Bioactive Lipodepsipeptides Produced by Bacteria and Fungi †
Abstract
1. Introduction
2. Source, Isolation, and Biological Activity of Bacterial Lipodepsipeptides
3. Source, Isolation, and Biological Activity of Fungal Lipodepsipeptides
4. Lipodepsipeptides Produced by Bacteria Pathogens of Mushrooms
5. Structure–Activity Relationship of SAR Studies
| Lpodepsipeptide | Source | Biological Activity | References |
|---|---|---|---|
| Lipodepsipeptides produced by bacteria | |||
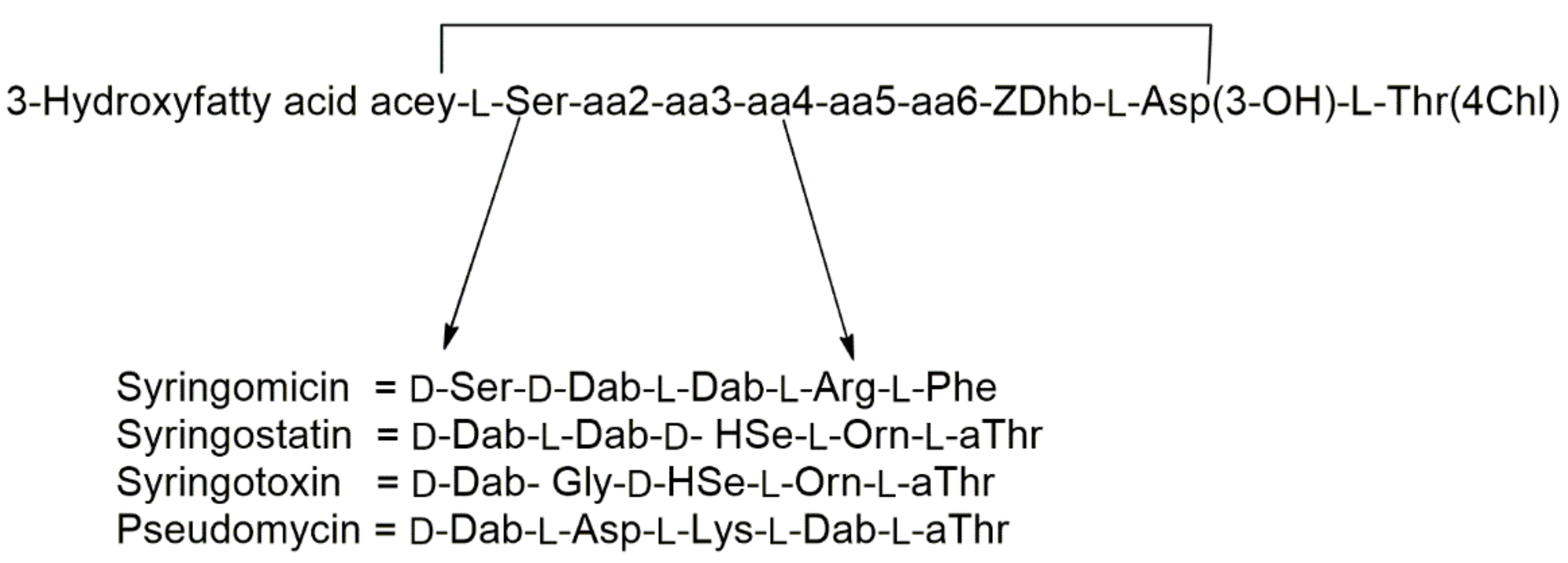
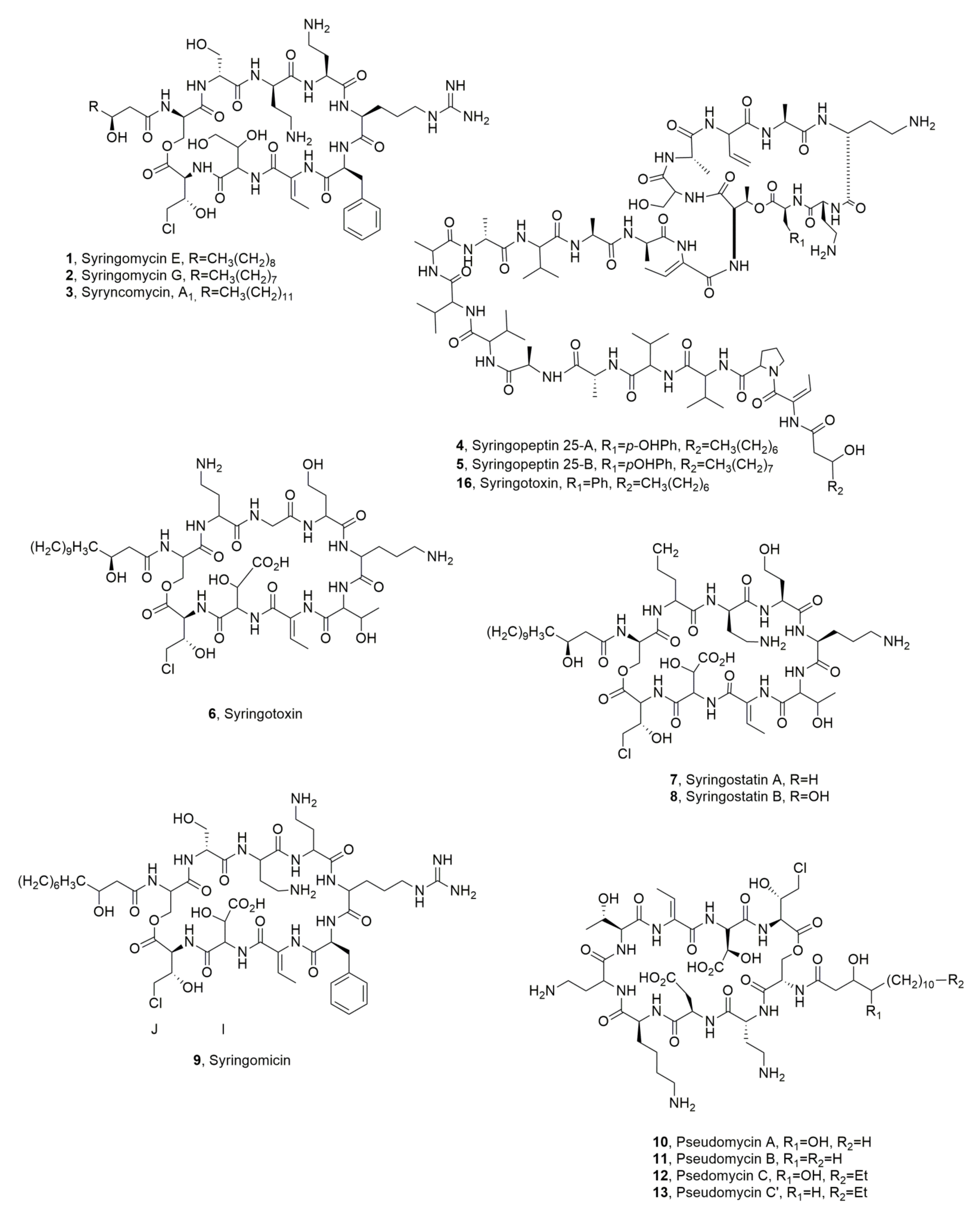
| Syringomicyn E (1) | Pseudomonas syrungae pv. syringae | Phytotoxic, Antifungal | [21] |
| Syringomicyn G (2) | “ | “ | “ |
| Syringomicyn A1 (3) | “ | “ | “ |
| Syringopeptin SP25A (4) | “ | Phytotoxic | [4] |
| Syringopeptin SP25B (5) | “ | “ | “ |
| Syringotoxin (6) | “ | No Activity | ” |
| Syringostatin A (7) | Pseudomonas syringae pv. syringae SY12 | Phytotoxic | [25] |
| Syringostatin B (8) | “ | “ | “ |
| Syrimgomicin (9) | Pseudomonas syringae pv. syringae SC1 | Antimicrobial | [26] |
| Pseudomycin A (10) | Pseudomonas syringae MSU 16H | Phytotoxic, Antifungal | [27,29] |
| Pseudomycin B (11) | “ | No Activity | [27] |
| Pseudomycin C (12) | “ | “ | “ |
| Pseudomycin C’ (13) | “ | “ | “ |
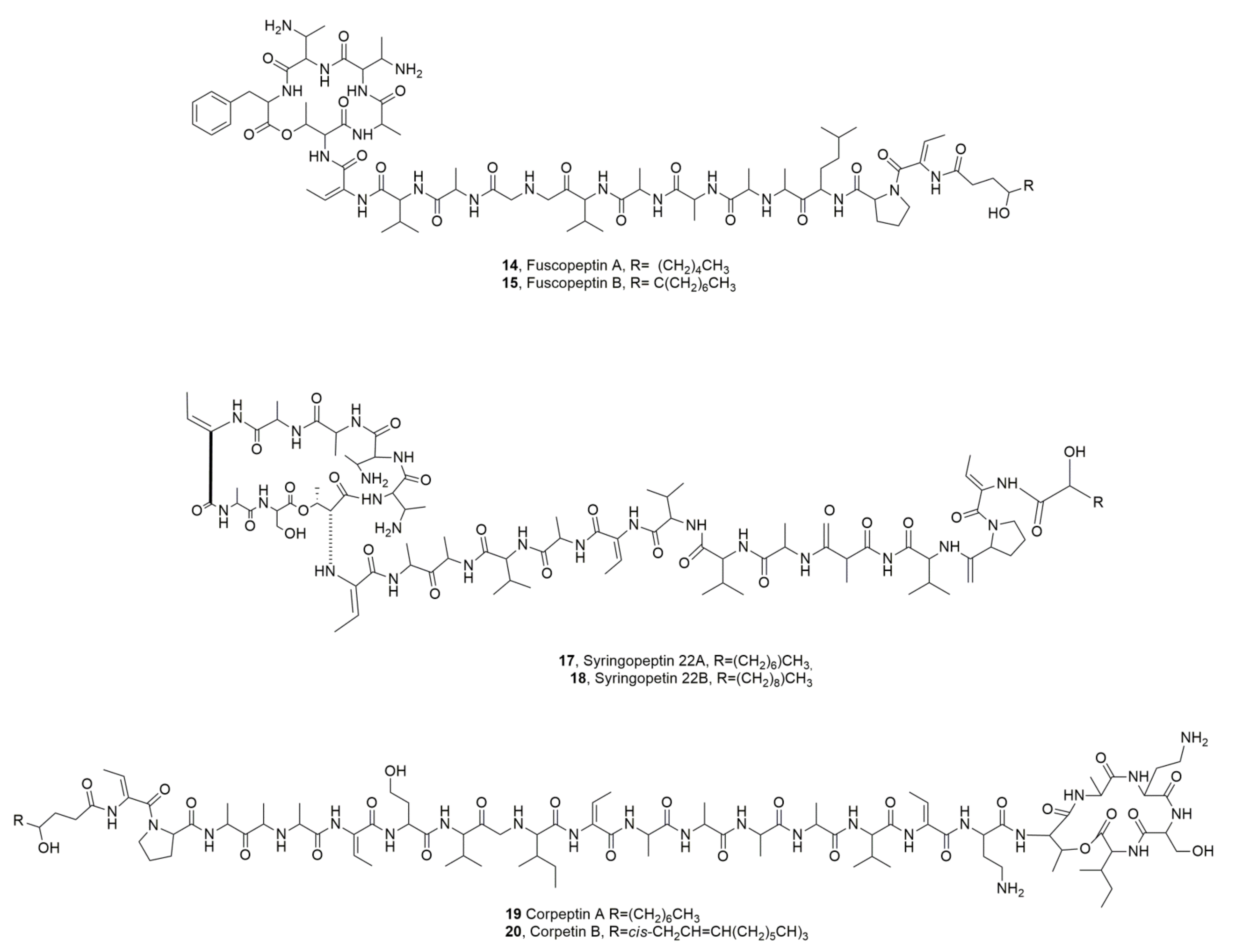
| Fuscopeptin A (14) | Pseudomonas fuscovaginae | Phytotoxic, Antifungal | [31] |
| Fuscopeptin B (15) | “ | “ | “ |
| Syringopeptin (16) | Pseudomonas syringae pv. syringae | Phytotoxic | [7] |
| Syringopeptin SP22A (17) | “ | Phytotoxic, Cytotoxic | [33] |
| Syringopeptin SP22B (18) | “ | “ | “ |
| Corpeptins A (19) | Pesudomonas corrugata | Phytotoxiv, Antibiotic | [35] |
| Corpeptins B (20) | “ | “ | |
| Putisolvin I (21) | Pseudomonas putida | Antibiofilm | [37] |
| Putisolvin II (22) | “ | “ | “ |
| Cormycin A (23) | Pseudomonas corrugata | Phytotoxic, Antibiotic, Red-blood-cell lysis | [36] |
| Syringopeptin 508A (24) | P. syringae pv. lachrymans | Antibiotic | [39] |
| Syringopeptin 508B (25) | “ | “ | “ |
| LPDs A54145 (26) | Streptomyces fradiae | “ | [40,41] |
| Pseudodesmin A (27) | Pseudomonas tolaassii | “ | [44] |
| Pseudodesmin A (28) | “ | “ | “ |
| Viscosamide (29) | Pseudomonas fluorescens | Biosurfactant, Antibiotic | [47] |
| Viscosin (30) | Pseudomonas viscosa, Pseudomonas fluorescens Pseudomonas libanensis | Antibiotic Phytotoxic, Surfactant Surfactant, Anticancer | [45] [51] [52] |
| Massetolide A (31) | Pseudomonas | Antibiotic | [53] |
| Massetolide B (32) | “ | No Activity | “ |
| Massetolide C (33) | “ | “ | “ |
| Massetolide D (34) | “ | “ | “ |
| Massetolide E (35) | “ | “ | “ |
| Massetolide F (36) | “ | “ | “ |
| Massetolide G (37) | “ | “ | “ |
| Massetolide H (38) | “ | “ | “ |
| Massetolide I (39) | “ | “ | “ |
| Massetolide J (40) | “ | “ | “ |
| Massetolide K (41) | “ | “ | “ |
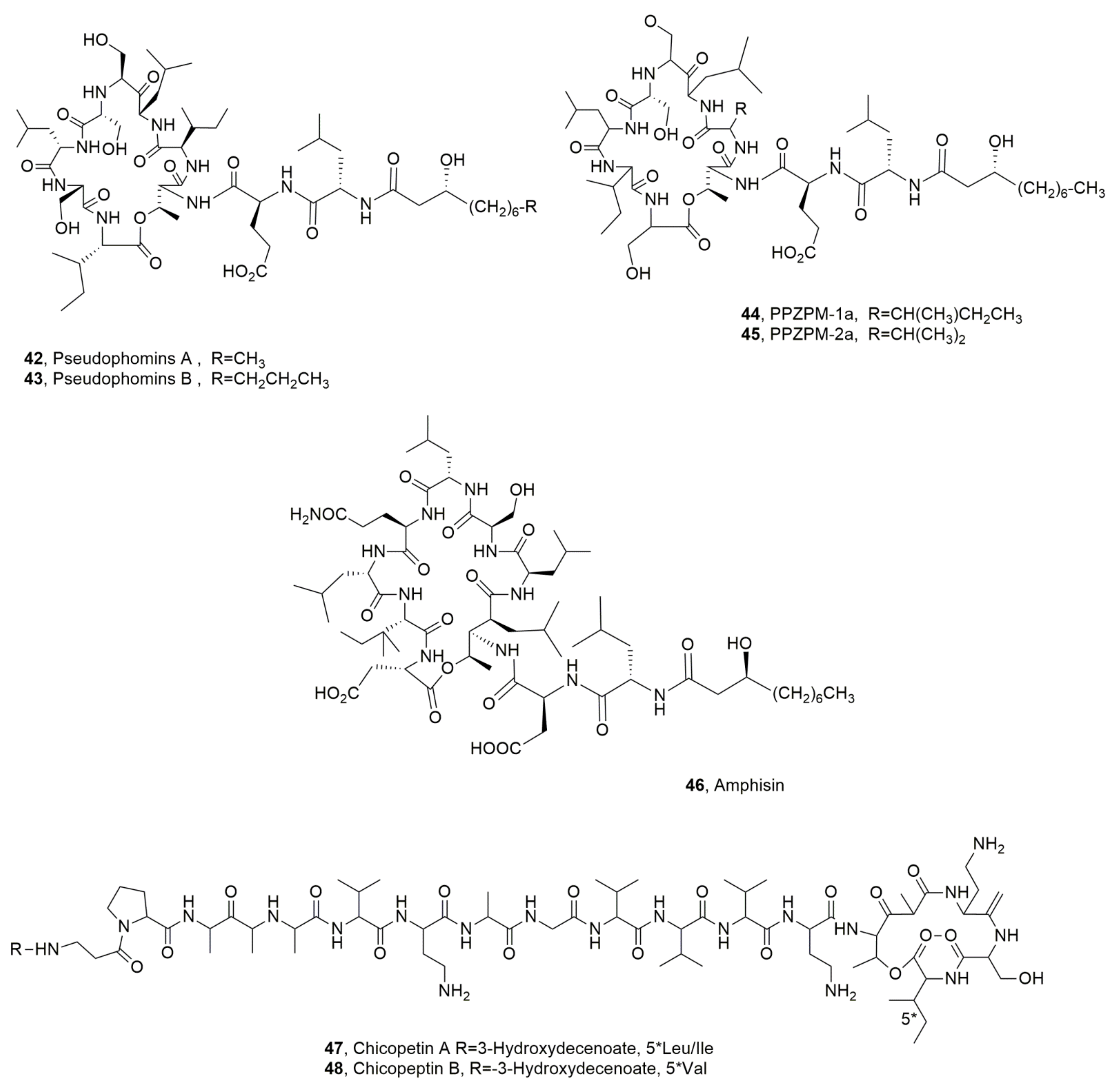
| Pseudophomin A (42) | Pseudomonas fluorescens | Phytotoxic, Antifungal | [49] |
| Pseudophomin B (43) | “ | “ | “ |
| PPZPM-1a (44) | Pseudomonas sp. JX090307 | No activity | [55] |
| PPZPM-2a (45) | “ | “ | “ |
| Amphisin (46) | Pseudomonas sp. | Biosurfactant, Antifungal | [56] [57] |
| Cichopeptin A (47) | Pseudomonas cichorii | Phytotoxic, Antibiotic | [67] |
| Cichopeptin B (48) | “ | “ | “ |
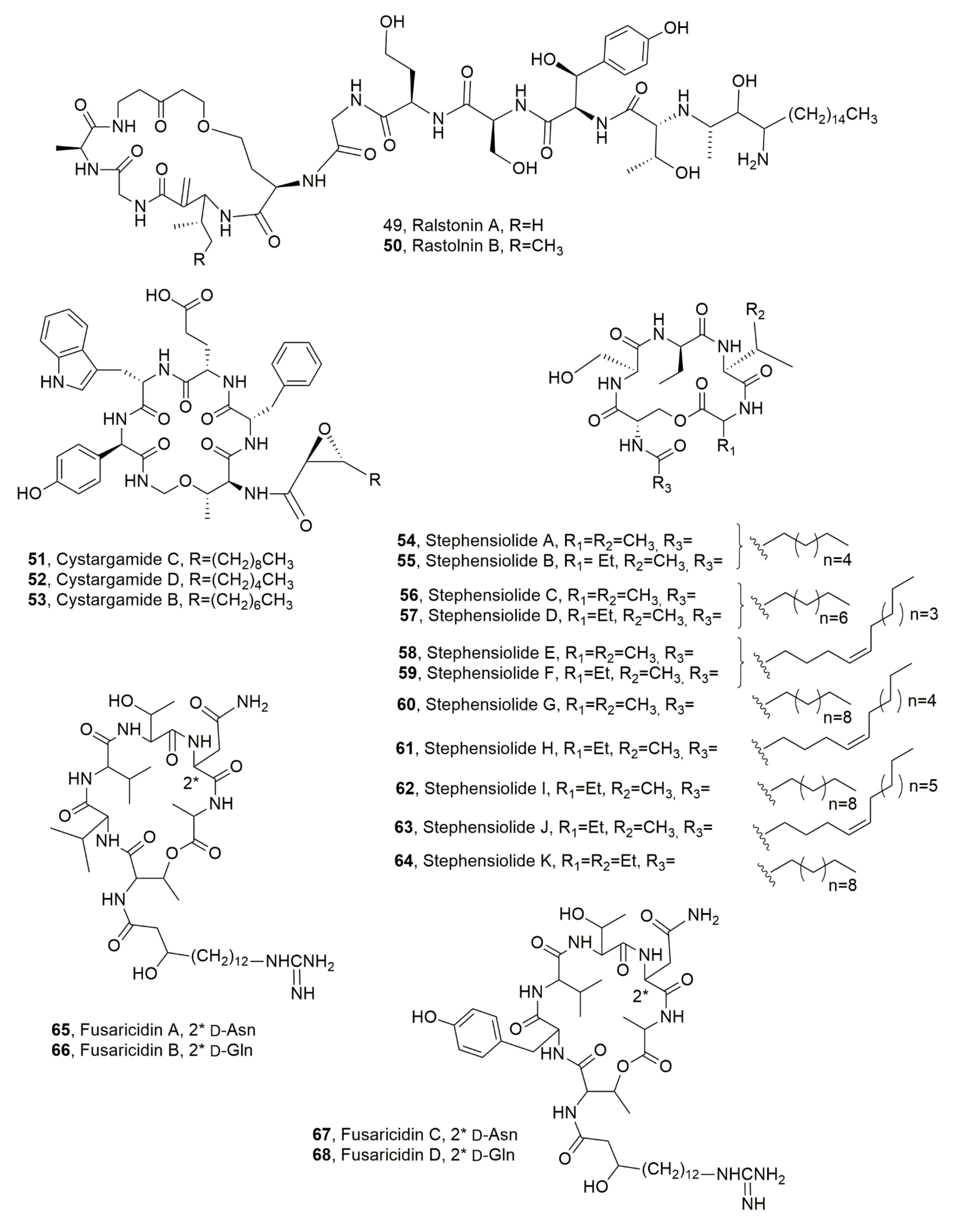
| Ralstonin A (49) | Ralstonia solanacearum | Chlamydospore-inducing activity, Phytotoxicity | [71] |
| Ralstonin B (50) | “ | “ | “ |
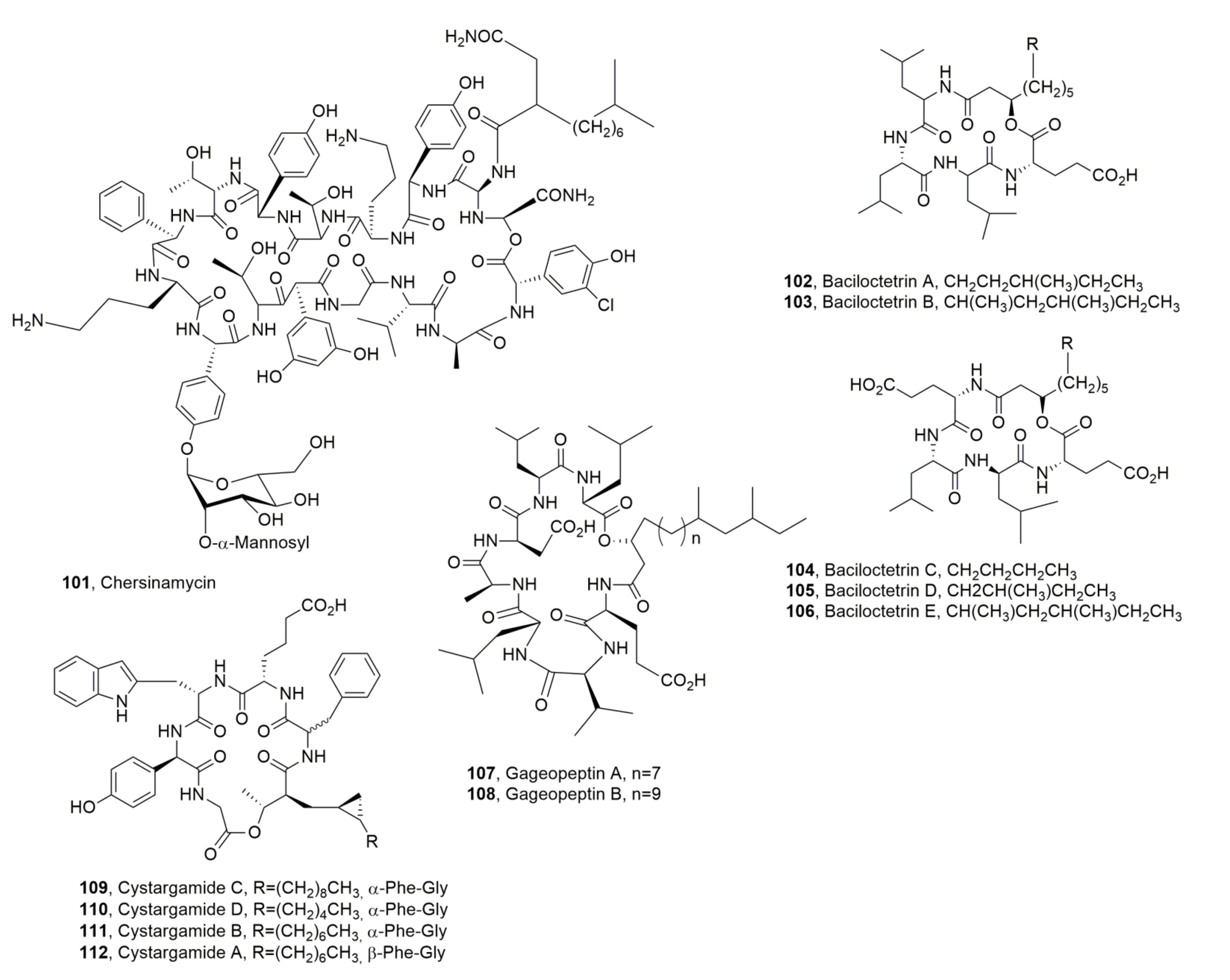
| Cystargamide C (51) | Streptomyces sp. | Antioxidant | [73] |
| Cystargamide D (52) | “ | “ | “ |
| Cystargamide B (53) | “ | “ | [72] |
| Stephensiolide A (54) | Serratia sp. | Antibiotic, Induction of bacterial motility | [74] |
| Stephensiolide B (55) | “ | “ | “ |
| Stephensiolide C (56) | “ | “ | “ |
| Stephensiolide D (57) | “ | “ | “ |
| Stephensiolide E (58) | “ | “ | “ |
| Stephensiolide F (59) | “ | “ | “ |
| Stephensiolide G (60) | “ | “ | “ |
| Stephensiolide H (61) | “ | “ | “ |
| Stephensiolide I (62) | “ | “ | “ |
| Stephensiolide J (63) | “ | “ | “ |
| Stephensiolide K (64) | “ | “ | “ |
| Fusaricidin A (65) | Paenibacillus polymyxa | Antimicrobial | [77] |
| Fusaricidin B (66) | “ | “ | [78] |
| Fusaricidin C (67) | “ | “ | “ |
| Fusaricidin D (68) | “ | “ | “ |
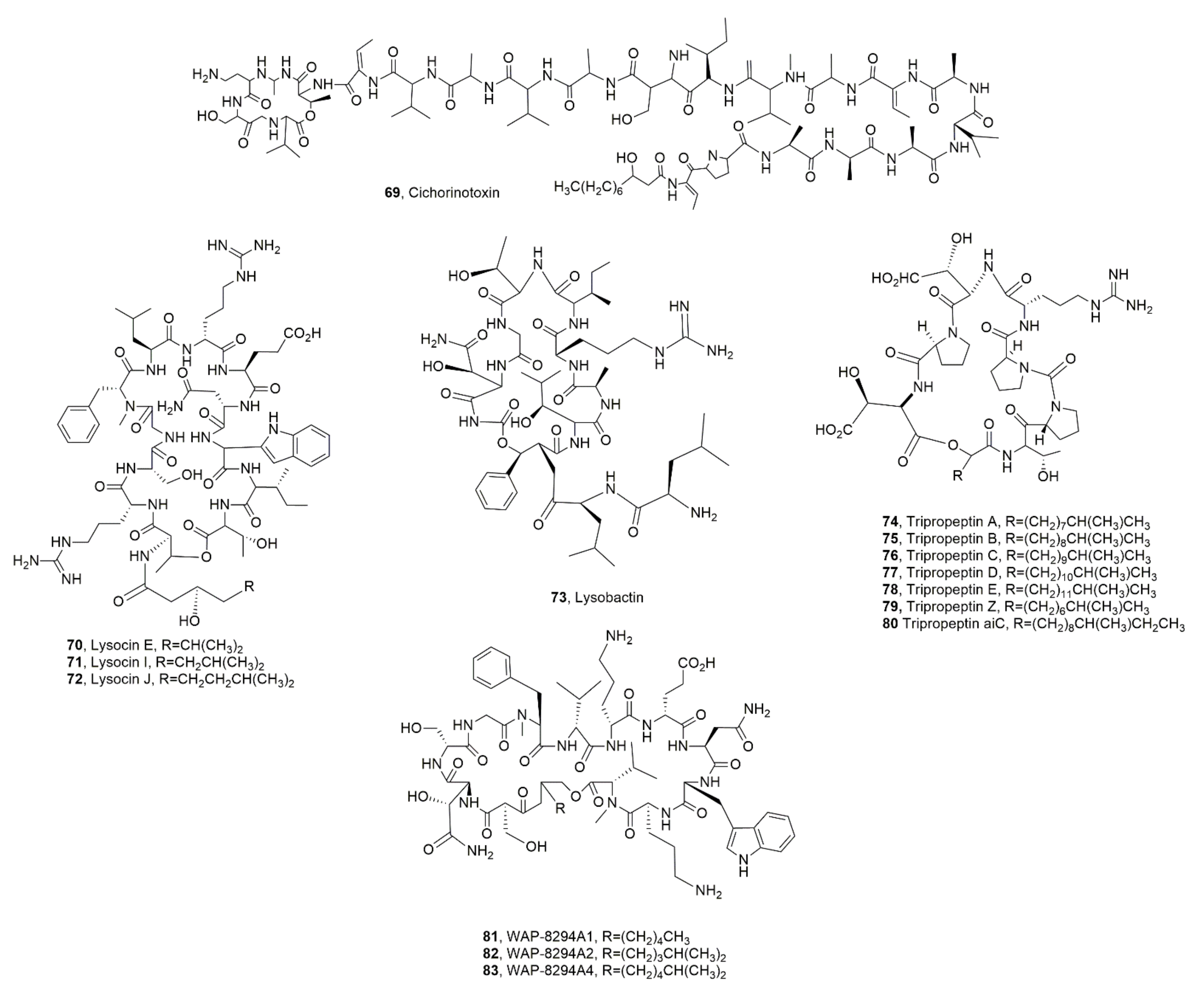
| Cichorinotoxin (69) | Pseudomonas cichorii | Phytotoxic | [85] |
| Lysocin E (70) | Lysobater sp. | Antibiotic | [86,89] |
| Lysocin I (71) | Lysobacter enzymogenes | “ | [89] |
| Lysocin J (72) | “ | “ | “ |
| Lysobactin (73) | Lysobacter sp. | “ | [99,100] |
| Tripropetin A (74) | “ | Antmicrobial | [92,93,101] |
| Tripropetin B (75) | “ | “ | “ |
| Tripropetin C (76) | “ | “ | “ |
| Tripropetin D (77) | “ | “ | “ |
| Tripropetin E (78) | “ | “ | “ |
| Tripropetin Z (79) | “ | “ | “ |
| Tripropetin aiC (80) | “ | “ | [102] |
| WAP-8294A1 (81) | “ Lysobacter antibioticus | Antibiotic “ | [104] [98] |
| WAP-8294A2 (82) | Lysobacter sp. | Antibiotic | [104] |
| WAP-8294A4 (83) | “ | “ | “ |
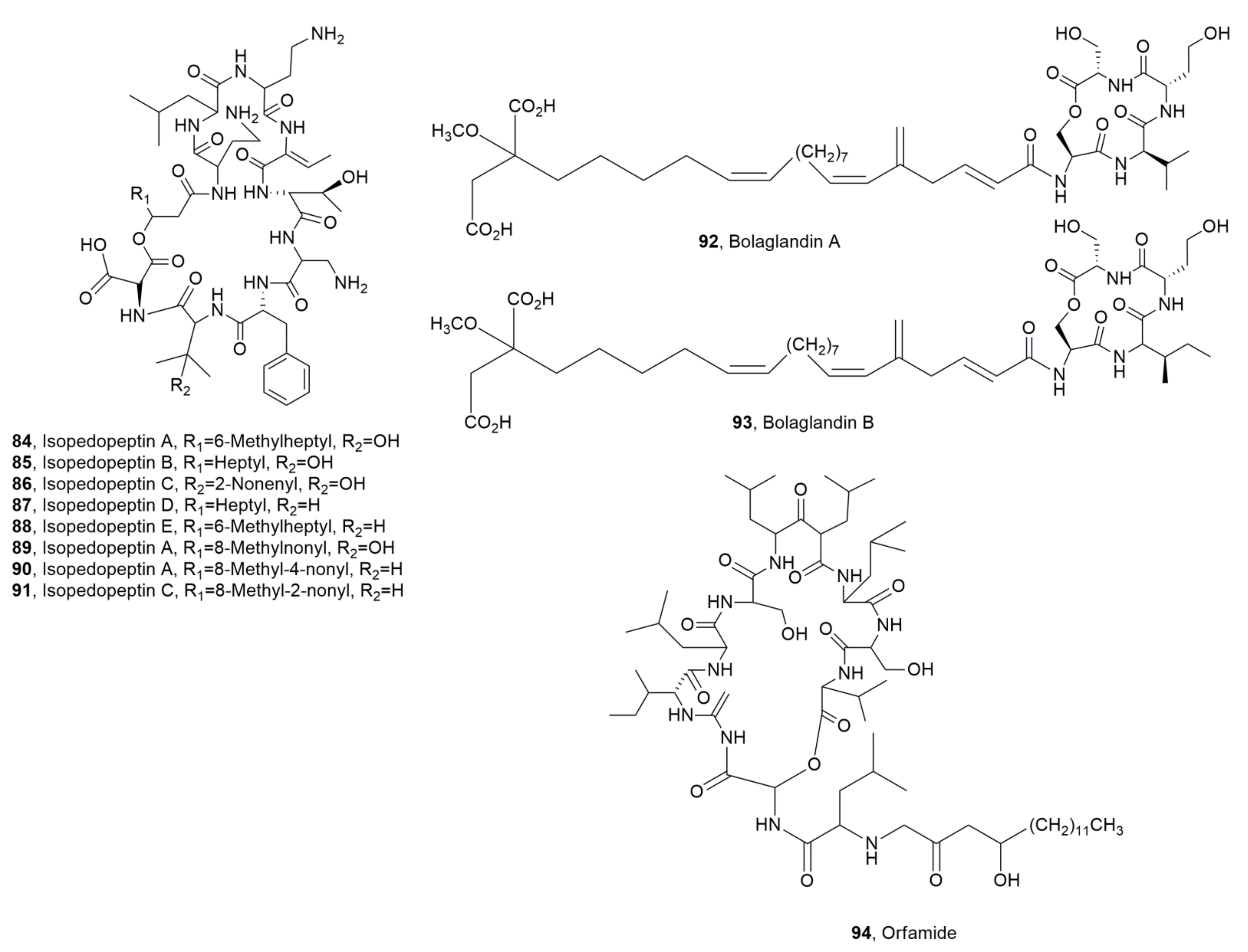
| Isopedopeptin A (84) | Antibiotic | [105] | |
| Isopedopeptin B (85) | Pedobacter cryoconitis | Antibiotic, Cytotoxic Hemolytic | “ |
| Isopedopeptin C (86) | “ | Antibiotic | “ |
| Isopedopeptin D (87) | “ | “ | “ |
| Isopedopeptin E (88) | “ | “ | “ |
| Isopedopeptin F (89) | “ | “ | “ |
| Isopedopeptin G (90) | “ | “ | “ |
| Isopedopeptin H (91) | “ | “ | “ |
| Bolagladin A (92) | Burkholderia gladioli | No activity | [106] |
| Bolagladin B (93) | “ | “ | “ |
| Orfamide H (94) | Pseudomonas protegens | Antifungal | [108] |
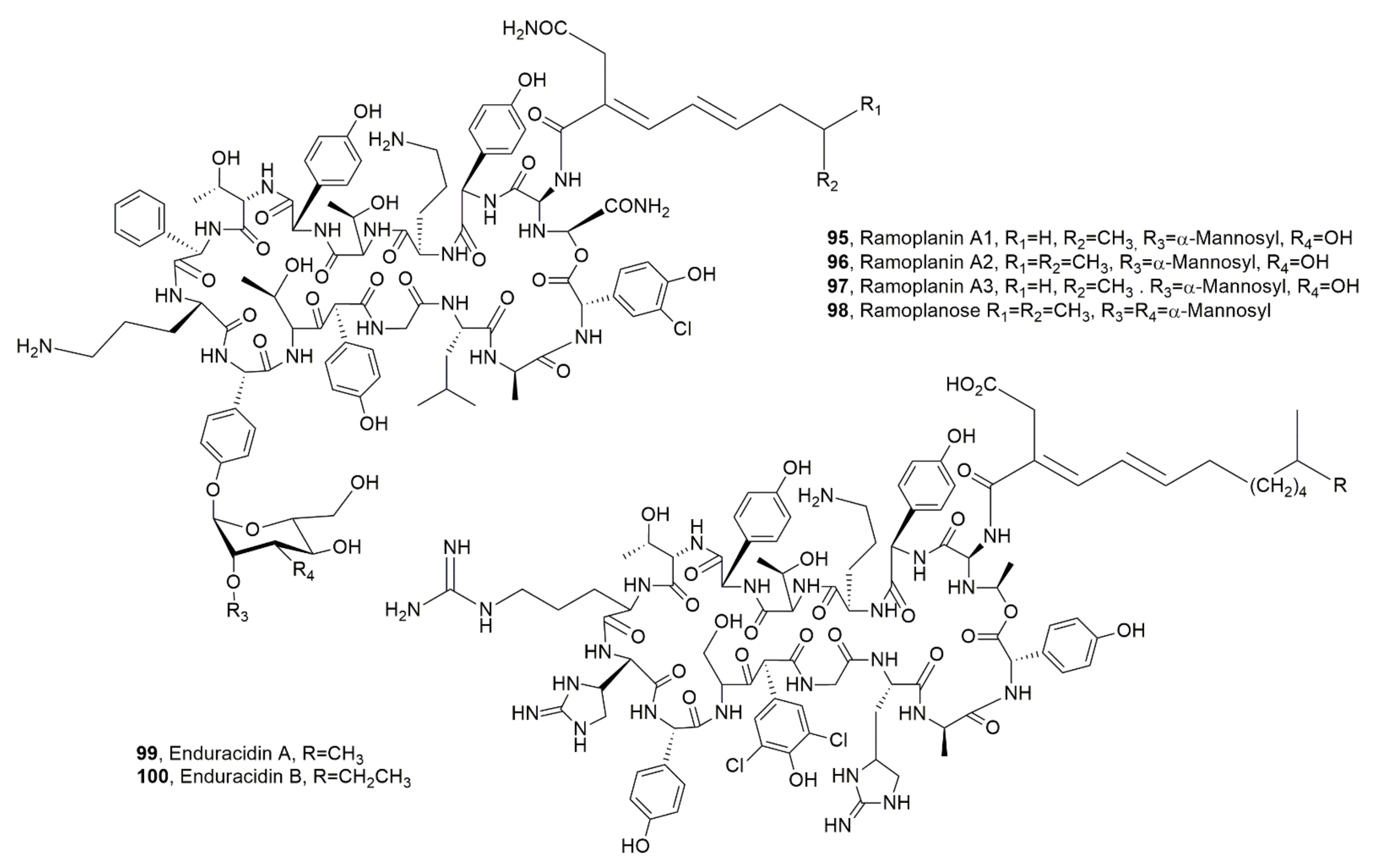
| Ramoplanin A1 (95) | Actinoplanes, Actinoplanes orientalis, Actinoplanes balhimycin, Micromonospora chersina | Antibiotic | [112] |
| Ramoplanin A2 (96) | “ | Antibiotic, Hemolytic | “ |
| Ramoplanin A3 (97) | “ | Antibotic | “ |
| Ramoplanose (98) | “ | “ | “ |
| Enduracidin A (99) | Streptomyces fungicidicus, A. orientalis, A. balhimycin, M. chersina | “ | “ |
| Enduracidin B (100) | “ | “ | “ |
| Chersinamycin (101) | Micromonospora chersina | “ | “ |
| Baciloctetrin A (102) | Bacillus subtilis | “ | [117] |
| Baciloctetrin B (103) | “ | “ | “ |
| Baciloctetrin C (104) | “ | Anti-micoplasma | [118] |
| Baciloctetrin D (105) | “ | “ | “ |
| Baciloctetrin E (106) | “ | “ | “ |
| Gageopeptin A (107) | “ | Impaire fungal zoospore motility, Antibiotiv | [119] |
| Gageopeptin B (108) | “ | “ | “ |
| Cystargamide C (109) | Streptomyces sp. | Antioxidant | [73] |
| Cystargamide D (110) | “ | “ | “ |
| Cystargamide B (111) | “ | “ | [72,73] |
| Lipodepsipeptides produced by fungi | |||
| Cystargamide A (112) | Kitasatospora cistarginea | No activity | [120] |
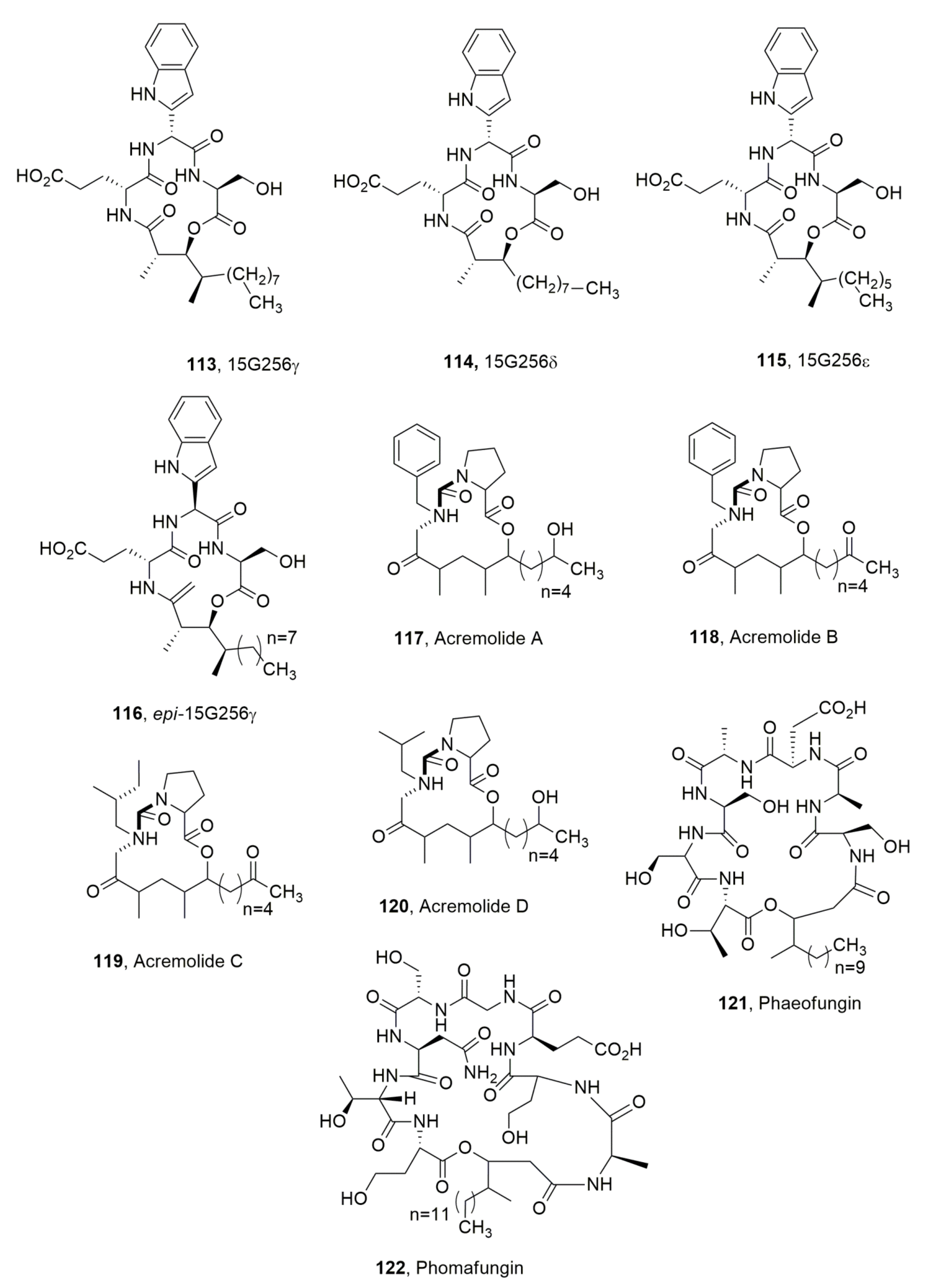
| 15G256γ (113) | Hypoxylon ocenicum | Antifungal | [113] |
| 15G256δ (114) | “ | “ | “ |
| 15G256ε (115) | “ | “ | “ |
| Epi-15G256ε (116) | Chemical derivative of 113 | No activity | “ |
| Acremolide A (117) | Acremonium sp. | “ | [123] |
| Acremolide B (118) | “ | “ | “ |
| Acremolide C (119) | “ | “ | “ |
| Acremolide D (120) | “ | “ | “ |
| Phaeofungin (121) | Phaeosphaeria sp. | Antifungal | [124] |
| Phomafungin (122) | Phoma sp. | “ | [125] |
| Verlamelin A (123) | Lecanicillium sp. | “ | [126] |
| Verlamelin B (124) | “ | “ | “ |
| Ophiotine (125) | Fungus of Ophiosphaerella genus | Nematocidal | [127] |
| Arthrichitin B (126) | No activity | “ | |
| Arthrichitin C (127) | Cytotoxic | “ | |
| Artrichitin (128) | No activity | “ | |
| Colletotrichamide A (129) | Colletotrichum gleosporioides | “ | [128] |
| Colletotrichamide B (130) | “ | Protective on HT22 cell hippocampal cell death | “ |
| Colletotrichamide C (131) | “ | “ | “ |
| Colletotrichamide D (132) | “ | No activity | “ |
| Colletotrichamide E (133) | “ | Protective om HT22 cell hippocampal cell death | “ |
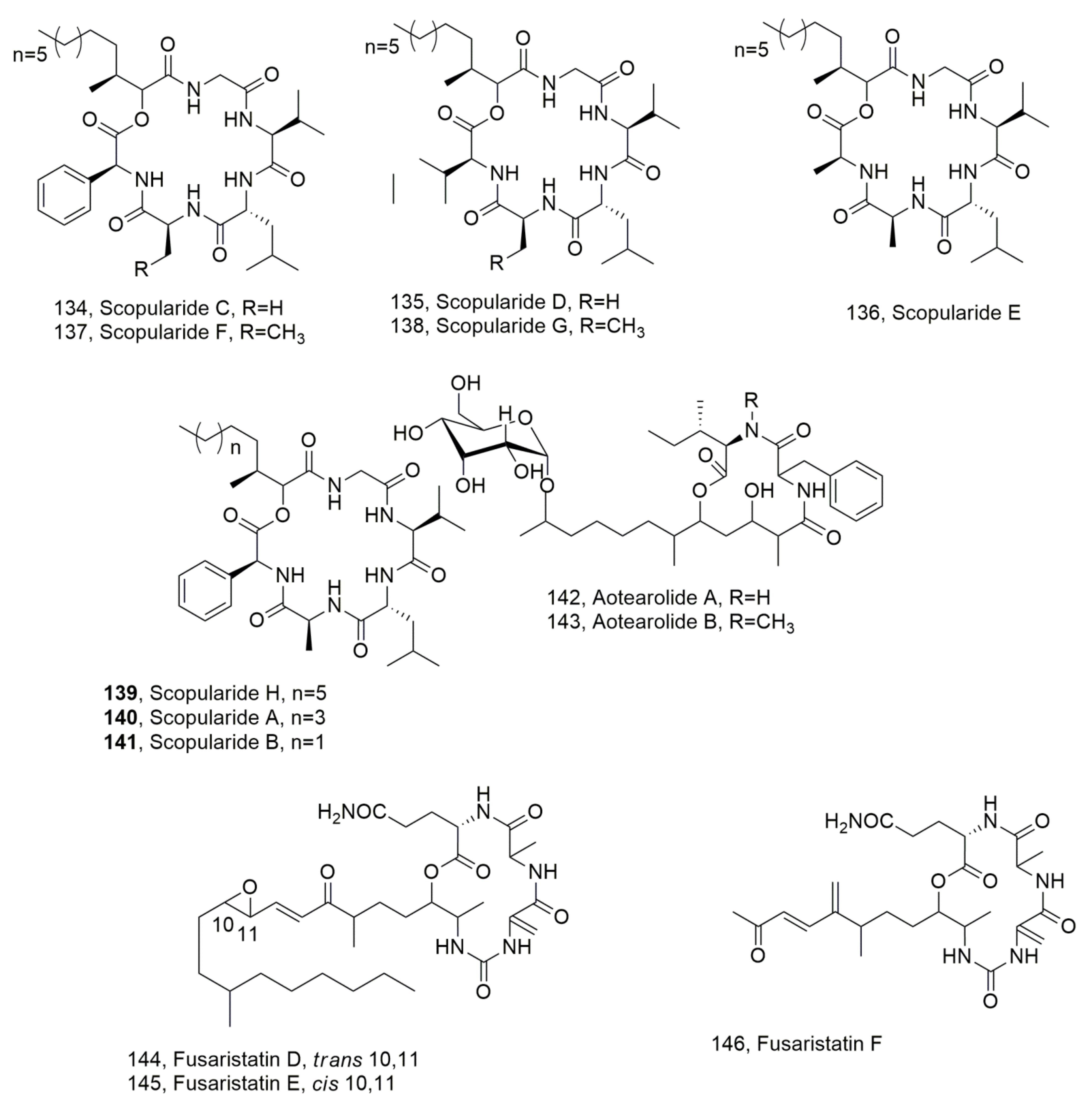
| Scopularide C (134) | Beauvaria sp. | No activity | [130] |
| Scopularide D (135) | “ | “ | “ |
| Scopularide E (136) | “ | “ | “ |
| Scopularide F (137) | “ | “ | “ |
| Scopularide G (138) | “ | “ | “ |
| Scopularide H (139) | Scopulariopsis sp. | “ | “ |
| Scopularide A (140) | Scopulariopsis brevicaulis | [129] | |
| Scopularide B (141) | “ | “ | |
| Aotearolide A (142) | Colletotrichum aotearoa | No activity | [131] |
| Aotearolide B (143) | “ | “ | “ |
| Fusaristatin D (144) | Fusarium sp. BZCB-CA | “ | [132] |
| Fusaristatin E (145) | “ | “ | “ |
| Fusaristatin F (146) | “ | “ | “ |
| Fusaristatin A (147) | Fusarium sp. YG-45 | Cytotoxic | [133] |
| Fusaristatin B (148) | “ | “ | “ |
| Fusaristatin C (149) | Pithomyces sp. | No activity | [134] |
| Lipodepsipeptides produced by bacteria pathogen of mushrooms | |||
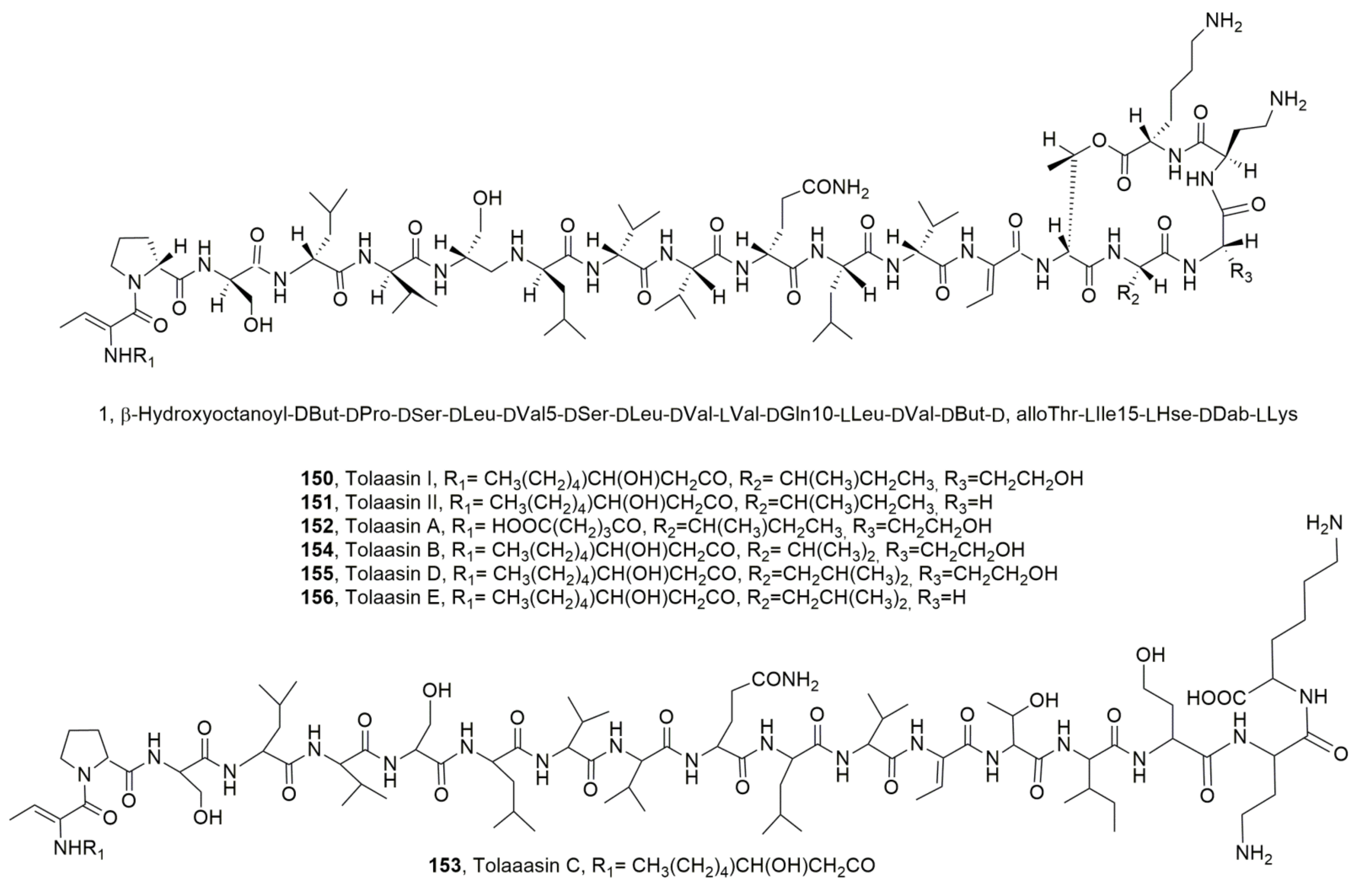
| Tolaasiin I (150) | Psudomonas tolaasii | Phytotoxic, Antimicrobial Hemolytic | [139] [140] [144] [137] |
| Tolaasiin II (151) | “ | “ | “ |
| Tolaasiin A (152) | “ | Antimicrobial | [140,144] |
| Tolaasiin B (153) | “ | “ | [140] |
| Tolaasiin C (154) | “ | “ | [140,144] |
| Tolaasiin D (155) | “ | “ | “ |
| Tolaasiin E (156) | “ | ||
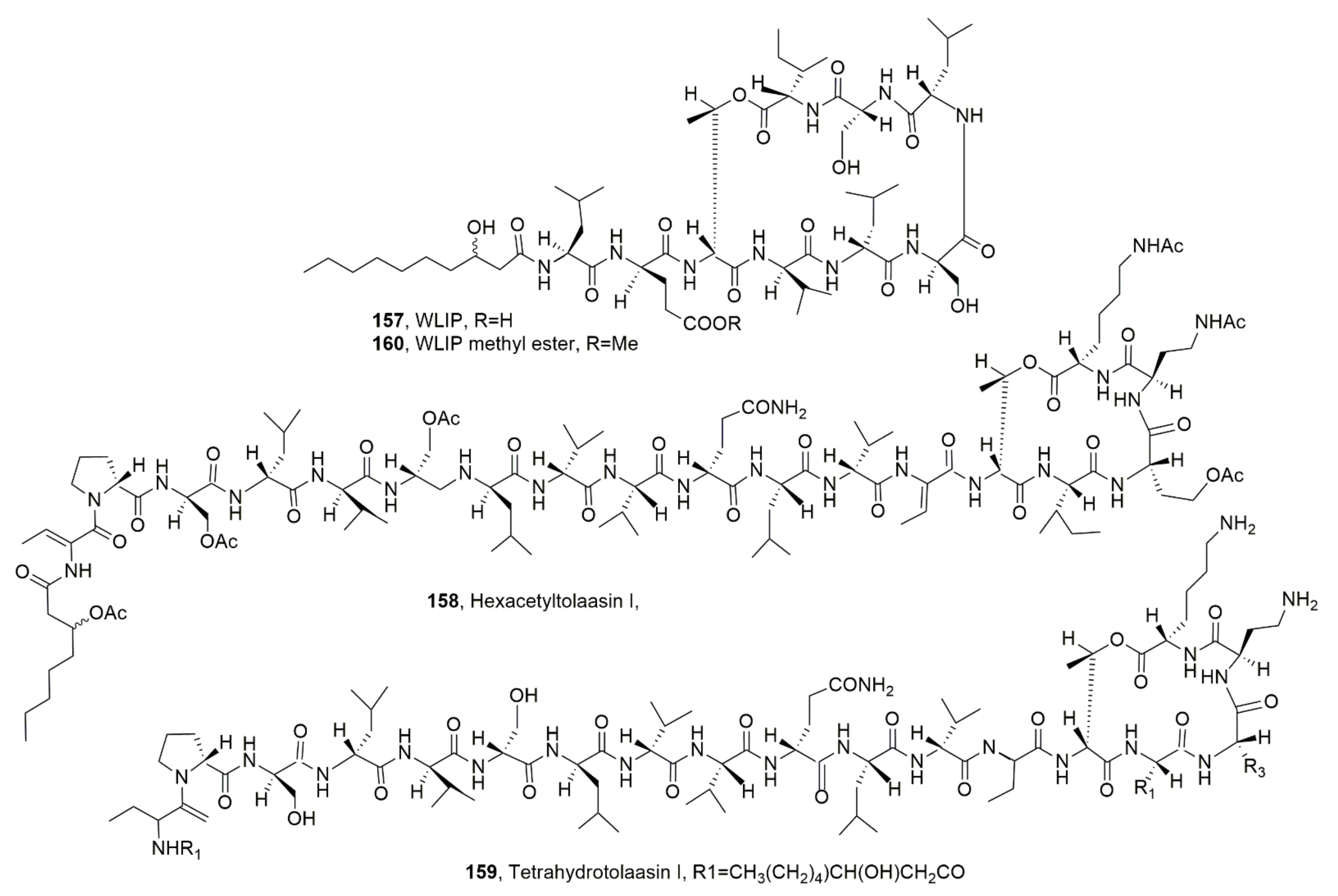
| WLIP (157) | Pseudomonas reactans | Phytotoxic, Antimicrobial, Hemolytic | [50] [137,145] [139] |
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Cimmino, A.; Masi, M.; Evidente, M.; Superchi, S.; Evidente, A. Fungal phytotoxins with potential herbicidal activity: Chemical and biological characterization. Nat. Prod. Rep. 2015, 32, 1629–1653. [Google Scholar] [CrossRef] [PubMed]
- Bender, C.L.; Alarcon-Chaidez, F.; Gross, D.C. Pseudomonas syringae phytotoxins: Mode of action, regulation, and biosynthesis by peptide and polyketide synthetases. Microbiol. Mol. Biol. Rev. 1999, 63, 266–292. [Google Scholar] [CrossRef] [PubMed]
- Ballio, A.; Barra, D.; Bossa, F.; Collina, A.; Grgurina, I.; Marino, G.; Moneti, G.; Paci, M.; Pucci, P.; Segre, A.; et al. Syringopeptins, new phytotoxic lipodepsipeptides of Pseudomonas syringae pv. syringae. FEBS Lett. 1991, 291, 109–112. [Google Scholar] [CrossRef]
- Ballio, A.; Bossa, F.; Di Giorgio, D.; Di Nola, A.; Manetti, C.; Paci, M.; Scaloni, A.; Segre, A.L. Solution conformation of the Pseudomonas syringae pv. syringae phytotoxic lipodepsipeptide syringopeptin 25-A: Twodimensional NMR, distance geometry and molecular dynamics. Eur. J. Biochem. 1995, 234, 747–758. [Google Scholar] [CrossRef] [PubMed]
- Jones, W.T.; Harvey, D.; Mitchell, R.E.; Ryan, G.B.; Bender, C.L.; Reynolds, P.H.S. Competitive ELISA employing monoclonal antibodies specific for coronafacoyl amino acid conjugates. Food Agric. Immunol. 1997, 9, 67–76. [Google Scholar] [CrossRef]
- Scaloni, A.; Camoni, L.; Di Giorgio, D.; Scortichini, M.; Cozzolino, R.; Ballio, A. A new syringopeptin produced by a Pseudomonas syringae pv. syringae strain isolated from diseased twigs of laurel. Physiol. Mol. Plant Pathol. 1997, 51, 259–264. [Google Scholar] [CrossRef]
- Isogai, A.; Iguchi, H.; Nakayama, J.; Kusai, A.; Takemoto, J.Y.; Suzuki, A. Structural analysis of new syringopeptins by tandem mass spectrometry. Biosci. Biotechnol. Biochem. 1995, 59, 1374–1376. [Google Scholar] [CrossRef]
- Bionda, N.; Cudic, P. Cyclic lipodepsipeptides in novel antimicrobial drug discovery. Croat. Chem. Acta 2011, 84, 315–329. [Google Scholar] [CrossRef]
- Guimarães, D.O.; Momesso, L.D.S.; Pupo, M.T. Antibiotics: Therapeutic importance and perspectives for the discovery and development of new agents. Quim. Nova 2010, 33, 667–679. [Google Scholar] [CrossRef]
- Schneider, T.; Sahl, H.-G. Lipid II and other bactoprenol-bound cell wall precursors as drug targets. Curr. Opin. Investig. Drugs 2010, 11, 157–164. [Google Scholar] [PubMed]
- Bionda, N.; Pitteloud, J.-P.; Cudic, P. Cyclic lipodepsipeptides: A new class of antibacterial agents in the battle against resistant bacteria. Future Med. Chem. 2013, 5, 1311–1330. [Google Scholar] [CrossRef] [PubMed]
- Roscetto, E.; Masi, M.; Esposito, M.; Di Lecce, R.; Delicato, A.; Maddau, L.; Calabrò, V.; Evidente, A.; Catania, M.R. Anti-biofilm activity of the fungal phytotoxin sphaeropsidin A against clinical isolates of antibiotic-resistant bacteria. Toxins 2020, 12, 444. [Google Scholar] [CrossRef]
- Cimmino, A.; Roscetto, E.; Masi, M.; Tuzi, A.; Radjai, I.; Gahdab, C.; Paolillo, R.; Guarino, A.; Catania, M.R.; Evidente, A. Sesquiterpene lactones from Cotula cinerea with antibiotic activity against clinical isolates of Enterococcus faecalis. Antibiotics 2021, 10, 819. [Google Scholar] [CrossRef]
- Masi, M.; Roscetto, E.; Cimmino, A.; Catania, M.R.; Surico, G.; Evidente, A. Farnesane-type sesquiterpenoids with antibiotic activity from Chiliadenus lopadusanus. Antibiotics 2021, 10, 148. [Google Scholar] [CrossRef] [PubMed]
- Balkovec, J.M. Section review: Anti-infectives: Lipopeptide antifungal agents. Expert Opin. Investig. Drugs 1994, 3, 65–82. [Google Scholar] [CrossRef]
- Sinden, S.L.; DeVay, J.E.; Backman, P.A. Properties of syringomycin, a wide spectrum antibiotic and phytotoxin produced by Pseudomonas syringae, and its role in the bacterial canker disease of peach trees. Physiol. Plant Pathol. 1971, 1, 199–213. [Google Scholar] [CrossRef]
- Deyay, J.E.; Lukezic, F.L.; Sinden, S.L.; English, H.; Coplin, D.L. A biocide produced by pathogenic isolates of Pseudomonas syringae and its possible role in the bacterial canker disease of peach trees. Phytopathology 1968, 58, 95–101. [Google Scholar]
- Ballio, A.; Barra, D.; Bossa, F.; DeVay, J.E.; Grgurina, I.; Iacobellis, N.S.; Marino, G.; Pucci, P.; Simmaco, M.; Surico, G. Multiple forms of syringomycin. Physiol. Mol. Plant Pathol. 1988, 33, 493–496. [Google Scholar] [CrossRef]
- Isogai, A.; Fukuchi, N.; Yamashita, S.; Suyama, K.; Suzuki, A. Syringostatins, novel phytotoxins produced by Pseudomonas syringae pv. syringae. Agric. Biol. Chem. 1989, 53, 3117–3119. [Google Scholar] [CrossRef][Green Version]
- Segre, A.; Bachmann, R.C.; Ballio, A.; Bossa, F.; Grgurina, I.; Iacobellis, N.S.; Marino, G.; Pucci, P.; Simmaco, M.; Takemoto, J.Y. The structure of syringomycins A1, E and G. FEBS Lett. 1989, 255, 27–31. [Google Scholar] [CrossRef]
- Adetuyi, F.C.; Isogai, A.; Di Giorgio, D.; Ballio, A.; Takemoto, J.Y. Saprophytic Pseudomonas syringae strain Ml of wheat produces cyclic lipodepsipeptides. FEMS Microbiol. Lett. 1995, 131, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Ballio, A.; Bossa, F.; Collina, A.; Gallo, M.; Iacobellis, N.S.; Paci, M.; Pucci, P.; Scaloni, A.; Simmaco, M. Structure of syringotoxin, a bioactive metabolite of Pseudomonas syringae pv. syringae. FEBS Lett. 1990, 269, 377–380. [Google Scholar] [CrossRef]
- Ballio, A.; Collina, A.; Nola, A.D.; Manetti, C.; Pad, M.; Segre, A. Determination of structure and conformation in solution of syringotoxin, a lipodepsipeptide from Pseudomonas syringae pv. syringae by 2D NMR and molecular dynamics. Struct. Chem. 1994, 5, 43–50. [Google Scholar] [CrossRef]
- Isogai, A.; Fukuchi, N.; Yamashita, S.; Suyama, K.; Suzuki, A. Structures of syringostatins A and B, novel phytotoxins produced by Pseudomonas syringae pv. syringae isolated from lilac blights. Tetrahedron Lett. 1990, 31, 695–698. [Google Scholar] [CrossRef]
- Fukuchi, N.; Isogai, A.; Yamashita, S.; Suyama, K.; Takemoto, J.Y.; Suzuki, A. Structure of phytotoxin syringomycin produced by a sugar cane isolate of Pseudomonas syringae pv. syringae. Tetrahedron Lett. 1990, 31, 1589–1592. [Google Scholar] [CrossRef]
- Harrison, L.; Teplow, D.B.; Rinaldi, M.; Strobel, G. Pseudomycins, a family of novel peptides from Pseudomonas syringae possessing broad-spectrum antifungal activity. J. Gen. Microbiol. 1991, 137, 2857–2865. [Google Scholar] [CrossRef]
- Lam, B.S.; Strobel, G.A.; Harrison, L.A.; Lam, S.T. Transposon mutagenesis and tagging of fluorescent Pseudomonas: Antimycotic production is necessary for control of Dutch elm disease. Proc. Natl. Acad. Sci. USA 1987, 84, 6447–6451. [Google Scholar] [CrossRef]
- Ballio, A.; Bossa, F.; Di Giorgio, D.; Ferranti, P.; Paci, M.; Pucci, P.; Scaloni, A.; Segre, A.; Strobel, G.A. Novel bioactive lipodepsipeptides from Pseudomonas syringae: The pseudomycins. FEBS Lett. 1994, 355, 96–100. [Google Scholar] [CrossRef]
- Duveiller, E.; Maraite, H. Bacterial sheath rot of wheat caused by Pseudomonas fuscovaginae in the highlands of Mexico. Plant Dis. 1990, 74, 932–935. [Google Scholar] [CrossRef]
- Ballio, A.; Bossa, F.; Camoni, L.; Di Giorgio, D.; Flamand, M.C.; Maraite, H.; Nitti, G.; Pucci, P.; Scaloni, A. Structure of fuscopeptins, phytotoxic metabolites of Pseudomonas fuscovaginae. FEBS Lett. 1996, 381, 213–216. [Google Scholar] [CrossRef]
- Baré, S.; Coiro, V.M.; Scaloni, A.; Di Nola, A.; Paci, M.; Segre, A.L.; Ballio, A. Conformations in solution of the fuscopeptins: Phytotoxic metabolites of Pseudomonas fuscovaginae. Eur. J. Biochem. 1999, 266, 484–492. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hutchison, M.L.; Gross, D.C. Lipopeptide phytotoxins produced by Pseudomonas syringae pv. syringae: Comparison of the biosurfactant and ion channel-forming activities of syringopeptin and syringomycin. Mol. Plant Microbe Interact. 1997, 10, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Lavermicocca, P.; Iacobellis, N.S.; Simmaco, M.; Graniti, A. Biological properties and spectrum of activity of Pseudomonas syringae pv. syringae toxins. Physiol. Mol. Plant Pathol. 1997, 50, 129–140. [Google Scholar] [CrossRef]
- Emanuele, M.C.; Scaloni, A.; Lavermicocca, P.; Jacobellis, N.S.; Camoni, L.; Di Giorgio, D.; Pucci, P.; Pace, M.; Segre, A.; Ballio, A. Corceptins, new bioactive lipodepsipeptides from cultures of Pseudomonas corrugata. FEBS Lett. 1998, 433, 317–320. [Google Scholar] [CrossRef]
- Scaloni, A.; Dalla Serra, M.; Amodeo, P.; Mannina, L.; Vitale, R.M.; Segre, A.L.; Cruciani, O.; Lodovichetti, F.; Greco, M.L.; Greco, M.L.; et al. Structure, conformation and biological activity of a novel lipodepsipeptide from Pseudomonas corrugata: Cormycin A. Biochem. J. 2004, 384, 25–36. [Google Scholar] [CrossRef]
- Kuiper, I.; Lagendijk, E.L.; Pickford, R.; Derrick, J.P.; Lamers, G.E.M.; Thomas-Oates, J.E.; Lugtenberg, B.J.J.; Bloemberg, G.V. Characterization of two Pseudomonas putida lipopeptide biosurfactants, putisolvin I and II, which inhibit biofilm formation and break down existing biofilms. Mol. Microbiol. 2004, 51, 97–113. [Google Scholar] [CrossRef]
- Burr, T.J.; Matteson, M.C.; Smith, C.A.; Corral-Garcia, M.R.; Huang, T.Z. Effectiveness of bacteria and yeasts from apple orchards as biological control agents of apple scab. Biol. Control 1996, 6, 151–157. [Google Scholar] [CrossRef]
- Grgurina, I.; Bensaci, M.; Pocsfalvi, G.; Mannina, L.; Cruciani, O.; Fiore, A.; Fogliano, V.; Sorensen, K.N.; Takemoto, J.Y. Novel cyclic lipodepsipeptide from Pseudomonas syringae pv. lachrymans strain 508 and syringopeptin antimicrobial activities. Antimicrob. Agents Chemother. 2005, 49, 5037–5045. [Google Scholar] [CrossRef]
- Boeck, L.D.; Papiska, H.R.; Wetzel, R.W.; Mynderse, J.S.; Fukuda, D.S.; Mertz, F.P.; Berry, D.M. A54145, a new lipopeptide antibiotic complex: Discovery, taxonomy, fermentation and HPLC. J. Antibiot. 1990, 43, 587–593. [Google Scholar] [CrossRef]
- Miao, V.; Brost, R.; Chapple, J.; She, K.; Gal, M.-F.C.-L.; Baltz, R.H. The lipopeptide antibiotic A54145 biosynthetic gene cluster from Streptomyces fradiae. J. Ind. Microbiol. Biotechnol. 2006, 33, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Counter, F.T.; Allen, N.E.; Fukuda, D.S.; Hobbs, J.N.; Ott, J.; Ensminger, P.W.; Mynderse, J.S.; Preston, D.A.; Wu, C.Y. A54145, a new lipopeptide antibiotic complex: Microbiological evaluation. J. Antibiot. 1990, 43, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Nybroe, O.; Sorensen, J. Production of cyclic lipopeptides by fluorescent pseudomonads. In Pseudomonas Macromolecules; Ramos, J.L., Ed.; Kluwer Academic/Plenum: New York, NY, USA, 2004; Volume IIId, pp. 147–172. [Google Scholar]
- Sinnaeve, D.; Michaux, C.; Van Hemel, J.; Vandenkerckhove, J.; Peys, E.; Borremans, F.A.; Sas, B.; Wouters, J.; Martins, J.C. Structure and X-ray conformation of pseudodesmins A and B, two new cyclic lipodepsipeptides from Pseudomonas bacteria. Tetrahedron 2009, 65, 4173–4181. [Google Scholar] [CrossRef]
- Kochi, M.; Weiss, D.W.; Pugh, L.H.; Groupé, V. Viscosin, a new antibiotic. Bacteriol. Proc. 1951, 1, 29–30. [Google Scholar]
- Laycock, M.V.; Hildebrand, P.D.; Thibault, P.; Walter, J.A.; Wright, J.L.C. Viscosin, a potent peptidolipid biosurfactant and phytopathogenic mediator produced by a pectolytic strain of Pseudomonas fluorescens. J. Agric. Food Chem. 1991, 39, 483–489. [Google Scholar] [CrossRef]
- Nielsen, T.H.; Christophersen, C.; Anthoni, U.; Sørensen, J. Viscosinamide, a new cyclic depsipeptide with surfactant and antifungal properties produced by Pseudomonas fluorescens DR54. J. Appl. Microbiol. 1999, 87, 80–90. [Google Scholar] [CrossRef]
- De Souza, J.T.; De Boer, M.; De Waard, P.; Van Beek, T.A.; Raaijmakers, J.M. Biochemical, genetic, and zoosporicidal properties of cyclic lipopeptide surfactants produced by Pseudomonas fluorescens. Appl. Environ. Microbiol. 2003, 69, 7161–7172. [Google Scholar] [CrossRef]
- Pedras, M.S.C.; Ismail, N.; Quail, J.W.; Boyetchko, S.M. Structure, chemistry, and biological activity of pseudophomins A and B, new cyclic lipodepsipeptides isolated from the biocontrol bacterium Pseudomonas fluorescens. Phytochemistry 2003, 62, 1105–1114. [Google Scholar] [CrossRef]
- Mortishire-Smith, R.J.; Nutkins, J.C.; Packman, L.C.; Brodey, C.L.; Rainey, P.B.; Johnstone, K.; Williams, D.H. Determination of the structure of an extracellular peptide produced by the mushroom saprotroph Pseudomonas reactans. Tetrahedron 1991, 47, 3645–3654. [Google Scholar] [CrossRef]
- Neu, T.R.; Härtner, T.; Poralla, K. Surface active properties of viscosin: A peptidolipid antibiotic. Appl. Microbiol. Biotechnol. 1990, 32, 518–520. [Google Scholar] [CrossRef]
- Saini, H.S.; Barragán-Huerta, B.E.; Lebrón-Paler, A.; Pemberton, J.E.; Vázquez, R.R.; Burns, A.M.; Marron, M.T.; Seliga, C.J.; Gunatilaka, A.A.L.; Maier, R.M. Efficient purification of the biosurfactant viscosin from Pseudomonas libanensis strain M9-3 and its physicochemical and biological properties. J. Nat. Prod. 2008, 71, 1011–1015. [Google Scholar] [CrossRef] [PubMed]
- Gerard, J.; Lloyd, R.; Barsby, T.; Haden, P.; Kelly, M.T.; Andersen, R.J. Massetolides A-H, antimycobacterial cyclic depsipeptides produced by two pseudomonads isolated from marine habitats. J. Nat. Prod. 1997, 60, 223–229. [Google Scholar] [CrossRef] [PubMed]
- De Bruijn, I.; de Kock, M.J.D.; de Waard, P.; van Beek, T.A.; Raaijmakers, J.M. Massetolide A biosynthesis in Pseudomonas fluorescens. J. Bacteriol. 2008, 190, 2777–2789. [Google Scholar] [CrossRef] [PubMed]
- Weißhoff, H.; Hentschel, S.; Zaspel, I.; Jarling, R.; Krause, E.; Pham, T.L.H. PPZPMs-A novel group of cyclic lipodepsipeptides produced by the Phytophthora alni associated strain Pseudomonas sp. JX090307-The missing link between the viscosin and amphisin group. Nat. Prod. Commun. 2014, 9, 989–996. [Google Scholar]
- Soerensen, D.; Nielsen, T.H.; Christophersen, C.; Soerensen, J.; Gajhede, M. Cyclic lipoundecapeptide amphisin from Pseudomonas sp. strain DSS73. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2001, 57, 1123–1124. [Google Scholar] [CrossRef]
- Groboillot, A.; Portet-Koltalo, F.; Le Derf, F.; Feuilloley, M.J.; Orange, N.; Poc, C.D. Novel application of cyclolipopeptide amphisin: Feasibility study as additive to remediate polycyclic aromatic hydrocarbon (PAH) contaminated sediments. Int. J. Mol. Sci. 2011, 12, 1787–1806. [Google Scholar] [CrossRef]
- Bradbury, J.F. Guide to Plant Pathogenic Bacteria; Oxford University Press: Oxford, UK, 1986. [Google Scholar]
- Jagger, I.C. A bacterial, leaf spot disease of celery (Abstract). Phytopathology 1914, 4, 395. [Google Scholar]
- Jones, J.B.; Raju, B.C.; Engelhard, A.W. Effects of temperature and leaf wetness on development of bacterial spot of geraniums and chrysanthemums incited by Pseudomonas cichorii. Plant Dis. 1984, 68, 248–251. [Google Scholar] [CrossRef]
- Wilkie, P.J.; Dye, D.W. Pseudomonas cichorii causing tomato and celery diseases in New Zealand. N. Z. J. Agric. Res. 1974, 17, 123–130. [Google Scholar] [CrossRef]
- Yu, S.-M.; Lee, Y.H. First report of Pseudomonas cichorii associated with leaf spot on soybean in South Korea. Plant Dis. 2012, 96, 142. [Google Scholar] [CrossRef]
- Hu, F.P.; Young, J.M.; Fletcher, M.J. Preliminary description of biocidal (syringomycin) activity in fluorescent plant pathogenic Pseudomonas species. J. Appl. Microbiol. 1998, 85, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Lazzaroni, S.; Evidente, A.; Surico, G. Toxic metabolites and lipopolysaccharides from Pseudomonas cichorii. In Pseudomonas syringae and Related Pathogens; Springer: Dordrecht, The Netherlands, 2003; pp. 233–243. [Google Scholar]
- Pauwelyn, E.; Huang, C.J.; Ongena, M.; Leclère, V.; Jacques, P.; Bleyaert, P.; Budzikiewicz, H.; Schäfe, M.; Höfte, M. New linear lipopeptides produced by Pseudomonas cichorii SF1-54 are involved in virulence, swarming motility, and biofilm formation. Mol. Plant Microbe Interact. 2013, 26, 585–598. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shirakawa, T.; Ozaki, K. Ultrastructural changes of lettuce tissue due to Pseudomonas cichorii and cichorin. Ann. Phytopathol. Soc. Jpn. 1993, 59, 316. [Google Scholar]
- Huang, C.-J.; Pauwelyn, E.; Ongena, M.; Debois, D.; Leclère, V.; Jacques, P.; Bleyaert, P.; Höfte, M. Characterization of cichopeptins, new phytotoxic cyclic lipodepsipeptides produced by Pseudomonas cichorii SF1-54 and their role in bacterial midrib rot disease of lettuce. Mol. Plant Microbe Interact. 2015, 28, 1009–1022. [Google Scholar] [CrossRef]
- Genin, S.; Denny, T.P. Pathogenomics of the Ralstonia solanacearum species complex. Annu. Rev. Phytopathol. 2012, 50, 67–89. [Google Scholar] [CrossRef]
- Brumbley, S.M.; Carney, B.F.; Denny, T.P. Phenotype conversion in Pseudomonas solanacearum due to spontaneous inactivation of PhcA, a putative LysR transcriptional regulator. J. Bacteriol. 1993, 175, 5477–5487. [Google Scholar] [CrossRef]
- Clough, S.J.; Schell, M.A.; Denny, T.P. Evidence for involvement of a volatile extracellular factor in Pseudomonas solanacearum virulence gene expression. Mol. Plant Microbe Interact. 1994, 7, 621–630. [Google Scholar] [CrossRef]
- Murai, Y.; Mori, S.; Konno, H.; Hikichi, Y.; Kai, K. Ralstonins A and B, lipopeptides with chlamydospore-inducing and phytotoxic activities from the plant pathogen Ralstonia solanacearum. Org. Lett. 2017, 19, 4175–4178. [Google Scholar] [CrossRef]
- Kitani, S.; Yoshida, M.; Boonlucksanawong, O.; Panbangred, W.; Anuegoonpipat, A.; Kurosu, T.; Ikuta, K.; Igarashi, Y.; Nihira, T. Cystargamide B, a cyclic lipodepsipeptide with protease inhibitory activity from Streptomyces sp. J. Antibiot. 2018, 71, 662–666. [Google Scholar] [CrossRef]
- Seo, J.; Shin, Y.-H.; Jo, S.J.; Du, Y.E.; Um, S.; Kim, Y.R.; Moon, K. Cystargamides C and D, new cyclic lipopeptides from a tidal mudflat-derived Streptomyces sp. JMS132. Front. Microbiol. 2022, 13, 904954. [Google Scholar] [CrossRef]
- Ganley, J.G.; Carr, G.; Ioerger, T.R.; Sacchettini, J.C.; Clardy, J.; Derbyshire, E.R. Discovery of antimicrobial lipodepsipeptides produced by a Serratia sp. within mosquito microbiomes. ChemBioChem 2018, 19, 1590–1594. [Google Scholar] [CrossRef] [PubMed]
- Haron, M.H.; Avula, B.; Shi, Q.; Li, X.C.; Ashfaq, M.K.; Bae, J.Y.; Shaohua Guanc, S.; Hincheec, M.; Khana, I.A.; Khan, S.I. Quantitative determination and pharmacokinetic study of fusaricidin A in mice plasma and tissues using ultra-high performance liquid chromatography-tandem mass spectrometry. J. Pharm. Biomed. Anal. 2019, 170, 187–192. [Google Scholar] [CrossRef]
- Kuroda, J.; Fukai, T.; Konishi, M.; Uno, J.; Kurusu, K.; Nomura, T. LI-F Antibiotics, a family of antifungal cyclic depsipeptides produced by Bacillus polymyxa L-1129. Heterocycles 2000, 53, 1533–1549. [Google Scholar] [CrossRef]
- Kajimura, Y.; Kaneda, M. Fusaricidin A, a new depsipeptide antibiotic producedby Bacillusa polymyxa KT-8: Taxonomy, fermentation, isolation, structureelucidation and biological activity. J. Antibiot. 1996, 49, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Kajimura, Y.; Kaneda, M. Fusaricidin B, C and D, new depsipeptide antibioticsproduced by Bacillusa Polymyxa KT-8: Isolation, structure elucidation and biological activity. J. Antibiot. 1997, 50, 220–228. [Google Scholar] [CrossRef]
- Kurusu, K.; Ohba, K.; Arai, T.; Fukushima, K. New peptide antibiotics LI-F03, F04, F05, F07, and F08,produced by Bacillus polymyxa. J. Antibiot. 1987, 40, 1506–1514. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-K.; Park, S.-Y.; Kim, R.; Lee, C.-H.; Kim, J.F.; Park, S.-H. Identification andfunctional analysis of the fusaricidin biosynthetic gene of Paenibacillus polymyxa E681. Biochem. Biophys. Res. Commun. 2008, 365, 89–95. [Google Scholar] [CrossRef]
- Cochrane, J.R.; McErlean, C.S.P.; Jolliffe, K.A. Total synthesis and assignment of the side chain stereochemistry of LI-F04a: An antimicrobial cyclicdepsipeptide. Org. Lett. 2010, 12, 3394–3397. [Google Scholar] [CrossRef]
- Beatty, P.H.; Jensen, S.E. Paenibacillus polymyxa produces fusaricidin-type antifungal antibiotics active against Leptosphaeria maculans, the causativeagent of blackleg disease of canola. Can. J. Microbiol. 2002, 48, 159–169. [Google Scholar] [CrossRef]
- Yu, W.-B.; Yin, C.-Y.; Zhou, Y.; Ye, B.-C. Prediction of the mechanism of action of fusaricidin on Bacillus subtilis. PLoS ONE 2012, 7, e50003. [Google Scholar] [CrossRef]
- Vater, J.; Herfort, S.; Doellinger, J.; Weydmann, M.; Dietel, K.; Faetke, S.; Lasch, P. Fusaricidins from Paenibacillus polymyxa M-1, a family of lipohexapeptides of unusual complexity-A mass spectrometric study. J. Mass Spectrom. 2017, 52, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, H.; Shirakawa, T.; Uchiyama, T.; Hoshino, T. Chemical structure of cichorinotoxin, a cyclic lipodepsipeptide that is produced by Pseudomonas cichorii and causes varnish spots on lettuce. Beilstein J. Org. Chem. 2019, 15, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Hamamoto, H.; Urai, M.; Ishii, K.; Yasukawa, J.; Paudel, A.; Murai, M.; Kaji, T.; Kuranaga, T.; Hamase, K.; Katsu, T.; et al. Lysocin E is a new antibiotic that targets menaquinone in the bacterial membrane. Nat. Chem. Biol. 2015, 11, 127–133. [Google Scholar] [CrossRef]
- Panthee, S.; Hamamoto, H.; Paudel, A.; Sekimizu, K. Lysobacter species: A potential source of novel antibiotics. Arch. Microbiol. 2016, 198, 839–845. [Google Scholar] [CrossRef]
- Yu, L.; Du, F.; Chen, X.; Zheng, Y.; Morton, M.; Liu, F.; Du, L. Identification of the biosynthetic gene cluster for the anti-MRSA lysocins through gene cluster activation using strong promoters of housekeeping genes and production of new analogs in Lysobacter sp. 3655. ACS Synth. Biol. 2020, 9, 1989–1997. [Google Scholar] [CrossRef]
- Santiago, M.; Lee, W.; Fayad, A.A.; Coe, K.A.; Rajagopal, M.; Do, T.; Hennessen, F.; Srisuknimit, V.; Müller, R.; Meredith, T.C.; et al. Genome-wide mutant profiling predicts the mechanism of a Lipid II binding antibiotic. Nat. Chem. Biol. 2018, 14, 601–608. [Google Scholar] [CrossRef]
- Christensen, P.; Cook, F.D. Lysobacter, a new genus of non-fruiting, gliding bacteria with a high base ratio. Int. J. Syst. Bacteriol. 1978, 28, 367–393. [Google Scholar] [CrossRef]
- Xie, Y.; Wright, S.; Shen, Y.; Du, L. Bioactive natural products from Lysobacter. Nat. Prod. Rep. 2012, 29, 1277–1287. [Google Scholar] [CrossRef]
- Hashizume, H.; Igarashi, M.; Hattori, S.; Hori, M.; Hamada, M.; Takeuchi, T. Tripropeptins, novel antimicrobial agents produced by Lysobacter sp. I. Taxonomy, isolation and biological activities. J. Antibiot. 2001, 54, 1054–1059. [Google Scholar] [CrossRef]
- Hashizume, H.; Hirosawa, S.; Sawa, R.; Muraoka, Y.; Ikeda, D.; Naganawa, H.; Igarashi, M. Tripropeptins, novel antimicrobial agents produced by Lysobacter sp. II. Structure elucidation. J. Antibiot. 2004, 57, 52–58. [Google Scholar] [CrossRef]
- Itoh, H.; Tokumoto, K.; Kaji, T.; Paudel, A.; Panthee, S.; Hamamoto, H.; Sekimizu, K.; Inoue, M. Total synthesis and biological mode of action of WAP-8294A2: A menaquinonetargeting antibiotic. J. Org. Chem. 2018, 83, 6924–6935. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, Y.; Qian, G.; Wang, Y.; Chen, H.; Li, Y.-Z.; Liu, F.; Shen, Y.; Du, L. Identification and characterization of the anti-methicillin-resistant Staphylococcus aureus WAP-8294A2 biosynthetic gene cluster from Lysobacter enzymogenes OH11. Antimicrob. Agents Chemother. 2011, 55, 5581–5589. [Google Scholar] [CrossRef]
- Murai, M.; Kaji, T.; Kuranaga, T.; Hamamoto, H.; Sekimizu, K.; Inoue, M. Total synthesis and biological evaluation of the antibiotic lysocin E and its enantiomeric, epimeric, and N-demethylated analogues. Angew. Chem. Int. Ed. Engl. 2015, 54, 1556–1560. [Google Scholar] [CrossRef] [PubMed]
- Itoh, H.; Tokumoto, K.; Kaji, T.; Paudel, A.; Panthee, S.; Hamamoto, H.; Sekimizu, K.; Inoue, M. Development of ahigh-throughput strategy for discovery of potent analogues of antibiotic lysocin E. Nat. Commun. 2019, 10, 2992. [Google Scholar] [CrossRef] [PubMed]
- Sang, M.; Wang, H.; Shen, Y.; de Almeida, N.R.; Conda-Sheridan, M.; Li, S.; Li, Y.; Du, L. Identification of an anti-MRSA cyclic lipodepsipeptide, WBP29479A1, by genome mining of Lysobacter antibioticus. Org. Lett. 2019, 21, 6432–6436. [Google Scholar] [CrossRef]
- Bonner, D.P.; O’Sullivan, J.; Tanaka, S.K.; Clark, J.M.; Whitney, R.R. Lysobactin, a novel antibacterial agent produced by Lysobacter sp. II. Biological properties. J. Antibiot. 1988, 41, 1745–1751. [Google Scholar] [CrossRef]
- Lee, W.; Schaefer, K.; Qiao, Y.; Srisuknimit, V.; Steinmetz, H.; Muller, R.; Kahne, D.; Walker, S. The mechanism ofaction of lysobactin. J. Am. Chem. Soc. 2016, 138, 100–103. [Google Scholar] [CrossRef]
- Hashizume, H.; Hattori, S.; Igarashi, M.; Akamatsu, Y. Tripropeptin E, a new tripropeptin group antibiotic produced by Lysobacter sp. BMK333-48F3. J. Antibiot. 2004, 57, 394–399. [Google Scholar] [CrossRef]
- Hashizume, H.; Sawa, R.; Harada, S.; Igarashi, M.; Adachi, H.; Nishimura, Y.; Nomoto, A. Tripropeptin C blocks the lipid cycle of cell wall biosynthesis by complex formation with undecaprenyl pyrophosphate. Antimicrob. Agents Chemother. 2011, 55, 3821–3828. [Google Scholar] [CrossRef]
- Hashizume, H.; Igarashi, M.; Sawa, R.; Adachi, H.; Nishimura, Y.; Akamatsu, Y. A new type of tripropeptin with anteiso-branched chain fatty acid from Lysobacter sp. BMK333-48F3. J. Antibiot. 2008, 61, 577–582. [Google Scholar] [CrossRef]
- Kato, A.; Nakaya, S.; Kokubo, N.; Aiba, Y.; Ohashi, Y.; Hirata, H. A new anti-MRSA antibiotic complex, WAP8294A. I. Taxonomy, isolation and biological activities. J. Antibiot. 1998, 51, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Nord, C.; Bjerketorp, J.; Levenfors, J.J.; Cao, S.; Stromstedt, A.A.; Guss, B.; Larsson, R.; Hughes, D.; Öberg, B.; Broberg, A. Isopedopeptins A–H: Cationic cyclic lipodepsipeptides from Pedobacter cryoconitis UP508 targeting WHO top-priority Carbapenem-resistant bacteria. ACS Chem. Biol. 2020, 15, 2937–2944. [Google Scholar] [CrossRef] [PubMed]
- Dashti, Y.; Nakou, I.T.; Mullins, A.J.; Webster, G.; Jian, X.; Mahenthiralingam, E.; Challis, G.L. Discovery and biosynthesis of bolagladins: Unusual lipodepsipeptides from Burkholderia gladioli clinical isolates. Angew. Chem. 2020, 132, 21737–21745. [Google Scholar] [CrossRef]
- Stutz, E.; Defago, G.; Kern, H. Naturally occurring fluorescent pseudomonads involved in suppression of black root rot of tobacco. Phytopathology 1986, 76, 181–185. [Google Scholar] [CrossRef]
- Ma, Z.; Zhang, S.; Liang, J.; Sun, K.; Hu, J. Isolation and characterization of a new cyclic lipopeptide orfamide H from Pseudomonas protegens CHA0. J. Antibiot. 2020, 73, 179–183. [Google Scholar] [CrossRef]
- Cavalleri, B.; Pagani, H.; Volpe, G.; Selva, E.; Parenti, F. A-16686, a new antibiotic from Actinoplanes I. Fermentation, isolation and preliminary physico-chemical characteristic. J. Antibiot. 1984, 37, 309–317. [Google Scholar] [CrossRef]
- Parenti, F.; Ciabatti, R.; Cavalleri, B.; Kettenring, J. Ramoplanin: A review of its discovery and its chemistry. Drug Exp. Clin. Res. 1990, 16, 451–455. [Google Scholar]
- Higashide, E.; Hatano, K.; Shibata, M.; Nakazawa, K. Enduradicinin, a new antiobiotioc. I Streptomyces fungicidicus no. B 5477, and enduracidin production organism. J. Antibiot. 1968, 21, 126–137. [Google Scholar] [CrossRef]
- Morgan, K.T.; Zheng, J.; McCafferty, D.G. Discovery of six ramoplanin family gene clusters and the lipoglycodepsipeptide chersinamycin. ChemBioChem 2021, 22, 176–185. [Google Scholar] [CrossRef]
- Cheng, M.; Huang, J.X.; Ramu, S.; Butler, M.S.; Cooper, M.A. Ramoplanin at bactericidal concentrations induces bacterial membrane depolarization in Staphylococcus aureus. Antimicrob. Agents Chemother. 2014, 58, 6819–6827. [Google Scholar] [CrossRef]
- Ciabatti, R.; Maffioli, S.I.; Panzone, G.; Canavesi, A.; Michelucci, E.; Tiseni, P.S.; Marzorati, E.; Checchia, A.; Giannone, M.; Jabes, D.; et al. Synthesis and preliminary biological characterization of new semisynthetic derivatives of ramoplanin. J. Med. Chem. 2007, 50, 3077–3085. [Google Scholar] [CrossRef] [PubMed]
- Goto, S.; Kuwahara, S.; Okubo, N.; Zenyoji, H. In vitro and in vitro evaluation of enduracidin, a new peptide antibiotic substance. J. Antibiot. 1968, 21, 119–125. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tsuchiya, K.; Kondo, M.; Oishi, T.; Yamazaki, I. Enduracidin, a new antibiotic. 3. In vitro and in vivo antimicrobial activity. J. Antibiot. 1968, 21, 147–153. [Google Scholar] [CrossRef]
- Tareq, F.S.; Shin, H.J. Bacilotetrins A and B, anti-staphylococcal cyclic-lipotetrapeptides from a marine-derived Bacillus subtilis. J. Nat. Prod. 2017, 80, 2889–2892. [Google Scholar] [CrossRef]
- Lee, H.-S.; Shin, H.J. Anti-mycoplasma activity of bacilotetrins C-E, cyclic lipodepsipeptides from the marine-derived Bacillus subtilis and structure revision of bacilotetrins A and B. Mar. Drugs 2021, 19, 528. [Google Scholar] [CrossRef]
- Tareq, F.S.; Hasan, C.M.; Lee, H.S.; Lee, Y.J.; Lee, J.S.; Surovy, M.Z.; Islam, M.T.; Shin, H.J. Gageopeptins A and B, new inhibitors of zoospore motility of the phytopathogen Phytophthora capsici from a marine-derived bacterium Bacillus sp. 109GGC020. Bioorganic Med. Chem. Lett. 2015, 25, 3325–3329. [Google Scholar] [CrossRef]
- Gill, K.A.; Berrué, F.; Arens, J.C.; Kerr, R.G. Isolation and structure elucidation of cystargamide, a lipopeptide from Kitasatospora cystarginea. J. Nat. Prod. 2014, 77, 1372–1376. [Google Scholar] [CrossRef]
- Schlingmann, G.; Milne, L.; Williams, D.R.; Carter, G.T. Cell wall active antifungal compounds produced by the marine fungus Hypoxylon oceanicum LL-15G256 II. Isolation and structure determination. J. Antibiot. 1998, 51, 303–316. [Google Scholar] [CrossRef]
- Osada, H.; Kakeya, H.; Hayashi, Y.; Shoji, M.; Uchida, S.P. Japanese Patent 101483, 2004.
- Ratnayake, R.; Fremlin, L.J.; Lacey, E.; Gill, J.H.; Capon, R.J. Acremolides A–D, lipodepsipeptides from an Australian marine-derived fungus, Acremonium sp. J. Nat. Prod. 2008, 71, 403–408. [Google Scholar] [CrossRef]
- Singh, S.B.; Ondeyka, J.; Harris, G.; Herath, K.; Zink, D.; Vicente, F.; Bills, G.; Collado, J.; Platas, G.; González del Val, A.; et al. Isolation, structure, and biological activity of phaeofungin, a cyclic lipodepsipeptide from a Phaeosphaeria sp. using the genome-wide Candida albicans fitness test. J. Nat. Prod. 2013, 76, 334–345. [Google Scholar] [CrossRef]
- Herath, K.; Harris, G.; Jayasuriya, H.; Zink, D.; Smith, S.; Vicente, F.; Bills, G.; Collado, J.; González, A.; Jiang, B.; et al. Isolation, structure and biological activity of phomafungin, a cyclic lipodepsipeptide from a widespread tropical Phoma sp. Bioorg. Med. Chem. 2009, 17, 1361–1369. [Google Scholar] [CrossRef] [PubMed]
- Ishidoh, K.I.; Kinoshita, H.; Igarashi, Y.; Ihara, F.; Nihira, T. Cyclic lipodepsipeptides verlamelin A and B, isolated from entomopathogenic fungus Lecanicillium sp. J. Antibiot. 2014, 67, 459–463. [Google Scholar] [CrossRef] [PubMed]
- Helaly, S.E.; Ashrafi, S.; Teponno, R.B.; Bernecker, S.; Dababat, A.A.; Maier, W.; Stadler, M. Nematicidal cyclic lipodepsipeptides and a xanthocillin derivative from a phaeosphariaceous fungus parasitizing eggs of the plant parasitic nematode Heterodera filipjevi. J. Nat. Prod. 2018, 81, 2228–2234. [Google Scholar] [CrossRef] [PubMed]
- Bang, S.; Lee, C.; Kim, S.; Song, J.H.; Kang, K.S.; Deyrup, S.T.; Nam, S.-J.; Xia, X.; Shim, S.H. Neuroprotective glycosylated cyclic lipodepsipeptides, colletotrichamides A–E, from a halophyte-associated fungus, Colletotrichum gloeosporioides JS419. J. Org. Chem. 2019, 84, 10999–11006. [Google Scholar] [CrossRef]
- Yu, Z.; Lang, G.; Kajahn, I.; Schmaljohann, R.; Imhoff, J.F. Scopularides A and B, cyclodepsipeptides from a marine sponge-derived fungus, Scopulariopsis brevicaulis. J. Nat. Prod. 2008, 71, 1052–1054. [Google Scholar] [CrossRef]
- Elbanna, A.H.; Khalil, Z.G.; Bernhardt, P.V.; Capon, R.J. Scopularides revisited: Molecular networking guided exploration of lipodepsipeptides in Australian marine fish gastrointestinal tract-derived fungi. Mar. Drugs 2019, 17, 475. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, J.; Xiang, W.; Wang, M.; Lu, C. Two new lipodepsipeptides from the Endophytic Fungus Colletotrichum aotearoa Y41. Rec. Nat. Prod. 2019, 13, 199–204. [Google Scholar] [CrossRef]
- Ariantari, N.P.; Frank, M.; Gao, Y.; Stuhldreier, F.; Kiffe-Delf, A.L.; Hartmann, R.; Simon-Patrick Hofert, S.P.; Christoph, J.; Wesselborg, S.; Müller, W.E.G.; et al. Fusaristatins D–F and (7S, 8R)-(−)-chlamydospordiol from Fusarium sp. BZCB-CA, an endophyte of Bothriospermum chinense. Tetrahedron 2021, 85, 132065. [Google Scholar] [CrossRef]
- Shiono, Y.; Tsuchinari, M.; Shimanuki, K.; Miyajima, T.; Murayama, T.; Koseki, T.; Laatsch, H.; Funakoshi, T.; Takanami, K.; Suzuki, K. Fusaristatins A and B, two new cyclic lipodepsipeptide from an endophytic Fusarium sp. J. Antbiot. 2007, 60, 309–316. [Google Scholar] [CrossRef]
- MacIntyre, L.W.; Marchbank, D.H.; Correa, H.; Kerr, R.G. Fusaristatin C, a cyclic lipodepsipeptide from Pithomyces sp. RKDO 1698. J. Nat. Prod. 2018, 81, 2768–2772. [Google Scholar] [CrossRef]
- Paine, P.B. Studies in bacteriosis II. A brown blotch disease of cultivated mushroom. Ann. Appl. Biol. 1919, 5, 206–219. [Google Scholar] [CrossRef]
- Munsch, P.; Alatossava, T. Several pseudomonads, associated with the cultivated mushrooms Agaricus bisporus or Pleurotus sp., are hemolytic. Microbiol. Res. 2002, 157, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Lo Cantore, P.; Lazzaroni, S.; Coraiola, M.; Serra, M.D.; Cafarchia, C.; Evidente, A.; Iacobellis, N.S. Biological characterization of white line-inducing principle (WLIP) produced by Pseudomonas reactans NCPPB1311. Mol. Plant Microbe Interact. 2006, 19, 1113–1120, (and literature therein cited). [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, P.R.; Heinemann, J.A. The general secretory pathway of Burkholderia gladioli pv. agaricicola BG164R is necessary for cavity disease in white button mushrooms. Appl. Environ. Microbiol. 2006, 72, 3558–3565. [Google Scholar] [CrossRef]
- Nutkins, J.C.; Mortishire-Smith, R.J.; Packman, L.C.; Brodey, C.L.; Rainey, P.B.; Johnstone, K.; Williams, D.H. Structure determination of tolaasin, an extracellular lipodepsipeptide produced by the mushroom pathogen, Pseudomonas tolaasii Paine. J. Am. Chem. Soc. 1991, 113, 2621–2627. [Google Scholar] [CrossRef]
- Bassarello, C.; Lazzaroni, S.; Bifulco, G.; Lo Cantore, P.; Iacobellis, N.S.; Riccio, R.; Gomez-Paloma, L.; Evidente, A. Tolaasins A–E, five new lipodepsipeptides produced by Pseudomonas tolaasii. J. Nat. Prod. 2004, 67, 811–816. [Google Scholar] [CrossRef]
- Coraiola, M.; Lo Cantore, P.; Lazzaroni, S.; Evidente, A.; Iacobellis, N.S.; Dalla Serra, M. WLIP and tolaasin I, lipodepsipeptides from Pseudomonas reactans and Pseudomonas tolaasii, permeabilise model membranes. Biochim. Biophys. Acta 2006, 1758, 1713–1722. [Google Scholar] [CrossRef]
- Hermenau, R.; Kugel, S.; Komor, A.J.; Hertweck, C. Helper bacteria halt and disarm mushroom pathogens by linearizing structurally diverse cyclolipopeptides. Proc. Natl. Acad. Sci. USA 2020, 117, 23802–23806. [Google Scholar] [CrossRef]
- Tomita, S.; Kajikawa, A.; Igimi, S.; Shinohara, H.; Yokota, K. Insights into detoxification of tolaasins, the toxins behind mushroom bacterial blotch, by Microbacterium foliorum NBRC 103072T. PhytoFrontiers 2021, 1, 267–275. [Google Scholar] [CrossRef]
- Castaldi, S.; Cimmino, A.; Masi, M.; Evidente, A. Bacterial lipodepsipeptides and some of their derivatives and cyclic dipeptides as potential agents for biocontrol of pathogenic bacteria and fungi of agrarian plants. J. Agric. Food Chem. 2022, 70, 4591–4598. [Google Scholar] [CrossRef]
- Bensaci, M.F.; Gurnev, P.A.; Bezrukov, S.M.; Takemoto, J.Y. Fungicidal activities and mechanisms of action of Pseudomonas syringae pv. syringae lipodepsipeptide syringopeptins 22A and 25A. Front. Microbiol. 2011, 2, 216. [Google Scholar] [CrossRef] [PubMed]
- Geudens, N.; De Vleeschouwer, M.; Fehr, K.; Rokni-Zadeh, H.; Ghequire, M.G.K.; Madder, A.; De Mot, R.; Jos Martins, J.C.; Davy Sinnaeve, D. Impact of a stereocentre inversion in cyclic lipodepsipeptides from the viscosin group: A comparative study of the viscosinamide an pconformation and self-assembly. ChemBioChem 2014, 15, 2736–2746. [Google Scholar] [CrossRef]
- Hirosawa, S.; Takahashi, Y.; Hashizume, H.; Miyake, T.; Akamatsu, Y. Synthesis and antibacterial activity of tripropeptin C derivatives modified at the carboxyl groups. J. Antibiot. 2014, 67, 265–268. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Evidente, A. Bioactive Lipodepsipeptides Produced by Bacteria and Fungi. Int. J. Mol. Sci. 2022, 23, 12342. https://doi.org/10.3390/ijms232012342
Evidente A. Bioactive Lipodepsipeptides Produced by Bacteria and Fungi. International Journal of Molecular Sciences. 2022; 23(20):12342. https://doi.org/10.3390/ijms232012342
Chicago/Turabian StyleEvidente, Antonio. 2022. "Bioactive Lipodepsipeptides Produced by Bacteria and Fungi" International Journal of Molecular Sciences 23, no. 20: 12342. https://doi.org/10.3390/ijms232012342
APA StyleEvidente, A. (2022). Bioactive Lipodepsipeptides Produced by Bacteria and Fungi. International Journal of Molecular Sciences, 23(20), 12342. https://doi.org/10.3390/ijms232012342






