The Effects of Genistein at Different Concentrations on MCF-7 Breast Cancer Cells and BJ Dermal Fibroblasts
Abstract
1. Introduction
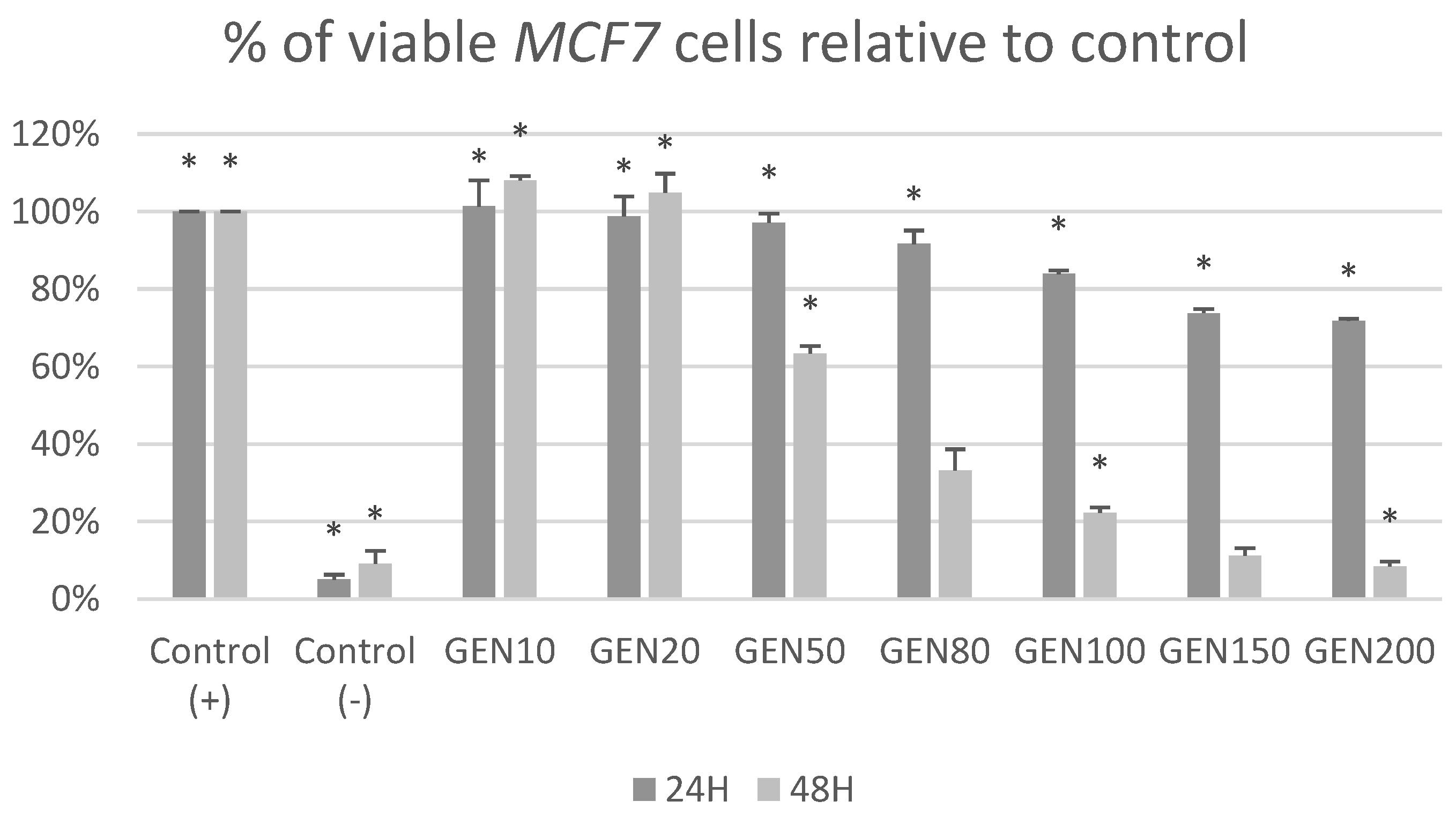
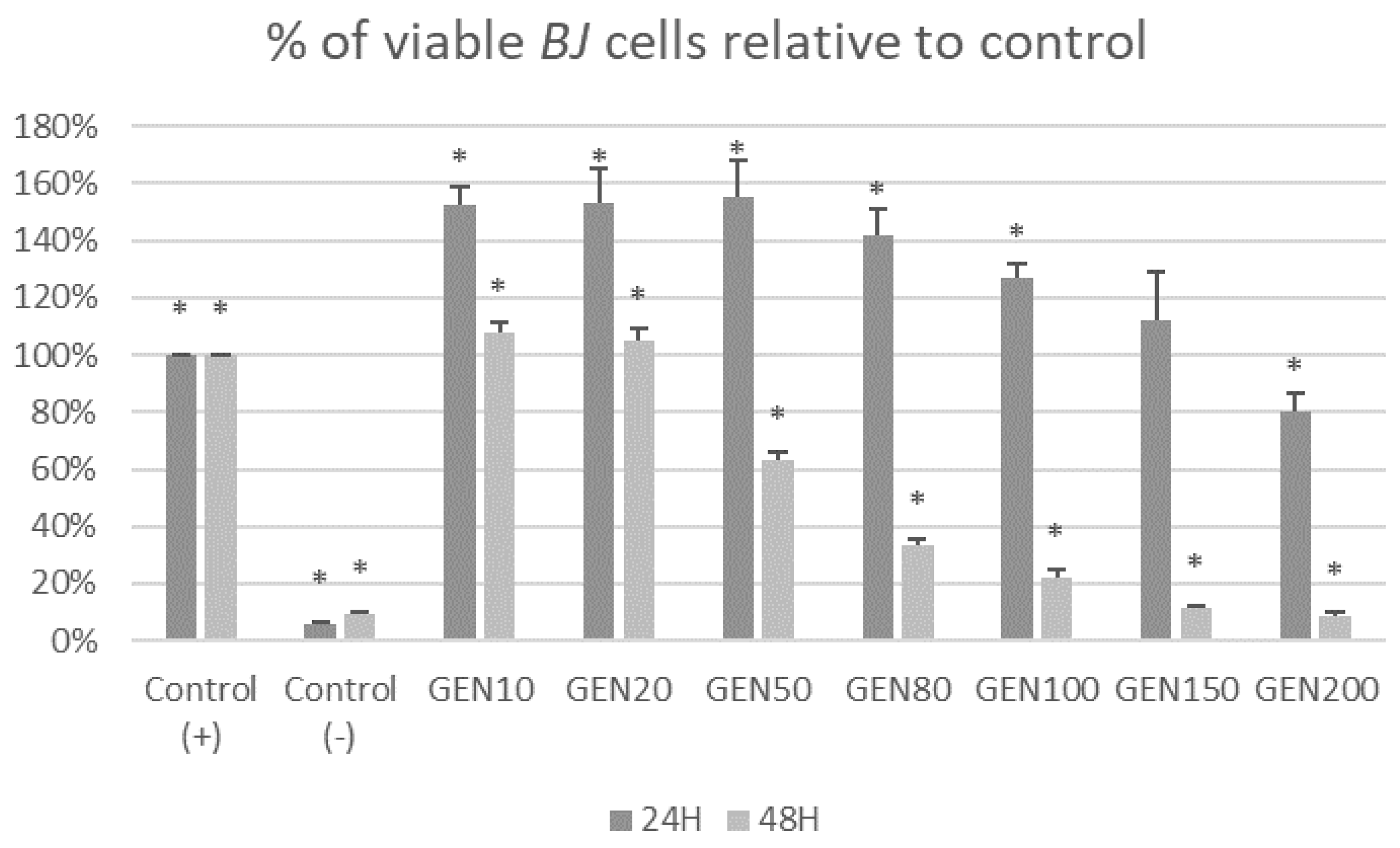
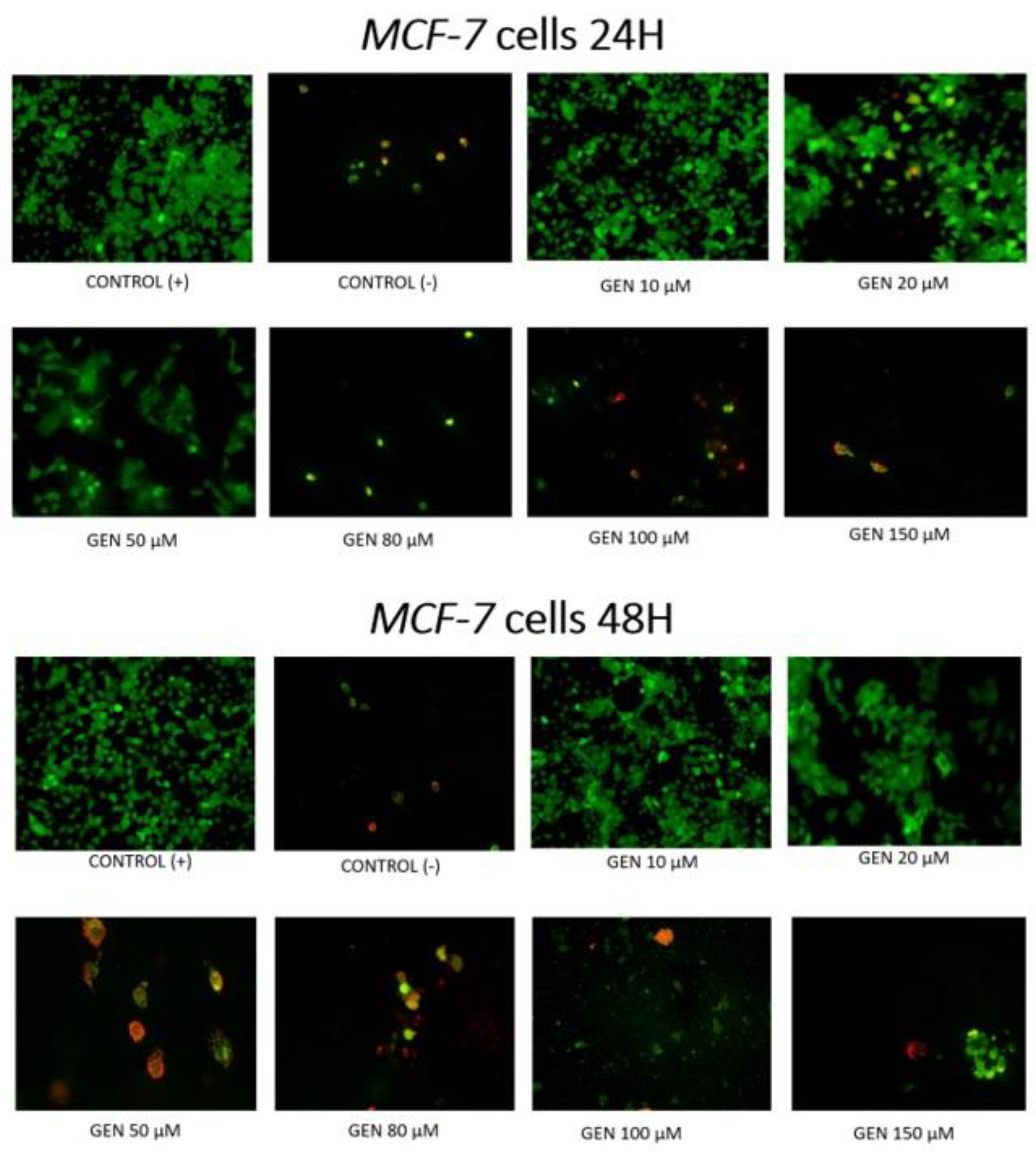
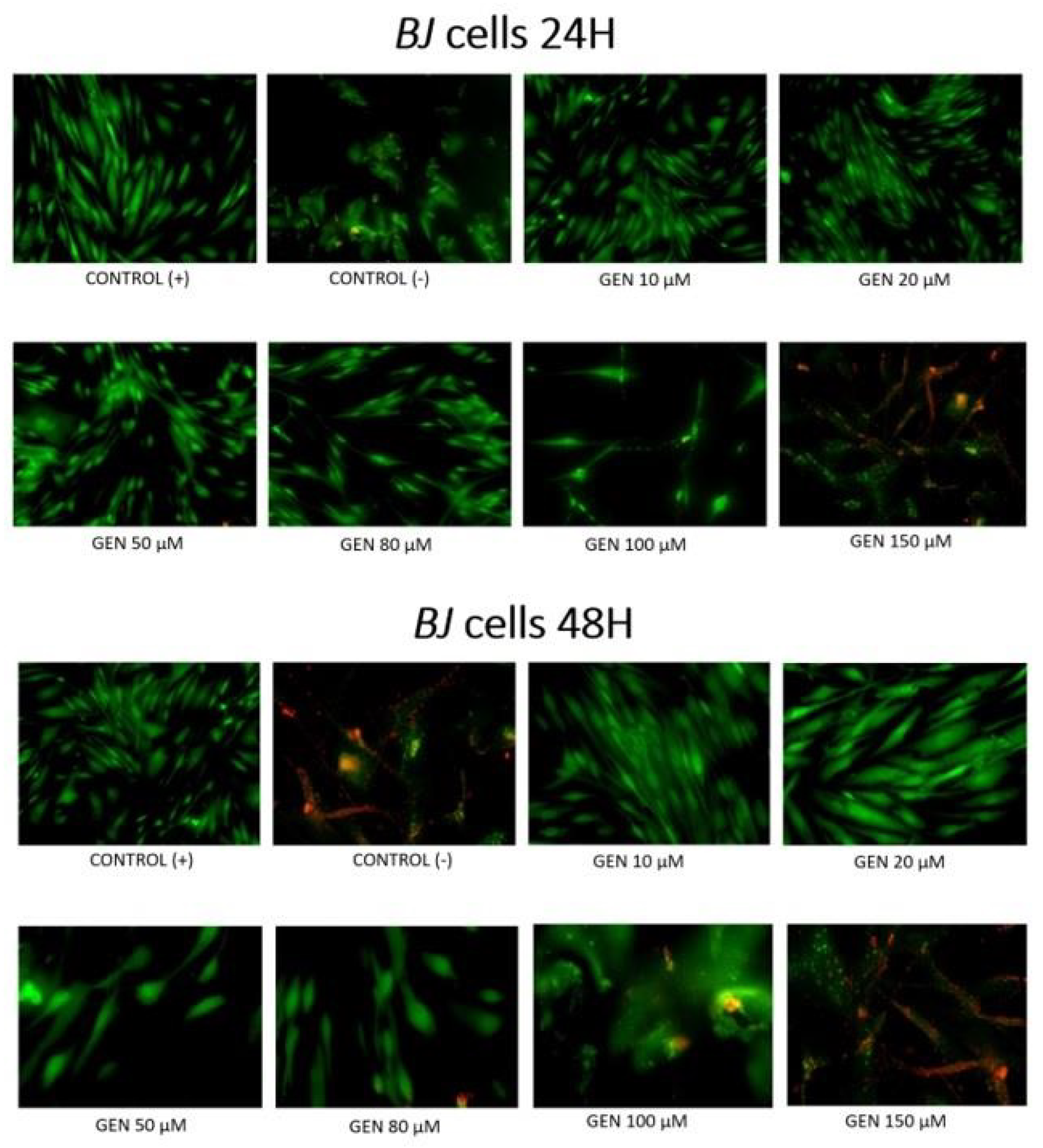
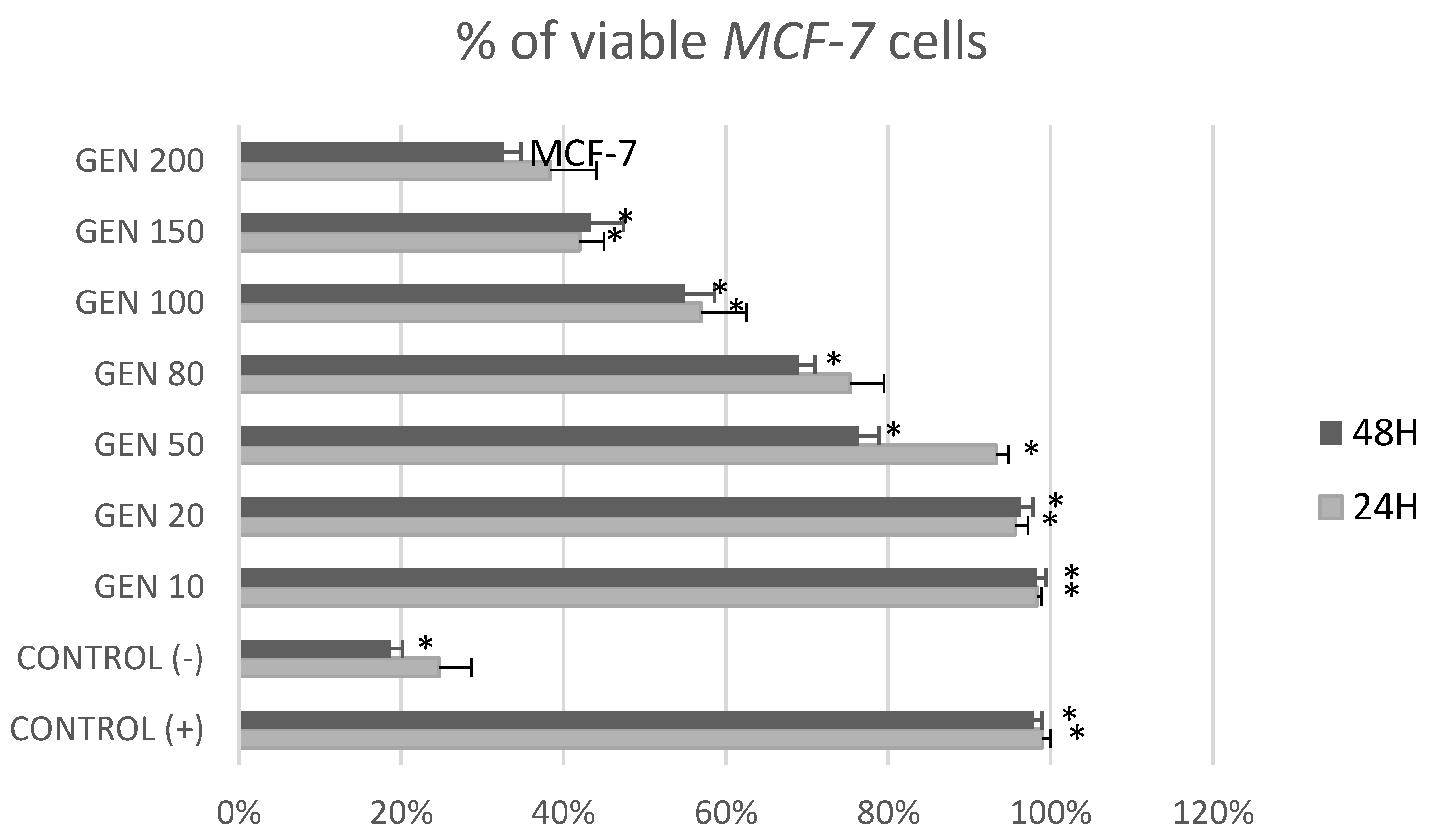
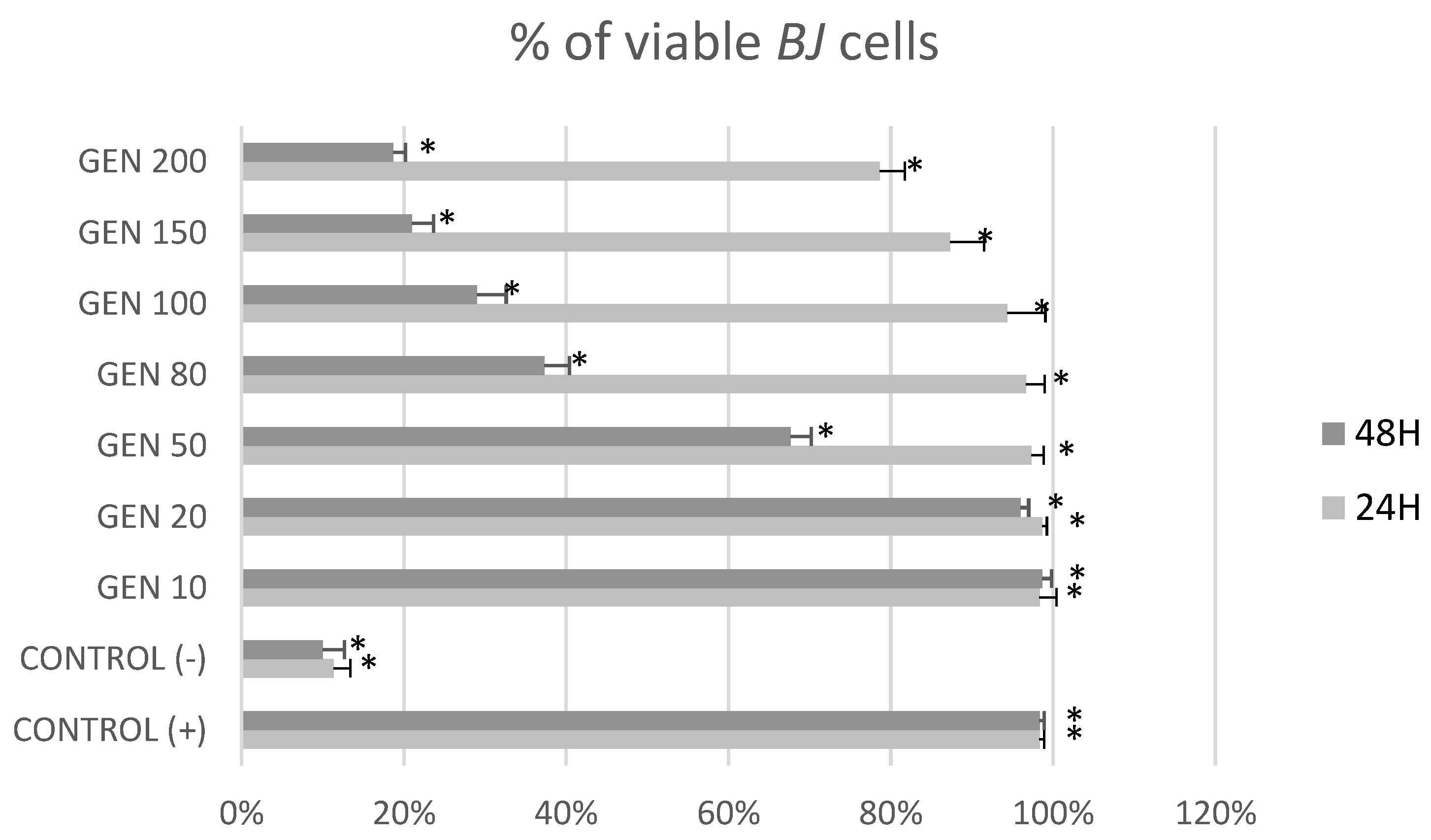
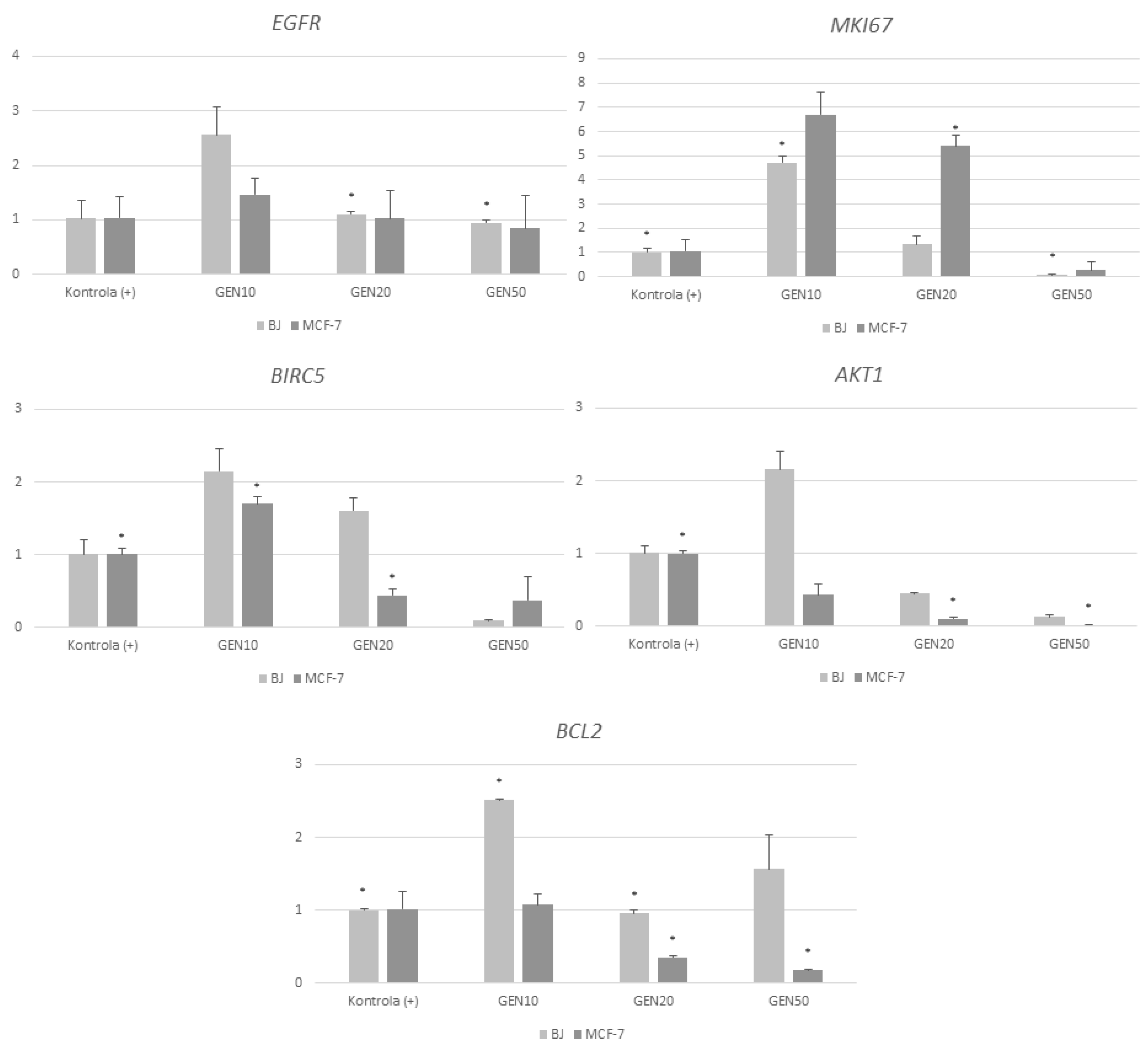
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Makki, J. Diversity of Breast Carcinoma: Histological Subtypes and Clinical Relevance. Clin. Med. Insights Pathol. 2015, 8, 23–31. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Global Health Estimates 2020: Deaths by Cause, Age, Sex, by Country and by Region, 2000–2019. 2020. Available online: https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates/ghe-leading-causes-of-death (accessed on 16 October 2021).
- Krzemieniecki, K.; Komorowski, K.; Wysocki. Interna Szczeklika. Podręcznik Chorób Wewnętrznych; Medycyna praktyczna: Cracow, Poland, 2013. [Google Scholar]
- Krzakowski, M.; Potemski, P.; Warzocha, K. Onkologia kliniczna tom 2; Via Medica: Gdansk, Poland, 2015. [Google Scholar]
- McDonald, E.; Clark, A.; Tchou, J.; Zhang, P.; Freedman, G. Clinical Diagnosis and Management of Breast Cancer. J. Nucl. Med. Febr. 2016, 57, 9S–16S. [Google Scholar] [CrossRef] [PubMed]
- Gamal, H.; Tawfik, W.; Fahmy, H.M.; El-Sayyad, H.H. Breakthroughs of using Photodynamic Therapy and Gold Nanoparticles in Cancer Treatment. In Proceedings of the 021 IEEE International Conference on Nanoelectronics, Nanophotonics, Nanomaterials, Nanobioscience & Nanotechnology (5NANO), Kottayam, Kerala, India, 29–30 April 2021; pp. 1–4. [Google Scholar] [CrossRef]
- Somogyi, R. Breast reconstruction. Can. Fam. Physician 2018, 64, 424–432. [Google Scholar]
- Gass, J.; Mitchell, S.; Hanna, M. How do breast cancer surgery scars impact survivorship? Findings from a nationwide survey in the United States. BMC Cancer 2019, 19, 342. [Google Scholar] [CrossRef] [PubMed]
- Jabłońska, B.; Brańka, J.; Lampe, P. Od Halsteda do leczenia oszczędzającego, czyli krótka historia chirurgii raka gruczołu piersiowego. Postępy Nauk. Med. 2011, 1, 29–32. [Google Scholar]
- Křížová, L.; Dadáková, K.; Kašparovská, J.; Kašparovský, T. Isoflavoes. Molecules 2019, 24, 1076. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.M.; Huh, J.S.; Lim, Y.; Cho, M. Soy Isoflavone Glycitin (4′-Hydroxy-6-Methoxyisoflavone-7-D-Glucoside) Promotes Human Dermal Fibroblast Cell Proliferation and Migration via TGF-β Signaling. Phytother. Res. 2015, 29, 757–769. [Google Scholar] [CrossRef] [PubMed]
- Czerpak, R.; Pietryczuk, A.; Jabłońska-Trypuć, A.; Obrębska, K. Aktywność biologiczna izoflawonoidów i ich znaczenie terapeutyczne i kosmetyczne. Borgis. Postępy Fitoter. 2009, 2, 113–121. [Google Scholar]
- Irrera, N.; Pizzino, G.; D’Anna, R.; Vaccaro, M.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bittol, A. Dietary Management of Skin Health: The Role of Genistein. Nutrients 2017, 9, 622. [Google Scholar] [CrossRef] [PubMed]
- Chin, G.S.; Liu, W.; Steinbrech, D.; Hsu, M.; Levinson, H.; Longaker, M.T. Cellular signaling by tyrosine phosphorylation in keloid and normal human dermal fibroblasts. Plast. Reconstr. Surg. 2000, 106, 1532–1540. [Google Scholar] [CrossRef]
- Cao, C.; Li, S.; Dai, X.; Chen, Y.; Feng, Z.; Zhao, Y.; Wu, J. Genistein inhibits proliferation and functions of hypertrophic scar fibroblasts. Burns 2009, 35, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Jurzak, M.; Adamczyk, K.; Antończak, P.; Garncarczyk, A.; Kuśmierz, D.; Latocha, M. Evaluation of genistein ability to modulate CTGF mRNA/protein expression, genes expression of TGFβ isoforms and expression of selected genes regulating cell cycle in keloid fibroblasts in vitro. Acta Pol. Pharm. 2014, 71, 972–986. [Google Scholar] [PubMed]
- Jurzak, M.; Adamczyk, K. Influence of genistein on c-Jun, c-Fos and Fos-B of AP-1 subunits expression in skin keratinocytes, fibroblasts and keloid fibroblasts cultured in vitro. Acta Pol. Pharm. 2013, 70, 205–213. [Google Scholar] [PubMed]
- Sienkiewicz, P.; Surazyński, A.; Pałka, J.; Miltyk, W. Nutritional concentration of genistein protects human dermal fibroblasts from oxidative stress-induced collagen biosynthesis inhibition through IGF-I receptor-mediated signaling. Acta Pol. Pharm. 2008, 65, 203–211. [Google Scholar] [PubMed]
- Park, E.; Lee, S.M.; Jung, I.K.; Lim, Y.; Kim, J.H. Effects of genistein on early-stage cutaneous wound healing. Biochem. Biophys. Res. Commun. 2011, 410, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Emmerson, E.; Campbell, L.; Ashcroft, G.S.; Hardman, M.J. The phytoestrogen genistein promotes wound healing by multiple independent mechanisms. Mol. Cell. Endocrinol. 2010, 321, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Marini, H.; Polito, F.; Altavilla, D.; Irrera, N.; Minutoli, L.; Calò, M.; Adamo, E.B.; Vaccaro, M.; Squadrito, F.; Bitto, A. Genistein aglycone improves skin repair in an incisional model of wound healing: A comparison with raloxifene and oestradiol in ovariectomized rats. Br. J. Pharmacol. 2010, 160, 1185–1194. [Google Scholar] [CrossRef] [PubMed]
- Polito, F.; Marini, H.; Bitto, A.; Irrera, N.; Vaccaro, M.; Adamo, E.B.; Micali, A.; Squadrito, F.; Minutoli, L.; Altavilla, D. Genistein aglycone, a soy-derived isoflavone, improves skin changes induced by ovariectomy in rats. Br. J. Pharmacol. 2012, 165, 994–1005. [Google Scholar] [CrossRef]
- Kloska, A.; Jakóbkiewicz-Banecka, J.; Narajczyk, M.; Banecka-Majkutewicz, Z.; Węgrzyn, G. Effects of flavonoids on glycosaminoglycan synthesis: Implications for substrate reduction therapy in Sanfilippo disease and other mucopolysaccharidoses. Metab. Brain Dis. 2011, 26, 1–8. [Google Scholar] [CrossRef]
- Isoherranen, K.; Punnonen, K.; Jansen, C.; Uotila, P. Ultraviolet irradiation induces cyclooxygenase-2 expression in keratinocytes. Br. J. Dermatol. 1999, 140, 1017–1022. [Google Scholar] [CrossRef]
- Iovine, B.; Iannella, M.L.; Gasparri, F.; Monfrecola, G.; Bevilacqua, M.A. Synergic Effect of Genistein and Daidzein on UVB-Induced DNA Damage: An Effective Photoprotective Combination. J. Biomed. Biotechnol. 2011, 2011, 692846. [Google Scholar] [CrossRef]
- Wang, Y.N.; Wu, W.; Chen, H.C.; Fang, H. Genistein protects against UVB-induced senescence-like characteristics in human dermal fibroblast by p66Shc down-regulation. J. Dermatol. Sci. 2010, 58, 19–27. [Google Scholar] [CrossRef]
- Kessel, B. Alternatives to estrogen for menopausal women. Soc. Exp. Biol. Med. 1998, 217, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Izumi, T.; Saito, M.; Obata, A.; Arii, M.; Yamaguchi, H.; Matsuyama, A. Oral intake of soy isoflavone aglycone improves the aged skin of adult women. J. Nutr. Sci. Vitaminol. Tokyo 2007, 53, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.A.; Ferraz Carbonel, A.A.; de Moraes, A.R.B.; Simões, R.S.; Sasso, G.R.D.S.; Goes, L.; Nunes, W.; Simões, M.J.; Patriarca, M.T. Collagen concentration on the facial skin of postmenopausal women after topical treatment with estradiol and genistein: A randomized double-blind controlled trial. Gynecol. Endocrinol. 2017, 33, 845–848. [Google Scholar] [CrossRef]
- Patriarca, M.T.; Barbosa de Moraes, A.R.; Nader, H.B.; Petri, V.; Martins, J.R.; Gomes, R.C.; Soares, J.M., Jr. Hyaluronic acid concentration in postmenopausal facial skin after topical estradiol and genistein treatment: A double-blind, randomized clinical trial of efficacy. Menopause 2013, 20, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Moraes, A.B.; Haidar, M.A.; Soares Júnior, J.M.; Simões, M.J.; Baracat, E.C.; Patriarca, M.T. The effects of topical isoflavones on postmenopausal skin: Double-blind and randomized clinical trial of efficacy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2009, 146, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Saladi, R.; Lu, Y.; Wang, Y.; Palep, S.R.; Moore, J.; Phelps, R.; Shyong, E.; Lebwohl, M.G. Isoflavone genistein: Photoprotection and clinical implications in dermatology. J. Nutr. 2003, 133 (Suppl. 1), 3811S–3819S. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Quispe, C.; Imran, M.; Rauf, A.; Nadeem, M.; Gondal, T.A.; Ahmad, B.; Atif, M.; Mubarak, M.S.; Sytar, O. Genistein: An Integrative Overview of Its Mode of Action, Pharmacological Properties, and Health Benefits. Oxidative Med. Cell. Longev. 2021, 2021, 3268136. [Google Scholar] [CrossRef]
- Kabała-Dzik, A.; Rzepecka-Stojko, A.; Kubina, R.; Iriti, M.; Wojtyczka, R.D.; Buszman, E.; Stojko, J. Flavonoids, bioactive components of propolis, exhibit cytotoxic activity and induce cell cycle arrest and apoptosis in human breast cancer cells MDA-MB-231 and MCF-7—A comparative study. Cell. Mol. Biol. 2018, 64, 1. [Google Scholar] [CrossRef]
- Choi, E.J.; Jung, J.Y.; Kim, G.H. Genistein inhibits the proliferation and differentiation of MCF-7 and 3T3-L1 cells via the regulation of ERα expression and induction of apoptosis. Exp. Ther. Med. 2014, 8, 454–458. [Google Scholar] [CrossRef] [PubMed]
- Tsuboy, M.S.; Marcarini, J.C.; de Souza, A.O.; de Paula, N.A.; Dorta, D.J.; Mantovani, M.S.; Ribeirol, L.R. Genistein at Maximal Physiologic Serum Levels Induces G0/G1 Arrest in MCF-7 and HB4a Cells, But Not Apoptosis. J. Med. Food. 2014, 17, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Uifălean, A.; Schneider, S.; Gierok, P.; Ionescu, C.; Iuga, C.A.; Lalk, M. The Impact of Soy Isoflavones on MCF-7 and MDA-MB-231 Breast Cancer Cells Using a Global Metabolomic Approach. Int. J. Mol. Sci. 2016, 17, 1443. [Google Scholar] [CrossRef] [PubMed]
- Lavigne, J.A.; Takahashi, Y.; Chandramouli, G.V.R.; Liu, H.; Perkins, S.N.; Hursting, S.D.; Wang, T.T.Y. Concentration-dependent effects of genistein on global gene expression in MCF-7 breast cancer cells: An oligo microarray study. Breast. Cancer Res. Treat. 2008, 110, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Chinni, S.R.; Alhasan, S.A.; Multani, A.S.; Pathak, S.; Sarkar, F.H. Pleotropic effects of genistein on MCF-7 breast cancer cells. Int. J. Mol. Med. 2003, 12, 29–34. [Google Scholar] [CrossRef]
- Prietsch, R.F.; Monte, L.G.; da Silva, F.A.; Beira, F.T.; del Pino, F.A.B.; Campos, V.F.; Collares, T.; Pinto, L.S.; Spanevello, R.M.; Gamaro, G.D.; et al. Genistein induces apoptosis and autophagy in human breast MCF-7 cells by modulating the expression of proapoptotic factors and oxidative stress enzymes. Mol. Cell Biochem. 2014, 390, 235–242. [Google Scholar] [CrossRef]
- Chen, J.; Lin, C.; Yong, W.; Ye, Y.; Huang, Z. Calycosin and genistein induce apoptosis by inactivation of HOTAIR/p-Akt signaling pathway in human breast cancer MCF-7 cells. Cell Physiol. Biochem. 2015, 35, 722–728. [Google Scholar] [CrossRef]
- Chen, J.; Duan, Y.; Zhang, X.; Ye, Y.; Ge, B.; Chen, J. Genistein induces apoptosis by the inactivation of the IGF-1R/p-Akt signaling pathway in MCF-7 human breast cancer cells. Food Funct. 2015, 6, 995–1000. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
| Gene | Forward Sequence | Reverse Sequence | Product Size (bp) |
|---|---|---|---|
| BCl2 | ATCGCCCTGTGGATGACTGAGT | GCCAGGAGAAATCAAACAGAGGC | 140 |
| MKI67 | GAAAGAGTGGCAACCTGCCTTC | GCACCAAGTTTTACTACATCTGCC | 151 |
| EGFR | AACACCCTGGTCTGGAAGTACG | TCGTTGGACAGCCTTCAAGACC | 106 |
| AKT1 | TGGACTACCTGCACTCGGAGAA | GTGCCGCAAAAGGTCTTCATGG | 154 |
| BIRC5 | CCACTGAGAACGAGCCAGACTT | GTATTACAGGCGTAAGCCACCG | 115 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pawlicka, M.A.; Zmorzyński, S.; Popek-Marciniec, S.; Filip, A.A. The Effects of Genistein at Different Concentrations on MCF-7 Breast Cancer Cells and BJ Dermal Fibroblasts. Int. J. Mol. Sci. 2022, 23, 12360. https://doi.org/10.3390/ijms232012360
Pawlicka MA, Zmorzyński S, Popek-Marciniec S, Filip AA. The Effects of Genistein at Different Concentrations on MCF-7 Breast Cancer Cells and BJ Dermal Fibroblasts. International Journal of Molecular Sciences. 2022; 23(20):12360. https://doi.org/10.3390/ijms232012360
Chicago/Turabian StylePawlicka, Magda Aleksandra, Szymon Zmorzyński, Sylwia Popek-Marciniec, and Agata Anna Filip. 2022. "The Effects of Genistein at Different Concentrations on MCF-7 Breast Cancer Cells and BJ Dermal Fibroblasts" International Journal of Molecular Sciences 23, no. 20: 12360. https://doi.org/10.3390/ijms232012360
APA StylePawlicka, M. A., Zmorzyński, S., Popek-Marciniec, S., & Filip, A. A. (2022). The Effects of Genistein at Different Concentrations on MCF-7 Breast Cancer Cells and BJ Dermal Fibroblasts. International Journal of Molecular Sciences, 23(20), 12360. https://doi.org/10.3390/ijms232012360








