Androgen-Responsive Oncogenic lncRNA RP11-1023L17.1 Enhances c-Myc Protein Stability in Prostate Cancer
Abstract
1. Introduction
2. Results
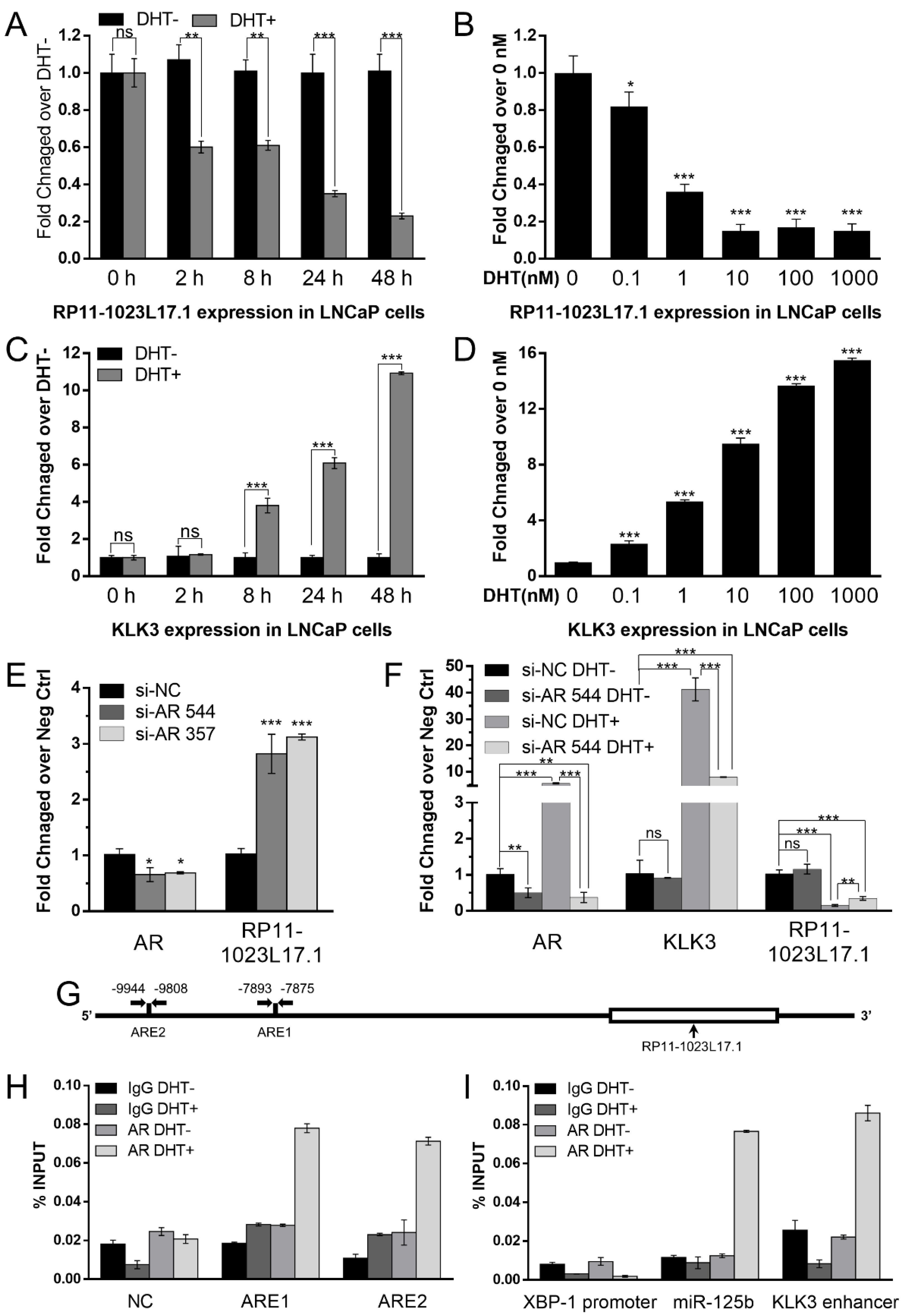
2.1. Androgen Responsive lncRNA RP11-1023L17.1 Is a Direct Target of AR
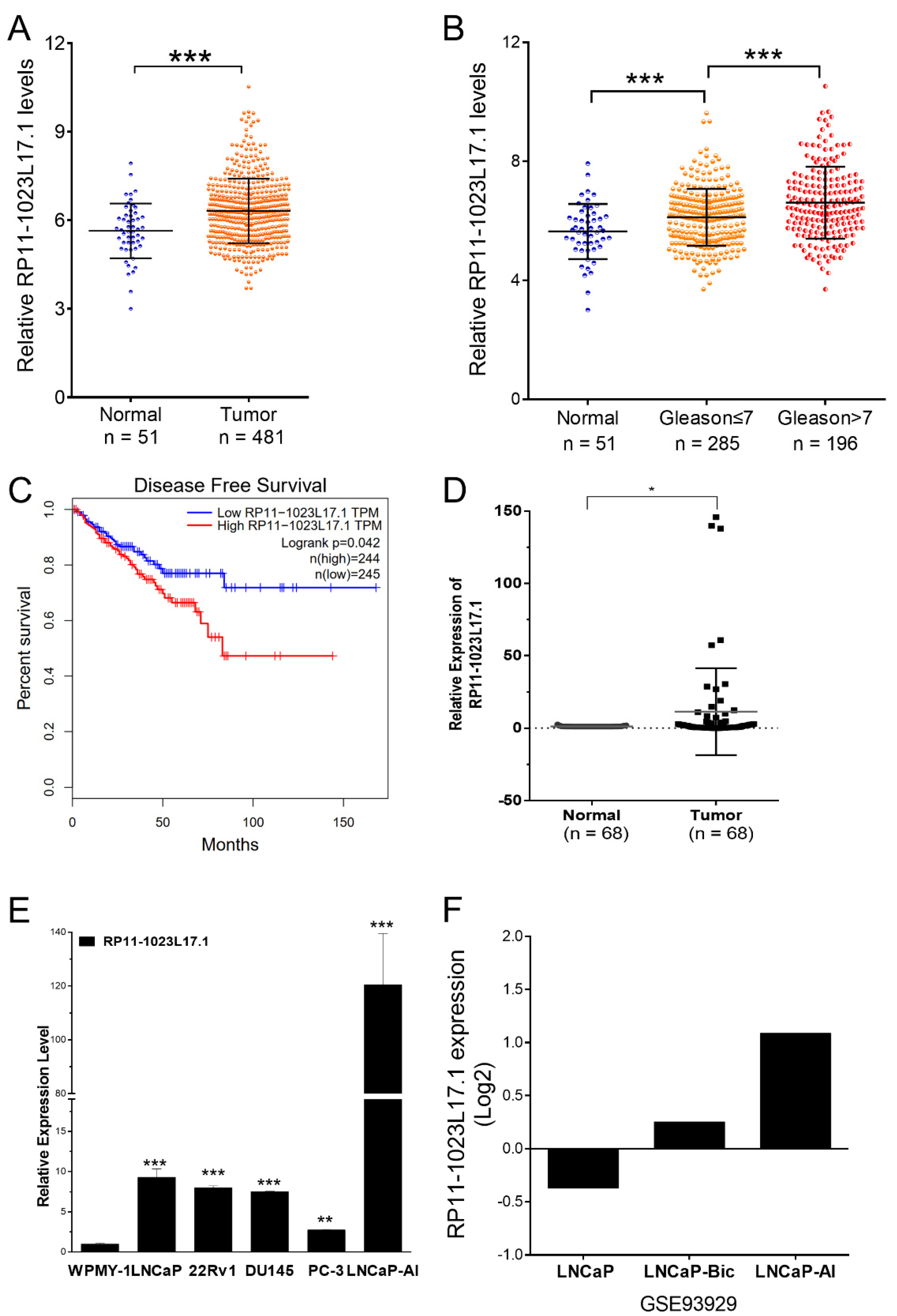
2.2. RP11-1023L17.1 Is Highly Expressed in PCa, and Its Expression Is Regulated by p38-MAPK Signaling Pathway
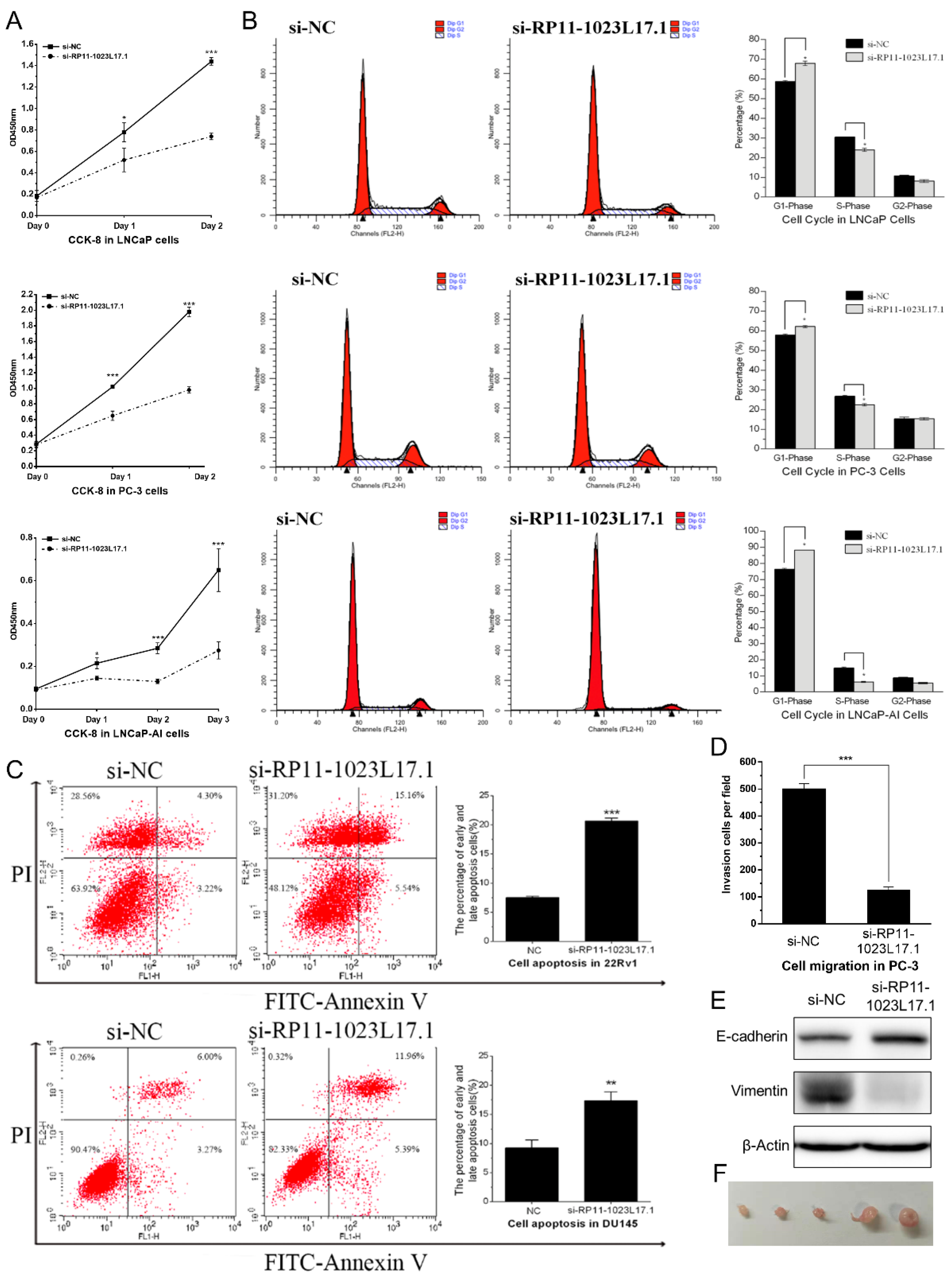
2.3. RP11-1023L17.1 Promotes Proliferation, Migration, and EMT of PCa Cells
2.4. RP11-1023L17.1 Does Not Function as a miRNA Sponge through ceRNA Mechanism
2.5. Identificaton of Genes Regulated by RP11-1023L17.1
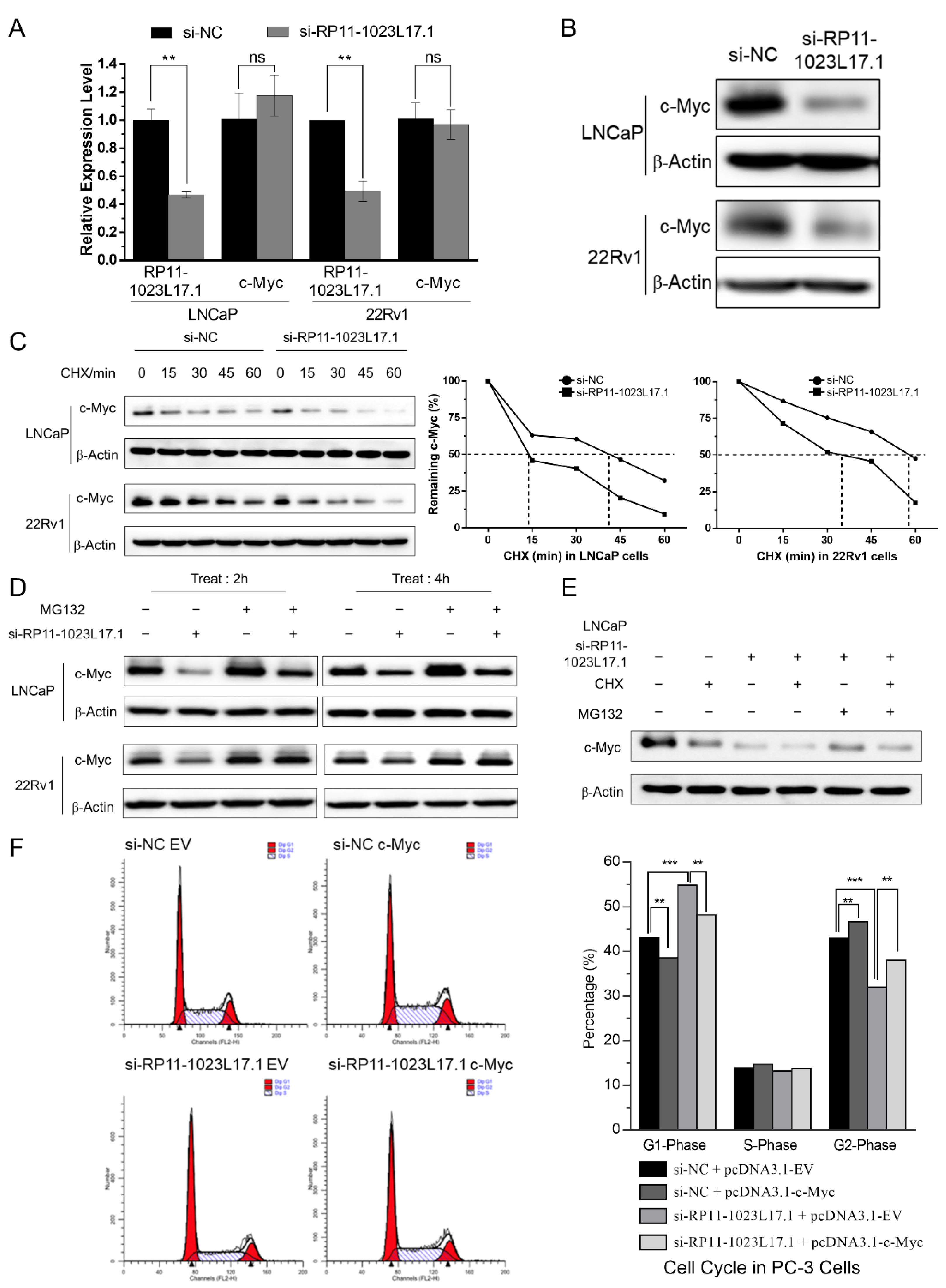
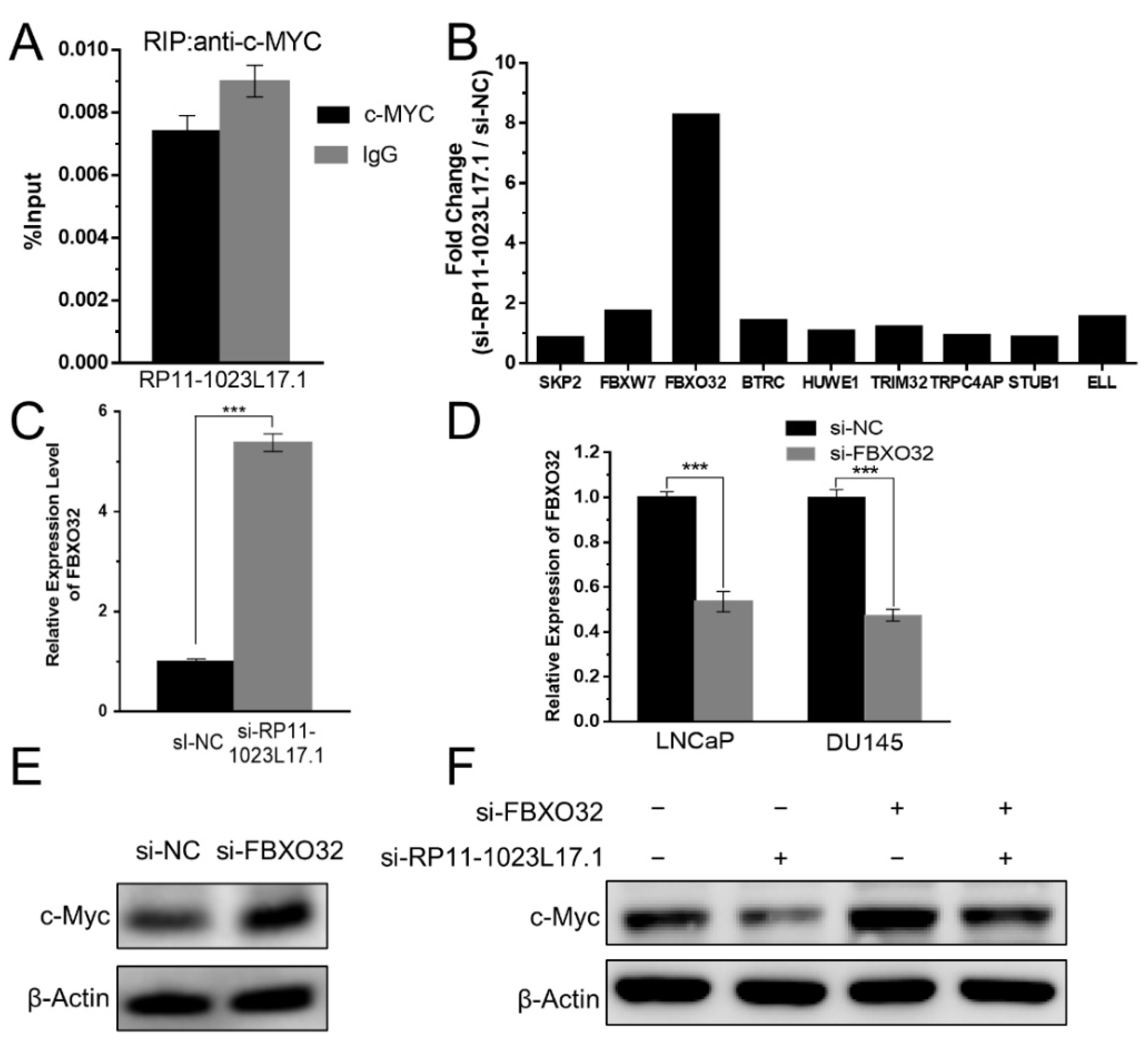
2.6. Post-Translational Regulation of c-Myc Protein Stability by RP11-1023L17.1 through Proteasomal Degradation
2.7. RP11-1023L17.1 Inhibits c-Myc Protein Degradation through Regulation of FBXO32
3. Discussion
4. Materials and Methods
4.1. Cell Culture, Drug Treatment, and Transfection
4.2. Cell Nuclear and Cytoplasmic Extraction, Quantitative Real-Time PCR (qRT–PCR), and Western Blot Analysis
4.3. Flow Cytometry Analysis, Cell Migration, and Cell Proliferation Assay
4.4. ChIP and RIP Assay
4.5. Microarray and Gene Ontology Assays
4.6. Nude Mice Xenograft Model
4.7. Patients and Tissue Samples
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Davey, R.A.; Grossmann, M. Androgen Receptor Structure, Function and Biology: From Bench to Bedside. Clin. Biochem Rev. 2016, 37, 3–15. [Google Scholar] [PubMed]
- Litwin, M.S.; Tan, H.-J. The Diagnosis and Treatment of Prostate Cancer: A Review. JAMA 2017, 317, 2532. [Google Scholar] [CrossRef] [PubMed]
- Rebello, R.J.; Oing, C.; Knudsen, K.E.; Loeb, S.; Johnson, D.C.; Reiter, R.E.; Gillessen, S.; Van der Kwast, T.; Bristow, R.G. Prostate Cancer. Nat. Rev. Dis. Prim. 2021, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Dykes, I.M.; Emanueli, C. Transcriptional and Post-Transcriptional Gene Regulation by Long Non-Coding RNA. Genom. Proteom. Bioinform. 2017, 15, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Prajapati, K.S.; Singh, A.K.; Kushwaha, P.P.; Shuaib, M.; Gupta, S. Long Non-Coding RNA Regulating Androgen Receptor Signaling in Breast and Prostate Cancer. Cancer Lett. 2021, 504, 15–22. [Google Scholar] [CrossRef]
- Zhang, A.; Zhao, J.C.; Kim, J.; Fong, K.; Yang, Y.A.; Chakravarti, D.; Mo, Y.-Y.; Yu, J. LncRNA HOTAIR Enhances the Androgen-Receptor-Mediated Transcriptional Program and Drives Castration-Resistant Prostate Cancer. Cell Rep. 2015, 13, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Ahmed, M.; Zhang, F.; Yao, C.Q.; Li, S.; Liang, Y.; Hua, J.; Soares, F.; Sun, Y.; Langstein, J.; et al. Modulation of Long Noncoding RNAs by Risk SNPs Underlying Genetic Predispositions to Prostate Cancer. Nat. Genet. 2016, 48, 1142–1150. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, K.Y.; Liu, Q.; Cao, Q. Androgen Receptor-Related Non-Coding RNAs in Prostate Cancer. Front. Cell Dev. Biol. 2021, 9, 660853. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Huang, W.; Yang, S.; Zhang, Y.; Pu, H.; Fu, F.; Huang, Y.; Wu, H.; Li, T.; Li, Y. Identification of Androgen-Responsive LncRNAs as Diagnostic and Prognostic Markers for Prostate Cancer. Oncotarget 2016, 7, 60503–60518. [Google Scholar] [CrossRef]
- Luo, J.; Wang, D.; Wan, X.; Xu, Y.; Lu, Y.; Kong, Z.; Li, D.; Gu, W.; Wang, C.; Li, Y.; et al. Crosstalk Between AR and Wnt Signaling Promotes Castration-Resistant Prostate Cancer Growth. Onco Targets Ther. 2020, 13, 9257–9267. [Google Scholar] [CrossRef] [PubMed]
- Crona, D.; Whang, Y. Androgen Receptor-Dependent and -Independent Mechanisms Involved in Prostate Cancer Therapy Resistance. Cancers 2017, 9, 67. [Google Scholar] [CrossRef] [PubMed]
- Jacob, A.; Raj, R.; Allison, D.B.; Myint, Z.W. Androgen Receptor Signaling in Prostate Cancer and Therapeutic Strategies. Cancers 2021, 13, 5417. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, A. Mathematical Modeling of CeRNA-Based Interactions. In Pseudogenes; Poliseno, L., Ed.; Methods in Molecular Biology; Springer: New York, NY, US, 2021; Volume 2324, pp. 105–114. ISBN 978-1-07-161502-7. [Google Scholar]
- Chen, Y.; Wang, X. MiRDB: An Online Database for Prediction of Functional MicroRNA Targets. Nucleic Acids Res. 2020, 48, D127–D131. [Google Scholar] [CrossRef]
- Huang, H.-Y.; Lin, Y.-C.-D.; Li, J.; Huang, K.-Y.; Shrestha, S.; Hong, H.-C.; Tang, Y.; Chen, Y.-G.; Jin, C.-N.; Yu, Y.; et al. MiRTarBase 2020: Updates to the Experimentally Validated MicroRNA-Target Interaction Database. Nucleic Acids Res. 2020, 48, D148–D154. [Google Scholar] [CrossRef]
- Duffy, M.J.; O’Grady, S.; Tang, M.; Crown, J. MYC as a Target for Cancer Treatment. Cancer Treat. Rev. 2021, 94, 102154. [Google Scholar] [CrossRef]
- Liu, J.-Y.; Chen, Y.-J.; Feng, H.-H.; Chen, Z.-L.; Wang, Y.-L.; Yang, J.-E.; Zhuang, S.-M. LncRNA SNHG17 Interacts with LRPPRC to Stabilize C-Myc Protein and Promote G1/S Transition and Cell Proliferation. Cell Death Dis. 2021, 12, 970. [Google Scholar] [CrossRef]
- Tang, J.; Yan, T.; Bao, Y.; Shen, C.; Yu, C.; Zhu, X.; Tian, X.; Guo, F.; Liang, Q.; Liu, Q.; et al. LncRNA GLCC1 Promotes Colorectal Carcinogenesis and Glucose Metabolism by Stabilizing C-Myc. Nat. Commun. 2019, 10, 3499. [Google Scholar] [CrossRef]
- Tong, L.; Qiu, Y.; Wang, H.; Qu, Y.; Zhao, Y.; Lin, L.; Wang, Y.; Xu, W.; Zhao, W.; He, H.; et al. Expression Profile and Function Analysis of Long Non-Coding RNAs in the Infection of Coxsackievirus B3. Virol. Sin. 2019, 34, 618–630. [Google Scholar] [CrossRef]
- Grosse, A.; Bartsch, S.; Baniahmad, A. Androgen Receptor-Mediated Gene Repression. Mol. Cell. Endocrinol. 2012, 352, 46–56. [Google Scholar] [CrossRef]
- Verras, M.; Lee, J.; Xue, H.; Li, T.-H.; Wang, Y.; Sun, Z. The Androgen Receptor Negatively Regulates the Expression of C-Met: Implications for a Novel Mechanism of Prostate Cancer Progression. Cancer Res. 2007, 67, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Hong, Y.; Zhang, F.; An, L.; Yang, Q.; Huang, X.; Xu, Q. Knockdown of COPS3 Inhibits the Progress of Prostate Cancer through Reducing Phosphorylated P38 MAPK Expression and Impairs the Epithelial-Mesenchymal Transition Process. Prostate 2019, 79, 1823–1831. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Mao, Y.; Huang, W.; Li, M.; Zhang, H.; Qing, Y.; Lu, S.; Xiao, H.; Li, K. Methylcrotonoyl-CoA Carboxylase 2 Promotes Proliferation, Migration and Invasion and Inhibits Apoptosis of Prostate Cancer Cells Through Regulating GLUD1-P38 MAPK Signaling Pathway. Onco Targets Ther. 2020, 13, 7317–7327. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lu, S.-Y.; Guo, R.; Ma, J.-X.; Tang, L.-Y.; Wang, J.-J.; Shen, C.-L.; Lu, L.-M.; Liu, J.; Wang, Z.-G.; et al. STK10 Knockout Inhibits Cell Migration and Promotes Cell Proliferation via Modulating the Activity of ERM and P38 MAPK in Prostate Cancer Cells. Exp. Ther. Med. 2021, 22, 851. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kwon, W.; Park, J.-K.; Baek, S.-M.; Lee, S.-W.; Cho, G.-J.; Ha, Y.-S.; Lee, J.N.; Kwon, T.G.; Kim, M.O.; et al. Suppression of Cathepsin a Inhibits Growth, Migration, and Invasion by Inhibiting the P38 MAPK Signaling Pathway in Prostate Cancer. Arch. Biochem. Biophys. 2020, 688, 108407. [Google Scholar] [CrossRef] [PubMed]
- Cheung, S.; Jain, P.; So, J.; Shahidi, S.; Chung, S.; Koritzinsky, M. P38 MAPK Inhibition Mitigates Hypoxia-Induced AR Signaling in Castration-Resistant Prostate Cancer. Cancers 2021, 13, 831. [Google Scholar] [CrossRef] [PubMed]
- Fang, K.; Hu, C.; Zhang, X.; Hou, Y.; Gao, D.; Guo, Z.; Li, L. LncRNA ST8SIA6-AS1 Promotes Proliferation, Migration and Invasion in Breast Cancer through the P38 MAPK Signalling Pathway. Carcinogenesis 2020, 41, 1273–1281. [Google Scholar] [CrossRef]
- Salmena, L.; Poliseno, L.; Tay, Y.; Kats, L.; Pandolfi, P.P. A CeRNA Hypothesis: The Rosetta Stone of a Hidden RNA Language? Cell 2011, 146, 353–358. [Google Scholar] [CrossRef]
- Mi, Y.-Y.; Sun, C.-Y.; Zhang, L.-F.; Wang, J.; Shao, H.-B.; Qin, F.; Xia, G.-W.; Zhu, L.-J. Long Non-Coding RNAs LINC01679 as a Competitive Endogenous RNAs Inhibits the Development and Progression of Prostate Cancer via Regulating the MiR-3150a-3p/SLC17A9 Axis. Front. Cell Dev. Biol. 2021, 9, 737812. [Google Scholar] [CrossRef]
- Peng, N.; Zhang, Z.; Wang, Y.; Yang, M.; Fan, J.; Wang, Q.; Deng, L.; Chen, D.; Cai, Y.; Li, Q.; et al. Down-Regulated LINC00115 Inhibits Prostate Cancer Cell Proliferation and Invasion via Targeting MiR-212-5p/FZD5/Wnt/β-Catenin Axis. J. Cell. Mol. Med. 2021, 25, 10627–10637. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhao, H.; Chen, Y.; Li, K.; Li, T.; Chen, J.; Zhang, B.; Guo, C.; Qing, L.; Shen, J.; et al. Exosomal Long Noncoding RNA HOXD-AS1 Promotes Prostate Cancer Metastasis via MiR-361-5p/FOXM1 Axis. Cell Death Dis. 2021, 12, 1129. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Gao, F.; He, Q.; Li, G.; Ding, G. LncRNA UCA1 Functions as a CeRNA to Promote Prostate Cancer Progression via Sponging MiR143. Mol. Ther. Nucleic Acids 2020, 19, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Vergani-Junior, C.A.; Tonon-da-Silva, G.; Inan, M.D.; Mori, M.A. DICER: Structure, Function, and Regulation. Biophys. Rev. 2021, 13, 1081–1090. [Google Scholar] [CrossRef] [PubMed]
- Stone, L. Antagonizing AR: MYC Affects Transcription. Nat. Rev. Urol. 2017, 14, 388. [Google Scholar] [CrossRef]
- Berger, A.; Brady, N.J.; Bareja, R.; Robinson, B.; Conteduca, V.; Augello, M.A.; Puca, L.; Ahmed, A.; Dardenne, E.; Lu, X.; et al. N-Myc–Mediated Epigenetic Reprogramming Drives Lineage Plasticity in Advanced Prostate Cancer. J. Clin. Investig. 2019, 129, 3924–3940. [Google Scholar] [CrossRef]
- Bernard, D.; Pourtier-Manzanedo, A.; Gil, J.; Beach, D.H. Myc Confers Androgen-Independent Prostate Cancer Cell Growth. J. Clin. Investig. 2003, 112, 1724–1731. [Google Scholar] [CrossRef]
- Hung, C.-L.; Wang, L.-Y.; Yu, Y.-L.; Chen, H.-W.; Srivastava, S.; Petrovics, G.; Kung, H.-J. A Long Noncoding RNA Connects C-Myc to Tumor Metabolism. Proc. Natl. Acad. Sci. USA 2014, 111, 18697–18702. [Google Scholar] [CrossRef]
- Mei, Z.; Zhang, D.; Hu, B.; Wang, J.; Shen, X.; Xiao, W. FBXO32 Targets C-Myc for Proteasomal Degradation and Inhibits c-Myc Activity. J. Biol. Chem. 2015, 290, 16202–16214. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, Y.; Zhu, R.; Ding, F.; Wan, Y.; Li, Y.; Liu, Z. FBXO32 Suppresses Breast Cancer Tumorigenesis through Targeting KLF4 to Proteasomal Degradation. Oncogene 2017, 36, 3312–3321. [Google Scholar] [CrossRef]
- Zhang, N.; Liao, Y.; Lv, W.; Zhu, S.; Qiu, Y.; Chen, N.; Xiao, M.; Zhang, H. FBXO32 Targets PHPT1 for Ubiquitination to Regulate the Growth of EGFR Mutant Lung Cancer. Cell Oncol. 2022, 45, 293–307. [Google Scholar] [CrossRef]
- Chou, J.-L.; Su, H.-Y.; Chen, L.-Y.; Liao, Y.-P.; Hartman-Frey, C.; Lai, Y.-H.; Yang, H.-W.; Deatherage, D.E.; Kuo, C.-T.; Huang, Y.-W.; et al. Promoter Hypermethylation of FBXO32, a Novel TGF-Beta/SMAD4 Target Gene and Tumor Suppressor, Is Associated with Poor Prognosis in Human Ovarian Cancer. Lab. Investig. 2010, 90, 414–425. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.; Zhang, H.; Li, J.; Shan, Y. LINC00494 Promotes Ovarian Cancer Development and Progression by Modulating NFκB1 and FBXO32. Front. Oncol. 2020, 10, 541410. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wan, X.; Huang, W.; Zhang, L.; Luo, J.; Li, D.; Huang, Y.; Li, Y.; Xu, Y. AC016745.3 Regulates the Transcription of AR Target Genes by Antagonizing NONO. Life 2021, 11, 1208. [Google Scholar] [CrossRef] [PubMed]
| Transcription Factor | The Number of Genes 1 |
|---|---|
| MYC (V$EBOX) | 169 |
| JUN (V$CREB, V$AP1F) | 89 |
| SP1 (V$SP1F) | 81 |
| E2F1 (V$E2FF) | 79 |
| EGR1 (V$EGRF) | 77 |
| FOS (V$AP1F) | 73 |
| STAT3 (V$STAT) | 56 |
| CEBPB (V$CEBP) | 55 |
| CREB1 (V$CREB) | 54 |
| ESR1 (V$EREF) | 44 |
| STAT1 (V$IRFF, V$STAT) | 41 |
| TBP (O$VTBP) | 38 |
| IRF1 (V$IRFF) | 36 |
| SOX2 (V$SORY, V$STEM) | 31 |
| YY1 (V$YY1F) | 29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, W.; Chen, Q.; Lu, Y.; Kong, Z.; Wan, X.; Huang, Y.; Qiu, M.; Li, Y. Androgen-Responsive Oncogenic lncRNA RP11-1023L17.1 Enhances c-Myc Protein Stability in Prostate Cancer. Int. J. Mol. Sci. 2022, 23, 12219. https://doi.org/10.3390/ijms232012219
Huang W, Chen Q, Lu Y, Kong Z, Wan X, Huang Y, Qiu M, Li Y. Androgen-Responsive Oncogenic lncRNA RP11-1023L17.1 Enhances c-Myc Protein Stability in Prostate Cancer. International Journal of Molecular Sciences. 2022; 23(20):12219. https://doi.org/10.3390/ijms232012219
Chicago/Turabian StyleHuang, Wenhua, Qin Chen, Yali Lu, Zhe Kong, Xuechao Wan, Yan Huang, Minyan Qiu, and Yao Li. 2022. "Androgen-Responsive Oncogenic lncRNA RP11-1023L17.1 Enhances c-Myc Protein Stability in Prostate Cancer" International Journal of Molecular Sciences 23, no. 20: 12219. https://doi.org/10.3390/ijms232012219
APA StyleHuang, W., Chen, Q., Lu, Y., Kong, Z., Wan, X., Huang, Y., Qiu, M., & Li, Y. (2022). Androgen-Responsive Oncogenic lncRNA RP11-1023L17.1 Enhances c-Myc Protein Stability in Prostate Cancer. International Journal of Molecular Sciences, 23(20), 12219. https://doi.org/10.3390/ijms232012219






