Genome-Wide Identification of the AGC Protein Kinase Gene Family Related to Photosynthesis in Rice (Oryza sativa)
Abstract
1. Introduction
2. Results
2.1. Identification of AGC Gene Family Members in Oryza Genus
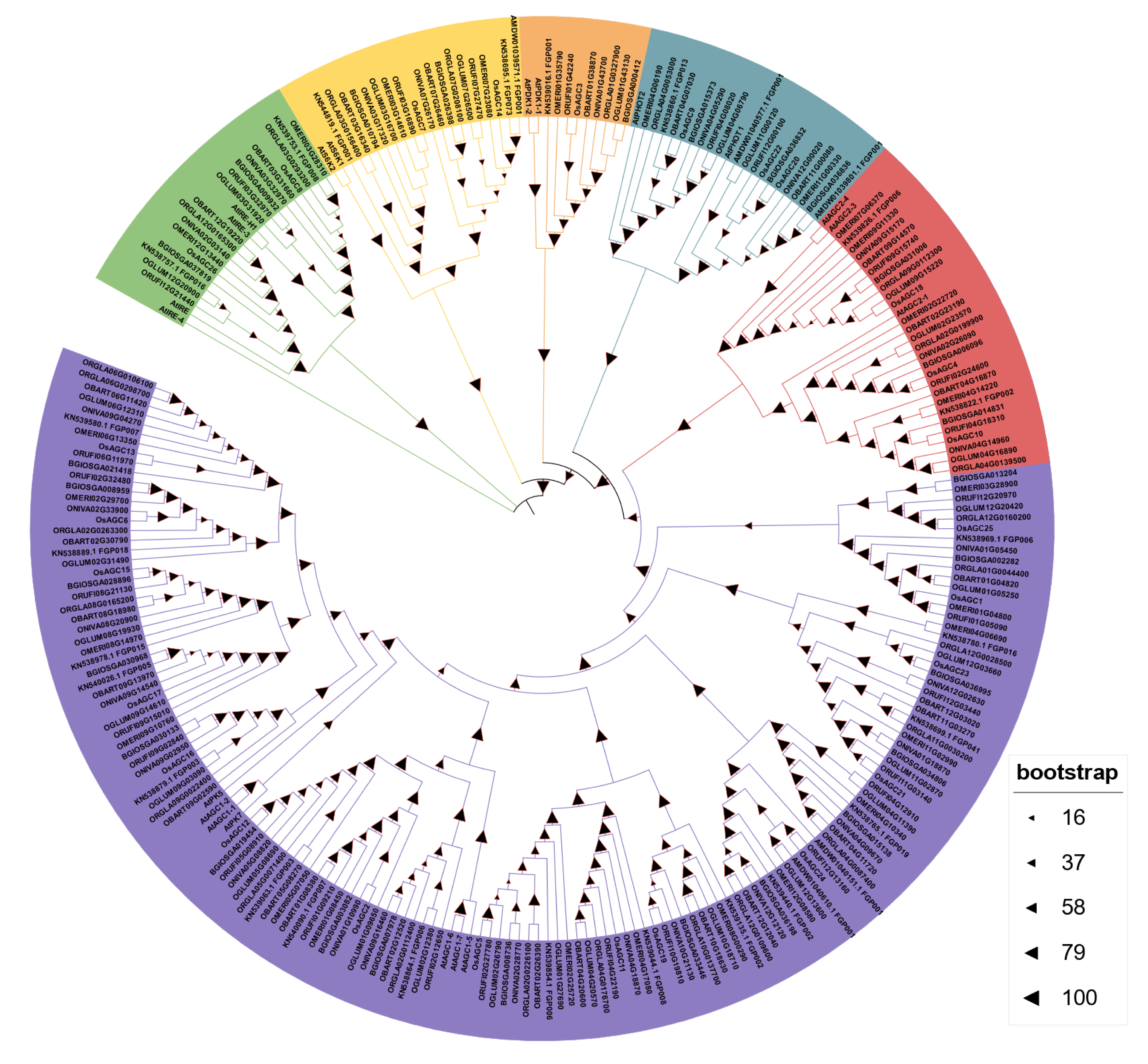
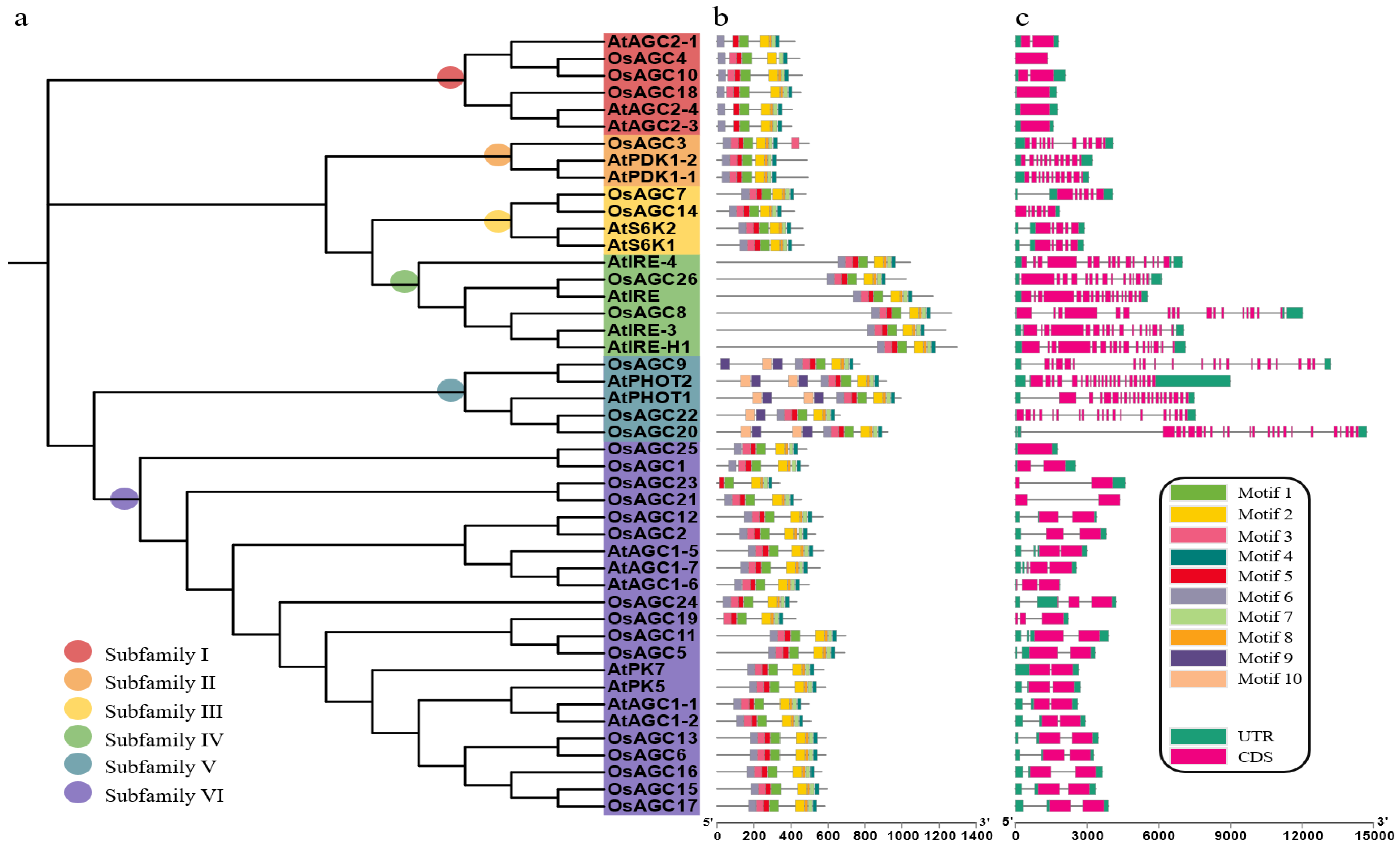
2.2. Characterization and Phylogenetic Relation of OsAGC Gene Family Members in Rice
2.3. Analysis of Gene Structures and Conserved Motifs
2.4. Collinearity Analysis of AGC Genes in Oryza Genus
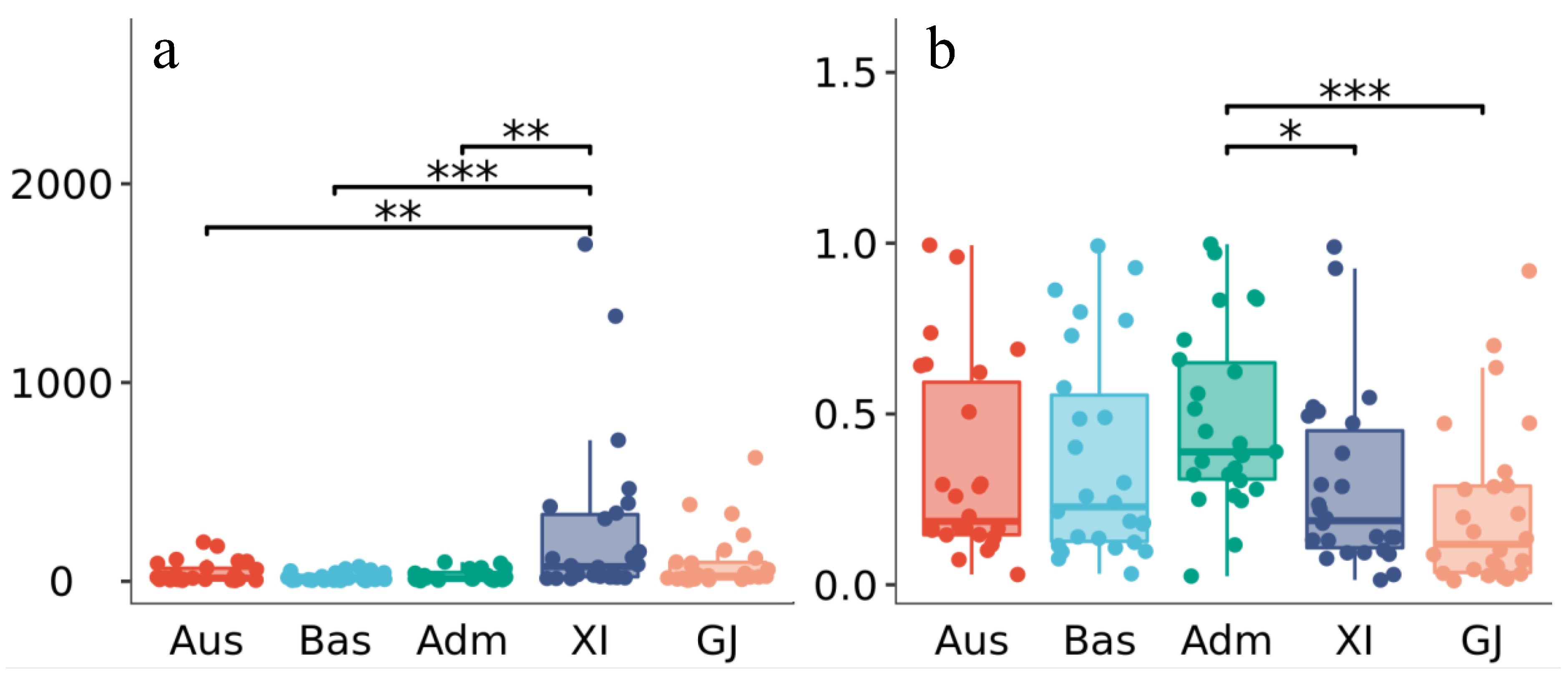
2.5. Gene–CDS–Haplotype (gcHap) Analysis of AGC Genes in 3010 Rice Genomes (3KRG)
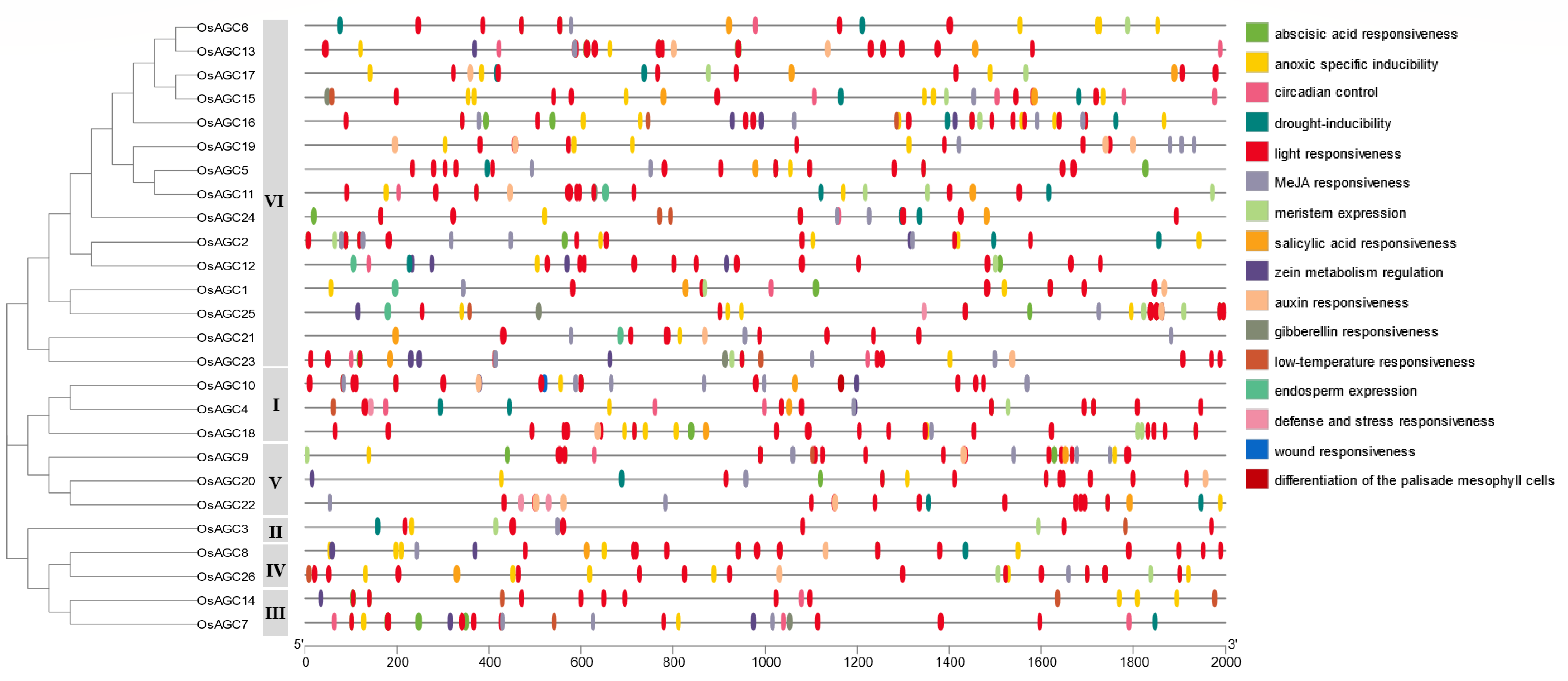
2.6. Analysis of Cis-Regulatory Elements (CREs) in the Promoters of OsAGCs
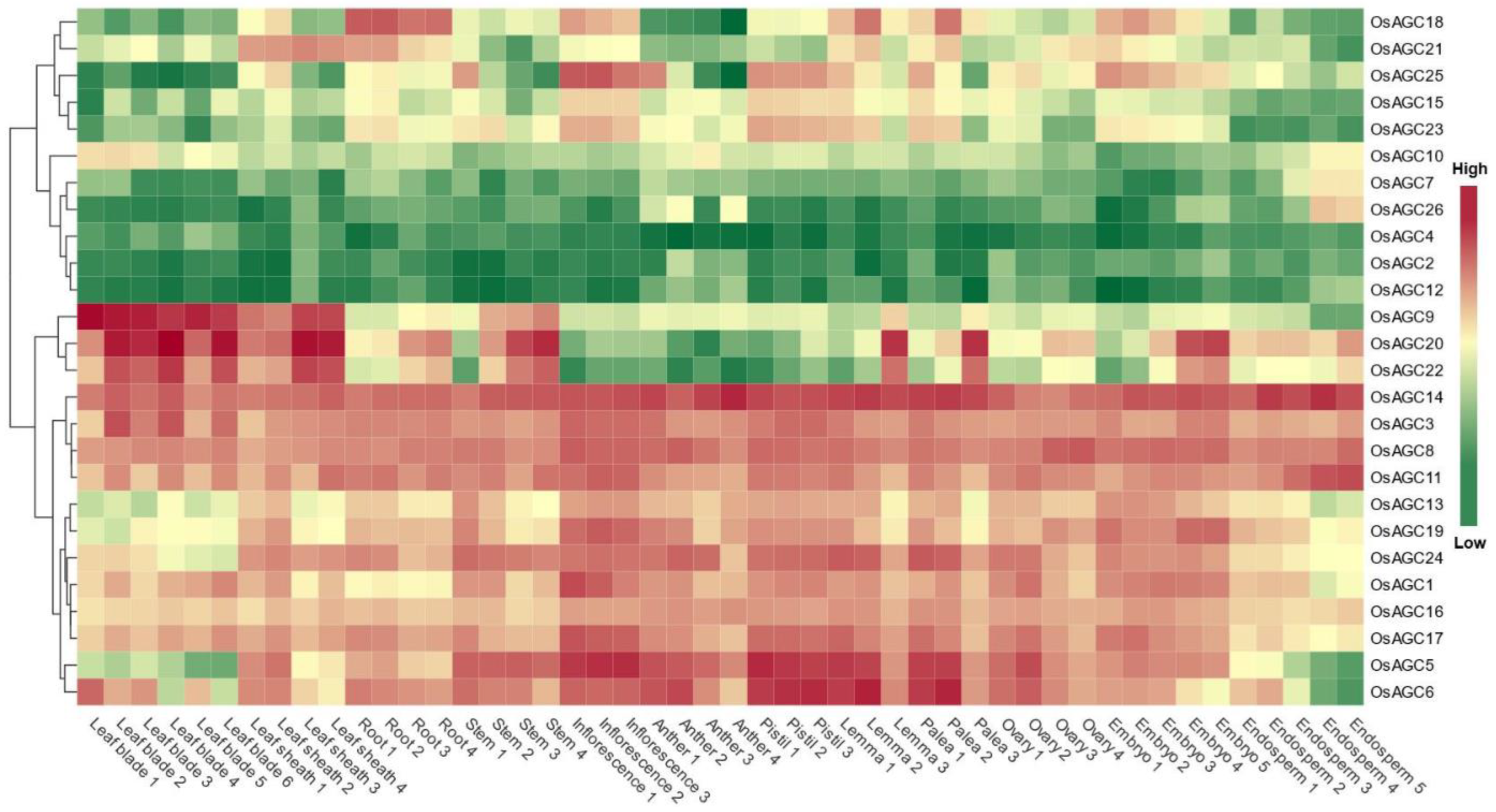
2.7. Tissue-Specific Expression Analysis of OsAGC Genes
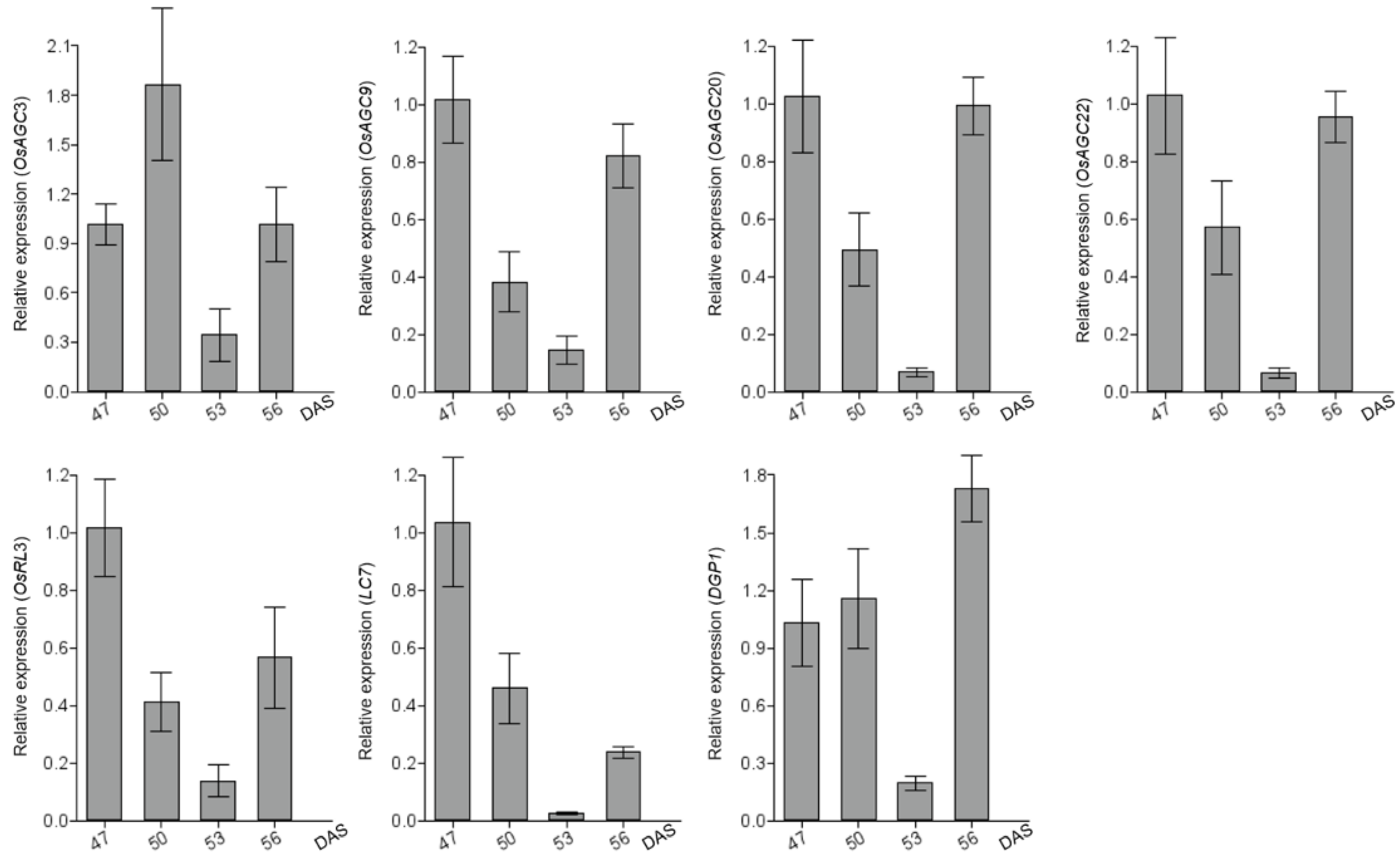
2.8. OsAGCs That May Function in Rice Photosynthesis
3. Discussion
4. Materials and Methods
4.1. Identification of AGC Genes from Rice
4.2. Characteristics, Gene Structure, Motif and Phylogenetic Relationship Analysis of AGC Gene Family
4.3. Collinearity Analysis of AGC Genes
4.4. gcHap Analysis of the AGC Genes in 3KRG
4.5. Identification of Cis-Regulatory Elements (CREs)
4.6. Expression and Interaction Analysis of OsAGC Gene Family
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bilbrough, T.; Piemontese, E.; Seitz, O. Dissecting the role of protein phosphorylation: A chemical biology toolbox. Chem. Soc. Rev. 2022, 51, 5691–5730. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Liu, J. Protein phosphorylation in plant cell signaling. Methods Mol. Biol. 2021, 2358, 45–71. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Han, X.; Lan, P. Emerging roles of protein phosphorylation in plant iron homeostasis. Trends Plant Sci. 2022, 27, 908–921. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.V.; Al-Yousif, M.; Hirt, H. Role of AGC kinases in plant growth and stress responses. Cell Mol. Life Sci. 2012, 69, 3259–3267. [Google Scholar] [CrossRef]
- Rademacher, E.H.; Offringa, R. Evolutionary adaptations of plant AGC kinases: From light signaling to cell polarity regulation. Front. Plant Sci. 2012, 3, 250. [Google Scholar] [CrossRef]
- Bogre, L.; Okresz, L.; Henriques, R.; Anthony, R.G. Growth signaling pathways in Arabidopsis and the AGC protein kinases. Trends Plant Sci. 2003, 8, 424–431. [Google Scholar] [CrossRef]
- Mahfouz, M.M.; Kim, S.; Delauney, A.J.; Verma, D.P.S. Arabidopsis TARGET OF RAPAMYCIN interacts with RAPTOR, which regulates the activity of S6 kinase in response to osmotic stress signals. Plant Cell 2006, 18, 477–490. [Google Scholar] [CrossRef]
- Zegzouti, H.; Anthony, R.G.; Jahchan, N.; Bogre, L.; Christensen, S.K. Phosphorylation and activation of PINOID by the phospholipid signaling kinase 3-phosphoinositide-dependent protein kinase 1 (PDK1) in Arabidopsis. Proc. Natl. Acad. Sci. USA 2006, 103, 6404–6409. [Google Scholar] [CrossRef]
- Henriques, R.; Magyar, Z.; Monardes, A.; Khan, S.; Zalejski, C.; Orellana, J.; Szabados, L.; de la Torre, C.; Koncz, C.; Bögre, L. Arabidopsis S6 kinase mutants display chromosome instability and altered RBR1-E2F pathway activity. EMBO J. 2010, 29, 2979–2993. [Google Scholar] [CrossRef]
- Lee, D.H.; Park, S.J.; Ahn, C.S.; Pai, H.S. MRF family genes are involved in translation control, especially under energy-deficient conditions, and their expression and functions are modulated by the TOR signaling pathway. Plant Cell 2017, 29, 2895–2920. [Google Scholar] [CrossRef]
- Tamaskovic, R.; Bichsel, S.J.; Hemmings, B.A. NDR family of AGC kinases—Essential regulators of the cell cycle and morphogenesis. FEBS Lett. 2003, 546, 73–80. [Google Scholar] [CrossRef]
- Oyama, T.; Shimura, Y.; Okada, K. The IRE gene encodes a protein kinase homologue and modulates root hair growth in Arabidopsis. Plant J. 2002, 30, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.F.; Qin, G.J.; Dai, X.H.; Zhao, Y.D. NPY genes and AGC kinases define two key steps in auxin-mediated organogenesis in Arabidopsis. Proc. Natl. Acad. Sci. USA 2008, 105, 21017–21022. [Google Scholar] [CrossRef] [PubMed]
- Christensen, S.K.; Dagenais, N.; Chory, J.; Weigel, D. Regulation of auxin response by the protein kinase PINOID. Cell 2000, 18, 469–478. [Google Scholar] [CrossRef]
- Glanc, M.; Van Gelderen, K.; Hoermayer, L.; Tan, S.; Naramoto, S.; Zhang, X.; Domjan, D.; Včelařová, L.; Hauschild, R.; Johnson, A.; et al. AGC kinases and MAB4/MEL proteins maintain PIN polarity by limiting lateral diffusion in plant cells. Curr. Biol. 2021, 31, 1918–1930. [Google Scholar] [CrossRef] [PubMed]
- Li, E.; Zhang, Y.L.; Shi, X.; Li, H.; Yuan, X.; Li, S.; Zhang, Y. A positive feedback circuit for ROP-mediated polar growth. Mol. Plant 2021, 14, 395–410. [Google Scholar] [CrossRef]
- Sukumar, P.; Edwards, K.S.; Rahman, A.; DeLong, A.; Muday, G.K. PINOID kinase regulates root gravitropism through modulation of PIN2-dependent basipetal auxin transport in Arabidopsis. Plant Physiol. 2009, 150, 722–735. [Google Scholar] [CrossRef]
- Zhang, Y.; He, J.; McCormick, S. Two Arabidopsis AGC kinases are critical for the polarized growth of pollen tubes. Plant J. 2009, 58, 474–484. [Google Scholar] [CrossRef]
- Eckstein, A.; Grzyb, J.; Hermanowicz, P.; Zglobicki, P.; Labuz, J.; Strzalka, W.; Dziga, D.; Banaś, A.K. Arabidopsis phototropins participate in the regulation of dark-induced leaf senescence. Int. J. Mol. Sci. 2021, 22, 1836. [Google Scholar] [CrossRef]
- Inoue, S.I.; Kaiserli, E.; Zhao, X.; Waksman, T.; Takemiya, A.; Okumura, M.; Takahashi, H.; Seki, M.; Shinozaki, K.; Endo, Y.; et al. CIPK23 regulates blue light-dependent stomatal opening in Arabidopsis thaliana. Plant J. 2020, 104, 679–692. [Google Scholar] [CrossRef]
- Scholz, S.; Plessmann, J.; Enugutti, B.; Huttl, R.; Wassmer, K.; Schneitz, K. The AGC protein kinase UNICORN controls planar growth by attenuating PDK1 in Arabidopsis thaliana. PLoS Genet. 2019, 15, e1007927. [Google Scholar] [CrossRef]
- Wang, J.; Liang, Y.P.; Zhu, J.D.; Wang, Y.X.; Yang, M.Y.; Yan, H.R.; Lv, Q.Y.; Cheng, K.; Zhao, X.; Zhang, X. Phototropin 1 mediates high-intensity blue light-induced chloroplast accumulation response in a root phototropism 2-Dependent manner in Arabidopsis phot2 mutant plants. Front. Plant Sci. 2021, 12, 704618. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wang, J.; Sheng, Y.; Tian, Y.; Zhang, Y.; Zhou, C.; Zhao, X.; Zhang, X. Phototropin2-mediated hypocotyl phototropism is negatively regulated by JAC1 and RPT2 in Arabidopsis. Plant Physiol. Biochem. 2021, 164, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Ek-Ramos, M.J.; Avila, J.; Cheng, C.; Martin, G.B.; Devarenne, T.P. The T-loop extension of the tomato protein kinase AvrPto-dependent Pto-interacting protein 3 (Adi3) directs nuclear localization for suppression of plant cell death. J. Biol. Chem. 2010, 285, 17584–17594. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Z.; Duan, J.; Ni, M.; Liu, Z.; Qiu, W.L.; Whitham, S.A.; Qian, W.J. S-Nitrosylation inhibits the kinase activity of tomato phosphoinositide-dependent kinase 1 (PDK1). J. Biol. Chem. 2017, 292, 19743–19751. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Yang, K.; Wei, X.; Zhang, Q.; Rong, W.; Du, L.; Ye, X.; Qi, L.; Zhang, Z. The wheat AGC kinase TaAGC1 is a positive contributor to host resistance to the necrotrophic pathogen Rhizoctonia cerealis. J. Exp. Bot. 2015, 66, 6591–6603. [Google Scholar] [CrossRef]
- Pislariu, C.I.; Dickstein, R. An IRE-like AGC kinase gene, MtIRE, has unique expression in the invasion zone of developing root nodules in Medicago truncatula. Plant Physiol. 2007, 144, 682–694. [Google Scholar] [CrossRef]
- Zhu, Y.K.; Wang, X.R.; Sun, B.; Tang, X.H.; Mao, Y.X. Cytological and transcriptional analysis reveal phosphatidylinositol signaling pathway plays key role in mitotic division of Pyropia yezoensis. J. Oceanol. Limnol. 2022, 40, 1148–1159. [Google Scholar] [CrossRef]
- Song, Y.; Wang, C.; Linderholm, H.W.; Fu, Y.; Cai, W.; Xu, J.; Zhuang, L.; Wu, M.; Shi, Y.; Wang, G.; et al. The negative impact of increasing temperatures on rice yields in southern China. Sci. Total Environ. 2022, 820, 153262. [Google Scholar] [CrossRef]
- Zhang, Z.; Gao, S.; Chu, C. Improvement of nutrient use efficiency in rice: Current toolbox and future perspectives. Theor. Appl. Genet. 2020, 133, 1365–1384. [Google Scholar] [CrossRef]
- He, Y.; Yan, L.; Ge, C.; Yao, X.F.; Han, X.; Wang, R.; Xiong, L.; Jiang, L.; Liu, C.-M.; Zhao, Y. PINOID is required for formation of the stigma and style in rice. Plant Physiol. 2019, 180, 926–936. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.M.; Xie, D.J.; Tang, Z.S.; Shi, D.Q.; Yang, W.C. PINOID regulates floral organ development by modulating auxin transport and interacts with MADS16 in rice. Plant Biotechnol. J. 2020, 18, 1778–1795. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Tang, D.; Cheng, X.J.; Zhang, J.X.; Tang, Y.J.; Tao, Q.; Shi, W.; You, A.; Gu, M.; Cheng, Z.; et al. OsPINOID regulates stigma and ovule initiation through maintenance of the floral meristem by auxin signaling. Plant Physiol. 2019, 180, 952–965. [Google Scholar] [CrossRef]
- Matsui, H.; Miyao, A.; Takahashi, A.; Hirochika, H. Pdk1 kinase regulates basal disease resistance through the OsOxi1-OsPti1a phosphorylation cascade in rice. Plant Cell Physiol. 2010, 51, 2082–2091. [Google Scholar] [CrossRef] [PubMed]
- Matsui, H.; Yamazaki, M.; Kishi-Kaboshi, M.; Takahashi, A.; Hirochika, H. AGC kinase OsOxi1 positively regulates basal resistance through suppression of OsPti1a-mediated negative regulation. Plant Cell Physiol. 2010, 51, 1731–1744. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Zou, T.; Zhang, K.; Xiong, P.; Zhou, F.; Chen, H.; Li, G.; Zheng, K.; Han, Y.; Peng, K.; et al. DEAP1 encodes a fasciclin-like arabinogalactan protein required for male fertility in rice. J. Integr. Plant Biol. 2022, 64, 1430–1447. [Google Scholar] [CrossRef]
- Park, D.Y.; Shim, Y.; Gi, E.; Lee, B.D.; An, G.; Kang, K.; Paek, N.C. The MYB-related transcription factor RADIALIS-LIKE3 (OsRL3) functions in ABA-induced leaf senescence and salt sensitivity in rice. Environ. Exp. Bot. 2018, 156, 86–95. [Google Scholar] [CrossRef]
- Yang, X.; Nian, J.; Xie, Q.; Feng, J.; Zhang, F.; Jing, H.; Zhang, J.; Dong, G.; Liang, Y.; Peng, J.; et al. Rice ferredoxin-dependent glutamate synthase regulates nitrogen-carbon metabolomes and is genetically differentiated between japonica and indica subspecies. Mol. Plant 2016, 9, 1520–1534. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, J.; Tang, Y.; Liu, K.; Liu, Y.; Tang, J.; Zhang, T.; Yu, H. DEEP GREEN PANICLE1 suppresses GOLDEN2-LIKE activity to reduce chlorophyll synthesis in rice glumes. Plant Physiol. 2020, 15, 469–477. [Google Scholar] [CrossRef]
- Wing, R.A.; Purugganan, M.D.; Zhang, Q. The rice genome revolution: From an ancient grain to green super rice. Nat. Rev. Genet. 2018, 19, 505–517. [Google Scholar] [CrossRef]
- Kong, W.; Zhang, Y.; Deng, X.; Li, S.; Zhang, C.; Li, Y. Comparative genomic and transcriptomic analysis suggests the evolutionary dynamic of GH3 genes in Gramineae crops. Front. Plant Sci. 2019, 15, 1297. [Google Scholar] [CrossRef]
- Tomar, S.; Subba, A.; Bala, M.; Singh, A.K.; Pareek, A.; Singla-Pareek, S.L. Genetic conservation of CBS domain containing protein family in Oryza species and their association with abiotic stress responses. Int. J. Mol. Sci. 2022, 23, 1687. [Google Scholar] [CrossRef]
- Zheng, K.; Pang, L.; Xue, X.; Gao, P.; Zhao, H.; Wang, Y.; Han, S. Genome-wide comprehensive survey of the subtilisin-like proteases gene family associated with rice caryopsis development. Front. Plant Sci. 2022, 13, 943184. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Guo, C.; Shan, H.; Kong, H. Divergence of duplicate genes in exon-intron structure. Proc. Natl. Acad. Sci. USA 2012, 109, 1187–1192. [Google Scholar] [CrossRef] [PubMed]
- Gotoh, E.; Suetsugu, N.; Yamori, W.; Ishishita, K.; Kiyabu, R.; Fukuda, M.; Higa, T.; Shirouchi, B.; Wada, M. Chloroplast accumulation response enhances leaf photosynthesis and plant biomass production. Plant Physiol. 2018, 178, 1358–1369. [Google Scholar] [CrossRef] [PubMed]
- Papatheodorou, I.; Moreno, P.; Manning, J.; Fuentes, A.M.; George, N.; Fexova, S.; Fonseca, N.A.; Füllgrabe, A.; Green, M.; Huang, N.; et al. Expression Atlas update: From tissues to single cells. Nucleic Acids Res. 2020, 48, D77–D83. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Takehisa, H.; Kamatsuki, K.; Minami, H.; Namiki, N.; Ikawa, H.; Ohyanagi, H.; Sugimoto, K.; Antonio, B.A.; Nagamura, Y. RiceXPro version 3.0: Expanding the informatics resource for rice transcriptome. Nucleic Acids Res. 2013, 41, D1206–D1213. [Google Scholar] [CrossRef] [PubMed]
- Potter, S.C.; Luciani, A.; Eddy, S.R.; Park, Y.; Lopez, R.; Finn, R.D. HMMER web server: 2018 update. Nucleic Acids Res. 2018, 46, W200–W204. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Savojardo, C.; Martelli, P.L.; Fariselli, P.; Profiti, G.; Casadio, R. BUSCA: An integrative web server to predict subcellular localization of proteins. Nucleic Acids Res. 2018, 46, W459–W466. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive Tree of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tang, H.; Debarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.; Jin, H.; Marler, B.; Guo, H.; et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Hu, W.; Wang, Y.; Liu, B.; Yan, H.; Xiang, Y. Genome-wide identification, classification, and expression of phytocyanins in Populus trichocarpa. Planta 2018, 247, 1133–1148. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wang, C.; Li, M.; Cui, Y.; Shi, Y.; Wu, Z.; Hu, Z.; Wang, W.; Xu, J.; Li, Z. The landscape of gene-CDS-haplotype diversity in rice (Oryza sativa L.): Properties, population organization, footprints of domestication and breeding, and implications in genetic improvement. Mol. Plant 2021, 14, 787–804. [Google Scholar] [CrossRef] [PubMed]
- Lescot, M.; Dehais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Gable, A.L.; Nastou, K.C.; Lyon, D.; Kirsch, R.; Pyysalo, S.; Doncheva, N.T.; Legeay, M.; Fang, T.; Bork, P.; et al. The STRING database in 2021: Customizable protein-protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021, 49, D605–D612. [Google Scholar] [CrossRef] [PubMed]

| Gene | Locus ID | Chr | Size (aa) | MW(Da) | pI | Subcellular Location |
|---|---|---|---|---|---|---|
| OsAGC1 | Os01g0174700 | 1 | 493 | 54,366 | 8.86 | Nucleus |
| OsAGC2 | Os01g0233800 | 1 | 532 | 57,906 | 9.14 | Nucleus |
| OsAGC3 | Os01g0872800 | 1 | 498 | 55,384 | 6.54 | Nucleus |
| OsAGC4 | Os02g0603000 | 2 | 447 | 47,166 | 8.37 | Nucleus |
| OsAGC5 | Os02g0654300 | 2 | 690 | 74,999 | 6.15 | endomembrane |
| OsAGC6 | Os02g0725000 | 2 | 588 | 65,088 | 8.06 | Nucleus |
| OsAGC7 | Os03g0334000 | 3 | 480 | 53,316 | 6.29 | Nucleus |
| OsAGC8 | Os03g0711800 | 3 | 1267 | 138,833 | 5.94 | endomembrane |
| OsAGC9 | Os04g0304200 | 4 | 771 | 86,407 | 8.46 | Nucleus |
| OsAGC10 | Os04g0488700 | 4 | 462 | 49,336 | 5.65 | Nucleus |
| OsAGC11 | Os04g0546300 | 4 | 695 | 75,638 | 6.38 | Nucleus |
| OsAGC12 | Os05g0237400 | 5 | 574 | 61,434 | 7.00 | Nucleus |
| OsAGC13 | Os06g0291600 | 6 | 589 | 64,808 | 8.38 | chloroplast |
| OsAGC14 | Os07g0680900 | 7 | 419 | 46,867 | 7.87 | Nucleus |
| OsAGC15 | Os08g0491200 | 8 | 594 | 65,034 | 8.74 | plasma membrane |
| OsAGC16 | Os09g0258500 | 9 | 567 | 61,832 | 8.21 | Nucleus |
| OsAGC17 | Os09g0478500 | 9 | 583 | 63,813 | 7.18 | Nucleus |
| OsAGC18 | Os09g0486700 | 9 | 455 | 49,179 | 6.34 | plasma membrane |
| OsAGC19 | Os10g0562500 | 10 | 426 | 46,678 | 9.21 | Nucleus |
| OsAGC20 | Os11g0102200 | 11 | 921 | 103,437 | 8.35 | Nucleus |
| OsAGC21 | Os11g0150700 | 11 | 458 | 49,130 | 6.43 | Nucleus |
| OsAGC22 | Os12g0101800 | 12 | 668 | 76,000 | 8.31 | chloroplast |
| OsAGC23 | Os12g0149700 | 12 | 338 | 36,565 | 6.28 | endomembrane |
| OsAGC24 | Os12g0480200 | 12 | 430 | 48,355 | 6.39 | Nucleus |
| OsAGC25 | Os12g0614600 | 12 | 484 | 51,769 | 9.50 | chloroplast outer membrane |
| OsAGC26 | Os12g0621500 | 12 | 1021 | 113,490 | 5.32 | Nucleus |
| Oryza Species | Gene Pairs | Ka | Ks | Ka/Ks | Date (Mya) | Type of Selection | Type of Duplication |
|---|---|---|---|---|---|---|---|
| O. sativa, japonica | OsAGC2/OsAGC12 | 0.23 | 0.66 | 0.35 | 36.26 | Purifying | Segmental/WGD |
| OsAGC4/OsAGC10 | 0.21 | 0.46 | 0.45 | 25.44 | Purifying | Segmental/WGD | |
| OsAGC5/OsAGC11 | 0.12 | 1.08 | 0.11 | 59.56 | Purifying | Segmental/WGD | |
| OsAGC15/OsAGC17 | 0.10 | 0.96 | 0.10 | 52.91 | Purifying | Segmental/WGD | |
| OsAGC21/OsAGC23 | 0.05 | 0.17 | 0.27 | 9.34 | Purifying | Segmental/WGD | |
| O. sativa, indica | BGIOSGA003082/BGIOSGA019454 | 0.23 | 0.65 | 0.34 | 35.93 | Purifying | Segmental/WGD |
| BGIOSGA034806/BGIOSGA036995 | 0.08 | 0.20 | 0.40 | 10.93 | Purifying | Segmental/WGD | |
| BGIOSGA006096/BGIOSGA014831 | 0.21 | 0.46 | 0.45 | 25.44 | Purifying | Segmental/WGD | |
| BGIOSGA010794/BGIOSGA026398 | 0.14 | 1.13 | 0.12 | 62.25 | Purifying | Segmental/WGD | |
| BGIOSGA028896/BGIOSGA030968 | 0.09 | 0.97 | 0.09 | 53.19 | Purifying | Segmental/WGD | |
| O. nivara | ONIVA01G18870/ONIVA12G02630 | 0.05 | 0.22 | 0.25 | 12.25 | Purifying | Segmental/WGD |
| ONIVA01G10990/ONIVA05G08820 | 0.22 | 0.64 | 0.34 | 35.39 | Purifying | Segmental/WGD | |
| ONIVA02G28770/ONIVA04G18870 | 0.12 | 1.08 | 0.11 | 59.34 | Purifying | Segmental/WGD | |
| ONIVA03G17320/ONIVA07G26170 | 0.11 | 1.06 | 0.10 | 58.30 | Purifying | Segmental/WGD | |
| ONIVA08G20900/ONIVA09G14540 | 0.09 | 0.97 | 0.10 | 53.08 | Purifying | Segmental/WGD | |
| O. rufipogon | ORUFI01G09210/ORUFI05G08910 | 0.23 | 0.63 | 0.37 | 34.56 | Purifying | Segmental/WGD |
| ORUFI11G03140/ORUFI12G03440 | 0.05 | 0.24 | 0.22 | 13.35 | Purifying | Segmental/WGD | |
| ORUFI02G24600/ORUFI04G18310 | 0.21 | 0.46 | 0.44 | 25.44 | Purifying | Segmental/WGD | |
| ORUFI02G27780/ORUFI04G22190 | 0.12 | 1.08 | 0.11 | 59.45 | Purifying | Segmental/WGD | |
| ORUFI03G16890/ORUFI07G27470 | 0.11 | 1.04 | 0.10 | 57.20 | Purifying | Segmental/WGD | |
| ORUFI08G21130/ORUFI09G15010 | 0.10 | 0.97 | 0.10 | 53.24 | Purifying | Segmental/WGD | |
| O. glaberrima | ORGLA11G0030200/ORGLA12G0028500 | 0.06 | 0.23 | 0.28 | 12.63 | Purifying | Segmental/WGD |
| ORGLA02G0226100/ORGLA04G0176700 | 0.12 | 1.08 | 0.11 | 59.09 | Purifying | Segmental/WGD | |
| ORGLA02G0199900/ORGLA04G0139500 | 0.20 | 0.47 | 0.43 | 25.72 | Purifying | Segmental/WGD | |
| ORGLA02G0263300/ORGLA06G0106100 | 0.13 | 1.34 | 0.10 | 73.37 | Purifying | Segmental/WGD | |
| ORGLA02G0263300/ORGLA08G0165200 | 0.18 | 2.50 | 0.07 | 137.23 | Purifying | Segmental/WGD | |
| O. meridionalis | OMERI02G25720/OMERI04G17080 | 0.12 | 1.07 | 0.12 | 58.74 | Purifying | Segmental/WGD |
| OMERI02G22720/OMERI04G14220 | 0.22 | 0.48 | 0.46 | 26.52 | Purifying | Segmental/WGD | |
| OMERI03G14610/OMERI07G23080 | 0.11 | 1.01 | 0.11 | 55.25 | Purifying | Segmental/WGD | |
| OMERI08G14970/OMERI09G10760 | 0.092 | 0.97 | 0.09 | 53.40 | Purifying | Segmental/WGD | |
| O. barthii | OBART01G08380/OBART05G08270 | 0.30 | 0.72 | 0.41 | 39.31 | Purifying | Segmental/WGD |
| OBART11G03270/OBART12G03020 | 0.09 | 0.23 | 0.38 | 12.69 | Purifying | Segmental/WGD | |
| OBART02G23190/OBART04G16870 | 0.35 | 0.51 | 0.70 | 27.86 | Purifying | Segmental/WGD | |
| OBART02G26390/OBART04G20600 | 0.12 | 1.08 | 0.11 | 59.09 | Purifying | Segmental/WGD | |
| OBART03G16340/OBART07G26460 | 0.11 | 1.06 | 0.10 | 58.29 | Purifying | Segmental/WGD | |
| OBART08G18980/OBART09G13970 | 0.09 | 0.96 | 0.10 | 53.02 | Purifying | Segmental/WGD | |
| OBART08G19720/OBART09G14570 | 0.20 | 0.47 | 0.44 | 25.11 | Purifying | Segmental/WGD | |
| O. glumaepatula | OGLUM01G09650/OGLUM05G08690 | 0.22 | 0.62 | 0.35 | 34.34 | Purifying | Segmental/WGD |
| OGLUM11G02870/OGLUM12G03660 | 0.06 | 0.22 | 0.26 | 12.23 | Purifying | Segmental/WGD | |
| OGLUM12G20420/OGLUM03G27960 | 0.20 | 0.54 | 0.38 | 29.76 | Purifying | Segmental/WGD | |
| OGLUM02G23570/OGLUM04G16890 | 0.20 | 0.46 | 0.44 | 25.18 | Purifying | Segmental/WGD | |
| OGLUM02G26790/OGLUM04G20570 | 0.12 | 1.08 | 0.11 | 59.49 | Purifying | Segmental/WGD | |
| OGLUM03G16700/OGLUM07G26500 | 0.11 | 1.07 | 0.10 | 59.05 | Purifying | Segmental/WGD | |
| OGLUM08G19930/OGLUM09G14610 | 0.10 | 0.96 | 0.10 | 52.78 | Purifying | Segmental/WGD | |
| OGLUM08G20880/OGLUM09G15220 | 0.21 | 0.42 | 0.50 | 23.32 | Purifying | Segmental/WGD |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Y.; Liu, X.; Zhou, M.; Yang, J.; Ke, S.; Li, Y. Genome-Wide Identification of the AGC Protein Kinase Gene Family Related to Photosynthesis in Rice (Oryza sativa). Int. J. Mol. Sci. 2022, 23, 12557. https://doi.org/10.3390/ijms232012557
Jiang Y, Liu X, Zhou M, Yang J, Ke S, Li Y. Genome-Wide Identification of the AGC Protein Kinase Gene Family Related to Photosynthesis in Rice (Oryza sativa). International Journal of Molecular Sciences. 2022; 23(20):12557. https://doi.org/10.3390/ijms232012557
Chicago/Turabian StyleJiang, Yifei, Xuhui Liu, Mingao Zhou, Jian Yang, Simin Ke, and Yangsheng Li. 2022. "Genome-Wide Identification of the AGC Protein Kinase Gene Family Related to Photosynthesis in Rice (Oryza sativa)" International Journal of Molecular Sciences 23, no. 20: 12557. https://doi.org/10.3390/ijms232012557
APA StyleJiang, Y., Liu, X., Zhou, M., Yang, J., Ke, S., & Li, Y. (2022). Genome-Wide Identification of the AGC Protein Kinase Gene Family Related to Photosynthesis in Rice (Oryza sativa). International Journal of Molecular Sciences, 23(20), 12557. https://doi.org/10.3390/ijms232012557





