The Central Nervous Mechanism of Stress-Promoting Cancer Progression
Abstract
1. Background
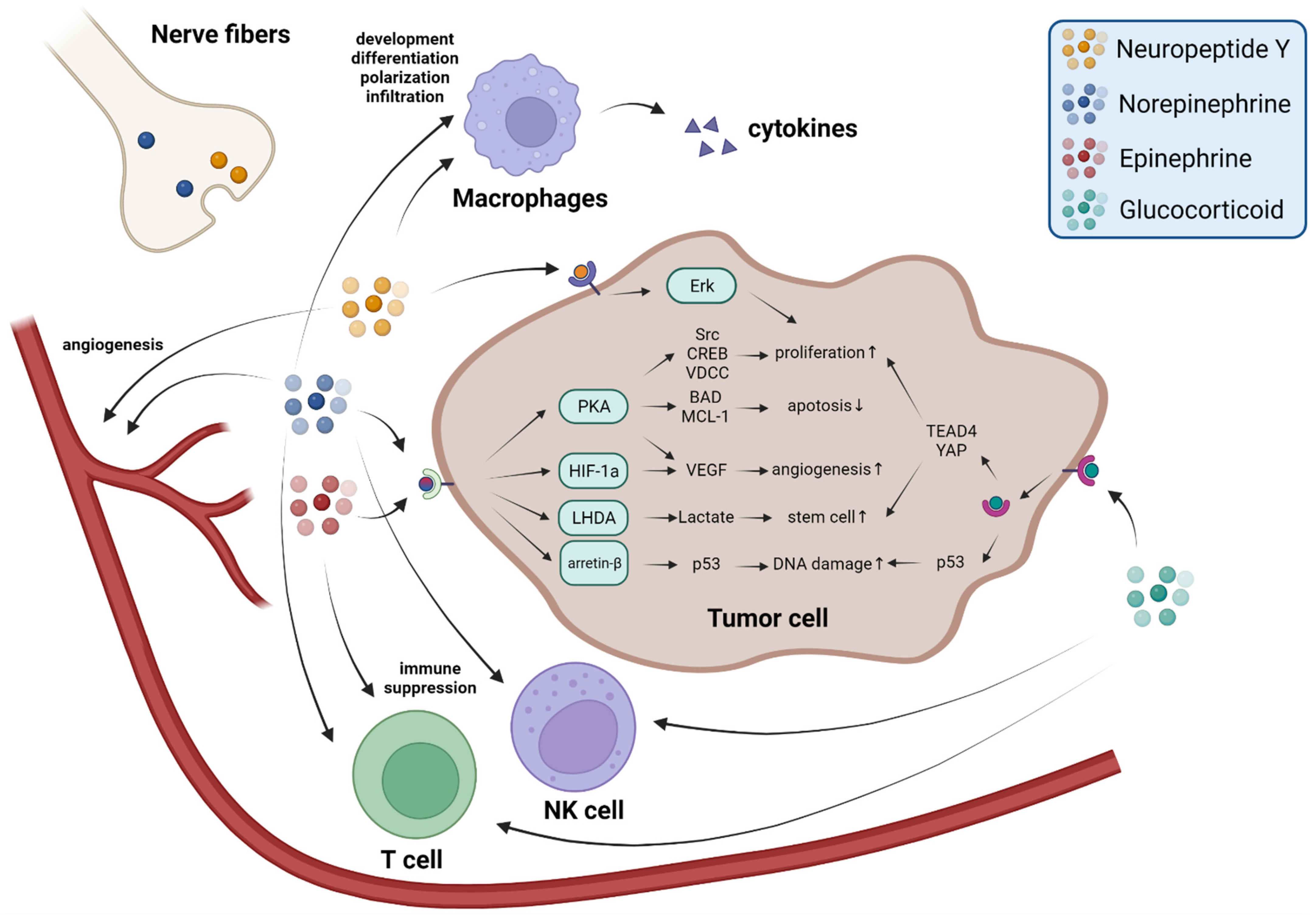
2. Stress Accelerates Tumor Progression via the Sympathetic Nervous System
2.1. Stress Activates SNS-Related Neural Circuits
2.2. Sympathetic Nerve Fibers Release Neurotransmitters to Promote Tumor Progression
2.3. The Adrenal Medulla Secretes Epinephrine to Promote Tumor Progression
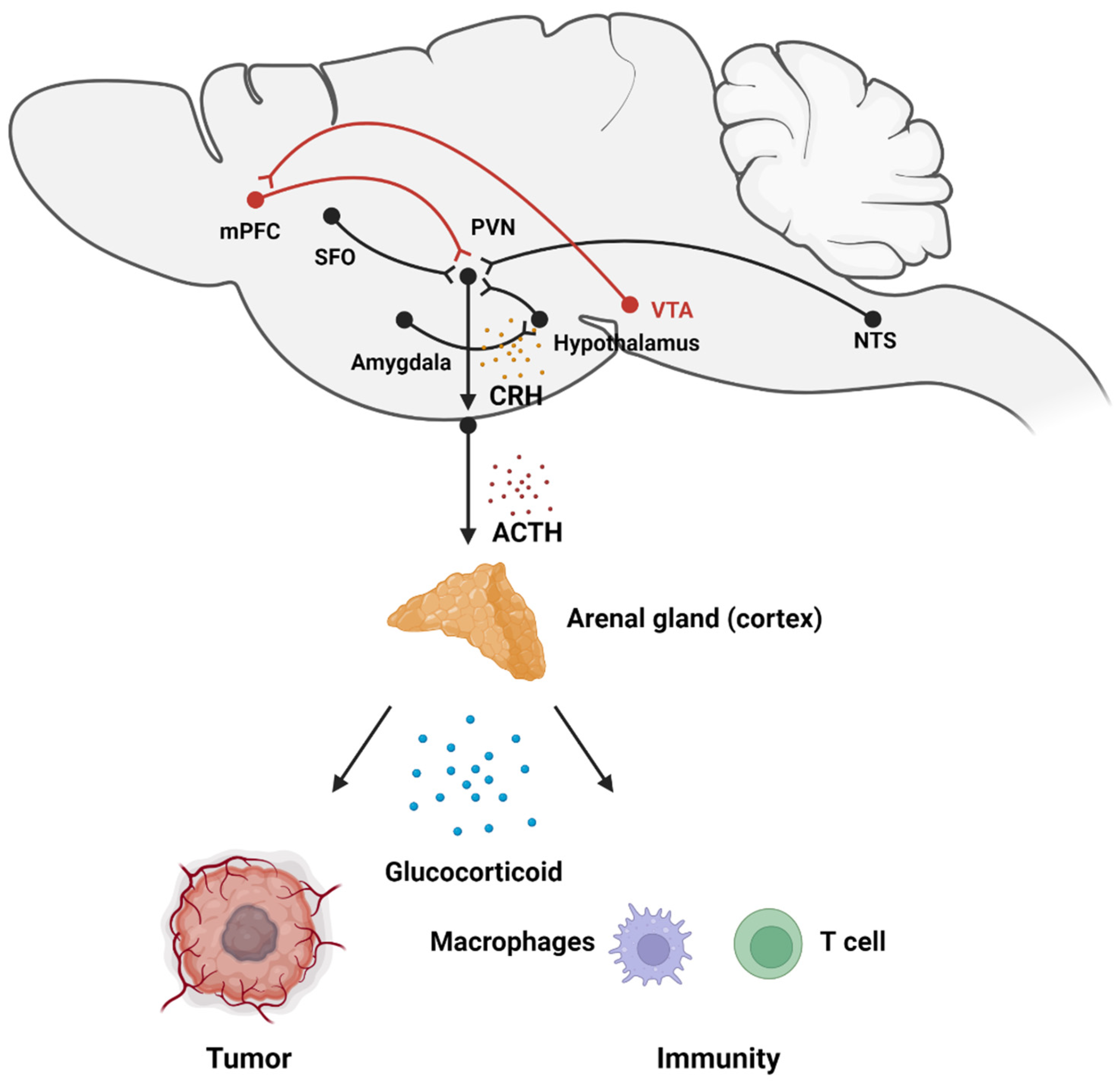
3. Stress Accelerates Tumor Progression via the HPA Axis
3.1. Stress Activates HPA Axis-Related Neural Circuits
3.2. The Adrenal Medulla Secretes Glucocorticoids to Promote Tumor Progression
4. Other Stressors
5. Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Src | Proto-oncogene tyrosine-protein kinase Src |
| CREB | cAMP response element-binding protein |
| NO | Nitric oxide |
| IL-4 | Interleukin-4 |
| IL-6 | Interleukin-6 |
| IL-12 | Interleukin-12 |
| TNF-α | Tumor necrosis factor α |
| BAD | Bcl-2-associated death promoter |
| MCL-1 | Myeloid leukemia 1 |
| LHDA | Lactate dehydrogenase A–dependent |
| VEGF | Vascular Endothelial Growth Factor |
| HIF-1α | Hypoxia-inducible factor 1a |
| IFN-γ | Interferon-γ |
| BRCA1 | Breast cancer type 1 gene |
| YAP | Yes-associated protein |
| TEAD4 | TEA Domain Transcription Factor 4 |
| PD-1/PD-L1 | Programmed Cell Death 1/Programmed Cell Death Ligand 1 |
| GABA | Gamma-aminobutyric acid |
| fMRI | Functional magnetic resonance imaging |
References
- McEwen, B.S.; Akil, H. Revisiting the Stress Concept: Implications for Affective Disorders. J. Neurosci. 2020, 40, 12–21. [Google Scholar] [CrossRef]
- Sapolsky, R.M. Stress and the brain: Individual variability and the inverted-U. Nat. Neurosci. 2015, 18, 1344–1346. [Google Scholar] [CrossRef]
- Calhoon, G.G.; Tye, K.M. Resolving the neural circuits of anxiety. Nat. Neurosci. 2015, 18, 1394–1404. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, Y.; Wang, H.; Liu, Z.; Yu, X.; Yan, J.; Yu, Y.; Kou, C.; Xu, X.; Lu, J.; et al. Prevalence of mental disorders in China: A cross-sectional epidemiological study. Lancet Psychiatry 2019, 6, 211–224. [Google Scholar] [CrossRef]
- McEwen, B.S. Neurobiological and Systemic Effects of Chronic Stress. Chronic Stress 2017, 1, 2470547017692328. [Google Scholar] [CrossRef] [PubMed]
- Clyne, M. Sexual dysfunction: Psychological inputs to sexual dysfunction. Nat. Rev. Urol. 2012, 9, 238. [Google Scholar] [CrossRef]
- Francois, A.; Low, S.A.; Sypek, E.I.; Christensen, A.J.; Sotoudeh, C.; Beier, K.T.; Ramakrishnan, C.; Ritola, K.D.; Sharif-Naeini, R.; Deisseroth, K.; et al. A Brainstem-Spinal Cord Inhibitory Circuit for Mechanical Pain Modulation by GABA and Enkephalins. Neuron 2017, 93, 822–839.e6. [Google Scholar] [CrossRef]
- Kim-Fuchs, C.; Le, C.P.; Pimentel, M.A.; Shackleford, D.; Ferrari, D.; Angst, E.; Hollande, F.; Sloan, E.K. Chronic stress accelerates pancreatic cancer growth and invasion: A critical role for beta-adrenergic signaling in the pancreatic microenvironment. Brain Behav. Immun. 2014, 40, 40–47. [Google Scholar] [CrossRef]
- Mayer, E.A.; Craske, M.; Naliboff, B.D. Depression, anxiety, and the gastrointestinal system. J. Clin. Psychiatry 2001, 62 (Suppl. S8), 28–36; Discussion 37. [Google Scholar]
- Ray, A.; Gulati, K.; Rai, N. Stress, Anxiety, and Immunomodulation: A Pharmacological Analysis. Vitam. Horm. 2017, 103, 1–25. [Google Scholar]
- Wang, Y.H.; Li, J.Q.; Shi, J.F.; Que, J.Y.; Liu, J.J.; Lappin, J.M.; Leung, J.; Ravindran, A.V.; Chen, W.Q.; Qiao, Y.L.; et al. Depression and anxiety in relation to cancer incidence and mortality: A systematic review and meta-analysis of cohort studies. Mol. Psychiatry 2020, 25, 1487–1499. [Google Scholar] [CrossRef] [PubMed]
- Cole, S.W.; Nagaraja, A.S.; Lutgendorf, S.K.; Green, P.A.; Sood, A.K. Sympathetic nervous system regulation of the tumour microenvironment. Nat. Rev. Cancer 2015, 15, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Lamotte, G.; Shouman, K.; Benarroch, E.E. Stress and central autonomic network. Auton. Neurosci. 2021, 235, 102870. [Google Scholar] [CrossRef] [PubMed]
- Joseph, D.N.; Whirledge, S. Stress and the HPA Axis: Balancing Homeostasis and Fertility. Int. J. Mol. Sci. 2017, 18, 2224. [Google Scholar] [CrossRef]
- Eckerling, A.; Ricon-Becker, I.; Sorski, L.; Sandbank, E.; Ben-Eliyahu, S. Stress and cancer: Mechanisms, significance and future directions. Nat. Rev. Cancer 2021, 21, 767–785. [Google Scholar] [CrossRef] [PubMed]
- Obradovic, M.M.S.; Hamelin, B.; Manevski, N.; Couto, J.P.; Sethi, A.; Coissieux, M.M.; Munst, S.; Okamoto, R.; Kohler, H.; Schmidt, A.; et al. Glucocorticoids promote breast cancer metastasis. Nature 2019, 567, 540–544. [Google Scholar] [CrossRef] [PubMed]
- Sloan, E.K.; Priceman, S.J.; Cox, B.F.; Yu, S.; Pimentel, M.A.; Tangkanangnukul, V.; Arevalo, J.M.; Morizono, K.; Karanikolas, B.D.; Wu, L.; et al. The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res. 2010, 70, 7042–7052. [Google Scholar] [CrossRef]
- Bernabe, D.G. Catecholamines Mediate Psychologic Stress-Induced Cancer Progression. Cancer Res. 2021, 81, 5144–5146. [Google Scholar] [CrossRef]
- Cui, B.; Peng, F.; Lu, J.; He, B.; Su, Q.; Luo, H.; Deng, Z.; Jiang, T.; Su, K.; Huang, Y.; et al. Cancer and stress: NextGen strategies. Brain Behav. Immun. 2021, 93, 368–383. [Google Scholar] [CrossRef]
- Dai, S.; Mo, Y.; Wang, Y.; Xiang, B.; Liao, Q.; Zhou, M.; Li, X.; Li, Y.; Xiong, W.; Li, G.; et al. Chronic Stress Promotes Cancer Development. Front. Oncol. 2020, 10, 1492. [Google Scholar] [CrossRef]
- Guyenet, P.G.; Stornetta, R.L. Rostral ventrolateral medulla, retropontine region and autonomic regulations. Auton. Neurosci. 2022, 237, 102922. [Google Scholar] [CrossRef] [PubMed]
- Won, E.; Kim, Y.K. Stress, the Autonomic Nervous System, and the Immune-kynurenine Pathway in the Etiology of Depression. Curr. Neuropharmacol. 2016, 14, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Zhang, C.; Lischinsky, J.E.; Jing, M.; Zhou, J.; Wang, H.; Zhang, Y.; Dong, A.; Wu, Z.; Wu, H.; et al. A Genetically Encoded Fluorescent Sensor for Rapid and Specific In Vivo Detection of Norepinephrine. Neuron 2019, 102, 745–761.e8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, Y.; Lv, X.; Zhao, L.; Wang, X. VLM catecholaminergic neurons control tumor growth by regulating CD8+ T cells. Proc. Natl. Acad. Sci. USA 2021, 118, e2103505118. [Google Scholar] [CrossRef]
- Magnon, C.; Hall, S.J.; Lin, J.; Xue, X.; Gerber, L.; Freedland, S.J.; Frenette, P.S. Autonomic nerve development contributes to prostate cancer progression. Science 2013, 341, 1236361. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, L.; Gao, W.; Hu, F.; Zhang, J.; Ren, Y.; Lin, R.; Feng, Q.; Cheng, M.; Ju, D.; et al. A Central Catecholaminergic Circuit Controls Blood Glucose Levels during Stress. Neuron 2017, 95, 138–152.e5. [Google Scholar] [CrossRef]
- Zhang, W.H.; Zhang, J.Y.; Holmes, A.; Pan, B.X. Amygdala Circuit Substrates for Stress Adaptation and Adversity. Biol. Psychiatry 2021, 89, 847–856. [Google Scholar] [CrossRef]
- Yoshikawa, E.; Matsuoka, Y.; Yamasue, H.; Inagaki, M.; Nakano, T.; Akechi, T.; Kobayakawa, M.; Fujimori, M.; Nakaya, N.; Akizuki, N.; et al. Prefrontal cortex and amygdala volume in first minor or major depressive episode after cancer diagnosis. Biol. Psychiatry 2006, 59, 707–712. [Google Scholar] [CrossRef]
- Leschak, C.J.; Dutcher, J.M.; Haltom, K.E.B.; Breen, E.C.; Bower, J.E.; Eisenberger, N.I. Associations between amygdala reactivity to social threat, perceived stress and C-reactive protein in breast cancer survivors. Soc. Cogn. Affect. Neurosci. 2020, 15, 1056–1063. [Google Scholar] [CrossRef]
- Muscatell, K.A.; Eisenberger, N.I.; Dutcher, J.M.; Cole, S.W.; Bower, J.E. Links between inflammation, amygdala reactivity, and social support in breast cancer survivors. Brain Behav. Immun. 2016, 53, 34–38. [Google Scholar] [CrossRef]
- Saha, S. Role of the central nucleus of the amygdala in the control of blood pressure: Descending pathways to medullary cardiovascular nuclei. Clin. Exp. Pharmacol. Physiol. 2005, 32, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Zhou, X.; Wei, P.; Xie, L.; Han, Y.; Wang, J.; Cai, A.; Xu, F.; Tu, J.; Wang, L. A new GABAergic somatostatin projection from the BNST onto accumbal parvalbumin neurons controls anxiety. Mol. Psychiatry 2021, 26, 4719–4741. [Google Scholar] [CrossRef]
- Gomes-de-Souza, L.; Costa-Ferreira, W.; Oliveira, L.A.; Benini, R.; Crestani, C.C. Cannabinoid receptor type 1 in the bed nucleus of the stria terminalis modulates cardiovascular responses to stress via local N-methyl-D-aspartate receptor/neuronal nitric oxide synthase/soluble guanylate cyclase/protein kinase G signaling. J. Psychopharmacol. 2020, 34, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.A.; Gomes-de-Souza, L.; Benini, R.; Crestani, C.C. Control of cardiovascular responses to stress by CRF in the bed nucleus of stria terminalis is mediated by local NMDA/nNOS/sGC/PKG signaling. Psychoneuroendocrinology 2018, 89, 168–176. [Google Scholar] [CrossRef]
- Nasimi, A.; Kafami, M. Vasopressin and sympathetic system mediate the cardiovascular effects of the angiotensin II in the bed nucleus of the stria terminalis in rat. Neurosci. Res. 2016, 108, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Kono, Y.; Yokota, S.; Fukushi, I.; Arima, Y.; Onimaru, H.; Okazaki, S.; Takeda, K.; Yazawa, I.; Yoshizawa, M.; Hasebe, Y.; et al. Structural and functional connectivity from the dorsomedial hypothalamus to the ventral medulla as a chronological amplifier of sympathetic outflow. Sci. Rep. 2020, 10, 13325. [Google Scholar] [CrossRef] [PubMed]
- Ulrich-Lai, Y.M.; Herman, J.P. Neural regulation of endocrine and autonomic stress responses. Nat. Rev. Neurosci. 2009, 10, 397–409. [Google Scholar] [CrossRef]
- Heydendael, W.; Sengupta, A.; Beck, S.; Bhatnagar, S. Optogenetic examination identifies a context-specific role for orexins/hypocretins in anxiety-related behavior. Physiol. Behav. 2014, 130, 182–190. [Google Scholar] [CrossRef]
- Borniger, J.C.; Walker II, W.H.; Surbhi; Emmer, K.M.; Zhang, N.; Zalenski, A.A.; Muscarella, S.L.; Fitzgerald, J.A.; Smith, A.N.; Braam, C.J.; et al. A Role for Hypocretin/Orexin in Metabolic and Sleep Abnormalities in a Mouse Model of Non-metastatic Breast Cancer. Cell Metab. 2018, 28, 118–129.e5. [Google Scholar] [CrossRef] [PubMed]
- Brechner, T.; Motyka, D.; Sherman, J. Growth enhancement of prolactin-sensitive mammary tumor by periaqueductal gray stimulation. Life Sci. 1983, 32, 525–530. [Google Scholar] [CrossRef]
- Ni, H.D.; Xu, L.S.; Wang, Y.; Li, H.; An, K.; Liu, M.; Liu, Q.; Deng, H.; He, Q.; Huang, B.; et al. Astrocyte activation in the periaqueductal gray promotes descending facilitation to cancer-induced bone pain through the JNK MAPK signaling pathway. Mol. Pain 2019, 15, 1744806919831909. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhang, Q.; Xiao, Z.; Yu, S.; Yan, Y.; Qin, Y. Activation of the P2X7 receptor in midbrain periaqueductal gray participates in the analgesic effect of tramadol in bone cancer pain rats. Mol. Pain 2018, 14, 1744806918803039. [Google Scholar] [CrossRef] [PubMed]
- Benarroch, E.E. Descending monoaminergic pain modulation: Bidirectional control and clinical relevance. Neurology 2008, 71, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Mei, L.; Sun, T.; Wang, Y.; Li, J.; Chen, C.; Farzinpour, Z.; Mao, Y.; Tao, W.; Li, J.; et al. A Central Amygdala-Ventrolateral Periaqueductal Gray Matter Pathway for Pain in a Mouse Model of Depression-like Behavior. Anesthesiology 2020, 132, 1175–1196. [Google Scholar] [CrossRef]
- de Menezes, R.C.; Zaretsky, D.V.; Fontes, M.A.; DiMicco, J.A. Cardiovascular and thermal responses evoked from the periaqueductal grey require neuronal activity in the hypothalamus. J. Physiol. 2009, 587 Pt 6, 1201–1215. [Google Scholar] [CrossRef]
- Adhikari, A.; Lerner, T.N.; Finkelstein, J.; Pak, S.; Jennings, J.H.; Davidson, T.J.; Ferenczi, E.; Gunaydin, L.A.; Mirzabekov, J.J.; Ye, L.; et al. Basomedial amygdala mediates top-down control of anxiety and fear. Nature 2015, 527, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Schaeuble, D.; Packard, A.E.B.; McKlveen, J.M.; Morano, R.; Fourman, S.; Smith, B.L.; Scheimann, J.R.; Packard, B.A.; Wilson, S.P.; James, J.; et al. Prefrontal Cortex Regulates Chronic Stress-Induced Cardiovascular Susceptibility. J. Am. Heart Assoc. 2019, 8, e014451. [Google Scholar] [CrossRef]
- Pastor, V.; Medina, J.H. Medial prefrontal cortical control of reward- and aversion-based behavioral output: Bottom-up modulation. Eur. J. Neurosci. 2021, 53, 3039–3062. [Google Scholar] [CrossRef]
- Whitton, A.E.; Treadway, M.T.; Pizzagalli, D.A. Reward processing dysfunction in major depression, bipolar disorder and schizophrenia. Curr. Opin. Psychiatry 2015, 28, 7–12. [Google Scholar] [CrossRef]
- Ben-Shaanan, T.L.; Schiller, M.; Azulay-Debby, H.; Korin, B.; Boshnak, N.; Koren, T.; Krot, M.; Shakya, J.; Rahat, M.A.; Hakim, F.; et al. Modulation of anti-tumor immunity by the brain’s reward system. Nat. Commun. 2018, 9, 2723. [Google Scholar] [CrossRef]
- Xu, X.R.; Xiao, Q.; Hong, Y.C.; Liu, Y.H.; Liu, Y.; Tu, J. Activation of dopaminergic VTA inputs to the mPFC ameliorates chronic stress-induced breast tumor progression. CNS Neurosci. Ther. 2021, 27, 206–219. [Google Scholar] [CrossRef] [PubMed]
- Ayala, G.E.; Dai, H.; Powell, M.; Li, R.; Ding, Y.; Wheeler, T.M.; Shine, D.; Kadmon, D.; Thompson, T.; Miles, B.J.; et al. Cancer-related axonogenesis and neurogenesis in prostate cancer. Clin. Cancer Res. 2008, 14, 7593–7603. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, A.; Hayama, Y.; Kato, S.; Shimomura, A.; Shimomura, T.; Irie, K.; Kaneko, R.; Yanagawa, Y.; Kobayashi, K.; Ochiya, T. Genetic manipulation of autonomic nerve fiber innervation and activity and its effect on breast cancer progression. Nat. Neurosci. 2019, 22, 1289–1305. [Google Scholar] [CrossRef]
- Thaker, P.H.; Han, L.Y.; Kamat, A.A.; Arevalo, J.M.; Takahashi, R.; Lu, C.; Jennings, N.B.; Armaiz-Pena, G.; Bankson, J.A.; Ravoori, M.; et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat. Med. 2006, 12, 939–944. [Google Scholar] [CrossRef]
- Gidron, Y.; Russ, K.; Tissarchondou, H.; Warner, J. The relation between psychological factors and DNA-damage: A critical review. Biol. Psychol. 2006, 72, 291–304. [Google Scholar] [CrossRef]
- Flint, M.S.; Baum, A.; Episcopo, B.; Knickelbein, K.Z.; Liegey Dougall, A.J.; Chambers, W.H.; Jenkins, F.J. Chronic exposure to stress hormones promotes transformation and tumorigenicity of 3T3 mouse fibroblasts. Stress 2013, 16, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Hara, M.R.; Kovacs, J.J.; Whalen, E.J.; Rajagopal, S.; Strachan, R.T.; Grant, W.; Towers, A.J.; Williams, B.; Lam, C.M.; Xiao, K.; et al. A stress response pathway regulates DNA damage through beta2-adrenoreceptors and beta-arrestin-1. Nature 2011, 477, 349–353. [Google Scholar] [CrossRef]
- Reeder, A.; Attar, M.; Nazario, L.; Bathula, C.; Zhang, A.; Hochbaum, D.; Roy, E.; Cooper, K.L.; Oesterreich, S.; Davidson, N.E.; et al. Stress hormones reduce the efficacy of paclitaxel in triple negative breast cancer through induction of DNA damage. Br. J. Cancer 2015, 112, 1461–1470. [Google Scholar] [CrossRef]
- Renz, B.W.; Takahashi, R.; Tanaka, T.; Macchini, M.; Hayakawa, Y.; Dantes, Z.; Maurer, H.C.; Chen, X.; Jiang, Z.; Westphalen, C.B.; et al. beta2 Adrenergic-Neurotrophin Feedforward Loop Promotes Pancreatic Cancer. Cancer Cell 2018, 33, 75–90.e7. [Google Scholar] [CrossRef]
- Armaiz-Pena, G.N.; Allen, J.K.; Cruz, A.; Stone, R.L.; Nick, A.M.; Lin, Y.G.; Han, L.Y.; Mangala, L.S.; Villares, G.J.; Vivas-Mejia, P.; et al. Src activation by beta-adrenoreceptors is a key switch for tumour metastasis. Nat. Commun. 2013, 4, 1403. [Google Scholar] [CrossRef]
- Jang, H.J.; Boo, H.J.; Lee, H.J.; Min, H.Y.; Lee, H.Y. Chronic Stress Facilitates Lung Tumorigenesis by Promoting Exocytosis of IGF2 in Lung Epithelial Cells. Cancer Res. 2016, 76, 6607–6619. [Google Scholar] [CrossRef] [PubMed]
- Zahalka, A.H.; Arnal-Estape, A.; Maryanovich, M.; Nakahara, F.; Cruz, C.D.; Finley, L.W.S.; Frenette, P.S. Adrenergic nerves activate an angio-metabolic switch in prostate cancer. Science 2017, 358, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Armaiz-Pena, G.N.; Gonzalez-Villasana, V.; Nagaraja, A.S.; Rodriguez-Aguayo, C.; Sadaoui, N.C.; Stone, R.L.; Matsuo, K.; Dalton, H.J.; Previs, R.A.; Jennings, N.B.; et al. Adrenergic regulation of monocyte chemotactic protein 1 leads to enhanced macrophage recruitment and ovarian carcinoma growth. Oncotarget 2015, 6, 4266–4273. [Google Scholar] [CrossRef]
- Heidt, T.; Sager, H.B.; Courties, G.; Dutta, P.; Iwamoto, Y.; Zaltsman, A.; von Zur Muhlen, C.; Bode, C.; Fricchione, G.L.; Denninger, J.; et al. Chronic variable stress activates hematopoietic stem cells. Nat. Med. 2014, 20, 754–758. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.F.; Jin, F.J.; Li, N.; Guan, H.T.; Lan, L.; Ni, H.; Wang, Y. Adrenergic receptor beta2 activation by stress promotes breast cancer progression through macrophages M2 polarization in tumor microenvironment. BMB Rep. 2015, 48, 295–300. [Google Scholar] [CrossRef]
- Zukowska-Grojec, Z.; Neuropeptide, Y. A novel sympathetic stress hormone and more. Ann. N. Y. Acad. Sci. 1995, 771, 219–233. [Google Scholar] [CrossRef]
- Medeiros, P.J.; Al-Khazraji, B.K.; Novielli, N.M.; Postovit, L.M.; Chambers, A.F.; Jackson, D.N. Neuropeptide Y stimulates proliferation and migration in the 4T1 breast cancer cell line. Int. J. Cancer 2012, 131, 276–286. [Google Scholar] [CrossRef]
- Lu, C.; Everhart, L.; Tilan, J.; Kuo, L.; Sun, C.C.; Munivenkatappa, R.B.; Jonsson-Rylander, A.C.; Sun, J.; Kuan-Celarier, A.; Li, L.; et al. Neuropeptide Y and its Y2 receptor: Potential targets in neuroblastoma therapy. Oncogene 2010, 29, 5630–5642. [Google Scholar] [CrossRef]
- Ekstrand, A.J.; Cao, R.; Bjorndahl, M.; Nystrom, S.; Jonsson-Rylander, A.C.; Hassani, H.; Hallberg, B.; Nordlander, M.; Cao, Y. Deletion of neuropeptide Y (NPY) 2 receptor in mice results in blockage of NPY-induced angiogenesis and delayed wound healing. Proc. Natl. Acad. Sci. USA 2003, 100, 6033–6038. [Google Scholar] [CrossRef]
- Lee, E.W.; Michalkiewicz, M.; Kitlinska, J.; Kalezic, I.; Switalska, H.; Yoo, P.; Sangkharat, A.; Ji, H.; Li, L.; Michalkiewicz, T.; et al. Neuropeptide Y induces ischemic angiogenesis and restores function of ischemic skeletal muscles. J. Clin. Investig. 2003, 111, 1853–1862. [Google Scholar] [CrossRef]
- Chandrasekharan, B.; Nezami, B.G.; Srinivasan, S. Emerging neuropeptide targets in inflammation: NPY and VIP. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 304, G949–G957. [Google Scholar] [CrossRef] [PubMed]
- Wurtman, R.J. Control of epinephrine synthesis by the pituitary and adrenal cortex: Possible role in the pathophysiology of chronic stress. Recent Adv. Biol. Psychiatry 1966, 9, 359–368. [Google Scholar] [PubMed]
- Feher, J. The Adrenal Medulla and Integration of Metabolic Control. In Quantitative Human Physiology; Academic Press: Cambridge, MA, USA, 2012; pp. 916–923. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; He, Z.; Yin, K.; Li, B.; Zhang, L.; Xu, Z. Chronic stress promotes gastric cancer progression and metastasis: An essential role for ADRB2. Cell Death Dis. 2019, 10, 788. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.; Karpova, Y.; Baiz, D.; Yancey, D.; Pullikuth, A.; Flores, A.; Register, T.; Cline, J.M.; D’Agostino, R., Jr.; Danial, N.; et al. Behavioral stress accelerates prostate cancer development in mice. J. Clin. Investig. 2013, 123, 874–886. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.; Pullikuth, A.; Nelson, K.C.; Flores, A.; Karpova, Y.; Baiz, D.; Zhu, S.; Sui, G.; Huang, Y.; Choi, Y.A.; et al. beta2-adrenoreceptor Signaling Increases Therapy Resistance in Prostate Cancer by Upregulating MCL1. Mol. Cancer Res. 2020, 18, 1839–1848. [Google Scholar] [CrossRef] [PubMed]
- Cui, B.; Luo, Y.; Tian, P.; Peng, F.; Lu, J.; Yang, Y.; Su, Q.; Liu, B.; Yu, J.; Luo, X.; et al. Stress-induced epinephrine enhances lactate dehydrogenase A and promotes breast cancer stem-like cells. J. Clin. Investig. 2019, 129, 1030–1046. [Google Scholar] [CrossRef]
- Shan, T.; Ma, J.; Ma, Q.; Guo, K.; Guo, J.; Li, X.; Li, W.; Liu, J.; Huang, C.; Wang, F.; et al. beta2-AR-HIF-1alpha: A novel regulatory axis for stress-induced pancreatic tumor growth and angiogenesis. Curr. Mol. Med. 2013, 13, 1023–1034. [Google Scholar] [CrossRef]
- Muthuswamy, R.; Okada, N.J.; Jenkins, F.J.; McGuire, K.; McAuliffe, P.F.; Zeh, H.J.; Bartlett, D.L.; Wallace, C.; Watkins, S.; Henning, J.D.; et al. Epinephrine promotes COX-2-dependent immune suppression in myeloid cells and cancer tissues. Brain Behav. Immun. 2017, 62, 78–86. [Google Scholar] [CrossRef]
- Xie, H.; Li, C.; He, Y.; Griffin, R.; Ye, Q.; Li, L. Chronic stress promotes oral cancer growth and angiogenesis with increased circulating catecholamine and glucocorticoid levels in a mouse model. Oral Oncol. 2015, 51, 991–997. [Google Scholar] [CrossRef]
- Ahmad, M.H.; Rizvi, M.A.; Fatima, M.; Mondal, A.C. Pathophysiological implications of neuroinflammation mediated HPA axis dysregulation in the prognosis of cancer and depression. Mol. Cell. Endocrinol. 2021, 520, 111093. [Google Scholar] [CrossRef]
- Zhang, R.; Asai, M.; Mahoney, C.E.; Joachim, M.; Shen, Y.; Gunner, G.; Majzoub, J.A. Loss of hypothalamic corticotropin-releasing hormone markedly reduces anxiety behaviors in mice. Mol. Psychiatry 2017, 22, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Wu, W.; Chen, M.; Cai, F.; Fan, C.; Shen, W.; Sun, W.; Hu, J. Reward Inhibits Paraventricular CRH Neurons to Relieve Stress. Curr. Biol. 2019, 29, 1243–1251.e4. [Google Scholar] [CrossRef] [PubMed]
- Schaeuble, D.; Myers, B. Cortical-Hypothalamic Integration of Autonomic and Endocrine Stress Responses. Front. Physiol. 2022, 13, 820398. [Google Scholar] [CrossRef] [PubMed]
- Nomura, K.; Hiyama, T.Y.; Sakuta, H.; Matsuda, T.; Lin, C.H.; Kobayashi, K.; Kobayashi, K.; Kuwaki, T.; Takahashi, K.; Matsui, S.; et al. [Na+] Increases in Body Fluids Sensed by Central Nax Induce Sympathetically Mediated Blood Pressure Elevations via H+-Dependent Activation of ASIC1a. Neuron 2019, 101, 60–75.e6. [Google Scholar] [CrossRef]
- Herman, J.P.; Figueiredo, H.; Mueller, N.K.; Ulrich-Lai, Y.; Ostrander, M.M.; Choi, D.C.; Cullinan, W.E. Central mechanisms of stress integration: Hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front. Neuroendocrinol. 2003, 24, 151–180. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, D.R.; Edwards, M.R.; Ulrich-Lai, Y.M.; Herman, J.P.; Cullinan, W.E. Brainstem origins of glutamatergic innervation of the rat hypothalamic paraventricular nucleus. J. Comp. Neurol. 2012, 520, 2369–2394. [Google Scholar] [CrossRef]
- Krause, E.G.; Melhorn, S.J.; Davis, J.F.; Scott, K.A.; Ma, L.Y.; de Kloet, A.D.; Benoit, S.C.; Woods, S.C.; Sakai, R.R. Angiotensin type 1 receptors in the subfornical organ mediate the drinking and hypothalamic-pituitary-adrenal response to systemic isoproterenol. Endocrinology 2008, 149, 6416–6424. [Google Scholar] [CrossRef][Green Version]
- Plotsky, P.M.; Sutton, S.W.; Bruhn, T.O.; Ferguson, A.V. Analysis of the role of angiotensin II in mediation of adrenocorticotropin secretion. Endocrinology 1988, 122, 538–545. [Google Scholar] [CrossRef]
- Ulrich-Lai, Y.M.; Jones, K.R.; Ziegler, D.R.; Cullinan, W.E.; Herman, J.P. Forebrain origins of glutamatergic innervation to the rat paraventricular nucleus of the hypothalamus: Differential inputs to the anterior versus posterior subregions. J. Comp. Neurol. 2011, 519, 1301–1319. [Google Scholar] [CrossRef]
- Myers, B.; Carvalho-Netto, E.; Wick-Carlson, D.; Wu, C.; Naser, S.; Solomon, M.B.; Ulrich-Lai, Y.M.; Herman, J.P. GABAergic Signaling within a Limbic-Hypothalamic Circuit Integrates Social and Anxiety-Like Behavior with Stress Reactivity. Neuropsychopharmacology 2016, 41, 1530–1539. [Google Scholar] [CrossRef]
- Nyhuis, T.J.; Masini, C.V.; Day, H.E.; Campeau, S. Evidence for the Integration of Stress-Related Signals by the Rostral Posterior Hypothalamic Nucleus in the Regulation of Acute and Repeated Stress-Evoked Hypothalamo-Pituitary-Adrenal Response in Rat. J. Neurosci. 2016, 36, 795–805. [Google Scholar] [CrossRef] [PubMed]
- Myers, B.; Mark Dolgas, C.; Kasckow, J.; Cullinan, W.E.; Herman, J.P. Central stress-integrative circuits: Forebrain glutamatergic and GABAergic projections to the dorsomedial hypothalamus, medial preoptic area, and bed nucleus of the stria terminalis. Brain Struct. Funct. 2014, 219, 1287–1303. [Google Scholar] [CrossRef] [PubMed]
- Cullinan, W.E.; Ziegler, D.R.; Herman, J.P. Functional role of local GABAergic influences on the HPA axis. Brain Struct. Funct. 2008, 213, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Herman, J.P.; Cullinan, W.E.; Ziegler, D.R.; Tasker, J.G. Role of the paraventricular nucleus microenvironment in stress integration. Eur. J. Neurosci. 2002, 16, 381–385. [Google Scholar] [CrossRef]
- Diorio, D.; Viau, V.; Meaney, M.J. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J. Neurosci. 1993, 13, 3839–3847. [Google Scholar] [CrossRef]
- Myers, B.; McKlveen, J.M.; Morano, R.; Ulrich-Lai, Y.M.; Solomon, M.B.; Wilson, S.P.; Herman, J.P. Vesicular Glutamate Transporter 1 Knockdown in Infralimbic Prefrontal Cortex Augments Neuroendocrine Responses to Chronic Stress in Male Rats. Endocrinology 2017, 158, 3579–3591. [Google Scholar] [CrossRef]
- Choi, D.C.; Furay, A.R.; Evanson, N.K.; Ostrander, M.M.; Ulrich-Lai, Y.M.; Herman, J.P. Bed nucleus of the stria terminalis subregions differentially regulate hypothalamic-pituitary-adrenal axis activity: Implications for the integration of limbic inputs. J. Neurosci. 2007, 27, 2025–2034. [Google Scholar] [CrossRef]
- Ahima, R.; Krozowski, Z.; Harlan, R. Type I corticosteroid receptor-like immunoreactivity in the rat CNS: Distribution and regulation by corticosteroids. J. Comp. Neurol. 1991, 313, 522–538. [Google Scholar] [CrossRef]
- McKlveen, J.M.; Myers, B.; Flak, J.N.; Bundzikova, J.; Solomon, M.B.; Seroogy, K.B.; Herman, J.P. Role of prefrontal cortex glucocorticoid receptors in stress and emotion. Biol. Psychiatry 2013, 74, 672–679. [Google Scholar] [CrossRef]
- Hermes, G.L.; Delgado, B.; Tretiakova, M.; Cavigelli, S.A.; Krausz, T.; Conzen, S.D.; McClintock, M.K. Social isolation dysregulates endocrine and behavioral stress while increasing malignant burden of spontaneous mammary tumors. Proc. Natl. Acad. Sci. USA 2009, 106, 22393–22398. [Google Scholar] [CrossRef]
- Feng, Z.; Liu, L.; Zhang, C.; Zheng, T.; Wang, J.; Lin, M.; Zhao, Y.; Wang, X.; Levine, A.J.; Hu, W. Chronic restraint stress attenuates p53 function and promotes tumorigenesis. Proc. Natl. Acad. Sci. USA 2012, 109, 7013–7018. [Google Scholar] [CrossRef] [PubMed]
- Antonova, L.; Mueller, C.R. Hydrocortisone down-regulates the tumor suppressor gene BRCA1 in mammary cells: A possible molecular link between stress and breast cancer. Genes Chromosomes Cancer 2008, 47, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Sorrentino, G.; Ruggeri, N.; Zannini, A.; Ingallina, E.; Bertolio, R.; Marotta, C.; Neri, C.; Cappuzzello, E.; Forcato, M.; Rosato, A.; et al. Glucocorticoid receptor signalling activates YAP in breast cancer. Nat. Commun. 2017, 8, 14073. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Yuan, L.; Sun, Y.; Wang, P.; Zhang, H.; Feng, X.; Wang, Z.; Zhang, W.; Yang, C.; Zeng, Y.A.; et al. Glucocorticoid Receptor Signaling Activates TEAD4 to Promote Breast Cancer Progression. Cancer Res. 2019, 79, 4399–4411. [Google Scholar] [CrossRef]
- Tuckermann, J.P.; Kleiman, A.; Moriggl, R.; Spanbroek, R.; Neumann, A.; Illing, A.; Clausen, B.E.; Stride, B.; Forster, I.; Habenicht, A.J.; et al. Macrophages and neutrophils are the targets for immune suppression by glucocorticoids in contact allergy. J. Clin. Investig. 2007, 117, 1381–1390. [Google Scholar] [CrossRef] [PubMed]
- Acharya, N.; Madi, A.; Zhang, H.; Klapholz, M.; Escobar, G.; Dulberg, S.; Christian, E.; Ferreira, M.; Dixon, K.O.; Fell, G.; et al. Endogenous Glucocorticoid Signaling Regulates CD8+ T Cell Differentiation and Development of Dysfunction in the Tumor Microenvironment. Immunity 2020, 53, 658–671.e6. [Google Scholar] [CrossRef]
- Yang, H.; Xia, L.; Chen, J.; Zhang, S.; Martin, V.; Li, Q.; Lin, S.; Chen, J.; Calmette, J.; Lu, M.; et al. Stress-glucocorticoid-TSC22D3 axis compromises therapy-induced antitumor immunity. Nat. Med. 2019, 25, 1428–1441. [Google Scholar] [CrossRef]
- Lopez-Otin, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- Aunan, J.R.; Cho, W.C.; Soreide, K. The Biology of Aging and Cancer: A Brief Overview of Shared and Divergent Molecular Hallmarks. Aging Dis. 2017, 8, 628–642. [Google Scholar] [CrossRef]
- Berben, L.; Floris, G.; Wildiers, H.; Hatse, S. Cancer and Aging: Two Tightly Interconnected Biological Processes. Cancers 2021, 13, 1400. [Google Scholar] [CrossRef]
- Berben, L.; Floris, G.; Kenis, C.; Dalmasso, B.; Smeets, A.; Vos, H.; Neven, P.; Antoranz Martinez, A.; Laenen, A.; Wildiers, H.; et al. Age-related remodelling of the blood immunological portrait and the local tumor immune response in patients with luminal breast cancer. Clin. Transl. Immunol. 2020, 9, e1184. [Google Scholar] [CrossRef] [PubMed]
- Koch, S.; Larbi, A.; Derhovanessian, E.; Ozcelik, D.; Naumova, E.; Pawelec, G. Multiparameter flow cytometric analysis of CD4 and CD8 T cell subsets in young and old people. Immun. Ageing 2008, 5, 6. [Google Scholar] [CrossRef] [PubMed]
- Jackaman, C.; Dye, D.E.; Nelson, D.J. IL-2/CD40-activated macrophages rescue age and tumor-induced T cell dysfunction in elderly mice. Age 2014, 36, 9655. [Google Scholar] [CrossRef] [PubMed]
- Jackaman, C.; Radley-Crabb, H.G.; Soffe, Z.; Shavlakadze, T.; Grounds, M.D.; Nelson, D.J. Targeting macrophages rescues age-related immune deficiencies in C57BL/6J geriatric mice. Aging Cell 2013, 12, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Onorati, A.; Havas, A.P.; Lin, B.; Rajagopal, J.; Sen, P.; Adams, P.D.; Dou, Z. Upregulation of PD-L1 in Senescence and Aging. Mol. Cell. Biol. 2022, e00171-22. [Google Scholar] [CrossRef]
- Harman, D. Aging: A theory based on free radical and radiation chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef]
- Klaunig, J.E.; Xu, Y.; Isenberg, J.S.; Bachowski, S.; Kolaja, K.L.; Jiang, J.; Stevenson, D.E.; Walborg, E.F., Jr. The role of oxidative stress in chemical carcinogenesis. Environ. Health Perspect. 1998, 106 (Suppl. S1), 289–295. [Google Scholar]
- Galadari, S.; Rahman, A.; Pallichankandy, S.; Thayyullathil, F. Reactive oxygen species and cancer paradox: To promote or to suppress? Free Radic. Biol. Med. 2017, 104, 144–164. [Google Scholar] [CrossRef]
- Prasad, S.; Gupta, S.C.; Tyagi, A.K. Reactive oxygen species (ROS) and cancer: Role of antioxidative nutraceuticals. Cancer Lett. 2017, 387, 95–105. [Google Scholar] [CrossRef]
- Maciag, A.; Sithanandam, G.; Anderson, L.M. Mutant K-rasV12 increases COX-2, peroxides and DNA damage in lung cells. Carcinogenesis 2004, 25, 2231–2237. [Google Scholar] [CrossRef]
- Hussain, S.P.; Aguilar, F.; Amstad, P.; Cerutti, P. Oxy-radical induced mutagenesis of hotspot codons 248 and 249 of the human p53 gene. Oncogene 1994, 9, 2277–2281. [Google Scholar] [PubMed]
- Toyokuni, S. Molecular mechanisms of oxidative stress-induced carcinogenesis: From epidemiology to oxygenomics. IUBMB Life 2008, 60, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Ushijima, T. Detection and interpretation of altered methylation patterns in cancer cells. Nat. Rev. Cancer 2005, 5, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Muniyan, S.; Chou, Y.W.; Tsai, T.J.; Thomes, P.; Veeramani, S.; Benigno, B.B.; Walker, L.D.; McDonald, J.F.; Khan, S.A.; Lin, F.F.; et al. p66Shc longevity protein regulates the proliferation of human ovarian cancer cells. Mol. Carcinog. 2015, 54, 618–631. [Google Scholar] [CrossRef] [PubMed]
- Salmeen, A.; Andersen, J.N.; Myers, M.P.; Meng, T.C.; Hinks, J.A.; Tonks, N.K.; Barford, D. Redox regulation of protein tyrosine phosphatase 1B involves a sulphenyl-amide intermediate. Nature 2003, 423, 769–773. [Google Scholar] [CrossRef]
- Jing, Y.; Liu, L.Z.; Jiang, Y.; Zhu, Y.; Guo, N.L.; Barnett, J.; Rojanasakul, Y.; Agani, F.; Jiang, B.H. Cadmium increases HIF-1 and VEGF expression through ROS, ERK, and AKT signaling pathways and induces malignant transformation of human bronchial epithelial cells. Toxicol. Sci. 2012, 125, 10–19. [Google Scholar] [CrossRef]
- Klimova, T.; Chandel, N.S. Mitochondrial complex III regulates hypoxic activation of HIF. Cell Death Differ. 2008, 15, 660–666. [Google Scholar] [CrossRef]
- Lee, K.H.; Kim, S.W.; Kim, J.R. Reactive oxygen species regulate urokinase plasminogen activator expression and cell invasion via mitogen-activated protein kinase pathways after treatment with hepatocyte growth factor in stomach cancer cells. J. Exp. Clin. Cancer Res. 2009, 28, 73. [Google Scholar] [CrossRef]
- OuYang, L.Y.; Wu, X.J.; Ye, S.B.; Zhang, R.X.; Li, Z.L.; Liao, W.; Pan, Z.Z.; Zheng, L.M.; Zhang, X.S.; Wang, Z.; et al. Tumor-induced myeloid-derived suppressor cells promote tumor progression through oxidative metabolism in human colorectal cancer. J. Transl. Med. 2015, 13, 47. [Google Scholar] [CrossRef]
- Wei, J.; Zhang, M.; Zhou, J. Myeloid-derived suppressor cells in major depression patients suppress T-cell responses through the production of reactive oxygen species. Psychiatry Res. 2015, 228, 695–701. [Google Scholar] [CrossRef]
- Weinberg, S.E.; Sena, L.A.; Chandel, N.S. Mitochondria in the regulation of innate and adaptive immunity. Immunity 2015, 42, 406–417. [Google Scholar] [CrossRef] [PubMed]
- Roux, C.; Jafari, S.M.; Shinde, R.; Duncan, G.; Cescon, D.W.; Silvester, J.; Chu, M.F.; Hodgson, K.; Berger, T.; Wakeham, A.; et al. Reactive oxygen species modulate macrophage immunosuppressive phenotype through the up-regulation of PD-L1. Proc. Natl. Acad. Sci. USA 2019, 116, 4326–4335. [Google Scholar] [CrossRef] [PubMed]
- Hinz, A.; Krauss, O.; Hauss, J.P.; Hockel, M.; Kortmann, R.D.; Stolzenburg, J.U.; Schwarz, R. Anxiety and depression in cancer patients compared with the general population. Eur. J. Cancer Care 2010, 19, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Fumagalli, C.; Maurizi, N.; Marchionni, N.; Fornasari, D. beta-blockers: Their new life from hypertension to cancer and migraine. Pharmacol. Res. 2020, 151, 104587. [Google Scholar] [CrossRef]
- Choi, C.H.; Song, T.; Kim, T.H.; Choi, J.K.; Park, J.Y.; Yoon, A.; Lee, Y.Y.; Kim, T.J.; Bae, D.S.; Lee, J.W.; et al. Meta-analysis of the effects of beta blocker on survival time in cancer patients. J. Cancer Res. Clin. Oncol. 2014, 140, 1179–1188. [Google Scholar] [CrossRef]
- De Giorgi, V.; Grazzini, M.; Benemei, S.; Marchionni, N.; Botteri, E.; Pennacchioli, E.; Geppetti, P.; Gandini, S. Propranolol for Off-label Treatment of Patients With Melanoma: Results From a Cohort Study. JAMA Oncol. 2018, 4, e172908. [Google Scholar] [CrossRef]
- Musselman, R.P.; Bennett, S.; Li, W.; Mamdani, M.; Gomes, T.; van Walraven, C.; Boushey, R.; Al-Obeed, O.; Al-Omran, M.; Auer, R.C. Association between perioperative beta blocker use and cancer survival following surgical resection. Eur. J. Surg. Oncol. 2018, 44, 1164–1169. [Google Scholar] [CrossRef]
- Na, Z.; Qiao, X.; Hao, X.; Fan, L.; Xiao, Y.; Shao, Y.; Sun, M.; Feng, Z.; Guo, W.; Li, J.; et al. The effects of beta-blocker use on cancer prognosis: A meta-analysis based on 319,006 patients. Onco Targets Ther. 2018, 11, 4913–4944. [Google Scholar] [CrossRef]
- Huttenrauch, M.; Salinas, G.; Wirths, O. Effects of Long-Term Environmental Enrichment on Anxiety, Memory, Hippocampal Plasticity and Overall Brain Gene Expression in C57BL6 Mice. Front. Mol. Neurosci. 2016, 9, 62. [Google Scholar] [CrossRef]
- Liu, C.; Yang, Y.; Chen, C.; Li, L.; Li, J.; Wang, X.; Chu, Q.; Qiu, L.; Ba, Q.; Li, X.; et al. Environmental eustress modulates beta-ARs/CCL2 axis to induce anti-tumor immunity and sensitize immunotherapy against liver cancer in mice. Nat. Commun. 2021, 12, 5725. [Google Scholar] [CrossRef]
- Bucsek, M.J.; Qiao, G.; MacDonald, C.R.; Giridharan, T.; Evans, L.; Niedzwecki, B.; Liu, H.; Kokolus, K.M.; Eng, J.W.; Messmer, M.N.; et al. beta-Adrenergic Signaling in Mice Housed at Standard Temperatures Suppresses an Effector Phenotype in CD8+ T Cells and Undermines Checkpoint Inhibitor Therapy. Cancer Res. 2017, 77, 5639–5651. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Mu, Y.; Huang, L.; Hu, Y.; Chen, Z.; Yang, Y.; Huang, X.; Fu, Y.; Xi, Y.; Lin, S.; et al. A visual circuit related to the periaqueductal gray area for the antinociceptive effects of bright light treatment. Neuron 2022, 110, 1712–1727.e7. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Xi, Y.; Peng, Y.; Yang, Y.; Huang, X.; Fu, Y.; Tao, Q.; Xiao, J.; Yuan, T.; An, K.; et al. A Visual Circuit Related to Habenula Underlies the Antidepressive Effects of Light Therapy. Neuron 2019, 102, 128–142.e8. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, Y.; Zhang, L.; Liu, N.; Xu, X.; Liu, D.; Tu, J. The Central Nervous Mechanism of Stress-Promoting Cancer Progression. Int. J. Mol. Sci. 2022, 23, 12653. https://doi.org/10.3390/ijms232012653
Hong Y, Zhang L, Liu N, Xu X, Liu D, Tu J. The Central Nervous Mechanism of Stress-Promoting Cancer Progression. International Journal of Molecular Sciences. 2022; 23(20):12653. https://doi.org/10.3390/ijms232012653
Chicago/Turabian StyleHong, Yuchuan, Lu Zhang, Nian Liu, Xirong Xu, Dan Liu, and Jie Tu. 2022. "The Central Nervous Mechanism of Stress-Promoting Cancer Progression" International Journal of Molecular Sciences 23, no. 20: 12653. https://doi.org/10.3390/ijms232012653
APA StyleHong, Y., Zhang, L., Liu, N., Xu, X., Liu, D., & Tu, J. (2022). The Central Nervous Mechanism of Stress-Promoting Cancer Progression. International Journal of Molecular Sciences, 23(20), 12653. https://doi.org/10.3390/ijms232012653





