Antimicrobial and Photoantimicrobial Activities of Chitosan/CNPPV Nanocomposites
Abstract
1. Introduction
2. Results and Discussion
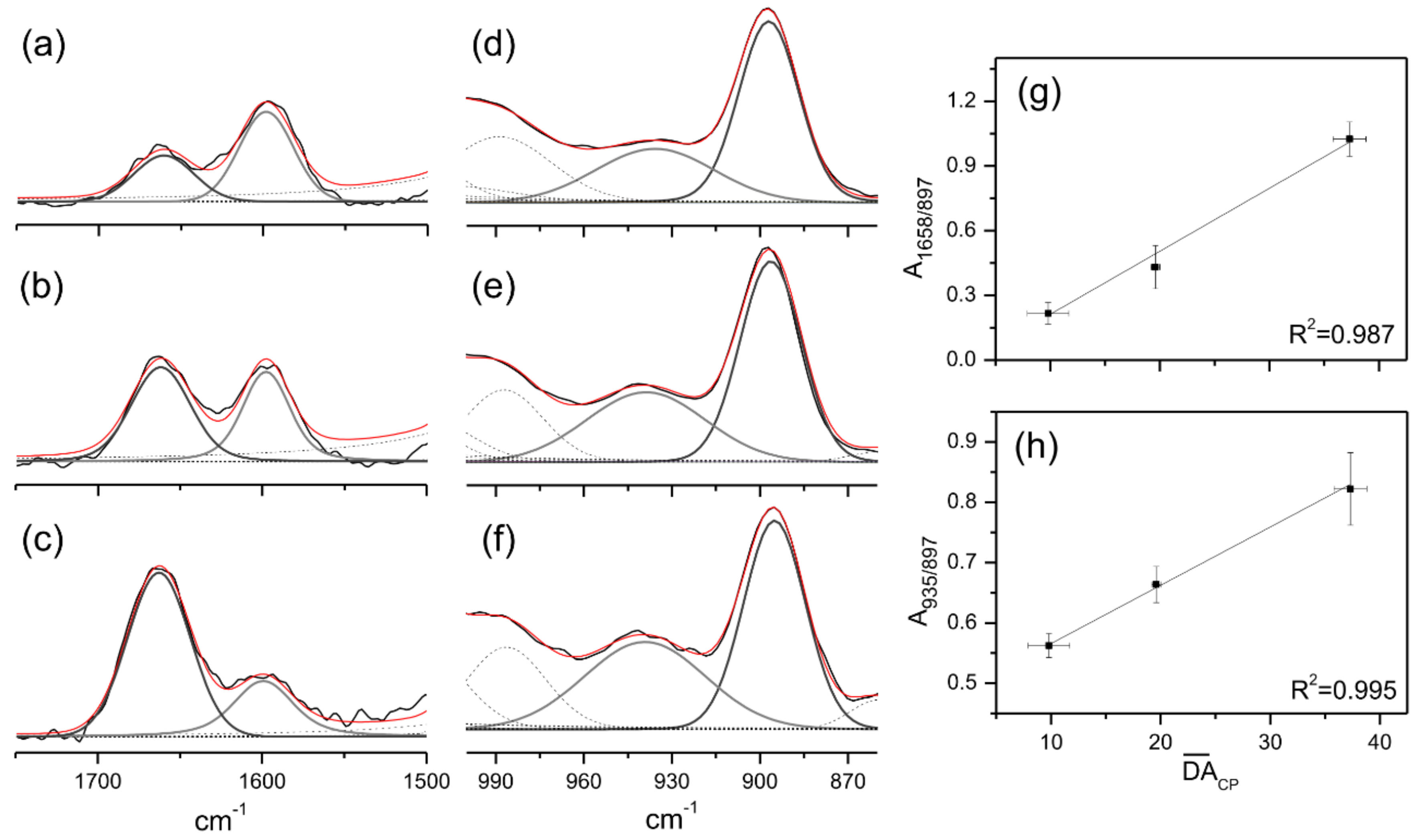
2.1. Spectroscopy Chacterization of Chitosans
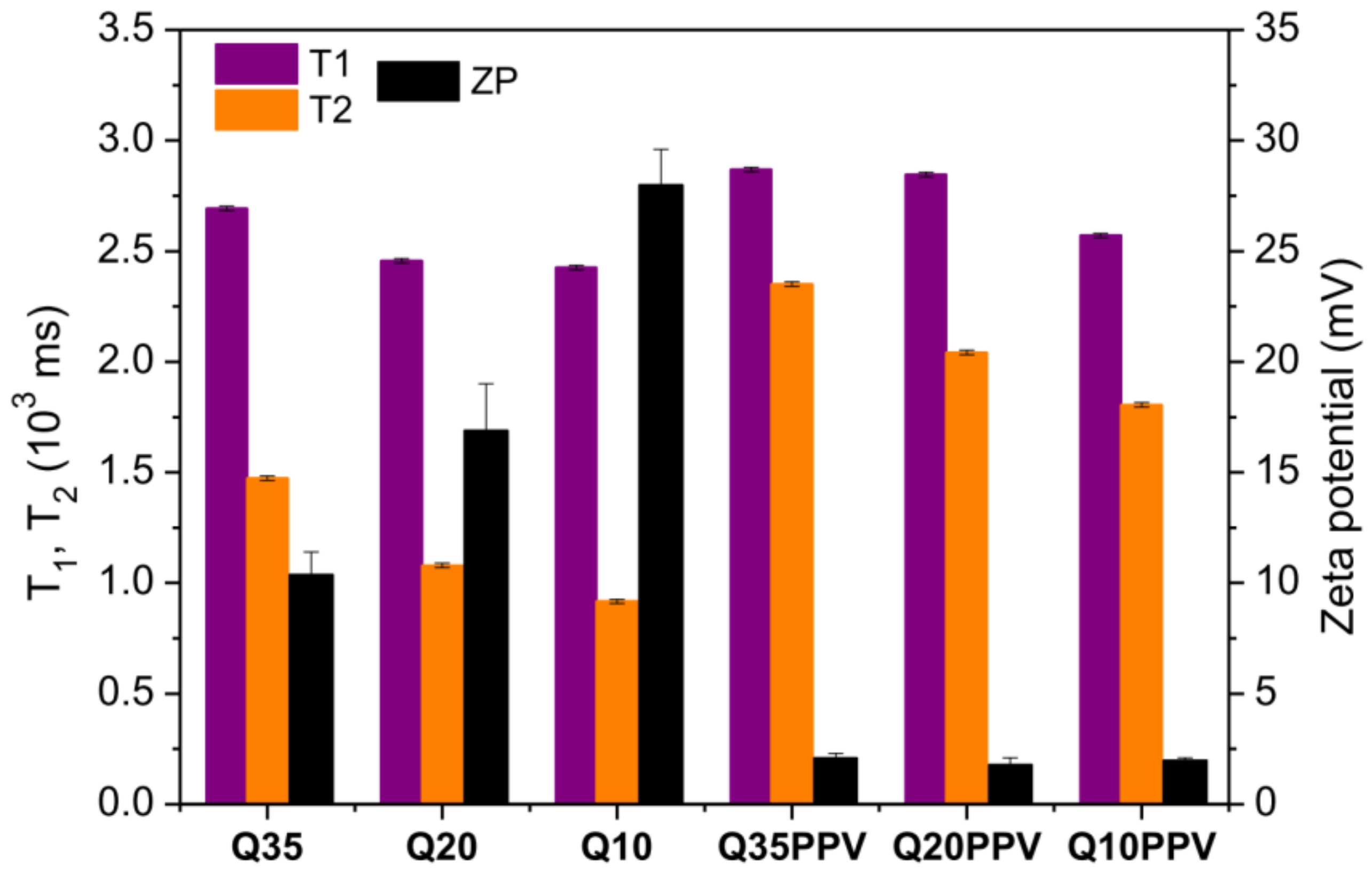
2.2. Physicochemical Characterization of Nanomaterials
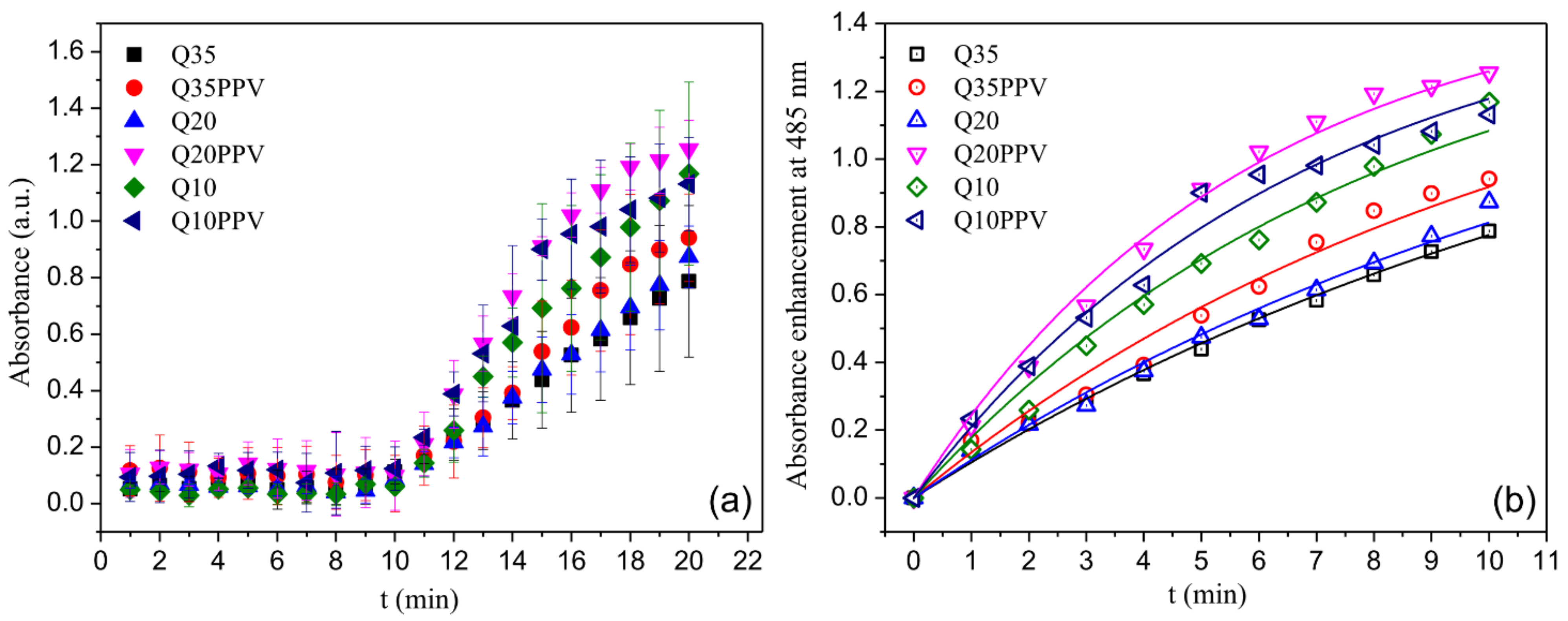
2.3. ROS Analysis
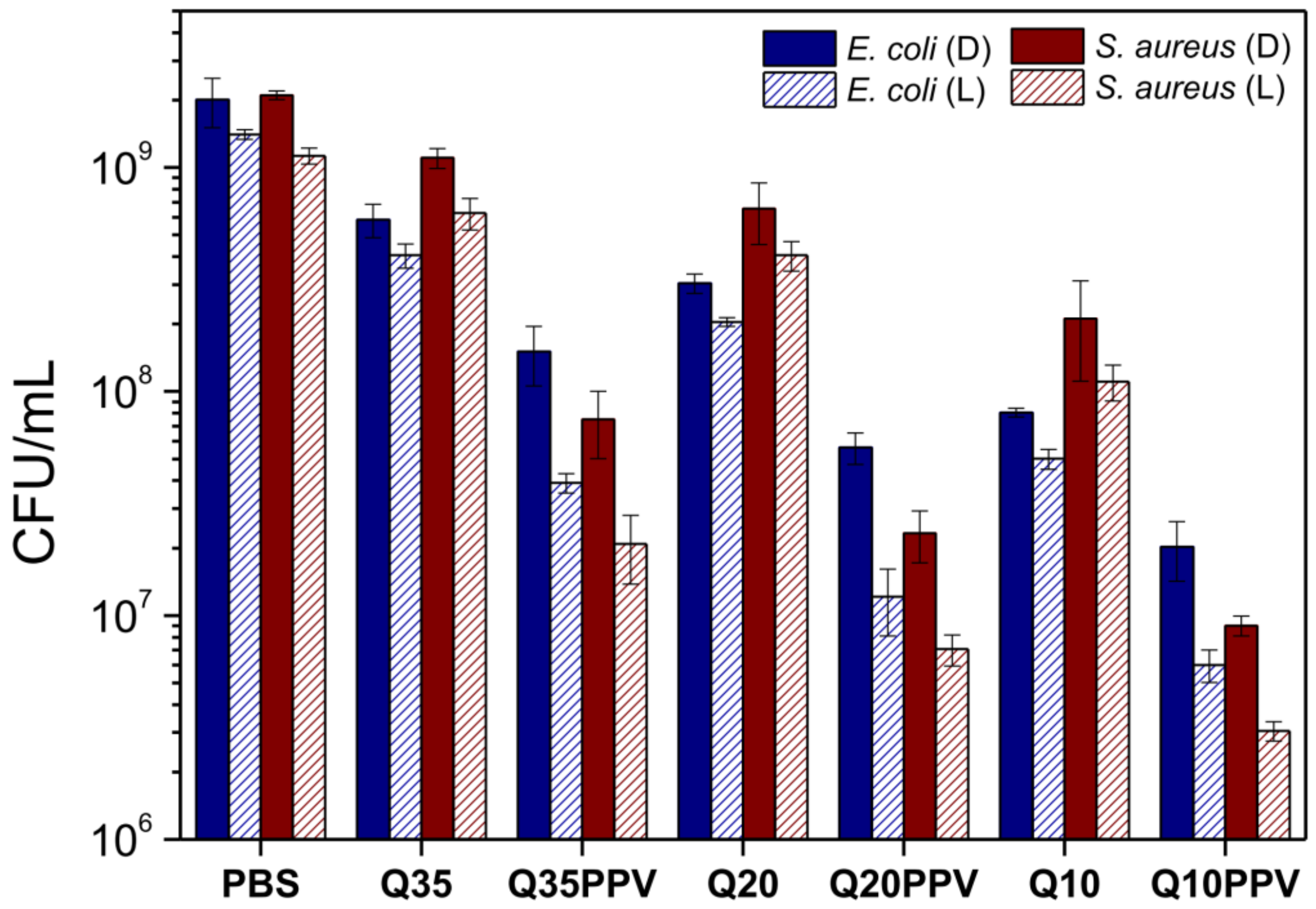
2.4. Bactericidal and Photobactericidal Activity
3. Materials and Methods
3.1. Materials
3.2. Preparation of Chitosan-Based Nanomaterials
3.3. Characterization of Chitosans
Fourier Transform Raman Spectroscopy
3.4. Characterization of Nanomaterials
3.4.1. Dynamic Light Scattering and Electrophoretic Behavior (DLS and ELS)
3.4.2. Transmission Electron Microscopy (TEM)
3.4.3. Time-Domain Nuclear Magnetic Resonance (TD-NMR)
3.5. Bactericidal and Photobactericidal Assays
3.6. Reactive Oxygen Species Experiments (ROS)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cole, L.A. Biology Life: Biochemistry, Physiology and Philosophy, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- O’neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations, A Review on Antimicobial Resistance; Wellcome Trust: London, UK, 2016; p. 80. Available online: https://amr-review.org/sites/default/files/160525_Final%20paper_with%20cover.pdf (accessed on 31 March 2022).
- World Health Organization. Global Action Plan on Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2017; pp. 1–28. [Google Scholar]
- Fair, R.J.; Tor, Y. Antibiotics and Bacterial Resistance in the 21st Century. Perspect. Medicin. Chem. 2014, 6, 25–64. [Google Scholar] [CrossRef] [PubMed]
- O’neill, J. Antimicrobial Resistance, Tackling a Crisis for the Health and Wealth of Nations, The Review on Antimicrobial Resistance; Wellcome Trust: London, UK, 2014; p. 20. Available online: https://amr-review.org/sites/default/files/AMR%20Review%20Paper%20-%20Tackling%20a%20crisis%20for%20the%20health%20and%20wealth%20of%20nations_1.pdf (accessed on 31 March 2022).
- Kashef, N.; Hamblin, M.R. Can Microbial Cells Develop Resistance to Oxidative Stress in Antimicrobial Photodynamic Inactivation? Drug Resist. Updat. 2017, 31, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Coradi Tonon, C.; Ashraf, S.; Hasan, T. Photodynamic and Antibiotic Therapy in Combination against Bacterial Infections: Efficacy, Determinants, Mechanisms, and Future Perspectives. Adv. Drug Deliv. Rev. 2021, 177, 113941. [Google Scholar] [CrossRef] [PubMed]
- Caires, C.S.A.; Leal, C.R.B.; Rodrigues, A.C.S.; Lima, A.R.; Silva, C.M.; Ramos, C.A.N.; Chang, M.R.; Arruda, E.J.; Oliveira, S.L.; Nascimento, V.A.; et al. Photoinactivation of Mcr-1 Positive Escherichia Coli. Laser Phys. Lett. 2018, 15, 015601. [Google Scholar] [CrossRef]
- Caires, C.S.A.; Silva, C.M.; Lima, A.R.; Alves, L.M.; Lima, T.H.N.; Rodrigues, A.C.S.; Chang, M.R.; Oliveira, S.L.; Whitby, C.; Nascimento, V.A.; et al. Photodynamic Inactivation of Methicillin-Resistant Staphylococcus Aureus by a Natural Food Colorant (E-141ii). Molecules 2020, 25, 4464. [Google Scholar] [CrossRef]
- Sarker, M.A.R.; Ahn, Y.H. Photodynamic Inactivation of Multidrug-Resistant Bacteria in Wastewater Effluent Using Green Phytochemicals as a Natural Photosensitizer. Environ. Pollut. 2022, 311, 120015. [Google Scholar] [CrossRef]
- Pucelik, B.; Dąbrowski, J.M. Photodynamic Inactivation (PDI) as a Promising Alternative to Current Pharmaceuticals for the Treatment of Resistant Microorganisms. Adv. Inorg. Chem. 2022, 79, 65–103. [Google Scholar] [CrossRef]
- O’Riordan, K.; Akilov, O.E.; Hasan, T. The Potential for Photodynamic Therapy in the Treatment of Localized Infections. Photodiagn. Photodyn. Ther. 2005, 2, 247–262. [Google Scholar] [CrossRef]
- Dosselli, R.; Millioni, R.; Puricelli, L.; Tessari, P.; Arrigoni, G.; Franchin, C.; Segalla, A.; Teardo, E.; Reddi, E. Molecular Targets of Antimicrobial Photodynamic Therapy Identified by a Proteomic Approach. J. Proteom. 2012, 77, 329–343. [Google Scholar] [CrossRef]
- Iluz, N.; Maor, Y.; Keller, N.; Malik, Z. The Synergistic Effect of PDT and Oxacillin on Clinical Isolates of Staphylococcus Aureus. Lasers Surg. Med. 2018, 50, 535–551. [Google Scholar] [CrossRef]
- Al-Mutairi, R.; Tovmasyan, A.; Batinic-Haberle, I.; Benov, L. Sublethal Photodynamic Treatment Does Not Lead to Development of Resistance. Front. Microbiol. 2018, 9, 1699. [Google Scholar] [CrossRef]
- Tim, M. Strategies to Optimize Photosensitizers for Photodynamic Inactivation of Bacteria. J. Photochem. Photobiol. B Biol. 2015, 150, 2–10. [Google Scholar] [CrossRef]
- Chen, J.; Fan, T.; Xie, Z.; Zeng, Q.; Xue, P.; Zheng, T.; Chen, Y.; Luo, X.; Zhang, H. Advances in Nanomaterials for Photodynamic Therapy Applications: Status and Challenges. Biomaterials 2020, 237, 119827. [Google Scholar] [CrossRef]
- Tada, D.B.; Baptista, M.S. Photosensitizing Nanoparticles and the Modulation of ROS Generation. Front. Chem. 2015, 3, 33. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, H. New Generation of PhotosensitizersPhotosensitizers Based on Inorganic Nanomaterials. In Photodynamic Therapy: Methods and Protocols; Broekgaarden, M., Zhang, H., Korbelik, M., Hamblin, M.R., Heger, M., Eds.; Springer: New York, NY, USA, 2022; pp. 213–244. ISBN 978-1-0716-2099-1. [Google Scholar]
- Liu, L.; Wang, X.; Ban, D.; Zhu, S.; Li, L. Controllable Accumulation of Conjugated Polymer Nanoparticles on the Surface of Adhesive Bacteria. Colloids Surf. A Physicochem. Eng. Asp. 2020, 591, 124569. [Google Scholar] [CrossRef]
- Zhang, H.; Liang, Y.; Zhao, H.; Qi, R.; Chen, Z.; Yuan, H.; Liang, H.; Wang, L. Dual-Mode Antibacterial Conjugated Polymer Nanoparticles for Photothermal and Photodynamic Therapy. Macromol. Biosci. 2020, 20, 1900301. [Google Scholar] [CrossRef]
- Chong, H.; Nie, C.; Zhu, C.; Yang, Q.; Liu, L.; Lv, F.; Wang, S. Conjugated Polymer Nanoparticles for Light-Activated Anticancer and Antibacterial Activity with Imaging Capability. Langmuir 2012, 28, 2091–2098. [Google Scholar] [CrossRef]
- Ren, R.; Leng, C.; Zhang, S. A Chronocoulometric DNA Sensor Based on Screen-Printed Electrode Doped with Ionic Liquid and Polyaniline Nanotubes. Biosens. Bioelectron. 2010, 25, 2089–2094. [Google Scholar] [CrossRef]
- Abelha, T.F.; Dreiss, C.A.; Green, M.A.; Dailey, L.A.; Abelha, T.F. Conjugated Polymers as Nanoparticle Probes for Fluorescence and Photoacoustic Imaging. J. Mater. Chem. B 2020, 8, 592–606. [Google Scholar] [CrossRef]
- Liu, L.; Wang, X.; Zhu, S.; Yao, C.; Ban, D.; Liu, R.; Li, L.; Wang, S. Controllable Targeted Accumulation of Fluorescent Conjugated Polymers on Bacteria Mediated by a Saccharide Bridge. Chem. Mater. 2020, 32, 438–447. [Google Scholar] [CrossRef]
- Zhou, S.; Wang, Z.; Wang, Y.; Feng, L. Near-Infrared Light-Triggered Synergistic Phototherapy for Antimicrobial Therapy. ACS Appl. Bio Mater. 2020, 3, 1730–1737. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Guo, L.; Wang, Y.; Feng, L. Bactericidal Activity-Tunable Conjugated Polymers as a Human-Friendly Bactericide for the Treatment of Wound Infections. Biomater. Sci. 2019, 7, 3788–3794. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.; He, R.; Liu, Q.; Dong, Y.; Lin, M.; Li, W.; Xu, F. Theranostics of Triple-Negative Breast Cancer Based on Conjugated Polymer Nanoparticles. ACS Appl. Mater. Interfaces 2018, 10, 10634–10646. [Google Scholar] [CrossRef] [PubMed]
- Cruz, R.A.; Catunda, T.; Facchinatto, W.M.; Balogh, D.T.; Faria, R.M. Absolute Photoluminescence Quantum Efficiency of P3HT/CHCl3 Solution by Thermal Lens Spectrometry. Synth. Met. 2013, 163, 38–41. [Google Scholar] [CrossRef]
- Facchinatto, W.M.; Torres, B.B.M.; Balogh, D.T. One-Pot Synthesis of Poly-(3-Hexylthiophene) with Variable Degrees of Molar Mass and Regioregularity. J. Polym. Res. 2016, 23, 187. [Google Scholar] [CrossRef]
- Fedatto Abelha, T.; Rodrigues Lima Caires, A. Light-Activated Conjugated Polymers for Antibacterial Photodynamic and Photothermal Therapy. Adv. NanoBiomed Res. 2021, 1, 2100012. [Google Scholar] [CrossRef]
- Huang, Y.; Pappas, H.C.; Zhang, L.; Wang, S.; Cai, R.; Tan, W.; Wang, S.; Whitten, D.G.; Schanze, K.S. Selective Imaging and Inactivation of Bacteria over Mammalian Cells by Imidazolium-Substituted Polythiophene. Chem. Mater. 2017, 29, 6389–6395. [Google Scholar] [CrossRef]
- Zhu, C.; Yang, Q.; Liu, L.; Lv, F.; Li, S.; Yang, G.; Wang, S. Multifunctional Cationic Poly(p-Phenylene Vinylene) Polyelectrolytes for Selective Recognition, Imaging, and Killing of Bacteria over Mammalian Cells. Adv. Mater. 2011, 23, 4805–4810. [Google Scholar] [CrossRef]
- Yuan, H.; Liu, Z.; Liu, L.; Lv, F.; Wang, Y.; Wang, S. Cationic Conjugated Polymers for Discrimination of Microbial Pathogens. Adv. Mater. 2014, 26, 4333–4338. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Shafi, M.E.; Zabermawi, N.M.; Arif, M.; Batiha, G.E.; Khafaga, A.F.; Abd El-Hakim, Y.M.; Al-Sagheer, A.A. Antimicrobial and Antioxidant Properties of Chitosan and Its Derivatives and Their Applications: A Review. Int. J. Biol. Macromol. 2020, 164, 2726–2744. [Google Scholar] [CrossRef]
- Goy, R.C.; De Britto, D.; Assis, O.B.G. A Review of the Antimicrobial Activity of Chitosan. Polímeros Ciência Tecnol. 2009, 19, 241–247. [Google Scholar] [CrossRef]
- Silva, D.S.; Facchinatto, W.M.; dos Santos, D.M.; Boni, F.I.; Valdes, T.A.; Leitão, A.; Gremião, M.P.D.; Colnago, L.A.; Campana-Filho, S.P.; Ribeiro, S.J.L. N-(2-Hydroxy)-Propyl-3-Trimethylammonium, O-Palmitoyl Chitosan: Synthesis, Physicochemical and Biological Properties. Int. J. Biol. Macromol. 2021, 178, 558–568. [Google Scholar] [CrossRef]
- Facchinatto, W.M.; Galante, J.; Mesquita, L.; Silva, D.S.; Martins dos Santos, D.; Moraes, T.B.; Campana-Filho, S.P.; Colnago, L.A.; Sarmento, B.; das Neves, J. Clotrimazole-Loaded N-(2-Hydroxy)-Propyl-3-Trimethylammonium, O-Palmitoyl Chitosan Nanoparticles for Topical Treatment of Vulvovaginal Candidiasis. Acta Biomater. 2021, 125, 312–321. [Google Scholar] [CrossRef]
- Cavalcante, L.L.R.; Tedesco, A.C.; Takahashi, L.A.U.; Curylofo-Zotti, F.A.; Souza-Gabriel, A.E.; Corona, S.A.M. Conjugate of Chitosan Nanoparticles with Chloroaluminium Phthalocyanine: Synthesis, Characterization and Photoinactivation of Streptococcus Mutans Biofilm. Photodiagn. Photodyn. Ther. 2020, 30, 101709. [Google Scholar] [CrossRef]
- Darabpour, E.; Kashef, N.; Mashayekhan, S. Chitosan Nanoparticles Enhance the Efficiency of Methylene Blue-Mediated Antimicrobial Photodynamic Inactivation of Bacterial Biofilms: An in Vitro Study. Photodiagn. Photodyn. Ther. 2016, 14, 211–217. [Google Scholar] [CrossRef]
- Chen, C.P.; Chen, C.T.; Tsai, T. Chitosan Nanoparticles for Antimicrobial Photodynamic Inactivation: Characterization and in Vitro Investigation. Photochem. Photobiol. 2012, 88, 570–576. [Google Scholar] [CrossRef]
- Kim, E.J.; Choi, J.H.; Yang, H.J.; Choi, S.S.; Lee, H.K.; Cho, Y.C.; Kim, H.K.; Kim, S.W.; Chae, H.S. Comparison of High and Low Molecular Weight Chitosan as In-Vitro Boosting Agent for Photodynamic Therapy against Helicobacter Pylori Using Methylene Blue and Endoscopic Light. Photodiagn. Photodyn. Ther. 2019, 26, 111–115. [Google Scholar] [CrossRef]
- Zheng, L.Y.; Zhu, J.F. Study on Antimicrobial Activity of Chitosan with Different Molecular Weights. Carbohydr. Polym. 2003, 54, 527–530. [Google Scholar] [CrossRef]
- Arjunan, N.; Singaravelu, C.M.; Kulanthaivel, J.; Kandasamy, J. A Potential Photocatalytic, Antimicrobial and Anticancer Activity of Chitosan-Copper Nanocomposite. Int. J. Biol. Macromol. 2017, 104, 1774–1782. [Google Scholar] [CrossRef]
- Karthikeyan, K.T.; Nithya, A.; Jothivenkatachalam, K. Photocatalytic and Antimicrobial Activities of Chitosan-TiO2 Nanocomposite. Int. J. Biol. Macromol. 2017, 104, 1762–1773. [Google Scholar] [CrossRef]
- Wang, H.; Yu, X.; Su, C.; Shi, Y.; Zhao, L. Chitosan Nanoparticles Triggered the Induction of ROS-Mediated Cytoprotective Autophagy in Cancer Cells. Artif. Cells Nanomed. Biotechnol. 2018, 46, 293–301. [Google Scholar] [CrossRef]
- Jiang, Y.; Yu, X.; Su, C.; Zhao, L.; Shi, Y. Chitosan Nanoparticles Induced the Antitumor Effect in Hepatocellular Carcinoma Cells by Regulating ROS-Mediated Mitochondrial Damage and Endoplasmic Reticulum Stress. Artif. Cells Nanomed. Biotechnol. 2019, 47, 747–756. [Google Scholar] [CrossRef]
- Sarangapani, S.; Patil, A.; Ngeow, Y.K.; Elsa Mohan, R.; Asundi, A.; Lang, M.J. Chitosan Nanoparticles’ Functionality as Redox Active Drugs through Cytotoxicity, Radical Scavenging and Cellular Behaviour. Integr. Biol. 2018, 10, 313–324. [Google Scholar] [CrossRef]
- Correia, H.M.G.; Ramos, M.M.D. Modeling Charge Transport Properties of Cyano-Substituted PPV. Mater. Sci. Eng. C 2003, 23, 773–777. [Google Scholar] [CrossRef][Green Version]
- Facchinatto, W.M.; Fiamingo, A.; dos Santos, D.M.; Campana-Filho, S.P. Characterization and Physical-Chemistry of Methoxypoly(Ethylene Glycol)-g-Chitosan. Int. J. Biol. Macromol. 2019, 124, 828–837. [Google Scholar] [CrossRef]
- Facchinatto, W.M.; Dos Santos, D.M.; Fiamingo, A.; Bernardes-Filho, R.; Campana-Filho, S.P.; de Azevedo, E.R.; Colnago, L.A. Evaluation of Chitosan Crystallinity: A High-Resolution Solid-State NMR Spectroscopy Approach. Carbohydr. Polym. 2020, 250, 116891. [Google Scholar] [CrossRef]
- Zając, A.; Hanuza, J.; Wandas, M.; Dymińska, L. Determination of N-Acetylation Degree in Chitosan Using Raman Spectroscopy. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2015, 134, 114–120. [Google Scholar] [CrossRef]
- Facchinatto, W.M.; dos Santos Garcia, R.H.; dos Santos, D.M.; Fiamingo, A.; Menezes Flores, D.W.; Campana-Filho, S.P.; de Azevedo, E.R.; Colnago, L.A. Fast-Forward Approach of Time-Domain NMR Relaxometry for Solid-State Chemistry of Chitosan. Carbohydr. Polym. 2021, 256, 117576. [Google Scholar] [CrossRef]
- Silvestro, I.; Francolini, I.; Di Lisio, V.; Martinelli, A.; Pietrelli, L.; Scotto, A.; Scoppio, A.; Piozzi, A. Preparation and Characterization of TPP-Chitosan Crosslinked Sca Ff Olds for Tissue Engineering. Materials 2020, 13, 3577. [Google Scholar] [CrossRef]
- Pontes, M.S.; Antunes, D.R.; Oliveira, I.P.; Forini, M.M.L.; Santos, J.S.; Arruda, G.J.; Caires, A.R.L.; Santiago, E.F.; Grillo, R. Chitosan/Tripolyphosphate Nanoformulation Carrying Paraquat: Insights on Its Enhanced Herbicidal Activity. Environ. Sci. Nano 2021, 8, 1336–1351. [Google Scholar] [CrossRef]
- Bootz, A.; Vogel, V.; Schubert, D. Comparison of Scanning Electron Microscopy, Dynamic Light Scattering and Analytical Ultracentrifugation for the Sizing of Poly (Butyl Cyanoacrylate) Nanoparticles. Eur. J. Pharm. Biopharm. 2004, 57, 369–375. [Google Scholar] [CrossRef]
- Filgueiras, J.G.; Cobo, M.F.; Faria, G.C.; Moraes, T.B.; De Azevedo, E.R. Chapter 13: Dipolar Based NMR Methods for Probing Intermediate Regime Motions in Polymers. In New Developments in NMR; Royal Society of Chemistry: London, UK, 2019; Volume 1, pp. 271–298. ISBN 9781782625391. [Google Scholar]
- Cooper, C.L.; Cosgrove, T.; van Duijneveldt, J.S.; Murray, M.; Prescott, S.W. The Use of Solvent Relaxation NMR to Study Colloidal Suspensions. Soft Matter 2013, 9, 7211–7228. [Google Scholar] [CrossRef]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New Colorimetric Cytotoxicity Assay for Anticancer-Drug Screening. JNCI J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhuang, S. Antibacterial Activity of Chitosan and Its Derivatives and Their Interaction Mechanism with Bacteria: Current State and Perspectives. Eur. Polym. J. 2020, 138, 109984. [Google Scholar] [CrossRef]
- Chung, Y.C.; Su, Y.P.; Chen, C.C.; Jia, G.; Wang, H.L.; Wu, J.C.G.; Lin, J.G. Relationship between Antibacterial Activity of Chitosan and Surface Characteristics of Cell Wall. Acta Pharmacol. Sin. 2004, 25, 932–936. [Google Scholar]
- Park, S.C.; Nah, J.W.; Park, Y. PH-Dependent Mode of Antibacterial Actions of Low Molecular Weight Water-Soluble Chitosan (LMWSC) against Various Pathogens. Macromol. Res. 2011, 19, 853–860. [Google Scholar] [CrossRef]
- Liu, X.F.; Guan, Y.L.; Yang, D.Z.; Li, Z.; Yao, K. De Antibacterial Action of Chitosan and Carboxymethylated Chitosan. J. Appl. Polym. Sci. 2001, 79, 1324–1335. [Google Scholar] [CrossRef]
- Ta, Q.; Ting, J.; Harwood, S.; Browning, N.; Simm, A.; Ross, K.; Olier, I.; Al-Kassas, R. Chitosan Nanoparticles for Enhancing Drugs and Cosmetic Components Penetration through the Skin. Eur. J. Pharm. Sci. 2021, 160, 105765. [Google Scholar] [CrossRef]
- Roller, S.; Covill, N. The Antifungal Properties of Chitosan in Laboratory Media and Apple Juice. Int. J. Food Microbiol. 1999, 47, 67–77. [Google Scholar] [CrossRef]
- Parthasarathy, A.; Pappas, H.C.; Hill, E.H.; Huang, Y.; Whitten, D.G.; Schanze, K.S. Conjugated Polyelectrolytes with Imidazolium Solubilizing Groups. Properties and Application to Photodynamic Inactivation of Bacteria. ACS Appl. Mater. Interfaces 2015, 7, 28027–28034. [Google Scholar] [CrossRef]
- Wang, Y.; Jett, S.D.; Crum, J.; Schanze, K.S.; Chi, E.Y.; Whitten, D.G. Understanding the Dark and Light-Enhanced Bactericidal Action of Cationic Conjugated Polyelectrolytes and Oligomers. Langmuir 2013, 29, 781–792. [Google Scholar] [CrossRef]
- Corbitt, T.S.; Ding, L.; Ji, E.; Ista, L.K.; Ogawa, K.; Lopez, G.P.; Schanze, K.S.; Whitten, D.G. Light and Dark Biocidal Activity of Cationic Poly(Arylene Ethynylene) Conjugated Polyelectrolytes. Photochem. Photobiol. Sci. 2009, 8, 998–1005. [Google Scholar] [CrossRef]
- Calvo, P.; Remunan-Lopez, C.; Vila-Jato, J.L.; Alonso, M.J. Novel Hydrophilic Chitosan-Polyethylene Oxide Nanoparticles as Protein Carriers. J. Appl. Polym. Sci. 1997, 63, 125–132. [Google Scholar] [CrossRef]
- Abelha, T.F.; Neumann, P.R.; Holthof, J.; Dreiss, C.A.; Alexander, C.; Green, M.; Dailey, L.A. Low Molecular Weight PEG-PLGA Polymers Provide a Superior Matrix for Conjugated Polymer Nanoparticles in Terms of Physicochemical Properties, Biocompatibility and Optical/Photoacoustic Performance. J. Mater. Chem. B 2019, 7, 5115–5124. [Google Scholar] [CrossRef]
- De Gonzaga, L.A.C.; Martins, M.C.F.; Correa, A.C.; Facchinatto, W.M.; da Silva, C.M.P.; Colnago, L.A.; Mattoso, L.H.C. Production of Carbon Nanofibers from PAN and Lignin by Solution Blow Spinning. J. Polym. Res. 2021, 28, 237. [Google Scholar] [CrossRef]
- Facchinatto, W.M.; dos Santos, D.M.; de Lacerda Bukzem, A.; Moraes, T.B.; Habitzreuter, F.; de Azevedo, E.R.; Colnago, L.A.; Campana-Filho, S.P. Insight into Morphological, Physicochemical and Spectroscopic Properties of β-Chitin Nanocrystalline Structures. Carbohydr. Polym. 2021, 273, 118563. [Google Scholar] [CrossRef]
- Moraes, T.B.; Monaretto, T.; Colnago, L.A. Rapid and Simple Determination of T 1 Relaxation Times in Time-Domain NMR by Continuous Wave Free Precession Sequence. J. Magn. Reson. 2016, 270, 1–6. [Google Scholar] [CrossRef]
- Meiboom, S.; Gill, D. Modified Spin-Echo Method for Measuring Nuclear Relaxation Times. Rev. Sci. Instrum. 1958, 29, 688–691. [Google Scholar] [CrossRef]
- Monaretto, T.; Souza, A.; Moraes, T.B.; Bertucci-Neto, V.; Rondeau-Mouro, C.; Colnago, L.A. Enhancing Signal-to-Noise Ratio and Resolution in Low-Field NMR Relaxation Measurements Using Post-Acquisition Digital Filters. Magn. Reson. Chem. 2019, 57, 616–625. [Google Scholar] [CrossRef]
- Caires, C.S.A.; Arias, L.A.S.; Gomes, L.E.; Pinto, B.P.; Gonçalves, D.A.; Zagonel, L.F.; Nascimento, V.A.; Alves, D.C.B.; Colbeck, I.; Whitby, C.; et al. Effective Killing of Bacteria under Blue-Light Irradiation Promoted by Green Synthesized Silver Nanoparticles Loaded on Reduced Graphene Oxide Sheets. Mater. Sci. Eng. C 2020, 113, 110984. [Google Scholar] [CrossRef]
- Pedruzzi, D.P.; Araujo, L.O.; Falco, W.F.; Machado, G.; Casagrande, G.A.; Colbeck, I.; Lawson, T.; Oliveira, S.L.; Caires, A.R.L. ZnO Nanoparticles Impact on the Photosynthetic Activity of Vicia Faba: Effect of Particle Size and Concentration. NanoImpact 2020, 19, 100246. [Google Scholar] [CrossRef]
- Sali, R.; Naik, L.R.; Bhajantri, R.F.; Kamanavalli, C.M.; Premakshi, S.G. Optical and Fluorescence Study of Ethidium Bromide Doped Poly (Vinyl Alcohol). Int. J. Luminesc. Appl. 2015, 5, 131–133. [Google Scholar]

| Samples | ||
|---|---|---|
| Q35 | 1.19 ± 0.02 | 3.49 ± 0.06 |
| Q35PPV | 1.54 ± 0.05 | 4.52 ± 0.14 |
| Q20 | 1.27 ± 0.25 | 3.73 ± 0.73 |
| Q20PPV | 2.91 ± 0.07 | 8.56 ± 0.21 |
| Q10 | 2.07 ± 0.06 | 6.07 ± 0.18 |
| Q10PPV | 2.47 ± 0.08 | 7.26 ± 0.22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Facchinatto, W.M.; Araujo, L.O.; Moraes, T.B.; Abelha, T.F.; Lima, T.H.N.; Santos, D.M.d.; Campana-Filho, S.P.; Colnago, L.A.; Caires, A.R.L. Antimicrobial and Photoantimicrobial Activities of Chitosan/CNPPV Nanocomposites. Int. J. Mol. Sci. 2022, 23, 12519. https://doi.org/10.3390/ijms232012519
Facchinatto WM, Araujo LO, Moraes TB, Abelha TF, Lima THN, Santos DMd, Campana-Filho SP, Colnago LA, Caires ARL. Antimicrobial and Photoantimicrobial Activities of Chitosan/CNPPV Nanocomposites. International Journal of Molecular Sciences. 2022; 23(20):12519. https://doi.org/10.3390/ijms232012519
Chicago/Turabian StyleFacchinatto, William M., Leandro O. Araujo, Tiago B. Moraes, Thais F. Abelha, Thalita H. N. Lima, Danilo M. dos Santos, Sérgio P. Campana-Filho, Luiz A. Colnago, and Anderson R. L. Caires. 2022. "Antimicrobial and Photoantimicrobial Activities of Chitosan/CNPPV Nanocomposites" International Journal of Molecular Sciences 23, no. 20: 12519. https://doi.org/10.3390/ijms232012519
APA StyleFacchinatto, W. M., Araujo, L. O., Moraes, T. B., Abelha, T. F., Lima, T. H. N., Santos, D. M. d., Campana-Filho, S. P., Colnago, L. A., & Caires, A. R. L. (2022). Antimicrobial and Photoantimicrobial Activities of Chitosan/CNPPV Nanocomposites. International Journal of Molecular Sciences, 23(20), 12519. https://doi.org/10.3390/ijms232012519










