Hybrid Coordination Networks for Removal of Pollutants from Wastewater
Abstract
1. Introduction
2. Results and Discussion
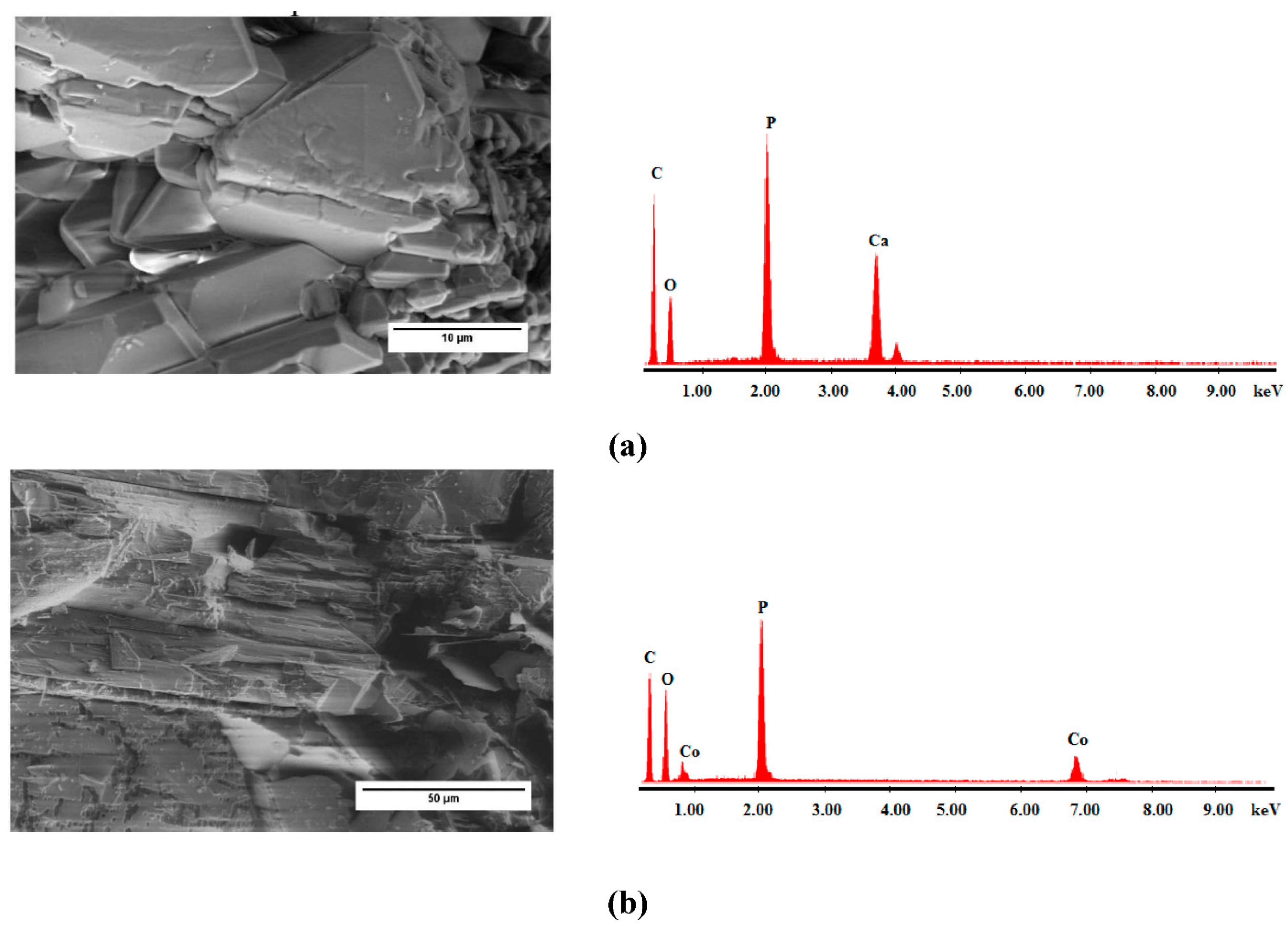
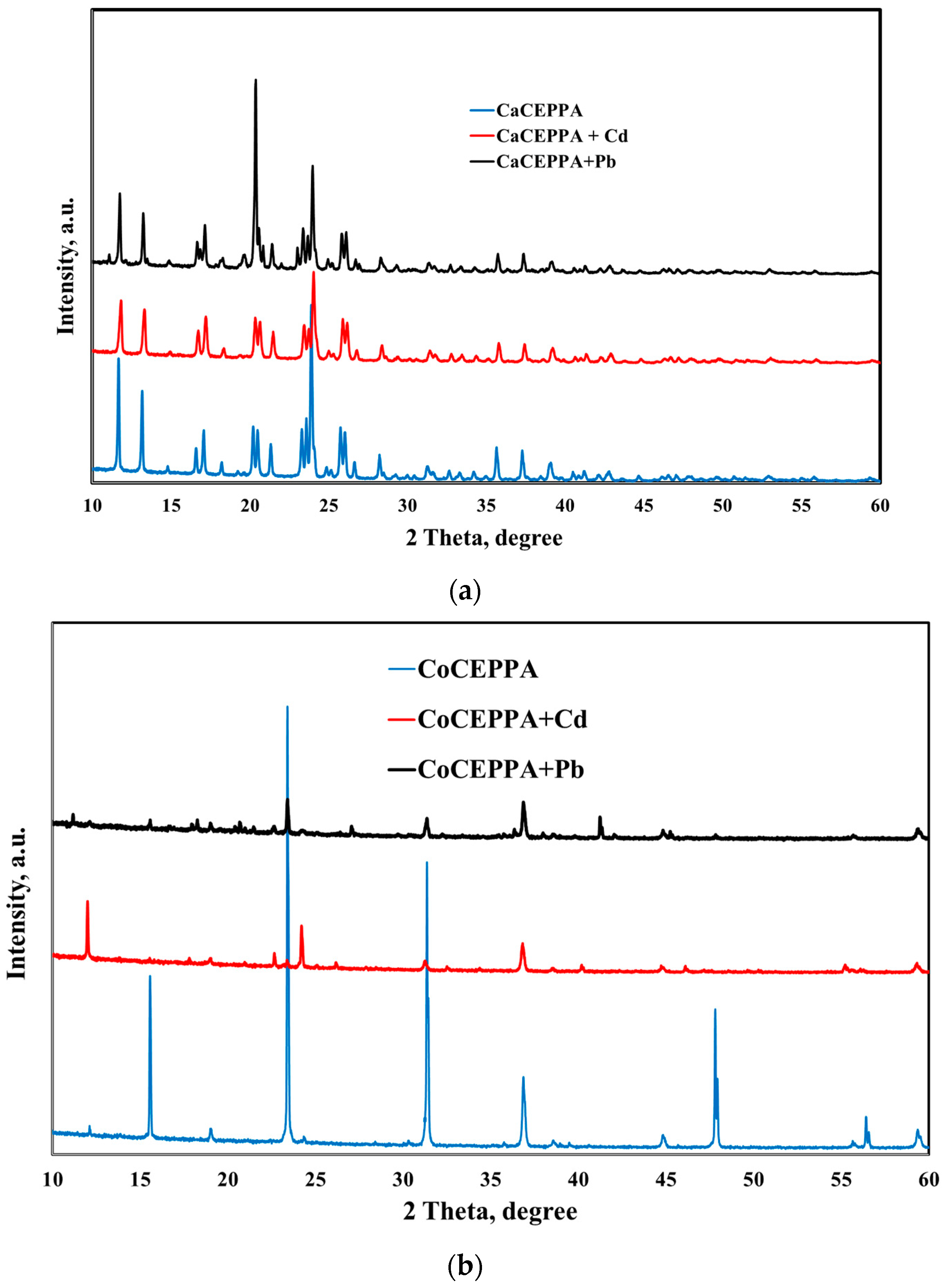
2.1. Characterization of Materials
2.2. Adsorption Studies
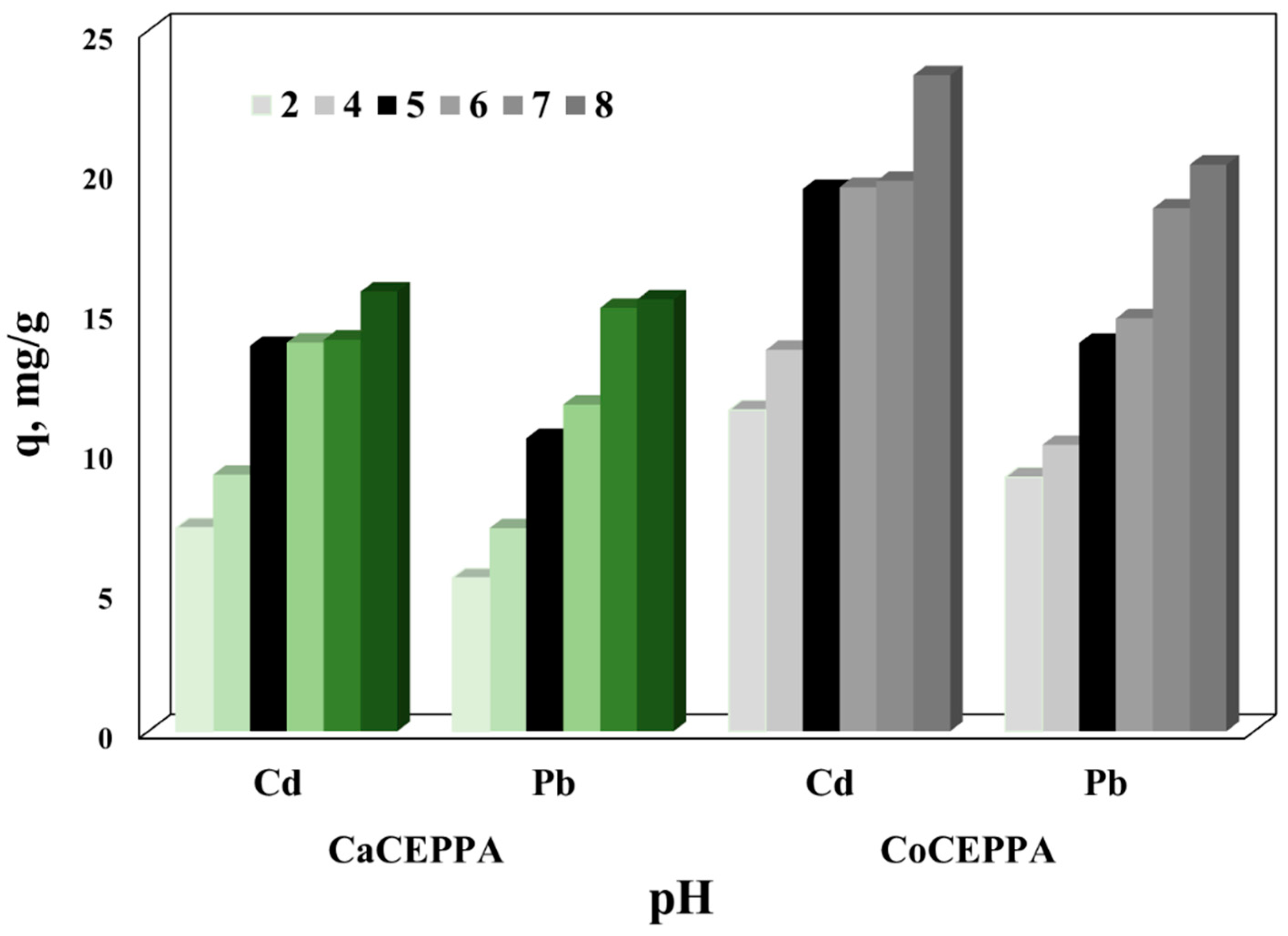
2.2.1. Influence of Initial pH of Aqueous Solutions upon the Adsorption Properties of CaCEPPA and CoCEPPA Materials
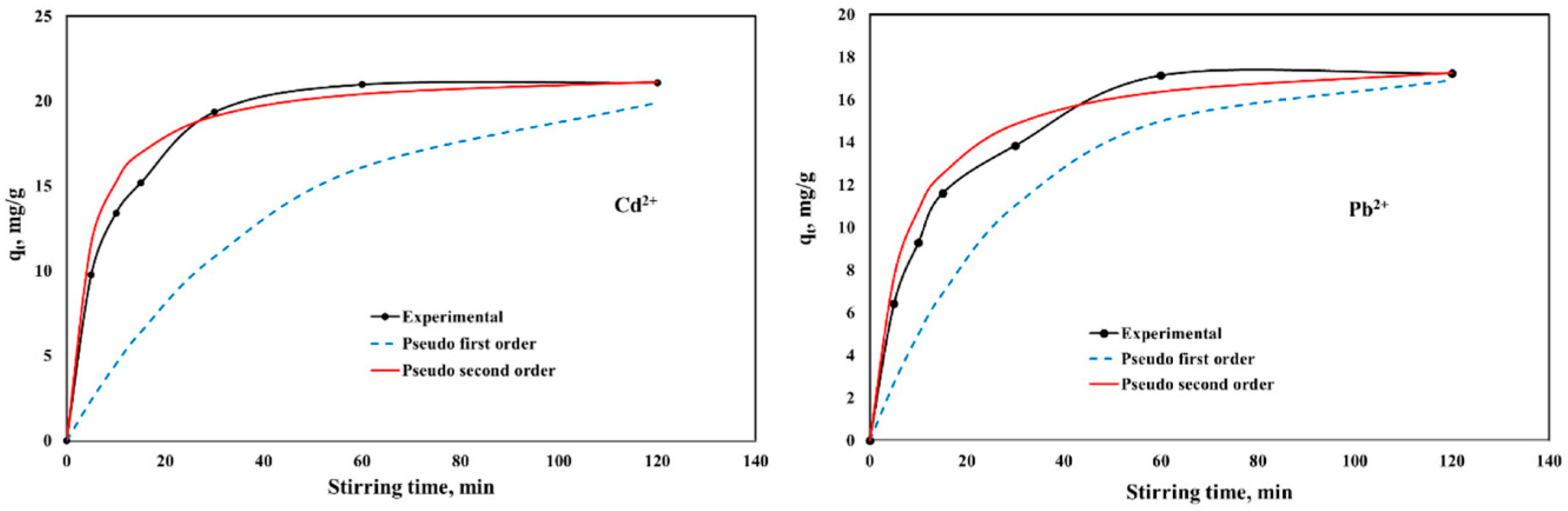
2.2.2. Stirring Time Influence on Adsorption Properties of CaCEPPA and CoCEPPA Materials
2.2.3. Influence of Equilibrium Concentration of Metal Ions upon the Adsorption Properties of CaCEPPA and CoCEPPA Materials
2.2.4. Comparison with Other Adsorbents
3. Materials and Methods
3.1. Materials
3.2. Instrumentation
3.3. Synthesis of Materials
Co2[(O2P(CH2CH2COO)(C6H5)(H2O)]2·2H2O (CoCEPPA) and Ca[O2P(CH2CH2COOH)(C6H5)]2 (CaCEPPA)
3.4. Adsorption Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dhahri, R.; Yılmaz, M.; Mechi, L.; Alsukaibi, A.K.D.; Alimi, F.; ben Salem, R.; Moussaoui, Y. Optimization of the Preparation of Activated Carbon from Prickly Pear Seed Cake for the Removal of Lead and Cadmium Ions from Aqueous Solution. Sustainability 2022, 14, 3245. [Google Scholar] [CrossRef]
- Flora, G.; Gupta, D.; Tiwari, A. Toxicity of lead: A review with recent updates. Interdiscip. Toxicol. 2012, 5, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Maranescu, B.; Lupa, L.; Visa, A. Synthesis, characterizations and Pb(II) sorption properties of cobalt phosphonate materials. Pure Appl. Chem. 2016, 88, 979–992. [Google Scholar] [CrossRef]
- Skipper, A.; Sims, J.N.; Yedjou, C.G.; Tchounwou, P.B. Cadmium chloride induces DNA damage and apoptosis of human liver carcinoma cells via oxidative stress. Int. J. Environ. Res. Public Health 2016, 13, 88. [Google Scholar] [CrossRef]
- Xu, M.Y.; Wang, P.; Sun, Y.J.; Yi-Jun Wu, Y.J. Disruption of kidney metabolism in rats after subchronic combined exposure to low-dose cadmium and chlorpyrifos. Chem. Res. Toxicol. 2019, 32, 122–129. [Google Scholar] [CrossRef]
- Abbas, A.; Al-Amer, A.M.; Laoui, T.; Al-Marri, M.J.; Nasser, M.S.; Khraisheh, M.; Atieh, M.A. Heavy metal removal from aqueous solution by advanced carbon nanotubes: Critical review of adsorption applications. Sep. Purif. Technol. 2016, 157, 141–161. [Google Scholar]
- Marques, J.P.; Vaz, C.M.P.; Sígolo, J.B.; Rodrigues, V.G.S. Soils of the Ribeira Valley (Brazil) as Environmental Protection Barriers: Characterization and Adsorption of Lead and Cadmium. Sustainability 2022, 14, 5135. [Google Scholar] [CrossRef]
- Kim, H.; Lee, B.I.; Byeon, S.H. The inner filter effect of Cr (VI) on Tb–doped layered rare earth hydroxychlorides: New fluorescent adsorbents for the simple detection of Cr (VI). Chem. Commun. 2015, 51, 725–728. [Google Scholar] [CrossRef]
- Lin, R.B.; Li, T.Y.; Zhou, H.L.; He, C.T.; Zhang, J.P.; Chen, X.M. Tuning fluorocarbon adsorption in new isoreticular porous coordination frameworks for heat transformation applications. Chem. Sci. 2015, 6, 2516–2521. [Google Scholar] [CrossRef]
- Wang, K.; Gu, J.; Yin, N. Efficient removal of Pb(II) and Cd(II) Using NH2-Functionalized Zr-MOFs via rapid microwave-promoted synthesis. Ind. Eng. Chem. Res. 2017, 56, 1880–1887. [Google Scholar] [CrossRef]
- Wang, Z.; Cohen, S.M. Postsynthetic modification of metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1315–1329. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [PubMed]
- Hu, M. Design, Synthesis and Applications of Metal Organic Frameworks. Ph.D. Thesis, Department of Chemistry and Biochemistry, Worcester Polytechnic Institute, Worcester, MA, USA, 2 September 2011. Available online: https://digitalcommons.wpi.edu/etd-theses/964/ (accessed on 8 March 2022).
- Sun, Y.; Zhou, H.C. Recent progress in the synthesis of metal–organic frameworks. Sci. Technol. Adv. Mater. 2015, 16, 054202. [Google Scholar] [CrossRef] [PubMed]
- Stock, N.; Biswas, S. Synthesis of Metal-Organic Frameworks (MOFs): Routes to Various MOF Topologies, Morphologies, and Composites. Chem. Rev. 2012, 112, 933–969. [Google Scholar] [CrossRef]
- Macarie, L.; Simulescu, V.; Ilia, G. Ultrasonic irradiation used in synthesis of aminophosphonates. Monatsh. Chem. 2019, 150, 163–171. [Google Scholar] [CrossRef]
- Khan, M.A.; Jhung, S.H. Synthesis of metal-organic frameworks (MOFs) with microwave or ultrasound: Rapid reaction, phase-selectivity, and size reduction. Coord. Chem. Rev. 2015, 285, 11–23. [Google Scholar] [CrossRef]
- Iliescu, S.; Ilia, G.; Pascariu, A.; Popa, A.; Plesu, N. Novel synthesis of phosphorus containing polymers under inverse phase transfer catalysis. Polymer 2006, 47, 6509–6512. [Google Scholar] [CrossRef]
- Visa, A.; Mracec, M.; Maranescu, B.; Maranescu, V.; Ilia, G.; Popa, A.; Mracec, M. Structure simulation into a lamellar supramolecular network and calculation of the metal ions/ligands ratio. Chem. Cent. J. 2012, 6, 91. [Google Scholar] [CrossRef]
- Popa, A.; Ilia, G.; Pascariu, A.; Iliescu, S.; Plesu, N. Grafted styrene-divinylbenzene copolymers containing benzaldehyde and their Wittig reactions with various phosphonium salts. Chin. J. Polym. Sci. 2005, 23, 651–656. [Google Scholar] [CrossRef]
- Maranescu, B.; Lupa, L.; Visa, A. Heavy metal removal from waste waters by phosphonate metal organic frameworks. Pure Appl. Chem. 2018, 90, 35–47. [Google Scholar] [CrossRef]
- Nistor, M.A.; Muntean, S.G.; Maranescu, B.; Visa, A. Phosphonate metal organic frameworks used as dyes removal materials from wastewaters. Appl. Organomet. Chem. 2020, 34, e5939. [Google Scholar] [CrossRef]
- Maranescu, B.; Popa, A.; Lupa, L.; Maranescu, V.; Visa, A. Use of chitosan complex with aminophosphonic groups and cobalt for the removal of Sr2+ ions. Sep. Sci. Technol. 2018, 53, 1058–1064. [Google Scholar] [CrossRef]
- Lupa, L.; Maranescu, B.; Visa, A. Equilibrium and kinetic studies of chromium ions adsorption on Co(II) based phosphonate metal organic frameworks. Sep. Sci. Technol. 2018, 53, 1017–1026. [Google Scholar] [CrossRef]
- Maranescu, B.; Lupa, L.; Tara Lunga Mihali, M.; Plesu, N.; Maranescu, V.; Visa, A. The Corrosion Inhibitor Behavior of Iron in Saline Solution by the Action of Magnesium Carboxyphosphonate. Pure Appl. Chem. 2018, 90, 1713–1722. [Google Scholar] [CrossRef]
- Popa, A.; Ilia, G.; Iliescu, S.; Dehelean, G.; Pascariu, A.; Bora, A.; Pacureanu, L. Mixed quaternary ammonium and phosphonium salts bound to macromolecular supports for removal bacteria from water. Mol. Cryst. Liq. Cryst. 2004, 418, 195–203. [Google Scholar] [CrossRef]
- Uemura, T.; Yanai, N.; Kitagawa, S. Polymerization reactions in porous coordination polymers. Chem. Soc. Rev. 2009, 38, 1228–1236. [Google Scholar] [CrossRef]
- Sharmin, E.; Zafar, F. (Eds.) Introductory Chapter: Metal Organic Frameworks (MOFs). In Metal-Organic Frameworks; IntechOpen Limited: London, UK, 2016; pp. 3–16. Available online: https://www.intechopen.com/books/metal-organic-frameworks (accessed on 12 October 2016).
- Shimizu, G.K.H.; Vaidhyanathan, R.; Taylor, J.M. Phosphonate and sulfonate metal organic frameworks. Chem. Soc. Rev. 2009, 38, 1430–1449. [Google Scholar] [CrossRef] [PubMed]
- Clearfield, A.; Demadis, K.D. Metal Phosphonate Chemistry: From Synthesis to Applications; Royal Society of Chemistry: London, UK, 2012. [Google Scholar]
- Maranescu, B.; Visa, A.; Mracec, M.; Ilia, G.; Maranescu, V.; Simon, Z.; Mracec, M. Lamellar Co2+ vinylphosphonate metal organic framework.PM3 semi-empirical analysis of structural properties. Rev. Roum. Chim. 2011, 56, 473–482. [Google Scholar]
- Maranescu, B.; Visa, A.; Ilia, G.; Simon, Z.; Demadis, K.; Colodrero, R.M.P.A.; Vallcorba, O.; Rius, J.; Choquesillo-Lazarte, D. Synthesis and characterization of styryl phosphonic acid and its use as new ligand for phosphonate metal organic framework. J. Coord. Chem. 2014, 67, 1562–1572. [Google Scholar] [CrossRef]
- Bataille, T.; Bracco, S.; Comotti, A.; Costantino, F.; Guerri, A.; Ienco, A.; Marmottini, F. Solvent dependent synthesis of micro- and nano- crystalline phosphinate based 1D tubular MOF: Structure and CO2 adsorption selectivity. Cryst. Eng. Comm. 2012, 14, 7170–7173. [Google Scholar] [CrossRef]
- Midollini, S.; Lorenzo-Luis, P.; Orlandini, A. Inorganic–organic hybrid materials of p,p0-diphenylmethylenediphosphinic acid (H2pcp) with magnesium and calcium ions: Synthesis and characterization of [Mg(Hpcp)2], [Mg(Hpcp)2(H2O)4], [Mg(pcp)(H2O)3](H2O), [Ca(Hpcp)2] and [Ca(pcp)(H2O)] complexes. Inorg. Chim. Acta 2006, 359, 3275–3282. [Google Scholar] [CrossRef]
- Ienco, A.; Midollini, S.; Orlandini, A.; Costantino, F. Synthesis and Structural Characterization of a Tetranuclear Zinc(II) Complex with P,P′ diphenylmethylenediphosphinate (pcp) and2,2′-Bipyridine (2,2′-bipy) Ligands. Z. Naturforsch. 2007, 62b, 1476–1480. [Google Scholar] [CrossRef]
- Costantino, F.; Ienco, A.; Midollini, S.; Orlandini, A.; Sorace, L.; Vacca, A. Copper(II) complexes with bridging diphosphinates—The effect of the elongation of the aliphatic chain on the structural arrangements around the metal centres. Eur. J. Inorg. Chem. 2008, 2008, 3046–3055. [Google Scholar] [CrossRef]
- Plinta, H.J.; Neda, I.; Schmutzler, R. 1.3-Dimethyl-l,3-diaza-2-R-5,6-benzo-2 λ3-phosphorinan-4-ones (R = F, Me2N, 2-Methylpiperidino, MeC(:O)NH-) as Ligands in Transition-Metal Complexes; Synthesis and Structure of DichloroPlatinum(II)- and Tetracarbonyl-Metal(0) Coordination Compounds (Metal = Cr, Mo and W). Z. Naturforsch. 1994, 49b, 100–110. [Google Scholar]
- Dieleman, C.B.; Matt, D.; Neda, I.; Schmutzler, R.; Harriman, A.; Yaftian, R. Hexahomotrioxacalix[3]arene: A scaffold for a C3-symmetric phosphine ligand that traps a hydridorhodium fragment inside a molecular funnel. Chem. Commun. 1999, 18, 1911–1912. [Google Scholar] [CrossRef]
- Vollbrecht, A.; Neda, I.; Thonnessen, H.; Jones, P.G.; Harris, R.K.; Crowe, L.A.; Schmutzler, R. Chemische Synthesis, structure, and reactivity of tetrakis(o,o-phosphorus)-bridged calix[4]resorcinols and their derivatives. Berichte 1997, 130, 1715–1720. [Google Scholar] [CrossRef]
- Hu, Q.S.; Zhang, X.Z.; Luo, S.F.; Sun, Y.H.; Du, Z.-Y. Two polymorphs of (2-carboxyethyl)-(phenyl)phosphinic acid. Acta Crystallogr. 2011, C67, o195–o197. [Google Scholar] [CrossRef]
- Bazaga-García, M.; Vílchez-Cózar, Á.; Maranescu, B.; Olivera-Pastor, P.; Marganovici, M.; Ilia, G.; Cabeza Díaz, A.; Visa, A.; Colodrero, R.M.P. Synthesis and electrochemical properties of metal(II)-carboxyethylphenylphosphinates. Dalton Trans. 2021, 50, 6539–6548. [Google Scholar] [CrossRef]
- You, L.; Hui, Y.; Shi, X.; Peng, Z. Study on the synthesis and characterization of a novel phosphorus-nitrogen containing intumescent flame retardant. Adv. Mater. Res. 2012, 399–401, 1376–1380. [Google Scholar] [CrossRef]
- Li, L.J.; Duan, R.T.; Zhang, J.B.; Wang, X.L.; Chen, L.; Yu-Zhong, W. Phosphorus-Containing Poly(ethylene terephthalate): Solid-State Polymerization and Its Sequential Distribution. Ind. Eng. Chem. Res. 2013, 52, 5326–5333. [Google Scholar] [CrossRef]
- Wang, G.; Jiang, M.; Zhang, Q.; Wang, P.; Qu, X.; Zhou, G. Poly(hexamethylene 2,5-furandicarboxylate) copolyesters containing phosphorus: Synthesis, crystallization behavior, thermal, mechanical and flame retardant properties. Polym. Degrad. Stab. 2018, 153, 272–280. [Google Scholar] [CrossRef]
- YiYi, D.; Huang, S.; Laird, D.A.; Wang, X.; Dong, C. Quantitative mechanisms of cadmium adsorption on rice straw- and swine manure-derived biochars. Environ. Sci. Pollut. Res. 2018, 25, 32418–32432. [Google Scholar]
- Sharma, R.; Sarswat, A.; Pittman, C.U.; Mohan, D. Cadmium and lead remediation using magnetic and non-magnetic sustainable biosorbents derived from Bauhinia purpurea pods. RSC Adv. 2017, 7, 8606–8624. [Google Scholar] [CrossRef]
- Xue, C.C.; Li, M.X.; Shao, M. Two novel 2D cadmium compounds with noncentrosymmetric or symmetric network dependent on different pH values. Russ. J. Coord. Chem. 2016, 42, 442–448. [Google Scholar] [CrossRef]
- Wang, X.; Wang, L.; Wang, Y.; Tan, R.; Ke, X.; Zhou, X.; Cheng, J.; Hou, H.; Zhou, M. Calcium sulfate hemihydrate whiskers obtained from flue gas desulfurization gypsum and used for the adsorption removal of lead. Crystals 2017, 7, 270. [Google Scholar] [CrossRef]
- Liang, Y.; Jun, M.; Liu, W. Enhanced removal of lead(II) and cadmium(II) from water in alum coagulation by ferrate(VI) pretreatment. Water Environ. Res. 2007, 79, 2420–2426. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Sheng, L.; Wang, Y.; Wyckoff, K.N.; He, C.; He, Q. Characteristics of Cadmium Sorption by Heat-Activated Red Mud in Aqueous Solution. Sci. Rep. 2018, 8, 13558. [Google Scholar] [CrossRef]
- Abbasi, A.; Moradpour, T.; Van Hecke, K. A new 3D cobalt (II) metal–organic framework nanostructure for heavy metal adsorption. Inorg. Chim. Acta 2015, 430, 261–267. [Google Scholar] [CrossRef]
- Rahimi, E.; Mohaghegh, N. Removal of Toxic Metal Ions from Sungun Acid Rock Drainage Using Mordenite Zeolite, Graphene Nanosheets, and a Novel Metal–Organic Framework. Mine Water Environ. 2016, 35, 18–28. [Google Scholar] [CrossRef]
- Waritu, H.H.; Aregahegn, D.A.; Abdisa, C.M.; Minaleshewa, A. High Performance Copper Based Metal Organic Framework for Removal of Heavy Metals From Wastewater. Front. Mater. 2022, 9, 840806. [Google Scholar]
- Soltani, R.; Pelalak, R.; Pishnamazi, M.; Marjani, A.; Albadarin, A.B.; Sarkar, M.S.; Shirazian, S. A novel and facile green synthesis method to prepare LDH/MOF nanocomposite for removal of Cd(II) and Pb(II). Sci Rep. 2021, 11, 1609. [Google Scholar] [CrossRef] [PubMed]
- Visa, A.; Plesu, N.; Maranescu, B.; Ilia, G.; Borota, A.; Crisan, L. Combined Experimental and Theoretical Insights into the Corrosion Inhibition Activity on Carbon Steel Iron of Phosphonic Acids. Molecules 2021, 26, 135. [Google Scholar] [CrossRef] [PubMed]
- Visa, A.; Maranescu, B.; Bucur, A.; Iliescu, S.; Demadis, K. Synthesis and characterization of a novel phosphonate metal organic framework starting from copper salts. Phosphorus Sulfur Silicon Relat. Elem. 2014, 189, 630–639. [Google Scholar] [CrossRef]
- Visa, A.; Maranescu, B.; Lupa, L.; Crisan, L.; Borota, A. New Efficient Adsorbent Materials for the Removal of Cd(II) from Aqueous Solutions. Nanomaterials 2020, 10, 899. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Wang, L. Comparison between linear and non-linear forms of pseudo-first-order and pseudo-second-order adsorption kinetic models for the removal of methylene blue by activated carbon. Front. Environ. Sci. Eng. China 2009, 3, 320–3244. [Google Scholar] [CrossRef]
- Kumar, K.V.; Porkodi, K.; Rocha, F. Comparison of various error functions in predicting the optimum isotherm by linear and non-linear regression analysis for the sorption of basic red 9 by activated carbon. J. Hazard. Mater. 2008, 150, 158–165. [Google Scholar] [CrossRef]
- Obayomi, K.S.; Bello, J.O.; Yahya, M.D.; Chukwunedum, E.; Adeoye, J.B. Statistical analyses on effective removal of cadmium and hexavalent chromium ions by multiwall carbon nanotubes (MWCNTs). Heliyon 2020, 6, e04174. [Google Scholar] [CrossRef]
- Tǎmaş, A.; Cozma, I.; Cocheci, L.; Lupa, L.; Rusu, G. Adsorption of orange II onto Zn2Al–layered double hydroxide prepared from zinc. Ash. Front. Chem. 2020, 8, 573535. [Google Scholar] [CrossRef]
- Azmi, S.N.H.; Al Lawati, W.M.; Al Hoqani, U.H.A.; Al Aufi, E.; Al Hatmi, K.; Al Zadjali, J.S.; Rahman, N.; Nasir, M.; Rahman, H.; Khan, S.A. Development of a Citric-Acid-Modified Cellulose Adsorbent Derived from Moringa peregrina Leaf for Adsorptive Removal of Citalopram HBr in Aqueous Solutions. Pharmaceuticals 2022, 15, 760. [Google Scholar] [CrossRef]

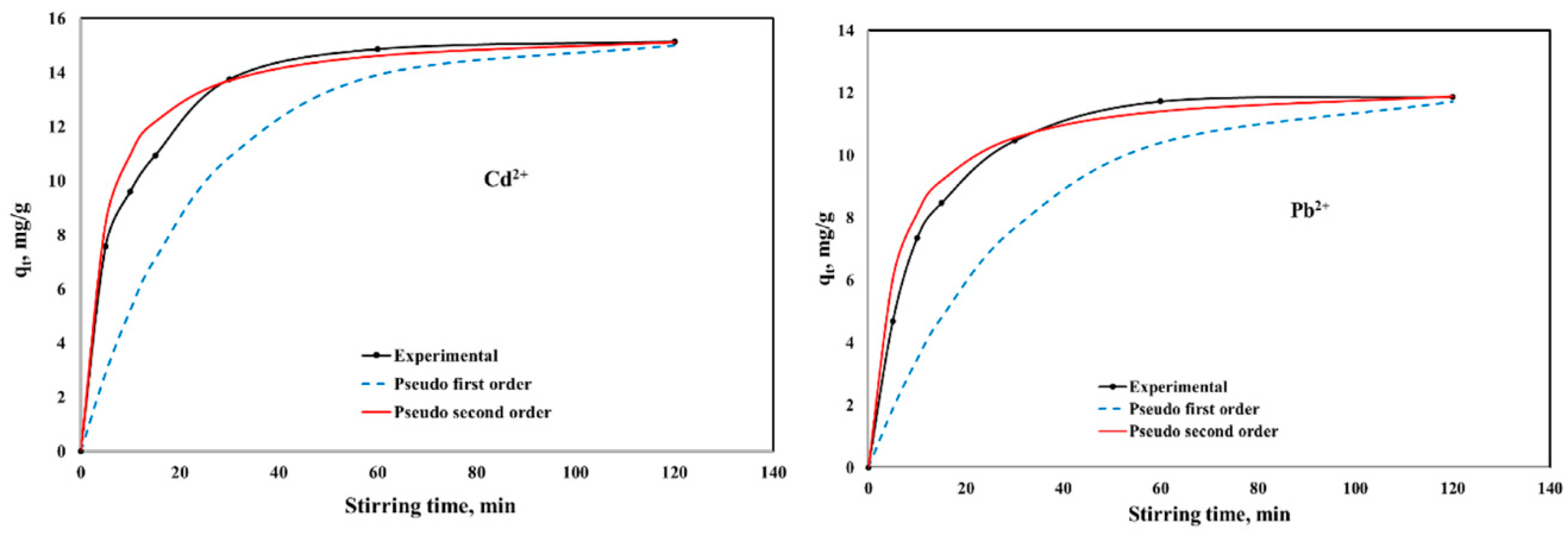


| Kinetic Model | Parameters | CaCEPPA | CoCEPPA | ||
|---|---|---|---|---|---|
| Cd | Pb | Cd | Pb | ||
| qe exp, mg/g | 15.14 | 11.85 | 21.08 | 17.23 | |
| Pseudo-first-order | qe calc, mg/g | 7.15 | 5.545 | 8.56 | 9.44 |
| k1, min−1 | 0.0424 | 0.0344 | 0.024 | 0.0342 | |
| R2 | 0.9674 | 0.8932 | 0.7840 | 0.8573 | |
| ERRSQ | 353 | 221 | 970 | 331 | |
| χ2 | 99.5 | 84.7 | 318 | 74.7 | |
| ARE, % | 228 | 253 | 458 | 183 | |
| Pseudo-second-order | qe calc, mg/g | 15.67 | 12.4 | 21.92 | 18.25 |
| k2, g mg−1 min−1 | 0.0149 | 0.0153 | 0.0104 | 0.0079 | |
| R2 | 0.9975 | 0.9959 | 0.9968 | 0.9928 | |
| ERRSQ | 4.21 | 3.09 | 10.4 | 6.39 | |
| χ2 | 0.389 | 0.45 | 0.727 | 0.59 | |
| ARE, % | 5.81 | 7.31 | 7.14 | 8.26 | |
| Isotherm Model | Parameters | CaCEPPA | CoCEPPA | ||
|---|---|---|---|---|---|
| Cd | Pb | Cd | Pb | ||
| qm exp, mg/g | 32.6 | 21.4 | 51.6 | 32.7 | |
| Langmuir | qm calc, mg/g | 36.5 | 23.52 | 54.9 | 35.8 |
| kL, L/mg | 0.0556 | 0.0483 | 0.0886 | 0.0739 | |
| R2 | 0.9964 | 0.9968 | 0.9909 | 0.9968 | |
| ERRSQ | 10.6 | 2.29 | 27.3 | 10.6 | |
| χ2 | 0.46 | 0.21 | 2.1 | 0.54 | |
| ARE, % | 5.3 | 5.99 | 18.1 | 5.78 | |
| Freundlich | kF, (mg/g) (L/mg)n | 3.106 | 2.188 | 6.65 | 3.509 |
| 1/n | 0.5183 | 0.4805 | 0.4590 | 0.5067 | |
| R2 | 0.9475 | 0.9448 | 0.9648 | 0.9193 | |
| ERRSQ | 165 | 38.5 | 253 | 259 | |
| χ2 | 4.86 | 2.38 | 5.23 | 8.01 | |
| ARE, % | 17.3 | 15.3 | 14.6 | 22.3 | |
| Temkin | b, J/mol | 353 | 563 | 275 | 352 |
| KT, L/g | 0.768 | 0.713 | 1.88 | 0.44 | |
| R2 | 0.9700 | 0.9931 | 0.9801 | 0.9628 | |
| ERRSQ | 22.9 | 1.83 | 36.0 | 29.9 | |
| χ2 | 1.82 | 0.422 | 7.59 | 1.85 | |
| ARE, % | 18.5 | 9.36 | 42.6 | 16.3 | |
| Dubinin–Raduschkevich | qm, mg/g | 21.6 | 14.1 | 31.0 | 22.3 |
| KDR, mol2/J2 | 2 × 10−6 | 2 × 10−6 | 4 × 10−7 | 1 × 10−6 | |
| R2 | 0.7985 | 0.7752 | 0.7785 | 0.7630 | |
| ERRSQ | 269 | 95.3 | 746 | 324 | |
| χ2 | 14.8 | 7.58 | 25.9 | 16.5 | |
| ARE, % | 43.8 | 24.6 | 37.7 | 34.0 | |
| Adsorbent | qm (mg g−1) | References |
|---|---|---|
| Functionalized Zr-MOFs | 41.32 | [10] |
| UiO-66-NHC(S)NHMe | 49 | [51] |
| TMU-5 | 43 | [52] |
| Cu-DPA MOF | 1.334 | [53] |
| LDH/MOF NC | 415.3 | [54] |
| CaCEPPA | 32.6 | This paper |
| CoCEPPA | 51.6 |
| Adsorbent | qm (mg g−1) | References |
|---|---|---|
| Functionalized Zr-MOFs | 50.51 | [10] |
| UiO-66-NHC(S)NHMe | 232 | [51] |
| TMU-5 | 251 | [52] |
| Cu-DPA MOF | 2.19 | [53] |
| LDH/MOF NC | 301.4 | [54] |
| CaCEPPA | 21.4 | This paper |
| CoCEPPA | 32.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marganovici, M.; Maranescu, B.; Visa, A.; Lupa, L.; Hulka, I.; Chiriac, V.; Ilia, G. Hybrid Coordination Networks for Removal of Pollutants from Wastewater. Int. J. Mol. Sci. 2022, 23, 12611. https://doi.org/10.3390/ijms232012611
Marganovici M, Maranescu B, Visa A, Lupa L, Hulka I, Chiriac V, Ilia G. Hybrid Coordination Networks for Removal of Pollutants from Wastewater. International Journal of Molecular Sciences. 2022; 23(20):12611. https://doi.org/10.3390/ijms232012611
Chicago/Turabian StyleMarganovici, Marko, Bianca Maranescu, Aurelia Visa, Lavinia Lupa, Iosif Hulka, Vlad Chiriac, and Gheorghe Ilia. 2022. "Hybrid Coordination Networks for Removal of Pollutants from Wastewater" International Journal of Molecular Sciences 23, no. 20: 12611. https://doi.org/10.3390/ijms232012611
APA StyleMarganovici, M., Maranescu, B., Visa, A., Lupa, L., Hulka, I., Chiriac, V., & Ilia, G. (2022). Hybrid Coordination Networks for Removal of Pollutants from Wastewater. International Journal of Molecular Sciences, 23(20), 12611. https://doi.org/10.3390/ijms232012611










