GDF11 Regulates PC12 Neural Stem Cells via ALK5-Dependent PI3K-Akt Signaling Pathway
Abstract
1. Introduction
2. Results
2.1. GDF11 Mitigated Cell Viability and Proliferation
2.2. GDF11 Promoted Differentiation of PC12 Cells
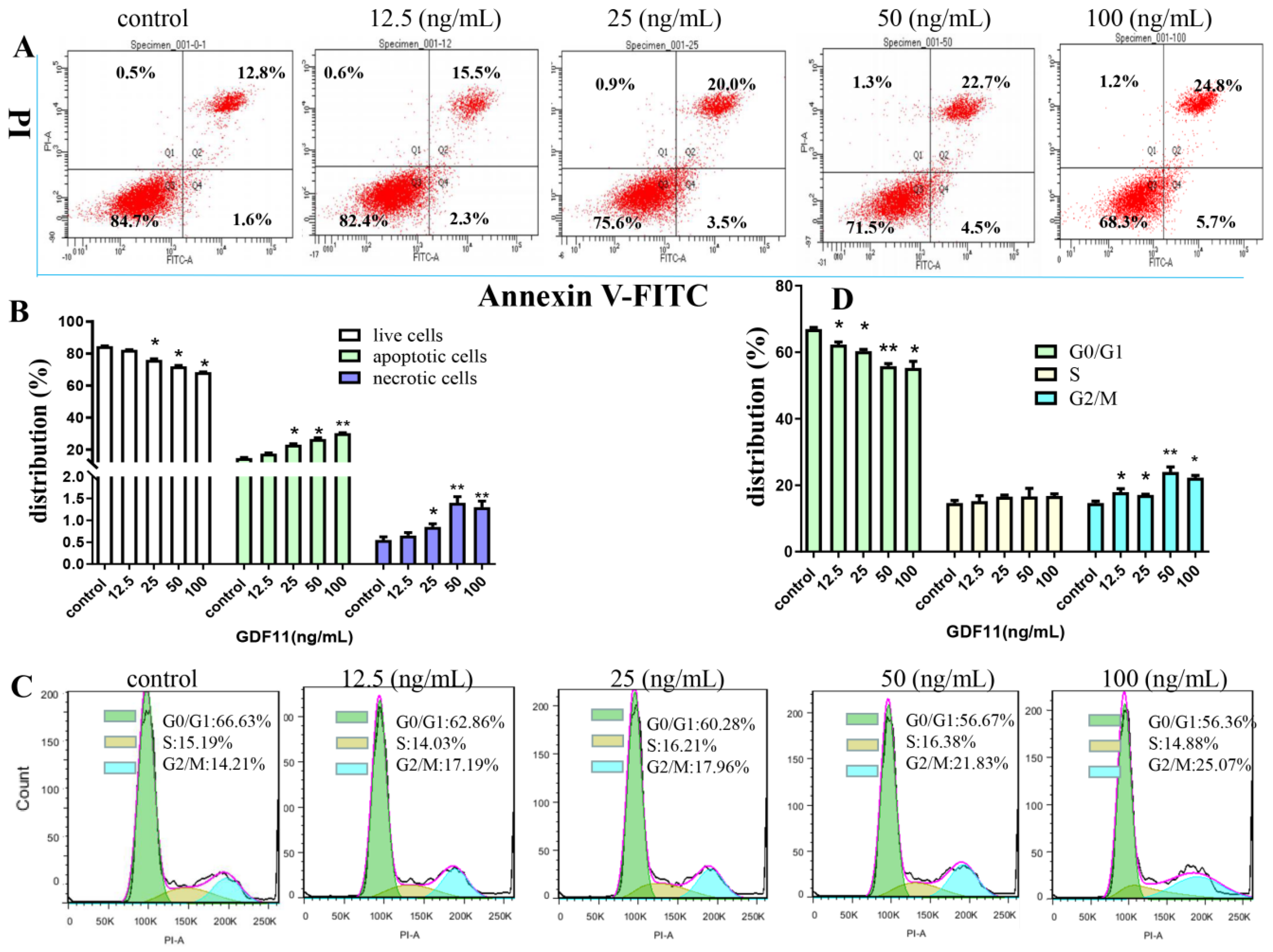
2.3. GDF11 Stimulated Apoptosis of PC12 Cells
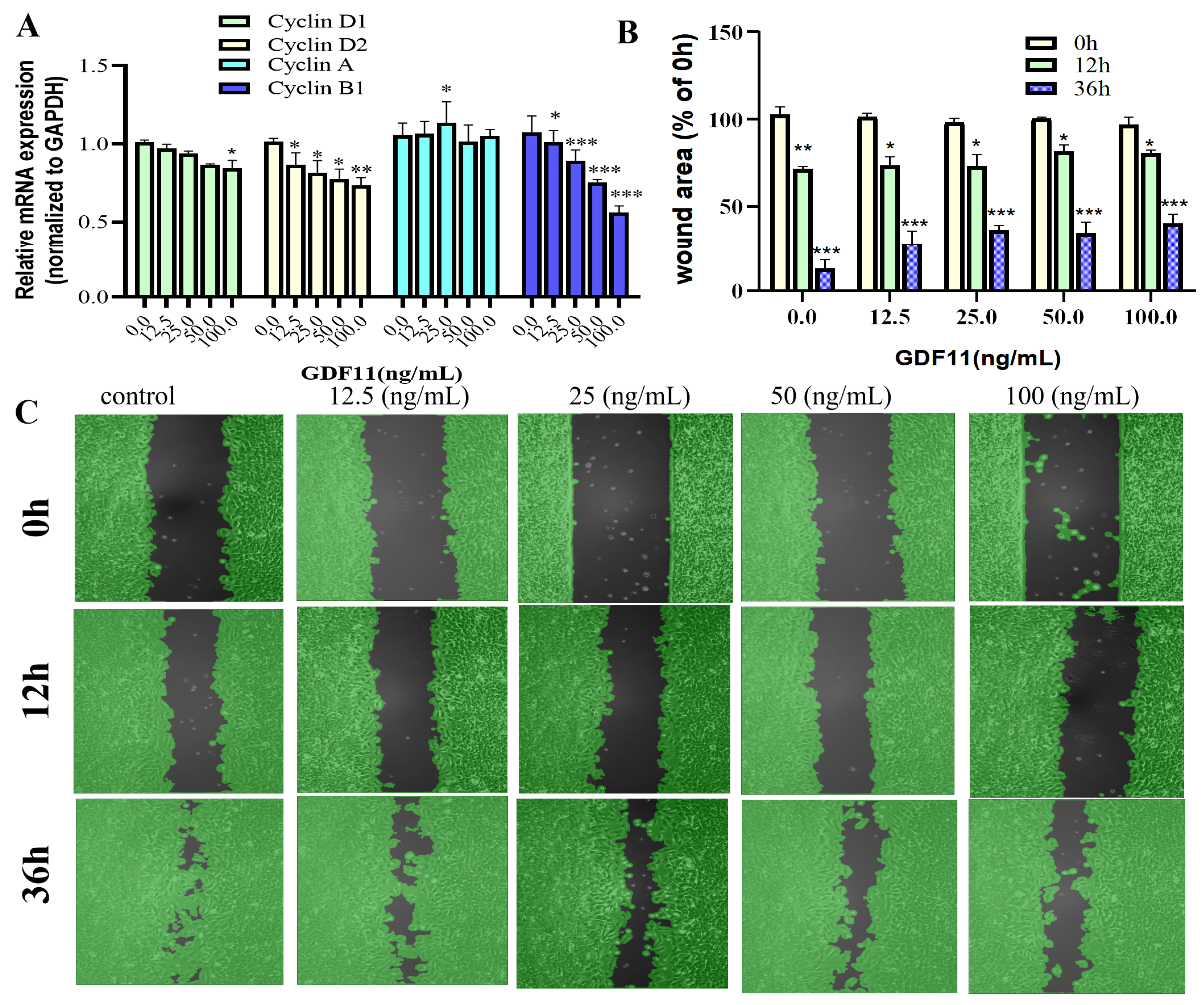
2.4. GDF11 Arrested Cell Cycle of PC12 Cells
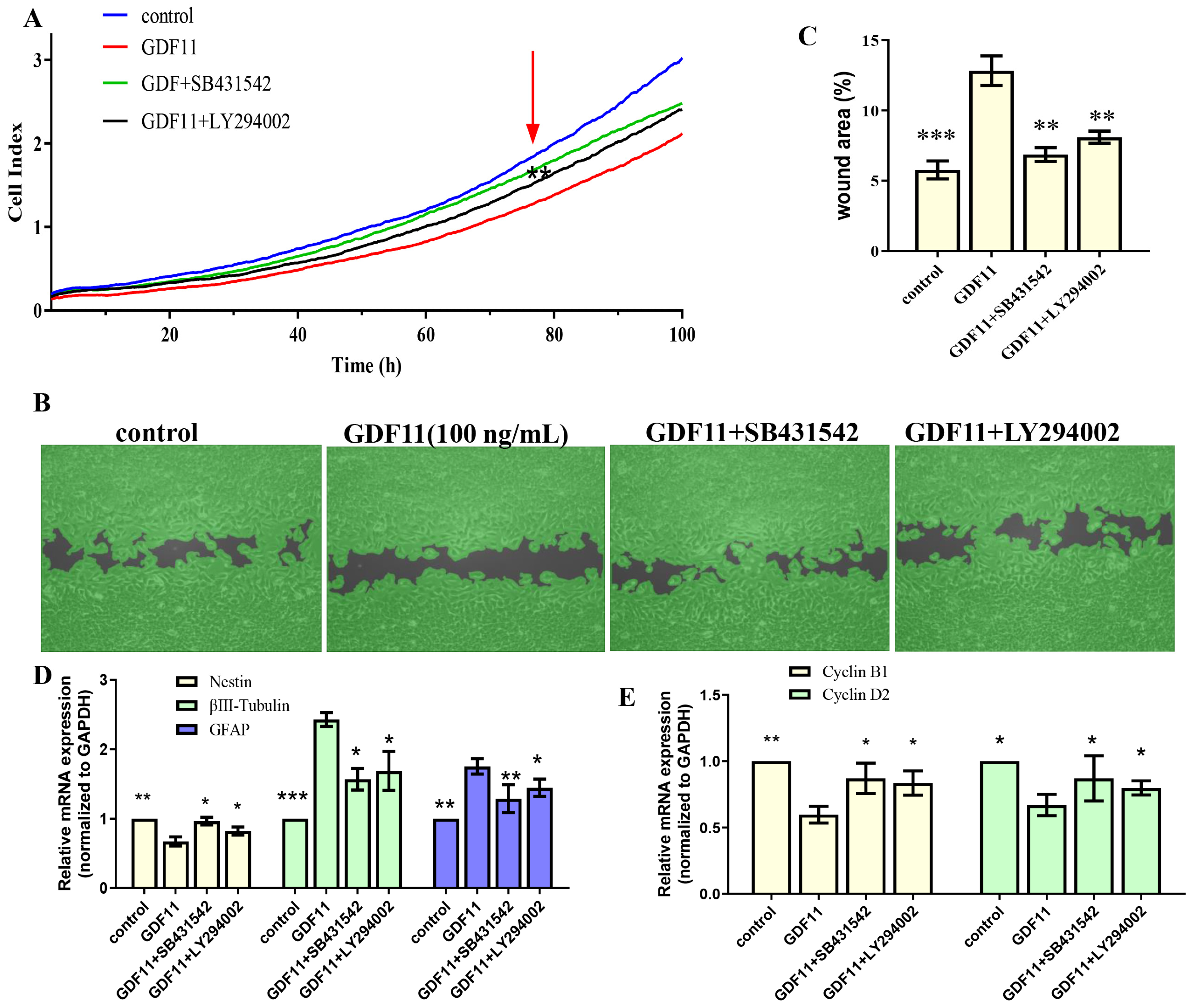
2.5. GDF11 Attenuated PC12 Cell Migration
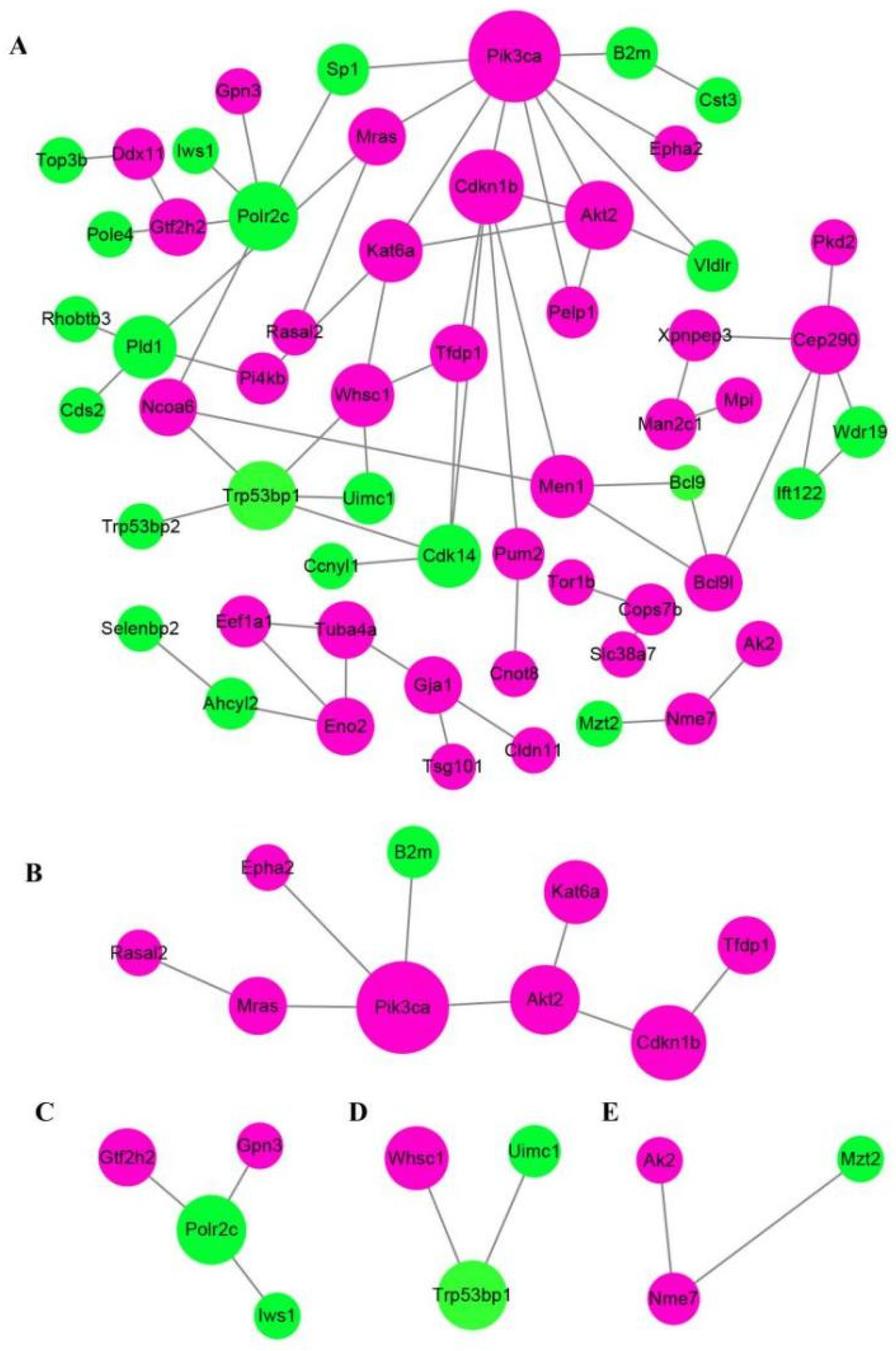
2.6. TMT–Based Protein Identification and Quantification
2.7. Annotation Analysis of the Differentially Expressed Proteins
2.8. PI3K-Akt Pathway Was Enriched by CSP100 PLUS Microarray
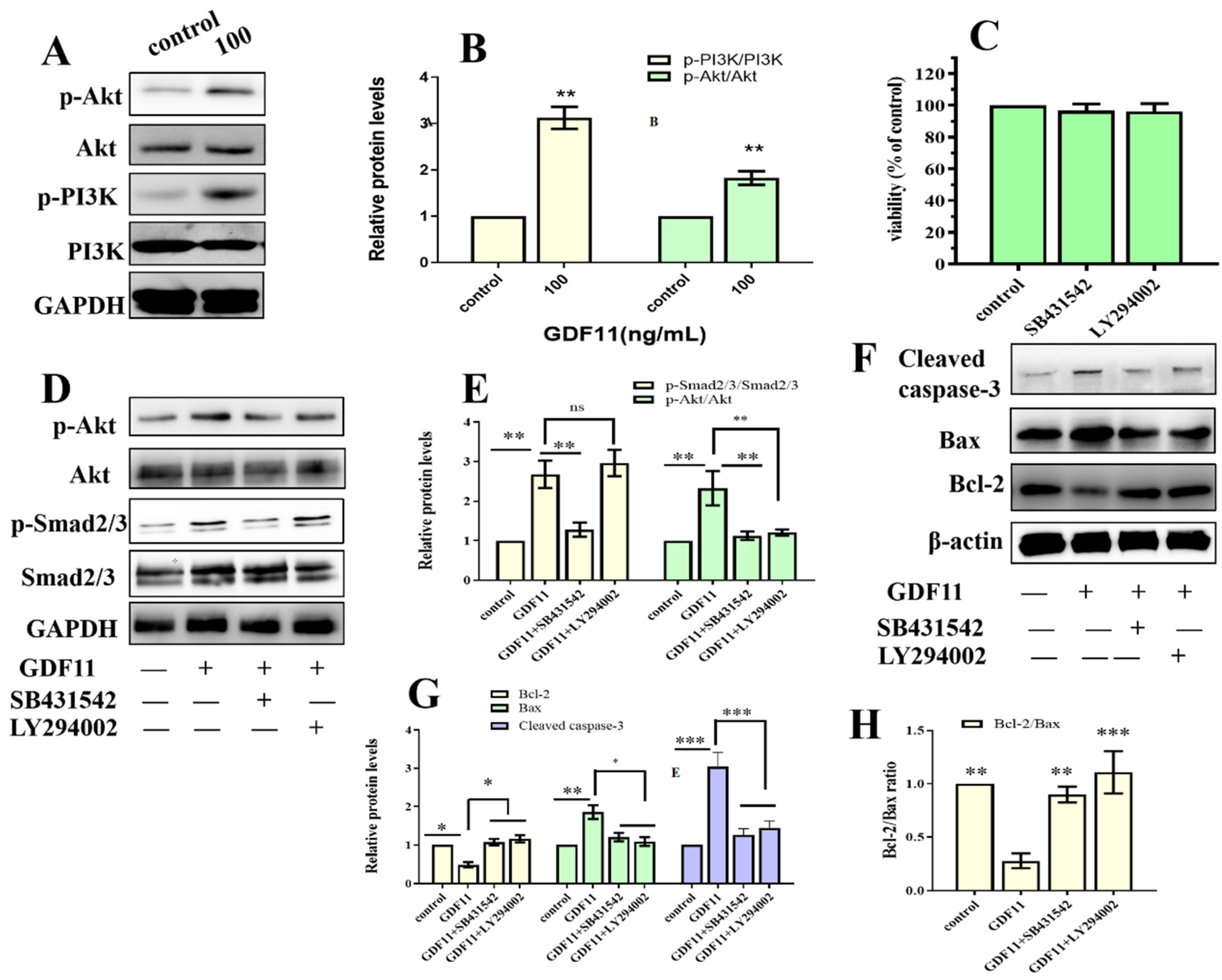
2.9. GDF11 Activated PI3K-Akt Signal Pathway
2.10. GDF11 Affected Neural Stem Cells through ALK5-Dependent PI3K-Akt Pathway
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Cell Viability/Proliferation Assay
4.3. Cell Apoptosis Assay
4.4. Wound Healing/Migration Assay
4.5. qRT-PCR Analysis
4.6. Western Blot Analysis and Validation
4.7. Sample Preparation, TMT-Labeling, and LC-MS/MS
4.8. MS/MS Data Analysis and Bioinformatics Analysis
4.9. Profiles of Key Signaling Pathway by an Antibody Array
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Flanagan, E.; Muller, M.; Hornberger, M.; Vauzour, D. Impact of Flavonoids on Cellular and Molecular Mechanisms Underlying Age-Related Cognitive Decline and Neurodegeneration. Curr. Nutr. Rep. 2018, 7, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, R.; Liu, F.; Jiang, P.; Xu, J.; Cao, H.; Du, X.; Ma, L.; Lin, F.; Cheng, L.; et al. TMT-Based Quantitative Proteomic Analysis Reveals Proteomic Changes Involved in Longevity. Proteom. Clin. Appl. 2019, 13, e1800024. [Google Scholar] [CrossRef] [PubMed]
- Rajan, K.B.; Weuve, J.; Barnes, L.L.; McAninch, E.A.; Wilson, R.S.; Evans, D.A. Population estimate of people with clinical Alzheimer’s disease and mild cognitive impairment in the United States (2020–2060). Alzheimer’s Dement. 2021, 17, 1966–1975. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Wei, C.; Chen, S.; Li, F.; Tang, Y.; Qin, W.; Zhao, L.; Jin, H.; Xu, H.; Wang, F.; et al. The cost of Alzheimer’s disease in China and re-estimation of costs worldwide. Alzheimer’s Dement. 2018, 14, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Ren, R.; Qi, J.; Lin, S.; Liu, X.; Yin, P.; Wang, Z.; Tang, R.; Wang, J.; Huang, Q.; Li, J.; et al. The China Alzheimer Report 2022. Gen. Psychiatr. 2022, 35, e100751. [Google Scholar] [CrossRef]
- Loffredo, F.S.; Steinhauser, M.L.; Jay, S.M.; Gannon, J.; Pancoast, J.R.; Yalamanchi, P.; Sinha, M.; Dall’Osso, C.; Khong, D.; Shadrach, J.L.; et al. Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell 2013, 153, 828–839. [Google Scholar] [CrossRef] [PubMed]
- Katsimpardi, L.; Litterman, N.K.; Schein, P.A.; Miller, C.M.; Loffredo, F.S.; Wojtkiewicz, G.R.; Chen, J.W.; Lee, R.T.; Wagers, A.J.; Rubin, L.L. Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science 2014, 344, 630–634. [Google Scholar] [CrossRef]
- Sinha, M.; Jang, Y.C.; Oh, J.; Khong, D.; Wu, E.Y.; Manohar, R.; Miller, C.; Regalado, S.G.; Loffredo, F.S.; Pancoast, J.R.; et al. Restoring systemic GDF11 levels reverses age-related dysfunction in mouse skeletal muscle. Science 2014, 344, 649–652. [Google Scholar]
- Biller-Andorno, N. Young blood for old hands? A recent anti-ageing trial prompts ethical questions. Swiss Med. Wkly. 2016, 146, w14359. [Google Scholar] [CrossRef][Green Version]
- Lavazza, A.; Garasic, M. Vampires 2.0? The ethical quandaries of young blood infusion in the quest for eternal life. Med. Health Care Phil. 2020, 23, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zhang, L.; Niu, T.; Ai, C.; Jia, G.; Jin, X.; Wen, L.; Zhang, K.; Zhang, Q.; Li, C. Growth differentiation factor 11 improves neurobehavioral recovery and stimulates angiogenesis in rats subjected to cerebral ischemia/reperfusion. Brain Res. Bull. 2018, 139, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Walker, R.G.; Poggioli, T.; Katsimpardi, L.; Buchanan, S.M.; Oh, J.; Wattrus, S.; Heidecker, B.; Fong, Y.W.; Rubin, L.L.; Ganz, P.; et al. Biochemistry and Biology of GDF11 and Myostatin: Similarities, Differences, and Questions for Future Investigation. Circ. Res. 2016, 118, 1125–1141. [Google Scholar] [CrossRef]
- Wang, Z.; Dou, M.; Liu, F.; Jiang, P.; Ye, S.; Ma, L.; Cao, H.; Du, X.; Sun, P.; Su, N.; et al. GDF11 induces differentiation and apoptosis and inhibits migration of C17.2 neural stem cells via modulating MAPK signaling pathway. PeerJ 2018, 6, e5524. [Google Scholar] [CrossRef] [PubMed]
- McPherron, A.C.; Lawler, A.M.; Lee, S.-J. Regulation of anterior/posterior patterning of the axial skeleton by growth/differentiation factor 11. Nat. Genet. 1999, 22, 260–264. [Google Scholar] [CrossRef]
- Wu, H.-H.; Ivkovic, S.; Murray, R.C.; Jaramillo, S.; Lyons, K.M.; Johnson, J.E.; Calof, A.L. Autoregulation of neurogenesis by GDF11. Neuron 2003, 37, 197–207. [Google Scholar] [CrossRef]
- Egerman, M.A.; Cadena, S.M.; Gilbert, J.A.; Meyer, A.; Nelson, H.N.; Swalley, S.E.; Mallozzi, C.; Jacobi, C.; Jennings, L.L.; Clay, I.; et al. GDF11 Increases with Age and Inhibits Skeletal Muscle Regeneration. Cell Metab. 2015, 22, 164–174. [Google Scholar] [CrossRef]
- Smith, S.C.; Zhang, X.; Zhang, X.; Gross, P.; Starosta, T.; Mohsin, S.; Franti, M.; Gupta, P.; Hayes, D.; Myzithras, M.; et al. GDF11 does not rescue aging-related pathological hypertrophy. Circ. Res. 2015, 117, 926–932. [Google Scholar] [CrossRef]
- Lu, Q.; Tu, M.L.; Li, C.J.; Zhang, L.; Jiang, T.J.; Liu, T.; Luo, X.H. GDF11 Inhibits Bone Formation by Activating Smad2/3 in Bone Marrow Mesenchymal Stem Cells. Calcif. Tissue Int. 2016, 99, 500–509. [Google Scholar] [CrossRef]
- Liu, W.; Zhou, L.; Zhou, C.; Zhang, S.; Jing, J.; Xie, L.; Sun, N.; Duan, X.; Jing, W.; Liang, X.; et al. GDF11 decreases bone mass by stimulating osteoclastogenesis and inhibiting osteoblast differentiation. Nat. Commun. 2016, 7, 12794. [Google Scholar] [CrossRef]
- Mayweather, B.A.; Buchanan, S.M.; Rubin, L.L. GDF11 expressed in the adult brain negatively regulates hippocampal neurogenesis. Mol. Brain 2021, 14, 134. [Google Scholar] [CrossRef] [PubMed]
- Frohlich, J.; Mazza, T.; Sobolewski, C.; Foti, M.; Vinciguerra, M. GDF11 rapidly increases lipid accumulation in liver cancer cells through ALK5-dependent signaling. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2021, 1866, 158920. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, M.; Toyono, T.; Akamine, A.; Joyner, A. Expression of growth/differentiation factor 11, a new member of the BMP/TGFβ superfamily during mouse embryogenesis. Mech. Develop. 1999, 80, 185–189. [Google Scholar] [CrossRef]
- Williams, G.; Zentar, M.P.; Gajendra, S.; Sonego, M.; Doherty, P.; Lalli, G. Transcriptional basis for the inhibition of neural stem cell proliferation and migration by the TGFbeta-family member GDF11. PLoS ONE 2013, 8, e78478. [Google Scholar] [CrossRef]
- Ozek, C.; Krolewski, R.C.; Buchanan, S.M.; Rubin, L.L. Growth Differentiation Factor 11 treatment leads to neuronal and vascular improvements in the hippocampus of aged mice. Sci. Rep. 2018, 8, 17293. [Google Scholar] [CrossRef]
- Xiao, A.; Chen, R.; Ren, Y.; You, C. Neuroprotective potential of GDF11 in experimental intracerebral hemorrhage in elderly rats. J. Clin. Neurosci. 2019, 63, 182–188. [Google Scholar]
- Walker, R.G.; Czepnik, M.; Goebel, E.J.; McCoy, J.C.; Vujic, A.; Cho, M.; Oh, J.; Aykul, S.; Walton, K.L.; Schang, G.; et al. Structural basis for potency differences between GDF8 and GDF11. BMC Biol. 2017, 15, 19. [Google Scholar] [CrossRef]
- Zhang, Y.-H.; Cheng, F.; Du, X.-T.; Gao, J.-L.; Xiao, X.-L.; Li, N.; Li, S.-L.; Dong, D.-L. GDF11/BMP11 activates both smad1/5/8 and smad2/3 signals but shows no significant effect on proliferation and migration of human umbilical vein endothelial cells. Oncotarget 2016, 7, 12063–12074. [Google Scholar] [CrossRef]
- Mei, W.; Zhu, B.; Shu, Y.; Liang, Y.; Lin, M.; He, M.; Luo, H.; Ye, J. GDF11 protects against glucotoxicity-induced mice retinal microvascular endothelial cell dysfunction and diabetic retinopathy disease. Mol. Cell Endocrinol. 2021, 537, 111422. [Google Scholar] [CrossRef]
- Jiao, L.; Shao, Y.; Yu, Q.; Li, M.; Wang, Y.; Gong, M.; Yang, X.; Liu, T.; Li, Z.; Liu, H.; et al. GDF11 replenishment protects against hypoxia-mediated apoptosis in cardiomyocytes by regulating autophagy. Eur. J. Pharmacol. 2020, 885, 173495. [Google Scholar] [CrossRef]
- Hernandez, S.; Simoni-Nieves, A.; Gerardo-Ramirez, M.; Torres, S.; Fucho, R.; Gonzalez, J.; Castellanos-Tapia, L.; Hernandez-Pando, R.; Tejero-Barrera, E.; Bucio, L.; et al. GDF11 restricts aberrant lipogenesis and changes in mitochondrial structure and function in human hepatocellular carcinoma cells. J. Cell Physiol. 2021, 236, 4076–4090. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Li, Y.; Yang, X.; Dong, W.; Yang, J.; Hu, Q.; Zhang, C.; Fang, H.; Liu, A. Growth differentiation factor 11 accelerates liver senescence through the inhibition of autophagy. Aging Cell 2022, 21, e13532. [Google Scholar] [PubMed]
- Zhang, C.; Lin, Y.; Zhang, K.; Meng, L.; Hu, X.; Chen, J.; Zhu, W.; Yu, H. GDF11 enhances therapeutic functions of mesenchymal stem cells for angiogenesis. Stem Cell Res. Ther. 2021, 12, 456. [Google Scholar] [PubMed]
- Seong, M.; Kang, H. Hypoxia-induced miR-1260b regulates vascular smooth muscle cell proliferation by targeting GDF11. BMB Rep. 2020, 53, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wang, H.; Feng, J.; Chen, L. Overexpression of circRNA circUCK2 Attenuates Cell Apoptosis in Cerebral Ischemia-Reperfusion Injury via miR-125b-5p/GDF11 Signaling. Mol. Ther. Nucleic Acids 2020, 22, 673–683. [Google Scholar] [PubMed]
- Wang, W.; Qu, R.; Wang, X.; Zhang, M.; Zhang, Y.; Chen, C.; Chen, X.; Qiu, C.; Li, J.; Pan, X.; et al. GDF11 Antagonizes Psoriasis-like Skin Inflammation via Suppression of NF-kappaB Signaling Pathway. Inflammation 2019, 42, 319–330. [Google Scholar] [CrossRef]
- Du, X.; Zhang, R.; Ye, S.; Liu, F.; Jiang, P.; Yu, X.; Xu, J.; Ma, L.; Cao, H.; Shen, Y.; et al. Alterations of Human Plasma Proteome Profile on Adaptation to High-Altitude Hypobaric Hypoxia. J. Proteome Res. 2019, 18, 2021–2031. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, F.; Ye, S.; Jiang, P.; Yu, X.; Xu, J.; Du, X.; Ma, L.; Cao, H.; Yuan, C.; et al. Plasma proteome profiling of high-altitude polycythemia using TMT-based quantitative proteomics approach. J. Proteom. 2019, 194, 60–69. [Google Scholar] [CrossRef]



| Total Spectrums | Matched Spectrums | Peptides | Unique Peptides | Identified Proteins | Quantifiable Proteins | DEPs |
|---|---|---|---|---|---|---|
| 784,796 | 79,915 | 18,504 | 17,878 | 5452 | 5209 | 165 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Jiang, P.; Liu, F.; Du, X.; Ma, L.; Ye, S.; Cao, H.; Sun, P.; Su, N.; Lin, F.; et al. GDF11 Regulates PC12 Neural Stem Cells via ALK5-Dependent PI3K-Akt Signaling Pathway. Int. J. Mol. Sci. 2022, 23, 12279. https://doi.org/10.3390/ijms232012279
Wang Z, Jiang P, Liu F, Du X, Ma L, Ye S, Cao H, Sun P, Su N, Lin F, et al. GDF11 Regulates PC12 Neural Stem Cells via ALK5-Dependent PI3K-Akt Signaling Pathway. International Journal of Molecular Sciences. 2022; 23(20):12279. https://doi.org/10.3390/ijms232012279
Chicago/Turabian StyleWang, Zongkui, Peng Jiang, Fengjuan Liu, Xi Du, Li Ma, Shengliang Ye, Haijun Cao, Pan Sun, Na Su, Fangzhao Lin, and et al. 2022. "GDF11 Regulates PC12 Neural Stem Cells via ALK5-Dependent PI3K-Akt Signaling Pathway" International Journal of Molecular Sciences 23, no. 20: 12279. https://doi.org/10.3390/ijms232012279
APA StyleWang, Z., Jiang, P., Liu, F., Du, X., Ma, L., Ye, S., Cao, H., Sun, P., Su, N., Lin, F., Zhang, R., & Li, C. (2022). GDF11 Regulates PC12 Neural Stem Cells via ALK5-Dependent PI3K-Akt Signaling Pathway. International Journal of Molecular Sciences, 23(20), 12279. https://doi.org/10.3390/ijms232012279







