Role of B Lymphocytes in the Pathogenesis of NAFLD: A 2022 Update
Abstract
1. Introduction
2. Liver Is Also an Immune Organ
3. B Cells and Autoimmune Diseases
4. B Cells and Metabolic Dysregulation
5. B Cells in Liver
6. The Role of BAFF in NAFLD
7. Role of Intrahepatic Regulatory B Cells in NAFLD
8. Autoantibodies and NAFLD
9. Roles of B Cells in Fibrosis and HCC
10. B Cells and Cholesterol Metabolism
11. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Ascha, M.S.; Hanouneh, I.A.; Lopez, R.; Tamimi, T.A.; Feldstein, A.F.; Zein, N.N. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology 2010, 51, 1972–1978. [Google Scholar] [CrossRef] [PubMed]
- Dalekos, G.N.; Gatselis, N.K.; Zachou, K.; Koukoulis, G.K. NAFLD and autoimmune hepatitis: Do not judge a book by its cover. Eur. J. Intern. Med. 2020, 75, 1–9. [Google Scholar] [CrossRef]
- Dyson, J.; Jaques, B.; Chattopadyhay, D.; Lochan, R.; Graham, J.; Das, D.; Aslam, T.; Patanwala, I.; Gaggar, S.; Cole, M.; et al. Hepatocellular cancer: The impact of obesity, type 2 diabetes and a multidisciplinary team. J. Hepatol. 2013, 60, 110–117. [Google Scholar] [CrossRef]
- Ferguson, D.; Finck, B.N. Emerging therapeutic approaches for the treatment of NAFLD and type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2021, 17, 484–495. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, S.A.; Mantzoros, C.S. Making progress in nonalcoholic fatty liver disease (NAFLD) as we are transitioning from the era of NAFLD to dys-metabolism associated fatty liver disease (DAFLD). Metabolism 2020, 111, 154318. [Google Scholar] [CrossRef]
- Horst, A.K.; Neumann, K.; Diehl, L.; Tiegs, G. Modulation of liver tolerance by conventional and nonconventional antigen-presenting cells and regulatory immune cells. Cell. Mol. Immunol. 2016, 13, 277–292. [Google Scholar] [CrossRef]
- Huang, Z.; Zhong, L.; Lee, J.T.H.; Zhang, J.; Wu, D.; Geng, L.; Wang, Y.; Wong, C.-M.; Xu, A. The FGF21-CCL11 Axis Mediates Beiging of White Adipose Tissues by Coupling Sympathetic Nervous System to Type 2 Immunity. Cell Metab. 2017, 26, 493–508. [Google Scholar] [CrossRef]
- Taylor, S.A.; Assis, D.N.; Mack, C.L. The Contribution of B Cells in Autoimmune Liver Diseases. Semin. Liver Dis. 2019, 39, 422–431. [Google Scholar] [CrossRef]
- Vazquez, M.I.; Catalan-Dibene, J.; Zlotnik, A. B cells responses and cytokine production are regulated by their immune microenvironment. Cytokine 2015, 74, 318–326. [Google Scholar] [CrossRef]
- Schultheiss, C.; Steinmann, S.; Lohse, A.W.; Binder, M. B cells in autoimmune hepatitis: Bystanders or central players? Semin Immunopathol 2022, 44, 411–427. [Google Scholar] [CrossRef]
- Zhu, Q.; Rui, K.; Wang, S.; Tian, J. Advances of Regulatory B Cells in Autoimmune Diseases. Front. Immunol. 2021, 12. [Google Scholar] [CrossRef]
- Shaikh, S.R.; Haas, K.M.; Beck, M.A.; Teague, H. The effects of diet-induced obesity on B cell function. Clin. Exp. Immunol. 2014, 179, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Quintela, A.; Alende, R.; Gude, F.; Campos-Franco, J.; Rey, J.; Meijide, L.M.; Fernandez-Merino, C.; Vidal, C. Serum levels of immunoglobulins (IgG, IgA, IgM) in a general adult population and their relationship with alcohol consumption, smoking and common metabolic abnormalities. Clin. Exp. Immunol. 2008, 151, 42–50. [Google Scholar] [CrossRef]
- Vieira, D.G.; Costa-Carvalho, B.T.; Hix, S.; Da Silva, R.; Correia, M.S.; Sarni, R.O.S. Higher Cardiovascular Risk in Common Variable Immunodeficiency and X-Linked Agammaglobulinaemia Patients. Ann. Nutr. Metab. 2015, 66, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, S.F.; Trøseid, M.; Kummen, M.; Anmarkrud, J.A.; Michelsen, A.E.; Osnes, L.T.; Holm, K.; Høivik, M.L.; Rashidi, A.; Dahl, C.P.; et al. Altered gut microbiota profile in common variable immunodeficiency associates with levels of lipopolysaccharide and markers of systemic immune activation. Mucosal Immunol. 2016, 9, 1455–1465. [Google Scholar] [CrossRef] [PubMed]
- Borba, E.F.; Carvalho, J.F.; Bonfá, E. Mechanisms of Dyslipoproteinemias in Systemic Lupus Erythematosus. Clin. Dev. Immunol. 2006, 13, 203–208. [Google Scholar] [CrossRef]
- Iurlo, A.; Orsi, E.; Cattaneo, D.; Resi, V.; Bucelli, C.; Orofino, N.; Sciume, M.; Elena, C.; Grancini, V.; Consonni, D.; et al. Effects of first- and second-generation tyrosine kinase inhibitor therapy on glucose and lipid metabolism in chronic myeloid leukemia patients: A real clinical problem? Oncotarget 2015, 6, 33944–33951. [Google Scholar] [CrossRef] [PubMed]
- Agostino, N.M.; Chinchilli, V.M.; Lynch, C.J.; Koszyk-Szewczyk, A.; Gingrich, R.; Sivik, J.; Drabick, J.J. Effect of the tyrosine kinase inhibitors (sunitinib, sorafenib, dasatinib, and imatinib) on blood glucose levels in diabetic and nondiabetic patients in general clinical practice. J. Oncol. Pharm. Pr. 2010, 17, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Carranza-Trejo, A.M.; Vetvicka, V.; Vistejnova, L.; Kralickova, M.; Montufar, E.B. Hepatocyte and immune cell crosstalk in non-alcoholic fatty liver disease. Expert Rev. Gastroenterol. Hepatol. 2021, 15, 783–796. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.M.; Liu, Y.S.; Davies, S.P.; Brown, R.M.; Kelly, D.A.; Scheel-Toellner, D.; Reynolds, G.M.; Stamataki, Z. The Role of B Cells in Adult and Paediatric Liver Injury. Front. Immunol. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Crispe, I.N.; Dao, T.; Klugewitz, K.; Mehal, W.Z.; Metz, D.P. The liver as a site of T-cell apoptosis: Graveyard, or killing field? Immunol. Rev. 2000, 174, 47–62. [Google Scholar] [CrossRef] [PubMed]
- Novobrantseva, T.I.; Majeau, G.R.; Amatucci, A.; Kogan, S.; Brenner, I.; Casola, S.; Shlomchik, M.J.; Koteliansky, V.; Hochman, P.S.; Ibraghimov, A. Attenuated liver fibrosis in the absence of B cells. J. Clin. Investig. 2005, 115, 3072–3082. [Google Scholar] [CrossRef]
- Thapa, M.; Chinnadurai, R.; Velazquez, V.M.; Tedesco, D.; Elrod, E.; Han, J.-H.; Sharma, P.; Ibegbu, C.; Gewirtz, A.; Anania, F.; et al. Liver fibrosis occurs through dysregulation of MyD88-dependent innate B-cell activity. Hepatology 2015, 61, 2067–2079. [Google Scholar] [CrossRef]
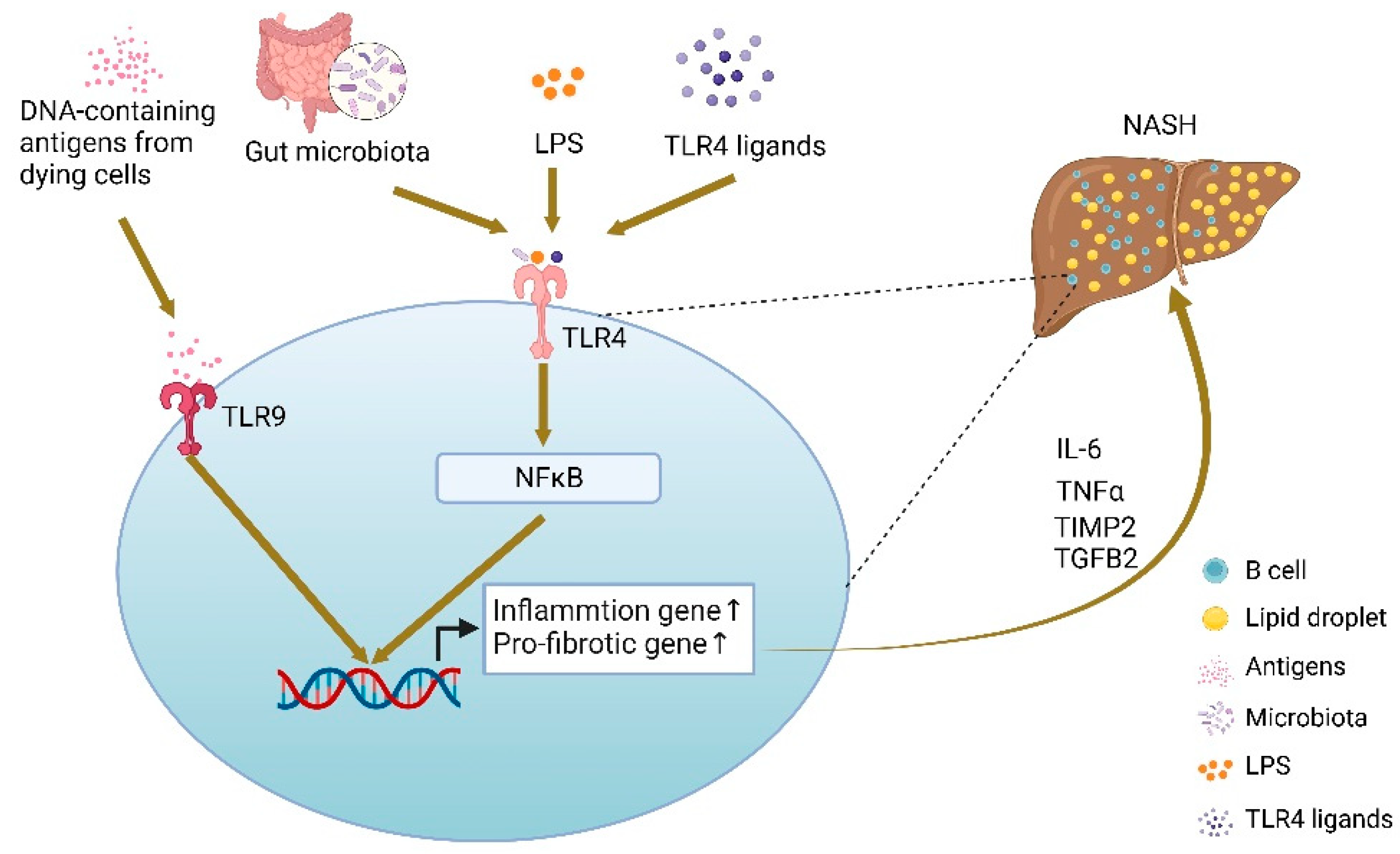
- Barrow, F.; Khan, S.; Fredrickson, G.; Wang, H.; Dietsche, K.; Parthiban, P.; Robert, S.; Kaiser, T.; Winer, S.; Herman, A.; et al. Microbiota-Driven Activation of Intrahepatic B Cells Aggravates NASH Through Innate and Adaptive Signaling. Hepatology 2021, 74, 704–722. [Google Scholar] [CrossRef]
- Sutti, S.; Albano, E. Adaptive immunity: An emerging player in the progression of NAFLD. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 81–92. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Y.; Yu, C. Identification of the Non-Alcoholic Fatty Liver Disease Molecular Subtypes Associated With Clinical and Immunological Features via Bioinformatics Methods. Front. Immunol. 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Puri, P.; Sanyal, A.J. The BAFFling problem of B cell-activating factor in nonalcoholic fatty liver disease. Hepatol. Int. 2013, 7, 309–312. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, Y.-H.; Choi, B.-H.; Cheon, H.-G.; Do, M.-S. B cell activation factor (BAFF) is a novel adipokine that links obesity and inflammation. Exp. Mol. Med. 2009, 41, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-H.; Do, M.-S. BAFF knockout improves systemic inflammation via regulating adipose tissue distribution in high-fat diet-induced obesity. Exp. Mol. Med. 2015, 47, e129. [Google Scholar] [CrossRef] [PubMed]
- Kato, A.; Truong-Tran, A.Q.; Scott, A.L.; Matsumoto, K.; Schleimer, R.P. Airway epithelial cells produce B cell-activating factor of TNF family by an IFN-beta-dependent mechanism. J. Immunol. 2006, 177, 7164–7172. [Google Scholar] [CrossRef]
- Miyake, T.; Abe, M.; Tokumoto, Y.; Hirooka, M.; Furukawa, S.; Kumagi, T.; Hamada, M.; Kawasaki, K.; Tada, F.; Ueda, T.; et al. B cell-activating factor is associated with the histological severity of nonalcoholic fatty liver disease. Hepatol. Int. 2012, 7, 539–547. [Google Scholar] [CrossRef]
- Kreuzaler, M.; Rauch, M.; Salzer, U.; Birmelin, J.; Rizzi, M.; Grimbacher, B.; Plebani, A.; Lougaris, V.; Quinti, I.; Thon, V.; et al. Soluble BAFF Levels Inversely Correlate with Peripheral B Cell Numbers and the Expression of BAFF Receptors. J. Immunol. 2011, 188, 497–503. [Google Scholar] [CrossRef]
- Samy, E.; Wax, S.; Huard, B.; Hess, H.; Schneider, P. Targeting BAFF and APRIL in systemic lupus erythematosus and other antibody-associated diseases. Int. Rev. Immunol. 2017, 36, 3–19. [Google Scholar] [CrossRef]
- Sánchez, D.C.V.; Castellanos, S.G.; Sandoval, M.E.V.; García, A.G. B-Cell Activating Factor Increases Related to Adiposity, Insulin Resistance, and Endothelial Dysfunction in Overweight and Obese Subjects. Life 2022, 12, 634. [Google Scholar] [CrossRef] [PubMed]
- Matthews, J.; Villescas, S.; Herat, L.; Schlaich, M.; Matthews, V. Implications of ADAM17 activation for hyperglycaemia, obesity and type 2 diabetes. Biosci. Rep. 2021, 41. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, L.; Vivanti, A.; Cavalera, M.; Marzano, V.; Ronci, M.; Fabrizi, M.; Menini, S.; Pugliese, G.; Menghini, R.; Khokha, R.; et al. Increased tumor necrosis factor α-converting enzyme activity induces insulin resistance and hepatosteatosis in mice. Hepatology 2009, 51, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Hamada, M.; Abe, M.; Miyake, T.; Kawasaki, K.; Tada, F.; Furukawa, S.; Matsuura, B.; Hiasa, Y.; Onji, M. B Cell-Activating Factor Controls the Production of Adipokines and Induces Insulin Resistance. Obesity 2011, 19, 1915–1922. [Google Scholar] [CrossRef]
- Kawasaki, K.; Abe, M.; Tada, F.; Tokumoto, Y.; Chen, S.; Miyake, T.; Furukawa, S.; Matsuura, B.; Hiasa, Y.; Onji, M. Blockade of B-cell-activating factor signaling enhances hepatic steatosis induced by a high-fat diet and improves insulin sensitivity. Lab. Investig. 2013, 93, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Abe, M.; Kawasaki, K.; Miyake, T.; Watanabe, T.; Yoshida, O.; Hirooka, M.; Matsuura, B.; Hiasa, Y. Depletion of B cell-activating factor attenuates hepatic fat accumulation in a murine model of nonalcoholic fatty liver disease. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Smulski, C.; Eibel, H. BAFF and BAFF-Receptor in B Cell Selection and Survival. Front. Immunol. 2018, 9, 2285. [Google Scholar] [CrossRef] [PubMed]
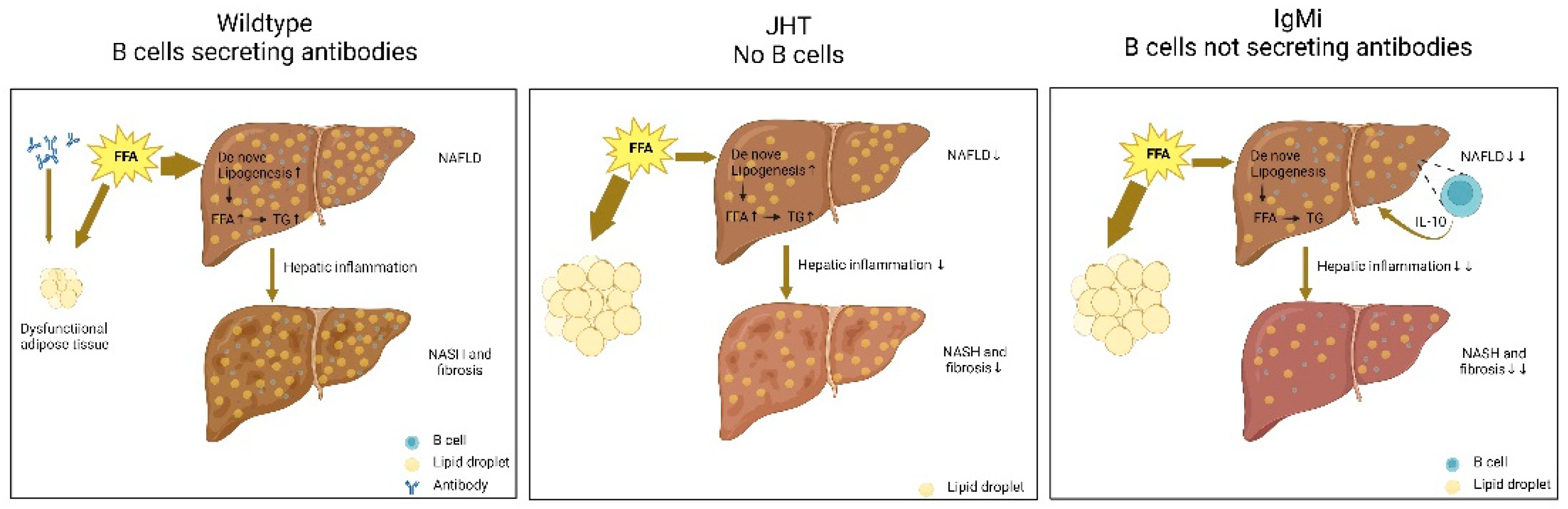
- Karl, M.; Hasselwander, S.; Zhou, Y.; Reifenberg, G.; Kim, Y.O.; Park, K.; Ridder, D.A.; Wang, X.; Seidel, E.; Hövelmeyer, N.; et al. Dual roles of B lymphocytes in mouse models of diet-induced nonalcoholic fatty liver disease. Hepatology 2022. [Google Scholar] [CrossRef]
- Waisman, A.; Kraus, M.; Seagal, J.; Ghosh, S.; Melamed, D.; Song, J.; Sasaki, Y.; Classen, S.; Lutz, C.; Brombacher, F.; et al. IgG1 B cell receptor signaling is inhibited by CD22 and promotes the development of B cells whose survival is less dependent on Ig alpha/beta. J. Exp. Med. 2007, 204, 747–758. [Google Scholar] [CrossRef] [PubMed]
- Sahputra, R.; Yam-Puc, J.C.; Waisman, A.; Muller, W.; Else, K.J. Evaluating the IgMi mouse as a novel tool to study B-cell biology. Eur. J. Immunol. 2018, 48, 2068–2071. [Google Scholar] [CrossRef]
- Gu, H.; Zou, Y.R.; Rajewsky, K. Independent control of immunoglobulin switch recombination at individual switch regions evidenced through Cre-loxP-mediated gene targeting. Cell 1993, 73, 1155–1164. [Google Scholar] [CrossRef]
- Trifunović, J.; Miller, L.; Debeljak, Ž.; Horvat, V. Pathologic patterns of interleukin 10 expression—A review. Biochem. medica 2015, 25, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.M.; Ricchetti, G.; Sarma, U.; Smallie, T.; Foxwell, B.M.J. Interleukin-10 suppression of myeloid cell activation—A continuing puzzle. Immunology 2004, 113, 281–292. [Google Scholar] [CrossRef]
- Wang, P.; Wu, P.; Siegel, M.I.; Egan, R.W.; Billah, M.M. Interleukin (IL)-10 inhibits nuclear factor kappa B (NF kappa B) activation in human monocytes. IL-10 and IL-4 suppress cytokine synthesis by different mechanisms. J. Biol. Chem. 1995, 270, 9558–9563. [Google Scholar] [CrossRef]
- Riley, J.K.; Takeda, K.; Akira, S.; Schreiber, R.D. Interleukin-10 receptor signaling through the JAK-STAT pathway. Requirement for two distinct receptor-derived signals for anti-inflammatory action. J. Biol. Chem. 1999, 274, 16513–16521. [Google Scholar] [CrossRef] [PubMed]
- Catalán, D.; Mansilla, M.A.; Ferrier, A.; Soto, L.; Oleinika, K.; Aguillón, J.C.; Aravena, O. Immunosuppressive Mechanisms of Regulatory B Cells. Front. Immunol. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Abebe, E.C.; Dejenie, T.A.; Ayele, T.M.; Baye, N.D.; Teshome, A.A.; Muche, Z.T. The Role of Regulatory B Cells in Health and Diseases: A Systemic Review. J. Inflamm. Res. 2021, 14, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Kohut, T.; Shah, A.; Russo, P.; Panganiban, J. Autoimmune Antibodies in Children and Adolescents With Nonalcoholic Fatty Liver Disease. J. Pediatr Gastroenterol. Nutr. 2022, 75, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhu, L.; Kinnear, D.; Triggs, N.; Quintanilla, N.M.; Himes, R. Clinical, Laboratory, and Histologic Correlates of Serum Antinuclear Antibody in Hispanic Pediatric Patients With Nonalcoholic Fatty Liver Disease. Am. J. Clin. Pathol. 2022, 158, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Ma, Q.; Lin, L.; Wang, H.; Ye, J.; Zhong, B. Prevalence and Significance of Antinuclear Antibodies in Biopsy-Proven Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Dis. Markers 2022, 2022, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Müller, P.; Messmer, M.; Bayer, M.; Pfeilschifter, J.M.; Hintermann, E.; Christen, U. Non-alcoholic fatty liver disease (NAFLD) potentiates autoimmune hepatitis in the CYP2D6 mouse model. J. Autoimmun. 2016, 69, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Frasca, D.; Ferracci, F.; Diaz, A.; Romero, M.; Lechner, S.; Blomberg, B.B. Obesity decreases B cell responses in young and elderly individuals. Obesity 2016, 24, 615–625. [Google Scholar] [CrossRef]
- Frasca, D.; Romero, M.; Garcia, D.; Diaz, A.; Blomberg, B.B. Obesity Accelerates Age-Associated Defects in Human B Cells Through a Metabolic Reprogramming Induced by the Fatty Acid Palmitate. Front. Aging 2022, 2. [Google Scholar] [CrossRef]
- Bruzzì, S.; Sutti, S.; Giudici, G.; Burlone, M.E.; Ramavath, N.N.; Toscani, A.; Bozzola, C.; Schneider, P.; Morello, E.; Parola, M.; et al. B2-Lymphocyte responses to oxidative stress-derived antigens contribute to the evolution of nonalcoholic fatty liver disease (NAFLD). Free Radic. Biol. Med. 2018, 124, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Binder, C.J.; Papac-Milicevic, N.; Witztum, J.L. Innate sensing of oxidation-specific epitopes in health and disease. Nat. Rev. Immunol. 2016, 16, 485–497. [Google Scholar] [CrossRef] [PubMed]
- Clement, C.C.; Osan, J.; Buque, A.; Nanaware, P.P.; Chang, Y.-C.; Perino, G.; Shetty, M.; Yamazaki, T.; Tsai, W.L.; Urbanska, A.M.; et al. PDIA3 epitope-driven immune autoreactivity contributes to hepatic damage in type 2 diabetes. Sci. Immunol. 2022, 7. [Google Scholar] [CrossRef] [PubMed]
- Thapa, M.; Tedesco, D.; Gumber, S.; Elrod, E.J.; Han, J.-H.; Kitchens, W.H.; Magliocca, J.F.; Adams, A.B.; Grakoui, A. Blockade of BAFF Reshapes the Hepatic B Cell Receptor Repertoire and Attenuates Autoantibody Production in Cholestatic Liver Disease. J. Immunol. 2020, 204, 3117–3128. [Google Scholar] [CrossRef] [PubMed]
- Faggioli, F.; Palagano, E.; Di Tommaso, L.; Donadon, M.; Marrella, V.; Recordati, C.; Mantero, S.; Villa, A.; Vezzoni, P.; Cassani, B. B lymphocytes limit senescence-driven fibrosis resolution and favor hepatocarcinogenesis in mouse liver injury. Hepatology 2018, 67, 1970–1985. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Ma, L.; Goswami, S.; Ma, J.; Zheng, B.; Duan, M.; Liu, L.; Zhang, L.; Shi, J.; Dong, L.; et al. Landscape of infiltrating B cells and their clinical significance in human hepatocellular carcinoma. OncoImmunology 2019, 8, e1571388. [Google Scholar] [CrossRef] [PubMed]
- Qin, M.; Wang, D.; Fang, Y.; Zheng, Z.; Liu, X.; Wu, F.; Wang, L.; Li, X.; Hui, B.; Ma, S.; et al. Current Perspectives on B Lymphocytes in the Immunobiology of Hepatocellular Carcinoma. Front. Oncol. 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, D.; Perucha, E. Cholesterol metabolism: A new molecular switch to control inflammation. Clin. Sci. 2021, 135, 1389–1408. [Google Scholar] [CrossRef]
- Duan, Y.; Gong, K.; Xu, S.; Zhang, F.; Meng, X.; Han, J. Regulation of cholesterol homeostasis in health and diseases: From mechanisms to targeted therapeutics. Signal Transduct. Target. Ther. 2022, 7, 1–29. [Google Scholar] [CrossRef]
- Fouchier, S.W.; Dallinga-Thie, G.M.; Meijers, J.C.; Zelcer, N.; Kastelein, J.J.; Defesche, J.C.; Hovingh, G.K. Mutations in STAP1 Are Associated With Autosomal Dominant Hypercholesterolemia. Circ. Res. 2014, 115, 552–555. [Google Scholar] [CrossRef] [PubMed]
- Brænne, I.; Kleinecke, M.; Reiz, B.; Graf, E.; Strom, T.; Wieland, T.; Fischer, M.; Kessler, T.; Hengstenberg, C.; Meitinger, T.; et al. Systematic analysis of variants related to familial hypercholesterolemia in families with premature myocardial infarction. Eur. J. Hum. Genet. 2015, 24, 191–197. [Google Scholar] [CrossRef]
- Hegele, R.A.; Knowles, J.W.; Horton, J.D. Delisting STAP1: The Rise and Fall of a Putative Hypercholesterolemia Gene. Arterioscler. Thromb Vasc. Biol. 2020, 40, 847–849. [Google Scholar] [CrossRef]
- Danyel, M.; Ott, C.-E.; Grenkowitz, T.; Salewsky, B.; Hicks, A.A.; Fuchsberger, C.; Steinhagen-Thiessen, E.; Bobbert, T.; Kassner, U.; DeMuth, I. Evaluation of the role of STAP1 in Familial Hypercholesterolemia. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef]
- Lamiquiz-Moneo, I.; Restrepo-Córdoba, M.A.; Mateo-Gallego, R.; Bea, A.M.; Alberiche-Ruano, M.D.P.; García-Pavía, P.; Cenarro, A.; Martín, C.; Civeira, F.; Sánchez-Hernández, R.M. Predicted pathogenic mutations in STAP1 are not associated with clinically defined familial hypercholesterolemia. Atherosclerosis 2019, 292, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Amor-Salamanca, A.; Castillo, S.; Gonzalez-Vioque, E.; Dominguez, F.; Quintana, L.; Lluís-Ganella, C.; Escudier, J.M.; Ortega, J.; Lara-Pezzi, E.; Alonso-Pulpon, L.; et al. Genetically Confirmed Familial Hypercholesterolemia in Patients With Acute Coronary Syndrome. J. Am. Coll. Cardiol. 2017, 70, 1732–1740. [Google Scholar] [CrossRef] [PubMed]
- Lamiquiz-Moneo, I.; Civeira, F.; Mateo-Gallego, R.; Laclaustra, M.; Moreno-Franco, B.; Tejedor, M.T.; Palacios, L.; Martín, C.; Cenarro, A. Diagnostic yield of sequencing familial hypercholesterolemia genes in individuals with primary hypercholesterolemia. Rev. Española Cardiol. (Engl. Ed.) 2020, 74, 664–673. [Google Scholar] [CrossRef]
- Blanco-Vaca, F.; Martín-Campos, J.M.; Pérez, A.; Fuentes-Prior, P. A rare STAP1 mutation incompletely associated with familial hypercholesterolemia. Clin. Chim. Acta 2018, 487, 270–274. [Google Scholar] [CrossRef]
- Mohebi, R.; Chen, Q.; Hegele, R.A.; Rosenson, R.S. Failure of cosegregation between a rare STAP1 missense variant and hypercholesterolemia. J. Clin. Lipidol. 2020, 14, 636–638. [Google Scholar] [CrossRef]
- Lee, D.S.W.; Rojas, O.L.; Gommerman, J.L. B cell depletion therapies in autoimmune disease: Advances and mechanistic insights. Nat. Rev. Drug Discov. 2020, 20, 179–199. [Google Scholar] [CrossRef]
- Barrow, F.; Khan, S.; Wang, H.; Revelo, X.S. The Emerging Role of B Cells in the Pathogenesis of NAFLD. Hepatology 2021, 74, 2277–2286. [Google Scholar] [CrossRef]
- MacParland, S.A.; Liu, J.C.; Ma, X.-Z.; Innes, B.T.; Bartczak, A.M.; Gage, B.K.; Manuel, J.; Khuu, N.; Echeverri, J.; Linares, I.; et al. Single cell RNA sequencing of human liver reveals distinct intrahepatic macrophage populations. Nat. Commun. 2018, 9, 1–21. [Google Scholar] [CrossRef]
| SNPs | Nucleotide | Amino Acid Change | Domain (Position) | Associated with FH | References |
|---|---|---|---|---|---|
| rs141647540 | c.35G> A | p.Arg12His | No | [70] | |
| No | [71] | ||||
| No | [72] | ||||
| rs201996284 | c.-60A > G | NA | No | [73] | |
| No | [71] | ||||
| No | [72] | ||||
| rs79388522 | c.139A> G | p. Thr47Ala | Pleckstrin homology domain (25-121) | Yes | [68] |
| rs938523789 | c.206T > C | p. Leu69Ser | Yes | [74] | |
| rs141647940 | c.212T > C | p. Ile71Thr | Yes | [74] | |
| rs199787258 | c.291G > C | p. Glu97Asp | Yes | [74] | |
| Yes | [72] | ||||
| No | [71] | ||||
| rs14983575 | c.414G> C | No changes | No | [70] | |
| rs199787258 | c.526C > T | p. Pro176Ser | Yes | [74] | |
| No | [70] | ||||
| No | [71] | ||||
| rs146545610 | c.619G > A | p. Asp207Asn | Src homology 2 domain (177-280) | Yes | [67] |
| No | [70] | ||||
| No | [75] | ||||
| rs12948217 | c.693C> T | No changes | No | [72] | |
| - | c.803T > C | p. Ile268Thr | No | [73] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, C.-J.; Lo, T.-H.; Chan, K.-Y.; Li, X.; Wu, M.-Y.; Xiang, Z.; Wong, C.-M. Role of B Lymphocytes in the Pathogenesis of NAFLD: A 2022 Update. Int. J. Mol. Sci. 2022, 23, 12376. https://doi.org/10.3390/ijms232012376
Deng C-J, Lo T-H, Chan K-Y, Li X, Wu M-Y, Xiang Z, Wong C-M. Role of B Lymphocytes in the Pathogenesis of NAFLD: A 2022 Update. International Journal of Molecular Sciences. 2022; 23(20):12376. https://doi.org/10.3390/ijms232012376
Chicago/Turabian StyleDeng, Chu-Jun, Tak-Ho Lo, Ka-Ying Chan, Xiang Li, Meng-Yao Wu, Zou Xiang, and Chi-Ming Wong. 2022. "Role of B Lymphocytes in the Pathogenesis of NAFLD: A 2022 Update" International Journal of Molecular Sciences 23, no. 20: 12376. https://doi.org/10.3390/ijms232012376
APA StyleDeng, C.-J., Lo, T.-H., Chan, K.-Y., Li, X., Wu, M.-Y., Xiang, Z., & Wong, C.-M. (2022). Role of B Lymphocytes in the Pathogenesis of NAFLD: A 2022 Update. International Journal of Molecular Sciences, 23(20), 12376. https://doi.org/10.3390/ijms232012376








