Phenol and Polyaromatic Hydrocarbons Are Stronger Drivers Than Host Plant Species in Shaping the Arbuscular Mycorrhizal Fungal Component of the Mycorrhizosphere
Abstract
1. Introduction
2. Results
2.1. Soil Physicochemical Features
2.2. PCR Results
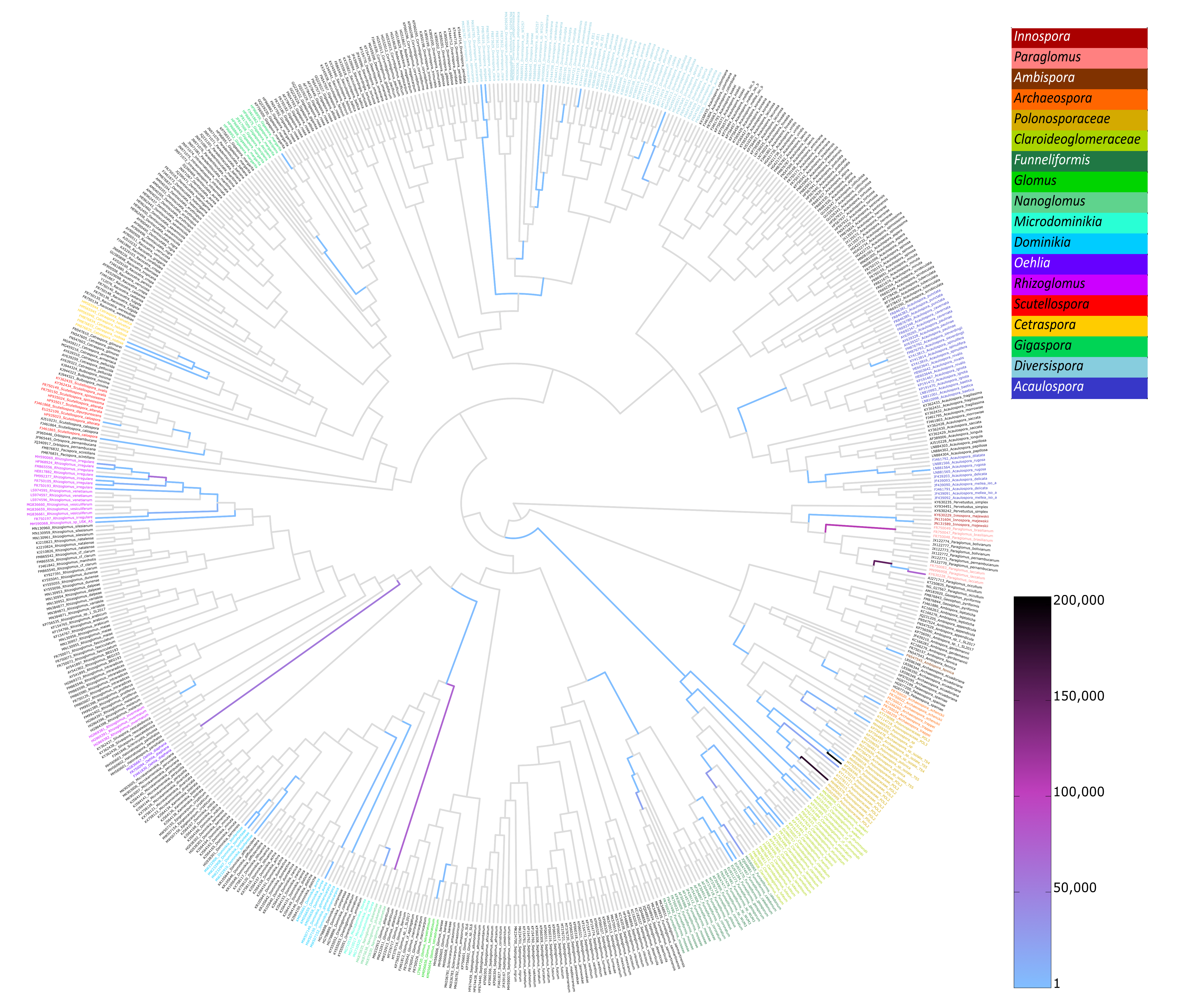
2.3. Sequences Processing
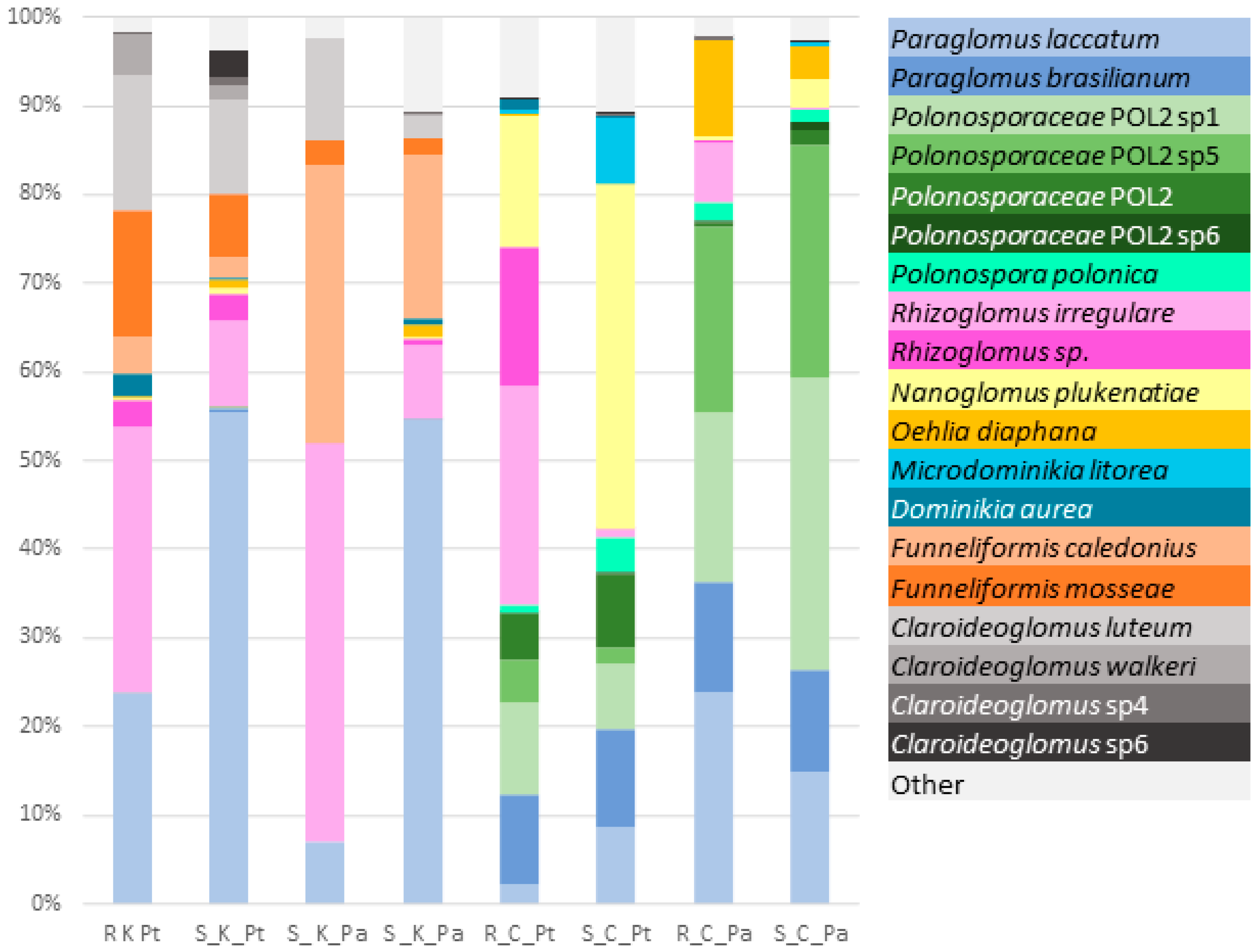
2.4. α-Biodiversity of AMF Communities
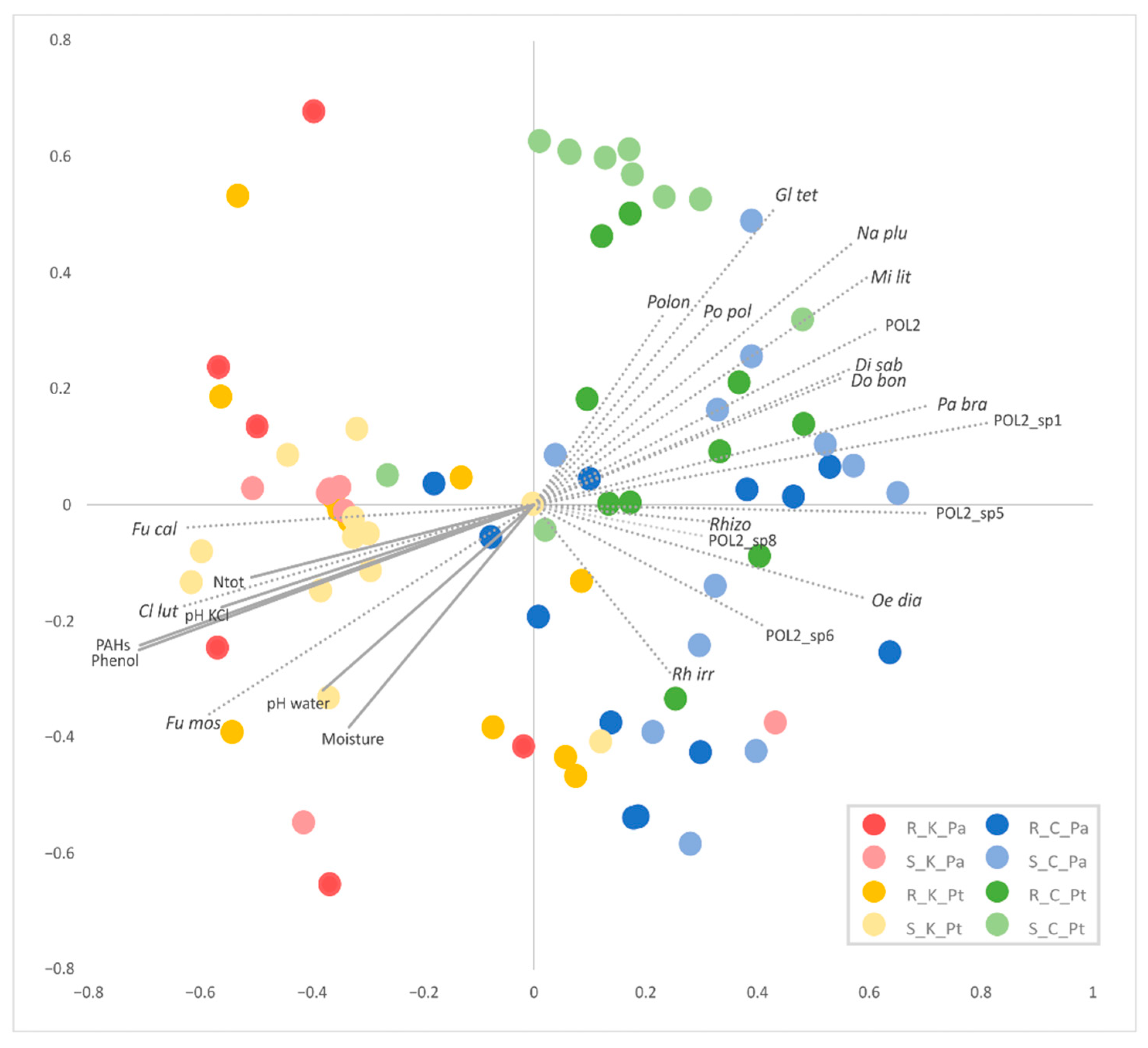
2.5. β-Biodiversity of the AMF Communities
3. Discussion
4. Materials and Methods
4.1. Sampling and DNA Extraction
4.2. Primer Design
4.3. PCR and Preparation of LSU rDNA Libraries for Sequencing
4.4. Bioinformatic Analysis
4.5. Analysis of AMF Communities Diversity
4.6. Accession Numbers
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Correa-García, S.; Pande, P.; Séguin, A.; St-Arnaud, M.; Yergeau, E. Rhizoremediation of petroleum hydrocarbons: A model system for plant microbiome manipulation. Microb. Biotechnol. 2018, 11, 819–832. [Google Scholar] [CrossRef]
- Zuzolo, D.; Guarino, C.; Tartaglia, M.; Sciarrillo, R. Plant-soil-microbiota combination for the removal of total petroleum hydrocarbons (TPH): An in-field experiment. Front. Microbiol. 2021, 11, 621581. [Google Scholar] [CrossRef]
- Gałązka, A.; Grządziel, J.; Gałązka, R.; Ukalska-Jaruga, A.; Strzelecka, J.; Smreczak, B. Genetic and functional diversity of bacterial microbiome in soils with long term impacts of petroleum hydrocarbons. Front. Microbiol. 2019, 9, 1923. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Garrido-Sanz, D.; Sansegundo-Lobato, P.; Redondo-Nieto, M.; Conlon, R.; Martin, M.; Mali, R.; Liu, X.; Dowling, D.N.; Rivilla, R.; et al. Soil microbiome structure and function in ecopiles used to remediate petroleum-contaminated soil. Front. Environ. Sci. 2021, 9, 624070. [Google Scholar] [CrossRef]
- Rich, M.K.; Nouri, E.; Courty, P.-E.; Reinhardt, D. Diet of arbuscular mycorrhizal fungi: Bread and butter. Trends Plant Sci. 2017, 22, 652–660. [Google Scholar] [CrossRef] [PubMed]
- López-García, Á.; Azcón-Aguilar, C.; Barea, J.M. The interactions between plant life form and fungal traits of arbuscular mycorrhizal fungi determine the symbiotic community. Oecologia 2014, 176, 1075–1086. [Google Scholar] [CrossRef]
- Millar, N.S.; Bennett, A.E. Stressed out symbiotes: Hypotheses for the influence of abiotic stress on arbuscular mycorrhizal fungi. Oecologia 2016, 182, 625–641. [Google Scholar] [CrossRef] [PubMed]
- Alguacil, M.M.; Torres, M.P.; Montesinos-Navarro, A.; Roldán, A. Soil characteristics driving arbuscular mycorrhizal fungal communities in semiarid mediterranean soils. Appl. Environ. Microbiol. 2013, 82, 3348–3356. [Google Scholar] [CrossRef] [PubMed]
- Entry, J.A.; Rygiewicz, P.T.; Watrud, L.S.; Donnelly, P.K. Influence of adverse soil conditions on the formation and function of Arbuscular mycorrhizas. Adv. Environ. Res. 2002, 7, 123–138. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, X.; Zhang, S.; Zhang, S.; Sun, Y. Interactions of microplastics and cadmium on plant growth and arbuscular mycorrhizal fungal communities in an agricultural soil. Chemosphere 2020, 254, 126791. [Google Scholar] [CrossRef]
- Kawahara, A.; An, G.H.; Miyakawa, S.; Sonoda, J.; Ezawa, T. Nestedness in arbuscular mycorrhizal fungal communities along soil pH gradients in early primary succession: Acid-tolerant fungi are pH generalists. PLoS ONE 2016, 11, e0165035. [Google Scholar] [CrossRef]
- Van Aarle, I.M.; Olsson, P.A.; Söderström, B. Arbuscular mycorrhizal fungi respond to the substrate pH of their extraradical mycelium by altered growth and root colonization. New Phytol. 2002, 155, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.E.D.; Bell, T.H.; Stefani, F.O.P.; Denis, D.; Hijri, M.; St-Arnaud, M. Contrasting the community structure of arbuscular mycorrhizal fungi from hydrocarbon-contaminated and uncontaminated soils following willow (Salix spp. L.) planting. PLoS ONE 2014, 9, e102838. [Google Scholar] [CrossRef] [PubMed]
- Dagher, D.J.; de la Providencia, I.E.; Pitre, F.E.; St-Arnaud, M.; Hijri, M. Arbuscular mycorrhizal fungal assemblages significantly shifted upon bacterial inoculation in non-contaminated and petroleum-contaminated environments. Microorganisms 2020, 8, 602. [Google Scholar] [CrossRef] [PubMed]
- Wipf, D.; Krajinski, F.; van Tuinen, D.; Recorbet, G.; Courty, P.-E. Trading on the arbuscular mycorrhiza market: From arbuscules to common mycorrhizal networks. New Phytol. 2019, 223, 1127–1142. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Tiwari, J.; Bauddh, K. Plant-mycorrhizal fungi interactions in phytoremediation of geogenic contaminated soils. Front. Microbiol. 2022, 13, 843415. [Google Scholar] [CrossRef]
- la Providencia, I.E.; Stefani, F.O.P.; Labridy, M.; St-Arnaud, M.; Hijri, M. Arbuscular mycorrhizal fungal diversity associated with Eleocharis obtusa and Panicum capillare growing in an extreme petroleum hydrocarbon-polluted sedimentation basin. FEMS Microbiol. Lett. 2015, 362, fnv081. [Google Scholar] [CrossRef]
- Iffis, B.; St-Arnaud, M.; Hijri, M. Petroleum hydrocarbon contamination, plant identity and arbuscular mycorrhizal fungal (AMF) community determine assemblages of the AMF spore-associated microbes. Environ. Microbiol. 2016, 18, 2689–2704. [Google Scholar] [CrossRef]
- Malicka, M.; Magurno, F.; Piotrowska-Seget, Z.; Chmura, D. Arbuscular mycorrhizal and microbial profiles of an aged phenol–polynuclear aromatic hydrocarbon-contaminated soil. Ecotoxicol. Environ. Saf. 2020, 192, 110299. [Google Scholar] [CrossRef]
- Malicka, M.; Magurno, F.; Posta, K.; Chmura, D.; Piotrowska-Seget, Z. Differences in the effects of single and mixed species of AMF on the growth and oxidative stress defense in Lolium perenne exposed to hydrocarbons. Ecotoxicol. Environ. Saf. 2021, 217, 112252. [Google Scholar] [CrossRef]
- Schoch, C.L.; Seifert, K.A.; Huhndorf, S.; Robert, V.; Spouge, J.L.; Levesque, C.A.; Chen, W.; Bolchacova, E.; Voigt, K.; Crous, P.W.; et al. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc. Natl. Acad. Sci. USA 2012, 109, 6241–6246. [Google Scholar] [CrossRef] [PubMed]
- Crossay, T.; Antheaume, C.; Redecker, D.; Bon, L.; Chedri, N.; Richert, C.; Guentas, L.; Cavaloc, Y.; Amir, H. New method for the identification of arbuscular mycorrhizal fungi by proteomic-based biotyping of spores using MALDI-TOF-MS. Sci. Rep. 2017, 7, 14306. [Google Scholar] [CrossRef] [PubMed]
- Öpik, M.; Vanatoa, A.; Vanatoa, E.; Moora, M.; Davison, J.; Kalwij, J.M.; Reier, Ü.; Zobel, M. The online database MaarjAM reveals global and ecosystemic distribution patterns in arbuscular mycorrhizal fungi (Glomeromycota). New Phytol. 2010, 188, 223–241. [Google Scholar] [CrossRef]
- Rosendahl, S. Communities, populations and individuals of arbuscular mycorrhizal fungi. New Phytol. 2008, 178, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Lumini, E.; Orgiazzi, A.; Borriello, R.; Bonfante, P.; Bianciotto, V. Disclosing arbuscular mycorrhizal fungal biodiversity in soil through a land-use gradient using a pyrosequencing approach. Environ. Microbiol. 2009, 12, 2165–2179. [Google Scholar] [CrossRef] [PubMed]
- Kohout, P.; Sudová, R.; Janoušková, M.; Čtvrtlíková, M.; Hejda, M.; Pánková, H.; Slavíková, R.; Štajerová, K.; Vosátka, M.; Sýkorová, Z. Comparison of commonly used primer sets for evaluating arbuscular mycorrhizal fungal communities: Is there a universal solution? Soil Biol. Biochem. 2014, 68, 482–493. [Google Scholar] [CrossRef]
- Krüger, M.; Stockinger, H.; Krüger, C.; Schüßler, A. DNA-based species level detection of Glomeromycota: One PCR primer set for all arbuscular mycorrhizal fungi. New Phytol. 2019, 183, 212–223. [Google Scholar] [CrossRef]
- Krüger, M.; Krüger, C.; Walker, C.; Stockinger, H.; Schüßler, A. Phylogenetic reference data for systematics and phylotaxonomy of arbuscular mycorrhizal fungi from phylum to species level. New Phytol. 2012, 193, 970–984. [Google Scholar] [CrossRef]
- Ravi, R.K.; Walton, K.; Khosroheidari, M. MiSeq: A next generation sequencing platform for genomic analysis. In Disease Gene Identification. Methods in Molecular Biology; DiStefano, J., Ed.; Humana Press: New York, NY, USA, 2018; Volume 1706, pp. 223–232. [Google Scholar]
- Pearman, W.S.; Freed, N.E.; Silander, O.K. Testing the advantages and disadvantages of short- and long- read eukaryotic metagenomics using simulated reads. BMC Bioinform. 2020, 21, 220. [Google Scholar] [CrossRef]
- Mueller, R.C.; Gallegos-Graves, L.V.; Kuske, C.R. A new fungal large subunit ribosomal RNA primer for high throughput sequencing surveys. FEMS Microbiol. 2015, 92, fiv153. [Google Scholar] [CrossRef]
- Garcés-Ruiz, M.; Senés-Guerrero, C.; Declerck, S.; Cranenbrouck, S. Arbuscular mycorrhizal fungal community composition in Carludovica palmata, Costus scaber and Euterpe precatoria from weathered oil ponds in the Ecuadorian Amazon. Front. Microbiol. 2017, 8, 2134. [Google Scholar] [CrossRef]
- Garcés-Ruiz, M.; Senés-Guerrero, C.; Declerck, S.; Cranenbrouck, S. Community composition of arbuscular mycorrhizal fungi associated with native plants growing in a petroleum-polluted soil of the Amazon region of Ecuador. MicrobiologyOpen 2019, 8, e00703. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-J.; Kong, M.; St-Arnaud, M.; Hijri, M. Arbuscular mycorrhizal fungal communities of native plant species under high petroleum hydrocarbon contamination highlights Rhizophagus as a key tolerant genus. Microorganisms 2020, 8, 872. [Google Scholar] [CrossRef]
- Yang, Y.; Han, X.; Liang, Y.; Ghosh, A.; Chen, J.; Tang, M. The combined effects of arbuscular mycorrhizal fungi (AMF) and lead (Pb) stress on Pb accumulation, plant growth parameters, photosynthesis, and antioxidant enzymes in Robinia pseudoacacia L. PLoS ONE 2015, 10, e0145726. [Google Scholar] [CrossRef] [PubMed]
- Oehl, F.; Laczko, E.; Oberholzer, H.-R.; Jansa, J.; Egli, S. Diversity and biogeography of arbuscular mycorrhizal fungi in agricultural soils. Biol. Fertil. Soils 2017, 53, 777–797. [Google Scholar] [CrossRef]
- Davison, J.; Öpik, M.; Daniell, T.J.; Moora, M.; Zobel, M. Arbuscular mycorrhizal fungal communities in plant roots are not random assemblages. FEMS Microbiol. Ecol. 2011, 78, 103–115. [Google Scholar] [CrossRef]
- Moora, M.; Berger, S.; Davison, J.; Öpik, M.; Bommarco, R.; Bruelheide, H.; Kühn, I.; Kunin, W.E.; Metsis, M.; Rortais, A.; et al. Alien plants associate with widespread generalist arbuscular mycorrhizal fungal taxa: Evidence from a continental-scale study using massively parallel 454 sequencing. J. Biogeogr. 2011, 38, 1305–1317. [Google Scholar] [CrossRef]
- Chagnon, P.L.; Bradley, R.L.; Maherali, H.; Klironomos, J.N. A trait-based framework to understand life history of mycorrhizal fungi. Trends Plant Sci. 2013, 18, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Gu, Z.; Cui, H.; Zhu, H.; Fu, S.; Yao, Q. Differences in arbuscular mycorrhizal fungal community composition in soils of three land use types in subtropical hilly area of Southern China. PLoS ONE 2015, 10, e0130983. [Google Scholar] [CrossRef] [PubMed]
- Lenoir, I.; Fontaine, J.; Lounès-Hadj Sahraoui, A. Arbuscular mycorrhizal fungal responses to abiotic stresses: A review. Phytochemistry 2016, 123, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Trigueros, C.A.; Hempel, S.; Powell, J.R.; Cornwell, W.K.; Rillig, M.C. Bridging reproductive and microbial ecology: A case study in arbuscular mycorrhizal fungi. ISME J. 2019, 13, 873–884. [Google Scholar] [CrossRef]
- Koltai, H.; Kapulnik, Y. Arbuscular Mycorrhizas: Physiology and Function; Springer: Dordrecht, The Netherlands, 2010. [Google Scholar]
- Marquez-Garcia, B.; Njo, M.; Beeckman, T.; Goormachtig, S.; Foyer, C.H. A new role for glutathione in the regulation of root architecture linked to strigolactones. Plant Cell Environ. 2014, 37, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Oehl, F.; Sieverding, E.; Ineichen, K.; Mäder, P.; Wiemken, A.; Boller, T. Distinct sporulation dynamics of arbuscular mycorrhizal fungal communities from different agroecosystems in long-term microcosms. Agric. Ecosyst. Environ. 2009, 134, 257–268. [Google Scholar] [CrossRef]
- Krüger, C.; Kohout, P.; Janoušková, M.; Püschel, D.; Frouz, J.; Rydlová, J. Plant communities rather than soil properties structure arbuscular mycorrhizal fungal communities along primary succession on a mine spoil. Front. Microbiol. 2017, 8, 719. [Google Scholar] [CrossRef] [PubMed]
- Baltruschat, H.; Santos, V.M.; da Silva, D.K.A.; Schellenberg, I.; Deubel, A.; Sieverding, E.; Oehl, F. Unexpectedly high diversity of arbuscular mycorrhizal fungi in fertile Chernozem croplands in Central Europe. Catena 2019, 182, 104135. [Google Scholar] [CrossRef]
- Błaszkowski, J.; Niezgoda, P.; Meller, E.; Milczarski, P.; Zubek, S.; Malicka, M.; Uszok, S.; Casieri, L.; Goto, B.T.; Magurno, F.; et al. New taxa in Glomeromycota: Polonosporaceae fam. nov., Polonospora gen. nov., and P. polonica comb. nov. Mycol. Prog. 2021, 20, 941–951. [Google Scholar] [CrossRef]
- Kolaříková, Z.; Slavíková, R.; Krüger, C.; Krüger, M.; Kohout, P. PacBio sequencing of Glomeromycota rDNA: A novel amplicon covering all widely used ribosomal barcoding regions and its applicability in taxonomy and ecology of arbuscular mycorrhizal fungi. New Phytol. 2021, 231, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Säle, V.; Palenzuela, J.; Azcón-Aguilar, C.; Sánchez-Castro, I.; da Silva, G.A.; Seitz, B.; Sieverding, E.; van der Heijden, M.G.A.; Oehl, F. Ancient lineages of arbuscular mycorrhizal fungi provide little plant benefit. Mycorrhiza 2021, 31, 559–576. [Google Scholar] [CrossRef]
- Morton, J.B.; Redecker, D. Two new families of Glomales, Archaeosporaceae and Paraglomaceae, with two new genera Archaeospora and Paraglomus, based on concordant molecular and morphological characters. Mycologia 2001, 93, 181. [Google Scholar] [CrossRef]
- van Geel, M.; Busschaert, P.; Honnay, O.; Lievens, B. Evaluation of six primer pairs targeting the nuclear rRNA operon for characterization of arbuscular mycorrhizal fungal (AMF) communities using 454 pyrosequencing. J. Microbiol. Methods 2014, 106, 93–100. [Google Scholar] [CrossRef]
- Hempel, S.; Renker, C.; Buscot, F. Differences in the species composition of arbuscular mycorrhizal fungi in spore, root and soil communities in a grassland ecosystem. Environ. Microbiol. 2007, 9, 1930–1938. [Google Scholar] [CrossRef] [PubMed]
- Higo, M.; Kang, D.-J.; Isobe, K. First report of community dynamics of arbuscular mycorrhizal fungi in radiocesium degradation lands after the Fukushima-Daiichi nuclear disaster in Japan. Sci. Rep. 2019, 9, 8240. [Google Scholar] [CrossRef]
- Purin, S.; Morton, J.B. In situ analysis of anastomosis in representative genera of arbuscular mycorrhizal fungi. Mycorrhiza 2011, 21, 505–514. [Google Scholar] [CrossRef]
- Malar, C.M.; Wang, Y.; Stajich, J.E.; Kokkoris, V.; Villeneuve-Laroche, M.; Yildirir, G.; Corradi, N. Early branching arbuscular mycorrhizal fungus Paraglomus occultum carries a small and repeat-poor genome compared to relatives in the Glomeromycotina. Microb. Genom. 2022, 8, 000810. [Google Scholar] [CrossRef]
- Turrini, A.; Avio, L.; Giovannetti, M.; Agnolucci, M. Functional complementarity of arbuscular mycorrhizal fungi and associated microbiota: The challenge of translational research. Front. Plant Sci. 2018, 9, 1407. [Google Scholar] [CrossRef] [PubMed]
- Senés-Guerrero, C.; Giménez, S.; Pacheco, A.; Gradilla-Hernández, M.S.; Schüßler, A. New MiSeq based strategy exposed plant-preferential arbuscular mycorrhizal fungal communities in arid soils of Mexico. Symbiosis 2020, 81, 235–246. [Google Scholar] [CrossRef]
- Delavaux, C.S.; Sturmer, S.L.; Wagner, M.R.; Schütte, U.; Morton, J.B.; Bever, J.D. Utility of large subunit for environmental sequencing of arbuscular mycorrhizal fungi: A new reference database and pipeline. New Phytol. 2021, 229, 3048–3052. [Google Scholar] [CrossRef]
- Delavaux, C.S.; Ramos, R.J.; Sturmer, S.L.; Bever, J.D. Environmental identification of arbuscular mycorrhizal fungi using the LSU rDNA gene region: An expanded database and improved pipeline. Mycorrhiza 2022, 32, 145–153. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal rna genes for phylogenetics. In PCR Protocols. A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Skinsky, J.J., White, T.J., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 315–322. [Google Scholar]
- van Tuinen, D.; Zhao, B.; Gianinazzi-Pearson, V. PCR in Studies of AM fungi: From primers to application. In Mycorrhiza Manual; Varma, A., Ed.; Springer: Berlin Heidelberg, Germany, 1998; pp. 387–400. [Google Scholar]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- 16S Metagenomic Sequencing Library Preparation. Available online: https://support.illumina.com/documents/documentation/chemistry_documentation/16s/16s-metagenomic-library-prep-guide-15044223-b.pdf (accessed on 25 January 2018).
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In Proceedings of the 2010 Gateway Computing Environments Workshop, New Orleans, LA, USA, 17 November 2010. [Google Scholar]
- Han, M.V.; Zmasek, C.M. PhyloXML: XML for evolutionary biology and comparative genomics. BMC Bioinform. 2009, 10, 356. [Google Scholar] [CrossRef] [PubMed]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef]
- Westcott, S.L.; Schloss, P.D. De novo clustering methods outperform reference-based methods for assigning 16S rRNA gene sequences to operational taxonomic units. PeerJ 2015, 3, e1487. [Google Scholar] [CrossRef]
- Berger, S.A.; Krompass, D.; Stamatakis, A. Performance, accuracy, and web server for evolutionary placement of short sequence reads under maximum likelihood. Syst. Biol. 2011, 60, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Czech, L.; Barbera, P.; Stamatakis, A. Genesis and Gappa: Processing, analyzing and visualizing phylogenetic (placement) data. Bioinformatics 2020, 36, 3263–3265. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.; Ryan, D.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 4. [Google Scholar]
- Yue, J.C.; Clayton, M.K. A similarity measure based on species proportions. Commun. Stat. Theory Methods 2005, 34, 2123–2131. [Google Scholar] [CrossRef]
- Paulson, J.N.; Pop, M.; Bravo, H.C. Metastats: An improved statistical method for analysis of metagenomic data. Genome Biol. 2011, 12, P17. [Google Scholar] [CrossRef]
- Holmes, I.; Harris, K.; Quince, C. Dirichlet multinomial mixtures: Generative models for microbial metagenomics. PLoS ONE 2012, 7, e30126. [Google Scholar] [CrossRef]
- Matsen, F.A.; Evans, S.N. Edge principal components and squash clustering: Using the special structure of phylogenetic placement data for sample comparison. PLoS ONE 2013, 8, e56859. [Google Scholar] [CrossRef]
- Czech, L.; Stamatakis, A. Scalable methods for analyzing and visualizing phylogenetic placement of metagenomic samples. PLoS ONE 2019, 14, e0217050. [Google Scholar]
- Washburne, A.D.; Silverman, J.D.; Leff, J.W.; Bennett, D.J.; Darcy, J.L.; Mukherjee, S.; Fierer, N.; David, L.A. Phylogenetic factorization of compositional data yields lineage-level associations in microbiome datasets. PeerJ 2017, 5, e2969. [Google Scholar] [CrossRef] [PubMed]


| Site (p < 0.001) | Plant (p = 0.232) | Sample (p = 0.013) | Species (p < 0.001) | Chao1 (p < 0.001) | ACE (p < 0.001) | Boneh | Shannon Index (p < 0.001) | Evenness Index (p = 0.052) |
|---|---|---|---|---|---|---|---|---|
| Cont. | Pt | Root | 5 ± 2 b | 5 ± 2 b | 6 ± 5 b | 0 ± 0 | 0.71 ± 0.38 c | 0.48 ± 0.19 |
| Soil | 7 ± 2 b | 8 ± 3 b | 10 ± 5 ab | 1 ± 0 | 1.15 ± 0.36 bc | 0.63 ± 0.12 | ||
| Pa | Root | 4 ± 2 b | 4 ± 2 b | 4 ± 3 b | 0 ± 0 | 0.77 ± 0.63 bc | 0.60 ± 0.26 | |
| Soil | 5 ± 2 b | 5 ± 3 b | 6 ± 4 b | 0 ± 0 | 0.84 ± 0.54 bc | 0.65 ± 0.24 | ||
| Uncont. | Pt | Root | 11 ± 2 a | 13 ± 3 a | 14 ± 3 a | 1 ± 1 | 1.77 ± 0.24 a | 0.74 ± 0.07 |
| Soil | 12 ± 4 a | 16 ± 6 a | 20 ± 6 a | 2 ± 1 | 1.57 ± 0.51 ab | 0.64 ± 0.16 | ||
| Pa | Root | 8 ± 1 ab | 9 ± 2 b | 12 ± 4 ab | 1 ± 0 | 1.36 ± 0.21 b | 0.68 ± 0.08 | |
| Soil | 9 ± 3 ab | 11 ± 5 ab | 18 ± 9 a | 1 ± 0 | 1.42 ± 0.44 ab | 0.66 ± 0.14 |
| Variance | AMOVA | HOMOVA | ||
|---|---|---|---|---|
| Group 1 | Group 2 | |||
| Site (K/C) | 0.311 | 0.336 | p < 0.001 | p = 0.240 |
| Plant (Pt/Pa) | 0.357 | 0.341 | p < 0.001 | p = 0.494 |
| Sample (R/S) | 0.381 | 0.346 | p = 0.012 | p = 0.122 |
| K_Pa/K_Pt | 0.302 | 0.383 | p = 0.119 | p = 0.169 |
| C_Pa/C_Pt | 0.277 | 0.229 | p < 0.001 | p = 0.059 |
| K_Pt/C_Pt | 0.277 | 0.302 | p < 0.001 | p = 0.264 |
| K_Pa/C_Pa | 0.229 | 0.383 | p < 0.001 | p = 0.007 |
| Primer | Sequences of Primers (5′–3′) | Position in Rhizophagus irregularis DAOM197198 Genome (BDIQ01000050) |
|---|---|---|
| FULF (forward) | GTGAAATTGTTGAAAGGGAAACG | 2,474,826 |
| FULR (reverse) | CCTTGGTCCGTGTTTCAAGAC | 2,475,206 |
| FULFN1ngs (forward) | * AGGGAAACGATTGAAGCCAGTC | 2,478,820 |
| FULFN2ngs (forward) | * GAAAGGGAAACGATTGAAGTCAGT | 2,478,817 |
| FULRNngs (reverse) | ** CGTGTTTCAAGACGGGTCGT | 2,475,199 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malicka, M.; Magurno, F.; Piotrowska-Seget, Z. Phenol and Polyaromatic Hydrocarbons Are Stronger Drivers Than Host Plant Species in Shaping the Arbuscular Mycorrhizal Fungal Component of the Mycorrhizosphere. Int. J. Mol. Sci. 2022, 23, 12585. https://doi.org/10.3390/ijms232012585
Malicka M, Magurno F, Piotrowska-Seget Z. Phenol and Polyaromatic Hydrocarbons Are Stronger Drivers Than Host Plant Species in Shaping the Arbuscular Mycorrhizal Fungal Component of the Mycorrhizosphere. International Journal of Molecular Sciences. 2022; 23(20):12585. https://doi.org/10.3390/ijms232012585
Chicago/Turabian StyleMalicka, Monika, Franco Magurno, and Zofia Piotrowska-Seget. 2022. "Phenol and Polyaromatic Hydrocarbons Are Stronger Drivers Than Host Plant Species in Shaping the Arbuscular Mycorrhizal Fungal Component of the Mycorrhizosphere" International Journal of Molecular Sciences 23, no. 20: 12585. https://doi.org/10.3390/ijms232012585
APA StyleMalicka, M., Magurno, F., & Piotrowska-Seget, Z. (2022). Phenol and Polyaromatic Hydrocarbons Are Stronger Drivers Than Host Plant Species in Shaping the Arbuscular Mycorrhizal Fungal Component of the Mycorrhizosphere. International Journal of Molecular Sciences, 23(20), 12585. https://doi.org/10.3390/ijms232012585








