Magnetic-Based Human Tissue 3D Cell Culture: A Systematic Review
Abstract
1. Introduction
1.1. Three-Dimensional (3D) Cell Culture Systems
1.2. Magnetic-Based 3D (m3D) Cell Culture Technology
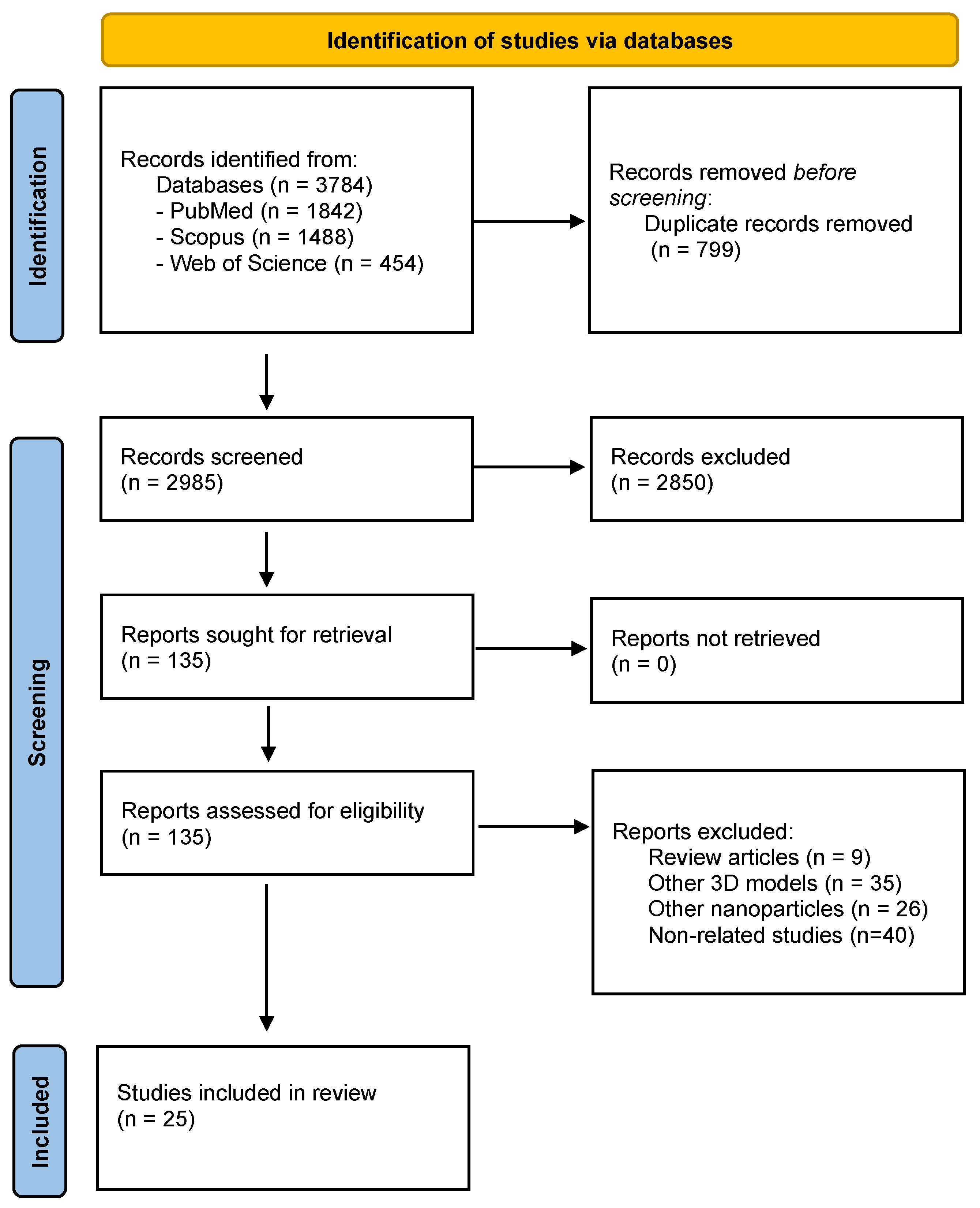
2. Materials and Methods
2.1. Literature Search Strategy
2.2. Study Selection
2.3. Data Extraction and Analysis
3. Results and Discussion
3.1. m3D Culture Method: Levitation, Bioprinting or Ring Formation
3.2. Composition of m3D Cultures: Homotypic or Heterotypic
3.3. Biocompatibility of Magnetic Nanoparticles (MNP)
3.4. Use of Scaffolds in m3d Cell Cultures
3.5. Formation Period of m3D Structure and Longevity in Culture
3.6. Tools and Methodologies Used for the Analysis of m3D Cultures
3.7. Size of the m3D Structures and Core Features
3.8. Cell Behavior and Intercommunication in m3D Cultures
3.9. m3D Cultures to Produce Tumor Spheroids
4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Appendix A
| Year | Reference | m3D Model | Heterotypic or Homotypic | Scaffold | 3D Mimicking Tissue or Disease | Cell Types | Applications | Observations Regarding m3D Model |
|---|---|---|---|---|---|---|---|---|
| 2010 | [22] | Levitation And Ring Formation | Homotypic and heterotypic | Yes– Magnetic Iron Oxide (MIO)-containing hydrogels | Glioblastoma |
|
|
|
| 2013 | [18] | Levitation | Homotypic | Yes–Polylysine-based hydrogel (MagPLL) | Vascular Smooth Muscle Cells System- |
|
|
|
| 2013 | [19] | Levitation | Homotypic and heterotypic | No | White adipose tissue (WAT) |
|
|
|
| 2013 | [13] | Levitation | Homotypic | No | Various- | General protocol for levitation of several cell types:
|
|
|
| 2013 | [38] | Ring Formation | Homotypic | No | Novel 3D assay for drug toxicity screening - |
|
|
|
| 2013 | [23] | Levitation | Homotypic and heterotypic | No | Bronchiole- |
|
|
|
| 2014 | [24] | Levitation | Homotypic and heterotypic | No | Aortic valve - |
|
|
|
| 2016 | [36] | Levitation and Bioprinting | Homotypic and heterotypic | No | Breast cancer |
|
|
|
| 2017 | [21] | Levitation | Homotypic | Yes-Poly(urethane acrylate)-poly(glycidyl methacrylate) thermoresponsive nanofabricated substratum (TNFS) | General 3D tissue architecture - |
|
|
|
| 2018 | [26] | Bioprinting | Homotypic | No | Pancreatic ductal adenocarcinoma (PDAC) |
|
|
|
| 2018 | [27] | Bioprinting | Homotypic | No | Innervated and bio-functional salivary glands (SG) epithelial cells |
|
|
|
| 2018 | [25] | Levitation | Homotypic and heterotypic | No | White adipose tissue (WAR)–adipospheres - |
|
|
|
| 2019 | [28] | Bioprinting | Homotypic and Heterotypic | Yes–Hydrogel (fabricated from 10% w/v PEGDA, 0.0001% w/v TEMPO and a 1.1mM LAP precursor solution using the DLP Pro4500) and Matrigel | Neural microphysiological system- |
|
|
|
| 2019 | [30] | Bioprinting | Homotypic and Heterotypic | No | Pancreatic ductal adenocarcinoma (PDAC) |
|
|
|
| 2019 | [39] | Levitation | Heterotypic | No | Human Hematopoietic Stem Cells (HSC) Microenvironment - |
|
|
|
| 2020 | [29] | Bioprinting | Homotypic | No | Early embryonic development - |
|
|
|
| 2020 | [15] | Bioprinting and Levitation | Homotypic and heterotypic | No |
|
|
|
|
| 2020 | [31] | Bioprinting | Heterotypic | No | Pancreatic niche - |
|
|
|
| 2021 | [17] | Levitation | Homotypic | No | Oral and maxillofacial tissues-mesenchymal stem cells (MSC) |
|
|
|
| 2021 | [14] | Bioprinting | Homotypic | No | Bone tissue- |
|
|
|
| 2021 | [37] | Levitation and Bioprinting | Homotypic and Heterotypic | No | Tuberculous granulomas–Human Tuberculosis |
|
|
|
| 2021 | [32] | Bioprinting | Homotypic | No | Squamous cell carcinoma, osteosarcoma |
|
|
|
| 2021 | [33] | Bioprinting | Homotypic | No | Wound Healing |
|
|
|
| 2021 | [35] | Bioprinting | Heterotypic | No | Hepatic inflammatory response - |
|
|
|
| 2021 | [34] | Bioprinting | Homotypic | No | Human skin and their extracellular matrix (ECM). |
|
|
|
References
- Caleffi, J.T.; Aal, M.C.E.; Gallindo, H.D.O.M.; Caxali, G.H.; Crulhas, B.P.; Ribeiro, A.O.; Souza, G.R.; Delella, F.K. Magnetic 3D Cell Culture: State of the Art and Current Advances. Life Sci. 2021, 286, 120028. [Google Scholar] [CrossRef] [PubMed]
- Jensen, C.; Teng, Y. Is It Time to Start Transitioning From 2D to 3D Cell Culture? Front. Mol. Biosci. 2020, 7, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Nunes, A.S.; Barros, A.S.; Costa, E.C.; Moreira, A.F.; Correia, I.J. 3D Tumor Spheroids as in Vitro Models to Mimic in Vivo Human Solid Tumors Resistance to Therapeutic Drugs. Biotechnol. Bioeng. 2019, 116, 206–226. [Google Scholar] [CrossRef]
- Brancato, V.; Oliveira, J.M.; Correlo, V.M.; Reis, R.L.; Kundu, S.C. Could 3D Models of Cancer Enhance Drug Screening? Biomaterials 2020, 232, 119744. [Google Scholar] [CrossRef]
- Edmondson, R.; Broglie, J.J.; Adcock, A.F.; Yang, L. Three-Dimensional Cell Culture Systems and Their Applications in Drug Discovery and Cell-Based Biosensors. Assay Drug Dev. Technol. 2014, 12, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, M.A.G.; Xavier, C.P.R.; Pereira, R.F.; Petrikaitė, V.; Vasconcelos, M.H. 3D Cell Culture Models as Recapitulators of the Tumor Microenvironment for the Screening of Anti-Cancer Drugs. Cancers 2021, 14, 190. [Google Scholar] [CrossRef]
- Lee, K.-H.; Kim, T.-H. Recent Advances in Multicellular Tumor Spheroid Generation for Drug Screening. Biosensors 2021, 11, 445. [Google Scholar] [CrossRef]
- Weiswald, L.B.; Bellet, D.; Dangles-Marie, V. Spherical Cancer Models in Tumor Biology. Neoplasia 2015, 17, 1–15. [Google Scholar] [CrossRef]
- Langhans, S.A. Three-Dimensional in Vitro Cell Culture Models in Drug Discovery and Drug Repositioning. Front. Pharmacol. 2018, 9, 1–14. [Google Scholar] [CrossRef]
- Chen, M.Y.; Skewes, J.; Desselle, M.; Wong, C.; Woodruff, M.A.; Dasgupta, P.; Rukin, N.J. Current Applications of Three-Dimensional Printing in Urology. BJU Int. 2020, 125, 17–27. [Google Scholar] [CrossRef]
- Shokoohmand, A.; Ren, J.; Baldwin, J.; Atack, A.; Shafiee, A.; Theodoropoulos, C.; Wille, M.-L.; Tran, P.A.; Bray, L.J.; Smith, D.; et al. Translational Models of Prostate Cancer Bone Metastasis. Biomaterials 2019, 7, 17535. [Google Scholar] [CrossRef][Green Version]
- Pinto, B.; Henriques, A.C.; Silva, P.M.A.; Bousbaa, H. Three-Dimensional Spheroids as in Vitro Preclinical Models for Cancer Research. Pharmaceutics 2020, 12, 1186. [Google Scholar] [CrossRef] [PubMed]
- Haisler, W.L.; Timm, D.M.; Gage, J.A.; Tseng, H.; Killian, T.C.; Souza, G.R. Three-Dimensional Cell Culturing by Magnetic Levitation. Nat. Protoc. 2013, 8, 1940–1949. [Google Scholar] [CrossRef]
- Gaitán-Salvatella, I.; López-Villegas, E.O.; González-Alva, P.; Susate-Olmos, F.; Álvarez-Pérez, M.A. Case Report: Formation of 3D Osteoblast Spheroid Under Magnetic Levitation for Bone Tissue Engineering. Front. Mol. Biosci. 2021, 8, 672518. [Google Scholar] [CrossRef] [PubMed]
- Natânia de Souza-Araújo, C.; Rodrigues Tonetti, C.; Cardoso, M.R.; Lucci de Angelo Andrade, L.A.; Fernandes da Silva, R.; Romani Fernandes, L.G.; Guimarães, F. Three-Dimensional Cell Culture Based on Magnetic Fields to Assemble Low-Grade Ovarian Carcinoma Cell Aggregates Containing Lymphocytes. Cells 2020, 9, 635. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. PLoS Med. 2021, 18, 1–15. [Google Scholar] [CrossRef]
- Chan, Y.-H.; Lee, Y.-C.; Hung, C.-Y.; Yang, P.-J.; Lai, P.-C.; Feng, S.-W. Three-Dimensional Spheroid Culture Enhances Multipotent Differentiation and Stemness Capacities of Human Dental Pulp-derived Mesenchymal Stem Cells by Modulating MAPK and NF-KB Signaling Pathways. Stem Cell Rev. Rep. 2021, 17, 1810–1826. [Google Scholar] [CrossRef]
- Castro-Chavez, F.; Vickers, K.C.; Lee, J.S.; Tung, C.-H.; Morrisett, J.D. Effect of Lyso-Phosphatidylcholine and Schnurri-3 on Osteogenic Transdifferentiation of Vascular Smooth Muscle Cells to Calcifying Vascular Cells in 3D Culture. Biochim. Biophys. Acta 2013, 1830, 3828–3834. [Google Scholar] [CrossRef]
- Daquinag, A.C.; Souza, G.R.; Kolonin, M.G. Adipose Tissue Engineering in Three-Dimensional Levitation Tissue Culture System Based on Magnetic Nanoparticles. Tissue Eng. Part C Methods 2013, 19, 336–344. [Google Scholar] [CrossRef]
- Barreto-Duran, E.; Mejia-Cruz, C.C.; Jaramillo-Garcia, L.F.; Leal-Garcia, E.; Barreto-Prieto, A.; Rodriguez-Pardo, V.M. 3d Multicellular Spheroid for the Study of Human Hematopoietic Stem Cells: Synergistic Effect between Oxygen Levels, Mesenchymal Stromal Cells and Endothelial Cells. J. Blood Med. 2021, 12, 517–528. [Google Scholar] [CrossRef]
- Penland, N.; Choi, E.; Perla, M.; Park, J.; Kim, D.-H. Facile Fabrication of Tissue-Engineered Constructs Using Nanopatterned Cell Sheets and Magnetic Levitation. Nanotechnology 2017, 28, 75103. [Google Scholar] [CrossRef] [PubMed]
- Souza, G.R.; Molina, J.R.; Raphael, R.M.; Ozawa, M.G.; Stark, D.J.; Levin, C.S.; Bronk, L.F.; Ananta, J.S.; Mandelin, J.; Georgescu, M.-M.M.; et al. Three-Dimensional Tissue Culture Based on Magnetic Cell Levitation. Nat. Nanotechnol. 2010, 5, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Tseng, H.; Gage, J.A.; Raphael, R.M.; Moore, R.H.; Killian, T.C.; Grande-Allen, K.J.; Souza, G.R. Assembly of a Three-Dimensional Multitype Bronchiole Coculture Model Using Magnetic Levitation. Tissue Eng. Part C Methods 2013, 19, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Tseng, H.; Balaoing, L.R.; Grigoryan, B.; Raphael, R.M.; Killian, T.C.; Souza, G.R.; Grande-Allen, K.J. A Three-Dimensional Co-Culture Model of the Aortic Valve Using Magnetic Levitation. Acta Biomater. 2014, 10, 173–182. [Google Scholar] [CrossRef]
- Tseng, H.; Daquinag, A.C.; Souza, G.R.; Kolonin, M.G. Three-Dimensional Magnetic Levitation Culture System Simulating White Adipose Tissue. In Methods in Molecular Biology (Clifton, N.J.); Humana Press: Totowa, NJ, USA, 2018; Volume 1773, pp. 147–154. [Google Scholar]
- Abou Ali, E.; Bordacahar, B.; Mestas, J.-L.; Batteux, F.; Lafon, C.; Camus, M.; Prat, F. Ultrasonic Cavitation Induces Necrosis and Impairs Growth in Three-Dimensional Models of Pancreatic Ductal Adenocarcinoma. PLoS ONE 2018, 13, e0209094. [Google Scholar] [CrossRef]
- Adine, C.; Ng, K.K.; Rungarunlert, S.; Souza, G.R.; Ferreira, J.N. Engineering Innervated Secretory Epithelial Organoids by Magnetic Three-Dimensional Bioprinting for Stimulating Epithelial Growth in Salivary Glands. Biomaterials 2018, 180, 52–66. [Google Scholar] [CrossRef]
- Bowser, D.A.; Moore, M.J. Biofabrication of Neural Microphysiological Systems Using Magnetic Spheroid Bioprinting. Biofabrication 2020, 12, 015002. [Google Scholar] [CrossRef]
- Chung, J.; Sriram, G.; Keefer, C.L. Nanoparticle Technology Improves In-Vitro Attachment of Cattle (Bos Taurus) Trophectoderm Cells. Biotechnol. Lett. 2020, 42, 2083–2089. [Google Scholar] [CrossRef]
- Leenhardt, R.; Camus, M.; Mestas, J.L.; Jeljeli, M.; Abou Ali, E.; Chouzenoux, S.; Bordacahar, B.; Nicco, C.; Batteux, F.; Lafon, C.; et al. Ultrasound-Induced Cavitation Enhances the Efficacy of Chemotherapy in a 3D Model of Pancreatic Ductal Adenocarcinoma with Its Microenvironment. Sci. Rep. 2019, 9, 18916. [Google Scholar] [CrossRef]
- Urbanczyk, M.; Zbinden, A.; Layland, S.L.; Duffy, G.; Schenke-Layland, K. Controlled Heterotypic Pseudo-Islet Assembly of Human β-Cells and Human Umbilical Vein Endothelial Cells Using Magnetic Levitation. Tissue Eng. Part A 2020, 26, 387–399. [Google Scholar] [CrossRef]
- Menegakis, A.; Klompmaker, R.; Vennin, C.; Arbusà, A.; Damen, M.; van den Broek, B.; Zips, D.; van Rheenen, J.; Krenning, L.; Medema, R.H. Resistance of Hypoxic Cells to Ionizing Radiation Is Mediated in Part via Hypoxia-Induced Quiescence. Cells 2021, 10, 610. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, L.S.; Henn, J.G.; Morás, A.M.; de Moura Sperotto, N.D.; Ferro, M.B.; Cao, Z.; Roehe, A.V.; Petry, A.U.S.; Nugent, M.; Moura, D.J. Plantago Australis Hydroethanolic Extract-Loaded Formulations: Promising Dressings for Wound Healing. Rev. Bras. Farmacogn. 2021, 31, 91–101. [Google Scholar] [CrossRef]
- Vu, B.; Souza, G.R.; Dengjel, J. Scaffold-Free 3D Cell Culture of Primary Skin Fibroblasts Induces Profound Changes of the Matrisome. Matrix Biol. Plus 2021, 11, 100066. [Google Scholar] [CrossRef] [PubMed]
- Sebők, C.; Tráj, P.; Vörösházi, J.; Mackei, M.; Papp, M.; Gálfi, P.; Neogrády, Z.; Mátis, G. Two Sides to Every Question: Attempts to Activate Chicken Innate Immunity in 2D and 3D Hepatic Cell Cultures. Cells 2021, 10, 1910. [Google Scholar] [CrossRef]
- Leonard, F.; Godin, B. 3D In Vitro Model for Breast Cancer Research Using Magnetic Levitation and Bioprinting Method. Methods Mol. Biol. 2016, 1406, 239–251. [Google Scholar] [CrossRef]
- Kotze, L.A.; Beltran, C.G.G.; Lang, D.; Loxton, A.G.; Cooper, S.; Meiring, M.; Koegelenberg, C.F.N.; Allwood, B.W.; Malherbe, S.T.; Hiemstra, A.M.; et al. Establishment of a Patient-Derived, Magnetic Levitation-Based, Three-Dimensional Spheroid Granuloma Model for Human Tuberculosis. mSphere 2021, 6, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Timm, D.M.; Chen, J.; Sing, D.; Gage, J.A.; Haisler, W.L.; Neeley, S.K.; Raphael, R.M.; Dehghani, M.; Rosenblatt, K.P.; Killian, T.C.; et al. A High-Throughput Three-Dimensional Cell Migration Assay for Toxicity Screening with Mobile Device-Based Macroscopic Image Analysis. Sci. Rep. 2013, 3, 3000. [Google Scholar] [CrossRef]
- Mejía-Cruz, C.C.; Barreto-Durán, E.; Pardo-Pérez, M.A.; Jimenez, M.C.; Rincón, J.; Vanegas, K.; Rodríguez, J.L.; Jaramillo-Garcia, L.F.; Ulloa, J.C.; Díaz, R.M.; et al. Generation of Organotypic Multicellular Spheres by Magnetic Levitation: Model for the Study of Human Hematopoietic Stem Cells Microenvironment. Int. J. Stem Cells 2019, 12, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Leek, R.; Grimes, D.R.; Harris, A.L.; McIntyre, A. Methods: Using Three-Dimensional Culture (Spheroids) as an In Vitro Model of Tumour Hypoxia. Adv. Exp. Med. Biol. 2016, 899, 167–196. [Google Scholar]


| Database | Search Query |
|---|---|
| Medline (via Pubmed) | Search: (“magnetic nanoparticle *” [Title/Abstract] OR “magnetic levitation *” [Title/Abstract] OR “nanoshuttle *” [Title/Abstract] OR “magnetic bioprint *” [Title/Abstract]) AND (“culture techniques” [MeSH Terms] OR “cells, cultured” [MeSH Terms] OR “Tissues” [MeSH Terms] OR “Organoids” [MeSH Terms] OR “printing, three dimensional” [MeSH Terms]) Filters: Full text, English |
| Scopus | TITLE-ABS-KEY ((“Magnetic * nanoparticle *” OR “Magnetic levitation *” OR “Nanoshuttle *” OR “Magnetic Bioprint *”) AND (“Cell Culture *” OR “Organ Culture *” OR “Tissue culture *” OR “organoids *” OR “spheroid *” OR “Patient-derived xenografts” OR “Primary Culture *” OR “three-dimensional model *” OR “Three-dimensional cell culture *” OR “levitated culture”)) AND (LIMIT-TO (LANGUAGE, “English”)) AND (LIMIT-TO (DOCTYPE, “ar”)) |
| Web of Science | TS = ((“Magnetic * nanoparticle *” OR “Magnetic levitation *” OR “Nanoshuttle *” OR “Magnetic Bioprint *”) AND (“Cell Culture *” OR “Organ Culture *” OR “Tissue culture *” OR “organoids *” OR “spheroid *” OR “Patient-derived xenografts” OR “Primary Culture *” OR “three-dimensional model *” OR “Three-dimensional cell culture *” OR “levitated culture”)) AND IDIOMA: (English) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marques, I.A.; Fernandes, C.; Tavares, N.T.; Pires, A.S.; Abrantes, A.M.; Botelho, M.F. Magnetic-Based Human Tissue 3D Cell Culture: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 12681. https://doi.org/10.3390/ijms232012681
Marques IA, Fernandes C, Tavares NT, Pires AS, Abrantes AM, Botelho MF. Magnetic-Based Human Tissue 3D Cell Culture: A Systematic Review. International Journal of Molecular Sciences. 2022; 23(20):12681. https://doi.org/10.3390/ijms232012681
Chicago/Turabian StyleMarques, Inês Alexandra, Carolina Fernandes, Nuno Tiago Tavares, Ana Salomé Pires, Ana Margarida Abrantes, and Maria Filomena Botelho. 2022. "Magnetic-Based Human Tissue 3D Cell Culture: A Systematic Review" International Journal of Molecular Sciences 23, no. 20: 12681. https://doi.org/10.3390/ijms232012681
APA StyleMarques, I. A., Fernandes, C., Tavares, N. T., Pires, A. S., Abrantes, A. M., & Botelho, M. F. (2022). Magnetic-Based Human Tissue 3D Cell Culture: A Systematic Review. International Journal of Molecular Sciences, 23(20), 12681. https://doi.org/10.3390/ijms232012681










