Collagen V α1 Chain Decrease in Papillary Dermis from Early Systemic Sclerosis: A New Proposal in Cutaneous Fibrosis Molecular Structure
Abstract
1. Introduction
2. Results
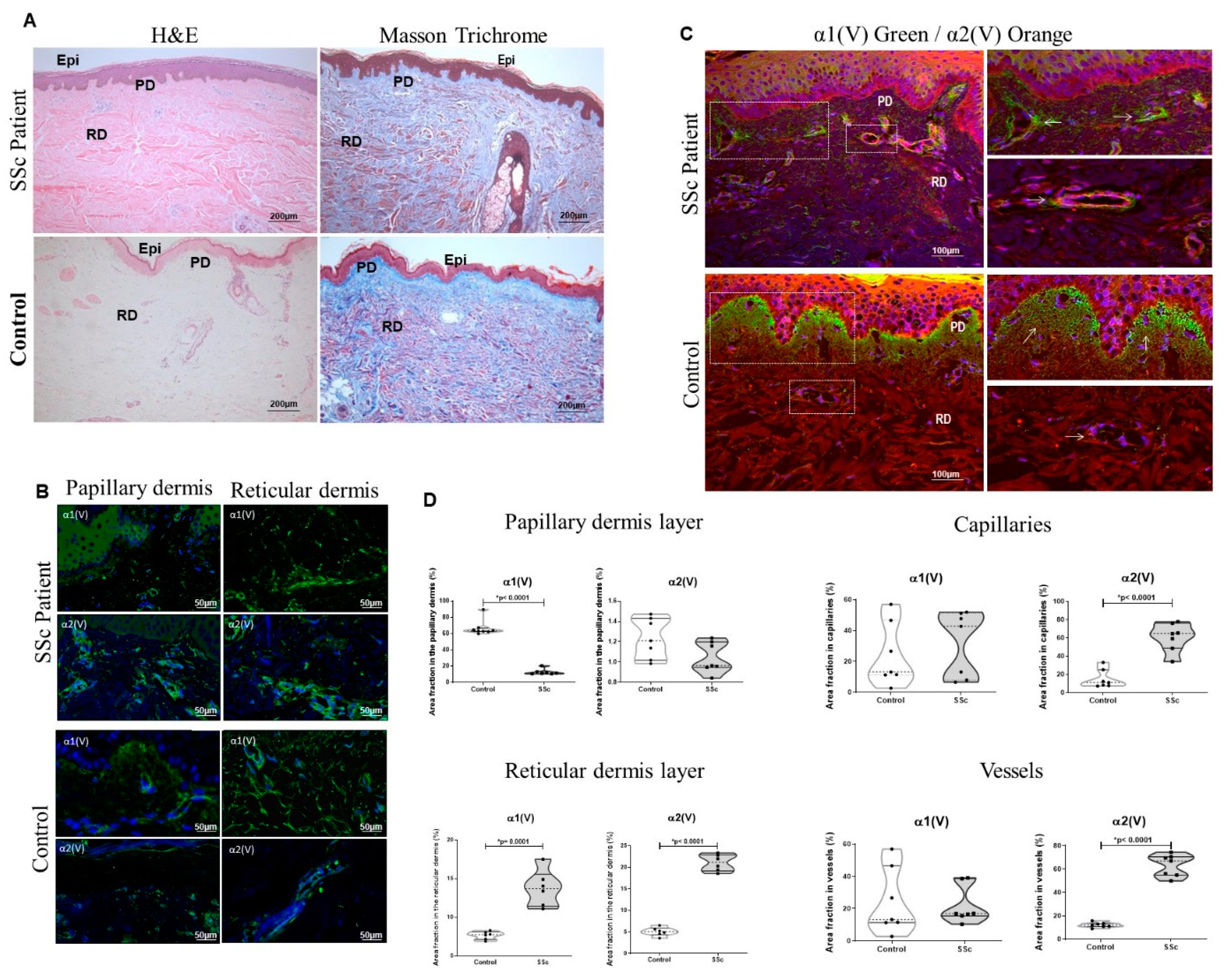
2.1. The Col V Alpha 1 Chain in Papillary Dermis Exhibits a Downregulation Pattern in Early SSc-Related Cutaneous Fibrosis
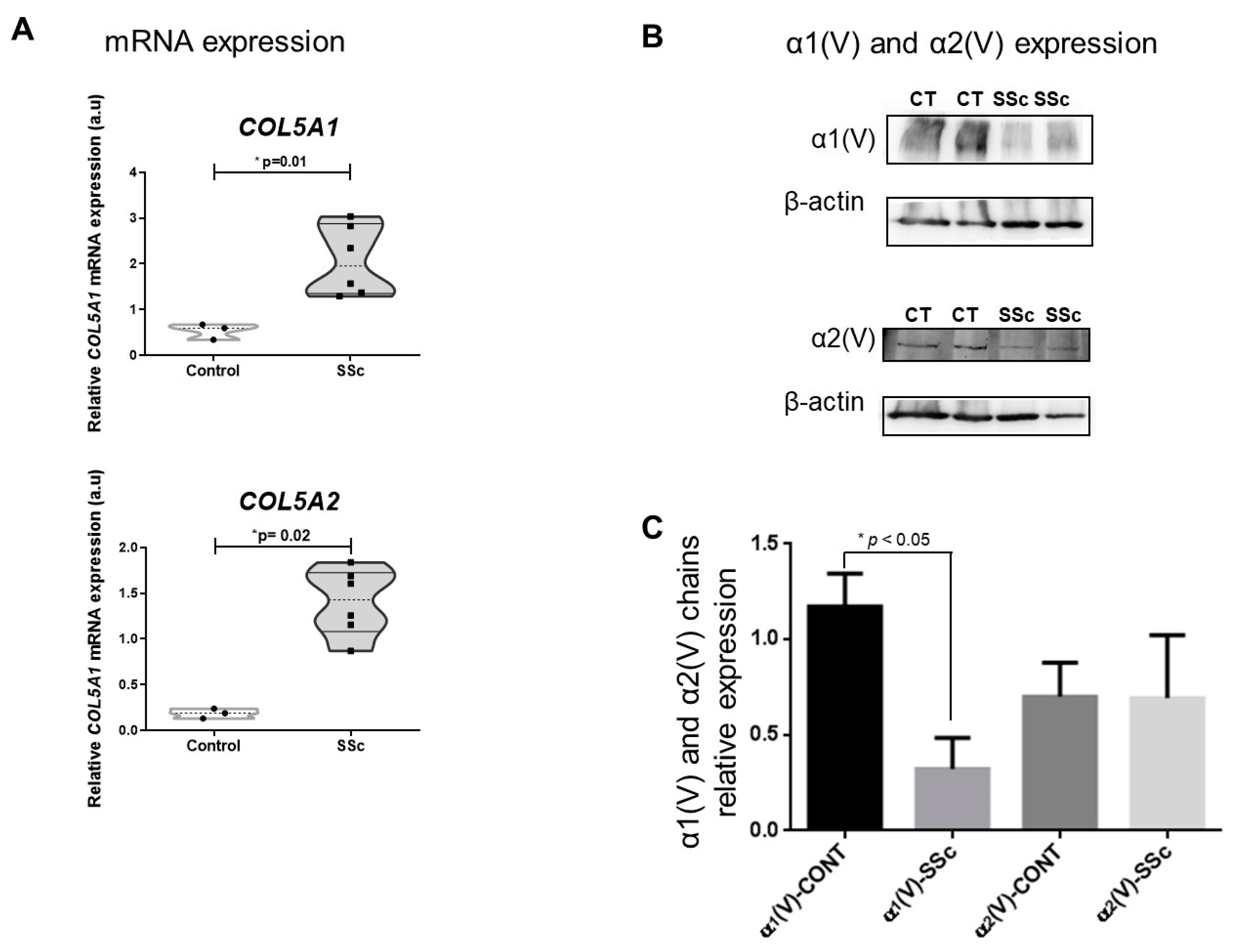
2.2. SSc Cutaneous Fibroblasts Express Decreased Col V Alpha 1 Chain despite the Overexpression of COL5A1 Gene
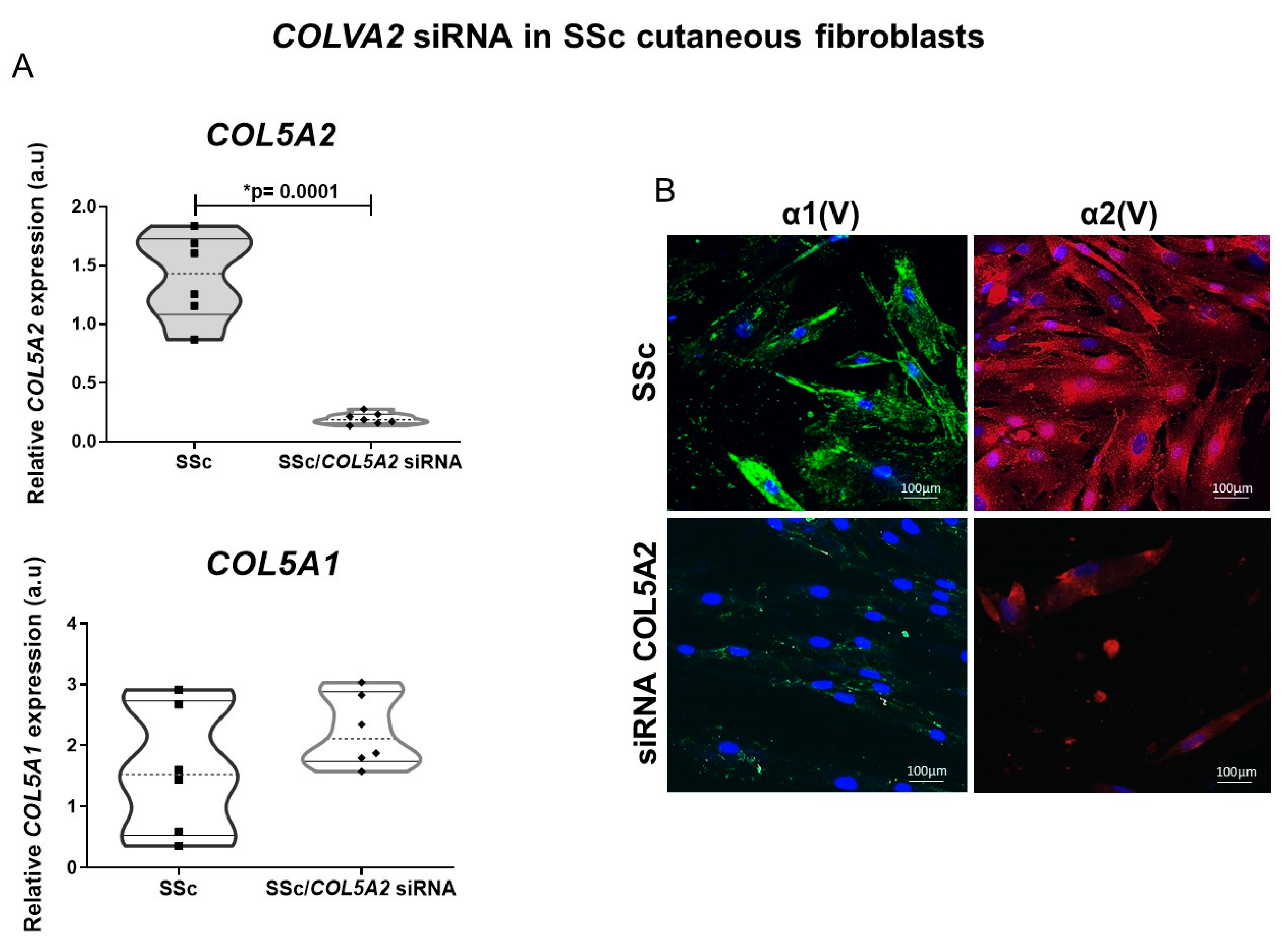
2.3. COLVA2 siRNA in SSc Cutaneous Fibroblasts Results in Decreased Col V Alpha 1 Chains Expression
3. Discussion
4. Materials and Methods
4.1. Study Participants
4.2. Histology
4.3. Immunofluorescence and Confocal Microscopy
4.4. Histomorphometry
4.5. Transmission Electron Microscopy
4.6. Immunogold Electron Microscopy
4.7. Cells Culture
4.8. Quantitative Reverse Transcription Polymerase Chain Reaction (RT-qPCR)
4.9. Western Blotting
4.10. siRNA Synthesis and Transfection
4.11. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Denton, C.P.; Khanna, D. Systemic sclerosis. Lancet 2017, 390, 1685–1699. [Google Scholar] [CrossRef]
- Varga, J.; Trojanowska, M.; Kuwana, M. Pathogenesis of systemic sclerosis: Recent insights of molecular and cellular mechanisms and therapeutic opportunities. J. Scleroderma Relat. Disord. 2017, 2, 137–152. [Google Scholar] [CrossRef]
- Tsou, P.-S.; Varga, J.; O’Reilly, S. Advances in epigenetics in systemic sclerosis: Molecular mechanisms and therapeutic potential. Nat. Rev. Rheumatol. 2021, 17, 596–607. [Google Scholar] [CrossRef] [PubMed]
- Steen, V.D.; Medsger, T.A. Severe organ involvement in systemic sclerosis with diffuse scleroderma. Arthritis Rheum. 2000, 43, 2437–2444. [Google Scholar] [CrossRef]
- Birk, D.E.; Fitch, J.M.; Babiarz, J.P.; Doane, K.J.; Linsenmayer, T.F. Collagen fibrillogenesis in vitro: Interaction of types I and V collagen regulates fibril diameter. J. Cell Sci. 1990, 95, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Bonod-Bidaud, C.; Roulet, M.; Hansen, U.; Elsheikh, A.; Malbouyres, M.; Ricard-Blum, S.; Faye, C.; Vaganay, E.; Rousselle, P.; Ruggiero, F. In vivo evidence for a bridging role of a collagen V subtype at the epidermis-dermis interface. J. Investig. Dermatol. 2012, 132, 1841–1849. [Google Scholar] [CrossRef]
- Milano, A.; Pendergrass, S.A.; Sargent, J.L.; George, L.K.; McCalmont, T.H.; Connolly, M.K.; Whitfield, M.L. Molecular subsets in the gene expression signatures of scleroderma skin. PLoS ONE 2008, 3, e2696. [Google Scholar] [CrossRef]
- Angiolilli, C.; Marut, W.; van der Kroef, M.; Chouri, E.; Reedquist, K.A.; Radstake, T.R.D.J. New insights into the genetics and epigenetics of systemic sclerosis. Nat. Rev. Rheumatol. 2018, 14, 657–673. [Google Scholar] [CrossRef]
- Martin, P.; Teodoro, W.R.; Velosa, A.P.P.; de Morais, J.; Carrasco, S.; Christmann, R.B.; Goldenstein-Schainberg, C.; Parra, E.R.; Katayama, M.L.; Sotto, M.N. Abnormal collagen V deposition in dermis correlates with skin thickening and disease activity in systemic sclerosis. Autoimmun. Rev. 2012, 11, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Parra, E.R.; Aguiar, A.C.; Teodoro, W.R.; de Souza, R.; Yoshinari, N.H.; Capelozzi, V.L. Collagen V and vascular injury promote lung architectural changes in systemic sclerosis. Clin. Respir. J. 2009, 3, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Teodoro, W.R.; de Jesus Queiroz, Z.A.; dos Santos, L.A.; Catanozi, S.; dos Santos Filho, A.; Bueno, C.; Vendramini, M.B.G.; Fernezlian, S.M.; Eher, E.M.; Sampaio-Barros, P.D.; et al. Proposition of a novel animal model of systemic sclerosis induced by type V collagen in C57BL/6 mice that reproduces fibrosis, vasculopathy and autoimmunity. Arthritis Res. Ther. 2019, 21, 278. [Google Scholar] [CrossRef]
- Xu, X.; Ramanujam, M.; Visvanathan, S.; Assassi, S.; Liu, Z.; Li, L. Transcriptional insights into pathogenesis of cutaneous systemic sclerosis using pathway driven meta-analysis assisted by machine learning methods. PLoS ONE 2020, 15, e0242863. [Google Scholar] [CrossRef] [PubMed]
- Skaug, B.; Khanna, D.; Swindell, W.R.; Hinchcliff, M.E.; Frech, T.M.; Steen, V.D.; Hant, F.N.; Gordon, J.K.; Shah, A.A.; Zhu, L.; et al. Global skin gene expression analysis of early diffuse cutaneous systemic sclerosis shows a prominent innate and adaptive inflammatory profile. Ann. Rheum. Dis. 2019, 79, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Sargent, J.L.; Li, Z.; Aliprantis, A.O.; Greenblatt, M.; Lemaire, R.; Wu, M.H.; Wei, J.; Taroni, J.; Harris, A.; Long, K.B.; et al. Identification of Optimal Mouse Models of Systemic Sclerosis by Interspecies Comparative Genomics. Arthritis Rheumatol. 2016, 68, 2003–2015. [Google Scholar] [CrossRef]
- Birk, D.E. Type V collagen: Heterotypic type I/V collagen interactions in the regulation of fibril assembly. Micron 2001, 32, 223–237. [Google Scholar] [CrossRef]
- Chanut-Delalande, H.; Bonod-Bidaud, C.; Cogne, S.; Malbouyres, M.; Ramirez, F.; Fichard, A.; Ruggiero, F. Development of a Functional Skin Matrix Requires Deposition of Collagen V Heterotrimers. Mol. Cell. Biol. 2004, 24, 6049–6057. [Google Scholar] [CrossRef] [PubMed]
- Mak, K.M.; Png, C.Y.M.; Lee, D.J. Type V Collagen in Health, Disease, and Fibrosis. Anat. Rec. 2016, 299, 613–629. [Google Scholar] [CrossRef]
- Wenstrup, R.J.; Smith, S.M.; Florer, J.B.; Zhang, G.; Beason, D.P.; Seegmiller, R.E.; Soslowsky, L.J.; Birk, D.E. Regulation of Collagen Fibril Nucleation and Initial Fibril Assembly Involves Coordinate Interactions with Collagens V and XI in Developing Tendon. J. Biol. Chem. 2011, 286, 20455–20465. [Google Scholar] [CrossRef] [PubMed]
- Hasebe, Y.; Hasegawa, S.; Date, Y.; Nakata, S.; Yagami, A.; Iwata, Y.; Sugiura, K.; Akamatsu, H. Localization of collagen type 5 in the papillary dermis and its role in maintaining stem cell functions. J. Dermatol. Sci. 2018, 89, 205–207. [Google Scholar] [CrossRef]
- Ritelli, M.; Dordoni, C.; Venturini, M.; Chiarelli, N.; Quinzani, S.; Traversa, M.; Zoppi, N.; Vascellaro, A.; Wischmeijer, A.; Manfredini, E.; et al. Clinical and molecular characterization of 40 patients with classic Ehlers-Danlos syndrome: Identification of 18 COL5A1 and 2 COL5A2 novel mutations. Orphanet J. Rare Dis. 2013, 8, 58. [Google Scholar] [CrossRef]
- Janson, D.; Saintigny, G.; Mahé, C.; el Ghalbzouri, A. Papillary fibroblasts differentiate into reticular fibroblasts after prolonged in vitro culture. Exp. Dermatol. 2013, 22, 48–53. [Google Scholar] [CrossRef]
- Driskell, R.R.; Lichtenberger, B.M.; Hoste, E.; Kretzschmar, K.; Simons, B.D.; Charalambous, M.; Ferron, S.R.; Herault, Y.; Pavlovic, G.; Ferguson-Smith, A.C.; et al. Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature 2013, 504, 277–281. [Google Scholar] [CrossRef]
- Zurita-Salinas, C.S.; Krötzsch, E.; Díaz De León, L.; Alcocer-Varela, J. Collagen turnover is diminished by different clones of skin fibroblasts from early- but not late-stage systemic sclerosis. Rheumatol. Int. 2003, 24, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Janson, D.; Saintigny, G.; Zeypveld, J.; Mahé, C.; el Ghalbzouri, A. TGF-β1 induces differentiation of papillary fibroblasts to reticular fibroblasts in monolayer culture but not in human skin equivalents. Eur. J. Dermatol. 2014, 24, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Rosendahl, A.H.; Schönborn, K.; Krieg, T. Pathophysiology of systemic sclerosis (scleroderma). Kaohsiung J. Med. Sci. 2022, 38, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Querfeld, C.; Eckes, B.; Huerkamp, C.; Krieg, T.; Sollberg, S. Expression of TGF-β1, -β2 and -β3 in localized and systemic scleroderma. J. Dermatol. Sci. 1999, 21, 13–22. [Google Scholar] [CrossRef]
- Pannu, J.; Gardner, H.; Shearstone, J.R.; Smith, E.; Trojanowska, M. Increased levels of transforming growth factor β receptor type I and up-regulation of matrix gene program: A model of scleroderma. Arthritis Care Res. 2006, 54, 3011. [Google Scholar] [CrossRef] [PubMed]
- Sargent, J.L.; Milano, A.; Bhattacharyya, S.; Varga, J.; Connolly, M.K.; Chang, H.Y.; Whitfield, M.L. A TGFΒ-responsive gene signature is associated with a subset of diffuse scleroderma with increased disease severity. J. Investig. Dermatol. 2010, 130, 694–705. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, L.; Ruppert, C.; Ochs, M. Tissue remodelling in pulmonary fibrosis. Cell Tissue Res. 2017, 367, 607–626. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, J. Expression and distribution of type I, III and V collagens in skin lesions from patients with systemic sclerosis. J. Sichuan Univ. Med. Sci. Ed. 2008, 39, 748–752. [Google Scholar]
- Ishikawa, Y.; Taga, Y.; Zientek, K.; Mizuno, N.; Salo, A.M.; Semenova, O.; Tufa, S.F.; Keene, D.R.; Holden, P.; Mizuno, K.; et al. Type I and type V procollagen triple helix uses different subsets of the molecular ensemble for lysine posttranslational modifications in the rER. J. Biol. Chem. 2021, 296, 100453. [Google Scholar] [CrossRef] [PubMed]
- Ellgaard, L.; McCaul, N.; Chatsisvili, A.; Braakman, I. Co- and Post-Translational Protein Folding in the ER. Traffic 2016, 17, 615–638. [Google Scholar] [CrossRef]
- Zeltz, C.; Gullberg, D. The integrin-collagen connection—A glue for tissue repair? J. Cell Sci. 2016, 129, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Chanut-Delalande, H.; Fichard, A.; Bernocco, S.; Garrone, R.; Hulmes, D.J.S.; Ruggiero, F. Control of Heterotypic Fibril Formation by Collagen V Is Determined by Chain Stoichiometry. J. Biol. Chem. 2001, 276, 24352–24359. [Google Scholar] [CrossRef] [PubMed]
- Stock, C.J.W.; Michaeloudes, C.; Leoni, P.; Durham, A.L.; Mumby, S.; Wells, A.U.; Chung, K.F.; Adcock, I.M.; Renzoni, E.A.; Lindahl, G.E. Bromodomain and Extraterminal (BET) Protein Inhibition Restores Redox Balance and Inhibits Myofibroblast Activation. BioMed Res. Int. 2019, 2019, 1484736. [Google Scholar] [CrossRef]
- Pachera, E.; Assassi, S.; Salazar, G.A.; Stellato, M.; Renoux, F.; Wunderlin, A.; Blyszczuk, P.; Lafyatis, R.; Kurreeman, F.; Vries-Bouwstra, J.; et al. Long noncoding RNA H19X is a key mediator of TGF-β–driven fibrosis. J. Clin. Investig. 2020, 130, 4888–4905. [Google Scholar] [CrossRef] [PubMed]
- Henderson, J.; Distler, J.; O’Reilly, S. The Role of Epigenetic Modifications in Systemic Sclerosis: A Druggable Target. Trends Mol. Med. 2019, 25, 395–411. [Google Scholar] [CrossRef]
- van den Hoogen, F.; Khanna, D.; Fransen, J.; Johnson, S.R.; Baron, M.; Tyndall, A.; Matucci-Cerinic, M.; Naden, R.P.; Medsger, T.A.; Carreira, P.E.; et al. classification criteria for systemic sclerosis: An American college of rheumatology/European league against rheumatism collaborative initiative. Ann. Rheum. Dis. 2013, 72, 1747–1755. [Google Scholar] [CrossRef] [PubMed]
- Clements, P.; Lachenbruch, P.; Siebold, J.; White, B.; Weiner, S.; Martin, R.; Weinstein, A.; Weinstein, M.; Mayes, M.; Collier, D.; et al. Inter and intraobserver variability of total skin thickness score (Modified Rodnan TSS) in systemic sclerosis. J. Rheumatol. 1995, 22, 1281–1285. [Google Scholar]
- Valentini, G.; Silman, A.J.; Veale, D. Assessment of disease activity. Clin. Exp. Rheumatol. 2003, 21, S39–S41. [Google Scholar] [PubMed]
- Keira, S.M.; Ferreira, L.M.; Gragnani, A.; Duarte, I.S.; Santos, I.A.N. Experimental model for fibroblast culture. Acta Cir. Bras. 2004, 19, 11–16. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]

| Clinical and Laboratory Features | |
|---|---|
| N (%) | 7 (100%) |
| Age, standard deviation (range) | 42 (12) |
| Sex, N (%) | Female, 4 (60%) 1 Male, 3 (40%) |
| Clinical subtype, N (%) | Limited, 3, (40%) Diffuse, 4 (60%) |
| Disease duration, median (range) | 12 (14–24 meses) |
| MRSS1, median (range) | 25 (11–40) |
| Valentini activity index 2, median (range) | 0.5 |
| FAN+, N (%) | 7 (100%) |
| Anti-Scl70+, N (%) | 2 (28.7%) |
| Anticentromere+, N (%) | 4 (51%) |
| Gene | Sense 3′—5′ | Antisense 5′—3′ | (pb) |
|---|---|---|---|
| ACTB | AGAAAATCTGGCACCACACC | AGAGGCGTACAGGGATAGCA | 175 |
| COL5A1 | GTGGCACAGAATTGCTCTCA | CTGGATGTCACCCTCAAACA | 175 |
| COL5A2 | GGAAATGTGGGCAAGACTGT | TTGATGGTGGTGCTCATTGT | 169 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Morais, J.; Velosa, A.P.P.; Andrade, P.C.; Frediani, D.; Carrasco, S.; Queiroz, Z.A.d.J.; Martin, P.; Saito, R.F.; Elias, V.; Goldenstein-Schainberg, C.; et al. Collagen V α1 Chain Decrease in Papillary Dermis from Early Systemic Sclerosis: A New Proposal in Cutaneous Fibrosis Molecular Structure. Int. J. Mol. Sci. 2022, 23, 12654. https://doi.org/10.3390/ijms232012654
de Morais J, Velosa APP, Andrade PC, Frediani D, Carrasco S, Queiroz ZAdJ, Martin P, Saito RF, Elias V, Goldenstein-Schainberg C, et al. Collagen V α1 Chain Decrease in Papillary Dermis from Early Systemic Sclerosis: A New Proposal in Cutaneous Fibrosis Molecular Structure. International Journal of Molecular Sciences. 2022; 23(20):12654. https://doi.org/10.3390/ijms232012654
Chicago/Turabian Stylede Morais, Jymenez, Ana Paula P. Velosa, Priscila C. Andrade, Denise Frediani, Solange Carrasco, Zelita A. de Jesus Queiroz, Patrícia Martin, Renata F. Saito, Vitória Elias, Cláudia Goldenstein-Schainberg, and et al. 2022. "Collagen V α1 Chain Decrease in Papillary Dermis from Early Systemic Sclerosis: A New Proposal in Cutaneous Fibrosis Molecular Structure" International Journal of Molecular Sciences 23, no. 20: 12654. https://doi.org/10.3390/ijms232012654
APA Stylede Morais, J., Velosa, A. P. P., Andrade, P. C., Frediani, D., Carrasco, S., Queiroz, Z. A. d. J., Martin, P., Saito, R. F., Elias, V., Goldenstein-Schainberg, C., Chammas, R., Sampaio-Barros, P. D., Capelozzi, V. L., & Teodoro, W. R. (2022). Collagen V α1 Chain Decrease in Papillary Dermis from Early Systemic Sclerosis: A New Proposal in Cutaneous Fibrosis Molecular Structure. International Journal of Molecular Sciences, 23(20), 12654. https://doi.org/10.3390/ijms232012654








