Polytopal Rearrangement Governing Stereochemistry of Bicyclic Oxime Ether Synthesis
Abstract
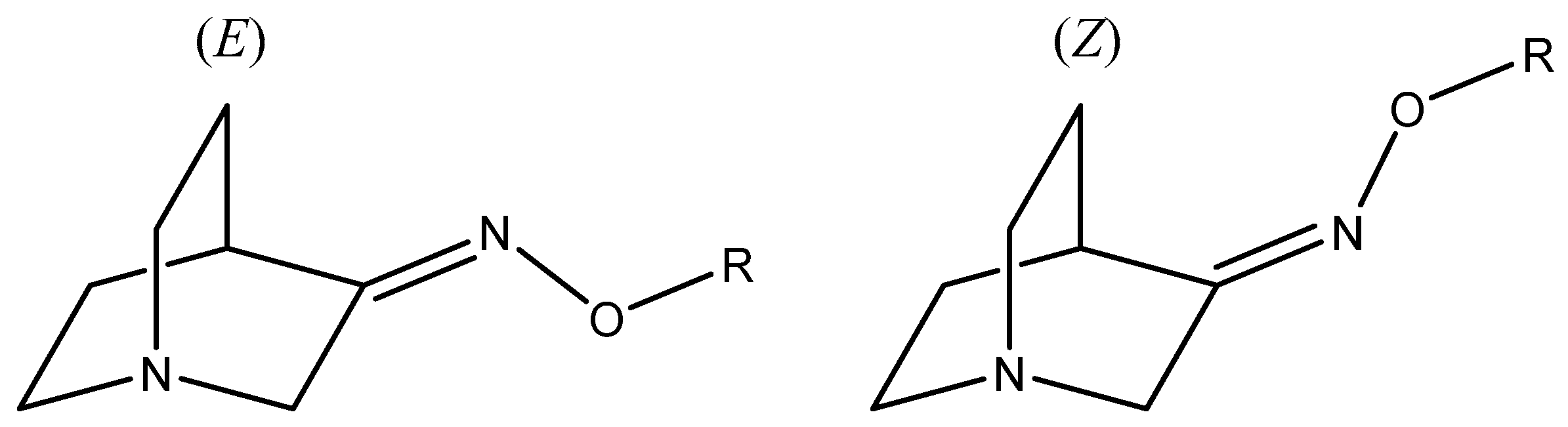
1. Introduction
2. Results and Discussion
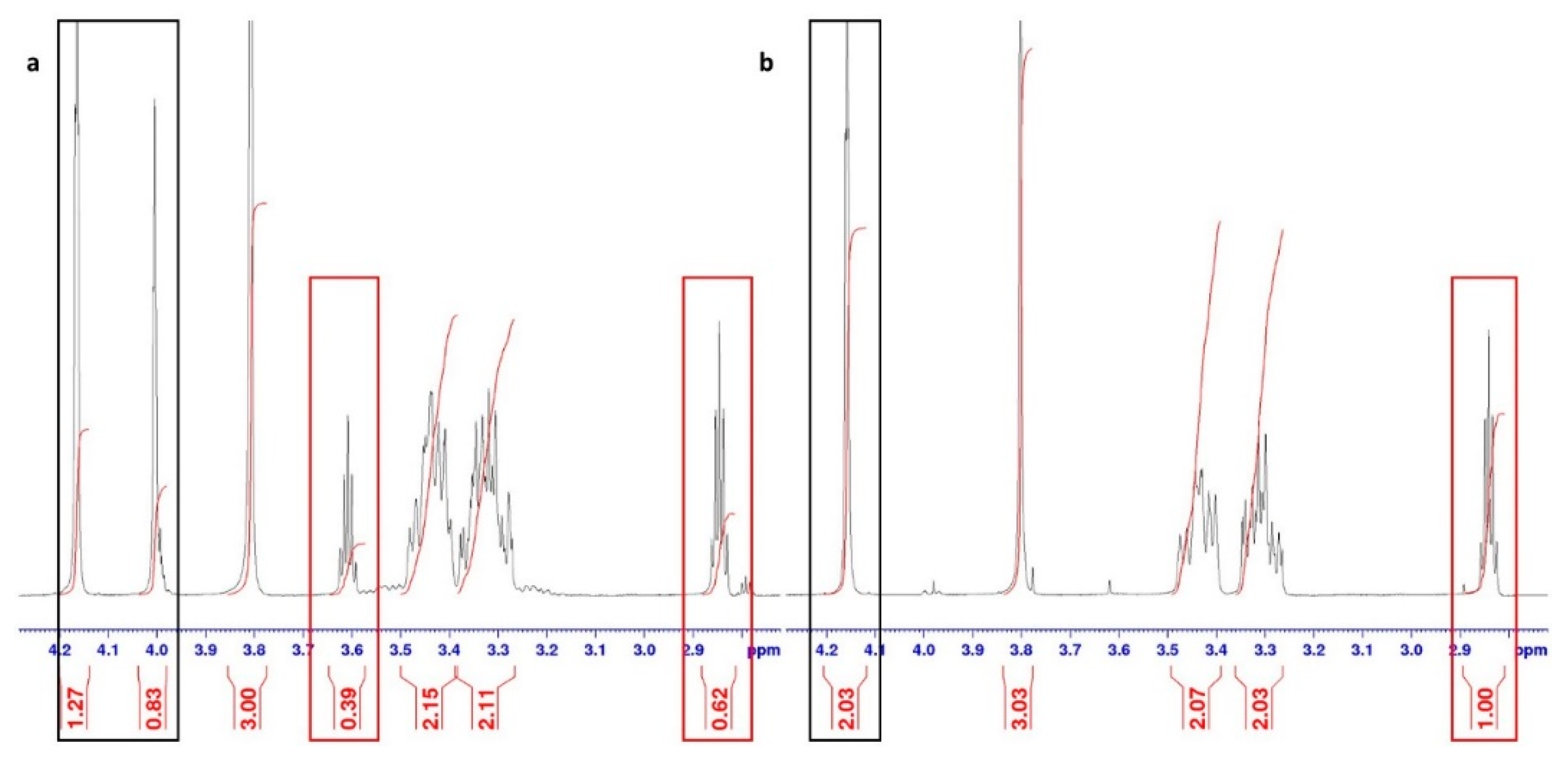
2.1. Synthesis of Compounds
2.2. Quantum Chemical Calculations
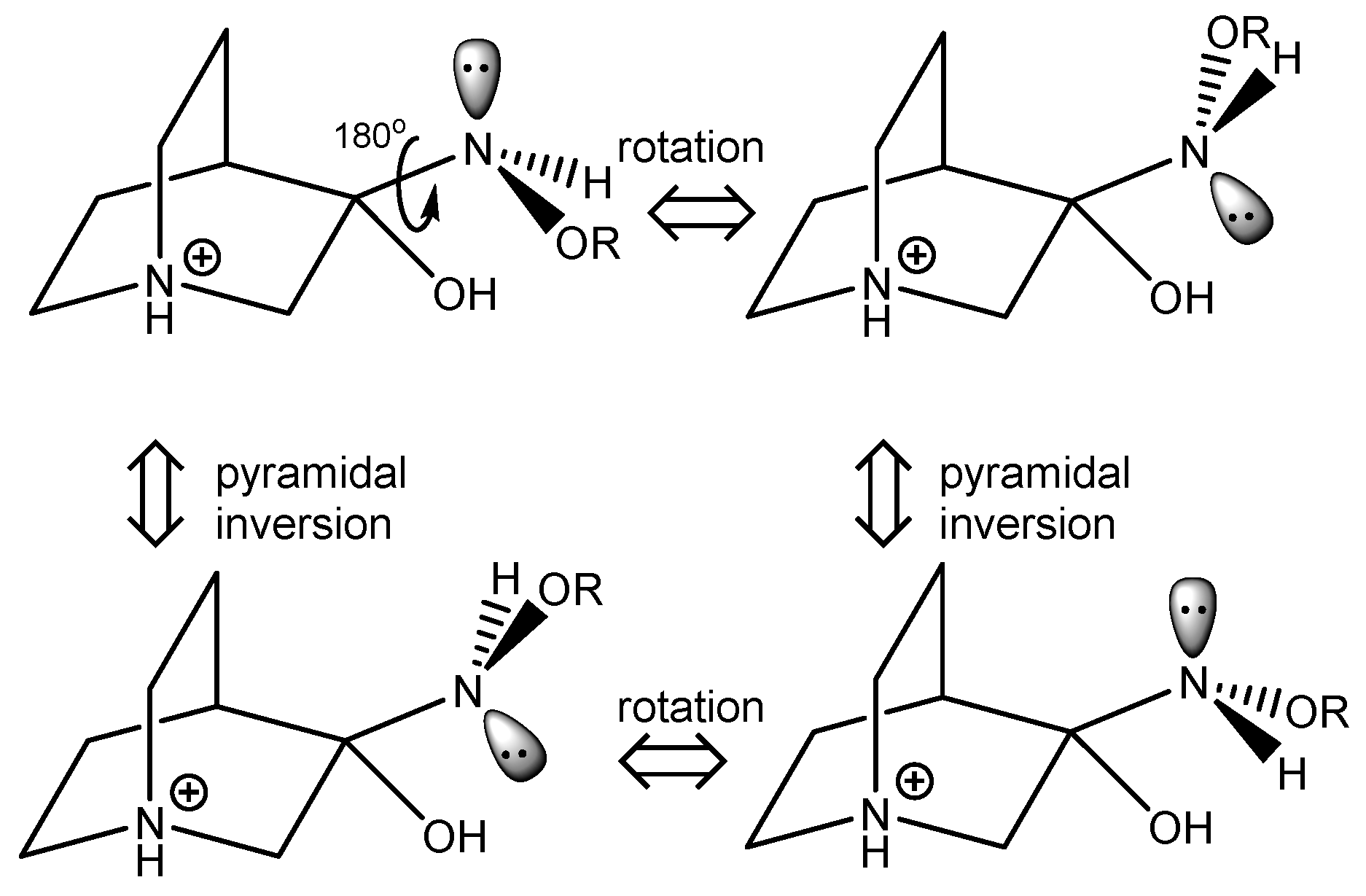
2.2.1. Reaction Mechanism
2.2.2. Conformational Analysis
2.2.3. Reaction Products and Stereoselectivity
3. Materials and Methods
3.1. Synthesis of Compounds
3.1.1. Quinuclidin-3-one O-Methyloxime (1)
3.1.2. Quinuclidin-3-one O-Tert-butyloxime (2)
3.1.3. Quinuclidin-3-one O-Benzyloxime (3)
3.1.4. Quinuclidin-3-one O-Phenyloxime (4)
3.2. Quantum Chemical Calculation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Odžak, R.; Skočibušić, M.; Maravić, A. Synthesis and antimicrobial profile of N-substituted imidazolium oximes and their monoquaternary salts against multidrug resistant bacteria. Bioorg. Med. Chem. 2013, 21, 7499–7506. [Google Scholar] [CrossRef] [PubMed]
- Zandona, A.; Katalinić, M.; Šinko, G.; Kastelic, A.R.; Primožič, I.; Kovarik, Z. Targeting Organophosphorus Compounds Poisoning by Novel quinuclidine-3 Oximes: Development of Butyrylcholinesterase-Based Bioscavengers. Arch. Toxicol. 2020, 94, 3157–3171. [Google Scholar] [CrossRef]
- Emami, S.; Foroumadi, A. 3-Imidazolyl-Substituted Flavans as Potential Antifungal Agents: Synthesis, Stereochemical Properties, and Antifungal Activity. Arch. Pharm. Chem. Life Sci. 2009, 342, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, A.; Iwamura, H.; Niwa, A.; Nakagawa, Y.; Fujita, T. Development of insect juvenile hormone active oxime O-ethers and carbamates. J. Agric. Food Chem. 1985, 33, 1034–1041. [Google Scholar] [CrossRef]
- Kato, M.; Nishino, S.; Ohno, M.; Fukuyama, S.; Kita, Y.; Hirasawa, Y.; Nakanishi, Y.; Takasugi, H.; Sakane, K. New reagents for controlled release of nitric oxide. Structure-stability relationships. Bioorg. Med. Chem. Lett. 1996, 6, 33–38. [Google Scholar] [CrossRef]
- Chandrasekhar, S.; Gopalaiah, K. Beckmann rearrangement in the solid state: Reaction of oxime hydrochlorides. Tetrahedron Lett. 2001, 42, 8123–8125. [Google Scholar] [CrossRef]
- Li, H.-Q.; Xiao, Z.-P.; Luo, Y.; Yan, T.; Lv, P.-C.; Zhu, H.-L. Amines and oximes derived from deoxybenzoins as Helicobacter pylori urease inhibitors. Eur. J. Med. Chem. 2009, 44, 2246–2251. [Google Scholar] [CrossRef]
- Mendelsohn, B.A.; Lee, S.; Kim, S.; Teyssier, F.; Aulakh, V.S.; Ciufolini, M.A. Oxidation of oximes to nitrile oxides with hypervalent iodine reagents. Org. Lett. 2009, 11, 1539–1542. [Google Scholar] [CrossRef]
- Olah, G.A.; Ramaiah, P.; Lee, C.-S.; Suryaprakash, G.K. Convenient oxidation of oximes to nitro compounds with sodium perborate in glacial acetic acid. Synlett 1992, 4, 337–339. [Google Scholar] [CrossRef]
- Furniss, B.S.; Hannaford, A.J.; Smith, P.W.G.; Tatchell, A.R. Vogel’s Textbook of Practical Organic Chemistry, 5th ed.; Longman Scietific and Techical: Essex, UK, 1989. [Google Scholar]
- Parthiban, P.; Aridoss, G.; Rathika, P.; Ramkumar, V.; Kabilan, S. Synthesis, stereochemistry and antimicrobial studies of novel oxime ethers of aza/diazabicycles. Bioorg. Med. Chem. Lett. 2009, 19, 6981–6985. [Google Scholar] [CrossRef]
- Primožič, I.; Hrenar, T.; Baumann, K.; Krišto, L.; Križić, I.; Tomić, S. Mechanochemical and conformational study of N-heterocyclic carbonyl-oxime transformations. Croat. Chem. Acta 2014, 87, 153–160. [Google Scholar] [CrossRef]
- Baláž, M.; Kudličková, Z.; Vilková, M.; Imrich, J.; Balážová, L.; Daneu, N. Mechanochemical synthesis and isomerization of N-substituted indole-3-carboxaldehyde oximes. Molecules 2019, 24, 3347. [Google Scholar] [CrossRef] [PubMed]
- Mehrez, A.; Mtat, D.; Touati, R. Microwave-assisted Synthesis of Chiral Oxime Ethers. Lett. Org. Chem. 2019, 16, 495–500. [Google Scholar] [CrossRef]
- Gašo-Sokač, D.; Bušić, V.; Cetina, M.; Jukić, M. An efficient synthesis of pyridoxal oxime derivatives under microwave irradiation. Molecules 2014, 19, 7610–7620. [Google Scholar] [CrossRef]
- Kad, G.L.; Bhandari, M.; Kaur, J.; Ratheea, R.; Singh, J. Solventless preparation of oximes in the solid state and via microwave irradiation. Green Chem. 2001, 3, 275–277. [Google Scholar] [CrossRef]
- Bozbey, I.; Uslu, H.; Türkmenoğlu, B.; Özdemir, Z.; Karakurt, A.; Levent, S. Conventional and microwave prompted synthesis of aryl(alkyl)azole oximes, 1H-NMR spectroscopic determination of E/Z isomer ratio and HOMO-LUMO analysis. J. Mol. Struct. 2022, 1251, 132077. [Google Scholar] [CrossRef]
- Nobuhiko, K.; Takeshi, S.; Osamu, O. 2D large-amplitude motions for the (Z)-isomer of n-butyraldehyde oxime. Chem. Phys. Lett. 2022, 803, 139826. [Google Scholar]
- Lukin, S.; Germann, L.S.; Friščić, T.; Halasz, I. Toward Mechanistic Understanding of Mechanochemical Reactions Using Real-Time In Situ Monitoring. Acc. Chem. Res. 2022, 55, 1262–1277. [Google Scholar] [CrossRef]
- Shi, Y.X.; Xu, K.; Clegg, J.K.; Ganguly, R.; Hirao, H.; Friščić, T.; García, F. The first synthesis of the sterically encumbered adamantoid phosphazane P4 (NtBu) 6: Enabled by mechanochemistry. Angew. Chemie Int. Ed. 2016, 55, 12736–12740. [Google Scholar] [CrossRef] [PubMed]
- Plate, R.; Plaum, M.J.; de Boer, T.; Andrews, J.S. Synthesis and muscarinic M3 pharmacological activities of 1-azabicyclo [2.2. 2] octan-3-one oxime derivatives. Bioorg. Med. Chem. 1996, 4, 239–245. [Google Scholar] [CrossRef]
- Schouten, A.; Kanters, J.A.; Kroon, J. Structure of Org 32763: 3-methoxyiminoquinuclidinium chloride. Acta Cryst. 1994, 50, 128–129. [Google Scholar] [CrossRef]
- Negi, S.; Matsukura, M.; Mizuno, M.; Miyake, K.; Minami, N. Synthesis of (2R)-1-(4-Chloro-2-pyridyl)-2-(2-pyridyl) ethylamine: A selective oxime reduction and crystallization-induced asymmetric transformation. Synthesis 1996, 1996, 991–996. [Google Scholar] [CrossRef]
- Ying, P.; Yu, J.; Su, W. Liquid-Assisted Grinding Mechanochemistry in the Synthesis of Pharmaceuticals. Adv. Synth. Catal. 2021, 363, 1246–1271. [Google Scholar] [CrossRef]
- Solvent Choice for Microwave Synthesis. Available online: https://cem.com/cn/microwave-chemistry/solvent-choice (accessed on 2 September 2022).
- Wang, G.; Chen, Y.; Zhong, A.; Du, H.; Lei, M. A DFT Study on Formation of Bisaryl Oxime Ether from Benzaldehyde and Phenoxyamine. Chem. Lett. 2008, 37, 656. [Google Scholar] [CrossRef]
- Kirmizialtin, S.; Yildiz, B.S.; Yildiz, I. A DFT-based mechanistic study on the formation of oximes. J. Phys. Org. Chem. 2017, 30, e3711. [Google Scholar] [CrossRef]
- Vogel, P.; Houk, K.N. Organic Chemistry; Wiley: Weinheim, Germany, 2019. [Google Scholar]
- Hrenar, T. Qcc. Quantum Chemistry Code (rev. 0.6826); University of Zagreb: Zagreb, Croatia, 2022. [Google Scholar]
- Hrenar, G.K.T.; Došlić, N. Hydrogen bonding in malonaldehyde: A density functional and reparametrized semiempirical approach. Chem. Phys. 2003, 293, 41–52. [Google Scholar]
- Stewart, J.J.P. Optimization of parameters for semiempirical methods VI: More modifications to the NDDO approximations and re-optimization of parameters. J. Mol. Model. 2013, 19, 1–32. [Google Scholar] [CrossRef]
- Hrenar, T. Moonee. Code for Manipulation and Analysis of Multi- and Univariate Big Data, rev. 0.68268; University of Zagreb: Zagreb, Croatia, 2022. [Google Scholar]
- Jović, O.; Smolić, T.; Primožič, I.; Hrenar, T. Spectroscopic and chemometric analysis of binary and ternary edible oil mixtures: Qualitative and quantitative study. Anal. Chem. 2016, 88, 4516. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16; Revision A.03; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
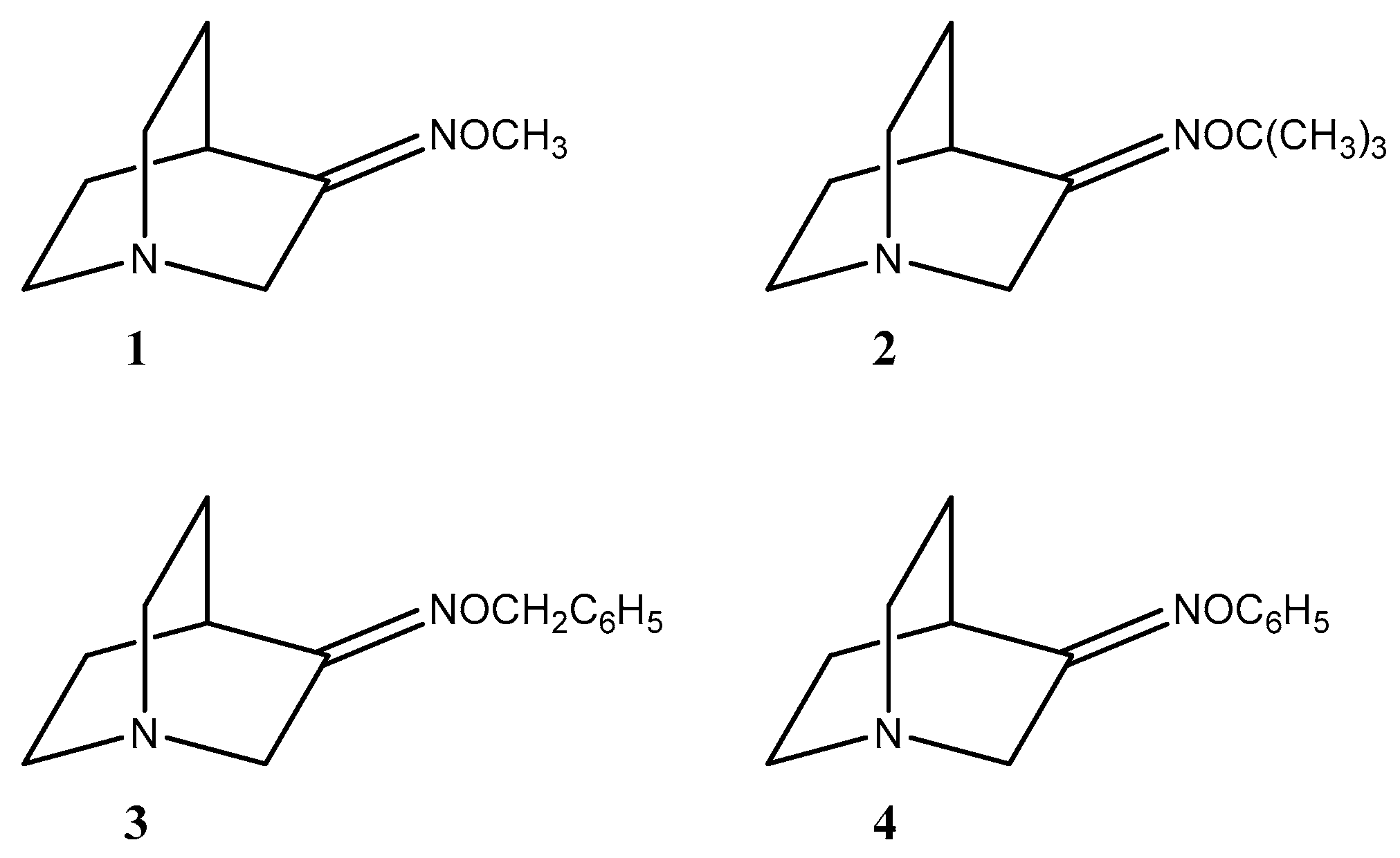


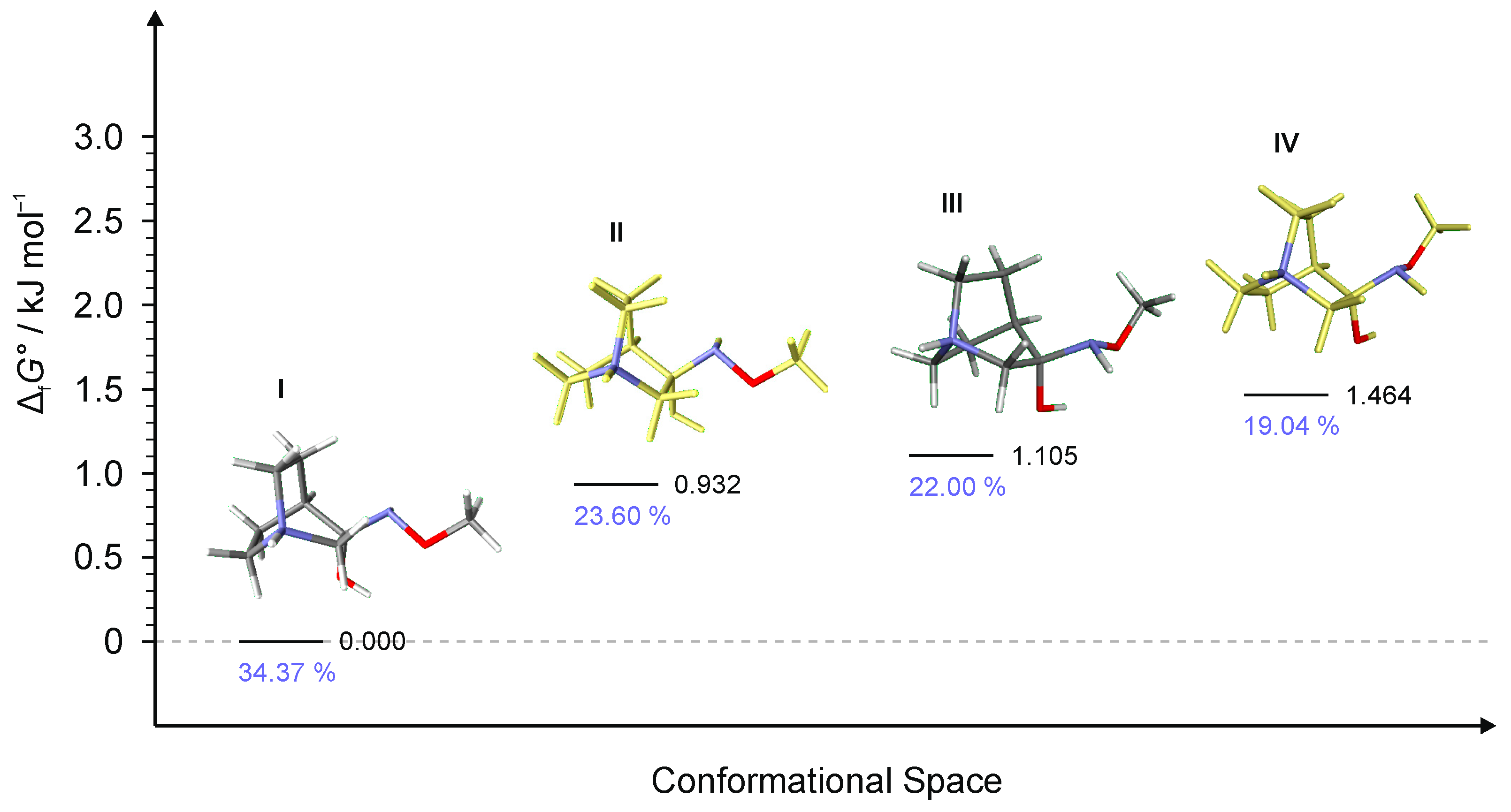



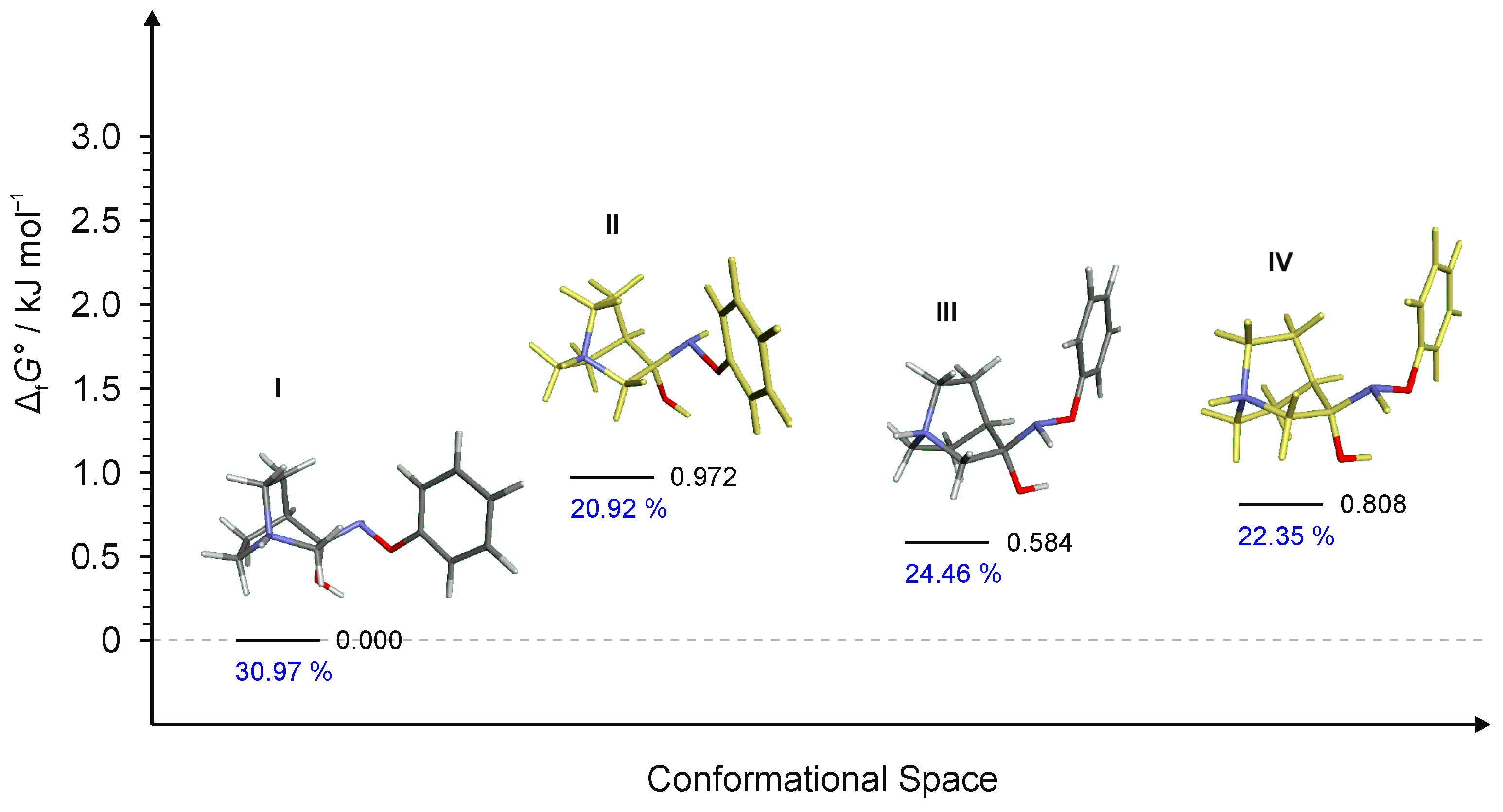
| Starting Compound | Reaction Time | Product | E/Z |
|---|---|---|---|
| Quinuclidin-3-one | 3 min | 1·HCl | 60:40 |
| 8 min | 2·HCl | 93:7 | |
| 5 min | 3·HCl | 70:30 | |
| 6 min | 4·HCl | 65:35 | |
| Quinuclidin-3-one hydrochloride | 24 h | 1·HCl | 85:15 |
| 48 h | 2·HCl | * | |
| 24 h | 3·HCl | 100:0 | |
| 48 h | 4·HCl | 100:0 |
| Starting Compound | LAG | Base | Reaction Time/Min | Product | E/Z |
|---|---|---|---|---|---|
| Quinuclidin-3-one | - | - | 60 | 1·HCl | 56:44 |
| EtOH | - | 5 | 1·HCl | 100:0 | |
| Hexane | - | 4 | 1·HCl | 100:0 | |
| Pyridine | - | 30 | 1·HCl | 50:50 | |
| Quinuclidin-3-one hydrochloride | - | - | * | 1·HCl | - |
| - | NaOH | 10 | 1·HCl | 100:0 | |
| EtOH | NaOH | 10 | 1·HCl | 61:39 | |
| - | K2CO3 | 5 | 1·HCl | 100 | |
| Quinuclidin-3-one | - | - | 8 | 2·HCl | 80:20 |
| EtOH | - | 3 | 2·HCl | 75:25 | |
| Hexane | - | 3 | 2·HCl | 78:22 | |
| Pyridine | - | 5 | 2·HCl | 75:25 | |
| Quinuclidin-3-one hydrochloride | Pyridine | - | 3 | 2·HCl | 75:25 |
| - | NaOH | 5 | 2·HCl | 100:0 | |
| EtOH | NaOH | 10 | 2·HCl | 70:30 | |
| - | K2CO3 | 5 | 2·HCl | 100:0 | |
| Quinuclidin-3-one | - | - | 5 | 3·HCl | 67:33 |
| EtOH | - | 5 | 3·HCl | 90:10 | |
| Hexane | - | 8 | 3·HCl | 75:25 | |
| Pyridine | - | 8 | 3·HCl | 80:20 | |
| Quinuclidin-3-one hydrochloride | Pyridine | - | 4 | 3·HCl | 100:0 |
| - | NaOH | 6 | 3·HCl | 100:0 | |
| EtOH | NaOH | 10 | 3·HCl | 96:4 | |
| - | K2CO3 | 4 | 3·HCl | 70:30 | |
| Quinuclidin-3-one | - | - | 6 | 4·HCl | 49:51 |
| EtOH | - | 3 | 4·HCl | 50:50 | |
| Hexane | - | 3 | 4·HCl | 60:40 | |
| Quinuclidin-3-one hydrochloride | - | - | * | 4·HCl | - |
| - | NaOH | 2 | 4·HCl | 100:0 | |
| EtOH | NaOH | 10 | 4·HCl | 72:28 | |
| - | K2CO3 | 6 | 4·HCl | 100:0 |
| Starting Compound | Solvent | Temperature/°C | Base | Time/Min | Product | E/Z |
|---|---|---|---|---|---|---|
| Quinuclidin-3-one | Acetonitrile | 140 | - | 3 | 1·HCl | 100 |
| Acetonitrile | 140 | - | 3 | 2·HCl | 100 | |
| Acetonitrile | 140 | - | 3 | 3·HCl | 100 | |
| Acetonitrile | 140 | - | 3 * | 4·HCl | - | |
| Acetonitrile | 140 | - | 1 | 1·HCl | 100 | |
| Acetonitrile | 80 | - | 1 | 1·HCl | 60:40 | |
| Acetone | 140 | - | 5 | 1·HCl | 100 | |
| Acetone | 140 | - | 1 * | 1·HCl | - | |
| Ethanol | 140 | - | 3 | 2·HCl | 90:10 | |
| Ethanol | 140 | - | 3 | 1·HCl | 65:35 | |
| Ethanol | 80 | - | 1 | 1·HCl | 60:40 | |
| Ethanol | 140 | - | 3 | 3·HCl | 85:15 | |
| Quinuclidin-3-one hydrochloride | Acetonitrile | 140 | K2CO3 | 3 | 1·HCl | 100 |
| Acetonitrile | 140 | - | 3 | 1·HCl | 100 | |
| Acetonitrile | 140 | NaOH | 3 | 1·HCl | 100 | |
| Pyridine | 140 | Pyridine | 3 | 1·HCl | 85:15 | |
| Ethanol | 140 | NaOH | 3 | 1·HCl | 55:45 |
| Compound | ΔΔfG°/kJ mol–1 |
|---|---|
| (E)-1·HCl | 0.00 |
| (Z)-1·HCl | 13.77 |
| (E)-2·HCl | 0.00 |
| (Z)-2·HCl | 10.34 |
| (E)-3·HCl | 0.00 |
| (Z)-3·HCl | 8.84 |
| (E)-4·HCl | 0.00 |
| (Z)-4·HCl | 10.41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spahić, Z.; Hrenar, T.; Primožič, I. Polytopal Rearrangement Governing Stereochemistry of Bicyclic Oxime Ether Synthesis. Int. J. Mol. Sci. 2022, 23, 12331. https://doi.org/10.3390/ijms232012331
Spahić Z, Hrenar T, Primožič I. Polytopal Rearrangement Governing Stereochemistry of Bicyclic Oxime Ether Synthesis. International Journal of Molecular Sciences. 2022; 23(20):12331. https://doi.org/10.3390/ijms232012331
Chicago/Turabian StyleSpahić, Zlatan, Tomica Hrenar, and Ines Primožič. 2022. "Polytopal Rearrangement Governing Stereochemistry of Bicyclic Oxime Ether Synthesis" International Journal of Molecular Sciences 23, no. 20: 12331. https://doi.org/10.3390/ijms232012331
APA StyleSpahić, Z., Hrenar, T., & Primožič, I. (2022). Polytopal Rearrangement Governing Stereochemistry of Bicyclic Oxime Ether Synthesis. International Journal of Molecular Sciences, 23(20), 12331. https://doi.org/10.3390/ijms232012331






