sJIA, and hub genes may play a role as biomarkers and future treatment targets for sJIA [27].
Due to the fact that sJIA is a severe disease, with an as-yet undetermined etiology and atypical manifestations at the onset, it requires diagnosis and effective treatment from the beginning. In this regard, Zhang et al. aimed to identify diagnostic biomarkers, immune cells, and pathways involved in the pathogenesis of sJIA, as well as possible treatment targets. The authors checked the gene expression profiles of GSE17590, GSE80060, and GSE112057 from the Gene Expression Omnibus (GEO) database to identify differentially expressed genes (DEGs) between sJIA and healthy controls; they analyzed whole-blood samples from 10 subjects, including 5 patients with sJIA (1 very recently diagnosed and 4 already undergoing treatment) and 5 healthy controls perfectly matched for age, race, and sex. The results identified 73 common DEGs, indicating the enrichment of neutrophil and platelet functions as well as the MAPK pathway in the pathogenesis of sJIA. Six hub genes were identified, three of which had high diagnostic sensitivity and specificity; ARG1 and PGLYRP1 were validated by quantitative reverse-transcription polymerase chain reaction (qRT-PCR) and microarray data from the GSE8361 dataset as potential markers for the early diagnosis of JIA, revealing new pathways and molecular targets for sJIA. Further studies with larger sample sizes and in-depth analysis are needed to confirm these results [28].
Ren et al. analyzed 70 active sJIA patients compared with 55 healthy controls to highlight differentially expressed genes (DEGs), hub gene sets, and patterns of immune system cell organization. The results showed 118 DEGs, of which 94 were upregulated (most: CD177, OLFM4, ARHGEF12, MMP8, PLOD2, CEACAM6, and CEACAM8) and 24 were downregulated (most: TCL1A, ALOX15 and HLA-DQB). A hub gene set of eight upregulated genes (ARG1, DEFA4, HP, MMP8, MMP9, MPO, OLFM4, and PGLYRP1) was found, and none were downregulated. Functional enrichment analysis clearly showed that these eight hub genes were mainly connected with the complex tasks of neutrophils, playing a decisive role in the pathogenesis of sJIA, while macrophages, CD8+ T cells, and naïve B cells were relevant drivers of disease progression. TPM2 and GZMB were identified as possible markers of positive short-term response to canakinumab treatment [29].
A summary of the abovementioned published studies on genetic biomarkers is shown in Table 1.

Table 1.
Genetic biomarkers proposed for the diagnosis, activity, and prognosis of sJIA and MAS.
2.2. Specific Cellular Biomarkers in sJIA and MAS
2.2.1. NK Cell Dysfunction in sJIA and MAS
Natural killer cells are large granular lymphocytes with cytotoxic activity that are crucial to the innate immune system, being a member of the rapidly growing innate lymphoid cell (ILC) family, which constitutes 5–20% of all circulating lymphocytes in humans and mediates antiviral and antitumor responses [30].
Innate lymphoid cells (ILCs) are the latest family of innate immune cells to be discovered from common lymphoid progenitors (CLPs). ILCs secrete signaling molecules and regulate both innate and adaptive immune cells. ILCs are mainly tissue-resident cells found in both lymphoid and non-lymphoid tissues, and not often in the blood [31].
ILCs are deeply embedded lymphocytes in tissues that do not express specialized antigen receptors for T and B cells. The study of ILCs has opened new perspectives for understanding immune regulation and maintenance of tissue homeostasis by the immune system. ILCs produce cytokines for various immune pathways expressed in relation to commensal agents and pathogens in mucosal barriers, while also stimulating adaptive immunity and adjusting inflammation in tissues, contributing to metabolic homeostasis, morphogenesis, remodeling, and tissue regeneration in direct association with the nervous system [32].
NK cells originate from hematopoietic stem cells that have recently been classified as ILCs in group 1 (ILC1s), comprising conventional NK (cNK) cells and subsets of “unconventional” NK cells. ILC1s are found in tissues and are also known as tissue-resident NK (trNK) cells, which are found in abundance in organs such as the liver, lungs, and uterus, whereas cNK cells circulate through the blood vessels. Although cNKs and ILC1s share interferon-gamma (IFN-γ) release when activated, these two categories of cells developed from different progenitors and have diverse tissue distributions and specific functions [32,33].
cNK cells have a much higher cytotoxic capacity and higher perforin expression compared to ILC1s, which have a low cytotoxic capacity but can release large amounts of IFN-γ, tumor necrosis factor alpha (TNF-α), and granulocyte–macrophage colony-stimulating factor (GM-CSF) [34,35].
NK cells’ numbers, growth, multiplication, and function are modulated and monitored by two pro-inflammatory cytokines: IL-6 and IL-18 [36,37].
Experimental studies showed that in mice with MAS, IL-6 levels were elevated in plasma and led to decreased perforin levels and granzyme B expression in NK cells, but without degranulation—phenomena that improved after targeted treatment with anti-IL-6. Similar attributes were found in patients with sJIA. Thus, IL-6 increases the inflammatory response and disrupts the function of NK cells, favoring the occurrence of MAS [38,39].
Among the first studies performed to inspect the functions of NK cells and their cytotoxicity in patients with sJIA complicated by MAS, Grom et al. used flow cytometry to investigate the expression of perforin in NK cells (CD56+/TCRαβ−), NK T cells (CD56+/TCRαβ+), and CD8+ cells. NK cells’ cytotoxic activity was quantified as measured after co-incubation with an NK-sensitive K562 cell line. The authors found two important categories of immunological dysfunctions: Some patients had reduced NK cell counts and activity, but slightly increased perforin expression in CD8+ and CD56+ cytotoxic cells. Other patients with sJIA and MAS had reduced NK activities associated with decreased perforin expression in all cytotoxic cells—a pattern similar to the perforin deficiency found in patients with familial hemophagocytic lymphohistiocytosis (fHLH). The authors’ conclusion was that in sJIA-MAS there is an NK cell dysfunction analogous to that in fHLH, which requires further investigation [40].
MAS has been described in association with many rheumatic disorders, but most often with sJIA. Clinical manifestations in MAS are similar to those of hemophagocytic lymphohistiocytosis (HLH)—a genetic disease accompanied by NK cell dysfunction. Villanueva et al. explored global NK cell dysfunction in JIA by studying peripheral blood mononuclear cells (PBMCs) from 40 patients with pauciarticular (n = 4), polyarticular (n = 16), and systemic (n = 20) subtypes of JIA. NK cells’ cytolytic function was determined after co-incubation of PBMCs with the NK-sensitive K562 cell line. NK cells (CD56+/T cell receptor [TCR]-αβ-), NK T cells (CD56+/TCR-αβ+), and CD8+ T cells were studied for perforin and granzyme B expression by flow cytometry. The results showed that NK cells’ cytolytic activity was much lower in sJIA patients than in other JIA groups or controls. The authors concluded that the subgroup of JIA patients who had not yet experienced any MAS event had low NK function and a lack of circulating CD56bright cells, similar to defects found in patients with MAS and HLH. These aspects were particularly prevalent in the sJIA subtype, which is known to be strongly associated with MAS [41].
Due to the connection between IL-18 and the activity of NK cells triggered by this cytokine, de Jager et al. analyzed the relationship between high IL-18 plasma concentrations and the phenotype and function of NK cells in 15 patients with sJIA, the same number of patients with polyarticular JIA, and 10 healthy controls, via in vitro staining and functional assays. Using fluorescence microscopy, they investigated the binding of the IL-18 ligand, and the phosphorylation reactions of several MAP kinases and the IL-18β receptor (IL-18Rβ) were highlighted by the Western blot technique. The results indicated that IL-18 from the plasma of patients diagnosed with sJIA stimulated the activation of NK cells from healthy controls and bound to its cognate receptor. However, after IL-18 excitation, NK cells from sJIA patients failed to upregulate killer molecules such as perforin and IFN-γ, nor did they trigger the phosphorylation of receptor-activated MAP kinases. As a result of an alternative stimulation of NK cells with IL-12, induction of cytotoxic activity was found. However, the authors did not find any adjunctive results of the combination of IL-18 and IL-12 in sJIA. Immunoprecipitation of IL-18Rβ revealed that NK cells from sJIA patients failed to phosphorylate this receptor following IL-18 stimulation, indicating that NK cell dysfunction in sJIA is directly correlated with a defect in IL-18Rβ phosphorylation, which has important implications for the understanding of the pathophysiological mechanisms and future therapy of sJIA [42].
Zhou et al. used flow cytometry to investigate the NK cell count, cytotoxicity, and expression of perforin, granzyme B, interferon (IFN)-γ, and tumor necrosis factor (TNF)-α in peripheral blood samples from 72 children with active JIA (systemic, 25; polyarticular, 24; pauciarticular, 23) and 25 controls. The authors used specimens from 220 children with JIA (systemic, 84; polyarticular, 72; pauciarticular, 64) and 150 controls for KIR2DS2, KIR2DS4, KIR3DS1, KIR2DL1, KIR2DL2, KIR2DL3, and KIR3DL1 typing by polymerase chain reaction with sequence-specific oligonucleotide probes. The results of the research proved that sJIA patients had lower NK cell counts, cytotoxicity, and expression of perforin and granzyme B compared to controls, whereas those with pauci- and polyarticular JIA subtypes expressed higher levels of perforin and granzyme B. In patients with sJIA, NK cells generated lower amounts of TNF-α and higher amounts of IFN-γ than in the pauci- and polyarticular JIA groups. No significant disparities were found in KIR gene frequencies between JIA subgroups and healthy controls, except for KIR2DS4, which was lower in the sJIA group. Finally, the authors concluded that in sJIA there was a decline in the activity of NK cells, which secreted more IFN-γ and less TNF-α, and the incidence of KIR2DS4 was reduced [43].
Put et al. investigated NK-cell-specific transcriptional changes via RNA sequencing of highly purified NK cells from six patients with active sJIA and six healthy controls. The stimulatory cytokines—mainly for NK cells—were analyzed from plasma (n = 18), evaluating the phenotype and cytotoxic activity of NK cells on tumor cells (n = 10), as well as the production of IFN-γ (n = 8). For patients with sJIA, an altered gene expression profile of NK cells was found compared to healthy controls, with increased immunoinflammatory pathways, innate gene expression (TLR4 and S100A9), and decreased expression of immunoregulatory genes (i.e., interleukin 10 receptor, alpha subunit (IL10RA), and granzyme K (GZMK)). In patients with sJIA, increased IL-18 levels and a decreased IFN-γ/IL-18 ratio were found. NK cells had an imbalance between inhibitory and activating receptors, with reduced G1 receptor expression and increased expression of natural cytotoxicity triggering receptor 2 (NKp44). A reduction in granzyme K expression was observed in CD56bright NK cells, along with defective IL-18-induced IFN-γ secretion and signaling. Hard-to-detect defects in the immune pathways governed by NK—such as the expression of granzyme K and the production of IFN-γ stimulated by IL-18—induce the immunoinflammatory dysfunctions that characterize sJIA [44].
The pathophysiology of sJIA and MAS still has many unclear elements, although it is known that there are high levels of IL-18 and reduced activity of NK cells in patients with active disease. Ohya et al. explored the phosphorylation of mitogen-activated protein kinase (MAPK) p38 and the enhancer of nuclear factor κ light chain of activated B cells (NF-κB) p65 in NK cells after being boosted by in vitro recombinant IL-18 (rIL-18) in 31 patients with sJIA and 6 healthy controls. The authors investigated the connections between clinical symptoms, serum IL-18 levels, and the strength of phosphorylation in NK cells. Patients were distributed in conformity with their disease activity into four groups: systemic characteristics (n = 8), chronic arthritis (n = 7), remission on medication (n = 10), and remission off medication (n = 6). The results showed that the phosphorylation of MAPK p38 and NF-κB p65 was more intense in the group of healthy patients than in those in remission without therapy, in remission on drugs, or with chronic arthritis, while there was a complete defect in phosphorylation in those with systemic manifestations. The serum concentration of IL-18 was highest in the group with systemic manifestations, followed by those with chronic arthritis, those in remission on medication, those in remission without medication and, finally, the healthy group. The authors concluded that the increased levels of IL-18 induce the phosphorylation defects of MAPK and NF-κB in NK cells, and that the inappropriate signaling of IL-18 in NK cells is directly related to the activity of sJIA [45].
2.2.2. Macrophages in sJIA and MAS
Macrophages are known as cells equipped for the detection, phagocytosis, and destruction of bacterial agents, as well as other microorganisms that are harmful to the body. They are antigen-presenting cells to T lymphocytes and produce molecules (cytokines) that develop the inflammatory process by activating other cells. Two types of activated macrophages are known: classically activated macrophages, i.e., macrophages that encourage inflammation (M1); and macrophages alternately activated by phages, i.e., macrophages that decrease inflammation and encourage tissue repair (M2). The activated or polarized type (M1) secrete interleukins with pro-inflammatory action (e.g., TNF-α, IL-1 beta, IL-6, IL-12), chemokine (C-X-C motif) ligands 9 and 10 (CXCL9, CXCL10, etc.), and low amounts of IL-10, which promotes and modulates the immune response of Th1 helper cells (CD4+ cells or CD4-positive cells), i.e., Th1 lymphocytes [46].
Feng et al. investigated the roles played by different macrophage subtypes in the evolutionary stages of sJIA. The study included 22 children diagnosed with sJIA, divided into two groups: 12 patients with active disease, recently diagnosed and still without treatment, as well as 10 with inactive disease, who were monitored for two years. The control was a group of 10 children with orthostatic proteinuria. For all subjects, peripheral blood was analyzed by flow cytometry to highlight the following macrophage subtypes: M1 (CD14+CD86+CD80+), M2a (CD14+CD206+CD301+), M2b (CD14+CD206+CD86+), and M2c (CD14+CD206+CD163+), as well as IL-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-17, INF-α, INF-γ, and TNF-α. The authors found the M1 phenotype marker CD80, M2 phenotype marker CD163, and CD301 to be strongly expressed in children with active sJIA—a group in which most macrophages were M1 and M2a. In contrast, in the group with inactive disease, M2 was polarized predominantly into M2b and M2c. IL-6 had high levels in the group with active disease, while in the other group IL-4, IL-10, and IL-17 had high values. The authors concluded that M1 induces inflammation in active sJIA, while M2a almost simultaneously triggers inflammation inhibition, whereas M2b and M2c play major roles in inhibiting inflammation in inactive disease [47].
Macrophage polarization is a biological phenomenon through which these cells are able to follow certain functional programs as they are guided by signals from the microenvironment. Polarization can be caused by chemical stimulation or by stiffness substrates on which the macrophage is grown [48].
M2 macrophages can be polarized by the action of the cytokines IL-10, IL-13, and IL-4 which, thus activated, can inhibit inflammation and promote tissue repair, angiogenesis, and fibrosis. Recent studies have shown that M2 macrophages can be divided into different subtypes, such as M2a, M2b, M2c, and M2d, which have activities specific to the stage of the disease. M2a macrophages are polarized by IL-4 and IL-13, lowering the levels of IL-10 and transforming growth factor beta (TGF-β), participating in tissue regeneration and incorporation of pro-inflammatory molecules, thereby preventing the inflammatory response. M2b macrophages secrete IL-1, IL-6, IL-10, and TNF-α under the influence of immune complexes or lipopolysaccharides (LPSs), resulting in the activation of Th2 cells and the reducing inflammation. M2c macrophages are activated by IL-10, TGF-β, and glucocorticoids; they secrete IL-10 and TGF-β, which suppress the inflammatory response. The M2a subtype, in tandem with Th2 cells, participates in allergic reactions, while M2b is involved in immune regulation and M2c can secrete anti-inflammatory cytokines with a role in reducing inflammation, reshaping the matrix and tissues. M2d macrophages are polarized by IL-6 and adenosine, and are known as tumor-associated macrophages (TAMs). In sJIA, the cluster of differentiation 163 (CD163), arginase-1 (Arg-1), and chemokine (C-C motif) ligand 2 (CCL2) or monocyte chemoattractant protein 1 (MCP1) genes are closely related to M2 macrophages and are expressed on the surface of monocytes [49,50,51].
CD163 is a potent receptor specific to monocytes and macrophages for haptoglobin–hemoglobin complexes. CD163 is strongly induced by the anti-inflammatory cytokine IL-10, as well as by glucocorticoids and IL-6. CD163 easily induces TNF-like weak inducer of apoptosis (TWEAK) and is a receptor for a variety of bacteria and viruses. CD163 is widely used as a marker for alternately activated macrophages. CD163 +-activated macrophages have an immunomodulatory profile and are valued as anti-inflammatory markers [52].
CD163 helps regulate and extinguish inflammation, eliminates free hemoglobin, and is strongly expressed in myeloid cells in patients with sJIA and MAS [53].
2.2.3. Neutrophils and Neutrophil Extracellular Traps
Neutrophils are cells that are part of the innate immune system, with an important role in the phagocytosis of pathogens; they originate from hematopoietic stem cells, have a short lifespan, and represent more than 50% of circulating white blood cells. The nuclear morphology of these cells gives them a phenotypic and functional heterogeneity. In addition to their ability to migrate, as well as their phagocytic and immunomodulatory functions, they can form neutrophil extracellular traps (NETs) in the form of a network of fibers consisting of cytoskeletal proteins, proteases, antimicrobial peptides, histones, and chromatin deoxyribonucleic acid [54,55,56].
It has been observed that in the early stages of sJIA’s onset, the innate immune cells with a defensive role—such as neutrophils and macrophages—are much more abundant in peripheral blood and play an essential role in the development of systemic inflammation. These cells produce several mediators involved in the pathogenesis of sJIA, of which IL-1 appears to play a pivotal role [57,58].
Ter Haar et al. investigated the role of neutrophils in 50 patients with sJIA receiving therapy with a recombinant IL-1 receptor antagonist (rIL-1Ra; anakinra). At the onset of the disease, the number of neutrophils increased significantly and was correlated directly with the pro-inflammatory factors, and after a few days of treatment with anakinra the neutrophil count returned to normal. RNA sequencing research has revealed an important adjustment of the inflammatory processes triggered by neutrophils in active disease patients, identical to the blood transcriptome analysis of patients with sepsis. Neutrophils from patients with active sJIA had a charged phenotype defined by increased reactive oxygen species (ROS) production, decreased L-selectin (CD62L) levels, and degranulation of secretory vesicles, which was completely changed after anakinra therapy in clinical remission patients. Those with a short period from the onset of sJIA had many neutrophils—especially immature ones—and a complete resolution of the disease under anti-rIL-1Ra, in contrast to those with a disease duration of more than one month, who had a normal number of neutrophils, but with an unsatisfactory response to anakinra treatment. These findings firmly suggest that neutrophils play an important role in sJIA’s onset and in the early inflammation stage, and that their counts and pro-inflammatory activity are influenced by IL-1 blockade [59].
To examine activated neutrophil subsets, the release of S100 alarmin, and gene expression signatures in patients with different spectra of sJIA activity, Brown et al. analyzed samples from 23 active sJIA patients and 22 children with clinically inactive disease (CID). Control samples were obtained from children undergoing evaluation for joint pain but found to have non-inflammatory disorders. The results indicated a higher percentage of CD16+CD62Llo neutrophils compared to controls. This subset of neutrophils was not observed in patients with CID or in those with active arthritis but without systemic features. CD16+CD62Llo neutrophils from sJIA-MAS patients showed increased nuclear hypersegmentation compared with CD16+CD62L+ neutrophils. Serum concentrations of S100A8/A9 and S100A12 were correlated very strongly with peripheral blood neutrophil counts. Whole-transcriptome analysis of highly purified neutrophils from children with active sJIA identified 214 differentially expressed genes (DEGs) compared with controls. In all samples, regardless of disease activity, increased inflammatory gene expression was found, including inflammasome components and S100A8. The authors’ conclusion was that in the case of activation of neutrophils in both active and inactive sJIA, a pro-inflammatory gene expression signature can be identified, indicating prolonged innate immune activation [60].
Known to be key phagocytic cells belonging to the innate immune system, neutrophils are endowed with the potential for oxidative and non-oxidative defense against pathogens. Their complex decision-shaping function, by which they instruct other leukocytes to adjust innate and adaptive immune responses, has recently been discovered. Under physiological conditions, they have a short life and are permanently set free by the bone marrow, starting from undifferentiated stem cells and going through multiple stages until maturation, in order to finally develop accelerated and intense immune responses. The human body needs many such cells during systemic inflammation in order to generate emergency granulopoiesis, but in excess they can cause tissue damage, as seen in sJIA. It is fundamental to better understand the activity and the types of neutrophils in the joints and blood of patients with sJIA. NET activation and release (NETosis) is a unique form of cell death in which neutrophils remove decondensed deoxyribonucleic acid (DNA), histones, cytokines, and granular proteins, and has never been studied directly in patients with sJIA. However, high serum histone levels in active sJIA, compared to inactive or healthy control patients, suggest increased NETosis, being well-correlated with sJIA disease activity. Increased high-mobility group box-1 (HMGB1) levels in patients with sJIA are associated with intensified NETosis, forming a positive feedback loop. Serum HMGB1 concentrations of NET molecules (including DNA without cells, myeloperoxidase (MPO)–DNA complexes, and α-defensin) were found to be increased in patients with adult-onset Still’s disease (AOSD) and sJIA, compared to healthy controls. NET molecules induce the release of HMGB1 and the production of IL-1β by monocytes, and as ligands of TLR4 they could be involved in activating the TLR signaling pathway in systemic arthritis [61,62,63].
In a very recent review, Kim et al. described the roles of neutrophils and the formation of NETs, which participate in the aggravation of inflammation in the pathogenesis of sJIA and AOSD. Activation of neutrophils and their surface receptors (including Fc receptors) by pathogen-associated molecular pattern (PAMP) or damage-associated molecular pattern (DAMP) molecules leads to their displacement in inflamed tissues and, subsequently, to the release of cytokines and chemokines with pro-inflammatory potential. Activated neutrophils and expelled mediators form a positive feedback loop that increases the recruitment of new neutrophils and exacerbates the inflammatory phenomena, on which the pathogenic mechanisms of AOSD and sJIA can be focused. Simultaneously, the activated neutrophils produce and release NETs and participate in the activation of macrophages in the inflamed area. At the same time, the levels of other DAMPs (e.g., HMGB-1, S100 proteins, LL-37, a human antimicrobial peptide from the cathelicidin group, and myeloperoxidase–DNA (MPO–DNA) complexes) increase in patients’ blood, accelerating systemic inflammation even more intensively. These novel results deepen the comprehension of the pathophysiological mechanisms involved in sJIA and AOSD, and can accelerate the patenting of neutrophil-associated biomarkers to confirm the diagnosis and likely course, as well as the development of original drugs to address the neutrophils and NETs [64].
2.2.4. Platelets
In 2016, a group of experts analyzed 115 sJIA-MAS patient profiles, starting from laboratory data before the onset of MAS, followed by those corresponding to the onset of MAS, with a particular focus on the changes between the two key moments. They selected and ordered the most important laboratory tests with the most dramatic changes for the timely diagnosis of sJIA-MAS, the relevance of which was debated and cataloged at an expert consensus conference. At the end of the analysis, platelet count, ferritin level, and aspartate aminotransferase (AST), followed by white blood cell count, neutrophil count, fibrinogen (Fg), and erythrocyte sedimentation rate (ESR), were declared to be the most valuable laboratory parameters with the most important changes at the onset of sJIA-MAS [65].
In a recent review, Crayne et al. noted that laboratory features of MAS include pancytopenia, hyperferritinemia, fibrinolytic coagulopathy, and liver dysfunction [66].
At the 2020 American College of Rheumatology Congress, De Matteis et al. highlighted platelet count and ferritin as two significant parameters with high specificity and sensibility, respectively, to diagnose MAS-sJIA. The authors stipulated that these biomarkers could be practical prognosticators of the clinical outcomes and the effectiveness of the administered therapy [67].
The important roles of platelets and the MAPK pathway in the pathogenesis of sJIA provide a new perspective for exploring potential molecular targets for the treatment of sJIA, as Zhang et al. revealed by integrated bioinformatics analysis [28].
2.2.5. Complete Blood Cell Count
Currently, there is a consensus that sJIA and AOSD are similar diseases but emerge at different ages [68].
Children with sJIA are particularly susceptible to developing MAS. In a multinational, multicenter study that included 95 pediatric rheumatologists and hemato-oncologists from 33 countries, data collected from 362 patients were retrospectively investigated and included 22% with MAS at the onset of sJIA. Clinical manifestations in order of frequency were as follows: fever (96%), hepatomegaly (70%), splenomegaly (58%), central nervous system dysfunction (35%), and hemorrhages (20%). In terms of laboratory data, platelet counts and levels of liver transaminases, ferritin, LDH, triglycerides, and D-dimers were the only laboratory biomarkers showing a change of more than 50% between the pre-MAS consultation and the onset of MAS. Macrophagic hemophagocytosis was detected by bone marrow aspiration in 60% of patients. Approximately one-third of patients required admission to the intensive care unit (ICU), and the mortality rate was 8%. MAS remains a grave complication, as an important percentage of children require mandatory admission to an ICU or will die [69].
A group of experts statistically analyzed 982 potential criteria for 428 profiles of patients with MAS-sJIA and obtained the best results for 37 of them, analyzed in comparison with eight criteria from the literature. With a consensus of 82%, they obtained the final MAS classification criteria, validated with a sensitivity of 0.73 and a specificity of 0.99. These points of reference could contribute to a better insight into MAS in sJIA, to the discovery of new active and safe therapies, and to a better selection of patients for future clinical studies [15].
The fact that systemic inflammation in sJIA includes elevated ESR, C-reactive protein (CRP), white blood cell count, platelet count, ferritin, transaminases, aldolases, and D-dimers may help define disease activity; according to Lee et al., primary care must closely monitor patients with sJIA to detect early complications and unexpected drug reactions [7].
Some of the studies on cellular biomarkers discussed above are presented in Table 2.

Table 2.
Cellular biomarkers proposed for the diagnosis, activity, and prognosis of sJIA and MAS.
2.3. Cytokines, Chemokines, and Other Biomarkers in sJIA and MAS
2.3.1. IL-1
The role of innate immune system involvement and the aberrant responses in the pathophysiology of sJIA have been documented in numerous clinical studies and publications [70].
The role of IL-1 in the pathogenesis of sJIA was first discovered by Virginia Pascual in 2005 [71]; since then, evidence has shown many other aspects of the involvement of this cytokine. Additional evidence includes clinical observations after the administration of biological agents blocking the IL-1 pathway via rIL-1Ra (anakinra) or prolonged blocking of IL-1 by the biological agent canakinumab [71,72,73,74,75].
Ling et al. highlighted that the flare versus quiescent signature in sJIA attests to the key role of IL-1 in the onset of the disease [76].
Several genetic alterations or mutations associated with dysregulated IL-1 activity and autoinflammatory disorders have been identified, and these findings have led to the successful use of IL-1 inhibitors in rheumatic diseases that were previously considered to be untreatable [77].
In a recent review on the role of IL-1 in general pathology, Kaneko et al. identified IL-1 as a key cytokine not only for cell-damage-related inflammation, but also for cell, tissue, and organ homeostasis in terms of overall pathology; thus, IL-1 has become an important molecular target for treating a wide range of diseases, such as autoinflammatory, autoimmune, and infectious diseases, malignant tumors, etc. [78].
Particular attention should be devoted to patients with recent-onset sJIA or pediatric Still’s disease—a syndrome with several clinical forms that may benefit from a treat-to-target strategy with a key role of IL-1 inhibition. Due to the heterogeneous and often difficult-to-treat nature of sJIA, the experience of expert teams is recommended [79].
At the onset of sJIA, it has been demonstrated that an early and correct treatment in the so-called “window of opportunity” by administering the use of IL-1 inhibitors—especially in steroid-naïve patients—can enable the interruption of the pathogenic cycle of the disease and induce a prompt and full remission. Ter Haar et al. conducted a prospective study of 42 patients diagnosed with sJIA at onset, but without an adequate response to non-steroidal anti-inflammatory drugs (NSAIDs), compared with 12 patients who did not have arthritis at onset, and both groups were followed for a mean period of 5.8 years. The first group received rIL-1Ra as monotherapy, as a targeted treatment. The results showed that the disease generally became inactive after 33 days, and after one year 76% of the patients had inactive disease, 52% of whom had not received any drug(s). It was observed that there was a correlation between the increased number of neutrophils at the beginning, the positive response after one month of treatment with rIL-1Ra, and the disappearance of symptoms one year after the initiation of the study. The monitoring of patients over a period of 5 years showed that 96% no longer had active disease, and 75% achieved inactive disease status without any medication. Articular and extra-articular damages were found in a small percentage (less than 5%) of cases, and only one-third of the patients required the administration of glucocorticoids. The authors also noted the effectiveness of therapy with rIL-1Ra in children with sJIA without arthritis at the onset. The use of rIL-1Ra as a first-line, short-term monotherapy may be a valuable option to interrupt the pathogenic cycle of sJIA at onset, in which IL-1 is the central component to be targeted [9].
Saccomanno et al. retrospectively analyzed the response to treatment with an IL-1 inhibitor (anakinra) for 62 patients diagnosed with sJIA over a 14-year period, including demographic, clinical, and laboratory data, as well as previous or concomitant therapies administered. The results of anakinra management were estimated by univariate and multivariable statistical analyses after one year of treatment, outlining the clinical patterns of patients with sJIA for whom IL-1 blockade may be a successful treatment. The authors recommend future in-depth studies on the efficacy of early therapy with IL-1 inhibitors, but also the detection of biomarkers that accurately predict the response to IL-1 or IL-6 antagonists [80].
Lainka et al. undertook a retrospective study on 202 patients diagnosed with sJIA and included in the German AID registry from 17 centers, of which 111 were children treated with IL-1 inhibitors, i.e., anakinra (ANA) (n = 84, 39 f) and/or canakinumab (CANA) (n = 27, 15 f). In the first year of therapy, 75/111 (ANA 55, CANA 20) patients were evaluated according to the Wallace criteria, and disease inactivation was achieved in 28/55 and 17/20, respectively, while remission over 6 months under medication was achieved in 13/55 and 7/20 patients, respectively. The clinical response to biological medication was maintained in most patients (ANA 54/80, CANA 20/27). The study acknowledged positive clinical results with both IL-1 inhibitors (anakinra and canakinumab), which were well-tolerated by patients and showed reasonable safety and efficiency when tested in a true clinical framework [81].
2.3.2. IL-1β
Pro-inflammatory cytokines in the innate immune system—such as IL-1, IL-6, IL-18, and TNF-α—are directly involved in the inflammatory process of sJIA. For example, IL-1β—a pleiotropic pro-inflammatory cytokine—is excessively released by mononuclear cells in the blood and adjusts not only its own transcription, but also that of IL-6. The fundamental role of IL-1β in the pathogenesis of sJIA and the perpetuation of chronic inflammation has been demonstrated by stopping its activity after the administration of biological agents [82,83,84,85,86].
In recent decades, special efforts have been made to establish complex consensus-based plans for the early diagnosis and management of sJIA as an autoinflammatory disease. These strategies also involve the implementation of expensive treatment methods that directly target the main initiating and supporting cytokines (i.e., IL-1 and IL-6) of the chronic inflammatory process [81,87].
It has already been shown that if the initial autoinflammation with the disturbance of innate immunity is not stopped, the phenotype changes with the disruption of the elements of adaptive immunity will have evolutionary consequences in the form of destructive arthritis. If biological treatment is applied early—that is, when the “window of opportunity” is open—and there is financial possibility of long-term continuation, patients with sJIA will be more likely to be diagnosed with inactive disease and even go into remission.
It is believed that the mutation in the nucleotide-binding oligomerization domain-like receptor pyrin domain-containing protein 3 (NLRP3) inflammasome leads to overexpression of the cytokine IL-1β. The NLRP3 gene codifies the multimeric protein complex cryopyrin, which monitors the activation of caspase-1 which, in turn, catalyzes the cleavage of pro-IL-1β into IL-1β [88].
Inflammasomes are cytosolic multiprotein oligomers responsible for the activation of inflammatory responses as important parts of the innate immune system. NLRP3 inflammasome activation during infections requires two signals: one priming signal, and one activation signal. Once assembled, the NLRP3 inflammasome activation leads to the auto-cleavage of pro-caspase-1, which mediates the proteolytic process of pro-IL-1β, pro-IL-18, and the propyroptotic factor gasdermin D [89,90].
Studies on the pathogenesis of sJIA have shown the biphasic nature of events—at the beginning of systemic manifestations, the elements of innate immunity participate with the key role of the cytokine IL-1β; and in the second phase, chronic arthritis interacts with the adaptive immunity through the cytokine IL-17A. On this basis, IL-1 blocking treatment was initiated in the early stages of sJIA [91].
Brunner et al. investigated the long-term benefits and safety of canakinumab and the predictability of responses in 123 patients with sJIA with or without fever (70 with fever, and 52 without fever) at the start of therapy. Only 84 subjects (68.3%) remained in the study to completion—a mean period of 1.8 years. Clinical–biological sJIA-ACR 50 (American College of Rheumatology score = at least 50% improvement in disease activity) responses occurring by day 15 were the most representative predictor of clinical remission, according to the JADAS (Juvenile Arthritis Disease Activity Score), as well as of steroid discontinuation. The biological agent, as a monoclonal antibody that inhibits IL-1β and can decrease the levels of IL-6 and the hepatic synthesis of CRP and Fg, rapidly and persistently improved the clinical condition of patients with active sJIA, regardless of whether or not they had fever at the initiation of therapy [92].
MAS, as a potentially fatal complication of sJIA, may not respond to conventional doses of biological cytokine inhibitors requiring dose escalation. It has been shown that when MAS-sJIA is triggered, it can be treated with anakinra—i.e., with IL-1Ra—and increasing the dose can be beneficial for the patient. Another IL-1 inhibitor, canakinumab—which is a monoclonal antibody of IL-1β—has been reported to effectively treat MAS-sJIA that is refractory to conventional treatment. Kostik et al. retrospectively analyzed the data from the electronic medical records of an academic center with respect to the use of canakinumab in eight children (five girls) diagnosed with MAS-sJIA, with an average age of 8.5 years, during the period 2011–2020. Among these patients, five children developed MAS at the onset of sJIA, while three developed it during the treatment with canakinumab. MAS remitted in all children included in the research, but when the dose of canakinumab was not sufficient, or when MAS developed during the canakinumab therapy, the dose of the administered drug was increased in the short term (2–3 times the normal dose), without observed side effects, attesting to the efficacy and safety of increased doses of canakinumab in MAS-sJIA, but posing the need for additional studies [93].
2.3.3. IL-6
IL-6 plays a special role in autoinflammatory diseases, and especially in sJIA, where it contributes to fever; increased acute-phase reactants (e.g., ESR, CRP, alpha-1 and alpha-2 globulins, Fg, haptoglobin, etc.), serum amyloid A (SAA), white blood cells, and platelets; and decreased red blood cells with chronic anemia. Elevated serum IL-6 concentrations have been found in several inflammatory diseases, such as AOSD and sJIA. IL-6 acts on B and T lymphocytes, hematopoietic progenitor cells, megakaryocytes, macrophages, hepatocytes, and neuronal cells [94].
In early research on the involvement of IL-6 in the pathophysiological mechanisms of sJIA, de Benedetti et al. evaluated serum IL-6 levels in 25 patients with systemic-onset juvenile arthritis using the B9 hybridoma cell line, finding significantly increased concentrations during active disease—correlated with the extent and severity of joint involvement and platelet counts—but not during remission. This study highlighted the important role of IL-6 in the pathogenesis of sJIA [95].
IL-6 levels are elevated in both serum and synovial fluid in patients with sJIA, directly related to fever, growth deficits, anemic syndrome, and thrombocytosis [96,97].
In a study on early changes in inflammatory gene and protein expression in sJIA managed with anti-IL-1β human monoclonal antibody therapy, Brachat et al. measured gene expression in febrile sJIA patients and matched healthy controls. Transcriptional response was assessed by changes in gene expression from baseline to day 3 using the adapted sJIA-ACR 50 response. The pro-inflammatory cytokines IL-6 and IL-18 were measured up to day 197. The strongest clinical response was seen in patients with higher baseline expression of dysregulated genes and a strong transcriptional response on day 3. IL-6 decreased on day 3 (≥8-fold decrease) and remained suppressed. IL-18 decreased at day 57 (≥1.5-fold decrease). The authors identified an important response signature to canakinumab for the majority of sJIA patients, with the transcript levels for sJIA overexpressed genes quickly returning to normal—including those associated with the IL-1 and IL-6 signaling pathways—as well as a marked decrease in transcription of the gene encoding the target, i.e., IL-1β, meaning that this treatment interrupts a positive feedback loop between IL-1β signaling and IL-1β production. Canakinumab treatment in sJIA led to downregulation of innate immune response genes and downregulation of IL-6, but also decreased the clinical symptoms, paving the way for further research [98].
IL-6 is a cytokine with a complex role in local and systemic inflammation in the pathogenesis of sJIA, as well as in complications of the disease. As it is not known exactly how it works, the choice of IL-6 inhibitors should be made in the context of genetic and biological factors, as well as the type and phase of the disease [99].
Tocilizumab (TCZ), a humanized monoclonal antibody against the human IL-6 receptor, has radically altered the course of patients with sJIA and MAS. However, MAS may occur in patients with sJIA under TCZ therapy. Thus, it could be concluded that blocking IL-6 with TCZ is not sufficient to inhibit the pathogenic mechanism of sJIA and MAS [100,101].
Bielak et al. reported the results of treatment with biological agents for 200 patients with sJIA from the German Registry of Autoinflammatory Diseases (AID), of whom 46 received TCZ therapy as an IL-6 blocker, causing the disease to become inactive and/or enter remission after one year from the initiation of the treatment [102].
In a study on the action of TCZ in sJIA-MAS, Shimizu et al. combined expert consensus with an analysis of clinical and laboratory data for 12 patients treated with TCZ, compared with 18 sJIA-MAS patients without treatment. Patients who received TCZ were less febrile and had significantly lower levels of ferritin, triglycerides, and CRP than the control group. TCZ-treated patients with MAS associated with sJIA were less likely to be classified as having MAS based on the MAS classification criteria, due to the absence of fever and lower ferritin levels compared to untreated patients, leading to the conclusion that TCZ could alter the clinical and laboratory features of MAS associated with sJIA. Thus, when patients with sJIA are evaluated during TCZ treatment, the criteria for MAS cannot be applied, and great care must be taken not to fall into the trap of underdiagnosing MAS [103].
Aiming to elucidate the immune profile of sJIA to identify clinical biomarkers correlated with new immune mechanisms, Qu et al. analyzed plasma samples from 21 patients diagnosed with sJIA, compared with 60 age- and sex-matched healthy controls, via a highly sensitive proteomic immunoassay and analysis of canonical pathways associated with cellular functions. The authors investigated well-known sJIA biomarkers such as IL-6, IL-18, and S100A12, which have been shown to be elevated during active sJIA compared to healthy controls. IL-18 was the only one of these biomarkers with elevated levels in inactive sJIA compared to healthy controls. Other novel factors—such as CASP8, CCL23, CD6, CXCL1, CXCL11, CXCL5, EIF4EBP1, KITLG, MMP1, OSM, SIRT2, SULT1A1, and TNFSF11—were found to be differentially expressed in active and/or inactive sJIA compared to the control group. HMGB1 levels were found to be higher in active than in inactive sJIA. The authors’ results contribute to a deeper understanding of the immune mechanisms in active sJIA, for new diagnostic and therapeutic plans of action [104].
2.3.4. IL-10
Interleukin 10 is a pleiotropic cytokine with various effects in immunoregulation and inflammation, secreted by almost all cells participating in immune defense, such as macrophages, mast cells, neutrophils, basophils, eosinophils, dendritic cells, B cells, and the various subtypes of T cells [105].
The main functions of IL-10 are performed through autocrine and paracrine mechanisms, which primarily give it an anti-inflammatory, inhibitory, or autoregulatory role through a negative feedback on inflammatory processes. IL-10 suppresses harmful inflammatory responses by preventing antigen presentation by dendritic cells and inhibiting the stimulation and penetration of macrophages in the lesion area, with the subsidiary consequence of reducing the expression of pro-inflammatory cytokines. Expanding the research areas for new cell-based therapies that benefit from the knowledge of IL-10 signaling pathways could bring important benefits to patients with chronic inflammatory phenomena and fibrotic consequences [106].
In a translational study using a comparative murine model of sJIA and samples from patients diagnosed with sJIA, Imbrechts et al. investigated whether the pathophysiological mechanisms of sJIA could be caused by defects in IL-10—a cytokine known to be crucial in controlling inflammation. The authors used a translational approach with an sJIA-like murine model, compared with samples from patients with sJIA. Freund’s complete adjuvant (CFA) containing heat-killed mycobacteria (1.5 mg/mL) was injected into IFN-γ-deficient BALB/c mice, as the corresponding wild-type (WT) mice are known to develop only a mild and transient inflammatory reaction. Diseased IFN-γ-deficient mice showed defective IL-10 production in CD4+ regulatory T cells, CD19+ B cells, and CD3-CD122+CD49b+NK cells, with B cells as the main IL-10 generators. Neutralization of IL-10 in WT mice generated a chronic immune inflammatory condition with an identical clinical and hematological picture to sJIA. The authors found plasma levels of IL-10 to be remarkably low compared to pro-inflammatory mediators in patients with sJIA, and CD19+ B cells from these patients showed decreased production of IL-10 both ex vivo and after stimulation in vitro. The authors concluded that neutralization of IL-10 in WT mice by CFA transformed a transient inflammatory reaction into a chronic disease and found cell-specific IL-10 imperfections in both mice and patients with sJIA, along with insufficient production of IL-10 to counterbalance the pro-inflammatory cytokines, indicating that the faulty production of IL-10 could contribute to the pathogenesis of sJIA [107].
Peng et al. investigated IL-10 concentrations in 21 patients with sJIA, compared with 35 patients with febrile illnesses suspected to be sJIA. The results showed that subjects with confirmed sJIA had significantly higher levels of IL-10 compared to those with other febrile illnesses; at the same time, serum concentrations of IL-10 were much higher in patients with active sJIA compared to those with inactive sJIA, with a direct relationship with other activity markers such as ESR, CRP, serum ferritin, and IL-6. Concomitantly, serum IL-10 levels at the time of diagnosis were observed to be much higher in sJIA patients with a non-monocyclic course than in those with a monocyclic pattern. IL-10 concentrations were more valuable in distinguishing monocyclic from non-monocyclic patterns in sJIA outcomes, in comparison with CRP, ESR, serum ferritin, and IL-6. The study showed that IL-10 levels were much higher in sJIA, leading to differentiation from other febrile diseases and a correct diagnosis, and were associated with the type of activity and disease progression. The authors proposed that serum IL-10 should be considered a reliable clinical marker in the diagnosis and prognosis of sJIA [108].
2.3.5. IL-18
IL-18, which belongs to the IL-1 cytokine family, is initially inactive and is presented as an integral membrane protein, and then under the action of caspase-1 it transforms into a form of active cytokine to induce IFN-γ. IL-18 can be found as a constitutive protein in almost all human cells and in healthy animals; it is buffered by its natural inhibitor—an IL-18-binding protein called IL-18BP, which has a high affinity and regulates IL-18 activity [109].
Lotito et al., in a group of 50 patients with JIA (13 systemic, 13 polyarticular, 24 oligoarticular) and 25 controls, were among the first to investigate the levels of IL-18 in synovial fluid (SF) and serum, in correlation with the activity and severity of the disease. The concentrations of the studied cytokines (i.e., IL-1, IL-1Ra, IL-6, and IL-18) were higher in the sera of patients with JIA than in controls. IL-18 levels were as high in serum as in SF and were positively correlated with IL-1, IL-1Ra, IL-6, and other disease activity parameters (i.e., CRP, number of active joints, and radiological score). However, the levels of IL-18 and IL-6 in SF and serum were much higher in patients with sJIA than in those with other types of the disease. The authors concluded that IL-18 is involved in the pathophysiology of JIA, reflects the severity of the disease, and could be a target for the treatment of arthritis [110].
Shimizu et al. studied serum levels of the cytokines IL-18, IL-6, neopterin, and TNF-α receptor types I and II, along with ferritin concentrations, in 5 subjects with sJIA complicated by MAS (sJIA-MAS), 10 patients with Epstein–Barr-virus-induced hemophagocytic lymphohistiocytosis (EBV-HLH), 22 patients with KD, and 28 healthy controls. Biological data were compared with clinical symptoms of sJIA-MAS. Serum samples revealed significantly higher levels of IL-18 in patients with sJIA-MAS compared to patients with EBV-HLH or KD. In contrast, IL-6 levels in KD subjects were significantly higher than those in EBV-HLH or sJIA-MAS subjects, but serum neopterin levels in EBV-HLH patients were higher than those in sJIA-MAS or KD patients. A positive correlation was observed between serum levels of IL-18 and parameters of disease activity such as CRP, ferritin, and LDH. Serum IL-18 also remained elevated in patients with sJIA in the inactive stage of the disease, while clinical parameters and other cytokines normalized. The serum level of IL-18 can be considered to be a biomarker of sJIA activity, and monitoring its profile could be beneficial for distinguishing MAS/HLH and estimating sJIA disease activity [111].
In another study, Shimizu et al. measured serum IL-6/IL-18 levels in 76 patients with sJIA, including 15 with MAS, and compared them with clinical symptoms. Depending on the IL-6/IL-18 serum concentrations, two distinct subsets were detected: The IL-6-dominant subset was more likely to develop much more severe joint disease. The IL-18-dominant subset included a larger number of patients who developed MAS and in whom IL-18 concentrations during the active phase were significantly higher, compared to those without MAS. The minimum serum level found for IL-18 was 47,750 pg/mL, which predicted the development of MAS. IL-18 could be involved in the pathophysiology of MAS, and serum concentrations > 47,750 pg/mL could be useful in predicting the initiation of MAS [112].
Xia et al. investigated whether IL-18 could be a valuable biomarker in the diagnosis of sJIA, comparatively investigating 23 patients with sJIA, 20 patients with acute lymphoblastic leukemia (ALL), 18 patients with severe infections (SIFs), 26 patients with Kawasaki disease (KD), 18 patients with JIA, and 25 healthy controls. Serum levels of IL-6, IL-18, S100A8, and S100A9 were measured. The results indicated significantly higher serum IL-6 concentrations in all patient groups, without significant differences between them, compared to healthy controls. S100A8 was significantly increased in sJIA vs. ALL, JIA, and healthy controls, but was not significantly different vs. the SIF and KD groups. S100A9 in sJIA was greatly increased compared to ALL and healthy controls, but not significantly different from SIF, KD, and JIA. The levels of IL-18 in sJIA were much higher than in groups with febrile diseases, allowing the authors to conclude that serum IL-18 could be a biomarker in the differential diagnosis of sJIA compared to other febrile diseases [113].
As an IFN-γ-stimulating production factor, IL-18 is a cytokine that plays an important role in Th1 and NK cell activation, but also in Th2, IL-17-producing γδ T cells, and macrophage activation in the processes of intracellular defense against bacteria and some viruses. IL-18 shares the same signaling pathway as IL-1 to initiate NF-κB and sets in motion some inflammatory mediators (e.g., adhesion molecules, chemokines, and Fas ligand (FasL)). IL-18, as a pleiotropic cytokine, plays an important role in various infectious, metabolic, and inflammatory diseases. IL-18 flows in the range of tens of nanograms/mL. Particularly high IL-18 concentrations in the sera of patients with active sJIA have been reported in several studies and correlated with elevated serum ferritin levels, predictive of MAS. sJIA and AOSD are characterized by high serum IL-18 concentrations and could be treated by IL-18BP [114].
Kudela et al. provided additional evidence in a study of 30 adult patients diagnosed with AOSD and 20 children diagnosed with sJIA with serum IL-18 levels up to 1000-fold higher during active disease compared to other rheumatic subtypes, proposing IL-18 as a biomarker for disease activity [115].
Yasin et al. investigated the total levels of IL-18, CXCL9, and S100 proteins in 40 patients with sJIA, comparing them between subjects to detect disease activity and history of MAS. Total IL-18 was greatly increased in patients with active sJIA and remained persistently elevated even in the vast majority of patients with inactive disease. Subjects with a history of MAS presented much higher concentrations of IL-18 compared to those who had never had MAS. Total IL-18 could predict disease activity and history of MAS. A moderate correlation was noted between IL-18 and CXCL9, as well as between S100A8/A9 and S100A12, being more pronounced for ferritin and, generally, for those with active disease. Elevated IL-18 may signal increased sJIA activity or the development of MAS [116].
Krei et al. conducted a systemic review of a total of 14 studies that included all HLH subtypes (and MAS subtypes) in both children and adults, investigating the role of IL-18 as a potential biomarker for the diagnosis and monitoring of HLH/MAS. Serum IL-18 was found to be elevated in both primary and secondary HLH (>1000 pg/mL), with a significantly higher level in MAS (IL-18 > 10 000 pg/mL) compared to other inflammatory conditions and healthy patients; therefore, serum IL-18 levels may differentiate between HLH subtypes and other inflammatory conditions. The authors concluded that IL-18 is a potential biomarker for HLH/MAS but is not currently part of the diagnostic criteria. IL-18 may help to differentiate between HLH subtypes and other inflammatory conditions. The potential of IL-18 to distinguish MAS from sJIA is more ambiguous, as IL-18 levels > 100,000 pg/mL were described in sJIA patients both with and without MAS [117].
Mizuta et al. investigated the significance of serum IL-18 concentrations for the diagnosis and prediction of sJIA’s progression; 116 patients with sJIA, 151 with other diseases, and 20 healthy controls were studied. IL-18 was measured in 41 patients with sJIA from the active period until remission. The minimum serum IL-18 concentration required for distinction from other diseases was 4800 pg/mL. IL-18 was steadily decreased during inactivity and remission in patients with monocyclic evolution. In contrast, in patients with polycyclic evolution, serum IL-18 concentrations were increased during the active period and normalized during the inactive phase. The cutoff value of serum IL-18 for remission in sJIA was 595 pg/mL. The authors noted that serum IL-18 levels > 4800 pg/mL may be useful in differentiating between sJIA and other autoimmune/autoinflammatory diseases; additionally, serum IL-18 levels may be useful in predicting progression and remission in sJIA [118].
Other recent studies have reported IL-18 as a predictive biomarker for the occurrence of MAS, along with the correlation of its high serum levels with the clinical parameters of MAS activity in patients treated with TCZ [103,119].
IL-18BP is abundant in the bloodstream under normal conditions and in many pathological disorders, stopping the negative systemic pro-inflammatory impact of IL-18. Severe clinical manifestations of sJIA are dependent on the levels of free IL-18 being elevated in the bloodstream, along with the imbalance between IL-18 and IL-18BP. The disequilibrium of IL-18/IL-18BP in favor of free-circulating IL-18 is known for its important role in the pathogenesis of sJIA, AOSD, and the severe complication of MAS. The use of recombinant IL-18BP in patients with AOSD or sJIA with MAS has shown promising results, making free IL-18 a biomarker and a therapeutic target in these diseases [120].
Recent findings support a pattern in which patients with high serum IL-18 levels (associated with pro-inflammatory activated neutrophils in the bloodstream) but low/normal CXCL9 levels are likely to respond well to IL-1 blockade. However, patients with sJIA who have low IL-18 (i.e., weak neutrophil marks) and low CXCL9 levels are unlikely to respond to IL-1 blockade, while patients with high IL-18 but also high CXCL9—reflecting the activation of IFN-γ (i.e., IL-18/CXCL9 ratio remains lower)—have a high risk of both MAS and failure of therapy [121].
Several studies have indicated that CXCL9, IL-18, neopterin, and the soluble TNF receptor (sTNFR) type II (sTNFR-II) could be useful in predicting MAS in patients with active sJIA. IFN-γ and the IFN-induced mediators are elevated during active MAS, and high blood levels of CXCL9 are predictive of future MAS. However, recent research has shown the opposite, where most patients with sJIA, despite significant increases in CXCL9, never developed MAS; this can be explained by the multiplex platform used in the laboratory determinations. Therefore, given these uncertainties, the use of CXCL9 and IL-18 to predict the outcome of IL-1 blocking treatment requires further study.
2.3.6. IFN-γ
IFN-γ is mainly regulated by NK cells and natural killer T (NKT) cells as a reaction of the innate immune system, and by CD4+Th1 and CD8+ cytotoxic T lymphocyte (CTL) effector T cells after the antigen-specific (i.e., adaptive) immunity develops [122].
IFN-γ attaches to and stimulates the specific receptors of antigen-presenting histiocytes and dendritic cells in tissues, causing the synthesis and release of CXCL9 and CXCL10 chemokines, which bind CTLs in the peripheral blood; when activated, these CTLs move to the site of inflammation, where they release more CTL-derived IFN-γ which, in turn, increase the inflammatory response [123].
On the other hand, IFN-γ, as a pro-inflammatory cytokine, plays an important role in regulating hematopoietic stem cells (HSCs) in physiological conditions. IFN-γ actively plays a special pathogenic role in various diseases that harm hematopoiesis, including HLH, aplastic anemia, liver cirrhosis, etc. Increased serum IFN-γ levels have been shown to negatively influence HSC balance by overstimulating differentiation and self-renewal until exhaustion of HSCs [124].
Antigen-presenting cells activate T lymphocytes and NK cells, which secrete IFN-γ which, in turn, activates monocytes and macrophages. IFN-γ-activated M1-type macrophages secrete large amounts of pro-inflammatory cytokines, including IL-6, IL-12, and IL-23, as well as IP-10 chemokines and monokines that recruit polarized Th1 lymphocytes and NK cells. At the same time, the continuous stimulation produced by IFN-γ activates the macrophages that participate in the phenomenon of hemophagocytosis [14].
There are studies that show that IFN-γ plays a key role in triggering primary hemophagocytic lymphohistiocytosis (pHLH) and MAS, because serum levels of IFN-γ and of the IFN-γ-induced chemokines are significantly elevated. Thus, the clinical manifestations of pHLH and MAS can be stopped by therapy with monoclonal antibodies directed against IFN-γ. The use of emapalumab—an anti-IFN-γ monoclonal antibody—for the treatment of patients with pHLH symptoms and disease that is refractory, recurrent, progressive, or intolerant to conventional therapy has been approved by the Food and Drug Administration (FDA) [125,126,127].
Put et al. undertook research that aimed to determine the role of IFN-γ in the pathogenesis of sJIA and HLH by discovering a profile of IFN-γ and its connection with other cytokines; 10 patients with active sJIA, 10 patients with inactive sJIA, and 5 patients with HLH—of whom 3 had associated sJIA-MAS—were included in the study, along with 16 healthy controls. The authors investigated the cytokines, the IFN-γ-induced genes, and the proteins from patients’ plasma and peripheral blood mononuclear cells (PBMCs), as well as the lymph node biopsies taken from a patient during two sJIA and MAS episodes. IFN-γ responses were also investigated in healthy donors’ PBMCs, primary fibroblasts, and endothelial cells. The results showed that plasma IFN-γ, IL-6, and IL-18 levels were increased in active sJIA and HLH. The concentrations of IFN-γ and IFN-γ-induced proteins (i.e., IP-10/CXCL-10, IL-18BP, and indoleamine 2,3-dioxygenase) in patients with HLH were significantly higher than those in patients with active sJIA, but the free IL-18 and IL-18/IFN-γ ratios were higher in active sJIA than in HLH. HLH PBMCs demonstrated hyposensitivity to IFN-γ in vitro compared with PBMCs from control subjects and those with sJIA. Both fibroblasts and endothelial cells expressed proteins induced by IFN-γ in situ—as evidenced by staining samples from the lymph nodes of a patient with MAS—and in vitro upon stimulation with IFN-γ. The authors found recognizably different cytokine profiles, with very high plasma levels of IFN-γ and IFN-γ-induced proteins, in patients with active sJIA and HLH/MAS. Histiocytes, endothelial cells, and fibroblast cells, along with PBMCs, may facilitate a distinct plasma IFN-γ profile. The high concentrations of IFN-γ, compared to those of IL-18, may indicate a presumptive progression of MAS in sJIA [128].
Bracaglia et al. hypothesized that IFN-γ would be the pivotal mediator in murine pHLH models and that there would be a similarity between primary and secondary HLH—including MAS—and investigated the involvement of the IFN-γ pathway in MAS by measuring IFN-γ levels and induced chemokines, along with their correlation with laboratory data, in patients with sJIA-MAS and in a murine MAS model. Serum levels of IL-1β, IL-6, IFN-γ, and the IFN-γ-induced chemokines CXCL9, CXCL10, and CXCL11 were evaluated in patients with sec-HLH (n = 11) and in patients with sJIA (n = 54), of whom 20 had active MAS at sampling. The results showed that IFN-γ and the IFN-γ-induced chemokines were significantly increased in active MAS and sec-HLH compared to active sJIA without MAS. In patients with active MAS, serum ferritin, alanine transferase, neutrophils, and platelet counts were significantly correlated with serum IFN-γ and CXCL9 levels. In murine MAS, serum ferritin was significantly correlated with CXCL9 mRNA concentrations in the liver and spleen. The authors concluded that elevated levels of IFN-γ and IFN-γ-induced chemokines, in combination with other severely altered laboratory parameters in MAS, support the significant involvement of IFN-γ in MAS [129].
In another study, Bracaglia et al. analyzed the levels of IFN-γ, CXCL9, CXCL10, and IL-18 in 24 patients with active sJIA and in 37 samples from MAS patients with different degrees of disease severity and under different treatments at the time of the study. The data obtained showed that the levels of IFN-γ, CXCL9, CXCL10, IL-18, neopterin, and soluble interleukin-2 receptor alpha chain (sIL2RA or sCD25) were significantly increased in MAS compared to active sJIA without MAS and were correlated with laboratory parameters of disease severity, except for IL-18, whose levels were only available for some of the samples. In patients with active sJIA without MAS, levels of IFN-γ-induced chemokine (i.e., CXCL9 and CXCL10) were significantly higher in those with a positive history of MAS compared to patients without a history of MAS. In conclusion, the increase in neopterin and CXCL9—under the influence of IFN-γ, and in direct connection with laboratory parameters—was indicative of the role of IFN-γ in MAS. Higher increases in serum CXCL9 and CXCL10 levels in patients with a history of MAS compared to patients who had never had MAS emphasized the activation of this pathway even in the absence of obvious clinical MAS, suggesting that these two chemokines could predict the risk of MAS. Concentrations of neopterin, IFN-γ, CXCL9, CXCL10, sCD25, and IL-18 can be used as biomarkers to discriminate MAS from active sJIA [130].
MAS and sec-HLH are hyperinflammatory disorders that can be triggered by a CS. Prompt detection and early therapy are necessary to avoid the unfortunate consequences of these diseases’ high mortality. As IFN-γ is already known to play a major role in triggering sec-HLH and sJIA-MAS, De Matteis et al. performed a study on 35 patients with sec-HLH, 21 patients with sJIA-MAS, and 25 patients with sJIA at three different intervals: active disease (T0), 7–10 days after initiation of therapy (T1), and when the disease was no longer active (T2), i.e., 1–3 months after onset. Serum biomarkers related to IFN-γ (i.e., CXCL9, CXCL10, and neopterin) from 265 samples were investigated. The results of the study showed that the concentrations of CXCL9, CXCL10, and neopterin at T0 were much higher in patients with MAS and sec-HLH compared to those with sJIA, while they were progressively reduced at T1 and normalized at T2. A more rapid decrease was observed for CXCL9 than for neopterin, similar to the drop in routine laboratory data. The study confirmed that platelet count and ferritin were the most distinctive laboratory parameters with high specificity and sensitivity for sJIA-MAS. At the same time, elevated LDH levels, which are not included in the 2016 classification criteria for sJIA-MAS, may be useful in predicting the occurrence of MAS. The results revealed increased levels of IFN-γ-related biomarkers in patients with MAS and sec-HLH, which could be useful together with traditional laboratory parameters for diagnosis, clinical follow-up, and response to therapy [67].
Guo et al. performed a retrospective cohort study of 149 patients with sJIA, of whom 27 had 31 episodes of MAS. Clinical and laboratory parameters of patients with sJIA-MAS were investigated and compared with those without MAS. Clinically, a high percentage of patients with MAS at onset did not have fever, arthritis, or central nervous system dysfunction; 35% of MAS patients had hypotension without shock, and 22.6% of patients had gastrointestinal symptoms. Subjects with MAS and hypotension had a higher rate of ICU hospitalization; they presented more frequently with arthritis, serositis, pneumonia, and gastrointestinal symptoms, accompanied by higher white blood cell and neutrophil counts, superior serum bilirubin concentrations, and lower total serum protein levels. The platelet count, LDH, and aspartate aminotransferase (AST), together with the ferritin/ESR ratio of approximately 20.0, had high sensitivity and specificity in the early diagnosis of MAS. The association between IFN-γ > 17.1 pg/mL and IL-10 > 7.8 pg/mL appears to be a valuable cytokine prototype to confirm the onset of MAS.
The sudden onset of arterial hypotension, the ferritin/ESR ratio, and the pattern of elevated IFN-γ and IL-10 cytokines are important parameters in predicting the early onset of sJIA-MAS [131].
2.3.7. TNF-α
TNF-α, recognized as TNF superfamily member 2 (TNFSF2), is a multifunctional cytokine with a well-determined role in innate and adaptive immunity, as well as in the normal physiology of the immune system. Abnormally high and sustained production of TNF-α is correlated with pathological manifestations of inflammatory diseases such as sepsis and chronic ADs. Physiologically, TNF-α is a crucial component for a normal immune response and can be considered a key factor in the development of infectious and autoimmune pathologies [132].
In the pathogenesis of MAS, it has been proposed that macrophages produce several cytokines, including TNF-α and pro-inflammatory interleukins (e.g., IL-6, IL-1, and IL-18), which unleash a cascade of inflammatory pathways and, ultimately, create a CS. TNF-α can be considered a key factor in the development of infectious and autoimmune pathologies. With advances in the development of many diagnostic tools to identify MAS in sJIA as well as other forms of MAS/sHLH, early management with cytokine-targeted therapies will likely save the lives of many patients. TNF-α, when produced in excess, participates in the spread of inflammatory phenomena by activating and concentrating fibroblasts, causing joint erosion, fibrosis, and joint deformity in rheumatic diseases. There are already five TNF-α receptor blockers (i.e., infliximab, adalimumab, etanercept, certolizumab pegol, and golimumab) currently used in the treatment of several inflammatory diseases, including in children [133,134].
Hügle et al. conducted a retrospective study of 32 patients for a period of 6 years, considering demographic and clinical data as well as the presence and titers of antinuclear antibodies (ANA) and rheumatoid factor (RF) at diagnosis, from the AID-Net database—a German registry and biobank that gathers information and biomaterials from patients with autoinflammatory syndromes, including periodic fever syndromes and sJIA; 8/32 patients had ANA titers ≥ 1:80 at diagnosis, 22/32 patients had rising ANA titers with titers ≥ 1:80 at their last visit, and 10/32 patients had a positive RF at least once during follow-up, compared to 0/32 at diagnosis. The authors concluded that a proportion of patients with sJIA developed increased ANA titers and positive RF over time, indicating the potential role of lymphocyte activation and autoimmune processes in this primarily autoinflammatory condition, independent of TNF antagonist treatment in sJIA [135].
Shimizu et al. measured the ratio between the levels of soluble TNF receptor (sTNFR) type II (sTNFR-II) and type I (sTNFR-I)—i.e., (sTNFR-II/I)—in 117 patients with sJIA, including 29 patients with MAS, 15 with EBV-HLH, 15 with KD, and 28 healthy controls, in correlation with the activity and severity of the disease. At the same time, they determined the serum levels of IL-18 in patients with EBV-HLH compared to MAS. The results showed that the ratio of sTNFR-II/I had a significantly higher level in patients with MAS and EBV-HLH compared to patients with active sJIA and KD. Serum IL-18 concentrations were also significantly increased in patients with MAS in comparison with EBV-HLH. Serum IL-18 and sTNFR-II/I could be used as biomarkers for the diagnosis of MAS and the differentiation between MAS and EBV-HLH [136].
Irabu et al. studied the serum concentrations of neopterin, IL-18, chemokine (C-X-C motif) ligand 9 (CXCL9), sTNFR-I, and sTNFR-II in a cohort of 36 patients with sJIA-MAS, including 12 patients under management with TCZ. The serum ratio of sTNFR-II/I was compared with the clinical characteristics of MAS. The results showed that the levels of all serum cytokines studied at the diagnosis of MAS were much lower in the TCZ group compared to those without this treatment, even if the serum sTNFR-II/I ratio at the diagnosis of MAS was similar between the compared groups. The serum ratio of sTNFR-II/I, which was elevated in sJIA-MAS patients, was positively correlated with disease activity, with cutoff values of 0.9722 and 4.71, respectively. The authors concluded that the serum sTNFR-II/I ratio could be a useful biomarker for assessing disease activity in sJIA-MAS, but also a predictive biomarker for the onset of MAS in the sJIA active phase—even in patients under TCZ [137].
Mizuta et al. comparatively studied the cytokines involved in the onset of MAS in order to detect the serum biomarkers for early prediction of MAS in different childhood rheumatic pathologies. Serum concentrations of neopterin, IL-6, IL-18, sTNFR-I, and sTNFR-II were investigated via the ELISA technique in 12 patients with SLE, including 5 with MAS; 12 patients with juvenile dermatomyositis (JDM), including 4 with MAS; 75 patients with KD, including 6 with MAS; and 179 patients with sJIA, including 43 with MAS. The results were analyzed in the context of the clinical symptoms of MAS. It was observed that the serum concentrations of neopterin, IL-18, and sTNFR-II were much higher during MAS compared to the active phase in all patients and all conditions studied. Serum concentrations of sTNFR-I were much higher during MAS compared to the active phase only in patients with SLE, KD, and sJIA. Depending on the disease, the curve analysis revealed the following cytokines at the highest levels: serum sTNFR-I for SLE, serum IL-18 for JDM, and serum sTNFR-II for KD and sJIA, which were positively correlated with the levels of serum ferritin. The authors assumed that the overproduction of IFN-γ, IL-18, and TNF-α could highlight the initiation of MAS, and that the serum concentrations of sTNFR-I for SLE, IL-18 for JDM, and sTNFR-II for KD and sJIA could be important biomarkers in the active phase of these diseases for the prediction of MAS onset [138].
The scientific studies discussed above to provide insight into the utility important cytokines as biomarkers are summarized in Table 3.

Table 3.
Important cytokines as biomarkers for the diagnosis, activity, and prognosis of sJIA and MAS.
2.3.8. CXCL9 Chemokine
Chemokines are a family of low-molecular-weight proteins that participate in inflammation as mediators, signaling molecules, migration stimulators and macrophage activators. There are four groups of ligands (XC, CC, CXC, and CX3C) for chemokines and over 20 rhodopsin-like receptors—7 of which are transmembrane—including CC receptors, CXC receptors, and receptors that exhibit non-G-protein-related signaling.
Chemokines act on macrophage differentiation and polarization in physiological and pathological conditions, especially in inflammatory diseases. M1-type macrophages expose CXCL9 and CXCL10 chemokines, resulting from the activation of the cytokines by IFN-γ and TNFα, which attract Th1 lymphocytes. At the same time, the chemokines CXCL9 and CXCL10 work as attractants of macrophages in various inflammatory diseases (stomach burns, joints, etc.). It has been shown that osteoclast precursors exhibit the chemokine CXCR3, which can cross-react with the chemokine ligand CXCL9 and stimulate migration and differentiation of osteoclasts [136,139,140,141,142,143,144,145].
To define the cytokines and serum biomarkers involved in the onset of sJIA-MAS, Mizuta et al. used a special antibody array that simultaneously detects 174 cytokines to investigate samples from 15 sJIA patients, including 5 patients on TCZ therapy. Concentrations of five cytokines were highly increased in MAS compared to active sJIA. The chemokine CXCL9 showed the greatest increase after the onset of sJIA-MAS. Serum levels of CXCL9 were measured comparatively in 56 patients with sJIA, including 20 with MAS, to highlight the clinical value of CXCL9 as a biomarker of sJIA-MAS. The authors proved that CXCL9 was positively correlated with disease activity and that monitoring the serum levels of this chemokine could enable surveillance of sJIA-MAS [146].
Hinze et al. aimed to examine the associations of baseline serum biomarkers with the responses and outcomes of 54 active sJIA patients without recent MAS after canakinumab therapy. Most biomarkers were elevated at baseline in canakinumab-naïve patients compared to healthy controls, and some rapidly decreased by day 15 (i.e., IL-1Ra, IL-6, IL-18, and S100A12). Patients who responded to treatment had higher IL-18 and IFN-γ levels and lower CXCL9 levels at baseline, as documented by IL-18/CXCL9 and IFN-γ/CXCL9 ratios, which had significant accuracy in predicting treatment responses. The authors observed a differential regulation of the IL-18–IFN-γ–CXCL9 axis in patients with sJIA, and the higher the IL-18/CXCL9 and IFN-γ/CXCL9 ratios, the better the clinical response to canakinumab in sJIA [147].
2.3.9. sCD163 and sCD25
In order to investigate MAS, which is a huge cascade of inflammatory phenomena triggered by the very intense activity of T cells and hemophagocytic macrophages, Bleesing et al. analyzed the concentrations of sIL2RA—also called sCD25 and soluble cluster of differentiation 163 (sCD163)—which reflected the levels of stimulation and expansion of these cells, for samples taken from 7 patients with sJIA-MAS compared with 16 patients with systemic sJIA at baseline without therapy, correlating these tests with serum ferritin levels and clinical symptoms. The mean level of sCD163 was 23,000 ng/mL in MAS, compared to only 5480 ng/mL in patients with active sJIA, while the mean level of sIL2RA was 19,646 pg/mL in MAS, compared to only 3787 pg/mL in active disease. In 5 out of 16 patients with sJIA, the serum concentrations of sIL2RA or sCD163 were similar in order of magnitude to those in MAS. It was concluded that sIL2RA and sCD163 could constitute sensitive biomarkers for the diagnosis of MAS, even in subclinical cases, and could also help identify patients with subclinical MAS [148].
Clinical and traditional laboratory markers of MAS, together with sCD25 and sCD163, were investigated by Reddy et al. in 33 patients with sJIA, 11 patients with active polyarticular JIA (polyJIA), and 2 patients with sJIA-MAS. Subjects with MAS had thrombocytopenia, leukopenia, and hypofibrinemia. The sCD25 value > 7500 pg/mL observed in MAS was also found in eight patients with active sJIA, who also had other highly altered laboratory values suggestive of MAS. Elevated levels of sCD63 (>1800 ng/mL) were detected in four patients with sJIA, and it was concluded that sCD25 > 7500 pg/mL could be a predictive biomarker of subclinical MAS [149].
Sakumura et al. conducted a study of 63 patients with sJIA, 4 with EBV-HLH, and 7 with KD, compared to 14 healthy controls, for whom the authors measured the serum concentrations of sCD163, neopterin, IL-18, and IL-6, and correlated them with the patients’ clinical symptoms. sCD163 was highly elevated in patients with sJIA-MAS and EBV-HLH compared to patients with only sJIA or KD in an acute phase. sCD163 increased dramatically with the evolution of MAS, being positively dependent on sJIA activity, even in patients under TCZ therapy. sCD163 showed a significant decrease in the inactive phase and later normalized, representing a potential diagnostic parameter for clinical remission in sJIA and an exclusive biomarker for the evaluation of disease activity, but also of MAS-sJIA [150].
Verweyen et al. conducted a study on gene expression in whole-blood samples taken from patients with sJIA at study entry and after 3 days of canakinumab therapy, compared to healthy controls. The authors found a distinct gene expression signature in patients with a very good clinical response to canakinumab compared to non-responders, mediated by upregulation of neutrophil-associated genes and IL-1, as well as an upregulated CD163 expression signature for the non-responders to biological therapy with canakinumab [151].
In a recent published review, Dik et al. concluded that in immune-mediated diseases where T-lymphocyte responses play an important role in the pathophysiological mechanisms, sCD25 could be a useful marker for prognosis or for monitoring the efficacy of immunosuppressive management [152].
2.3.10. Neopterin
In a recent study, Takakura et al. comparatively investigated the serum concentrations of neopterin, IL-18, CXCL9, and sTNFR-I and II in 78 children diagnosed with sJIA, including 21 with MAS, to draw conclusions regarding the determination of the most accurate biomarkers for establishing the diagnosis of sJIA-MAS. From the performed analyses, the authors finally indicated neopterin as having the highest concentrations in sJIA-MAS and being positively correlated with transition to MAS from active sJIA; thus, neopterin could be a valuable future biomarker in sJIA-MAS [143].
2.3.11. S100 Proteins
Various biomarkers are currently being researched for the diagnosis, monitoring of disease activity, predicting response to treatment, or the risk of recurrence in sJIA, AOSD, MAS, and other autoinflammatory diseases [57].
S100 proteins are a subgroup of the Ca2+-binding protein family; they are engaged in cell signal transduction, cell differentiation, motility regulation, transcription, and cell cycle progression, and extracellularly they perform a similar role to cytokines. The proteins S100A8/A9 (MRP8/14 or calprotectin) and S100A12 (calgranulin C) are secreted by phagocytic cells and may act as endogenous agonists of the toll-like receptor-4 and stimulate the innate immune response by releasing alarmins. These proteins have been identified as being more valuable than ESR and CRP as inflammatory biomarkers of the activity levels of rheumatic diseases. It is already known that the proteins S100A8/S100A9 and IL-1β play a special role in the pathogenesis of sJIA by activating phagocytic cells via two major signaling pathways of innate immunity [153,154,155].
S100 proteins, after being released by activated cells of the innate immune system (i.e., monocytes or neutrophils), play a special role in extending inflammation by binding to TLR4 and RAGE. TLR4 is known as a multiligand receptor for PAMPs of pathogenic bacteria, as well as DAMPs, while RAGE is a multiligand member of the cell surface molecule superfamily and interacts with various other molecules involved in homeostasis and inflammation. Both TLR4 and RAGE are expressed on the surface of immunological cells, endothelial cells, and other extravascular cells, so they participate in the onset and spread of systemic diseases during infection or inflammation [156,157,158].
Calprotectin, or CP—also known as MRP-8/MRP-14 or S100A8/A9 heterocomplex—released primarily by activated neutrophils, has been investigated in feces and used as a biomarker of inflammatory bowel disease and chronic rheumatic diseases in adults and children, including sJIA. CP functions through a mechanism termed “nutritional immunity”, by retaining transition metals essential for growth and vitality. Overaccumulation of CP could play a crucial role in inflammatory diseases, different types of arthritis, metastatic cancer, and septicemia. S100A8/A9 and S100A12 are valuable biomarkers for diagnosis, treatment response predictions, recurrence risk, and disease activity surveillance in sJIA [159,160,161,162].
Upon activation of neutrophils or monocyte endothelial adhesion, CP is secreted by an alternative microtubule-mediated, calcium-dependent pathway, along with protein kinase C (PKC), necrotic cells, and NETs, and may act as a marker for mononuclear phagocyte crowding at the site of inflammation [163].
Since CP is released at the site of inflammation, plasma CP concentrations have been proposed as a biomarker that represents the local activity of inflammatory disease, as opposed to CRP, which is produced mainly by hepatocytes during the nonspecific systemic inflammatory process. Being a relatively stable product, CP is more advantageous than IL-6, TNF-α, or IL-1β [164].
Altobelli et al. carried out a systematic review and meta-analysis of the role of CP as a potential biomarker of inflammation in JIA. The results showed a statistically significant difference between patients with the active systemic subtype compared to other subtypes of JIA, inactive disease, and healthy controls; the mean values of ESR and CRP were not found to be statistically significant. In conclusion, the evaluation of serum PC could provide us with data on the more accurate stratification of disease activity and the administration of more tailored and appropriate therapy in JIA [165].
Wittkowski et al. investigated the serum concentrations of the phagocytic pro-inflammatory protein S100A12 in serum samples taken from 240 patients with various pathologies with fever of unknown origin (FUO), diagnosed as follows: 60 with sJIA, 17 with familial Mediterranean fever (FMF), 18 with neonatal-onset multisystem inflammatory disease, 17 with Muckle–Wells syndrome, 40 with acute lymphoblastic leukemia, 5 with acute myeloblastic leukemia, and 83 with systemic infections, compared with 45 healthy controls. The samples were taken at the introduction to the study, before starting any kind of therapy. The results demonstrated a sensitivity of 66% and a specificity of 94% for S100A12 in differentiating sJIA from infections. The authors concluded that S100A12, as a biomarker of granulocyte activation, is highly overexpressed in patients with sJIA or FMF and can be a very valuable parameter in the evaluation and differential diagnosis of FUO, helping the doctor to make the correct decision to treat the patient with antibiotics or immunosuppressive agents [166].
Holzinger et al. studied the serum concentrations of myeloid-related protein complex 8 and 14 (MRP8/14) in 52 patients with sJIA to monitor disease activity and attempt a classification of patients at risk of relapse. The results indicated highly elevated serum concentrations of MRP8/14 in active disease and significantly reduced concentrations in the case of positive-response therapies. MRP8/14 faithfully reflected the clinical activity of sJIA, along with its inactivity or relapses, representing a valuable parameter for monitoring the response to treatment, being the first predictive biomarker for the subclinical activity of the disease, and allowing the stratification of patients at risk of relapse during periods of clinical inactivity of sJIA [167].
Starting from the hypothesis that the expression of programmed death ligand-1 (PD-L1)—an important immunoregulator (i.e., a glycoprotein expressed on antigen-presenting cells to limit T-lymphocyte activation during an inflammatory response)—is deregulated in sJIA monocytes, Shenoi et al. analyzed it in a pilot study on 20 patients (10 with active sJIA and 10 febrile non-sJIA). The authors compared PD-L1 expression in myeloid cells with other possible predictive biomarkers of sJIA, such as: CRP, ESR, leukocyte counts, S100A12, S100A8, S100A9, calprotectin, and procalcitonin, each used as a predictor independent of the other parameters in the analysis. The results highlighted that PD-L1 was significantly lower in sJIA, while S100 proteins were significantly increased with >80% sensitivity and >90% specificity in sJIA. The other investigated biomarkers were not found to be specific to sJIA, and the exploratory gene analysis indicated 106 genes as being important for sJIA, several of which were associated with immune response pathways. Although performed in a small cohort, S100 proteins stood out as specific diagnostic biomarkers for febrile patients with sJIA, and the decreased PD-L1 expression on the surface of circulating myeloid cells in sJIA could indicate a mechanism of loss of regulation of peripheral immunity [168].
Aljaberi et al. retrospectively investigated the usefulness of S100 protein testing in sJIA and other autoinflammatory and fever syndromes, in a pediatric clinical setting, to monitor the activity of each disease and support the diagnosis in 136 patients with the following pathologies: sJIA, non-systemic JIA (nsJIA), other defined autoinflammatory diseases (AIDs), and systemic undifferentiated recurring fever syndromes (SURFSs). In the case of sJIA, the concentrations of S100A8/9 and S100A12 were higher in active disease compared to inactive disease and had much higher values compared to all other categories included in the research; by comparison with CRP, they proved to be clearly superior in differentiating sJIA from AID and SURFS, and they were not associated with disease activity in nsJIA, AIDs, or SURFSs. The authors concluded that the significantly higher S100A8/9 and S100A12 protein levels in sJIA can support the correct diagnosis in this pathology, compared to other autoinflammatory and febrile diseases, and are superior to CRP in establishing the positive diagnosis of sJIA compared to other diagnoses, as well as when monitoring sJIA activity [169].
Because the differential diagnosis of sJIA from other infectious pathologies is sometimes very difficult in cases of prolonged fever in children, Park et al. investigated which would be the most useful biomarkers in diagnosis and tried to determine the usefulness of myeloid protein 8/14 (MRP8/14) measurements in order to transfer them from the laboratory to the bedside in clinical practice, in order to develop new methods of diagnosis for sJIA patients. Laboratory data from 1110 children and adolescents were divided into two cohorts: group A, to validate the performance of the MRP8/14 test by experimental ELISA, commercial ELISA, and an innovative lateral flow immunoassay (LFIA); and group B, to validate the accuracy of the diagnosis only with the last two tests. The authors found that MRP8/14 was increased in group A (n = 940) in sJIA compared to other conditions—including infections and autoinflammatory diseases—regardless of fever or the anti-inflammatory treatment received by the patient. In children and adolescents with fever, but without treatment (n = 195), serum concentrations of MRP8/14 in sJIA (19740 ± 5080 ng/mL) were much higher compared to other diagnoses, having the highest precision compared to all other parameters measured—such as ferritin, IL-18, ESR, soluble IL-2 receptor, and procalcitonin. The authors highlighted that serum tests of MRP8/14 can be useful for the positive diagnosis of sJIA in febrile patients, and commercial ELISA and LFIA allow accurate testing for rapid diagnostic screening at the point of care [18].
The lack of highly accurate biomarkers for monitoring disease activity—and as valuable tools for classifying and prognosticating treatment response or risk of flares in JIA patients—led La et al. to investigate serum calprotectin (sCP) concentrations in a multicenter cohort of 81 JIA patients, compared with 11 healthy controls. As compared to the controls, the researchers found that sCP values increased 8-fold in active sJIA and 2-fold in inactive disease, with greater specificity than CRP values and even stronger than ESH, allowing the differentiation of forms such as oligoarthritis, polyarthritis, and systemic forms, and recommending the future applications of sCP as a biomarker not only in the diagnosis and follow-up of JIA, but also for predicting relapses after stopping the treatment with non-steroidal anti-inflammatory drugs, methotrexate, or etanercept; thus, sCP should be implemented as soon as possible in clinical settings [170].
2.3.12. Procalcitonin
First identified in the 1970s [171] as a precursor of calcitonin (a hormone involved in calcium homeostasis), procalcitonin (PCT) is a 116-amino-acid peptide generated by the parafollicular cells of the thyroid and the neuroendocrine cells of the intestine and the lungs. Plasma concentrations in healthy subjects are below the detection limit (0.01 µg/L) of clinical tests [172], increasing in response to pro-inflammatory stimuli—especially in bacterial infections—and considered to be an acute-phase reactant. In pediatric pathologies with high fever of unknown origin, an elevated plasma PCT level at the threshold of 0.5 ng/mL has a sensitivity and specificity of over 80% for the diagnosis of sepsis in infants and children [173].
Ninety-two subjects aged between 6 months and 18 years with untreated active JIA, inactive JIA, and healthy elective preoperative patients were included in a cohort study by Trachtman et al. to test the hypothesis that serum procalcitonin (sPCT) levels would differ between active JIA, inactive JIA, and healthy controls, by measuring their serum PCT and other common laboratory parameters of inflammation. The results indicated increased values for ESR, CRP, and PCT in patients with bacteremia compared to the other groups, with high rates of overlap between groups for ESR and CRP. The PCT concentration was equal to or greater than 0.15 μg/mL in only 1 out of 59 patients with JIA, whereas it was equal to or less than 0.15 μg/mL in only 1 patient with bacteremia. The authors’ conclusions were that although ESR, CRP, and PCT may all be biomarkers of choice for distinguishing JIA at presentation from serious bacterial infection (SBI), the most suitable biomarker appeared to be PCT, which was the most accurate in distinguishing between patients with infection and those with non-infectious inflammatory arthritis, which can greatly help clinicians to initiate the proper management immediately [174].
2.3.13. sRAGE
Receptor for advanced glycation end products (RAGE; UniProtKB-Q15109) is a multiligand transmembrane receptor that is part of the immunoglobulin gene superfamily; 35 kD soluble cleaved RAGE—or soluble receptor for advanced glycation end products (sRAGE)—can be quantified in plasma or serum, and it has been found that high levels are associated with an increased risk of chronic diseases such as diabetes, atherosclerosis, coronary heart disease, chronic lung disease, etc. Increased sRAGE is positively associated with other pro-inflammatory advanced glycation end products (AGEs), and negatively with leukocytosis and increased levels of CRP and Fg [175,176,177,178].
Geroldi et al. presented an overview of the state of the art in the use of sRAGE as a biomarker and discussed its therapeutic potential for inflammatory diseases such as Alzheimer’s disease, atherosclerosis, and arthritis [179].
Elevated plasma levels of sRAGE may account for the potential anti-inflammatory and atheroprotective properties of this biomarker. sRAGE levels are inversely correlated with white blood cell counts and high-sensitivity C-reactive protein (hsCRP), and could indicate a slower rate of progression and a better prognosis of carotid artery disease [180].
In a recently published study, Prasad claimed that a low serum percentage of sRAGE cannot be considered a universal biomarker for patients with different pathologies compared to healthy control subjects. However, evidence supports the validity of a high AGE/sRAGE ratio as a universal biomarker/risk marker for diseases [181].
In an experimental murine model of cardiac ischemia/reperfusion (I/R), Zhang et al. demonstrated that sRAGE protected the heart from I/R lesions that could be induced by infiltration and proliferation of M1-type macrophages, which release IFN-γ following activation of the NF-κB signaling pathway by sRAGE in the differentiated M1 macrophages. The recruitment and proliferation of macrophages increased significantly in the presence of sRAGE [182].
2.3.14. HMGB1
The high-mobility group box-1 (HMGB1; also called HMG1 or amphoterin) protein is the most plentiful member of the HMG family of DNA-binding proteins—a prototype of alarmins or DAMPs that are released into the extracellular space by activated or necrotic cells in viral and bacterial infections, as well as in several diseases with acute or chronic inflammation, such as cancer and ADs, and that work as key molecules connecting tissue damage and stress with innate immunity. In addition to its pro-inflammatory functions, HMGB1 also has effects in repairing and regenerating tissues. HMGB1 could be a therapeutic target in inflammatory chronic diseases. Recently generated HMGB1-specific inhibitors have been proposed as an interesting therapeutic target in inflammatory conditions that currently lack efficient therapies [183,184,185].
Together with extrinsic factors, intrinsic components can trigger an unpredictable immune response in sJIA. HMGB1 takes part in the pathogenesis of inflammatory diseases through interactions with pivotal transmembrane receptors, including RAGE and toll-like receptor-4 (TLR4). Macrophages and monocytes that are highly activated, along with disrupted TLR signaling and its undertrained ligands, are implicated in sJIA and AOSD. Extracellular HMGB1 has the ability to amplify inflammation and modulate immune responses in conjunction with RAGE. HMGB1 is an extracellular DAMP that binds to RAGE and TLR4 and spreads inflammatory signals. HMGB1 is involved in systemic inflammation from septicemic infections, arthritis, SLE, and liver damage [186,187,188,189].
The role of extracellular HMGB1 was discovered in 1999 as an important secreted protein in inflammation and infection. Extracellular HMGB1 transporting nucleic acids binds to RAGE and is internalized via endocytosis, so that the transferred nucleic acids interact with intracellular receptors and generate interferon and cytokine responses. HMGB1, as a pro-inflammatory mediator, binds several pattern-recognition receptors (PRRs); in addition to RAGE, members of the Toll-like receptor (TLR) family—i.e., TLR2, TLR4, and TLR9—as well as the CXC chemokine receptor 4 (CXCR4) and T-cell immunoglobulin mucin-3 (TIM-3) are released during apoptosis or necrosis, acting as DAMPs, while the inflammatory cytokines (i.e., TNF-α and IFN-γ) improve their release. When the concentration of the HMGB1 ligand increases, transmembrane RAGE is overexpressed in monocytes and macrophages and triggers an inflammatory immune response. sRAGE was shown to decrease the pro-inflammatory effects of HMGB1 [63,190,191,192].
Extracellular HMGB1 levels are increased in the inflamed joints of JIA patients and could be a biomarker of inflammatory activity and a target for therapy [193].
Bobek et al. investigated HMGB1 and sRAGE in a total of 144 enrolled children (97 with different subtypes of JIA, including sJIA; 19 with juvenile SLE; and 28 healthy controls), collecting consecutive samples of peripheral blood as well as synovial fluid (SF) at the time of diagnosis—prior to the initiation of treatment—and studying them in correlation with other common acute-phase reactants and clinical markers. The obtained results indicated much higher serum concentrations of HMGB1 and lower concentrations of sRAGE for children with JIA and those with SLE, respectively, compared to healthy controls, with positive correlations between HMGB1 and ESR, CRP, and α2 globulin, while the concentrations of sRAGE were inversely proportional to the same inflammatory markers in children with JIA. The median serum HMGB1 concentration in sJIA (17,402 pg/mL) was 15-fold higher than that in the control group (1149 pg/mL) and was also much higher than those in oligoarticular (3552 pg/mL), polyarticular (4374 pg/mL), and SLE patients (8523 pg/mL). Serum concentrations of HMGB1 were correlated with hepatosplenomegaly or serositis in sJIA. All JIA subgroups, as well as the SLE group, were characterized by lower serum levels of sRAGE, but the difference was significant only in patients with sJIA and in those with SLE. The inverse relationship between HMGB1 and its receptor sRAGE in blood and SF reflected that the inflammatory processes triggered by alarmins could play an important role in both investigated pathogeneses, and that HMGB1 could be an inflammatory biomarker to track in these patients as a potential target in the framework of biological therapy and for monitoring the activity of the disease [194].
Xu et al. conducted a prospective longitudinal study of 64 children with a mean age of 9.25 years and a mean duration of illness of 2.38 months, of whom 48.4% were female, and collected blood samples at the first visit and after 1 month, 3 months, and 6 months, for comparison to samples from healthy controls as well as from patients with reactive arthritis at the first visit. HMGB1 levels, routine laboratory data, and clinical disease characteristics were analyzed according to the protocol. The results at the first visit indicated significantly increased serum concentrations of HMGB1 in patients with sJIA compared to the other groups, as well as in enthesitis-associated arthritis compared to healthy controls. They also highlighted important correlations at the first visit between HMGB1 levels and disease duration, C-reactive protein, neutrophil percentage, and ferritin, suggesting that serum HMGB1 could be a sensitive inflammatory biomarker in sJIA. The authors recommended that further large-scale studies would be useful to explore the full range of HMGB1-related properties in JIA [195].
2.3.15. Key Laboratory Data in sJIA-MAS
In order to confront the concentrations of CRP and ESR in children with JIA, and to trace their influence on diagnostic variables and prognostic factors, Wu et al. investigated 107 patients, of whom 18 had sJIA. At the time of diagnosis, the prevalence of ESR was much higher than that of CRP (86.8% vs. 47.2%) and was positively correlated with good response to therapy. On the other hand, the high concentrations of CRP at the beginning of the treatment were correlated with an inadequate response to the therapy and generally proved to be a predictive parameter of the failure of the first remission. This study demonstrated that ESR is useful in the diagnosis of sJIA—much more valuable than CRP—whereas a high initial CRP value may predict failure of remission [196].
Bloom et al. investigated 31 children with sJIA who were at high risk of developing severe disease based on their first measured D-dimer concentration. Ten children who initially had their risk category classified as “high” developed a severe outcome. The authors proved the paradigm that the risk of severe disease indicated by persistently elevated levels of fibrin D-dimer could be a prognostic parameter indicating a poor longer-term outcome in sJIA [197].
Follistatin-like protein 1 (FSTL-1) is known as a glycoprotein that is overexpressed in certain inflammatory diseases. Gorelik et al. investigated FSTL-1 concentrations in 28 patients with sJIA, including 7 patients who developed MAS, and compared them to 30 healthy controls, in correlation with already known biomarkers for the evaluation of sJIA activity. FSTL-1 gene expression concentrations were investigated in peripheral blood mononuclear cells (PBMCs) in correlation with ESR, ferritin, and sIL2RA. Serum concentrations of FSTL-1 were high in patients with sJIA at the beginning of the study, and they returned to normal over the following 24 months. The same high concentrations of FSTL-1 were also found in patients with acute MAS, which normalized after treatment. FSTL-1 was correlated with several serum markers of inflammation, including sIL2RA and ferritin. The ferritin/ESR ratio was superior to ferritin, sIL2RA, and FSTL-1 in differentiating MAS from new-onset sJIA. High serum concentrations of FSTL-1 detected before sJIA therapy are accompanied by dysregulated gene expression that may predict the presence of hidden MAS and progression to observable MAS. It has been shown that the serum ferritin/ESR ratio may be more valuable than ferritin alone in differentiating MAS from new-onset sJIA [198].
Minoia et al. conducted extensive research within a multinational collaborative project on 766 patients, of whom 362 had sJIA-MAS and 404 had active sJIA without MAS, in an attempt to develop and validate a score for the diagnosis of sJIA-MAS. The score for sJIA-MAS, named MS score, comprised seven variables: central nervous system (CNS) dysfunction, hemorrhagic manifestations, active arthritis, platelet count, Fg, LDH, and ferritin. The cutoff value ≥ −2.1 was the most reliable in differentiating MAS from active sJIA, with a sensitivity of 0.85 and a specificity of 0.95. The MS score could be a valuable practical instrument to be used by clinicians in the early diagnosis of sJIA-MAS [199].
Eloseily et al. investigated the value of the serum ferritin/ESR ratio for the diagnosis of sJIA-MAS and ferritin alone as a screening parameter for detecting MAS of other febrile etiologies. For sJIA patients with MAS (n = 362), without MAS (n = 404), and subjects (n = 345) hospitalized with systemic infection (SI), the ferritin/ESR ratio and ferritin alone were investigated to detect MAS among sJIA patients.
Meanwhile, the same parameters were investigated on a smaller number of cases with MAS of various causes, but also in patients hospitalized for fever. The ferritin/ESR ratio of 21.5 was 82% sensitive and 78% specific for the diagnosis of sJIA-MAS versus active sJIA without MAS. Only ferritin as a single laboratory parameter had a sensitivity of 95% (screening tool) and a specificity of 89.3% for differentiating patients with MAS from subjects with other febrile conditions. It was concluded that the serum ferritin/ESR ratio is a valuable parameter for the diagnosis of sJIA-MAS, and that the serum ferritin level alone can be used in screening to detect MAS among febrile patients [200].
In a multicenter study of 80 patients with MAS, Zou et al. included 53 cases of sJIA-MAS, 10 cases of KD-MAS, and 17 cases of connective tissue disease (CTD-MAS) and investigated the clinical symptoms and laboratory data before (pre-), at the onset, and during the complete phase of MAS. Among all patients, 51.2% presented MAS for the first time at the diagnosis of the underlying disease. Among patients with sJIA, 22.6% had hypotension before the onset of sJIA-MAS; in these patients, an increase in aspartate aminotransferase (AST) and lactate dehydrogenase (LDH) was observed, along with a decrease in serum albumin. Serum ferritin levels and ferritin/ESR ratios were greatly increased in subjects with active sJIA-MAS compared to the pre-MAS period. At the same time, a very high serum ferritin concentration and a significantly increased ferritin/ESR ratio were found in sJIA subjects compared to KD and CTD patients. In conclusion, almost half of the cases of MAS occurred when sJIA, KD, and CTD were present at first diagnosis. Hypotension was present in almost 30% of cases of MAS at onset, which could constitute a predictive aspect of the diagnosis. Increased serum ferritin, ferritin/ESR ratio, AST, and LDH, along with decreased serum albumin, could be predictive elements of the emergence of sJIA-MAS [201].
Ganeva et al. investigated biomarkers and their relationships with disease progression in the first year after diagnosis in a cohort of 266 JIA patients. Clinical symptoms and laboratory data were recorded quarterly in the first year and semiannually thereafter. The investigated biomarkers were increased in most JIA patients compared to healthy controls. In 10 subjects with sJIA, high concentrations of CRP, ESR, IL-18, S100A8/A9, and S100A12 were recorded compared to subjects with other JIA subtypes. Higher levels of ESR, G-CSF, IL-6, IL-17A, and TNF at baseline were predictive of a high risk of uninterrupted disease still active after 12 months. Elevated serum levels of CRP, S100A8/A9, and S100A12, as well as high ESR at baseline, were correlated with the necessity of intensified management with biological DMARDs in the first year of follow-up [202].
2.3.16. Serum Amyloid A
Serum amyloid A is an acute-phase protein belonging to the group of apolipoproteins, synthesized by macrophages, adipocytes, synoviocytes, and especially hepatocytes. During the acute inflammatory process, it can increase significantly and has a chemotactic capacity for neutrophils and mast cells. SAA participates in the release of TNF-α, IL-1, IL-6, and granulocyte colony-stimulating factor (G-CSF); it stimulates angiogenesis, matrix metalloproteinases, and tissue factors. It is validated as a biomarker of the activity of diseases such as RA, JIA, rheumatic polymyalgia, ankylosing spondylitis, and sarcoidosis [203,204,205].
The main role of SAA in the pathogenesis of rheumatic inflammatory diseases is recognized by contemporary studies demonstrating its involvement in activating the inflammasome cascade and attracting IL-17-producing T-helper lymphocytes. From the earliest findings, it was clear that SAA could be used to monitor and assess the severity of disease activity in patients with rheumatoid arthritis and secondary amyloidosis. SAA has been observed to be a valuable biomarker in the immune pathology of rheumatic diseases, especially in the case of subclinical inflammation. Unlike other biomarkers, recent research has recognized the benefits of SAA monitoring in biological immunotherapy, especially after the implementation of proteomic techniques. Modern techniques encourage the research of SAA and its isoforms as sensitive biomarkers in rheumatic diseases—and even the development of therapeutic agents with SAA as a target. Another argument for recognizing its potential is given by recent findings that have shown that SAA is a very useful biomarker in monitoring severe coronavirus disease [206,207,208].
The clinical utility of SAA in both predicting and monitoring the response to therapy in patients with JIA has been less explored in recent decades. Dev et al. conducted a study on 50 newly diagnosed cases of JIA of all subtypes, along with 40 healthy controls, to find out whether SAA could be a biomarker of disease activity in JIA. The results showed that SAA concentrations were significantly higher in patients with JIA compared to healthy controls. Elevated SAA levels were positively correlated with disease activity in JIA, but not with CRP or ESR levels. The study showed that SAA is a more sensitive laboratory marker than ESR and CRP for assessing the presence of active joints [209].
2.3.17. Leucine-Rich α2-Glycoprotein
Leucine-rich α2-glycoprotein (LRG) is a plasma glycoprotein whose physiological role is being studied. Recent data have highlighted the role of LRG in the differentiation and proliferation of Th17 lymphocytes and in the process of neovascularization by acting on the TGF-β in endothelial cells [210,211].
LRG is synthesized by the hepatocytes, and its concentration increases during inflammation under the action of the pro-inflammatory cytokines IL-1β, IL-6, and TNF-α, making it useful in diagnosing a multitude of inflammatory diseases (e.g., intestinal, rheumatoid arthritis, AOSD, sJIA, etc.). As part of the family of acute-phase proteins, similar to CRP and SAA, proteomic studies have shown that LRG is a sensitive biomarker in patients with rheumatoid arthritis, which increases during disease activity and can be reduced after anti-TNF-α therapy [212,213,214].
Leucine-rich α-2 glycoprotein 1 (LRG1) is an important member of the leucine-rich repetitive sequence protein family and is implicated in many human conditions, including cancer, diabetes, cardiovascular disease, neurological disease, inflammatory disorders, etc. It has recently been proven that LRG1 is compacted into the granules of human neutrophils and secreted upon the neutrophils’ stimulation to adjust the micro-medium. Serum LRG1 has been recognized as a practical biomarker for monitoring disease activity in patients with rheumatoid arthritis, lupus nephritis, adult-onset Still’s disease, and vasculitis. LRG1 is a recently detected important upstream signaling molecule of TGF-β that influences multiple pathophysiological mechanisms through the TGF-β signaling pathway [215,216,217].
In an early study, Shimizu et al. investigated the attributes of LRG as a biomarker for controlling sJIA activity during IL-6-targeting therapy. Serum LRG concentrations were investigated in four JIA patients treated with TCZ and correlated with clinical parameters and pro-inflammatory cytokines, including IL-18, IL-6, neopterin, and TNF-α receptor types I and II. Serum LRG concentrations increased simultaneously with the progression of active sJIA and the onset of MAS. Even when the disease was no longer active, serum LRG concentrations remained higher than normal values. A significant positive reciprocity was observed between serum levels of LRG and pro-inflammatory cytokines. Serum concentrations of LRG could probably be used as a unique biomarker for monitoring the progress of sJIA activity during IL-6 blocking therapy [218].
In another study, Shimizu et al. explored serum levels of LRG, IL-6, IL-18, sTNFR-I, and sTNFR-II in 59 patients with sJIA, 15 with other subtypes of JIA, 7 with KD, 7 with influenza A infection, 7 with infection with enterohemorrhagic Escherichia coli (EHEC), and 20 healthy controls (HCs) to whom the clinical symptoms were also compared. The serum LRG concentrations in active sJIA were much higher compared to other subtypes of JIA, EHEC, influenza patients, and HCs. Serum LRG values were normalized in patients with inactive sJIA after treatment and were positively correlated with those of serum CRP and serum ferritin. The authors claimed that serum LRG concentrations reflected the level of sJIA activity and could be useful as a biomarker for monitoring sJIA disease activity [219].
2.3.18. Adenosine Deaminase 2
Lee et al. evaluated the serum levels of adenosine deaminase 2 (ADA2)—a protein with as-yet unknown function released mainly by monocytes and macrophages—as a novel biomarker of MAS in 324 healthy children and adults, and compared these serum levels with those of 173 children with inflammatory and immune-mediated diseases, including sJIA and nsJIA, KD, SLE, and JDM. The results showed increased levels of ADA2 in the peripheral blood of patients with sJIA and MAS; the activity of this protein was strongly correlated with the levels of ferritin, IL-18, and CXCL9 inducible by IFN-γ, but inconsistent with inflammatory markers (i.e., CRP and ESR). IL-18 and IFN-γ generated increased production of ADA2 by peripheral blood mononuclear cells, and ADA2 was abundant in MAS hemophagocytes. In conclusion, the authors noted the usefulness of research on serum ADA2 levels as a future biomarker for the diagnosis of sJIA-MAS [220].
The chemokines and other biomarkers discussed above are highlighted in Table 4.

Table 4.
Chemokines and other biomarkers for the diagnosis, activity, and prognosis of sJIA and MAS.
3. Final Remarks and Conclusions
The COVID-19 pandemic—along with the associated CS—was and still is a challenge for 21st century medicine. Concretely, in the current crisis, solutions were sought, and initially an approach was even tried using some drugs inspired by rheumatology, including pediatric rheumatology. The emergence of multisystem inflammatory syndrome in children (MIS-C) was another test for pediatricians, because children and adolescents exposed to the SARS-CoV-2 virus—who, even if they initially only had mild forms of the disease, or were only contacts of people who were infected— developed a fulminant progression of a disease very similar to MAS after about 2–4 weeks, which put their lives in danger [11,221].
Being increasingly common, hyperinflammatory syndromes in pediatrics—including sJIA-MAS—rely on new biological disease-modifying anti-rheumatic drugs (bDMARDs) targeting various cytokines involved in the pathophysiological mechanisms of the diseases, which pediatric rheumatologists have begun to administer successfully more and more often. A CS, or hypercytokinemia, is initially a physiological response in which the innate immune system triggers an exaggerated and extreme discharge of pro-inflammatory signaling molecules, which would normally represent the immune system’s response to infection or immunotherapy, but the unexpected huge amounts of cytokines can cause multiorgan failure and death. There is still no single universally accepted definition of CS, as it differs depending on the corresponding inflammatory response [222].
CSS is an umbrella term for a systemic inflammatory condition [223] that results in the killing of both healthy and diseased cells, manifested by fever, fatigue, headache, rash, and muscle and/or joint aches, and may advance to more serious symptoms such as hypotension, tachycardia, vasodilatation, edema, hypoxemia, seizures, and even coma. Prompt and correct diagnosis, as well as the appropriate emergency management through immunosuppressive drugs, can hinder the irreversible multiorgan failure and save the patient’s life.
In pediatric pathology, within CSS, MAS can appear relatively frequently in children with sJIA—considered a prototype for this major complication that can even threaten their lives, just like MIS-C after infection with the SAR-CoV-2 virus during the COVID-19 pandemic. The major difficulty for pediatric rheumatologists is to make the correct differential diagnosis between active sJIA and MAS that has recently started or is already in progress, because these two entities have common clinical characteristics such as high fever, skin eruptions, and other systemic signs of inflammation which, if not identified in time, will evolve into hepatosplenic dysfunction, pancytopenia, consumption coagulopathy, hyperferritinemia and, finally, multiorgan failure [16,223,224].
sJIA-MAS continues to be a major problem in pediatric rheumatology due to its morbidity, mortality, and lack of standardization for early and successful diagnosis.
To be as close as possible to reality and clinical requirements, new model biomarkers are needed for an individualized approach to ensure the accurate assessment and management of patients with sJIA. In the current clinical practice generalized to date, a certain number of specific biomarkers for JIA have already been adopted by expert guidelines and are currently applied, such as ESR, CRP, serum ferritin, RF, HLA-B27 molecule, ANA, etc., which can help clinicians to better discriminate between the different subtypes and anticipate the response to the applied therapy, as well as relapses, which ideally should indicate the most appropriate medication for that patient and the point at which the therapy can be safely stopped after remission [225].
Already, in many clinics around the world that have significant funding for research and patient care—but especially those with ultrahigh-performance equipment and special analysis kits—new biomarkers are used to diagnose sJIA-MAS as quickly and accurately as possible and to predict the response to the established emergency treatment in this aggravating, disastrous, and potentially deadly complication of sJIA, which may be misleading for pediatric clinicians because it is so hard to diagnose and manage.
According to clinical studies, more than one-third of patients with sJIA may suffer from a subclinical form of MAS which, if recognized in time, could be managed long before reaching fully developed sJIA-MAS.
In children with subclinical sJIA-MAS, some biomarkers that highlight the extent of T-cell activation and are a measure for the escalation of phagocytic macrophages—such as sCD25 (sIL2RA) and sCD163—should be investigated to support the recognition of MAS.
In 2007, Bleesing et al. showed that elevated serum concentrations of sCD25 and sCD163 may be predictive biomarkers of sJIA-MAS and could help detect patients with subclinical MAS [148].
A few years later, Reddy et al. indicated that the presence of sCD25 levels > 7500 pg/mL can be considered to be a useful biomarker in the detection of patients with subclinical MAS [149].
In 2018, Sakumura et al., studying 63 patients with sJIA, concluded that sCD163 is a potential biomarker for the evaluation of disease activity and remission, faithfully reflecting macrophage activation in sJIA—even for patients treated with TCZ; its levels increased dramatically with the progression of MAS in sJIA [150].
In 2021, Verweyen et al. studied how distinct gene expression signatures are able to characterize strong clinical responders versus non-responders to canakinumab therapy in sJIA patients, and indicated that a signature including upregulated CD163 expression was associated with the non-response to this treatment [151].
Two other important biomarkers are the chemokines CXCL9 and CXCL10, which play an important role in the local recruitment of CD8+ T cells to tissues. Some scientific research has shown high serum concentrations of IFN-γ and IFN-γ-induced cytokines such as IL-18BP, CXCL9, and CXCL10 in sJIA-MAS, compared to their concentrations in active sJIA, inactive disease, or healthy controls. Serum values of CXCL9 and CXCL10 became smaller with remission of sJIA-MAS. Thus, these two biomarkers highlight the tissue activity of the IFN-γ pathway, and their ability to recruit CD8+ T cells—i.e., CXCL9 and CXCL10—correlates very well with the laboratory parameters of MAS, providing a strongly evidenced reason to act for in the vent of an IFN-γ blockage in sJIA-MAS [16,21,128,129].
According to other recent studies, CXCL9 has been shown to display the most significant increase after the onset of sJIA-MAS, and monitoring its serum levels would allow physicians to conduct surveillance of sJIA-MAS.
Concerning the response to canakinumab in sJIA, a differential regulation of the IL-18–IFN-γ–CXCL9 axis has been proven in patients with sJIA, and the higher the IL-18/CXCL9 and IFN-γ/CXCL9 ratios, the better the clinical response to canakinumab treatment [146,147].
Neopterin is released by macrophages stimulated by IFN-γ and could be a useful biomarker of immune system activation that can indicate disease activity in sJIA-MAS, while also allowing the diagnosis of the transition to MAS in active sJIA [143].
Neopterin could be an accurate biomarker for the evaluation of underlying inflammatory processes and the flares of sJIA, together with traditional laboratory parameters for diagnosis, clinical follow-up, and response to therapy. Concentrations of neopterin, IFN-γ, CXCL9, CXCL10, sCD25, and IL-18 can be used as biomarkers to determine the onset of MAS in active sJIA—even in children who have been prescribed TCZ [67,130,137,138].
Serum concentrations of MRP8/14 have been confirmed as an important biomarker for the diagnosis of sJIA, ensuring early discrimination from other inflammatory conditions so that targeted therapy for the patient’s benefit can be started immediately when the “window of opportunity” is open, stopping a possible persistent chronic outcome which, until the discovery of bDMARDs, was inevitable in more than 50% of patients [17,18,58,153].
The role of NK cells in sJIA-MAS has been widely investigated, but is still difficult to fully understand due to the small size or insufficient number of studies and conflicting conclusions. However, for sJIA-MAS, no strong genetic or transcriptional impairments in NK cells’ killing pathways were detected. Functional deficits of NK cells have been identified in sJIA-MAS due to inflammation-induced NK cell exhaustion. The high constitutive levels of cytokines (e.g., IL-6 and IL-18) could trigger the cytokine-induced NK cell dysfunction that fails to stop the immune response; thus, continuous inflammatory processes are generated [226].
The dramatic decrease in the number of figurative elements of the blood, coagulation abnormalities, significant liver cytolysis, and the increase in LDH as well as serum ferritin are important laboratory data for the identification of patients with sJIA-MAS. A concentration above 3500 μg/L has 85% sensitivity and 97% predictability for sJIA-MAS, and extreme hyperferritinemia detected at the time of sJIA diagnosis may be a biomarker to foreshadow sJIA-MAS [227].
Serum ferritin is accepted as an acute-phase reactant along with CRP, ESR, Fg, etc. In sJIA, high levels of ferritin and low levels of fibrinogen are inceptive evidence of inevitable sJIA-MAS [228].
High levels of platelets, CRP, and serum ferritin are specific biomarkers for the diagnosis MAS. Elevated serum ferritin concentrations in sJIA patients were correlated with active disease during progression, even when the disease activity decreased. Its status as a biomarker of disease activity is well-known, but the serum ferritin/ESR ratio may be a more valuable biomarker than ferritin alone in differentiating MAS from new-onset sJIA [200,229,230].
In discriminating between sJIA with and without MAS, the importance of the ferritin/ESR ratio, as well as the early identification of significant changes in serum ferritin and neutrophil, leukocyte, and platelet counts, is essential in the early stages of MAS, as evidenced by other recent studies [231].
Because many proposed new biomarkers will be difficult to implement in current clinical use due to their high cost or difficulties in implementation, an ideal candidate could be chosen from among the conventional laboratory biomarkers used already for assessing the evolution of clinical symptoms, such as the ferritin/ESR ratio, neutrophil/lymphocyte ratio, etc.
A neutrophil/lymphocyte ratio greater than 5.23 predicts the need for early biological treatment in sJIA and MAS [232].
Although sJIA-MAS was classified by expert consensus in 2016, today, after 6 years, new research insights are essential.
Biomarkers in sJIA-MAS could be relevant to differentiate disease subtypes, to assess disease status and activity, and to predict responses to initial treatment. They can also be very valuable tools in the pediatric rheumatologist’s decision to stop medication in a patient who has entered clinical remission.
There is no updated consensus regarding the validation, standardization, and reproducibility of methods for the determination and implementation of the most predictive biomarkers for the diagnosis of sJIA-MAS, the progress of MAS, the emergency treatment to be applied, the follow-up of the response, and the change of treatment in the event of emergency to save the life of the patient.
It was suggested that the limitations imposed by each method of determining a single biomarker could be overcome by using a comprehensive picture of the pathological aspects regarding the evolution of MAS and the very rapid use of several clinical parameters and laboratory data to include many more biomarkers.
A clinically useful biomarker, in addition to being correctly measured, must have diagnostic and prognostic value in order to correlate well with the disease extent. It must also be reasonably stable, present in an easily accessible sample, and its measurement should be cost-effective. As of more recently, a multiple factor analysis that allows for simultaneous examination of multiple parameters—classified according to their physiological meaning—could be applied. On the other hand, in sJIA-MAS, the choice of biomarkers to be considered in the global assessment score should dictate their clinical relevance in the examined patients.
Machine learning analysis was efficient in differentiating between JIA patients and healthy controls, with ~90% precision. Analysis of the immune profiles could reveal immunological disturbances in patients with different JIA subtypes, but was much more important in patients with sJIA and in those with active disease. Such results open new perspectives for large-scale immunophenotyping in longitudinal studies of JIA, which will be able to better distinguish between JIA patients and healthy subjects, while the addition of machine learning could foretell the response to treatment [233].
The clinical significance of the biomarkers in sJIA and sJIA-MAS results from a comprehensive analysis of the biomarkers revealed in all of the abovementioned revised studies, and is highlighted as a synthesis in the following diagram (Figure 1):
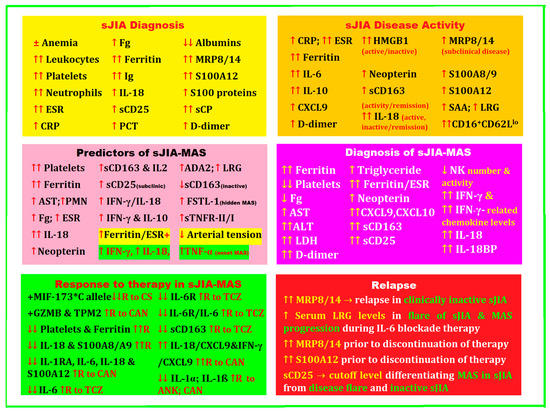
Figure 1.
Biomarkers in sJIA and sJIA-MAS: diagnosis; disease activity; predictors of sJIA-MAS; diagnosis of sJIA-MAS; response to therapy in sJIA-MAS; and relapse. (Figure 1 was conceptualized and drawn by L.M.A. using Microsoft Paint 3D for Windows 10). Legend: [↑ = increasing/high; ↑↑ = very high; ↓ = decreasing/low; ↓↓ = very low; “+” = present; ± = present/absent; R = response].
Since the processes in sJIA-MAS take place extremely rapidly, it is imperative that the responsible pediatrician has adequate biomarkers to label the disease, initiate therapy, test correctly and quickly for response, and immediately prescribe a new drug if the patient does not respond.
sJIA and sJIA-MAS remain a call to participate in an open competition for clinicians to solve all of the questions that do not yet have a satisfactory answer, e.g., what are the most valuable biomarkers for an immediate and correct diagnosis? Which is the pathogenic pathway that needs to be blocked first? What is the best management practice? How should treatment be individualized and balanced?
The clinical significance of biomarkers in sJIA-MAS should be delineated by the latest scientific research, reanalyzed by a group of experts to draw new guidelines in clinical practice through an objective analysis of all of the relevant predictive biomarkers with great sensitivity and specificity, and to offer real support to clinicians to make the best decisions and choices under certain conditions—especially in the COVID-19 era.
Author Contributions
Conceptualization, L.M.A. and C.A.; writing—original draft preparation, L.M.A.; review and editing, G.L. and L.M.A. All authors have read and agreed to the published version of the manuscript.
Funding
There was no special funding for this review article.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data supporting reported results can be found by the first author L.M.A.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| ALL | Acute lymphoblastic leukemia |
| ADA2 | Adenosine deaminase 2 |
| AOSD | Adult-onset Still’s disease |
| AGEs | Advanced glycation end products |
| AHSP | Alpha hemoglobin-stabilizing protein |
| ACR | American College of Rheumatology |
| ACR 50 | American College of Rheumatology score = at least 50% improvement in disease activity |
| ANK | Anakinra |
| ANAs | Antinuclear antibodies |
| Arg-1 | Arginase-1 |
| AST | Aspartate aminotransferase |
| ADs | Autoimmune diseases |
| AID | Autoinflammatory disease |
| bDMARDs | Biological disease-modifying anti-rheumatic drugs |
| S100A12 | Calgranulin C |
| CP, or S100A8/A9, or MRP8/14 | Calprotectin |
| CAN | Canakinumab |
| CASP8 | Caspase-8 |
| CCL23 | C-C motif chemokine ligand 23 |
| CDC | Centers for Disease Control and Prevention |
| CNS | Central nervous system |
| CXCL1…11 | Chemokine (C-X-C motif) ligand 1…11 |
| CCL2 | Chemokine (C-C motif) ligand 2 |
| CXCL9 | Chemokine (C-X-C motif) ligand 9 |
| CXCL10 | Chemokine (C-X-C motif) ligand 10 |
| CX3CR1 | Chemokine (C-X3-C motif) receptor 1 |
| CID | Clinically inactive disease |
| CD | Cluster of differentiation |
| CD163 | Cluster of differentiation 163 |
| CLPs | Common lymphoid progenitors |
| CBC | Complete blood count |
| CTD | Connective tissue disease |
| cNK | Conventional NK |
| csDMARDs | Conventional synthetic disease-modifying anti-rheumatic drugs |
| CS | Corticosteroid |
| CRP | C-reactive protein |
| CSS | Cytokine storm syndrome |
| DAMP | Damage-associated molecular pattern |
| DNA | Deoxyribonucleic acid |
| DEGs | Differentially expressed genes |
| DMARDs | Disease-modifying anti-rheumatic drugs |
| EHEC | Enterohemorrhagic Escherichia coli |
| ELISA | Enzyme-linked immunosorbent assay |
| EBV | Epstein–Barr virus |
| EBV-HLH | Epstein–Barr-virus-induced hemophagocytic lymphohistiocytosis |
| ESR | Erythrocyte sedimentation rate |
| fHLH | Familial hemophagocytic lymphohistiocytosis |
| FMF | Familial Mediterranean fever |
| FasL | Fas ligand |
| FUO | Fever of unknown origin |
| Fg | Fibrinogen |
| FSTL-1 | Follistatin-like protein 1 |
| FDA | Food and Drug Administration |
| CFA | Freund’s complete adjuvant |
| GEO | Gene Expression Omnibus |
| G-CSF | Granulocyte colony-stimulating factor |
| GM-CSF | Granulocyte–macrophage colony-stimulating factor |
| GZMK | Granzyme K |
| HCs | Healthy controls |
| HSC | Hematopoietic stem cell |
| HLH | Hemophagocytic lymphohistiocytosis |
| HMGB1 | High-mobility group box-1 |
| hsCRP | High-sensitivity C-reactive protein |
| HLA | Human leukocyte antigen |
| Igs | Immunoglobulins |
| ILCs | Innate lymphoid cells |
| ILC1 | “Unconventional” NK cells |
| ICAM-5 | Intercellular adhesion molecule 5 |
| IFN-α | Interferon-alpha |
| IFN-γ | Interferon-gamma |
| IRF5 | Interferon regulatory factor 5 |
| IL-1, IL-6, IL-18 | Interleukin 1, 6, 18 |
| IL-18Rβ | IL-18β receptor |
| IL-1 alpha, IL-1α; IL-1 beta, IL-1β | Interleukin 1 alpha, beta |
| IL-1Ra | Interleukin-1 receptor antagonist |
| IL10RA | Interleukin-10 receptor, alpha subunit |
| IL6ST | Interleukin-6 signal transducer |
| IL-18BP | IL-18-binding protein |
| ICAM-1 | Intracellular adhesion molecule 1 |
| IV | Intravenous |
| JDM | Juvenile dermatomyositis |
| JIA | Juvenile idiopathic arthritis |
| JADAS | Juvenile Arthritis Disease Activity Score |
| KD | Kawasaki disease |
| LDH | Lactate dehydrogenase |
| LRG | Leucine-rich α2-glycoprotein |
| LRG1 | Leucine-rich α-2 glycoprotein 1 |
| LPSs | Lipopolysaccharides |
| CD62L | L-selectin |
| LCLs | Lymphoblastoid cell lines |
| M | Macrophage |
| M1 | M1 macrophages (encourage inflammation) |
| M2 | M2 macrophages (decrease inflammation and encourage tissue repair) |
| MAS | Macrophage activation syndrome |
| sJIA-MAS | Macrophage activation syndrome in sJIA |
| MIF | Macrophage migration inhibitory factor |
| MHC | Major histocompatibility complex |
| MMP1 | Matrix metalloproteinase 1 |
| MMP3 | Matrix metalloproteinase 3 |
| MEFV | Mediterranean fever |
| MTX | Methotrexate |
| MAPKs | Mitogen-activated protein kinases |
| MCP1 | Monocyte chemoattractant protein 1 |
| MIS-C | Multisystem inflammatory syndrome in children |
| MPO–DNA complexes | Myeloperoxidase–DNA complexes |
| MRP | Myeloid-related protein |
| MRP8 | Myeloid-related protein 8 |
| MRP14 | Myeloid-related protein 14 |
| NCR2 or NKp44 | Natural cytotoxicity-triggering receptor 2 |
| NK | Natural killer |
| NKT | Natural killer T |
| NCAM or CD56 | Neural cell adhesion molecule, also called CD56 |
| NETs | Neutrophil extracellular traps |
| NETosis | NET activation and release |
| NLR | NOD-like receptor |
| NSAIDs | Non-steroidal anti-inflammatory drugs |
| nsJIA | Non-systemic forms of JIA |
| NFIL3 | Nuclear factor, interleukin 3-regulated gene |
| NF-κB or NF-kappaB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NOD | Nucleotide-binding oligomerization |
| NLRP | Nucleotide-binding oligomerization domain, leucine-rich repeat and Pyrin domain |
| NLRP3 | Nucleotide-binding oligomerization domain-like receptor pyrin domain-containing protein 3 |
| NLRs | Nucleotide-binding oligomerization domain-like receptors = NOD-like receptors |
| No. | Number; Latin “numero”, the ablative case of “numerus” |
| PAMP | Pathogen-associated molecular pattern |
| PRRs | Pattern-recognition receptors |
| PRINTO | Pediatric Rheumatology International Trials Organization |
| PRF1 | Perforin 1 |
| PBMC | Peripheral blood mononuclear cell |
| PMLs or PMNLs | Polymorphonuclear leukocytes |
| PMN | Polymorphonuclear neutrophil |
| + | Present |
| ± | Present/absent |
| pHLH | Primary hemophagocytic lymphohistiocytosis |
| PCT | Procalcitonin |
| PD-L1 | Programmed death ligand-1 |
| PKC | Protein kinase C |
| qRT-PCR | Quantitative reverse-transcription polymerase chain reaction |
| ROS | Reactive oxygen species |
| TNFSF11 | Receptor activator of nuclear factor-κB ligand, RANKL |
| RAGE | Receptor for advanced glycation end products |
| rIL-1Ra | Recombinant IL-1 receptor antagonist |
| rr | Reference range |
| R | Response |
| RMD | Rheumatic and musculoskeletal disease |
| RF | Rheumatoid factor |
| S100A9 | S100 calcium-binding protein A9 |
| MS score | Score for sJIA-MAS |
| sec-HLH | Secondary hemophagocytic lymphohistiocytosis |
| SAA | Serum amyloid A |
| sCP | Serum calprotectin |
| sPCT | Serum procalcitonin |
| SIF | Severe infections |
| SNP | Single-nucleotide polymorphism |
| sCD163 | Soluble cluster of differentiation 163 |
| sCD25 or sIL2RA | Soluble IL-2 receptor alpha chain, or soluble IL2RA |
| sRAGE | Soluble receptor for advanced glycation end products |
| sTNFR-I | Soluble tumor necrosis factor receptor I |
| sTNFR-II | Soluble tumor necrosis factor receptor II |
| sTNF-R II/I | Soluble TNF receptor II/I |
| SF | Synovial fluid |
| sJIA | Systemic juvenile idiopathic arthritis |
| SLE | Systemic lupus erythematosus |
| TCR | T-cell receptor |
| Th cells, CD4+ or CD4-positive | T-helper cells, or CD4+ cells, or CD4-positive cells |
| trNK | Tissue-resident (tr)NK |
| TWEAK | TNF-like weak inducer of apoptosis |
| TCZ | Tocilizumab |
| TLR | Toll-like receptor |
| TGF-β | Transforming growth factor beta |
| TAMs | Tumor-associated macrophages |
| TNF-α | Tumor necrosis factor alpha |
| TNFR1 | Tumor necrosis factor receptor 1 |
| FcγRIII | Type III Fcγ receptor |
| WGCNA | Weighted gene co-expression network analysis |
| WT | Wild type |
| Increasing/high | ↑ |
| Very high | ↑↑ |
| Decreasing/low | ↓ |
| Very low | ↓↓ |
References
- Hinze, C.; Gohar, F.; Foell, D. Management of juvenile idiopathic arthritis: Hitting the target. Nat. Rev. Rheumatol. 2015, 11, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Ravelli, A.; Consolaro, A.; Horneff, G.; Laxer, R.M.; Lovell, D.J.; Wulffraat, N.M.; Akikusa, J.D.; Al-Mayouf, S.M.; Antón, J.; Avcin, T.; et al. Treating juvenile idiopathic arthritis to target: Recommendations of an international task force. Ann. Rheum. Dis. 2018, 77, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Ailioaie, L.M.; Litscher, G. Molecular and Cellular Mechanisms of Arthritis in Children and Adults: New Perspectives on Applied Photobiomodulation. Int. J. Mol. Sci. 2020, 21, 6565. [Google Scholar] [CrossRef] [PubMed]
- Guzman, J.; Oen, K.; Huber, A.M.; Watanabe Duffy, K.; Boire, G.; Shiff, N.; Berard, R.A.; Levy, D.M.; Stringer, E.; Scuccimarri, R.; et al. The risk and nature of flares in juvenile idiopathic arthritis: Results from the ReACCh-Out cohort. Ann. Rheum. Dis. 2016, 75, 1092–1098. [Google Scholar] [CrossRef] [PubMed]
- Horton, D.B.; Salas, J.; Wec, A.; Kohlheim, M.; Kapadia, P.; Beukelman, T.; Boneparth, A.; Haverkamp, K.; Mannion, M.L.; Moorthy, L.N.; et al. Making Decisions About Stopping Medicines for Well-Controlled Juvenile Idiopathic Arthritis: A Mixed-Methods Study of Patients and Caregivers. Arthritis Care Res. 2021, 73, 374–385. [Google Scholar] [CrossRef]
- Gerss, J.; Tedy, M.; Klein, A.; Horneff, G.; Miranda-Garcia, M.; Kessel, C.; Holzinger, D.; Stanevica, V.; Swart, J.F.; Cabral, D.A.; et al. Prevention of disease flares by risk-adapted stratification of therapy withdrawal in juvenile idiopathic arthritis: Results from the PREVENT-JIA trial. Ann. Rheum. Dis. 2022, 81, 990–997. [Google Scholar] [CrossRef]
- Lee, J.; Schneider, R. Systemic juvenile idiopathic arthritis. Pediatr Clin. N. Am. 2018, 65, 691–709. [Google Scholar] [CrossRef]
- Barut, K.; Adrovic, A.; Sahin, S.; Tarcin, G.; Tahaoglu, G.; Koker, O.; Yildiz, M.; Kasapcopur, O. Prognosis, complications and treatment response in systemic juvenile idiopathic arthritis patients: A single-center experience. Int. J. Rheum. Dis. 2019, 22, 1661–1669. [Google Scholar] [CrossRef]
- Ter Haar, N.M.; van Dijkhuizen, E.H.P.; Swart, J.F.; van Royen-Kerkhof, A.; El Idrissi, A.; Leek, A.P.; de Jager, W.; de Groot, M.C.H.; Haitjema, S.; Holzinger, D. Treatment to Target Using Recombinant Interleukin-1 Receptor Antagonist as First-Line Monotherapy in New-Onset Systemic Juvenile Idiopathic Arthritis: Results From a Five-Year Follow-Up Study. Arthritis Rheumatol. 2019, 71, 1163–1173. [Google Scholar] [CrossRef]
- Newswise. The American College of Rheumatology Releases Two Updated Guidelines for Treatment of Juvenile Idiopathic Arthritis. Available online: https://www.newswise.com/articles/the-american-college-of-rheumatology-releases-two-updated-guidelines-for-treatment-of-juvenile-idiopathic-arthritis (accessed on 10 July 2022).
- Ailioaie, L.M.; Ailioaie, C.; Litscher, G. Implications of SARS-CoV-2 Infection in Systemic Juvenile Idiopathic Arthritis. Int. J. Mol. Sci. 2022, 23, 4268. [Google Scholar] [CrossRef]
- Moradinejad, M.H.; Ziaee, V. The incidence of macrophage activation syndrome in children with rheumatic disorders. Minerva Pediatr. 2011, 63, 459–466. [Google Scholar] [PubMed]
- Ravelli, A.; Grom, A.A.; Behrens, E.M.; Cron, R.Q. Macrophage activation syndrome as part of systemic juvenile idiopathic arthritis: Diagnosis, genetics, pathophysiology and treatment. Genes Immun. 2012, 13, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Schulert, G.S.; Grom, A.A. Pathogenesis of macrophage activation syndrome and potential for cytokine-directed therapies. Annu. Rev. Med. 2015, 66, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Ravelli, A.; Minoia, F.; Davì, S.; Horne, A.; Bovis, F.; Pistorio, A.; Aricò, M.; Avcin, T.; Behrens, E.M.; De Benedetti, F.; et al. 2016 Classification Criteria for Macrophage Activation Syndrome Complicating Systemic Juvenile Idiopathic Arthritis: A European League Against Rheumatism/American College of Rheumatology/Paediatric Rheumatology International Trials Organisation Collaborative Initiative. Arthritis Rheumatol. 2016, 68, 566–576. [Google Scholar] [CrossRef]
- Schneider, R.; Canny, S.P.; Mellins, E.D. Cytokine Storm Syndrome Associated with Systemic Juvenile Idiopathic Arthritis. In Cytokine Storm Syndrome, 1st ed.; Cron, R.Q., Behrens, E.M., Eds.; Springer Nature Switzerland AG: Cham, Switzerland, 2019; pp. 349–379. [Google Scholar] [CrossRef]
- Pardeo, M.; Vastert, S.J.; De Benedetti, F. It is about time: The first validated biomarker for early diagnosis of systemic juvenile idiopathic arthritis. Rheumatology 2021, 61, 2724–2725. [Google Scholar] [CrossRef]
- Park, C.; Miranda-Garcia, M.; Berendes, R.; Horneff, G.; Kuemmerle-Deschner, J.; Ganser, G.; Huppertz, H.I.; Minden, K.; Haas, J.P.; Jansson, A.F.; et al. MRP8/14 serum levels as diagnostic markers for systemic juvenile idiopathic arthritis in children with prolonged fever. Rheumatology 2022, 61, 3082–3092. [Google Scholar] [CrossRef]
- Ruperto, N.; Brunner, H.I.; Quartier, P.; Constantin, T.; Wulffraat, N.; Horneff, G.; Brik, R.; McCann, L.; Kasapcopur, O.; Rutkowska-Sak, L.; et al. Two randomized trials of canakinumab in systemic juvenile idiopathic arthritis. N. Engl. J. Med. 2012, 367, 2396–2406. [Google Scholar] [CrossRef]
- De Benedetti, F.; Meazza, C.; Vivarelli, M.; Rossi, F.; Pistorio, A.; Lamb, R.; Lunt, M.; Thomson, W.; Ravelli, A.; Donn, R.; et al. Functional and prognostic relevance of the -173 polymorphism of the macrophage migration inhibitory factor gene in systemic-onset juvenile idiopathic arthritis. Arthritis Rheum. 2003, 48, 1398–1407. [Google Scholar] [CrossRef]
- Yanagimachi, M.; Naruto, T.; Miyamae, T.; Hara, T.; Kikuchi, M.; Hara, R.; Imagawa, T.; Mori, M.; Sato, H.; Goto, H.; et al. Association of IRF5 polymorphisms with susceptibility to macrophage activation syndrome in patients with juvenile idiopathic arthritis. J. Rheumatol. 2011, 38, 769–774. [Google Scholar] [CrossRef]
- Canna, S.W.; de Jesus, A.A.; Gouni, S.; Brooks, S.R.; Marrero, B.; Liu, Y.; DiMattia, M.A.; Zaal, K.J.; Sanchez, G.A.; Kim, H.; et al. An activating NLRC4 inflammasome mutation causes autoinflammation with recurrent macrophage activation syndrome. Nat. Genet. 2014, 46, 1140–1146. [Google Scholar] [CrossRef]
- Ombrello, M.J.; Remmers, E.F.; Tachmazidou, I.; Grom, A.; Foell, D.; Haas, J.P.; Martini, A.; Gattorno, M.; Özen, S.; Prahalad, S.; et al. International Childhood Arthritis Genetics (INCHARGE) Consortium. HLA-DRB1*11 and variants of the MHC class II locus are strong risk factors for systemic juvenile idiopathic arthritis. Proc. Natl. Acad. Sci. USA 2015, 112, 15970–15975. [Google Scholar] [CrossRef]
- De Silvestri, A.; Capittini, C.; Poddighe, D.; Marseglia, G.L.; Mascaretti, L.; Bevilacqua, E.; Scotti, V.; Rebuffi, C.; Pasi, A.; Martinetti, M.; et al. HLA-DRB1 alleles and juvenile idiopathic arthritis: Diagnostic clues emerging from a meta-analysis. Autoimmun. Rev. 2017, 16, 1230–1236. [Google Scholar] [CrossRef] [PubMed]
- Arthur, V.L.; Shuldiner, E.; Remmers, E.F.; Hinks, A.; Grom, A.A.; Foell, D.; Martini, A.; Gattorno, M.; Özen, S.; Prahalad, S.; et al. IL1RN Variation Influences Both Disease Susceptibility and Response to Recombinant Human Interleukin-1 Receptor Antagonist Therapy in Systemic Juvenile Idiopathic Arthritis. Arthritis Rheumatol. 2018, 70, 1319–1330. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Qu, H.; Zhang, S.; Qi, X.; Hakonarson, H.; Xia, Q.; Li, J. The Multi-Omics Architecture of Juvenile Idiopathic Arthritis. Cells 2020, 9, 2301. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Guo, R.; Wang, Y.F.; Yang, W.; Li, R.; Lu, L. Application of Weighted Gene Coexpression Network Analysis to Identify Key Modules and Hub Genes in Systemic Juvenile Idiopathic Arthritis. BioMed Res. Int. 2021, 2021, 957569. [Google Scholar] [CrossRef]
- Zhang, M.; Dai, R.; Zhao, Q.; Zhou, L.; An, Y.; Tang, X.; Zhao, X. Identification of Key Biomarkers and Immune Infiltration in Systemic Juvenile Idiopathic Arthritis by Integrated Bioinformatic Analysis. Front. Mol. Biosci. 2021, 8, 681526. [Google Scholar] [CrossRef]
- Ren, Y.; Labinsky, H.; Palmowski, A.; Bäcker, H.; Müller, M.; Kienzle, A. Altered molecular pathways and prognostic markers in active systemic juvenile idiopathic arthritis: Integrated bioinformatic analysis. Bosn. J. Basic Med. Sci. 2022, 22, 247–260. [Google Scholar] [CrossRef]
- Perera Molligoda Arachchige, A.S. Human NK cells: From development to effector functions. Innate Immunity 2021, 27, 212–229. [Google Scholar] [CrossRef]
- Spits, H.; Cupedo, T. Innate lymphoid cells: Emerging insights in development, lineage relationships, and function. Annu. Rev. Immunol. 2012, 30, 647–675. [Google Scholar] [CrossRef]
- Vivier, E.; Artis, D.; Colonna, M.; Diefenbach, A.; Di Santo, J.P.; Eberl, G.; Koyasu, S.; Locksley, R.M.; McKenzie, A.N.J.; Mebius, R.E.; et al. Innate Lymphoid Cells: 10 Years On. Cell 2018, 174, 1054–1066. [Google Scholar] [CrossRef]
- Fuchs, A. ILC1s in Tissue Inflammation and Infection. Front. Immunol. 2016, 7, 104. [Google Scholar] [CrossRef] [PubMed]
- Daussy, C.; Faure, F.; Mayol, K.; Viel, S.; Gasteiger, G.; Charrier, E.; Bienvenu, J.; Henry, T.; Debien, E.; Hasan, U.A.; et al. T-bet and Eomes instruct the development of two distinct natural killer cell lineages in the liver and in the bone marrow. J. Exp. Med. 2014, 211, 563–577. [Google Scholar] [CrossRef]
- Sojka, D.K.; Plougastel-Douglas, B.; Yang, L.; Pak-Wittel, M.A.; Artyomov, M.N.; Ivanova, Y.; Zhong, C.; Chase, J.M.; Rothman, P.B.; Yu, J.; et al. Tissue-resident natural killer (NK) cells are cell lineages distinct from thymic and conventional splenic NK cells. eLife 2014, 3, e01659. [Google Scholar] [CrossRef] [PubMed]
- Takakura, M.; Shimizu, M.; Yakoyama, T.; Mizuta, M.; Yachie, A. Transient Natural Killer Cell Dysfunction Associated with Interleukin-18 Overproduction in Systemic Juvenile Idiopathic Arthritis. Pediatr. Int. 2018, 60, 984–985. [Google Scholar] [CrossRef] [PubMed]
- Avau, A.; Put, K.; Wouters, C.H.; Matthys, P. Cytokine balance and cytokine-driven natural killer cell dysfunction in systemic juvenile idiopathic arthritis. Cytokine Growth Factor Rev. 2015, 26, 35–45. [Google Scholar] [CrossRef]
- Cifaldi, L.; Prencipe, G.; Caiello, I.; Bracaglia, C.; Locatelli, F.; De Benedetti, F.; Strippoli, R. Inhibition of natural killer cell cytotoxicity by interleukin-6: Implications for the pathogenesis of macrophage activation syndrome. Arthritis Rheumatol. 2015, 67, 3037–3046. [Google Scholar] [CrossRef]
- Shimizu, M. Macrophage activation syndrome in systemic juvenile idiopathic arthritis. Immunol. Med. 2021, 44, 237–245. [Google Scholar] [CrossRef]
- Grom, A.A.; Villanueva, J.; Lee, S.; Goldmuntz, E.A.; Passo, M.H.; Filipovich, A. Natural killer cell dysfunction in patients with systemic-onset juvenile rheumatoid arthritis and macrophage activation syndrome. J. Pediatr. 2003, 142, 292–296. [Google Scholar] [CrossRef]
- Villanueva, J.; Lee, S.; Giannini, E.H.; Graham, T.B.; Passo, M.H.; Filipovich, A.; Grom, A.A. Natural killer cell dysfunction is a distinguishing feature of systemic onset juvenile rheumatoid arthritis and macrophage activation syndrome. Arthritis Res. Ther. 2005, 7, R30–R37. [Google Scholar] [CrossRef]
- de Jager, W.; Vastert, S.J.; Beekman, J.M.; Wulffraat, N.M.; Kuis, W.; Coffer, P.J.; Prakken, B. Defective phosphorylation of interleukin-18 receptor beta causes impaired natural killer cell function in systemic-onset juvenile idiopathic arthritis. Arthritis Rheum. 2009, 60, 2782–2793. [Google Scholar] [CrossRef]
- Zhou, J.; Tang, X.; Ding, Y.; An, Y.; Zhao, X. Natural killer cell activity and frequency of killer cell immunoglobulin-like receptors in children with different forms of juvenile idiopathic arthritis. Pediatr. Allergy Immunol. 2013, 24, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Put, K.; Vandenhaute, J.; Avau, A.; van Nieuwenhuijze, A.; Brisse, E.; Dierckx, T.; Rutgeerts, O.; Garcia-Perez, J.E.; Toelen, J.; Waer, M.; et al. Inflammatory Gene Expression Profile and Defective Interferon-γ and Granzyme K in Natural Killer Cells From Systemic Juvenile Idiopathic Arthritis Patients. Arthritis Rheumatol. 2017, 69, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Ohya, T.; Nishimura, K.; Murase, A.; Hattori, S.; Ohara, A.; Nozawa, T.; Hara, R.; Ito, S. Impaired Interleukin-18 Signaling in Natural Killer Cells From Patients With Systemic Juvenile Idiopathic Arthritis. ACR Open Rheumatol. 2022, 4, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Fukui, S.; Iwamoto, N.; Takatani, A.; Igawa, T.; Shimizu, T.; Umeda, M.; Nishino, A.; Horai, Y.; Hirai, Y.; Koga, T.; et al. M1 and M2 monocytes in rheumatoid arthritis: A contribution of imbalance of M1/M2 monocytes to osteoclastogenesis. Front. Immunol. 2017, 8, 1958. [Google Scholar] [CrossRef]
- Feng, D.; Huang, W.Y.; Niu, X.L.; Hao, S.; Zhang, L.N.; Hu, Y.J. Significance of Macrophage Subtypes in the Peripheral Blood of Children with Systemic Juvenile Idiopathic Arthritis. Rheumatol. Ther. 2021, 8, 1859–1870. [Google Scholar] [CrossRef]
- Sridharan, R.; Cavanagh, B.; Cameron, A.R.; Kelly, D.J.; O’Brien, F.J. Material stiffness influences the polarization state, function and migration mode of macrophages. Acta Biomater. 2019, 89, 47–59. [Google Scholar] [CrossRef]
- Wang, Q.; Ni, H.; Lan, L.; Wei, X.; Xiang, R.; Wang, Y. Fra-1 protooncogene regulates IL-6 expression in macrophages and promotes the generation of M2d macrophages. Cell Res. 2010, 20, 701–712. [Google Scholar] [CrossRef]
- Funes, S.C.; Rios, M.; Escobar-Vera, J.; Kalergis, A.M. Implications of macrophage polarization in autoimmunity. Immunology 2018, 154, 186–195. [Google Scholar] [CrossRef]
- Wang, L.X.; Zhang, S.X.; Wu, H.J.; Rong, X.L.; Guo, J. M2b macrophage polarization and its roles in diseases. J. Leukoc. Biol. 2019, 106, 345–358. [Google Scholar] [CrossRef]
- Fischer-Riepe, L.; Daber, N.; Schulte-Schrepping, J.; Véras De Carvalho, B.C.; Russo, A.; Pohlen, M.; Fischer, J.; Chasan, A.I.; Wolf, M.; Ulas, T.; et al. CD163 expression defines specific, IRF8-dependent, immune-modulatory macrophages in the bone marrow. J. Allergy Clin. Immunol. 2020, 146, 1137–1151. [Google Scholar] [CrossRef]
- Thornton, S.; Tan, R.; Sproles, A.; Do, T.; Schick, J.; Grom, A.A.; DeLay, M.; Schulert, G.S. A Multiparameter Flow Cytometry Analysis Panel to Assess CD163 mRNA and Protein in Monocyte and Macrophage Populations in Hyperinflammatory Diseases. J. Immunol. 2019, 202, 1635–1643. [Google Scholar] [CrossRef] [PubMed]
- Silvestre-Roig, C.; Fridlender, Z.G.; Glogauer, M.; Scapini, P. Neutrophil diversity in health and disease. Trends Immunol. 2019, 40, 565–583. [Google Scholar] [CrossRef] [PubMed]
- Fousert, E.; Toes, R.; Desai, J. Neutrophil extracellular traps (NETs) take the central stage in driving autoimmune responses. Cells 2020, 9, 915. [Google Scholar] [CrossRef] [PubMed]
- Ng, L.G.; Ostuni, R.; Hidalgo, A. Heterogeneity of neutrophils. Nat. Rev. Immunol. 2019, 19, 255–265. [Google Scholar] [CrossRef]
- Gohar, F.; Kessel, C.; Lavric, M.; Holzinger, D.; Foel, D. Review of biomarkers in systemic juvenile idiopathic arthritis: Helpful tools or just playing tricks? Arthritis Res. Ther. 2016, 18, 163. [Google Scholar] [CrossRef]
- Nigrovic, P.A. Review: Is there a window of opportunity for treatment of systemic juvenile idiopathic arthritis? Arthritis Rheumatol. 2014, 66, 1405–1413. [Google Scholar] [CrossRef]
- Ter Haar, N.M.; Tak, T.; Mokry, M.; Scholman, R.C.; Meerding, J.M.; de Jagerz, W.; Verwoerd, A.; Foell, D.; Vogl, T.; Roth, J.; et al. Reversal of Sepsis-Like Features of Neutrophils by Interleukin-1 Blockade in Patients with Systemic-Onset Juvenile Idiopathic Arthritis. Arthritis Rheumatol. 2018, 70, 943–956. [Google Scholar] [CrossRef]
- Brown, R.A.; Henderlight, M.; Do, T.; Yasin, S.; Grom, A.A.; DeLay, M.; Thornton, S.; Schulert, G.S. Neutrophils from Children with Systemic Juvenile Idiopathic Arthritis Exhibit Persistent Proinflammatory Activation Despite Long-Standing Clinically Inactive Disease. Front. Immunol. 2018, 9, 2995. [Google Scholar] [CrossRef]
- Malengier-Devlies, B.; Metzemaekers, M.; Wouters, C.; Proost, P.; Matthys, P. Neutrophil Homeostasis and Emergency Granulopoiesis: The Example of Systemic Juvenile Idiopathic Arthritis. Front. Immunol. 2021, 12, 766620. [Google Scholar] [CrossRef]
- Torres-Ruiz, J.; Carrillo-Vázquez, D.A.; Tapia-Rodríguezc, M.; Garcia-Galicia, J.A.; Alcocer-Varela, J.; Gómez-Martín, D. The role of low-density granulocytes and NETosis in the pathogenesis of adult-onset Still’s Disease. Clin. Exp. Rheumatol. 2019, 37, 74–82. [Google Scholar] [PubMed]
- Jung, J.Y.; Kim, J.W.; Suh, C.H.; Kim, H.A. Roles of Interactions Between Toll-Like Receptors and Their Endogenous Ligands in the Pathogenesis of Systemic Juvenile Idiopathic Arthritis and Adult-Onset Still’s Disease. Front. Immunol. 2020, 11, 583513. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-W.; Ahn, M.H.; Jung, J.Y.; Suh, C.H.; Kim, H.A. An Update on the Pathogenic Role of Neutrophils in Systemic Juvenile Idiopathic Arthritis and Adult-Onset Still’s Disease. Int. J. Mol. Sci. 2021, 22, 13038. [Google Scholar] [CrossRef] [PubMed]
- Ravelli, A.; Minoia, F.; Davì, S.; Horne, A.; Bovis, F.; Pistorio, A.; Aricò, M.; Avcin, T.; Behrens, E.M.; De Benedetti, F.; et al. Expert consensus on dynamics of laboratory tests for diagnosis of macrophage activation syndrome complicating systemic juvenile idiopathic arthritis. RMD Open 2016, 2, e000161. [Google Scholar] [CrossRef] [PubMed]
- Crayne, C.B.; Albeituni, S.; Nichols, K.E.; Cron, R.Q. The Immunology of Macrophage Activation Syndrome. Front. Immunol. 2019, 10, 119. [Google Scholar] [CrossRef] [PubMed]
- De Matteis, A.; Pires Marafon, D.; Caiello, I.; Pardeo, M.; Marucci, G.; Sacco, E.; Prencipe, G.; De Benedetti, F.; Bracaglia, C. Traditional Laboratory Parameters and New Biomarkers in Macrophage Activation Syndrome and Secondary Hemophagocytic Lymphohistiocytosis [abstract]. Arthritis Rheumatol. 2020, 72 (Suppl. S4). Available online: https://acrabstracts.org/abstract/traditional-laboratory-parameters-and-new-biomarkers-in-macrophage-activation-syndrome-and-secondary-hemophagocytic-lymphohistiocytosis/ (accessed on 21 August 2022).
- Jamilloux, Y.; Georgin-Lavialle, S.; Sève, P.; Belot, A.; Fautrel, B. Le temps est venu de réconcilier l’arthrite juvénile idiopathique systémique et la maladie de Still de l’adulte [It is time to reconcile systemic juvenile idiopathic arthritis and adult-onset Still’s disease]. Rev. Med. Interne 2019, 40, 635–636. [Google Scholar] [CrossRef]
- Minoia, F.; Davì, S.; Horne, A.; Demirkaya, E.; Bovis, F.; Li, C.; Lehmberg, K.; Weitzman, S.; Insalaco, A.; Wouters, C.; et al. Clinical features, treatment, and outcome of macrophage activation syndrome complicating systemic juvenile idiopathic arthritis: A multinational, multicenter study of 362 patients. Arthritis Rheumatol. 2014, 66, 3160–3169. [Google Scholar] [CrossRef]
- Vastert, S.; Prakken, B. Update on research and clinical translation on specific clinical areas: From bench to bedside: How insight in immune pathogenesis can lead to precision medicine of severe juvenile idiopathic arthritis. Best practice & research. Clin. Rheumatol. 2014, 28, 229–246. [Google Scholar] [CrossRef]
- Pascual, V.; Allantaz, F.; Arce, E.; Punaro, M.; Banchereau, J. Role of interleukin-1 (IL-1) in the pathogenesis of systemic onset juvenile idiopathic arthritis and clinical response to IL-1 blockade. J. Exp. Med. 2005, 201, 1479–1486. [Google Scholar] [CrossRef]
- Ogilvie, E.M.; Khan, A.; Hubank, M.; Kellam, P.; Woo, P. Specific gene expression profiles in systemic juvenile idiopathic arthritis. Arthritis Rheum. 2007, 56, 1954–1965. [Google Scholar] [CrossRef]
- Fall, N.; Barnes, M.; Thornton, S.; Luyrink, L.; Olson, J.; Ilowite, N.T.; Gottlieb, B.S.; Griffin, T.; Sherry, D.D.; Thompson, S.; et al. Gene expression profiling of peripheral blood from patients with untreated new-onset systemic juvenile idiopathic arthritis reveals molecular heterogeneity that may predict macrophage activation syndrome. Arthritis Rheum. 2007, 56, 3793–3804. [Google Scholar] [CrossRef] [PubMed]
- Vastert, S.J.; Jamilloux, Y.; Quartier, P.; Ohlman, S.; Osterling Koskinen, L.; Kullenberg, T.; Franck-Larsson, K.; Fautrel, B.; de Benedetti, F. Anakinra in children and adults with Still’s disease. Rheumatology 2019, 58, vi9–vi22. [Google Scholar] [CrossRef]
- Ruperto, N.; Brunner, H.I.; Quartier, P.; Constantin, T.; Wulffraat, N.M.; Horneff, G.; Kasapcopur, O.; Schneider, R.; Anton, J.; Barash, J.; et al. Canakinumab in patients with systemic juvenile idiopathic arthritis and active systemic features: Results from the 5-year long-term extension of the phase III pivotal trials. Ann. Rheum. Dis. 2018, 77, 1710–1719. [Google Scholar] [CrossRef] [PubMed]
- Ling, X.B.; Park, J.L.; Carroll, T.; Nguyen, K.D.; Lau, K.; Macaubas, C.; Chen, E.; Lee, T.; Sandborg, C.; Milojevic, D.; et al. Plasma profiles in active systemic juvenile idiopathic arthritis: Biomarkers and biological implications. Proteomics 2010, 10, 4415–4430. [Google Scholar] [CrossRef] [PubMed]
- Palomo, J.; Dietrich, D.; Martin, P.; Palmer, G.; Gabay, C. The interleukin (IL)-1 cytokine family—Balance between agonists and antagonists in inflammatory diseases. Cytokine 2015, 66, 25–37. [Google Scholar] [CrossRef]
- Kaneko, N.; Kurata, M.; Yamamoto, T.; Morikawa, S.; Masumoto, J. The role of interleukin-1 in general pathology. Inflamm. Regen. 2019, 39, 12. [Google Scholar] [CrossRef]
- Quartier, P. Systemic Juvenile Idiopathic Arthritis/Pediatric Still’s Disease, a Syndrome but Several Clinical Forms: Recent Therapeutic Approaches. J. Clin. Med. 2022, 11, 1357. [Google Scholar] [CrossRef]
- Saccomanno, B.; Tibaldi, J.; Minoia, F.; Bagnasco, F.; Pistorio, A.; Guariento, A.; Caorsi, R.; Consolaro, A.; Gattorno, M.; Ravelli, A. Predictors of Effectiveness of Anakinra in Systemic Juvenile Idiopathic Arthritis. J. Rheumatol. 2019, 46, 416–421. [Google Scholar] [CrossRef]
- Lainka, E.; Baehr, M.; Raszka, B.; Haas, J.P.; Hügle, B.; Fischer, N.; Foell, D.; Hinze, C.; Weissbarth-Riedel, E.; Kallinich, T.; et al. Experiences with IL-1 blockade in systemic juvenile idiopathic arthritis—Data from the German AID-registry. Pediatr. Rheumatol. Online J. 2021, 19, 38. [Google Scholar] [CrossRef]
- Wilson, D.C.; Marinov, A.D.; Blair, H.C.; Bushnell, D.S.; Thompson, S.D.; Chaly, Y.; Hirsch, R. Follistatin-like protein 1 is a mesenchyme-derived inflammatory protein and may represent a biomarker for systemic-onset juvenile rheumatoid arthritis. Arthritis Rheum. 2010, 62, 2510–2516. [Google Scholar] [CrossRef]
- Mellins, E.D.; Macaubas, C.; Grom, A.A. Pathogenesis of systemic juvenile idiopathic arthritis: Some answers, more questions. Nat. Rev. Rheumatol. 2011, 7, 416–426. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Blocking IL-1 in systemic inflammation. J. Exp. Med. 2005, 201, 1355–1359. [Google Scholar] [CrossRef] [PubMed]
- Monteagudo, L.A.; Boothby, A.; Gertner, E. Continuous Intravenous Anakinra Infusion to Calm the Cytokine Storm in Macrophage Activation Syndrome. ACR Open Rheumatol. 2020, 2, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-Y.; Yang, H.-Y.; Huang, J.-L.; Lai, J.-H. Signals and Mechanisms Regulating Monocyte and Macrophage Activation in the Pathogenesis of Juvenile Idiopathic Arthritis. Int. J. Mol. Sci. 2021, 22, 7960. [Google Scholar] [CrossRef] [PubMed]
- Hinze, C.H.; Holzinger, D.; Lainka, E.; Haas, J.P.; Speth, F.; Kallinich, T.; Rieber, N.; Hufnagel, M.; Jansson, A.F.; Hedrich, C.; et al. Practice and consensus-based strategies in diagnosing and managing systemic juvenile idiopathic arthritis in Germany. Pediatr. Rheumatol. 2018, 16, 7. [Google Scholar] [CrossRef]
- Toplak, N.; Blazina, Š.; Avčin, T. The role of IL-1 inhibition in systemic juvenile idiopathic arthritis: Current status and future perspectives. Drug Des. Dev. Ther. 2018, 12, 1633–1643. [Google Scholar] [CrossRef]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef]
- Zhao, C.; Zhao, W. NLRP3 Inflammasome-A Key Player in Antiviral Responses. Front. Immunol. 2020, 11, 211. [Google Scholar] [CrossRef]
- Kessel, C.; Lippitz, K.; Weinhage, T.; Hinze, C.; Wittkowski, H.; Holzinger, D.; Fall, N.; Grom, A.A.; Gruen, N.; Foell, D. Proinflammatory Cytokine Environments Can Drive Interleukin-17 Overexpression by γ/δ T Cells in Systemic Juvenile Idiopathic Arthritis. Arthritis Rheumatol. 2017, 69, 1480–1494. [Google Scholar] [CrossRef]
- Brunner, H.I.; Quartier, P.; Alexeeva, E.; Constantin, T.; Kone-Paut, I.; Marzan, K.; Schneider, R.; Wulffraat, N.M.; Chasnyk, V.; Tirosh, I.; et al. Efficacy and Safety of Canakinumab in Patients with Systemic Juvenile Idiopathic Arthritis With and Without Fever at Baseline: Results From an Open-Label, Active-Treatment Extension Study. Arthritis Rheumatol. 2020, 72, 2147–2158. [Google Scholar] [CrossRef]
- Kostik, M.M.; Isupova, E.A.; Belozerov, K.; Likhacheva, T.S.; Suspitsin, E.N.; Raupov, R.; Masalova, V.V.; Chikova, I.A.; Dubko, M.F.; Kalashnikova, O.V.; et al. Standard and increased canakinumab dosing to quiet macrophage activation syndrome in children with systemic juvenile idiopathic arthritis. Front. Pediatr. 2022, 10, 894846. [Google Scholar] [CrossRef]
- Vilaiyuk, S.; Lerkvaleekul, B.; Soponkanaporn, S.; Setthaudom, C.; Buranapraditkun, S. Correlations between serum interleukin 6, serum soluble interleukin 6 receptor, and disease activity in systemic juvenile idiopathic arthritis patients treated with or without tocilizumab. Cent. Eur. J. Immunol. 2019, 44, 150–158. [Google Scholar] [CrossRef]
- de Benedetti, F.; Massa, M.; Robbioni, P.; Ravelli, A.; Burgio, G.R.; Martini, A. Correlation of serum interleukin-6 levels with joint involvement and thrombocytosis in systemic juvenile rheumatoid arthritis. Arthritis Rheum. 1991, 34, 1158–1163. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, M.; Kendirl, S.G.; Altintas, D.; Bingol, G.; Antmen, B. Cytokine levels in serum of patients with juvenile rheumatoid arthritis. Clin. Rheumatol. 2001, 20, 30–35. [Google Scholar] [CrossRef]
- de Jager, W.; Hoppenreijs, E.P.; Wulffraat, N.M.; Wedderburn, L.R.; Kuis, W.; Prakken, B.J. Blood and synovial fluid cytokine signatures in patients with juvenile idiopathic arthritis: A cross-sectional study. Ann. Rheum. Dis. 2007, 66, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Brachat, A.H.; Grom, A.A.; Wulffraat, N.; Brunner, H.I.; Quartier, P.; Brik, R.; McCann, L.; Ozdogan, H.; Rutkowska-Sak, L.; Schneider, R.; et al. Early changes in gene expression and inflammatory proteins in systemic juvenile idiopathic arthritis patients on canakinumab therapy. Arthritis Res. Ther. 2017, 19, 13. [Google Scholar] [CrossRef] [PubMed]
- Akioka, S. Interleukin-6 in juvenile idiopathic arthritis. Mod. Rheumatol. 2019, 29, 275–286. [Google Scholar] [CrossRef]
- Schulert, G.S.; Minoia, F.; Bohnsack, J.; Cron, R.Q.; Hashad, S.; KonÉ-Paut, I.; Kostik, M.; Lovell, D.; Maritsi, D.; Nigrovic, P.A.; et al. Effect of biologic therapy on clinical and laboratory features of macrophage activation syndrome associated with systemic juvenile idiopathic arthritis. Arthritis Care Res. 2018, 70, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Chiran, D.A.; Weber, M.; Ailioaie, L.M.; Moraru, E.; Ailioaie, C.; Litscher, D.; Litscher, G. Intravenous laser blood irradiation and tocilizumab in a patient with juvenile arthritis. Case Rep. Med. 2014, 2014, 923496. [Google Scholar] [CrossRef]
- Bielak, M.; Husmann, E.; Weyandt, N.; Haas, J.P.; Hügle, B.; Horneff, G.; Neudorfc, U.; Lutz, T.; Lilienthal, E.; Kallinich, T.; et al. IL-6 blockade in systemic juvenile idiopathic arthritis—Achievement of inactive disease and remission (data from the German AID-registry). Pediatr. Rheumatol. 2018, 16, 22. [Google Scholar] [CrossRef]
- Shimizu, M.; Mizuta, M.; Okamoto, N.; Yasumi, T.; Iwata, N.; Umebayashi, H.; Okura, Y.; Kinjo, N.; Kubota, T.; Nakagishi, Y.; et al. Tocilizumab modifies clinical and laboratory features of macrophage activation syndrome complicating systemic juvenile idiopathic arthritis. Pediatr. Rheumatol 2020, 18, 2. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.; Sundberg, E.; Aulin, C.; Neog, M.; Palmblad, K.; Horne, A.C.; Granath, F.; Ek, A.; Melén, E.; Olsson, M.; et al. Immunoprofiling of active and inactive systemic juvenile idiopathic arthritis reveals distinct biomarkers: A single-center study. Pediatr. Rheumatol. 2021, 19, 173. [Google Scholar] [CrossRef] [PubMed]
- O’Garra, A.; Barrat, F.J.; Castro, A.G.; Vicari, A.; Hawrylowicz, C. Strategies for use of IL-10 or its antagonists in human disease. Immunol. Rev. 2008, 223, 114–131. [Google Scholar] [CrossRef] [PubMed]
- Steen, E.H.; Wang, X.; Balaji, S.; Butte, M.J.; Bollyky, P.L.; Keswani, S.G. The Role of the Anti-Inflammatory Cytokine Interleukin-10 in Tissue Fibrosis. Adv. Wound Care 2020, 9, 184–198. [Google Scholar] [CrossRef]
- Imbrechts, M.; Avau, A.; Vandenhaute, J.; Malengier-Devlies, B.; Put, K.; Mitera, T.; Berghmans, N.; Burton, O.; Junius, S.; Liston, A.; et al. Insufficient IL-10 Production as a Mechanism Underlying the Pathogenesis of Systemic Juvenile Idiopathic Arthritis. J. Immunol. 2018, 201, 2654–2663. [Google Scholar] [CrossRef]
- Peng, Y.; Liu, X.; Duan, Z.; Duan, J.; Zhou, Y. The Association of Serum IL-10 Levels with the Disease Activity in Systemic-Onset Juvenile Idiopathic Arthritis Patients. Mediat. Inflamm. 2021, 2021, 6650928. [Google Scholar] [CrossRef]
- Novick, D.; Kim, S.; Kaplanski, G.; Dinarello, C.A. Interleukin-18, more than a Th1 cytokine. Semin. Immunol. 2013, 25, 439–448. [Google Scholar] [CrossRef]
- Lotito, A.P.; Campa, A.; Silva, C.A.; Kiss, M.H.; Mello, S.B. Interleukin 18 as a marker of disease activity and severity in patients with juvenile idiopathic arthritis. J. Rheumatol. 2007, 34, 823–830. [Google Scholar] [PubMed]
- Shimizu, M.; Yokoyama, T.; Yamada, K.; Kaneda, H.; Wada, H.; Wada, T.; Toma, T.; Ohta, K.; Kasahara, Y.; Yachie, A. Distinct cytokine profiles of systemic-onset juvenile idiopathic arthritis-associated macrophage activation syndrome with particular emphasis on the role of interleukin-18 in its pathogenesis. Rheumatology 2010, 49, 1645–1653. [Google Scholar] [CrossRef]
- Shimizu, M.; Nakagishi, Y.; Inoue, N.; Mizuta, M.; Ko, G.; Saikawa, Y.; Kubota, T.; Yamasaki, Y.; Takei, S.; Yachie, A. Interleukin-18 for predicting the development of macrophage activation syndrome in systemic juvenile idiopathic arthritis. Clin. Immunol. 2015, 160, 277–281. [Google Scholar] [CrossRef]
- Xia, Y.; Cui, P.; Li, Q.; Liang, F.; Li, C.; Yang, J. Extremely elevated IL-18 levels may help distinguish systemic-onset juvenile idiopathic arthritis from other febrile diseases. Braz. J. Med. Biol. Res. 2017, 50, e5958. [Google Scholar] [CrossRef] [PubMed]
- Kaplanski, G. Interleukin-18: Biological properties and role in disease pathogenesis. Immunol. Rev. 2018, 281, 138–153. [Google Scholar] [CrossRef] [PubMed]
- Kudela, H.; Drynda, S.; Lux, A.; Horneff, G.; Kekow, J. Comparative study of Interleukin-18 (IL-18) serum levels in adult-onset Still’s disease (AOSD) and systemic onset juvenile idiopathic arthritis (sJIA) and its use as a biomarker for diagnosis and evaluation of disease activity. BMC Rheumatol. 2019, 3, 4. [Google Scholar] [CrossRef] [PubMed]
- Yasin, S.; Fall, N.; Brown, R.A.; Henderlight, M.; Canna, S.W.; Girard-Guyonvarc’h, C.; Gabay, C.; Grom, A.A.; Schulert, G.S. IL-18 as a biomarker linking systemic juvenile idiopathic arthritis and macrophage activation syndrome. Rheumatology 2020, 59, 361–366. [Google Scholar] [CrossRef]
- Krei, J.M.; Møller, H.J.; Larsen, J.B. The role of interleukin-18 in the diagnosis and monitoring of hemophagocytic lymphohistiocytosis/macrophage activation syndrome—A systematic review. Clin. Exp. Immunol. 2021, 203, 174–182. [Google Scholar] [CrossRef]
- Mizuta, M.; Shimizu, M.; Inoue, N.; Ikawa, Y.; Nakagishi, Y.; Yasuoka, R.; Iwata, N.; Yachie, A. Clinical significance of interleukin-18 for the diagnosis and prediction of disease course in systemic juvenile idiopathic arthritis. Rheumatology 2021, 60, 2421–2426. [Google Scholar] [CrossRef]
- Girard-Guyonvarc’h, C.; Palomo, J.; Martin, P.; Rodriguez, E.; Troccaz, S.; Palmer, G.; Gabay, C. Unopposed IL-18 signaling leads to severe TLR9-induced macrophage activation syndrome in mice. Blood 2018, 131, 1430–1441. [Google Scholar] [CrossRef]
- Girard-Guyonvarc’h, C.; Harel, M.; Gabay, C. The Role of Interleukin 18/Interleukin 18-Binding Protein in Adult-Onset Still’s Disease and Systemic Juvenile Idiopathic Arthritis. J. Clin. Med. 2022, 11, 430. [Google Scholar] [CrossRef]
- Schulert, G.S. The IL-18/IFN-γ axis in systemic JIA and MAS—New answers, more questions. Rheumatology 2021, 60, 3045–3047. [Google Scholar] [CrossRef]
- Burke, J.D.; Young, H.A. IFN-γ: A cytokine at the right time, is in the right place. Semin. Immunol. 2019, 43, 101280. [Google Scholar] [CrossRef]
- Merli, P.; Quintarelli, C.; Strocchio, L.; Locatelli, F. The role of interferon-gamma and its signaling pathway in pediatric hematological disorders. Pediatr. Blood Cancer 2021, 68, e28900. [Google Scholar] [CrossRef]
- Morales-Mantilla, D.E.; King, K.Y. The Role of Interferon-Gamma in Hematopoietic Stem Cell Development, Homeostasis, and Disease. Curr. Stem Cell Rep. 2018, 4, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, F.; Jordan, M.B.; Allen, C.; Cesaro, S.; Rizzari, C.; Rao, A.; Degar, B.; Garrington, T.P.; Sevilla, J.; Putti, M.C.; et al. Emapalumab in Children with Primary Hemophagocytic Lymphohistiocytosis. N. Engl. J. Med. 2020, 382, 1811–1822. [Google Scholar] [CrossRef] [PubMed]
- Merli, P.; Algeri, M.; Gaspari, S.; Locatelli, F. Novel Therapeutic Approaches to Familial HLH (Emapalumab in FHL). Front. Immunol. 2020, 11, 608492. [Google Scholar] [CrossRef]
- Garonzi, C.; Chinello, M.; Cesaro, S. Emapalumab for adult and pediatric patients with hemophagocytic lymphohistiocytosis. Expert Rev. Clin. Pharmacol. 2021, 14, 527–534. [Google Scholar] [CrossRef]
- Put, K.; Avau, A.; Brisse, E.; Mitera, T.; Put, S.; Proost, P.; Bader-Meunier, B.; Westhovens, R.; Eynde, B.J.V.D.; Orabona, C.; et al. Cytokines in systemic juvenile idiopathic arthritis and haemophagocytic lymphohistiocytosis: Tipping the balance between interleukin-18 and interferon-γ. Rheumatology 2015, 54, 1507–1517. [Google Scholar] [CrossRef] [PubMed]
- Bracaglia, C.; De Graaf, K.; Marafon, D.P.; Guilhot, F.; Ferlin, W.; Prencipe, G.; Caiello, I.; Davì, S.; Schulert, G.; Ravelli, A.; et al. Elevated circulating levels of interferon-γ and interferon-γ-induced chemokines characterise patients with macrophage activation syndrome complicating systemic juvenile idiopathic arthritis. Ann. Rheum. Dis. 2017, 76, 166–172. [Google Scholar] [CrossRef]
- Bracaglia, C.; Pires Marafon, D.; Caiello, I.; de Graaf, K.; Ballabio, M.; Ferlin, W.; Davì, S.; Schulert, G.; Ravelli, A.; Grom, A.A.; et al. Biomarkers for the Diagnosis and the Identification of Risk of Macrophage Activation Syndrome (MAS) in Systemic Juvenile Idiopathic Arthritis (sJIA) [abstract]. Arthritis Rheumatol. 2017, 69 (Suppl. S10). Available online: https://acrabstracts.org/abstract/biomarkers-for-the-diagnosis-and-the-identification-of-risk-of-macrophage-activation-syndrome-mas-in-systemic-juvenile-idiopathic-arthritis-sjia/ (accessed on 21 August 2022).
- Guo, L.; Xu, Y.; Qian, X.; Zou, L.; Zheng, R.; Teng, L.; Zheng, Q.; Leung Jung, L.K.; Lu, M. Sudden Hypotension and Increased Serum Interferon-γ and Interleukin-10 Predict Early Macrophage Activation Syndrome in Patients with Systemic Juvenile Idiopathic Arthritis. J. Pediatr. 2021, 235, 203–211.e3. [Google Scholar] [CrossRef]
- Sethi, J.K.; Hotamisligil, G.S. Metabolic Messengers: Tumour necrosis factor. Nat. Metab. 2021, 3, 1302–1312. [Google Scholar] [CrossRef]
- Crayne, C.; Cron, R.Q. Pediatric macrophage activation syndrome, recognizing the tip of the Iceberg. Eur. J. Rheumatol. 2019, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Jang, D.I.; Lee, A.H.; Shin, H.Y.; Song, H.R.; Park, J.H.; Kang, T.B.; Lee, S.R.; Yang, S.H. The Role of Tumor Necrosis Factor Alpha (TNF-α) in Autoimmune Disease and Current TNF-α Inhibitors in Therapeutics. Int. J. Mol. Sci. 2021, 22, 2719. [Google Scholar] [CrossRef] [PubMed]
- Hügle, B.; Hinze, C.; Lainka, E.; Fischer, N.; Haas, J.P. Development of positive antinuclear antibodies and rheumatoid factor in systemic juvenile idiopathic arthritis points toward an autoimmune phenotype later in the disease course. Pediatr. Rheumatol. 2014, 12, 28. [Google Scholar] [CrossRef]
- Shimizu, M.; Inoue, N.; Mizuta, M.; Nakagishi, Y.; Yachie, A. Characteristic elevation of soluble TNF receptor II: I ratio in macrophage activation syndrome with systemic juvenile idiopathic arthritis. Clin. Exp. Immunol. 2018, 191, 349–355. [Google Scholar] [CrossRef]
- Irabu, H.; Shimizu, M.; Kaneko, S.; Inoue, N.; Mizuta, M.; Nakagishi, Y.; Yachie, A. Comparison of serum biomarkers for the diagnosis of macrophage activation syndrome complicating systemic juvenile idiopathic arthritis during tocilizumab therapy. Pediatr. Res. 2020, 88, 934–939. [Google Scholar] [CrossRef]
- Mizuta, M.; Shimizu, M.; Irabu, H.; Usami, M.; Inoue, N.; Nakagishi, Y.; Wada, T.; Yachie, A. Comparison of serum cytokine profiles in macrophage activation syndrome complicating different background rheumatic diseases in children. Rheumatology 2021, 60, 231–238. [Google Scholar] [CrossRef]
- Silva, T.A.; Garlet, G.P.; Lara, V.S.; Martins, W., Jr.; Silva, J.S.; Cunha, F.Q. Differential expression of chemokines and chemokine receptors in inflammatory periapical diseases. Oral Microbiol. Immunol. 2005, 20, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Kwak, H.B.; Lee, S.W.; Jin, H.M.; Ha, H.; Lee, S.H.; Takeshita, S.; Tanaka, S.; Kim, H.M.; Kim, H.H.; Lee, Z.H. Monokine induced by interferon-gamma is induced by receptor activator of nuclear factor kappa B ligand and is involved in osteoclast adhesion and migration. Blood 2005, 105, 2963–2969. [Google Scholar] [CrossRef]
- Raman, D.; Sobolik-Delmaire, T.; Richmond, A. Chemokines in health and disease. Exp. Cell Res. 2011, 317, 575–589. [Google Scholar] [CrossRef]
- Ruytinx, P.; Proost, P.; Van Damme, J.; Struyf, S. Chemokine-Induced Macrophage Polarization in Inflammatory Conditions. Front. Immunol. 2018, 9, 1930. [Google Scholar] [CrossRef]
- Takakura, M.; Shimizu, M.; Irabu, H.; Sakumura, N.; Inoue, N.; Mizuta, M.; Nakagishi, Y.; Yachie, A. Comparison of serum biomarkers for the diagnosis of macrophage activation syndrome complicating systemic juvenile idiopathic arthritis. Clin. Immunol. 2019, 208, 108252. [Google Scholar] [CrossRef]
- House, I.G.; Savas, P.; Lai, J.; Chen, A.X.Y.; Oliver, A.J.; Teo, Z.L.; Todd, K.L.; Henderson, M.A.; Giuffrida, L.; Petley, E.V.; et al. Macrophage-Derived CXCL9 and CXCL10 Are Required for Antitumor Immune Responses Following Immune Checkpoint Blockade. Clin. Cancer Res. 2020, 26, 487–504. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, T.; Venkata Suresh, V.; Yahata, Y.; Nakano, M.; Suzuki, S.; Suzuki, S.; Yamada, S.; Kitaura, H.; Mizoguchi, I.; Noiri, Y.; et al. Inhibition of the CXCL9-CXCR3 axis suppresses the progression of experimental apical periodontitis by blocking macrophage migration and activation. Sci. Rep. 2021, 11, 2613. [Google Scholar] [CrossRef] [PubMed]
- Mizuta, M.; Shimizu, M.; Inoue, N.; Nakagishi, Y.; Yachie, A. Clinical Significance of Serum CXCL9 Levels as a Biomarker for Systemic Juvenile Idiopathic Arthritis Associated Macrophage Activation Syndrome. Cytokine 2019, 119, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Hinze, T.; Kessel, C.; Hinze, C.H.; Seibert, J.; Gram, H.; Foell, D. A dysregulated interleukin-18-interferon-γ-CXCL9 axis impacts treatment response to canakinumab in systemic juvenile idiopathic arthritis. Rheumatology 2021, 60, 5165–5174. [Google Scholar] [CrossRef]
- Bleesing, J.; Prada, A.; Siegel, D.M.; Villanueva, J.; Olson, J.; Ilowite, N.T.; Brunner, H.I.; Griffin, T.; Graham, T.B.; Sherry, D.D.; et al. The diagnostic significance of soluble CD163 and soluble interleukin-2 receptor alpha-chain in macrophage activation syndrome and untreated new-onset systemic juvenile idiopathic arthritis. Arthritis Rheum. 2007, 56, 965–971. [Google Scholar] [CrossRef]
- Reddy, V.V.; Myles, A.; Cheekatla, S.S.; Singh, S.; Aggarwal, A. Soluble CD25 in serum: A potential marker for subclinical macrophage activation syndrome in patients with active systemic onset juvenile idiopathic arthritis. Int. J. Rheum. Dis. 2014, 17, 261–267. [Google Scholar] [CrossRef]
- Sakumura, N.; Shimizu, M.; Mizuta, M.; Inoue, N.; Nakagishi, Y.; Yachie, A. Soluble CD163, a unique biomarker to evaluate the disease activity, exhibits macrophage activation in systemic juvenile idiopathic arthritis. Cytokine 2018, 110, 459–465. [Google Scholar] [CrossRef]
- Verweyen, E.L.; Pickering, A.; Grom, A.A.; Schulert, G.S. Distinct Gene Expression Signatures Characterize Strong Clinical Responders Versus Nonresponders to Canakinumab in Children with Systemic Juvenile Idiopathic Arthritis. Arthritis Rheumatol. 2021, 73, 1334–1340. [Google Scholar] [CrossRef]
- Dik, W.A.; Heron, M. Clinical significance of soluble interleukin-2 receptor measurement in immune-mediated diseases. Neth. J. Med. 2020, 78, 220–231. [Google Scholar] [PubMed]
- Frosch, M.; Ahlmann, M.; Vogl, T.; Wittkowski, H.; Wulffraat, N.; Foell, D.; Roth, J. The myeloid-related proteins 8 and 14 complex, a novel ligand of toll-like receptor 4, and interleukin-1 β form a positive feedback mechanism in systemic-onset juvenile idiopathic arthritis. Arthritis Rheum. 2009, 60, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Nirmala, N.; Grom, A.; Gram, H. Biomarkers in systemic juvenile idiopathic arthritis: A comparison with biomarkers in cryopyrin-associated periodic syndromes. Curr. Opin. Rheumatol. 2014, 26, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.G. Role of Biomarkers in Juvenile Idiopathic Arthritis. J. Rheum. Dis. 2020, 27, 233–240. [Google Scholar] [CrossRef]
- Miller, Y.I.; Choi, S.-H.; Wiesner, P.; Fang, L.; Harkewicz, R.; Hartvigsen, K.; Boullier, A.; Gonen, A.; Diehl, C.J.; Que, X.; et al. Oxidation-specific epitopes are danger-associated molecular patterns recognized by pattern recognition receptors of innate immunity. Circ. Res. 2011, 108, 235–248. [Google Scholar] [CrossRef]
- Wang, S.; Song, R.; Wang, Z.; Jing, Z.; Wang, S.; Ma, J. S100A8/A9 in Inflammation. Front. Immunol. 2018, 9, 1298. [Google Scholar] [CrossRef]
- Marongiu, L.; Gornati, L.; Artuso, I.; Zanoni, I.; Granucci, F. Below the surface: The inner lives of TLR4 and TLR9. J. Leukoc. Biol. 2019, 106, 147–160. [Google Scholar] [CrossRef]
- Jukic, A.; Bakiri, L.; Wagner, E.F.; Tilg, H.; Adolph, T.E. Calprotectin: From biomarker to biological function. Gut 2021, 70, 1978–1988. [Google Scholar] [CrossRef]
- Degraeuwe, P.L.; Beld, M.P.; Ashorn, M.; Canani, R.B.; Day, A.S.; Diamanti, A.; Fagerberg, U.L.; Henderson, P.; Kolho, K.L.; Van de Vijver, E.; et al. Faecal calprotectin in suspected paediatric inflammatory bowel disease. J. Pediatr. Gastroenterol. Nutr. 2015, 60, 339–346. [Google Scholar] [CrossRef]
- Austermann, J.; Spiekermann, C.; Roth, J. S100 Proteins in Rheumatic Diseases. Nat. Rev. Rheumatol. 2018, 14, 528–541. [Google Scholar] [CrossRef]
- Mylemans, M.; Nevejan, L.; Van Den Bremt, S.; Stubbe, M.; Cruyssen, B.V.; Moulakakis, C.; Berthold, H.; Konrad, C.; Bossuyt, X.; Van Hoovels, L. Circulating calprotectin as a biomarker in neutrophil-associated inflammation: Pre-analytical recommendations and reference values depending on the type of sample. Clin. Chim. Acta 2021, 517, 149–155. [Google Scholar] [CrossRef]
- Ometto, F.; Friso, L.; Astorri, D.; Botsios, C.; Raffeiner, B.; Punzi, L.; Doria, A. Calprotectin in rheumatic diseases. Exp. Biol. Med. 2017, 242, 859–873. [Google Scholar] [CrossRef] [PubMed]
- Romand, X.; Bernardy, C.; Nguyen, M.V.C.; Courtier, A.; Trocme, C.; Clapasson, M.; Paclet, M.H.; Toussaint, B.; Gaudin, P.; Baillet, A. Systemic calprotectin and chronic inflammatory rheumatic diseases. Jt. Bone Spine 2019, 86, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Altobelli, E.; Angeletti, P.M.; Petrocelli, R.; Lapergola, G.; Farello, G.; Cannataro, G.; Breda, L. Serum Calprotectin a Potential Biomarker in Juvenile Idiopathic Arthritis: A Meta-Analysis. J. Clin. Med. 2021, 10, 4861. [Google Scholar] [CrossRef] [PubMed]
- Wittkowski, H.; Frosch, M.; Wulffraat, N.; Goldbach-Mansky, R.; Kallinich, T.; Kuemmerle-Deschner, J.; Frühwald, M.C.; Dassmann, S.; Pham, T.H.; Roth, J.; et al. S100A12 is a novel molecular marker differentiating systemic-onset juvenile idiopathic arthritis from other causes of fever of unknown origin. Arthritis Rheum. 2008, 58, 3924–3931. [Google Scholar] [CrossRef] [PubMed]
- Holzinger, D.; Frosch, M.; Kastrup, A.; Prince, F.H.; Otten, M.H.; Van Suijlekom-Smit, L.W.; ten Cate, R.; Hoppenreijs, E.P.; Hansmann, S.; Moncrieffe, H.; et al. The Toll-like receptor 4 agonist MRP8/14 protein complex is a sensitive indicator for disease activity and predicts relapses in systemic-onset juvenile idiopathic arthritis. Ann. Rheum. Dis. 2012, 71, 974–980. [Google Scholar] [CrossRef] [PubMed]
- Shenoi, S.; Ou, J.N.; Ni, C.; Macaubas, C.; Gersuk, V.H.; Wallace, C.A.; Mellins, E.D.; Stevens, A.M. Comparison of biomarkers for systemic juvenile idiopathic arthritis. Pediatr. Res. 2015, 78, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Aljaberi, N.; Tronconi, E.; Schulert, G.; Grom, A.A.; Lovell, D.J.; Huggins, J.L.; Henrickson, M.; Brunner, H. The use of S100 proteins testing in juvenile idiopathic arthritis and autoinflammatory diseases in a pediatric clinical setting: A retrospective analysis. Pediatr. Rheumatol. 2020, 18, 7. [Google Scholar] [CrossRef]
- La, C.; Lê, P.Q.; Ferster, A.; Goffin, L.; Spruyt, D.; Lauwerys, B.; Durez, P.; Boulanger, C.; Sokolova, T.; Rasschaert, J.; et al. Serum calprotectin (S100A8/A9): A promising biomarker in diagnosis and follow-up in different subgroups of juvenile idiopathic arthritis. RMD Open 2021, 7, e001646. [Google Scholar] [CrossRef]
- Deftos, L.J.; Roos, B.A.; Parthemore, J.G. Calcium and skeletal metabolism. West. J. Med. 1975, 123, 447–458. [Google Scholar]
- Dandona, P.; Nix, D.; Wilson, M.F.; Aljada, A.; Love, J.; Assicot, M.; Bohuon, C. Procalcitonin increase after endotoxin injection in normal subjects. J. Clin. Endocrinol. Metab. 1994, 79, 1605–1608. [Google Scholar] [CrossRef]
- Trippella, G.; Galli, L.; De Martino, M.; Lisi, C.; Chiappini, E. Procalcitonin performance in detecting serious and invasive bacterial infections in children with fever without apparent source: A systematic review and meta-analysis. Expert Rev. Anti-Infect. Ther. 2017, 15, 1041–1057. [Google Scholar] [CrossRef] [PubMed]
- Trachtman, R.; Murray, E.; Wang, C.M.; Szymonifka, J.; Toussi, S.S.; Walters, H.; Nellis, M.E.; Onel, K.B.; Mandl, L.A. Procalcitonin Differs in Children With Infection and Children With Disease Flares in Juvenile Idiopathic Arthritis. J. Clin. Rheumatol. 2021, 27, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Park, S.; Lee, M.J.; Song, Y.R.; Han, S.H.; Kim, S.G.; Kang, S.W.; Choi, K.H.; Kim, H.J.; Yoo, T.H. Plasma levels of soluble receptor for advanced glycation end products (sRAGE) and proinflammatory ligand for RAGE (EN-RAGE) are associated with carotid atherosclerosis in patients with peritoneal dialysis. Atherosclerosis 2012, 220, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Mendez, J.D.; Mendez-Valenzuela, V.; Aguilar-Hernandez, M.M. Cellular signalling of the receptor for advanced glycation end products (RAGE). Cell Signal. 2013, 25, 2185–2197. [Google Scholar] [CrossRef] [PubMed]
- Selvin, E.; Halushka, M.K.; Rawlings, A.M.; Hoogeveen, R.C.; Ballantyne, C.M.; Coresh, J.; Astor, B.C. sRAGE and risk of diabetes, cardiovascular disease, and death. Diabetes 2013, 62, 2116–2121. [Google Scholar] [CrossRef]
- Pratte, K.A.; Curtis, J.L.; Kechris, K.; Couper, D.; Cho, M.H.; Silverman, E.K.; DeMeo, D.L.; Sciurba, F.C.; Zhang, Y.; Ortega, V.E.; et al. Soluble receptor for advanced glycation end products (sRAGE) as a biomarker of COPD. Respir. Res. 2021, 22, 127. [Google Scholar] [CrossRef]
- Geroldi, D.; Falcone, C.; Emanuele, E. Soluble receptor for advanced glycation end products: From disease marker to potential therapeutic target. Curr. Med. Chem. 2006, 13, 1971–1978. [Google Scholar] [CrossRef]
- Grauen Larsen, H.; Marinkovic, G.; Nilsson, P.M.; Nilsson, J.; Engström, G.; Melander, O.; Orho-Melander, M.; Schiopu, A. High Plasma sRAGE (Soluble Receptor for Advanced Glycation End Products) Is Associated With Slower Carotid Intima-Media Thickness Progression and Lower Risk for First-Time Coronary Events and Mortality. Arter. Thromb. Vasc. Biol. 2019, 39, 925–933. [Google Scholar] [CrossRef]
- Prasad, K. Is there any evidence that AGE/sRAGE is a universal biomarker/risk marker for diseases? Mol. Cell. Biochem. 2019, 451, 139–144. [Google Scholar] [CrossRef]
- Zhang, X.; Cao, X.; Dang, M.; Wang, H.; Chen, B.; Du, F.; Li, H.; Zeng, X.; Guo, C. Soluble receptor for advanced glycation end-products enhanced the production of IFN-γ through the NF-κB pathway in macrophages recruited by ischemia/reperfusion. Int. J. Mol. Med. 2019, 43, 2507–2515. [Google Scholar] [CrossRef]
- Klune, J.R.; Dhupar, R.; Cardinal, J.; Billiar, T.R.; Tsung, A. HMGB1: Endogenous danger signaling. Mol. Med. 2008, 14, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Andersson, U.; Rauvala, H. Introduction: HMGB1 in inflammation and innate immunity. J. Intern. Med. 2011, 270, 296–300. [Google Scholar] [CrossRef]
- Yang, H.; Wang, H.; Andersson, U. Targeting Inflammation Driven by HMGB1. Front. Immunol. 2020, 11, 484. [Google Scholar] [CrossRef] [PubMed]
- Andersson, U.; Harris, H.E. The role of HMGB1 in the pathogenesis of rheumatic disease. Biochim. Biophys. Acta 2010, 1799, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Harris, H.E.; Andersson, U.; Pisetsky, D.S. HMGB1: A multifunctional alarmin driving autoimmune and inflammatory disease. Nat. Rev. Rheumatol. 2012, 8, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Magna, M.; Pisetsky, D.S. The role of HMGB1 in the pathogenesis of inflammatory and autoimmune diseases. Mol. Med. 2014, 20, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Paudel, Y.N.; Angelopoulou, E.; Piperi, C.; Balasubramaniam, V.; Othman, I.; Shaikh, M.F. Enlightening the role of high mobility group box 1 (HMGB1) in inflammation: Updates on receptor signalling. Eur. J. Pharmacol. 2019, 858, 172487. [Google Scholar] [CrossRef]
- Moser, B.; Hudson, B.I.; Schmidt, A.M. Soluble RAGE: A hot new biomarker for the hot joint? Arthritis Res. Ther. 2005, 7, 142. [Google Scholar] [CrossRef][Green Version]
- Yang, H.; Wang, H.; Chavan, S.S.; Andersson, U. High Mobility Group Box Protein 1 (HMGB1): The Prototypical Endogenous Danger Molecule. Mol. Med. 2015, 21, S6–S12. [Google Scholar] [CrossRef]
- Zhong, H.; Li, X.; Zhou, S.; Jiang, P.; Liu, X.; Ouyang, M.; Nie, Y.; Chen, X.; Zhang, L.; Liu, Y.; et al. Interplay between RAGE and TLR4 Regulates HMGB1-Induced Inflammation by Promoting Cell Surface Expression of RAGE and TLR4. J. Immunol. 2020, 205, 767–775. [Google Scholar] [CrossRef]
- Schierbeck, H.; Pullerits, R.; Pruunsild, C.; Fischer, M.; Holzingerc, D.; Laestadius, Å.; Sundberg, E.; Harris, H.E. HMGB1 levels are increased in patients with juvenile idiopathic arthritis, correlated with early onset of disease, and are independent of disease duration. J. Rheumatol. 2013, 40, 1604–1613. [Google Scholar] [CrossRef] [PubMed]
- Bobek, D.; Grčević, D.; Kovačić, N.; Lukić, I.K.; Jelušić, M. The presence of high mobility group box-1 and soluble receptor for advanced glycation end-products in juvenile idiopathic arthritis and juvenile systemic lupus erythematosus. Pediatr. Rheumatol. 2014, 12, 50. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Zhang, Y.; Zhang, Z.Y.; Tang, X.M. Association between high mobility group box 1 protein and juvenile idiopathic arthritis: A prospective longitudinal study. Pediatr. Rheumatol. 2021, 19, 112. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.F.; Yang, Y.H.; Wang, L.C.; Lee, J.H.; Shen, E.Y.; Chiang, B.L. Comparative usefulness of C-reactive protein and erythrocyte sedimentation rate in juvenile rheumatoid arthritis. Clin. Exp. Rheumatol. 2007, 25, 782–785. [Google Scholar] [PubMed]
- Bloom, B.J.; Alario, A.J.; Miller, L.C. Persistent elevation of fibrin d-dimer predicts long term outcome in systemic juvenile idiopathic arthritis. J. Rheumatol. 2009, 36, 422–426. [Google Scholar] [CrossRef]
- Gorelik, M.; Fall, N.; Altaye, M.; Barnes, M.G.; Thompson, S.D.; Grom, A.A.; Hirsch, R. Follistatin-like protein 1 and the ferritin/erythrocyte sedimentation rate ratio are potential biomarkers for dysregulated gene expression and macrophage activation syndrome in systemic juvenile idiopathic arthritis. J. Rheumatol. 2013, 40, 1191–1199. [Google Scholar] [CrossRef]
- Minoia, F.; Bovis, F.; Davì, S.; Horne, A.; Fischbach, M.; Frosch, M.; Huber, A.; Jelusic, M.; Sawhney, S.; McCurdy, D.K.; et al. Pediatric Rheumatology International Trials Organization, the Childhood Arthritis & Rheumatology Research Alliance, the Pediatric Rheumatology Collaborative Study Group and the Histiocyte Society. Development and initial validation of the MS score for diagnosis of macrophage activation syndrome in systemic juvenile idiopathic arthritis. Ann. Rheum. Dis. 2019, 78, 1357–1362. [Google Scholar] [CrossRef]
- Eloseily, E.M.; Minoia, F.; Crayne, C.B.; Beukelman, T.; Ravelli, A.; Cron, R.Q. Ferritin to erythrocyte sedimentation rate ratio: Simple measure to identify macrophage activation syndrome in systemic juvenile idiopathic arthritis. ACR Open Rheumatol. 2019, 1, 345–349. [Google Scholar] [CrossRef]
- Zou, L.X.; Zhu, Y.; Sun, L.; Ma, H.H.; Yang, S.R.; Zeng, H.S.; Xiao, J.H.; Yu, H.G.; Guo, L.; Xu, Y.P.; et al. Clinical and laboratory features, treatment, and outcomes of macrophage activation syndrome in 80 children: A multi-center study in China. World J. Pediatr. 2020, 16, 89–98. [Google Scholar] [CrossRef]
- Ganeva, M.; Fuehner, S.; Kessel, C.; Klotsche, J.; Niewerth, M.; Minden, K.; Foell, D.; Hinze, C.H.; Wittkowski, H. Trajectories of disease courses in the inception cohort of newly diagnosed patients with JIA (ICON-JIA): The potential of serum biomarkers at baseline. Pediatr. Rheumatol. 2021, 19, 64. [Google Scholar] [CrossRef]
- Shimojima, Y.; Matsuda, M.; Gono, T.; Ishii, W.; Ikeda, S. Serum amyloid A as a potent therapeutic marker in a refractory patient with polymyalgia rheumatica. Intern. Med. 2005, 44, 1009–1012. [Google Scholar] [CrossRef] [PubMed]
- Cantarini, L.; Giani, T.; Fioravanti, A.; Iacoponi, F.; Simonini, G.; Pagnini, I.; Spreafico, A.; Chellini, F.; Galeazzi, M.; Cimaz, R. Serum amyloid A circulating levels and disease activity in patients with juvenile idiopathic arthritis. Yonsei Med. J. 2012, 53, 1045–1048. [Google Scholar] [CrossRef]
- Feyzkhanova, G.U.; Voloshin, S.A.; Novikov, A.A.; Aleksandrova, E.N.; Smoldovskaya, O.V.; Rubina, A.Y. Analysis of rheumatoid factor and acute phase proteins using microarrays in patients with rheumatoid arthritis. Klin. Lab. Diagn. 2022, 67, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Sorić Hosman, I.; Kos, I.; Lamot, L. Serum Amyloid A in Inflammatory Rheumatic Diseases: A Compendious Review of a Renowned Biomarker. Front. Immunol. 2021, 11, 631299. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xiang, X.; Ren, H.; Xu, L.; Zhao, L.; Chen, X.; Long, H.; Wang, Q.; Wu, Q. Serum Amyloid A is a biomarker of severe Coronavirus Disease and poor prognosis. J. Infect. 2020, 80, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Yang, J.Z.; Bai, W.H.; Li, Z.Y.; Sun, L.F.; Yan, J.J.; Zhou, C.L.; Tang, B.P. Prognostic value of serum amyloid A in patients with COVID-19. Infection 2020, 48, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Dev, S.; Singh, A. Study of role of serum amyloid A (SAA) as a marker of disease activity in juvenile idiopathic arthritis. J. Fam. Med. Prim. Care 2019, 8, 2129–2133. [Google Scholar] [CrossRef]
- Wang, X.; Abraham, S.; McKenzie, J.A.G.; Jeffs, N.; Swire, M.; Tripathi, V.B.; Luhmann, U.F.O.; Lange, C.A.K.; Zhai, Z.; Arthur, H.M.; et al. LRG1 promotes angiogenesis by modulating endothelial TGF-β signalling. Nature 2013, 499, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Urushima, H.; Fujimoto, M.; Mishima, T.; Ohkawara, T.; Honda, H.; Lee, H.; Kawahata, H.; Serada, S.; Naka, T. Leucine-rich alpha 2 glycoprotein promotes Th17 differentiation and collagen-induced arthritis in mice through enhancement of TGF-β-Smad2 signaling in naïve helper T cells. Arthritis Res. Ther. 2017, 19, 137. [Google Scholar] [CrossRef] [PubMed]
- Serada, S.; Fujimoto, M.; Ogata, A.; Terabe, F.; Hirano, T.; Iijima, H.; Shinzaki, S.; Nishikawa, T.; Ohkawara, T.; Iwahori, K.; et al. iTRAQ-based proteomic identification of leucine-rich alpha-2 glycoprotein as a novel inflammatory biomarker in autoimmune diseases. Ann. Rheum. Dis. 2010, 69, 770–774. [Google Scholar] [CrossRef]
- Yoshimura, T.; Mitsuyama, K.; Sakemi, R.; Takedatsu, H.; Yoshioka, S.; Kuwaki, K.; Mori, A.; Fukunaga, S.; Araki, T.; Morita, M.; et al. Evaluation of Serum Leucine-Rich Alpha-2 Glycoprotein as a New Inflammatory Biomarker of Inflammatory Bowel Disease. Mediat. Inflamm. 2021, 2021, 8825374. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, H.; Nakajima, K.; Takaishi, M.; Ohko, K.; Serada, S.; Fujimoto, M.; Naka, T.; Sano, S. The Skin-Liver Axis Modulates the Psoriasiform Phenotype and Involves Leucine-Rich α-2 Glycoprotein. J. Immunol. 2021, 206, 1469–1477. [Google Scholar] [CrossRef]
- Druhan, L.J.; Lance, A.; Li, S.; Price, A.E.; Emerson, J.T.; Baxter, S.A.; Gerber, J.M.; Avalos, B.R. Leucine Rich α-2 Glycoprotein: A Novel Neutrophil Granule Protein and Modulator of Myelopoiesis. PLoS ONE 2017, 12, e0170261. [Google Scholar] [CrossRef]
- Camilli, C.; Hoeh, A.E.; De Rossi, G.; Moss, S.E.; Greenwood, J. LRG1: An emerging player in disease pathogenesis. J. Biomed. Sci. 2022, 29, 6. [Google Scholar] [CrossRef]
- Zou, Y.; Xu, Y.; Chen, X.; Wu, Y.; Fu, L.; Lv, Y. Research Progress on Leucine-Rich Alpha-2 Glycoprotein 1: A Review. Front. Pharmacol. 2022, 12, 809225. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M.; Nakagishi, Y.; Inoue, N.; Mizuta, M.; Yachie, A. Leucine-rich α2-glycoprotein as the acute-phase reactant to detect systemic juvenile idiopathic arthritis disease activity during anti-interleukin-6 blockade therapy: A case series. Mod. Rheumatol. 2017, 27, 833–837. [Google Scholar] [CrossRef]
- Shimizu, M.; Inoue, N.; Mizuta, M.; Nakagishi, Y.; Yachie, A. Serum Leucine-Rich α2-Glycoprotein as a Biomarker for Monitoring Disease Activity in Patients with Systemic Juvenile Idiopathic Arthritis. J. Immunol. Res. 2019, 2019, 3140204. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.Y.; Schulert, G.S.; Canna, S.W.; Huang, Y.; Sundel, J.; Li, Y.; Hoyt, K.J.; Blaustein, R.B.; Wactor, A.; Do, T.; et al. Adenosine deaminase 2 as a biomarker of macrophage activation syndrome in systemic juvenile idiopathic arthritis. Ann. Rheum. Dis. 2020, 79, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Nakra, N.A.; Blumberg, D.A.; Herrera-Guerra, A.; Lakshminrusimha, S. Multi-System Inflammatory Syndrome in Children (MIS-C) Following SARS-CoV-2 Infection: Review of Clinical Presentation, Hypothetical Pathogenesis, and Proposed Management. Children 2020, 7, 69. [Google Scholar] [CrossRef] [PubMed]
- Fajgenbaum, D.C.; June, C.H. Cytokine Storm. N. Engl. J. Med. 2020, 383, 2255–2273. [Google Scholar] [CrossRef] [PubMed]
- Henderson, L.A. Deciphering the cytokine fingerprint of macrophage activation syndrome. Lancet Rheumatol. 2021, 3, e535–e538. [Google Scholar] [CrossRef]
- Tang, S.; Li, S.; Zheng, S.; Ding, Y.; Zhu, D.; Sun, C.; Hu, Y.; Qiao, J.; Fang, H. Understanding of cytokines and targeted therapy in macrophage activation syndrome. Semin. Arthritis Rheum. 2021, 51, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Consolaro, A.; Varnier, G.C.; Martini, A.; Ravelli, A. Advances in biomarkers for paediatric rheumatic diseases. Nat. Rev. Rheumatol. 2015, 11, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Vandenhaute, J.; Wouters, C.H.; Matthys, P. Natural Killer Cells in Systemic Autoinflammatory Diseases: A Focus on Systemic Juvenile Idiopathic Arthritis and Macrophage Activation Syndrome. Front. Immunol 2020, 10, 3089. [Google Scholar] [CrossRef]
- Javaux, C.; El-Jammal, T.; Neau, P.-A.; Fournier, N.; Gerfaud-Valentin, M.; Perard, L.; Fouillet-Desjonqueres, M.; Le Scanff, J.; Vignot, E.; Durupt, S.; et al. Detection and Prediction of Macrophage Activation Syndrome in Still’s Disease. J. Clin. Med. 2022, 11, 206. [Google Scholar] [CrossRef]
- Çakan, M.; Karadağ, Ş.G.; Tanatar, A.; Ayaz, N.A. The frequency of macrophage activation syndrome and disease course in systemic juvenile idiopathic arthritis. Mod. Rheumatol. 2020, 30, 900–904. [Google Scholar] [CrossRef]
- Zaripova, L.N.; Midgley, A.; Christmas, S.E.; Beresford, M.W.; Baildam, E.M.; Oldershaw, R.A. Juvenile idiopathic arthritis: From aetiopathogenesis to therapeutic approaches. Pediatr. Rheumatol. 2021, 19, 135. [Google Scholar] [CrossRef] [PubMed]
- Das, G.; Sarkar, S.; Datta, S. Evaluation of serum ferritin and its correlation with disease activity in non-systemic juvenile idiopathic arthritis. Int. J. Contemp. Pediatr. 2021, 8, 625–630. [Google Scholar] [CrossRef]
- Høeg, P.E.; Glerup, M.; Mahler, B.; Høst, C.; Herlin, T. Evaluation of Macrophage Activation Syndrome in Patients with Systemic Juvenile Idiopathic Arthritis: A Single Center Experience. Int. J. Rheumatol. 2022, 2022, 1784529. [Google Scholar] [CrossRef] [PubMed]
- Adiguzel Dundar, H.; Acari, C.; Turkucar, S.; Unsal, E. Treatment of systemic JIA: When do we need a biologic? Real world data of a single center. Mod. Rheumatol. 2021, 31, 684–690. [Google Scholar] [CrossRef]
- Van Nieuwenhove, E.; Lagou, V.; Van Eyck, L.; Dooley, J.; Bodenhofer, U.; Roca, C.; Vandebergh, M.; Goris, A.; Humblet-Baron, S.; Wouters, C.; et al. Machine learning identifies an immunological pattern associated with multiple juvenile idiopathic arthritis subtypes. Ann. Rheum. Dis. 2019, 78, 617–628. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).