Abstract
Aspongopus chinensis Dallas is used as a traditional Chinese medicine as well as an edible insect. Although its anti-tumor effects have been observed, the anti-tumor active component(s) in the hemolymph of A. chinensis remains unknown. In this study, a combination usage of ultrafiltration, gel filtration chromatography, FPLC and RP-HPLC to separate and purify active peptides was performed based on the proliferation of the human gastric cancer SGC-7901 cell line treated with candidates. One peptide (MW = 2853.3 Da) was isolated from the hemolymph of A. chinensis. A total of 24 amino acid residues were continuously determined for the active peptide: N′-ECGYCAEKGIRCDDIHCCTGLKKK-C′. In conclusion, a peptide that can inhibit the proliferation of gastric cancer SGC-7901 cells in the hemolymph of A. chinensis was purified in this study, which is homologous to members of the spider toxin protein family. These results should facilitate further works for this peptide, such as the cloning of genes, expression in vitro by prokaryotic or eukaryotic systems, more specific tests of anti-tumor activity, and so on.
1. Introduction
Cancers are a serious threat to human health. There were 19.3 million new cancer cases and 10 million cancer deaths in 2020 [1]. Lung cancer, colorectal cancer, breast cancer, gastric cancer, and liver cancer are the top five cancers with the highest morbidity and mortality worldwide [2]. However, there are no adequate efficacious medicines for cancer treatments. Meanwhile, many disadvantages are associated with current medicines, such as high price, severe adverse reactions, drug resistance, relapse after drug cessation, and so on [3]. Therefore, the development of new anti-cancer drugs is a major issue at present.
Development of anti-cancer chemicals should be based on chemical design and screening according to targets, or based on the screening of natural chemicals. For natural anti-cancer chemical screening, medicinal insects are important resources for active component isolation. It has been proven that cantharidin and its derivatives from meloid insects possess inhibitory effects on various cancer cells, such as laryngeal cancer, gastric cancer, leukemia, esophageal cancer, liver cancer, lung cancer, cervical carcinoma and prostatic cancer [4,5,6,7,8,9]. Bee venom and propolis (Apidae and Vespidae, Hymenoptera), which are traditionally utilized as medicines, also have inhibitory effects on the proliferation of leukemia, liver cancer, and esophageal carcinoma cells [10,11]. Mastoparan is an α-helical and amphipathic tetradecapeptide obtained from the venom of the wasp Vespula lewisii that exhibits tumor cell cytotoxicity [12]. It induced caspase-dependent apoptosis in melanoma cells through the intrinsic mitochondrial pathway, protecting mice against tumor development. In addition, chemicals originating from Bombyx mori, Chrysomya megacephala, Musca domestica, and Holotrichia diomphalia larvae also have certain inhibitory effects on various tumor cells [13,14,15,16].
Anti-cancer peptides are novel natural anti-cancer chemicals [17,18]. Insects are important sources of anti-cancer peptides, such as mastoparan mentioned above from V. lewisii [19]; Mastoparan-C from Vespa crabro, which can inhibit the proliferation of non-small cell lung cancer H157, melanocyte MDA-MB-435S, human prostate carcinoma PC-3, human glioblastoma astrocytoma U251MG, and human breast cancer MCF-7 cells [20]; SK84 from Drosophila virilis larvae, with a specific inhibitory effect on the proliferation of various cancer cell lines such as human leukemia THP-1, liver cancer HepG2, and breast cancer MCF-7 cells [21]. Contents of biological active peptides in organisms are usually very low [22]. Moreover, these peptides are usually mixed with sophisticated components in various tissues. Common techniques to purify these peptides include gel filtration chromatography, ion exchange chromatography, reversed-phase high performance liquid chromatography (RP-HPLC), affinity chromatography, and so on [23].
Aspongopus chinensis Dallas 1851 is a Traditional Chinese Medicine and also acts as edible material in China. It was found that the hemolymph of A. chinensis could significantly inhibit the growth of human gastric cancer cell SGC-7901 and human breast cancer cells in a time- and dose-dependent manner [24,25,26,27,28]. To ascertain the active protein/peptide component(s) in hemolymph of A. chinensis for cancer resistance, ultrafiltration, gel filtration chromatography, ion exchange chromatography, and RP-HPLC were sequentially used in this study. Inhibition of cell proliferation (human gastric cancer cells SGC-7901) was tested for sections isolated at each stage. The anti-tumor peptide isolated from A. chinensis is named as AcATP (anti-tumor peptide of A. chinensis). Furthermore, the molecular weight of AcATP was determined by Matrix-Assisted Laser Desorption/Ionization-Time of Flight Mass Spectrometry (MALDI-TOF-MS), and its amino acid sequence was determined based on the Edman chemical degradation method. The results of this study should facilitate research on the cloning of active peptides, biological active tests, recombinant protein production, and so on.
2. Results
2.1. Inhibiting Effect of Ultrafiltration Components in Hemolymph of A. chinensis on SGC-7901 Cells
The hemolymph of A. chinensis was divided into three sections: Sect. I (molecular weights < 3 kD), Sect. II (3–50 kD), and Sect. III (>50 kD). The proliferation inhibiting effect of each section on SGC-7901 cells was observed by an inverted microscope. Except for reductions of cell density, cultured cells that were treated with separated components also showed other abnormal phenomena: shrinkage, smaller volume, nucleus pyknosis, shedding, and floating (Figure 1a). Cytotoxic effects to SGC-7901 cells resulting from these three sections (treatments with 100, 200, 400 μg/mL, respectively) are shown in Table 1. For each section, the inhibition on cell proliferation became more effective with an increase of each section supplement. At the same concentration, treatment with Sect. III resulted in less inhibition of cell proliferation (significantly or non-significantly). At a concentration of 400 μg/mL, Sect. II had the highest inhibition rate (56.85 ± 3.79%) on SGC-7901 cells; the IC50 (50% inhibitory concentration) of Sect. II was 358.600 μg/mL (Figure 1b). Furthermore, cell morphological changes treated with Sect. I or II were more obvious than Sect. III. Therefore, Sect. II was chosen to separate active peptides in the following steps.
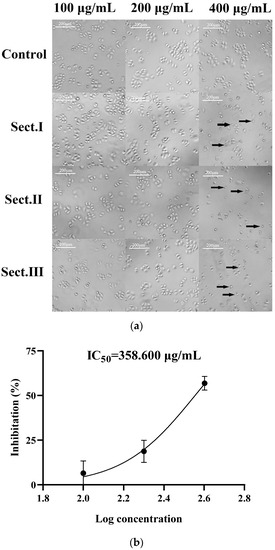
Figure 1.
(a) Effects of ultra-filtration components in hemolymph of A. chinensis on SGC-7901 cells after 48 h treatment. Control: cell group without drug; Sect. I: molecular weights of the section < 3 kD; Sect. II: molecular weights of the section between 3 kD and 50 kD; Sect. III: molecular weights of the section > 50 Kd, the cells indicated by the arrow are morphologically altered cells. (b) IC50 curve fitting of Sect. II.

Table 1.
Inhibitory rates of cell proliferation (SGC-7901 cells) induced by various components of A. chinensis hemolymph separated by ultrafiltration after 48 h treatment.
2.2. Inhibiting Effect of Components Separated by Gel Filtration Chromatography
Five fractions in Sect. II (3–50 kD, ultrafiltration) were discriminated and eluted (Figure 2a): peaks 1–5 were denoted as Sect. II-1–5, respectively. Each fraction (differential peak collection) was desalted, freeze-dried, and re-dissolved to various concentrations to test the inhibition effects on SGC-7901 cells. Except for Sect. II-5 (peak 5) at lower concentration (25 μg/mL), the fractions expressed inhibitory effects (Figure 2b). Although Sect. II-3 (peak 3) and Sect. II-2 (peak 2) both expressed stronger inhibition, there was not further enhancement of the inhibition effect along with increasing dosage for Sect. II-2. Sect. II-3 had more effective inhibition on proliferation of SGC-7901 cells (with a dosage-dependent manner) than other fractions, especially at high concentrations (100 μg/mL), and the IC50 value was 155.200 μg/mL (Figure 2c). Therefore, Sect. II-3 was used for further separation or purification.
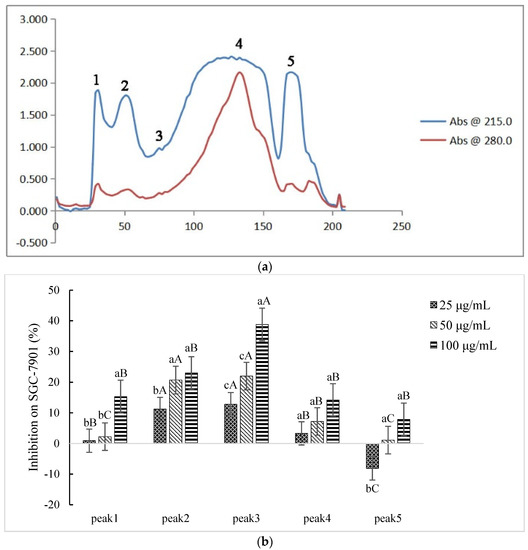
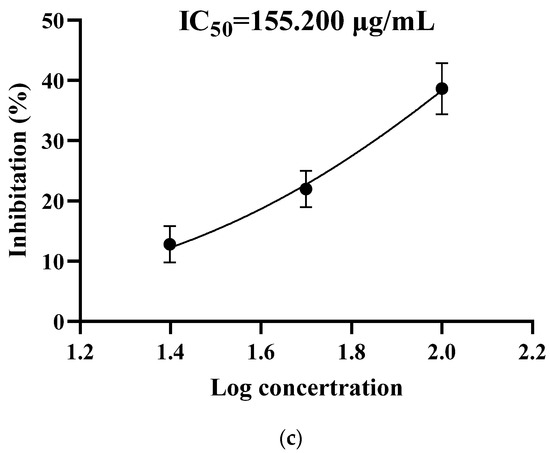
Figure 2.
Inhibiting effect of components separated by gel filtration chromatography. (a) The elution curve of gel filtration chromatography. (b) Inhibitory rates of cell proliferation (SGC-7901 cells) induced by Sect. II-1–5 that were separated by gel filtration chromatography after 48 h treatment. a, b, c means p < 0.05; A, B, C means p < 0.01. (c) IC50 curve fitting of Sect. II-3.
2.3. Inhibition Effect of Components Separated by FPLC
The peptides/proteins bound to the Resource Q strong anion exchange resin (ÄKTA Purifier FPLC system) were linearly eluted using 0–1 M NaCl elution buffer for 50 min. Four fractions in Sect. II-3 (gel filtration chromatography) were discriminated and eluted (Figure 3a): peaks 1–4 were denoted as Sects. II-3-1-4, respectively. Each fraction (differential peak collection) was treated the same as above. Except for Sect. II-3-4 (peak 4) at a lower concentration (10 μg/mL), the fractions expressed inhibitory effects (Figure 3b). It was found that Sect. II-3-3. (peak 3) was more effective than the other fractions at any concentration, and its IC50 value for SGC-7901 cells was 70.900 μg/mL (Figure 3c). Therefore, Sect. II-3-3 was collected for further separation or purification.
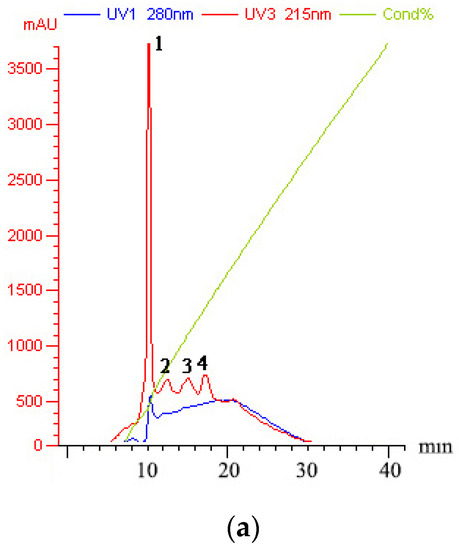
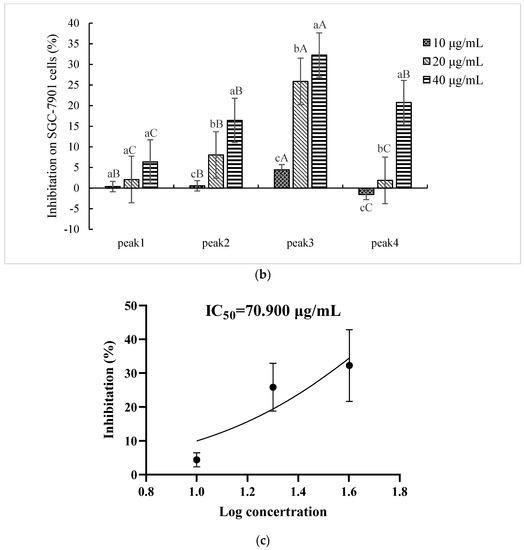
Figure 3.
Inhibition effect of components separated by FPLC. (a) The elution curve of FPLC, linear electrical conductivity showed correctly linear elution. (b) Inhibitory rates of cell proliferation (SGC-7901 cells) induced by Sect. II-3-1-4 that were separated by FPLC after 48 h treatment. a, b, c means p < 0.05; A, B, C means p < 0.01. (c) IC50 curve fitting of Sect. II-3-3.
2.4. Inhibition Effect of Components Separated by RP-HPLC
Six fractions in Sect. II-3-3 (FPLC) were discriminated and eluted by RP-HPLC (Figure 4a): peaks 1–6 were denoted as Sect. II-3-3-1-6, respectively. Each fraction (differential peak collection) was treated the same as above. Sect. II-3-3-2 (peak 2), Sect. II-3-3-3 (peak 3), and Sect. II-3-3-5 (peak 5) expressed inhibitory effects on the proliferation of SGC-7901. In particular, a dosage-dependent action manner was shown in Sect. II-3-3-2 and Sect. II-3-3-5 (Figure 4b). Sect. II-3-3-5 had the highest inhibition effect at a high concentration treatment (30 μg/mL), and its IC50 value was 51.290 μg/mL (Figure 4c). Therefore, Sect. II-3-3-5 was collected to determine molecular weight and amino acid sequence.
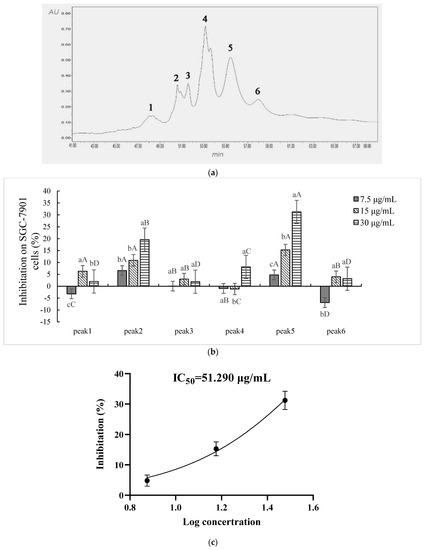
Figure 4.
Inhibition effect of components separated by RP-HPLC. (a) The elution curve of RP-HPLC. (b) Inhibitory rates of cell proliferation (SGC-7901 cells) induced by Sect. II-3-3-1~6 that were separated by RP-HPLC after 48 h treatment. a, b, c means p < 0.05; A, B, C, D means p < 0.01. (c) IC50 curve fitting of Sect. II-3-3-5.
Although the raw product (Sect. II, isolated by ultrafiltration) originating from the hemolymph of A. chinensis had a relatively high quantity, the component(s) with anti-cancer activity was had a low yield rate (Table 2). The final yield rate of active peptide(s) was 0.020% compared to peptides/proteins in the raw product (Sect. II). Compared to the last step, the yield rate for each dissection operation increased along with separation and purification, step by step. Along with the purification and enrichment of active peptide(s), the IC50 value of isolated fractions decreased sequentially.

Table 2.
Effectiveness of various separation operations denoted by protein yield and IC50.
2.5. Molecular Weight and Amino Acid Sequence of Active Peptide
Molecular weight of the active peptide (Sect. II-3-3-5) that inhibited proliferation of gastric cancer SGC-7901 cells was determined as 2853.3 Da by MALDI-TOF-MS (Figure 5a). Amino acid sequence of the active peptide was determined by the ‘Edman chemical degradation method’. Together, 24 amino acid residues were continuously determined for the active peptide (Figure 5b, Supplementary Information): N′-EKHYAAEKGIRRDDIIHTTTLKKK-C′.
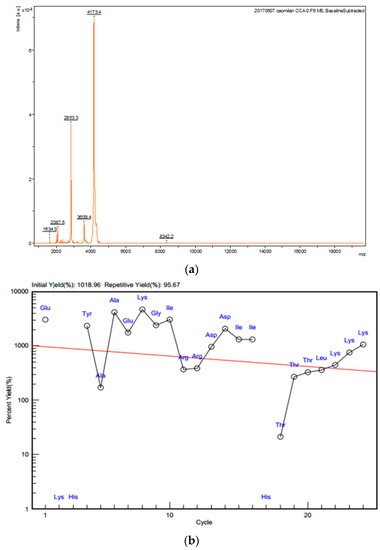
Figure 5.
Molecular weight and amino acid sequence of active peptide. (a) Molecular weight of the active peptide determined by MALDI-TOF-MS. (b) N-terminal amino acid sequence of active protein.
3. Discussion
Cancer is the second leading cause of death after cardiovascular and cerebrovascular diseases and poses serious threats to human life and health. Therefore, scholars at home and abroad have been working to explore ways to treat cancer. In recent years, due to the continuous research on anti-cancer traditional Chinese medicine, the advantages of the comprehensive conditioning function, lower toxicity, and fewer side effects of anti-cancer medicinal insects have attracted more and more attention. At present, several drugs have been developed and used in the treatment of cancer. Medicinal insects have a significant inhibitory effect on a variety of cancer cells, with different types of anti-cancer mechanisms, such as signaling pathways regulating apoptosis of tumor cells, blocking of the tumor cell cycle, and inhibition of the migration and invasion of tumor cells. The diversity of anticancer mechanisms not only facilitates the development and utilization of medicinal insects from multiple angles but also reduces the possibility of drug resistance in tumor cells. However, there are still many problems to be solved to realize the widespread application of anti-cancer insects. Meanwhile, the components of anti-cancer insects are complex, and their anti-cancer functions and mechanisms are not yet fully understood.
A. chinensis is an anti-cancer medicinal insect. Previous experiments have shown its significant anti-cancer activity of inhibiting proliferation of human gastric cancer SGC-7901 cells and breast cancer MCF-7 cells [27]. While, its anti-cancer active component(s) is unclear. In this study, the combination of ultrafiltration, gel filtration chromatography, FPLC, and RP-HPLC is utilized to separate and purify the active peptide based on proliferation of human gastric cancer SGC-7901 cells. The active peptide possesses a molecular weight of 2853.3 Da. As seen in the results in this study, the active peptide from A. chinensis inhibits cell proliferation of SGC-7901 cells. It is suggested that this peptide is one of the active substances of A. chinensis and may participate in the anticancer effect of the hemolymph of the A. chinensis. The results of this study also provide an experimental basis for the development of anti-tumor substances with hemolymph activity of the nine incense worms, and provide a new idea for the development of anti-tumor aversion of the nine incense worms.
The hemolymph is more effective for inhibition of SGC-7901 cells than other tissues of A. chinensis. The protein/peptide section is more effective for inhibition of SGC-7901 cells than other constituents in the hemolymph [29]. However, the content of the protein/peptide in the hemolymph of A. chinensis is very low. Separation and purification of the peptide (antitumor active component) in this study are valid, which is indicated by the decreasing of IC50 values for sequential separating products (Table 2). The final yield rate of the peptide (compared to 3–50 kD product by ultrafiltration) is very low (0.020%). The very low yield rate of active components for each separation/purification operation is not consistent with the change of IC50 values for the respective product (Table 2). This result could be as a result of the partial recovery of active components in each operation or from losses of active components that possess minor or auxiliary active components in each separation/purification step (Figure 3a, Figure 4a and Figure 5a,b).
In summary, this study was the first to isolate and purify an active peptide that inhibits the proliferation of gastric cancer cells from the hemolymph of the worm, which lays an important foundation for the development of the medicinal insect resources and the research and development of new anti-tumor drugs.
4. Materials and Methods
4.1. Materials
A. chinensis was collected from Xiasi Town, Kaili City, Guizhou Province, China (107°80′ E, 26°52′ N), and stored in −80 °C refrigerators. Their bodies were placed into a 10 mL syringe after removing heads, chests, and wings and then squeezed to release the hemolymph. The hemolymph was transferred into centrifuge tubes. After centrifugation at 12,000× g for 30 min at 4 °C, the top liquid layer (containing oil) was gently scraped off. The aqueous solution was centrifuged again at 12,000× g for 30 min at 4 °C, and the supernatant was collected for further research.
4.2. Protein Concentration Determination by BCA
Measurements of protein concentration for the original hemolymph of A. chinensis and separated products in were carried out using the BCA method and the BCA protein concentration assay kit instructions (Cat. No.: BL521A, Biosharp). A standard curve (absorbance value at 562 nm) for determination of protein concentrations was made with a series of bovine serum solutions dissolved with PBS.
4.3. Cell Culture and MTT Assay
Human gastric cancer SGC-7901 cells (Shanghai Cell Bank of Chinese Academy of Sciences) were cultured in a DMEM (high glucose) medium containing 10% fetal bovine serum (FCS) and 1% streptomycin in a carbon dioxide incubator (37 °C, 5% CO2). The medium was renewed every other day during culture, and sub-cultures of cells were carried out when cells were spread to 80% on the bottom of flask (in the logarithmic growth phase).
MTT [3-(4,5-dimethyl thiazol-2-yl)-2,5-diphenyl tetrazolium bromide] assay was carried out to detect the inhibition effect resulting from the hemolymph of A. chinensis from Section 2.1 or other dissected products that were added into the cultural medium after sub-cultured cells, adherent with four replicates per group. Inhibition rate = [1 − (P1-O)/(P0-O)] × 100%. P1 is a value of the experiment group (SGC-7901 cells treated with eluted fraction); P0 is a value of the cell group without the drug (SGC-7901 cells without treatment); O is a value of the blank group (medium without cells or drug, only cell culture fluid). The experiment was repeated three times. The half-inhibitory concentration (IC50 value) was calculated by curve fitting.
4.4. Separation and Purification of Active Component in Hemolymph of A. chinensis
4.4.1. Ultrafiltration
The hemolymph of A. chinensis was diluted with PBS at a ratio of 1:4 and then centrifuged for 1 h (12,000× g, 4 °C) to collect the supernatant. The supernatant was transferred to MWCO 50 kD and MWCO 3 kD ultrafiltration tubes (Millipore) and centrifuged for 1–2 h (4500× g, 4 °C). Components in the hemolymph were separated into three sections: >50 kD, 3–50 kD, and <3 kD. After freeze-drying, these components were redissolved in 1 mL PBS.
4.4.2. Gel Filtration Chromatography
The Sephadex G-50 cross-linked dextran gel was selected as the filler (GE Healthcare of American) to separate components in the product using ultrafiltration. The equilibration/elution buffer was 50 mmol/L Tris-HCl (pH 9.0). The OD280/OD215 was measured with a spectrophotometer (Ultrospec 2100 pro UV/Vis, Amersham Bioscience; the same as below) along with elution. Eluted samples were collected and combined according to the peak shape and then lyophilized. After freeze-drying, collections for each peak component were redissolved in 1 mL PBS.
4.4.3. Fast Protein Liquid Chromatography (FPLC)
To separate components in the product that resulted from gel filtration chromatography, FPLC (ÄKTA Explorer 10S Purifier FPLC, GE) was used. The following were used in the experiment: chromatography column: resource Q (6 mL) strong anion exchange column; equilibration buffer (solution A): 50 mmol/L Tris-HCl (pH 9.0); elution buffer (solution B): 50 mmol/L Tris-HCl + 1 mol/L NaCl (pH 9.0); pressure limit: 0.6 MPa. A linear gradient elution was performed with 0–1 mol/L NaCl elution buffer at a flow rate of 1.5 mL/min for 50 min. The OD215/OD280 was measured along with elution. Eluted samples were collected and combined according to the peak shape and then lyophilized. Further treatments for collections are the same as above.
4.4.4. Reverse-Phase High Performance Liquid Chromatography (RP-HPLC)
To separate components in the product that resulted from FPLP, RP-HPLC (Waters 1525 series Binary HPLC Pump, Waters) was used. The following were used: preparation column: Waters X BridgeTM C18 Size LC Column (4.6 × 250 mm); equilibration buffer (solution A): 99.9% ultrapure water + 0.1% trifluoroacetic acid (TFA); elution buffer (solution B): 99.9% acetonitrile + 0.1% trifluoroacetic acid (TFA). The procedure for gradient elution is shown in Table 3. The OD215/OD280 was measured along with elution. The UV absorbance values of OD215 and OD280 were measured using a Waters 2489 Vis/UV detector. Eluted samples were collected and combined according to the peak shape and then lyophilized. Further treatments for collections are the same as above.

Table 3.
Elution procedure of RP-HPLC.
4.5. Determination of Molecular Weight and Amino Acid Sequencing for Active Peptide in Hemolymph of A. chinensis
4.5.1. Determination of Molecular Weight for Peptide(s)
The desalting active peptide(s) (10 µL, >4 pmol/µL) resulting from RP-HPLC was used to detect the purity and then was used for molecular weight measurement by matrix-assisted laser desorption time-of-flight mass spectrometry [30].
4.5.2. Amino Acid Sequencing
The pure active peptide(s) (about 100 µg) resulting from RP-HPLC was used to determine its amino acid sequence with the Edman chemical degradation method [19].
4.6. Data Analysis
Data were analyzed by one-way ANOVA analysis and homogeneity testing of variance using statistical software package SPSS 19.0. The variance t-test was used to compare the data of multiple groups. All data were expressed by ± SD (Standard Deviation), and the difference was statistically significant at p < 0.05.
5. Conclusions
In conclusion, a peptide could inhibit the proliferation of gastric cancer SGC-7901 cells in the hemolymph of A. chinensis, separated and purified by ultrafiltration, gel filtration chromatography, FPLC, and RP-HPLC in this study. We determined that the sequence of the active peptide is N′-EKHYAAEKGIRRDDIIHTTTLKK-C′, and its molecular weight is 2853.3 Da. These results should facilitate further works for this peptide, such as the cloning of genes, expression in vitro by prokaryotic or eukaryotic systems, additional testing of anti-tumor activity, and so on.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms232012535/s1.
Author Contributions
Conceptualization, methodology, writing—original draft, validation, data curation, software: X.-M.C., S.-Q.Z. and M.-L.C.; funding: J.-J.G.; supervision: J.-J.G. and R.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (NSFC 82160743), Guizhou Provincial Science and Technology Projects (Qiankehe Pingtai Rencai-GCC [2022]029-1).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data are available within the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.C.; Li, Z.X.; Zhang, Y.; Zhou, T.; Zhang, J.Y.; You, W.C.; Pan, K.F.; Li, W.Q. Interpretation on the report of Global Cancer Statistics 2020. J. Multidiscip. Cancer Manag. (Electron. Version) 2021, 7, 1–14. [Google Scholar]
- Li, W. Optimization on the Synthesis of Anticancer New Drug LY989-03. Master’s Thesis, Huaqiao University, Quanzhou, China, 2018. [Google Scholar]
- Wei, C.; Deng, X.G.; Gao, S.D.; Wan, X.M.; Chen, J. Cantharidin inhibits proliferation of liver cancer by inducing DNA damage via KDM4A-dependent histone H3K36 demethylation. Evid.-Based Complement. Altern. Med. 2022, 2022, 2197071. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Bao, H.; Feng, X.D.; Wang, Y.; Han, Z.F.; Li, H.B. Superoxide anion induced by NCTD promoting apoptosis in HeLa cells. Chin. Arch. Tradit. Chin. Med. 2021, 39, 97–100. [Google Scholar]
- Yu, Z.Y.; Li, L.; Wang, C.Q.; He, H.; Liu, G.; Ma, H.Y.; Peng, L.; Jiang, M.D.; Lu, Q.W.; Li, P.; et al. Cantharidin induces apoptosis and promotes differentiation of AML cells through nuclear receptor Nur77-mediated signaling pathway. Front. Pharmacol. 2020, 11, 1321. [Google Scholar] [CrossRef]
- Lin, L.H.; Huang, H.S.; Lin, C.C.; Lee, L.W.; Lin, P.Y. Effects of cantharidinimides on human carcinoma cells. Chem. Pharm. Bull. 2004, 52, 855–857. [Google Scholar] [CrossRef]
- Li, X.F.; Hou, X.H.; Chen, X.S. Inhibitory effect of cantharidin from meloid on laryngeal carcinoma cell lines and gastric carcinoma cell lines. Acta Entomol. Sin. 2009, 52, 946–951. [Google Scholar]
- Luo, X.N.; Wang, W.J.; Li, K.; Du, H.H. Effect of norcantharidin on proliferation and apoptosis of androgen independent prostate cancer PC-3 cells. Shaanxi Med. J. 2020, 49, 928–931. [Google Scholar]
- Guo, W.J.; Han, L.T.; Wang, C.M.; Wei, Z.X.; Gao, J.H.; Qiu, Y.G.; Li, X.W. Morphologic study of inhibitory effects of propolis on cell growth of transplantation tumor in mice. China Pharm. 2003, 14, 206–207. [Google Scholar]
- Zhou, C.; Ma, J.; Lu, Y.H.; Zhao, W.; Xu, B.X.; Lin, J.; Ma, Y.J.; Tian, Y.F.; Zhang, Q.; Wang, W.; et al. TERT promoter regulating melittin expression induces apoptosis and G0/G1 cell cycle arrest in esophageal carcinoma cells. Oncol. Lett. 2021, 21, 16. [Google Scholar]
- Azevedo, R.A.; Figueiredo, C.R.; Ferreira, A.K.; Matsuo, A.L.; Massaoka, M.H.; Girola, N.; Auada, A.V.V.; Farias, C.F.; Pasqualoto, K.F.M.; Rodrigues, C.P.; et al. Mastoparan induces apoptosis in B16F10-Nex2 melanoma cells via the intrinsic mitochondrial pathway and displays antitumor activity in vivo. Peptides 2014, 68, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, S.; Hassab, E.S.; Hawash, A.; Elseedi, H.R.; Khalifa, S.A.M.; Ullah, S.; AlSehemi, A.G.; ElGarawani, L.M. curcumin-injected musca domestica larval hemolymph: Cecropin upregulation and potential anticancer effect. Molecules 2022, 27, 1570. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Wu, Y.; Ma, J.I.; Yang, J.H.; Zhang, F.C. The antibacterial peptide from Bombyx mori cecropinXJ induced growth arrest and apoptosis in human hepatocellular carcinoma cells. Oncol. Lett. 2016, 12, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Song, L.L.; Sun, S.; Liu, S.S.; Jin, G.Z.; Jin, L.L.; Yang, W.S. Morphological observation of hela human cervical carcinoma cells by the raw grub extract. Lishizhen Med. Mater. Med. Res. 2006, 17, 673–675. [Google Scholar]
- Hua, Y.F.; Wu, J.L.; Qian, J.Q. Inhibitory effect of fatty acids from specifically-cultivated Chrysomya megacephala (Fabricius) (Diptera: Calliphoridae) larvae on tumor cells and HIV-1 integrase in vitroand their ingredient analysis. Acta Entomol. Sin. 2008, 51, 137–142. [Google Scholar]
- Sun, H.L.; Gu, X.L.; Zhang, Q.; Xu, H.; Zhong, Z.Y.; Deng, C. Cancer nanomedicines based on synthetic polypeptides. Biomacromolecules 2019, 20, 4299–4311. [Google Scholar] [CrossRef]
- Yue, S.H.; Tian, C.; Hu, Y.Z.; Zhang, J.L. Research progress and prospects on anticancer peptides. Biotechnol. Bull. 2017, 33, 41–47. [Google Scholar]
- Arcangeli, A.; Crociani, O.; Lastraioli, E.; Masi, A.; Pillozzi, S.; Becchetti, A. Targeting ion channels in cancer: A novel frontier in antineoplastic therapy. Curr. Med. Chem. 2009, 16, 66–93. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, L.; Wu, Y.; Wang, L.; Ma, C.B.; Xi, X.P.; Bininde-Emonds, O.R.P.; Shaw, C.; Chen, T.B.; Zhou, M. Evaluation of the bioactivity of a mastoparan peptide from wasp venom and of its analogues designed through targeted engineering. Int. J. Biol. Sci. 2018, 14, 599–607. [Google Scholar] [CrossRef]
- Lu, J.; Chen, Z. Isolation, characterization and anti-cancer activity of SK84, a novel glycine-rich antimicrobial peptide from Drosophila virilis. Peptides 2010, 31, 44–50. [Google Scholar] [CrossRef]
- Sun, Y.; Mo, F.; Dai, Z.R. Research progress in preparation, isolation and purication of bioactive peptides. J. Beibu Gulf Univ. 2017, 35, 35–40. [Google Scholar]
- Guo, X.J.; Yuan, K.S.; Jan, J.Y.; Bao, Z.J.; You, J.X.; Wang, Z.T.; Hu, Z.W.; Li, H.B.; Jiang, Z.J.; Zhou, H.B. The research progress on the purification, separation and analysis of antimicrobial peptides. World Notes Antibiot. 2017, 38, 9–12. [Google Scholar]
- Tan, J.; Guo, J.J.; Wei, C.; Yang, J.Q. The inhibitory effects of harmolymph from Aspongopus chinensis Dallas on in vitro proliferation of gastric carcinoma cell SGC-7901. J. Mt. Agric. Biol. 2013, 32, 119–122. [Google Scholar]
- Yang, J.Q.; Long, J.L.; Tan, J.; Jin, D.C.; Guo, J.J. Effects of hemolymph of Aspongopus chinensis Dallas on the expression of apoptosis-related proteins in tumor cells. Biot. Resour. 2017, 39, 360–365. [Google Scholar]
- Yang, J.Q.; Tan, J.; Cao, M.L.; Jin, D.C.; Guo, J.J. The inhibitory effect of hemolymph from Aspongopus chinensis Dallas on growth of human breast cancer MCF-7 by CCK-8. J. Environ. Entomol. 2017, 39, 193–197. [Google Scholar]
- Yang, J.Q.; Tan, J.; Cao, M.L.; Jin, D.C.; Song, X.; Guo, J.J. Apoptosis rules of MCF-7 and SGC-7901 cell line induced by hemolymph from Aspomgopus chinensis detected by ELISA. J. Southwest China Norm. Univ. Nat. Sci. Ed. 2017, 42, 48–52. [Google Scholar]
- Zhao, S.; Tan, J.; Yu, H.M.; Tian, Y.; Guo, J.J. In vivo and in vitro antiproliferative and antimetastatic effects of hemolymph of Aspongopus chinensis Dallas on breast cancer cells. J. Tradit. Chin. Med. 2021, 41, 523–552. [Google Scholar]
- Cao, M.L.; Tan, J.; Jin, D.C.; Guo, J.J. The proliferation effects of three kinds of biological molecules from Aspongopus Chinensis hemolymph on the gastric cancer SGC-7901 cells. J. Mt. Agric. Biol. 2018, 37, 41–45. [Google Scholar]
- Jiang, L.P.; Liu, C.J.; Duan, C.J.; Deng, M.C.; Tang, X.; Liang, S.P. Transcriptome analysis of venom glands from a single fishing spider Dolomedes mizhoanus. Toxicon 2013, 73, 23–32. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).