Abstract
HR4, a member of the nuclear receptor family, has been extensively studied in insect molting and development, but reports on crustaceans are still lacking. In the current study, the MnHR4 gene was identified in Macrobrachium nipponense. To further improve the molting molecular mechanism of M. nipponense, this study investigated whether MnHR4 functions during the molting process of M. nipponense. The domain, phylogenetic relationship and 3D structure of MnHR4 were analyzed by bioinformatics. Quantitative real-time PCR (qRT-PCR) analysis showed that MnHR4 was highly expressed in the ovary. In different embryo stages, the highest mRNA expression was observed in the cleavage stage (CS). At different individual stages, the mRNA expression of MnHR4 reached its peak on the fifteenth day after hatching (L15). The in vivo injection of 20-hydroxyecdysone (20E) can effectively promote the expression of the MnHR4 gene, and the silencing of the MnHR4 gene increased the content of 20E in M. nipponense. The regulatory role of MnHR4 in 20E synthesis and 20E signaling was further investigated by RNAi. Finally, the function of the MnHR4 gene in the molting process of M. nipponense was studied by counting the molting frequency. After knocking down MnHR4, the molting frequency of M. nipponense decreased significantly. It was proved that MnHR4 plays a pivotal role in the molting process of M. nipponense.
1. Introduction
Endocrine signals play a central role in animal growth and maturation. Although vertebrate growth and development are primarily controlled by thyroid hormones and sex steroids, insect development is controlled by several key hormones and neuropeptides, of which the steroid hormone ecdysteroid is the main regulator [1]. 20-Hydroxyecdysone (20E) is a steroid hormone that was originally discovered in plants and arthropods [2]. The accumulation of research over the past two decades has shown that cholesterol is catalyzed into 20E by the Halloween family of genes (i.e., Spook, Phantom, Disembodied, Shadow and Shade) [3,4,5]. 20E converts hormonal signals into transcriptional responses through members of the nuclear receptor family, and when 20E binds to the EcR/USP complex, the transcriptional cascade leads to the onset of ecdysis and metamorphosis [6,7]. Drosophila, as a classic model organism, has become a model for elucidating the regulatory role of 20E. The 20E pulse directs each molt and metamorphosis of the Drosophila melanogaster’s life cycle [8]. Many downstream nuclear receptor transcription factors activated by 20E have been identified in insects [9].
Nuclear receptors contain two common structural elements—a DNA-binding domain (DBD) and a ligand-binding domain (LBD)—which are involved in the regulation of myriad biological processes [10]. In the holometabolous insect D. melanogaster, some genes, such as nuclear receptor E75 (E75), nuclear receptor E78 (E78), hormone receptor 3 (HR3), hormone receptor 4 (HR4), ecdysis-triggering hormone receptor (ETHR) and Fushi tarazu factor-1 (Ftz-f1), are transcriptionally regulated by 20E and play a central role in transducing the molting signals [11,12]. The expressions of HR3 and E75B increased with the increase in the 20E titer during D. melanogaster pupation, and βFtz-f1 was activated after the decrease in 20E [13]. In the hemimetabolous insect Blattella germanica, the regulatory relationship between nuclear receptor genes E75, HR3, HR4 and Ftz-f1 changed with the fluctuation in the 20E titer [14]. In insects, the function of nuclear receptors activated by 20E has been reported in detail. For example, an E75A mutation causes developmental arrest and molting defects in Drosophila and the E75C-mutant Drosophila die in adulthood, whereas E75B-mutant individuals survive and reproduce normally [15]. The knockdown of BgE75 results in B. germanica prothoracic gland degeneration and ecdysteroid deficiency [14]. HR3 is involved in the regulation of chitin synthesis and degradation during Locusta migratoria molting [16]. Silencing of BgFtz-f1 prevents normal molting and development of B. germanica [17], and Ftz-f1 is involved in the regulation of Leptinotarsa decemlineata’s pupation by regulating 20E and JH titers [18]. HR4 plays an important role in Tribolium castaneum molting and ovulation [19] and plays a central role in coordinating growth and the maturation of D. melanogaster [20]. In addition, the HR4 gene has been identified in some crustacean transcriptomes. In Litopenaeus vannamei and Daphnia, HR4 was identified as an ecdysone signaling response gene [21,22]. In Callinectes sapidus, increased ecdysteroid concentration induced HR4 expression [23]. Altogether, nuclear receptor genes have been extensively studied in insect molting and development, but reports on crustaceans are still lacking.
Macrobrachium nipponense (Crustacea, Decapoda) is an important freshwater economic prawn in China [24]. Molting is a pivotal event in the growth of crustaceans [25]. M. nipponense grows by molting, but the mechanism of molting is still poorly understood. Therefore, it is of great scientific significance to study the molting mechanism of M. nipponense for breeding and increasing production. We previously demonstrated the functions of Spook and Ftz-f1 genes in the molting and ovarian development of M. nipponense [5,26]. Transcriptome analysis of the different molting stages of M. nipponense revealed that MnHR4 is an important differential gene. To further improve the molting molecular mechanism of M. nipponense, this study investigated whether MnHR4 functions during the molting process of M. nipponense. In the current study, the MnHR4 gene was identified in M. nipponense. The domain, phylogenetic relationship and 3D structure of MnHR4 were analyzed by bioinformatics. The expression patterns of the MnHR4 gene in different tissues and developmental stages of M. nipponense were detected by qRT-PCR. The expression of the MnHR4 gene was detected by qRT-PCR after a 20E injection in vivo. After knockdown of the MnHR4 gene by the RNA interference, the content of 20E in M. nipponense was detected by an ELISA. The regulatory role of MnHR4 in 20E synthesis and 20E signaling was further investigated by RNAi. Finally, the function of the MnHR4 gene in the molting process of M. nipponense was studied by counting the molting frequency.
2. Results
2.1. Sequence Analysis and Phylogeny of MnHR4
The rapid amplification of the cDNA ends (RACE) (TaKaRa, Kyoto, Japan) of the HR4 fragment yielded a cDNA sequence with a putative open reading frame of 2901 bp, encoding a total of 966 amino acids, named MnHR4 (Figure 1). A phylogenetic tree of the HR4 amino acids of different species was constructed, indicating that insecta and crustacea form two independent clades, which is consistent with the traditional taxonomy of species. M. nipponense is the most closely related to Penaeus chinensis, followed by Armadillidium vulgare (Figure 2). A comparison of the HR4 amino acid sequences between M. nipponense and other crustaceans using DNAMAN 6.0 showed that MnHR4 contained a conserved C4 zinc finger (ZnF_C4) domain. Zinc finger domains are relatively small protein motifs containing different binding-specific finger-like protrusions, usually in clusters. The HR4 amino acid identity of M. nipponense and P. chinensis was 49.64%, followed by A. vulgare at 30.18% (Figure 3). The amino acid sequence of MnHR4 was analyzed using the iterative threading assembly refinement (I-TASSER) server. Figure 4A shows a three-dimensional (3D) atom model of M. nipponense’s MnHR4 generated by I-TASSER. The predicted binding ligands are the pale-green spheres, and the binding residues are the blue ball and stick (Figure 4B).
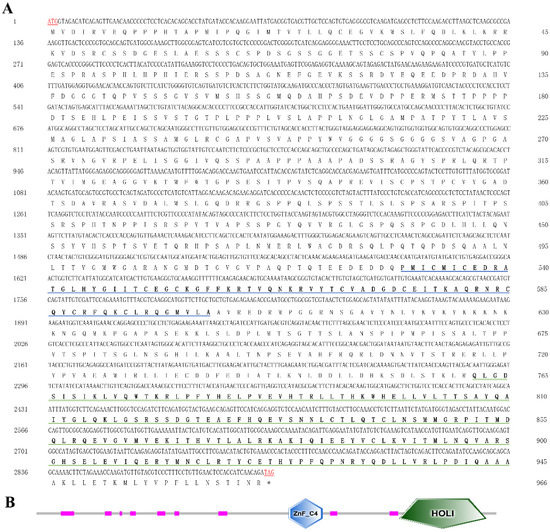
Figure 1.
(A) Nucleotide and deduced amino acid sequences of MnHR4 in M. nipponense. The numbers on the left and right refer to the coordinates of the nucleotide and amino acid sequences, respectively. Blue and green underscores represent the ZnF_C4 and HOLI domains, respectively. The termination signals are indicated with an asterisk (*). (B) Domain architecture organization of MnHR4 as predicted by SMART (http://smart.embl-heidelberg.de/ (accessed on 20 April 2022)). The conserved domains (i.e., ZnF_C4 and HOLI) of MnHR4 are shown by the different shapes and colors.
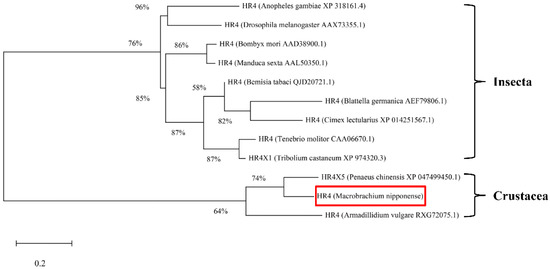
Figure 2.
Phylogenetic tree of the amino acid sequences of HR4 from various species. The numbers shown at the branches indicate the bootstrap values (%). The red rectangles indicate the position of M. nipponense in the phylogenetic tree. The species and GenBank accession numbers for constructing the phylogenetic tree are listed below: Anopheles gambiae (XP_318161.4), D. melanogaster (AAX73355.1), Bombyx mori (AAD38900.1), Manduca sexta (AAL50350.1), Bemisia tabaci (QJD20721.1), B. germanica (AEF79806.1), Cimex lectularius (XP_014251567.1), Tenebrio molitor (CAA06670.1), Tribolium castaneum (XP_974320.3), P. chinensis (XP_047499450.1), M. nipponense and A. vulgare (RXG72075.1).
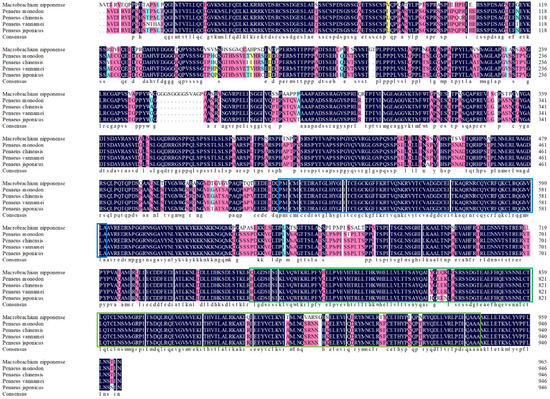
Figure 3.
Sequence alignment of the HR4 amino acids between M. nipponense and other crustaceans. The blue and green underscores represent the ZnF_C4 and HOLI domains, respectively.
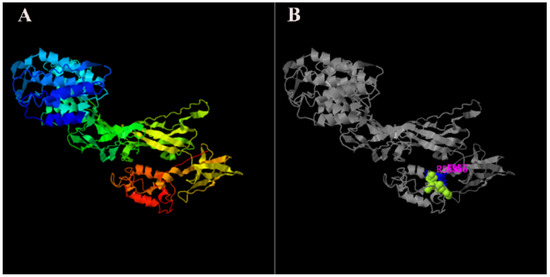
Figure 4.
The 3D structures of MnHR4 predicted by I-TASSER: (A) predicted function of MnHR4 3D structures using COFACTOR and COACH, where the pale-green sphere represents the predicted binding ligand; (B) molecules and ions that bind to anchoring proteins are called ligands.
2.2. Expression of the MnHR4 Gene in Different Tissues and Different Developmental Stages
The expression levels of MnHR4 mRNA in different tissues and different developmental stages of M. nipponense were investigated by qRT-PCR. The highest mRNA expression of MnHR4 was observed in the ovary, followed by the eyestalk, with the lowest expression in the hepatopancreas. The expression levels of MnHR4 mRNA in the ovary and eyestalk were significantly higher than those in other tissues, and the expression level in the ovary was 4.47-fold higher than that in the eyestalk (p < 0.05) (Figure 5A). The expression of MnHR4 mRNA showed no significant difference in different ovary stages (p > 0.05) (Figure 5B). In different embryo stages, the highest mRNA expression was observed in the cleavage stage (CS), followed by the blastula stage (BS), and the lowest was observed in the zoea stage (ZS). The expression level in the CS was 4.53-fold that in the BS and 23.22-fold that in the ZS (p < 0.05) (Figure 5C). In different individual stages, the mRNA expression of MnHR4 gradually decreased from the first day after hatching (L1) to L10 and reached a peak at L15. MnHR4 mRNA expression was lowest on the first day post-larvae (PL1) and showed a significant difference (p < 0.05) (Figure 5D).
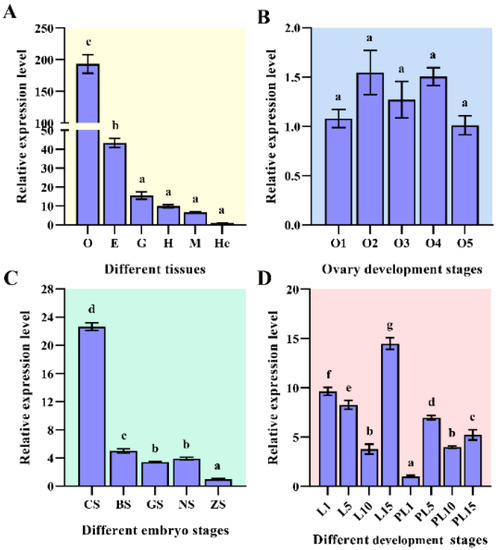
Figure 5.
Expression of MnHR4 mRNA in different tissues and different developmental stages of M. nipponense. Samples (at different developmental stages) were collected at the experimental site in Dapu, according to previous criteria [27,28]. The expression of MnHR4 mRNA was normalized to the EIF transcript level. (A) Different tissues: O, ovary; E, eyestalk; G, gill; H, heart; M, muscle; He, hepatopancreas. (B) Different ovary stages: O1, undeveloped stage; O2, developing stage; O3, nearly ripe stage; O4, ripe stage; O5, spent stage. (C) Different embryo stages: CS, cleavage stage; BS, blastula stage; GS, gastrula stage; NS, nauplius stage; ZS, zoea stage. (D) Different development stages: L1, the first day after hatching; PL1, the first day post-larvae, etc. Statistical analyses were performed by one-way ANOVA. Data are shown as the mean ± SEM (n = 6). Bars with different letters indicate significant differences (p < 0.05).
2.3. Interaction between 20E and MnHR4
Referring to previous studies, 20E (5 μg/g) was injected into M. nipponense [26]. The effect of 20E on the expression of MnHR4 was detected using qRT-PCR (Figure 6A). The results show that there was no significant difference in the expression level of the MnHR4 gene between the experimental group and the control group at 0 h (no injection) and 3 h after injection (p > 0.05). The expression level of the MnHR4 gene in the experimental group was significantly higher than that in the control group at 6 and 12 h after injection, and it reached a peak at 12 h in the experimental group (p < 0.05). There was no significant difference in the expression of MnHR4 between the two groups at the 24th hour after injection (p > 0.05). After knockdown of MnHR4, the content of 20E in M. nipponense was measured by an ELISA (Figure 6B). The results showed that there was no significant difference in the content of 20E in M. nipponense on the first day (p > 0.05). The content of 20E in the experimental group of M. nipponense was significantly higher than that in the control group on the 5th day. Compared with the control group, the content of 20E in the experimental group increased by 37.79% (p < 0.05).
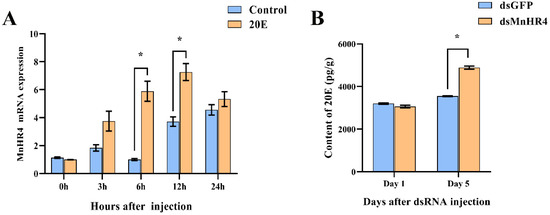
Figure 6.
(A) Expression of MnHR4 mRNA in the ovary under the influence of 20E. The expression of MnHR4 mRNA was normalized to the EIF transcript level. (B) The content of 20E in M. nipponense after knockdown of MnHR4. Control, injection of solvent; 20E, injection of 20E. Data are expressed as the mean ± SEM (n = 6). Significant differences between the experimental group and the control group were determined using Student’s t-test (* p < 0.05).
2.4. Effects of RNAi MnHR4 on the Expression of Mn-Spook, Phantom, HR3, E75b, ETHR and Mnftz-f1
The regulatory role of MnHR4 in 20E synthesis and 20E signaling was further investigated by RNAi. After knockdown of MnHR4, the expression levels of genes catalyzing 20E synthesis (i.e., Mn-Spook and Phantom) and downstream genes conducting 20E signaling (i.e., HR3, E75b, ETHR and MnFtz-f1) were detected by qRT-PCR (Figure 7). Compared to the control, the expression of MnHR4 mRNA in the experimental group decreased by 17.45%, 69.25%, 73.88% and 69.97% at 24, 48, 96 and 120 h after dsMnHR4 administration, respectively (p < 0.05) (Figure 7A). After the knockdown of MnHR4, the expression levels of Mn-Spook and Phantom in the experimental group significantly increased. At the 120th hour after the knockdown of MnHR4, the expression levels of Mn-Spook and Phantom in the experimental group were 24.4-fold and 2.1-fold higher than those in the control group, respectively (Figure 7B,C). The results show that the expression levels of HR3 and E75b in the experimental group also significantly increased at the 24th and 48th hours after MnHR4 gene silencing. At the 120th hour after silencing, the expression of HR3 in the experimental group was 20.55-fold that of the control group, and the expression of E75b in the experimental group was 2.49-fold that of the control group (Figure 7D,E). On the contrary, the expressions of ETHR and MnFtz-f1 in the experimental group decreased to different degrees compared with the control group after MnHR4 gene silencing. The expression of ETHR in the experimental group decreased by 31.13% and 38.91% at the 96th and 120th hour of MnHR4 gene silencing, respectively (Figure 7F). Compared with the control group, the expression of MnFtz-f1 in the experimental group decreased by 79.36% at the 120th hour (Figure 7G).
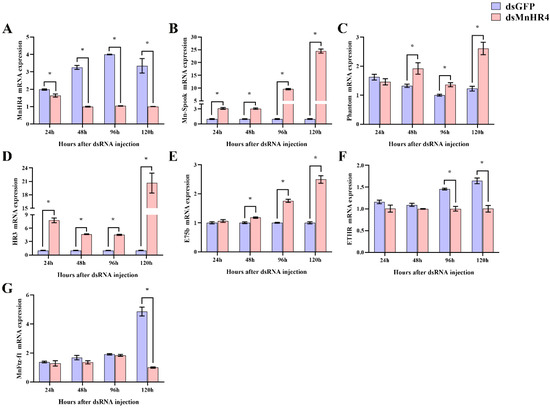
Figure 7.
Knockdown of MnHR4 on the expression of other 20E-related genes in the ovary of M. nipponense. The expression of MnHR4 mRNA was normalized to the EIF transcript level: (A) MnHR4; (B) Mn-Spook; (C) Phantom; (D) HR3; (E) E75b; (F) ETHR; (G) Mn-Ftz-f1. Data are expressed as the mean ± SEM (n = 6). Significant differences between the experimental group and the control group were determined using Student’s t-test (* p < 0.05).
2.5. Effect of MnHR4 Knockdown on the Molting Frequency of M. nipponense
Figure 8 shows the molting frequency of M. nipponense in the control and experimental groups after MnHR4 knockdown. M. nipponense starts molting on the second day and completes one round of molting on the 12th day. The results show that there was no significant difference in the frequency of molting between the experimental and control groups during the first round of molting (p > 0.05). From the 21st day, the control group of M. nipponense began the second round of concentrated molting, which was significantly higher than the experimental group of M. nipponense’s molting frequency (p < 0.05).
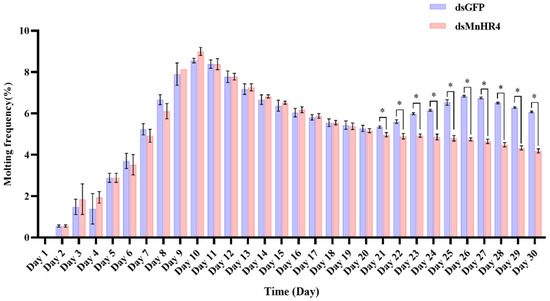
Figure 8.
Molting frequency of M. nipponense in the experimental and control groups after knocking down MnHR4. Molting frequency = (Nm/Ns)/D, where Nm is the total number of molts; Ns is the number of prawns in the aquarium; D is the number of experimental days. Data are expressed as the mean ± SEM. Significant differences between the experimental group and the control group were determined using Student’s t-test (* p < 0.05).
3. Discussion
Nuclear receptor genes function in many biological processes such as embryonic development, sex determination, insect metamorphosis and molting [29,30,31]. Using RNAi, we previously demonstrated that MnFtz-f1 played a pivotal role in the molting and ovulation process of M. nipponense [26]. Previous studies have demonstrated that HR4 plays an important role in both holometabolous and hemimetabolous insects [11,14], but its function in M. nipponense is still unclear.
In the present study, we identified the nuclear receptor gene MnHR4 from the transcriptome of M. nipponense at different molting stages. The predicted MnHR4 coding region encodes a total of 966 amino acids, including two conserved domains: the c4 zinc finger in nuclear hormone receptors (ZnF_C4) and ligand-binding domain of hormone receptors (HOLI). Zinc finger domains are relatively small protein motifs with a stable structure and are involved in a wide range of physiological functions including controlling embryonic development and cell differentiation [32,33]. HOLI is located in the LBD region of the nuclear receptor gene, which acts as a molecular switch to turn on transcriptional activity by binding to ligands [34]. In the phylogenetic tree, there is a clear boundary between crustaceans and insects, indicating that HR4 is more conserved among its class.
MnHR4 was expressed in multiple tissues of M. nipponense, with higher expression levels in the ovary, indicating that MnHR4 has different functions in M. nipponense. In insects, HR4 expression was also detected in multiple tissues [35]. The extremely high expression of HR4 in the ovaries is similar to other studies on Drosophila. Recent studies on Drosophila have shown that HR4 is strongly expressed in the ovaries and is required for Drosophila to oogenesis [36]. There was no significant difference in the MnHR4 in different ovarian development stages of M. nipponense. Therefore, we speculate that MnHR4 may be required to function in the whole ovarian development cycle. The expression of MnHR4 in the cleavage stage was significantly higher than that in other stages of embryonic development, suggesting it has a function during cell division. This result is similar to the expression trend of nuclear receptor gene MnFtz-f1 in M. nipponense [26]. Additionally, MnHR4 expression levels were the highest at L15 during the larval developmental stages, suggesting that MnHR4 may function during the metamorphosis of M. nipponense [37].
To explore the relationship between 20E and MnHR4, the expression level of MnHR4 was detected after 20E injection in M. nipponense, and the content of 20E in M. nipponense was measured after MnHR4 knockdown. The expression of MnHR4 significantly increased after 20E injection in vivo, which proves that 20E can induce the expression of MnHR4 in M. nipponense. King-jones et al. demonstrated that feeding 20E for 3–4 h induced the peak expression of HR4 in Drosophila [20]. Similarly, the injection of 20E upregulated HR4’s expression in B. germanica and L. decemlineata [38,39]. We further investigated the effect of the knockdown of MnHR4 on the 20E content. The results show that the knockdown of MnHR4 increased the 20E titer in M. nipponense. In L. decemlineata, 20E titers also increased after HR4 knockdown, which is consistent with our conclusions [39]. Conversely, 20E titers decreased after silencing HR4 in L. migratoria [35], whereas 20E titers were not affected by silencing HR4 in B. germanica [38]. In conclusion, HR4 may have different regulatory effects on 20E in different species, and its molecular mechanism needs to be further investigated.
RNAi is an effective method to explore the regulatory relationship between genes. We further investigated the molecular mechanism of MnHR4 in 20E synthesis and signaling by RNAi (Figure 9). Spook and Phantom are Halloween family member genes that function in the 20E biosynthetic pathway [5,40]. To investigate the molecular mechanism of MnHR4 on 20E synthesis in M. nipponense, the expressions of Spook and Phantom were detected after knockdown of MnHR4. The expressions of Spook and Phantom significantly increased after knockdown of MnHR4, indicating that MnHR4 has an inhibitory effect on Spook and Phantom. Similar studies have shown that HR4 inhibits ecdysone synthesis by regulating cytochrome P450 genes [41]. HR4 is a repressor of early ecdysone-induced regulatory genes [20]. There are many nuclear receptor genes in the 20E signaling pathway, whose main function is to transmit upstream signals [42]. To further investigate the role of MnHR4 in 20E signaling, the expression of other genes (i.e., HR3, E75b, ETHR and MnFtz-f1) was examined after knockdown of MnHR4. The results show that the expressions of HR3 and E75b significantly increased after silencing MnHR4, indicating that MnHR4 had an inhibitory effect on HR3 and E75b. In L. decemlineata, the expressions of HR3 and E75 were also affected by HR4, and the knockdown of HR4 significantly upregulated the expressions of HR3 and E75 [39]. In addition, the expressions of ETHR and MnFtz-f1 were decreased after knockdown of MnHR4, indicating that MnHR4 had a promoting effect on them. Previous studies have demonstrated that ETHR functions during the molting process of M. nipponense [43], but the regulatory relationship between HR4 and ETHR is rarely reported. Our results demonstrate that MnHR4 positively regulates ETHR, which provides a reference for future research. Ftz-f1 plays a central role in coordinating different molting processes [44]. Consistent with our conclusion, the abundant results demonstrate that HR4 can induce the expression of Ftz-f1 [20,38,41]. In conclusion, MnHR4 plays a regulatory role in 20E synthesis and signaling. We further investigated whether the molting of the M. nipponense occurred after the knockdown of MnHR4. It was proved that MnHR4 plays a pivotal role in the molting process of M. nipponense. The molting function of HR4 has been demonstrated in some insects. In B. Germanica, interference with HR4 resulted in molting failure and eventual death [38]. HR4 is required for pupal molting and adult oogenesis of Tribolium castaneum [19]. The knockdown of MnHR4 increased 20E titers, which significantly inhibited the molting of M. nipponense. Our previous study showed that knockdown of MnFtz-f1 reduced 20E titers, similarly, leading to the failure of M. nipponense’s molting [26]. In B. mori, increasing or decreasing 20E titers could affect the normal physiological phenomena of the larvae or even lead to death [45]. Precise regulation of 20E titers is also important for the molting process in D. melanogaster [1]. This suggests that an appropriate 20E titer is important for regulating successful molting in insects or crustaceans.
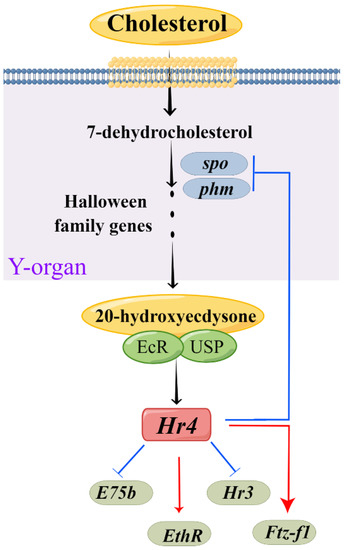
Figure 9.
Model for MnHR4 function, developed using Figdraw (www.figdraw.com). The Y-organ is a pair of secretory glands of crustaceans, the site of the synthesis of cholesterol into ecdysone.
In summary, the MnHR4 gene was identified in M. nipponense. The MnHR4 gene was comprehensively analyzed using bioinformatics, qRT-PCR, RNAi, ELISA, etc. Our results strongly demonstrate that MnHR4 functions in the molting process of M. nipponense by regulating 20E synthesis and 20E signaling. This study further enriched the molecular regulatory mechanism of molting in M. nipponense and could be useful for future gene-editing breeding.
4. Materials and Methods
4.1. Experimental Prawns and Conditions
Experimental prawns (2.15 ± 0.63 g) were obtained from the Dapu experimental base, Freshwater Fisheries Research Center, Chinese Academy of Fishery Sciences. Briefly, the experimental prawns were transferred from Dapu to the laboratory’s constant-temperature water-circulation system and allowed to acclimatize for a week. Tissues (i.e., ovaries, muscles, gills, hepatopancreas, eyestalks and hearts) were harvested, frozen in liquid nitrogen and stored at −80 ℃ for RNA extraction. Samples were also collected at different stages of embryo, individual and ovarian development according to previous criteria [27,28]. All sampling was performed in triplicate (n = 6). The prawns in this study were handled according to the guidelines of the Institutional Animal Care and Use Ethics Committee of the Freshwater Fisheries Research Center, Chinese Academy of Fishery Sciences (Wuxi, China).
4.2. Nucleotide Sequence and Bioinformatics Analysis of MnHR4
Total RNA was extracted using the RNAiso Plus kit (TaKaRa, Shiga, Japan), as described previously [26]. DNase I (Sangon, Shanghai, China) was used to eliminate possible DNA contamination, and 1.2% agarose gel and a NanoDrop ND2000 (NanoDrop Technologies, Wilmington, DE, USA) were used to detect RNA quality and concentration, respectively, with an A260/A280 ratio of 1.9–2.0.
The MnHR4 cDNA fragments were screened from the transcriptome of M. nipponense at different molting stages. The MnHR4 cDNA fragments were analyzed using the GenBank BLASTX and BLASTN programs (https://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 20 April 2022)). The open reading frame (ORF) of MnHR4 was predicted by using ORF Finder (https://www.ncbi.nlm.nih.gov/orffinder/ (accessed on 20 April 2022)). Molecular Evolutionary Genetics Analysis (MEGA-X) software was used to construct a phylogenetic tree, and the bootstrapping replications were 1000 [46,47]. DNAMAN 6.0 was used for translating and aligning amino acid sequences. The spatial structure and function of MnHR4 amino acids were predicted by I-TASSER (https://zhanglab.ccmb.med.umich.edu/I-TASSER/ (accessed on 22 April 2022)) [48]. The HR4 amino acid sequences of other species investigated in this study were downloaded from the GenBank database (http://www.ncbi.nlm.nih.gov/ (accessed on 20 April 2022)).
4.3. Quantitative Real-Time PCR (qRT-PCR) Analysis
Gene expression patterns were evaluated using qRT-PCR on a Bio-Rad iCycler iQ5 Real-Time PCR System (Bio-Rad, Carlsbad, CA, USA). The primers were designed using NCBI’s Primer-Blast tool (http://www.ncbi.nlm.nih.gov/tools/primer-blast/ (accessed on 25 April 2022)) and synthesized by exsyn-bio Technology Co., Ltd. (Shanghai, China). The reaction system and procedure of qRT-PCR have been described in previous studies [5]. The internal reference gene, eukaryotic translation initiation factor 5A (EIF), was used as a control for data normalization [49], and the relative expression levels of the genes were calculated with the 2−ΔΔCT method [50].
4.4. RNA Interference (RNAi)
The design and synthesis of MnHR4-interfering primers followed a method used in previous studies [26]. The green fluorescent protein gene (GFP) was used as a control [51]. A total of 180 healthy female prawns in the pre-molting stage were randomly divided into two groups (i.e., experimental group and control group) in triplicate (n = 30). The experimental group and control group were injected with MnHR4 dsRNA and dsGFP, respectively (8 µg/g of body weight). Prawn tissues were collected in triplicate at 24, 48, 96 and 120 h after injection for RNA extraction and an interference efficiency assessment (n = 6). On the basis of the significant interference efficiency of the experimental group and control group, 300 prawns in the pre-molting stage were divided into two groups using the same method and injected with MnHR4 dsRNA and dsGFP, respectively (n = 50). The number of molting prawns per day was counted, and the molting frequency was calculated. The number of molts was counted for 30 days, and dsRNA was injected every five days. Molting frequency = (Nm/Ns)/D, where Nm is the total number of molts; Ns is the number of prawns in the aquarium; and D is the number of experimental days [5,26].
4.5. ELISA
After knocking down the MnHR4 gene, the Shrimp EH ELISA Kit (lot number: m1963525-J; Meibo, Shanghai, China) was used to detect the content of 20E in M. nipponense, according to the manufacturer’s protocols. In brief, the tissue was first washed with pre-cooled PBS to remove the residual blood. Next, the tissue was weighed and cut into pieces, and it was added to the PBS at a ratio of 1:9. Then, it was put into a glass homogenizer and fully ground on ice to lyse the tissue’s cells. The tissue homogenate was centrifuged at 5000 rpm for 5–10 min, and the supernatant was removed until use. All reagents and samples were prepared before the experiment: (1) The standard wells and sample wells were set on the microtiter plate. Fifty microliters of different concentrations of standards (i.e., 2000, 1000, 500, 250, 125 and 62.5 pg/mL) were added to the standard wells, and 50 μL of the samples to be tested were added to the sample wells; blank wells were not added. (2) Then, 100 μL of enzyme ligands was added to the standard and sample wells, and the reaction wells were sealed with a closure plate membrane and incubated for 60 min at 37 ℃ in a water bath or incubator. (3) The microtiter plate was rinsed 4–5 times, and then, 50 μL of substrate A and B was added to each well. They were mixed gently and incubated at 37 °C for 15 min. (4) After adding 50 μL of stop solution to each well and tapping the plate to ensure adequate mixing, the OD values of each well were measured at a wavelength of 450 nm within 15 min. (5) Taking the OD value of the standard as the abscissa and the concentration value of the standard as the ordinate, the standard curve was drawn using Excel software, and the linear regression equation was obtained. The OD value of the sample was substituted into the equation to calculate the concentration of the sample.
4.6. 20E Treatments
A total of 120 pre-molting M. nipponense were divided into the experimental group and the control group, in triplicate (n = 20). M. nipponense in the experimental group was injected with 20E (Sigma-Aldrich, St. Louis, MO, USA; 5 μg/g), and the control group was injected with an equal volume of solvent (ethanol) [26]. Prawn tissues were collected at 3, 6, 12, 24 and 48 h after injection of 20E and stored at −80 °C after liquid nitrogen quick-freezing for mRNA extraction. The relative expression levels of the MnHR4 gene in the experimental and control groups were evaluated by qRT-PCR.
4.7. Data Analysis
Statistical analyses were performed using SPSS 20.0 software (IBM, New York, NY, USA). One-way ANOVA was used for comparisons between multiple sample means. The differences between the two groups were compared using the independent sample t-test. All data are presented as the mean ± SEM. The significance level for the data was set at p < 0.05.
Author Contributions
Investigation, methodology, conceptualization, and writing—original draft, H.Y.; formal analysis and data curation, W.Z., H.Q. and S.J. (Shubo Jin); resources and investigation, S.J. (Sufei Jiang), Y.X. and Y.G.; supervision, funding acquisition, and writing—review and editing, H.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by grants from the Central Public-interest Scientific Institution Basal Research Fund CAFS (2020TD36); the seed industry revitalization project of Jiangsu province (JBGS [2021] 118); Jiangsu Agricultural Industry Technology System; the earmarked fund for CARS-48; and the New cultivar breeding Major Project of Jiangsu province (PZCZ201745).
Acknowledgments
Our thanks goes to the Germplasm Bank of Jiangsu Provincial Science and Technology Resources (Agricultural Germplasm Resources) Coordination Service Platform for providing the Macrobrachium nipponense.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yamanaka, N.; Rewitz, K.F.; O’Connor, M.B. Ecdysone control of developmental transitions: Lessons from Drosophila research. Annu. Rev. Entomol. 2013, 58, 497–516. [Google Scholar] [CrossRef] [PubMed]
- Festucci-Buselli, R.A.; Contim, L.A.; Barbosa, L.C.A.; Stuart, J.; Otoni, W.C. Biosynthesis and potential functions of the ecdysteroid 20-hydroxyecdysone—A review. Botany 2008, 86, 978–987. [Google Scholar] [CrossRef]
- Petryk, A.; Warren, J.T.; Marqués, G.; Jarcho, M.P.; Gilbert, L.I.; Kahler, J.; Parvy, J.P.; Li, Y.; Dauphin-Villemant, C.; O’Connor, M.B. Shade is the Drosophila P450 enzyme that mediates the hydroxylation of ecdysone to the steroid insect molting hormone 20-hydroxyecdysone. Proc. Natl. Acad. Sci. USA 2003, 100, 13773–13778. [Google Scholar] [CrossRef] [PubMed]
- Rewitz, K.; Rybczynski, R.; Warren, J.T.; Gilbert, L.I. The Halloween genes code for cytochrome P450 enzymes mediating synthesis of the insect moulting hormone. Biochem. Soc. Trans. 2006, 34, 1256–1260. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Qiao, H.; Fu, Y.; Fu, H.; Zhang, W.; Jin, S.; Gong, Y.; Jiang, S.; Xiong, Y.; Hu, Y. RNA interference shows that Spook, the precursor gene of 20-hydroxyecdysone (20E), regulates the molting of Macrobrachium nipponense. J. Steroid Biochem. Mol. Biol. 2021, 213, 105976. [Google Scholar] [CrossRef] [PubMed]
- Yao, T.P.; Forman, B.M.; Jiang, Z.; Cherbas, L.; Chen, J.; McKeown, M.; Cherbas, P.; Evans, R.M. Functional ecdysone receptor is the product of EcR and Ultraspiracle genes. Nature 1993, 366, 476–479. [Google Scholar] [CrossRef]
- Koelle, M.R.; Talbot, W.S.; Segraves, W.A.; Bender, M.T.; Cherbas, P.; Hogness, D.S. The Drosophila EcR gene encodes an ecdysone receptor, a new member of the steroid receptor superfamily. Cell 1991, 67, 59–77. [Google Scholar] [CrossRef]
- Riddiford, L.M. Hormones and Drosophila development. Dev. Drosoph. Melanogaster 1993, 2, 899–939. [Google Scholar]
- Ou, Q.; King-Jones, K. What goes up must come down: Transcription factors have their say in making ecdysone pulses. Curr. Top. Dev. Biol. 2013, 103, 35–71. [Google Scholar]
- Bain, D.L.; Heneghan, A.F.; Connaghan-Jones, K.D.; Miura, M.T. Nuclear receptor structure: Implications for function. Annu. Rev. Physiol. 2007, 69, 201–220. [Google Scholar] [CrossRef]
- King-Jones, K.; Thummel, C.S. Nuclear receptors—A perspective from Drosophila. Nat. Rev. Genet. 2005, 6, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Broadus, J.; McCabe, J.R.; Endrizzi, B.; Thummel, C.S.; Woodard, C.T. The Drosophila βFTZ-F1 orphan nuclear receptor provides competence for stage-specific responses to the steroid hormone ecdysone. Mol. Cell 1999, 3, 143–149. [Google Scholar] [CrossRef]
- White, K.P.; Hurban, P.; Watanabe, T.; Hogness, D.S. Coordination of Drosophila metamorphosis by two ecdysone-induced nuclear receptors. Science 1997, 276, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Mané-Padrós, D.; Cruz, J.; Vilaplana, L.; Pascual, N.; Bellés, X.; Martín, D. The nuclear hormone receptor BgE75 links molting and developmental progression in the direct-developing insect Blattella germanica. Dev. Biol. 2008, 315, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Bialecki, M.; Shilton, A.; Fichtenberg, C.; Segraves, W.A.; Thummel, C.S. Loss of the ecdysteroid-inducible E75A orphan nuclear receptor uncouples molting from metamorphosis in Drosophila. Dev. Cell 2002, 3, 209–220. [Google Scholar] [CrossRef]
- Zhao, X.; Qin, Z.; Liu, W.; Liu, X.; Moussian, B.; Ma, E.; Li, S.; Zhang, J. Nuclear receptor HR3 controls locust molt by regulating chitin synthesis and degradation genes of Locusta migratoria. Insect Biochem. Mol. Biol. 2018, 92, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cruz, J.; Nieva, C.; Mané-Padrós, D.; Martín, D.; Bellés, X. Nuclear receptor BgFTZ-F1 regulates molting and the timing of ecdysteroid production during nymphal development in the hemimetabolous insect Blattella germanica. Dev. Dyn. 2008, 237, 3179–3191. [Google Scholar] [CrossRef]
- Vickers, N.J. Animal communication: When I’m calling you, will you answer too? Curr. Biol. 2017, 27, R713–R715. [Google Scholar] [CrossRef]
- Tan, A.; Palli, S.R. Identification and characterization of nuclear receptors from the red flour beetle, Tribolium castaneum. Insect Biochem. Mol. Biol. 2008, 38, 430–439. [Google Scholar] [CrossRef]
- King-Jones, K.; Charles, J.P.; Lam, G.; Thummel, C.S. The ecdysone-induced DHR4 orphan nuclear receptor coordinates growth and maturation in Drosophila. Cell 2005, 121, 773–784. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, T.; Wang, C.; Chan, S.; Wang, W. Deciphering the molecular regulatory mechanism orchestrating ovary development of the Pacific whiteleg shrimp Litopenaeus vannamei through integrated transcriptomic analysis of reproduction-related organs. Aquaculture 2021, 533, 736160. [Google Scholar] [CrossRef]
- Miyakawa, H.; Sato, T.; Song, Y.; Tollefsen, K.E.; Iguchi, T. Ecdysteroid and juvenile hormone biosynthesis, receptors and their signaling in the freshwater microcrustacean Daphnia. J. Steroid Biochem. Mol. Biol. 2018, 184, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Legrand, E.; Bachvaroff, T.; Schock, T.B.; Chung, J.S. Understanding molt control switches: Transcriptomic and expression analysis of the genes involved in ecdysteroidogenesis and cholesterol uptake pathways in the Y-organ of the blue crab, Callinectes sapidus. PLoS ONE 2021, 16, e0256735. [Google Scholar] [CrossRef] [PubMed]
- Hongtuo, F.; Sufei, J.; Yiwei, X. Current status and prospects of farming the giant river prawn (Macrobrachium rosenbergii) and the oriental river prawn (Macrobrachium nipponense) in China. Aquac. Res. 2012, 43, 993–998. [Google Scholar] [CrossRef]
- Chang, E.S.; Mykles, D.L. Regulation of crustacean molting: A review and our perspectives. Gen. Comp. Endocrinol. 2011, 172, 323–330. [Google Scholar] [CrossRef]
- Yuan, H.; Zhang, W.; Fu, Y.; Jiang, S.; Xiong, Y.; Zhai, S.; Gong, Y.; Qiao, H.; Fu, H.; Wu, Y. MnFtz-f1 Is Required for Molting and Ovulation of the Oriental River Prawn Macrobrachium nipponense. Front. Endocrinol. 2021, 12, 798577. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Z.; Chen, H.; Zhu, X.; Cui, Z.; Qiu, G. The morphological and histological observation of embryonic development in the oriental river prawn Macrobrachium nipponense. J. Shanghai Ocean Univ. 2012, 21, 33–40. [Google Scholar]
- Qiao, H.; Fu, H.; Xiong, Y.; Jiang, S.; Sun, S.; Jin, S.; Gong, Y.; Wang, Y.; Shan, D.; Li, F. Molecular insights into reproduction regulation of female Oriental River prawns Macrobrachium nipponense through comparative transcriptomic analysis. Sci. Rep. 2017, 7, 12161. [Google Scholar] [CrossRef]
- Kastner, P.; Mark, M.; Chambon, P. Nonsteroid nuclear receptors: What are genetic studies telling us about their role in real life? Cell 1995, 83, 859–869. [Google Scholar] [CrossRef]
- Thummel, C.S. From embryogenesis to metamorphosis: The regulation and function of Drosophila nuclear receptor superfamily members. Cell 1995, 83, 871–877. [Google Scholar] [CrossRef]
- Iyer, A.K.; McCabe, E.R. Molecular mechanisms of DAX1 action. Mol. Genet. Metab. 2004, 83, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Gronemeyer, H.; Laudet, V. Transcription factors 3: Nuclear receptors. Protein Profile 1995, 2, 1173–1308. [Google Scholar] [PubMed]
- Schwabe, J.W.; Rhodes, D. Beyond zinc fingers: Steroid hormone receptors have a novel structural motif for DNA recognition. Trends Biochem. Sci. 1991, 16, 291–296. [Google Scholar] [CrossRef]
- Edwards, D.P. The role of coactivators and corepressors in the biology and mechanism of action of steroid hormone receptors. J. Mammary Gland Biol. Neoplasia 2000, 5, 307–324. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, J.; Sun, Y.; Liang, X.; Zhang, R.; Zhao, X.; Zhang, M.; Zhang, J. A nuclear receptor HR4 is essential for the formation of epidermal cuticle in the migratory locust, Locusta migratoria. Insect Biochem. Mol. Biol. 2022, 143, 103740. [Google Scholar] [CrossRef] [PubMed]
- Weaver, L.N.; Drummond-Barbosa, D. Hormone receptor 4 is required in muscles and distinct ovarian cell types to regulate specific steps of Drosophila oogenesis. Development 2021, 148, dev198663. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Qiao, H.; Zhang, W.; Sun, S.; Jiang, S.; Gong, Y.; Xiong, Y.; Jin, S.; Fu, H. Molecular cloning and expression analysis of two sex-lethal homolog genes during development in the oriental river prawn, Macrobrachium nipponense. Genet. Mol. Res. 2013, 12, 4698–4711. [Google Scholar] [CrossRef]
- Mané-Padrós, D.; Borràs-Castells, F.; Belles, X.; Martín, D. Nuclear receptor HR4 plays an essential role in the ecdysteroid-triggered gene cascade in the development of the hemimetabolous insect Blattella germanica. Mol. Cell. Endocrinol. 2012, 348, 322–330. [Google Scholar] [CrossRef]
- Xu, Q.Y.; Meng, Q.W.; Deng, P.; Guo, W.C.; Li, G.Q. Leptinotarsa hormone receptor 4 (HR4) tunes ecdysteroidogenesis and mediates 20-hydroxyecdysone signaling during larval-pupal metamorphosis. Insect Biochem. Mol. Biol. 2018, 94, 50–60. [Google Scholar] [CrossRef]
- Niwa, R.; Matsuda, T.; Yoshiyama, T.; Namiki, T.; Mita, K.; Fujimoto, Y.; Kataoka, H. CYP306A1, a cytochrome P450 enzyme, is essential for ecdysteroid biosynthesis in the prothoracic glands of Bombyx and Drosophila. J. Biol. Chem. 2004, 279, 35942–35949. [Google Scholar] [CrossRef]
- Ou, Q.; Magico, A.; King-Jones, K. Nuclear receptor DHR4 controls the timing of steroid hormone pulses during Drosophila development. PLoS Biol. 2011, 9, e1001160. [Google Scholar] [CrossRef] [PubMed]
- Fahrbach, S.E.; Smagghe, G.; Velarde, R.A. Insect nuclear receptors. Annu. Rev. Entomol. 2012, 57, 83–106. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.X.; Fu, H.T.; Qiao, H.; Sun, S.M.; Zhang, W.Y.; Jin, S.B.; Gong, Y.S.; Jiang, S.F.; Xiong, Y.W.; Wu, Y. Molecular Cloning and Characterization of a Putative Ecdysis-triggering Hormone Receptor (ETHR) Gene from Macrobrachium nipponense. J. World Aquacult. Soc. 2018, 49, 1081–1094. [Google Scholar] [CrossRef]
- Pick, L.; Anderson, W.R.; Shultz, J.; Woodard, C.T. The Ftz-F1 family: Orphan nuclear receptors regulated by novel protein–protein interactions. Adv. Dev. Biol. 2006, 16, 255–296. [Google Scholar]
- You, L.; Li, Z.; Zhang, Z.; Hu, B.; Yu, Y.; Yang, F.; Tan, A. Two dehydroecdysone reductases act as fat body-specific 20E catalyzers in Bombyx mori. Insect Sci. 2022, 29, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Bairoch, A.; Bucher, P.; Hofmann, K. The PROSITE database, its status in 1997. Nucleic Acids Res. 1997, 25, 217–221. [Google Scholar] [CrossRef]
- Combet, C.; Blanchet, C.; Geourjon, C.; Deleage, G. NPS@: Network protein sequence analysis. Trends Biochem. Sci. 2000, 25, 147–150. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Y. I-TASSER server: New development for protein structure and function predictions. Nucleic Acids Res. 2015, 43, W174–W181. [Google Scholar] [CrossRef]
- Hu, Y.; Fu, H.; Qiao, H.; Sun, S.; Zhang, W.; Jin, S.; Jiang, S.; Gong, Y.; Xiong, Y.; Wu, Y. Validation and evaluation of reference genes for quantitative real-time PCR in Macrobrachium nipponense. Int. J. Mol. Sci. 2018, 19, 2258. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Qian, Z.; He, S.; Liu, T.; Liu, Y.; Hou, F.; Liu, Q.; Wang, X.; Mi, X.; Wang, P.; Liu, X. Identification of ecdysteroid signaling late-response genes from different tissues of the Pacific white shrimp, Litopenaeus vannamei. Comp. Biochem. Physiol. A-Mol. Integr. Physiol. 2014, 172, 10–30. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).