Using Computational Drug-Gene Analysis to Identify Novel Therapeutic Candidates for Retinal Neuroprotection
Abstract
1. Introduction
2. Results
3. Discussion
Limitations
4. Materials and Methods
4.1. Literature Search and Data Extraction
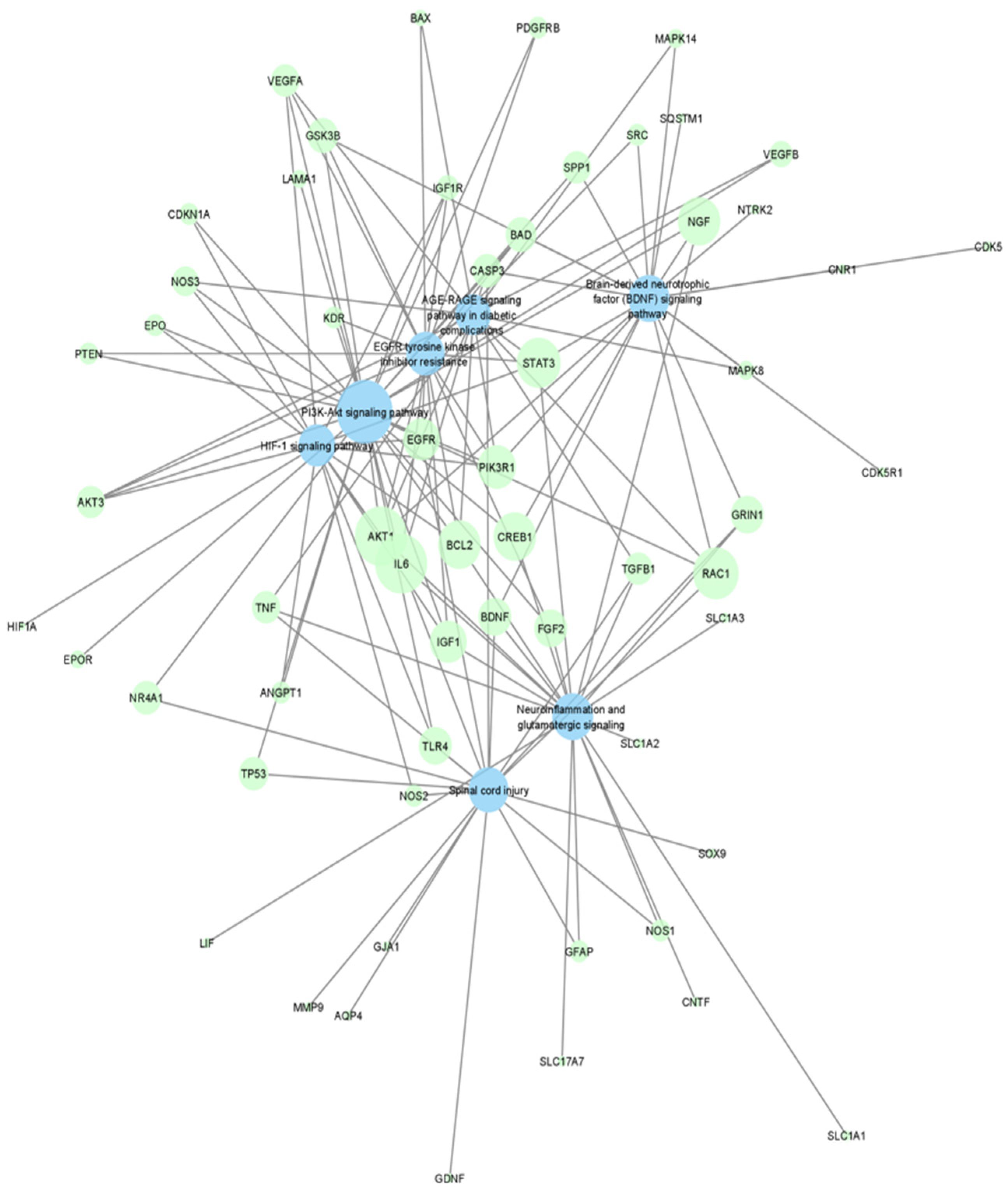
4.2. Discovering Potential Neuroprotection Therapeutic Targets via Enrichment Analysis
4.3. Narrowing down Drugs/Chemicals Useful in Neuroprotection
4.4. Visualization of Networks
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weber, A.J.; Harman, C.D.; Viswanathan, S. Effects of optic nerve injury, glaucoma, and neuroprotection on the survival, structure, and function of ganglion cells in the mammalian retina. J. Physiol. 2008, 586, 4393–4400. [Google Scholar] [CrossRef] [PubMed]
- Pardue, M.T.; Allen, R.S. Neuroprotective strategies for retinal disease. Prog. Retin. Eye Res. 2018, 65, 50–76. [Google Scholar] [CrossRef] [PubMed]
- Yonekawa, Y.; Miller, J.W.; Kim, I.K. Age-Related Macular Degeneration: Advances in Management and Diagnosis. J. Clin. Med. 2015, 4, 343–359. [Google Scholar] [CrossRef]
- Bernstein, P.S.; Li, B.; Vachali, P.P.; Gorusupudi, A.; Shyam, R.; Henriksen, B.S.; Nolan, J.M. Lutein, zeaxanthin, and meso-zeaxanthin: The basic and clinical science underlying carotenoid-based nutritional interventions against ocular disease. Prog. Retin. Eye Res. 2015, 50, 34–66. [Google Scholar] [CrossRef] [PubMed]
- Morrone, L.A.; Rombolà, L.; Corasaniti, M.T.; Bagetta, G.; Nucci, C.; Russo, R. Natural compounds and retinal ganglion cell neuroprotection. Prog. Brain Res. 2015, 220, 257–281. [Google Scholar] [CrossRef] [PubMed]
- Handa, J.T.; Rickman, C.B.; Dick, A.D.; Gorin, M.B.; Miller, J.W.; Toth, C.A.; Ueffing, M.; Zarbin, M.; Farrer, L.A. A systems biology approach towards understanding and treating non-neovascular age-related macular degeneration. Nat. Commun. 2019, 10, 3347. [Google Scholar] [CrossRef]
- Pool, F.M.; Kiel, C.; Serrano, L.; Luthert, P.J. Repository of proposed pathways and protein–protein interaction networks in age-related macular degeneration. NPJ Aging Mech. Dis. 2020, 6, 2. [Google Scholar] [CrossRef]
- Oulas, A.; Minadakis, G.; Zachariou, M.; Sokratous, K.; Bourdakou, M.M.; Spyrou, G.M. Systems Bioinformatics: Increasing precision of computational diagnostics and therapeutics through network-based approaches. Brief. Bioinform. 2019, 20, 806–824. [Google Scholar]
- Chu, H.; Sun, P.; Yin, J.; Liu, G.; Wang, Y.; Zhao, P.; Zhu, Y.; Yang, X.; Zheng, T.; Zhou, X.; et al. Integrated network analysis reveals potentially novel molecular mechanisms and therapeutic targets of refractory epilepsies. PLoS ONE 2017, 12, e0174964. [Google Scholar] [CrossRef]
- Chen, X.; Zang, W.; Xue, F.; Shen, Z.; Zhang, Q. Bioinformatics analysis reveals potential candidate drugs for different subtypes of glioma. Neurol. Sci. 2012, 34, 1139–1143. [Google Scholar] [CrossRef]
- Hurgobin, B.; de Jong, E.; Bosco, A. Insights into respiratory disease through bioinformatics. Respirology 2018, 23, 1117–1126. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, Z.; Shen, X.; Cui, X.; Guo, Y. Identification of novel biomarkers and small molecule drugs in human colorectal cancer by microarray and bioinformatics analysis. Mol. Genet. Genom. Med. 2019, 7, e00713. [Google Scholar] [CrossRef] [PubMed]
- Siavelis, J.C.; Bourdakou, M.M.; Athanasiadis, E.I.; Spyrou, G.M.; Nikita, K.S. Bioinformatics methods in drug repurposing for Alzheimer’s disease. Brief. Bioinform. 2016, 17, 322–335. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Yuan, M.; Xin, J.; Liu, X.; Wang, J. Screening novel drug candidates for Alzheimer’s disease by an integrated network and transcriptome analysis. Bioinformatics 2020, 36, 4626–4632. [Google Scholar] [CrossRef]
- Nadeem, U.; Xie, B.; Xie, E.F.; D’Souza, M.; Dao, D.; Sulakhe, D.; Skondra, D. Using Advanced Bioinformatics Tools to Identify Novel Therapeutic Candidates for Age-Related Macular Degeneration. Transl. Vis. Sci. Technol. 2022, 11, 10. [Google Scholar] [CrossRef]
- Platania, C.B.M.; Leggio, G.M.; Drago, F.; Salomone, S.; Bucolo, C. Computational systems biology approach to identify novel pharmacological targets for diabetic retinopathy. Biochem. Pharmacol. 2018, 158, 13–26. [Google Scholar] [CrossRef]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug repurposing: Progress, challenges and recommendations. Nat. Rev. Drug Discov. 2019, 18, 41–58. [Google Scholar] [CrossRef]
- Gesualdo, C.; Balta, C.; Platania, C.B.M.; Trotta, M.C.; Herman, H.; Gharbia, S.; Rosu, M.; Petrillo, F.; Giunta, S.; Della Corte, A.; et al. Fingolimod and Diabetic Retinopathy: A Drug Repurposing Study. Front. Pharmacol. 2021, 12, 718902. [Google Scholar] [CrossRef]
- Platania, C.B.M.; Ronchetti, S.; Riccardi, C.; Migliorati, G.; Marchetti, M.C.; Di Paola, L.; Lazzara, F.; Drago, F.; Salomone, S.; Bucolo, C. Effects of protein-protein interface disruptors at the ligand of the glucocorticoid-induced tumor necrosis factor receptor-related gene (GITR). Biochem. Pharmacol. 2020, 178, 114110. [Google Scholar] [CrossRef]
- Nosengo, N. Can you teach old drugs new tricks? Nature 2016, 534, 314–316. [Google Scholar] [CrossRef]
- López-Malo, D.; Villarón-Casares, C.A.; Alarcón-Jiménez, J.; Miranda, M.; Díaz-Llopis, M.; Romero, F.J.; Villar, V.M. Curcumin as a Therapeutic Option in Retinal Diseases. Antioxidants 2020, 9, 48. [Google Scholar] [CrossRef] [PubMed]
- Akinleye, A.; Furqan, M.; Mukhi, N.; Ravella, P.; Liu, D. MEK and the inhibitors: From bench to bedside. J. Hematol. Oncol. 2013, 6, 27. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.Y. Neuroprotection signaling of nuclear akt in neuronal cells. Exp. Neurobiol. 2014, 23, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Beatty, S.; Koh, H.; Phil, M.; Henson, D.; Boulton, M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv. Ophthalmol. 2000, 45, 115–134. [Google Scholar] [CrossRef]
- Levkovitch-Verbin, H. Retinal ganglion cell apoptotic pathway in glaucoma: Initiating and downstream mechanisms. Prog. Brain Res. 2015, 220, 37–57. [Google Scholar] [CrossRef]
- Frank, R.N. Diabetic retinopathy. N. Engl. J. Med. 2004, 350, 48–58. [Google Scholar] [CrossRef]
- Datta, S.; Cano, M.; Ebrahimi, K.; Wang, L.; Handa, J.T. The impact of oxidative stress and inflammation on RPE degeneration in non-neovascular AMD. Prog. Retin. Eye Res. 2017, 60, 201–218. [Google Scholar] [CrossRef]
- Kang, Q.; Yang, C. Oxidative stress and diabetic retinopathy: Molecular mechanisms, pathogenetic role and therapeutic implications. Redox Biol. 2020, 37, 101799. [Google Scholar] [CrossRef]
- Agus, D.B.; Gambhir, S.S.; Pardridge, W.M.; Spielholz, C.; Baselga, J.; Vera, J.C.; Golde, D.W. Vitamin C crosses the blood-brain barrier in the oxidized form through the glucose transporters. J. Clin. Investig. 1997, 100, 2842–2848. [Google Scholar] [CrossRef]
- Sano, H.; Namekata, K.; Kimura, A.; Shitara, H.; Guo, X.; Harada, C.; Mitamura, Y.; Harada, T. Differential effects of N-acetylcysteine on retinal degeneration in two mouse models of normal tension glaucoma. Cell Death Dis. 2019, 10, 75. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, X.L.; Zhu, B.F.; Ding, Y.N. Effect of antioxidant N-acetylcysteine on diabetic retinopathy and expression of VEGF and ICAM-1 from retinal blood vessels of diabetic rats. Mol. Biol. Rep. 2011, 39, 3727–3735. [Google Scholar] [CrossRef] [PubMed]
- Ajith, T.A. Alpha-lipoic acid: A possible pharmacological agent for treating dry eye disease and retinopathy in diabetes. Clin. Exp. Pharmacol. Physiol. 2020, 47, 1883–1890. [Google Scholar] [CrossRef] [PubMed]
- Monti, D.A.; Zabrecky, G.; Kremens, D.; Liang, T.W.; Wintering, N.A.; Bazzan, A.J.; Zhong, L.; Bowens, B.K.; Chervoneva, I.; Intenzo, C.; et al. N-Acetyl Cysteine Is Associated With Dopaminergic Improvement in Parkinson’s Disease. Clin. Pharmacol. Ther. 2019, 106, 884–890. [Google Scholar] [CrossRef] [PubMed]
- Voloboueva, L.A.; Liu, J.; Suh, J.H.; Ames, B.N.; Miller, S.S. (R)-alpha-lipoic acid protects retinal pigment epithelial cells from oxidative damage. Investig. Opthalmol. Vis. Sci. 2005, 46, 4302–4310. [Google Scholar] [CrossRef][Green Version]
- Kowluru, R.A.; Odenbach, S. Role of interleukin-1beta in the development of retinopathy in rats: Effect of antioxidants. Investig. Opthalmol. Vis. Sci. 2004, 45, 4161–4166. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.F.; Zou, H.D. PEDF in diabetic retinopathy: A protective effect of oxidative stress. J. Biomed. Biotechnol. 2012, 2012, 580687. [Google Scholar] [CrossRef]
- Lee, S.Y.; Usui, S.; Zafar, A.B.; Oveson, B.C.; Jo, Y.J.; Lu, L.; Masoudi, S.; Campochiaro, P.A. N-Acetylcysteine promotes long-term survival of cones in a model of retinitis pigmentosa. J. Cell. Physiol. 2011, 226, 1843–1849. [Google Scholar] [CrossRef]
- Campochiaro, P.A.; Iftikhar, M.; Hafiz, G.; Akhlaq, A.; Tsai, G.; Wehling, D.; Lu, L.; Wall, G.M.; Singh, M.S.; Kong, X. Oral N-acetylcysteine improves cone function in retinitis pigmentosa patients in phase I trial. J. Clin. Investig. 2020, 130, 1527–1541. [Google Scholar] [CrossRef]
- Pringsheim, T.; Davenport, W.; Mackie, G.; Worthington, I.; Aubé, M.; Christie, S.N.; Gladstone, J.; Becker, W.J. Canadian Headache Society Prophylactic Guidelines Development Group. Canadian Headache Society guideline for migraine prophylaxis. Can. J. Neurol. Sci. 2012, 39 (Suppl. S2), S1–S59. [Google Scholar]
- Dinte, E.; Vostinaru, O.; Samoila, O.; Sevastre, B.; Bodoki, E. Ophthalmic Nanosystems with Antioxidants for the Prevention and Treatment of Eye Diseases. Coatings 2020, 10, 36. [Google Scholar] [CrossRef]
- Garcia-Medina, J.J.; Rubio-Velazquez, E.; Lopez-Bernal, M.D.; Cobo-Martinez, A.; Zanon-Moreno, V.; Pinazo-Duran, M.D.; Del-Rio-Vellosillo, M. Glaucoma and Antioxidants: Review and Update. Antioxidants 2020, 9, 1031. [Google Scholar] [CrossRef] [PubMed]
- Haritoglou, C.; Gerss, J.; Hammes, H.P.; Kampik, A.; Ulbig, M.W. RETIPON Study Group. Alpha-lipoic acid for the prevention of diabetic macular edema. Ophthalmologica 2011, 226, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Nebbioso, M.; Federici, M.; Rusciano, D.; Evangelista, M.; Pescosolido, N. Oxidative stress in preretinopathic diabetes subjects and antioxidants. Diabetes Technol. Ther. 2012, 14, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Medina, J.J.; Pinazo-Duran, M.D.; Garcia-Medina, M.; Zanon-Moreno, V.; Pons-Vazquez, S. A 5-year follow-up of antioxidant supplementation in type 2 diabetic retinopathy. Eur. J. Ophthalmol. 2011, 21, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kręgiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B.; Novellino, E.; et al. The Therapeutic Potential of Apigenin. Int. J. Mol. Sci. 2019, 20, 1305. [Google Scholar] [CrossRef]
- McCormack, D.; McFadden, D. A review of pterostilbene antioxidant activity and disease modification. Oxidative Med. Cell. Longev. 2013, 2013, 575482. [Google Scholar] [CrossRef]
- Al-Dosari, D.I.; Ahmed, M.M.; Al-Rejaie, S.S.; Alhomida, A.S.; Ola, M.S. Flavonoid Naringenin Attenuates Oxidative Stress, Apoptosis and Improves Neurotrophic Effects in the Diabetic Rat Retina. Nutrients 2017, 9, 1161. [Google Scholar] [CrossRef]
- Mandal, M.N.; Patlolla, J.M.; Zheng, L.; Agbaga, M.P.; Tran, J.T.; Wicker, L.; Kasus-Jacobi, A.; Elliott, M.H.; Rao, C.V.; Anderson, R.E. Curcumin protects retinal cells from light-and oxidant stress-induced cell death. Free Radic. Biol. Med. 2009, 46, 672–679. [Google Scholar] [CrossRef]
- Kowluru, R.A.; Kanwar, M. Effects of curcumin on retinal oxidative stress and inflammation in diabetes. Nutr. Metab. 2007, 4, 8. [Google Scholar] [CrossRef]
- Liu, L.; Zuo, Z.; Lu, S.; Liu, A.; Liu, X. Naringin attenuates diabetic retinopathy by inhibiting inflammation, oxidative stress and NF-κB activation in vivo and in vitro. Iran J. Basic Med. Sci. 2017, 20, 813–821. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Y.; Yu, H.; Li, M.; Hang, L.; Xu, X. Apigenin Protects Mouse Retina against Oxidative Damage by Regulating the Nrf2 Pathway and Autophagy. Oxid. Med. Cell. Longev. 2020, 2020, 9420704. [Google Scholar] [CrossRef] [PubMed]
- Muangnoi, C.; Ratnatilaka Na Bhuket, P.; Jithavech, P.; Supasena, W.; Paraoan, L.; Patumraj, S.; Rojsitthisak, P. Curcumin diethyl disuccinate, a prodrug of curcumin, enhances anti-proliferative effect of curcumin against HepG2 cells via apoptosis induction. Sci. Rep. 2019, 9, 11718. [Google Scholar] [CrossRef] [PubMed]
- Mazzolani, F.; Togni, S. Oral administration of a curcumin-phospholipid delivery system for the treatment of central serous chorioretinopathy: A 12-month follow-up study. Clin. Ophthalmol. 2013, 7, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R. Effect of Oral Curcumin Supplementation in Dry Age-Related Macular Degeneration (AMD) Patient. Available online: https://clinicaltrials.gov/ct2/show/NCT04590196 (accessed on 22 August 2022).
- Han, Y.; Xie, H.; Liu, Y.; Gao, P.; Yang, X.; Shen, Z. Effect of metformin on all-cause and cardiovascular mortality in patients with coronary artery diseases: A systematic review and an updated meta-analysis. Cardiovasc Diabetol. 2019, 18, 96. [Google Scholar] [CrossRef]
- Kasznicki, J.; Sliwinska, A.; Drzewoski, J. Metformin in cancer prevention and therapy. Ann. Transl. Med. 2014, 2, 57. [Google Scholar] [CrossRef]
- Samaras, K.; Makkar, S.; Crawford, J.D.; Kochan, N.A.; Wen, W.; Draper, B.; Trollor, J.N.; Brodaty, H.; Sachdev, P.S. Metformin Use Is Associated With Slowed Cognitive Decline and Reduced Incident Dementia in Older Adults With Type 2 Diabetes: The Sydney Memory and Ageing Study. Diabetes Care 2020, 43, 2691–2701. [Google Scholar] [CrossRef]
- Han, J.; Li, Y.; Liu, X.; Zhou, T.; Sun, H.; Edwards, P.; Gao, H.; Yu, F.S.; Qiao, X. Metformin suppresses retinal angiogenesis and inflammation in vitro and in vivo. PLoS ONE 2018, 13, e0193031. [Google Scholar] [CrossRef]
- Mori, A.; Ishikawa, E.; Amano, T.; Sakamoto, K.; Nakahara, T. Anti-diabetic drug metformin dilates retinal blood vessels through activation of AMP-activated protein kinase in rats. Eur. J. Pharmacol. 2017, 798, 66–71. [Google Scholar] [CrossRef]
- Altmann, C.; Schmidt, M.H. The Role of Microglia in Diabetic Retinopathy: Inflammation, Microvasculature Defects and Neurodegeneration. Int. J. Mol. Sci. 2018, 19, 110. [Google Scholar] [CrossRef]
- Zeng, X.X.; Ng, Y.K.; Ling, E.A. Neuronal and microglial response in the retina of streptozotocin-induced diabetic rats. Vis. Neurosci. 2000, 17, 463–471. [Google Scholar] [CrossRef]
- Luodan, A.; Zou, T.; He, J.; Chen, X.; Sun, D.; Fan, X.; Xu, H. Rescue of Retinal Degeneration in rd1 Mice by Intravitreally Injected Metformin. Front. Mol. Neurosci. 2019, 12, 102. [Google Scholar] [CrossRef]
- Fan, Y.P.; Wu, C.T.; Lin, J.L.; Hsiung, C.A.; Liu, H.Y.; Lai, J.N.; Yang, C.C. Metformin Treatment Is Associated with a Decreased Risk of Nonproliferative Diabetic Retinopathy in Patients with Type 2 Diabetes Mellitus: A Population-Based Cohort Study. J. Diabetes Res. 2020, 2020, 9161039. [Google Scholar] [CrossRef] [PubMed]
- Blitzer, A.L.; Ham, S.A.; Colby, K.A.; Skondra, D. Association of Metformin Use With Age-Related Macular Degeneration: A Case-Control Study. JAMA Ophthalmol. 2021, 139, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Evangelho, K.; Mogilevskaya, M.; Losada-Barragan, M.; Vargas-Sanchez, J.K. Pathophysiology of primary open-angle glaucoma from a neuroinflammatory and neurotoxicity perspective: A review of the literature. Int. Ophthalmol. 2019, 39, 259–271. [Google Scholar] [CrossRef]
- Rossino, M.G.; Dal Monte, M.; Casini, G. Relationships Between Neurodegeneration and Vascular Damage in Diabetic Retinopathy. Front. Neurosci. 2019, 13, 1172. [Google Scholar] [CrossRef]
- Watanabe, K.; Asano, D.; Ushikubo, H.; Morita, A.; Mori, A.; Sakamoto, K.; Ishii, K.; Nakahara, T. Metformin Protects against NMDA-Induced Retinal Injury through the MEK/ERK Signaling Pathway in Rats. Int. J. Mol. Sci. 2021, 22, 4439. [Google Scholar] [CrossRef]
- Hasanvand, A. The role of AMPK-dependent pathways in cellular and molecular mechanisms of metformin: A new perspective for treatment and prevention of diseases. Inflammopharmacology 2022, 30, 775–788. [Google Scholar] [CrossRef]
- Li, Q.; Zhuang, Q.K.; Yang, J.N.; Zhang, Y.Y. Statins excert neuroprotection on cerebral ischemia independent of their lipid-lowering action: The potential molecular mechanisms. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 1113–1126. [Google Scholar]
- Trapani, L.; Segatto, M.; Pallottini, V. New compounds able to control hepatic cholesterol metabolism: Is it possible to avoid statin treatment in aged people? World J. Hepatol. 2013, 5, 676–684. [Google Scholar] [CrossRef]
- Pfrieger, F.W. Outsourcing in the brain: Do neurons depend on cholesterol delivery by astrocytes? Bioessays 2003, 25, 72–78. [Google Scholar] [CrossRef]
- Mysore, Y.; del Amo, E.M.; Loukovaara, S.; Hagström, M.; Urtti, A.; Kauppinen, A. Author Correction: Statins for the prevention of proliferative vitreoretinopathy: Cellular responses in cultured cells and clinical statin concentrations in the vitreous. Sci. Rep. 2021, 11, 980. [Google Scholar] [CrossRef] [PubMed]
- Vavvas, D.G.; Daniels, A.B.; Kapsala, Z.G.; Goldfarb, J.W.; Ganotakis, E.; Loewenstein, J.I.; Young, L.H.; Gragoudas, E.S.; Eliott, D.; Kim, I.K.; et al. Regression of Some High-risk Features of Age-related Macular Degeneration (AMD) in Patients Receiving Intensive Statin Treatment. eBioMedicine 2016, 5, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Loukovaara, S.; Sahanne, S.; Takala, A.; Haukka, J. Statin use and vitreoretinal surgery: Findings from a Finnish population-based cohort study. Acta Ophthalmol. 2018, 96, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Tuuminen, R.; Yegutkin, G.G.; Jalkanen, S.; Loukovaara, S. Simvastatin use associated with low intraocular ADP levels in patients with sight-threatening diabetic retinopathy. Graefes Arch. Clin. Exp. Ophthalmol. 2016, 254, 1643–1644. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tuuminen, R.; Sahanne, S.; Loukovaara, S. Low intravitreal angiopoietin-2 and VEGF levels in vitrectomized diabetic patients with simvastatin treatment. Acta Ophthalmol. 2014, 92, 675–681. [Google Scholar] [CrossRef]
- Kang, E.Y.; Chen, T.H.; Garg, S.J.; Sun, C.C.; Kang, J.H.; Wu, W.C.; Hung, M.J.; Lai, C.C.; Cherng, W.J.; Hwang, Y.S. Association of Statin Therapy With Prevention of Vision-Threatening Diabetic Retinopathy. JAMA Ophthalmol. 2019, 137, 363–371. [Google Scholar] [CrossRef]
- Yuan, Y.; Wang, W.; Shang, X.; Xiong, R.; Ha, J.; Zhang, L.; Zhu, Z.; He, M. Association between statin use and the risks of glaucoma in Australia: A 10-year cohort study. Br. J. Ophthalmol. 2021; ahead of print. [Google Scholar] [CrossRef]
- Sang, N.; Stiehl, D.P.; Bohensky, J.; Leshchinsky, I.; Srinivas, V.; Caro, J. MAPK signaling up-regulates the activity of hypoxia-inducible factors by its effects on p300. J. Biol. Chem. 2003, 278, 14013–14019. [Google Scholar] [CrossRef]
- Ruan, Y.; Jiang, S.; Gericke, A. Age-Related Macular Degeneration: Role of Oxidative Stress and Blood Vessels. Int. J. Mol. Sci. 2021, 22, 1296. [Google Scholar] [CrossRef]
- Baynes, J.W. Role of oxidative stress in development of complications in diabetes. Diabetes. 1991, 40, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Kowluru, R.A.; Chan, P.S. Oxidative stress and diabetic retinopathy. Exp. Diabetes Res. 2007, 2007, 43603. [Google Scholar] [CrossRef] [PubMed]
- Bruschi, M.; Bartolucci, M.; Petretto, A.; Calzia, D.; Caicci, F.; Manni, L.; Traverso, C.E.; Candiano, G.; Panfoli, I. Differential expression of the five redox complexes in the retinal mitochondria or rod outer segment disks is consistent with their different functionality. FASEB Bioadv. 2020, 2, 315–324. [Google Scholar] [CrossRef]
- Calzia, D.; Barabino, S.; Bianchini, P.; Garbarino, G.; Oneto, M.; Caicci, F.; Diaspro, A.; Tacchetti, C.; Manni, L.; Candiani, S.; et al. New findings in ATP supply in rod outer segments: Insights for retinopathies. Biol. Cell. 2013, 105, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Calzia, D.; Oneto, M.; Caicci, F.; Bianchini, P.; Ravera, S.; Bartolucci, M.; Diaspro, A.; Degan, P.; Manni, L.; Traverso, C.E.; et al. Effect of polyphenolic phytochemicals on ectopic oxidative phosphorylation in rod outer segments of bovine retina. Br. J. Pharmacol. 2015, 172, 3890–3903. [Google Scholar] [CrossRef] [PubMed]
- Ravera, S.; Caicci, F.; Degan, P.; Maggi, D.; Manni, L.; Puddu, A.; Nicolò, M.; Traverso, C.E.; Panfoli, I. Inhibitory Action of Antidiabetic Drugs on the Free Radical Production by the Rod Outer Segment Ectopic Aerobic Metabolism. Antioxidants 2020, 9, 1133. [Google Scholar] [CrossRef]
- Gu, H.; Huang, Z.; Chen, G.; Zhou, K.; Zhang, Y.; Chen, J.; Xu, J.; Yin, X. Network and pathway-based analyses of genes associated with osteoporosis. Medicine 2020, 99, e19120. [Google Scholar] [CrossRef]
- Hu, Y.; Pan, Z.; Hu, Y.; Zhang, L.; Wang, J. Network and Pathway-Based Analyses of Genes Associated with Parkinson’s Disease. Mol. Neurobiol. 2017, 54, 4452–4465. [Google Scholar] [CrossRef]
- Tao, C.; Sun, J.; Zheng, W.J.; Chen, J.; Xu, H. Colorectal cancer drug target prediction using ontology-based inference and network analysis. Database 2015, 2015, bav015. [Google Scholar] [CrossRef]
- Boyle, E.; Shuai Weng, J.G.; Sherlock, G. GO: TermFinder–open Source Software for Accessing Gene Ontology Information and Finding Significantly Enriched Gene Ontology Terms Associated with a List of Genes. Bioinformatics 2004, 20, 3710–3715. [Google Scholar] [CrossRef]
- Chen, J.; Bardes, E.E.; Aronow, B.J.; Jegga, A.G. ToppGene Suite for gene list enrichment analysis and candidate gene prioritization. Nucleic Acids Res. 2009, 37, W305–W311. [Google Scholar] [CrossRef]
- Davis, A.P.; Grondin, C.J.; Johnson, R.J.; Sciaky, D.; Wiegers, J.; Wiegers, T.C.; Mattingly, C.J. The Comparative Toxicogenomics Database: Update 2021. Nucleic Acids Res. 2021, 49, D1138–D1143. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, M.; von Mering, C.; Campillos, M.; Jensen, L.J.; Bork, P. STITCH: Interaction networks of chemicals and proteins. Nucleic Acids Res. 2007, 36, D684–D688. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Sulakhe, D.; Balasubramanian, S.; Xie, B.; Feng, B.; Taylor, A.; Wang, S.; Berrocal, E.; Dave, U.; Xu, J.; Börnigen, D.; et al. Lynx: A database and knowledge extraction engine for integrative medicine. Nucleic Acids Res. 2013, 42, D1007–D1012. [Google Scholar] [CrossRef]
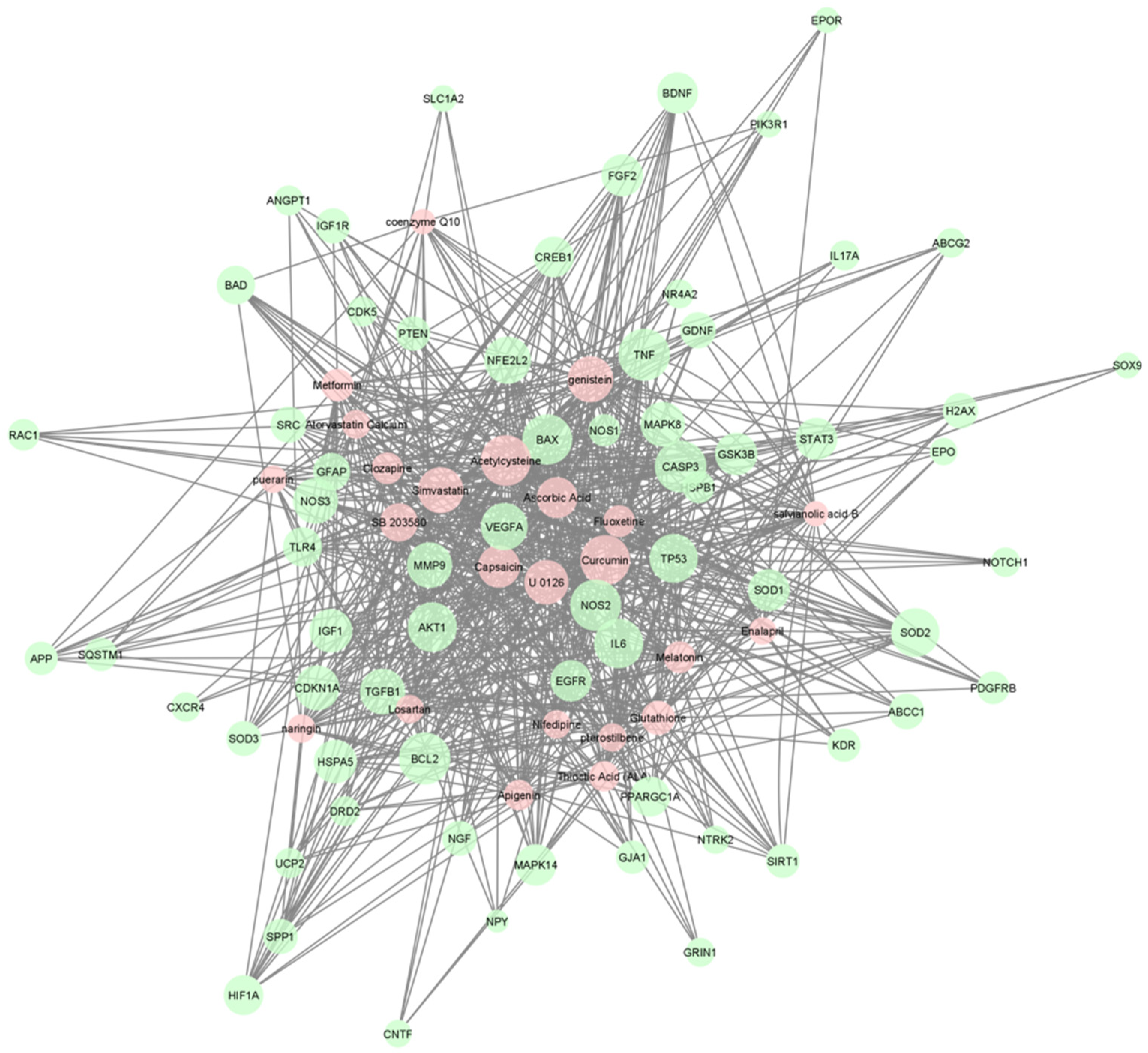
| FILTERED POSITION | UNFILTERED POSITION | NAME | SOURCE | P-VALUE | Q-VALUE FDR B&H | HIT COUNT IN QUERY LIST | HIT COUNT IN GENOME |
|---|---|---|---|---|---|---|---|
| 1 | 2 | U 0126 | CTD | 2.51 × 10−52 | 3.64 × 10−52 | 51 | 444 |
| 2 | 3 | Acetylcysteine | CTD | 3.67 × 10−55 | 3.55 × 10−51 | 59 | 781 |
| 3 | 5 | Simvastatin | CTD | 2.72 × 10−53 | 1.58 × 10−49 | 53 | 581 |
| 4 | 7 | Curcumin | CTD | 1.66 × 10−51 | 6.86 × 10−48 | 58 | 851 |
| 5 | 9 | Capsaicin | CTD | 1.97 × 10−49 | 6.36 × 10−46 | 48 | 488 |
| 6 | 18 | SB 203580 | CTD | 1.18 × 10−44 | 1.90 × 10−41 | 42 | 388 |
| 7 | 20 | Ascorbic Acid | CTD | 2.56 × 10−41 | 3.72 × 10−38 | 46 | 627 |
| 8 | 29 | Genistein | Stitch | 2.20 × 10−38 | 2.21 × 10−35 | 53 | 1117 |
| 9 | 43 | Glutathione | CTD | 3.34 × 10−36 | 2.25 × 10−33 | 35 | 339 |
| 10 | 44 | Thioctic Acid | CTD | 2.51 × 10−35 | 1.65 × 10−32 | 28 | 163 |
| 11 | 45 | Melatonin | CTD | 6.47 × 10−35 | 4.17 × 10−32 | 31 | 243 |
| 12 | 55 | Nifedipine | CTD | 8.36 × 10−33 | 4.41 × 10−30 | 24 | 112 |
| 13 | 61 | Apigenin | CTD | 2.96 × 10−32 | 1.41 × 10−29 | 28 | 207 |
| 14 | 64 | Deferoxamine | CTD | 5.7 × 10−32 | 2.59×10−29 | 27 | 186 |
| 15 | 72 | Pterostilbene | CTD | 4.13 × 10−31 | 1.66 × 10−28 | 24 | 130 |
| 16 | 84 | Salvianolic acid | CTD | 1.24 × 10−31 | 4.28 × 10−28 | 17 | 35 |
| 17 | 83 | Fluoxetine | CTD | 1.18 × 10−30 | 4.12 × 10−28 | 31 | 331 |
| 18 | 86 | Puerarin | CTD | 1.34 × 10−30 | 4.54 × 10−28 | 22 | 98 |
| 19 | 98 | Naringin | CTD | 8.18 × 10−30 | 2.42 × 10−27 | 24 | 146 |
| 20 | 101 | Metformin | CTD | 1.83 × 10−29 | 5.26 × 10−27 | 32 | 400 |
| 21 | 111 | Atorvastatin Calcium | CTD | 9.68 × 10−29 | 2.53 × 10−26 | 25 | 186 |
| 22 | 113 | Losartan | CTD | 1.82 × 10−28 | 4.69 × 10−26 | 24 | 165 |
| 23 | 114 | Clozapine | CTD | 1.92 × 10−28 | 4.89 × 10−26 | 31 | 390 |
| 24 | 127 | Coenzyme Q10 | CTD | 1.10 × 10−27 | 2.51 × 10−25 | 17 | 48 |
| 25 | 128 | Enalapril | CTD | 1.38 × 10−27 | 3.14 × 10−25 | 22 | 131 |
| ID | GO DESCRIPTION | P-VALUE | Q-VALUE FDR B&H | HIT COUNT IN QUERY LIST | HIT COUNT IN GENOME |
|---|---|---|---|---|---|
| GO:1901701 | cellular response to oxygen-containing compound | 6.24 × 10−55 | 3.95 × 10−51 | 78 | 1790 |
| GO:0043067 | regulation of programmed cell death | 1.58 × 10−53 | 4.99 × 10−50 | 79 | 1944 |
| GO:0009628 | response to abiotic stimulus | 1.04 × 10−52 | 1.65 × 10−49 | 77 | 1839 |
| GO:0042981 | regulation of apoptotic process | 1.06 × 10−51 | 1.35 × 10−48 | 77 | 1897 |
| GO:0010243 | response to organonitrogen compound | 4.00 × 10−49 | 4.22 × 10−46 | 71 | 1605 |
| GO:1901214 | regulation of neuron death | 4.75 × 10−47 | 3.76 × 10−44 | 47 | 462 |
| GO:0014070 | response to organic cyclic compound | 7.90 × 10−47 | 5.56 × 10−44 | 69 | 1591 |
| GO:0048666 | neuron development | 2.23 × 10−39 | 6.42 × 10−37 | 64 | 1673 |
| GO:0051094 | positive regulation of developmental process | 4.93 × 10−39 | 1.25 × 10−36 | 64 | 1695 |
| GO:0042327 | positive regulation of phosphorylation | 1.02 × 10−38 | 2.47 × 10−36 | 55 | 1113 |
| GO:0031175 | neuron projection development | 1.18 × 10−37 | 2.67 × 10−35 | 59 | 1424 |
| GO:0070482 | response to oxygen levels | 1.87 × 10−37 | 3.94 × 10−35 | 45 | 647 |
| GO:0031399 | regulation of protein modification process | 3.24 × 10−37 | 6.61 × 10−35 | 66 | 1976 |
| GO:0051247 | positive regulation of protein metabolic process | 4.57 × 10−37 | 9.05 × 10−35 | 64 | 1827 |
| GO:0009611 | response to wounding | 8.65 × 10−37 | 1.66 × 10−34 | 50 | 919 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, E.; Nadeem, U.; Xie, B.; D’Souza, M.; Sulakhe, D.; Skondra, D. Using Computational Drug-Gene Analysis to Identify Novel Therapeutic Candidates for Retinal Neuroprotection. Int. J. Mol. Sci. 2022, 23, 12648. https://doi.org/10.3390/ijms232012648
Xie E, Nadeem U, Xie B, D’Souza M, Sulakhe D, Skondra D. Using Computational Drug-Gene Analysis to Identify Novel Therapeutic Candidates for Retinal Neuroprotection. International Journal of Molecular Sciences. 2022; 23(20):12648. https://doi.org/10.3390/ijms232012648
Chicago/Turabian StyleXie, Edward, Urooba Nadeem, Bingqing Xie, Mark D’Souza, Dinanath Sulakhe, and Dimitra Skondra. 2022. "Using Computational Drug-Gene Analysis to Identify Novel Therapeutic Candidates for Retinal Neuroprotection" International Journal of Molecular Sciences 23, no. 20: 12648. https://doi.org/10.3390/ijms232012648
APA StyleXie, E., Nadeem, U., Xie, B., D’Souza, M., Sulakhe, D., & Skondra, D. (2022). Using Computational Drug-Gene Analysis to Identify Novel Therapeutic Candidates for Retinal Neuroprotection. International Journal of Molecular Sciences, 23(20), 12648. https://doi.org/10.3390/ijms232012648







