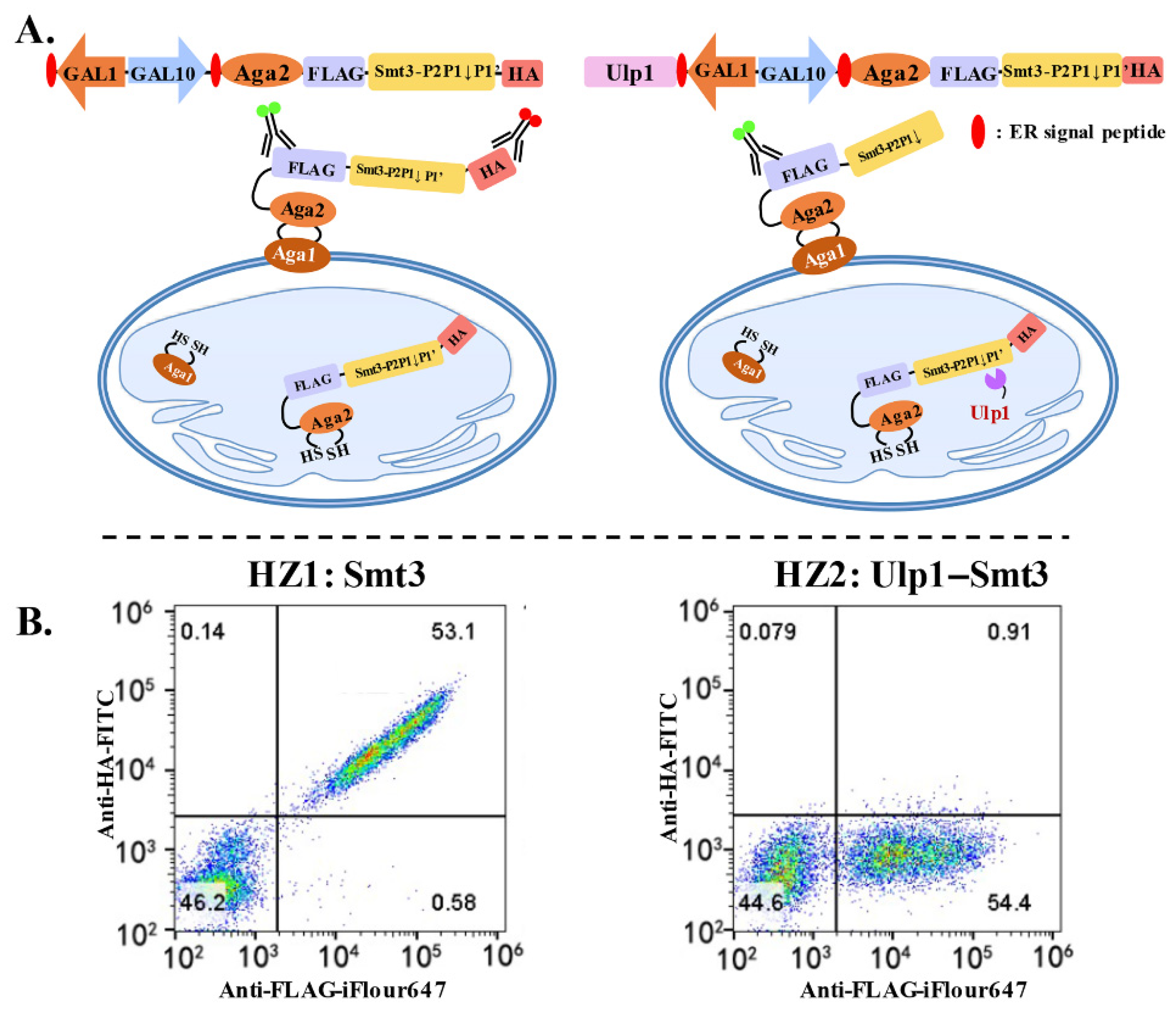
2.1. Verification of the YESS–PSSC System for Quantitatively Characterizing Ulp1–Smt3 Specificity
Conventional gel-electrophoresis-based in vitro methods for studying the protease–substrate specificity are always complicated and time-consuming due to the large number of tested substrates [
20]. In contrast, in vivo analytical platforms such as the YESS–PSSC system, which is used for studying the substrate specificity of Ulp1 against Smt3, are particularly attractive for their advantages on simplicity and high-throughput [
18]. Based on the YESS–PSSC concept, Ulp1 and the bi-epitope tag fused substrate cassette (Aga2-FLAG-Smt3↓-HA) were simultaneously expressed under the control of a divergent promoter (
GAL1-
GAL10), followed by being escorted into yeast ER for proteolytic reactions. The proteolytic substrate part (Aga2-FLAG-Smt3↓) was then displayed on the cell surface for fluorophore-conjugated antibody labelling and flow cytometry analysis (
Figure 1A).
The display efficiency of the substrate cassette determined the efficacy of the YESS–PSSC system. Considering that Smt3 is a globular-structure protein containing 101 residues [
15], the display efficiency of the Smt3 substrate cassette (Aga2-FLAG-Smt3-HA) was evaluated first (
Figure 1B). The Smt3 substrate cassette was displayed on the yeast cell surface with an efficiency of about 50–60%, which was slightly lower than the peptide–substrate display system’s efficiency of 70% [
18]. However, the clearly-separated cell population and the utter dual anti-FLAG-iFluor 647/anti-HA-FITC fluorescence intensities implied that the Smt3 substrate cassette was well-expressed, cell-surface-displayed and not interfered by the
S. cerevisiae endogenous Ulp1. This might be because the substrate part was escorted into yeast ER through the ER signal peptide, while the endogenous Ulp1 was usually localized in the nucleus, guided by its N-terminal nuclear-localization signal (NLS) [
24]. Subsequently, we proceeded to evaluate Ulp1 activity towards the Smt3 substrate cassette in the YESS–PSSC system.
S. cerevisiae Ulp1 contains an N-terminal regulation domain (1–402) and a highly conserved C-terminal protease domain (403–621), among which the C-terminal protease domain is sufficient to recognize and cleave Smt3 with comparably high activity to full-length Ulp1 [
24]. The C-terminal protease domain was used in our studies to evaluate its substrate specificity. In principle, if Smt3 was recognized and cleaved by Ulp1, the C-terminal HA tag of the substrate cassette would be removed to generate the dominating display of Aga2-FLAG-Smt3GG↓ on the cell surface, resulting in high anti-FLAG-iFluor 647 and no–low anti-HA-FITC fluorescence intensities. However, if the substrate cassette was not cleaved, the intact Aga2-FLAG-Smt3-HA would be displayed and labeled with both high anti-FLAG-iFluor 647 and anti-HA-FITC fluorescence intensities. The stronger anti-HA-FITC fluorescence intensity indicated a more inefficient cleavage. As expected, only anti-FLAG-iFluor 647 fluorescence intensity was observed in the cells co-expressing Ulp1 and the Smt3 substrate cassette, suggesting nearly 100% cleavage efficiency (
Figure 1B). These results collectively verified that the YESS–PSSC system is the proper system to study Ulp1 substrate specificity.
Not only can it be applied to characterize the substrate specificity of Ulp1, the YESS–PSSC system also showed great potential in dissecting the substrate specificity of other SUMO protease homologs as well as protein–substrate proteases, with several unique advantages. Firstly, the in vivo proteolytic reaction enabled the evaluation of multiple substrates without the need to purify the protease and substrates, so the process was time- and labor-efficient [
18,
19]. Secondly, compartmentalizing the proteolytic reaction of protease in the endoplasmic reticulum (ER) through the ER signal peptide largely maintained the physiological properties of proteases and substrates due to the existence of various molecular chaperones in the ER [
25]. Thirdly, the powerful secretory system of
S. cerevisiae enabled the proteolytic protein products to be quickly transported to the cell surface for fluorophore-conjugated antibody labelling and flow cytometry analysis [
26,
27]. Besides, the ER separated the proteolytic reaction from complex endogenous biological processes, thus barely being disturbed by the endogenous regulating system.
2.3. Single Mutation of Smt3 in the Smt3–Ulp1 Interacting Interface Would Not Affect Ulp1 Recognition Specificity
The crystal structure of the Ulp1–Smt3 complex revealed that the Ulp1–Smt3 interacting interface could be roughly described as six structural motifs of Ulp1 interacting with the exposed β sheet and C-terminal strand of Smt3 [
15] (
Figure 3A). Several conservative sites in the Ulp1 motif were, respectively, mutated to identify the essential roles of central regions such as motif 2, motif 3 and motif 5 in maintaining Ulp1–Smt3 recognition specificity [
15]. In our work, the key residues of Smt3 interacting with Ulp1 in every motif were mutated to nonpolar amino acids to explore the significance of each site for Ulp1–Smt3 recognition specificity. Guided by the structural analysis, R64G, R68G, G69A, R71G, D82G, E94G, Q95G, I96G and G98A mutations were, respectively, introduced to eliminate the major salt bridge or/and hydrogen bond contact with Ulp1.
The mutated Smt3
I cassettes (Aga2-FLAG-Smt3
I GG↓A-HA, where Smt3
I represents the Smt3 interface mutation) were, respectively, co-expressed with Ulp1 in the YESS–PSSC system. Flow cytometry analysis showed that the wild-type Smt3 and eight mutated Smt3
I substrates were all cleaved by Ulp1 with more than 98% efficiency except the Smt3
I–G98A mutant whose cleavage efficiency was 94.65%, indicating that a single mutation in Smt3 had almost no effect on its interactions with Ulp1 (
Figure 3B). This can be explained by the recognition of Ulp1 towards Smt3 being dependent on an extensive interface consisting of multiple residues, thereby the influence caused by a single mutation could be neglected. It is worth noting that the Smt3–G98A mutation, a position that is usually considered highly-conserved in SUMO proteins (G
G98th↓A), exhibited a cleavage efficiency that was about 5% lower than the wild-type Smt3 and other mutations. This confirmed that Gly98 is indeed important for the recognition and cleavage of Ulp1 against Smt3, and, more importantly, it also implied that the Gly–Gly motif is not strictly required for the Ulp1 cleavage of Smt3. Therefore, we further characterized the specificity of Ulp1 against the conserved Gly–Gly motif (P2-P1↓ position) of Smt3.
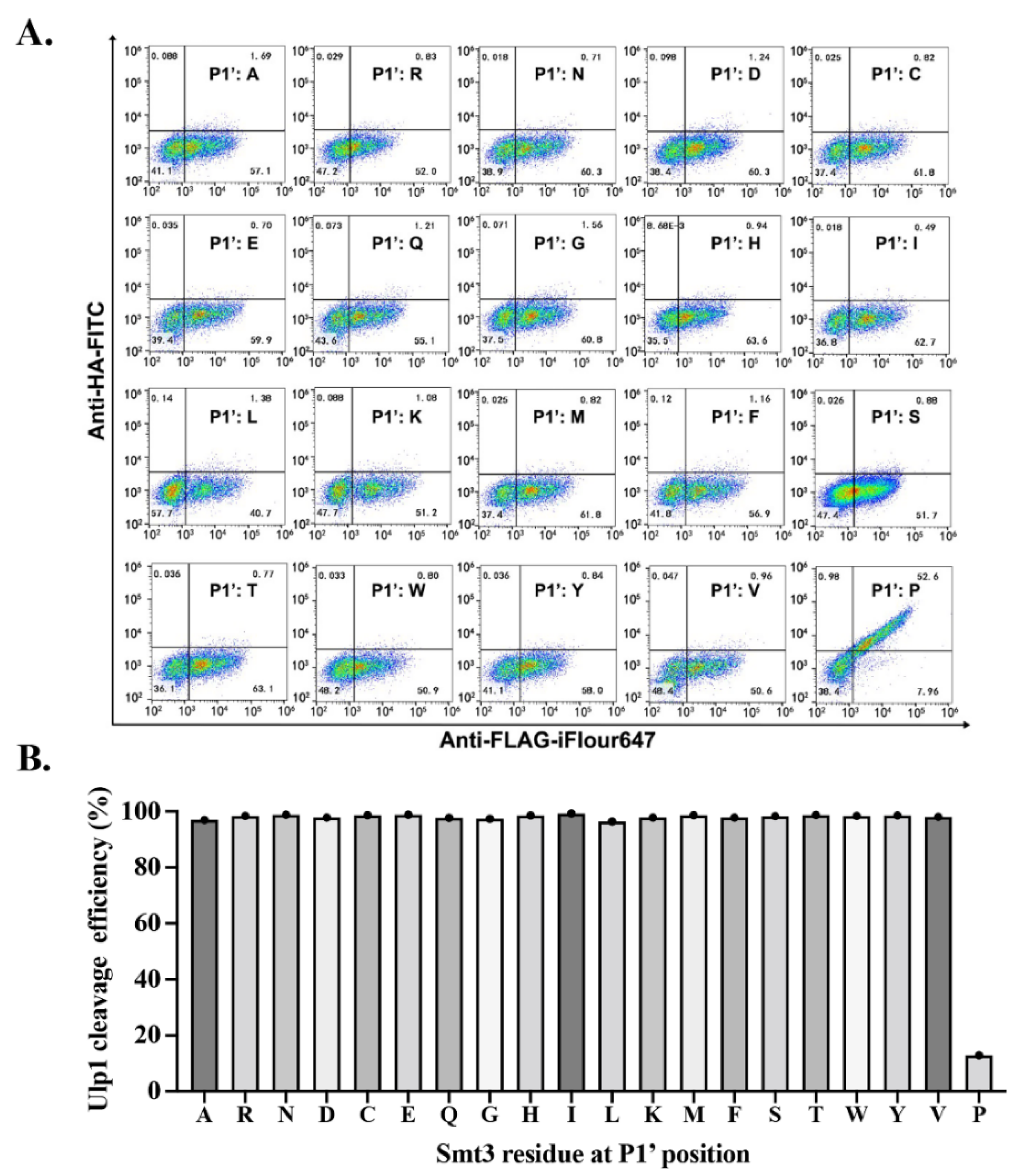
2.4. Characterizing Residue Specificity of Ulp1 against Smt3 at P1 and P2 Positions
To explore the residue specificity of Ulp1 against Smt3 at the P1 and P2 positions (the conserved Gly–Gly motif), a series of mutated Smt3
P–G
X1↓A and –
X2G↓A substrates (where X is an arbitrary amino acid, and Smt3
P represents the Smt3 P1 or P2 mutation) were constructed and analyzed in the YESS–PSSC system. It needs to be emphasized that we mutated each Gly into larger amino acids because these two sites are both located in the narrow active tunnel of Ulp1 (
Figure 4B) [
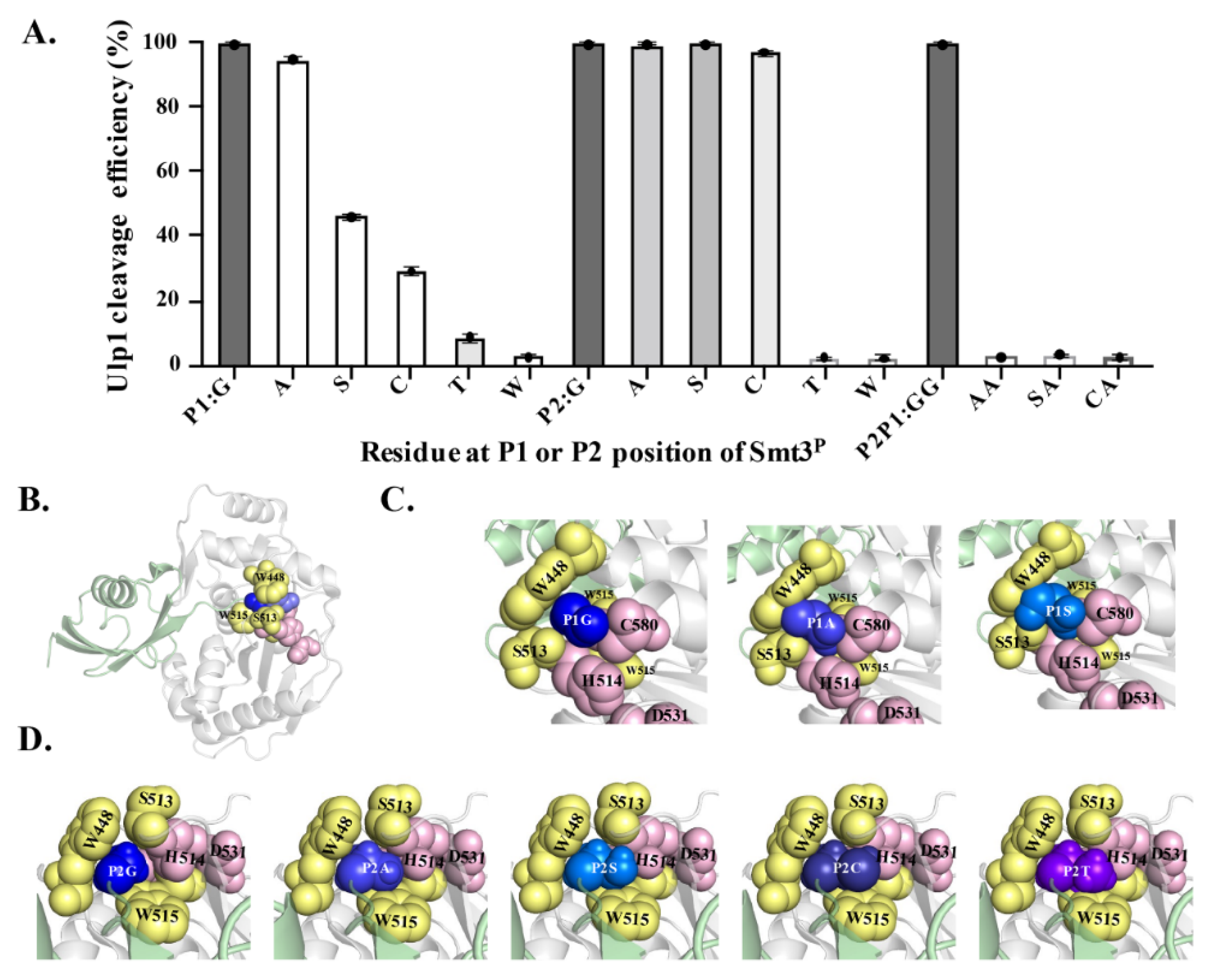
15]. At the P1 position, Ulp1 presented a high activity of 94.65% against the Smt3
P–G
A↓A substrate, which is only slightly lower than the 99.48% cleavage efficiency against the wild-type Smt3–GG↓A substrate. Comparably, when replacing Gly at the P1 position with residues that have bulky side chains, the cleavage efficiency of Ulp1 against the Smt3
P1 substrate gradually decreased, showing a cleavage efficiency of 46.07%, 29.04%, 8.60% and 2.59% for Smt3
P–G
S↓A, Smt3
P–G
C↓A, Smt3
P–G
T↓A and Smt3
P–G
W↓A substrates, respectively (
Figure 4A). Other residue substitutions were not analyzed because it could be speculated that amino acids having bulkier side chains than Thr could possibly provoke steric hindrance, preventing Smt3
P from entering the substrate tunnel. At the P2 position, Ulp1 cleavage efficiency against the Smt3
P–
AG↓A, Smt3
P–
SG↓A and Smt3
P–
CG↓A substrates were, respectively, measured to 99.39%, 99.42% and 96.98% (
Figure 4A). Similar to that of the P1 position, Ulp1 presented no detectable cleavage against Smt3 with Thr or Trp at the P2 position (
Figure 4A). Based on these results, three dual-mutation substrates, including Smt3
P–
AA↓A, Smt3
P–
SA↓A and Smt3
P–
CA↓A, were further constructed to explore whether Ulp1 could accept dual mutations at the Gly–Gly motif. No detectable cleavage was observed on these substrates, even though each single-mutated substrate could be efficiently cleaved by Ulp1 with high efficiency (
Figure 4A), implying the significance of the role of the smallest residue Gly at the P1 or P2 position for Ulp1 cleavage.
The C-terminal Gly–Gly motif is highly-conserved in all reported SUMO proteins from yeast to human, whereas our work suggested that Ulp1 was sufficient to cleave mutated substrates with preferential activity of Gly > Ala > Ser > Cys at the P1 position and Gly = Ala = Ser = Cys at the P2 position. These findings further supported the previous observation that a SUMO protein with similar residue substitutions at the Gly–Gly motif, such as directly-expressed human SUMO-1/3–AA, –GS and –GA mutants, could also undergo endogenous SUMOylation as effectively as the wild-type SUMO-1/3–GG protein [
16]. These results together indicated that the Gly–Gly motif is not strictly required for SUMO precursor activation and SUMO protein modification.
2.5. Structural Modeling to Explore the Possible Mechanism of the Substrate Specificity of Ulp1
A clear correlation was observed between the residue size at the P1 and P2 positions and the cleavage efficiency. Therefore, structural modeling of the Ulp1–Smt3
P complex was performed to prompt the molecular understanding of the Ulp1-unique substrate specificity of Smt3 at the P1 and P2 positions (
GG↓A motif). In the Ulp1–Smt3 complex, the Ulp1 catalytic triad C580–H514–D531 and residues W448, S513 and W515 jointly constructed a tight and shallow active tunnel, resembling a tapered structure wrapping around the Gly–Gly motif of Smt3 (
Figure 4B–D). The side-chain-free diglycine motif retained extra free space, enabling the C-terminal tail to be able to flexibly pass through the active tunnel and, finally, place the P1–P1’ target bond above the active site without encountering any steric hindrance in the tunnel (
Figure 4C,D). However, this changed when the Gly was replaced with residues possessing bulky side chains. When Gly at the P1 position was replaced with Ala, the side chain from Ala occupied more space in the Ulp1 substrate tunnel but still did not cause a significant blockage. Comparably, the -CH
2OH side chain of Ser occupied the entire cavity and formed a serious conflict with the side chains of Ulp1–S513, –W448 and –C580, causing inefficient movement in the P1–P1’ peptide bond (G
S↓A) in the active site for cleavage. Besides, the interaction also limited the orientation of S513 in the channel, thus greatly impairing the activity of Ulp1 against the Smt3
P–G
S↓A substrate (
Figure 4C). For other residues with even bulkier side chains, such as Thr and Trp, the substrates could not even be docked in the substrate tunnel.
Different than at the P1 position, Ulp1 retained more free space at the P2 position to accommodate amino acids with certain side chains. Smt3
P2 substrates with the substitution of Ala, Ser and Cys could all be easily introduced into the Ulp1 substrate pocket (
Figure 4D). In the simulated structures, Gly and Ala could smoothly enter the substrate tunnel and retained some free space due to their small side chains. Ser and Cys have slightly bulkier side chains which almost occupied the cavity though this was without obvious conflict with the residues in the tunnel. Because of the ability of free orientation, these substrates could all be effectively cleaved by Ulp1. Comparably, when the P2 position was mutated to Thr, its large side chain group filled the substrate tunnel, making Thr difficult to move to the correct position for substrate cleavage. The bulky side chain of Thr may evoke obvious steric conflict to Ulp1 channel residues like S513 and W448 as well as to the catalytic residue H514 (
Figure 4D).
Overall, the structural analysis indicated that it is the special taper-resembling active pocket of Ulp1 that conferred the distinguishing selectivity against residues of Smt3 at the P1 and P2 positions, in which only small amino acids without steric conflict with the Ulp1 substrate tunnel could flexibly adjust in the tunnel and approximate the active sites for proteolytic cleavage.
2.6. Gly in P1’ Position Expands Inclusivity of Ulp1 against Smt3
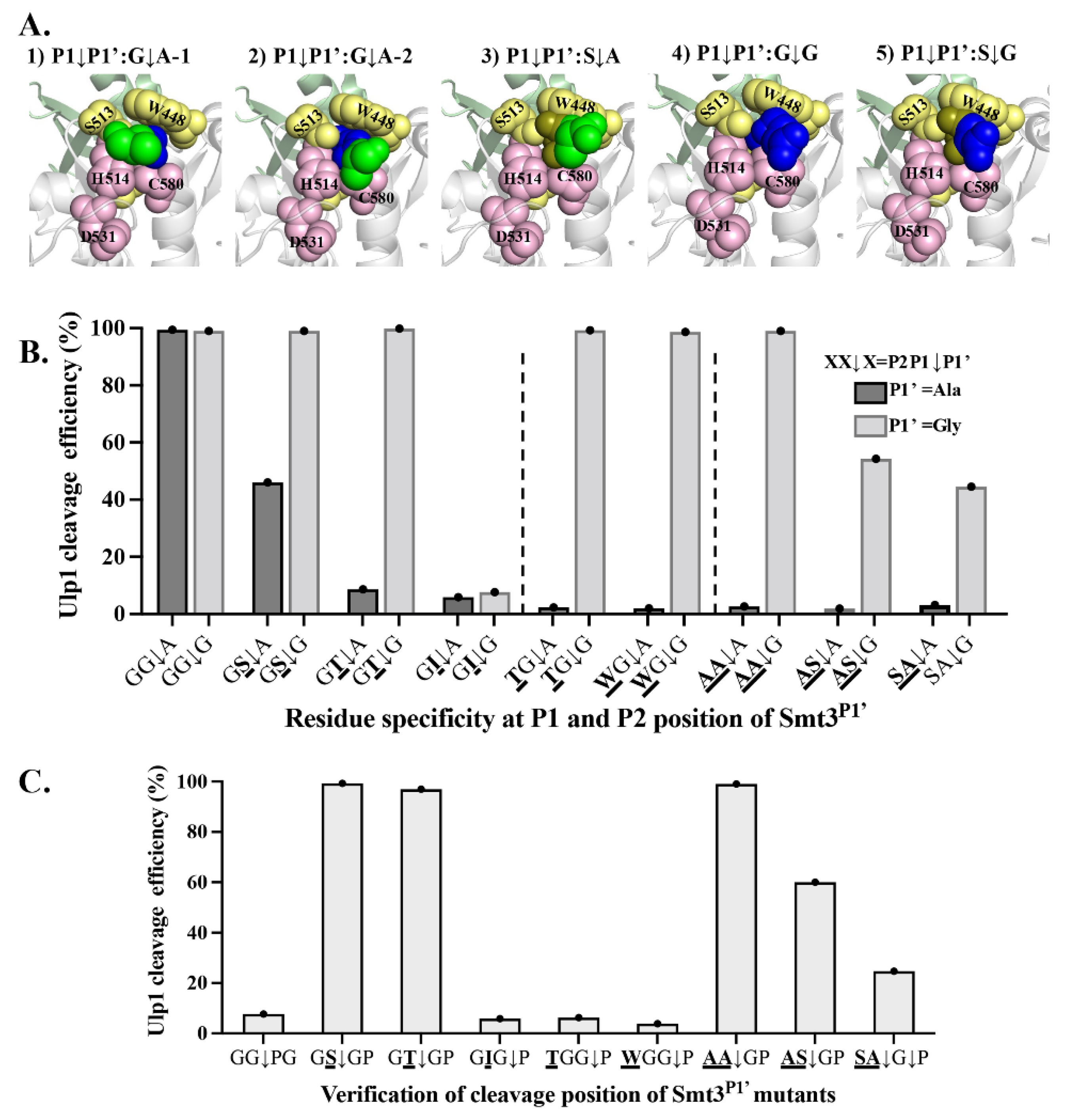
The structural analysis of the Ulp1–Smt3 complex indicated that extra moving space in the substrate tunnel was required to enable the P1–P1’ target peptide bond (G
G↓A) to flexibly approach the nucleophilic residue C580 for cleavage. During this process, the side chain group of Ala at the P1’ position might form obvious steric hindrance between the Ulp1 catalytic residues of H514 and C580, thus affecting Ulp1 catalytic efficiency (
Figure 5A). In the wild-type Smt3, the Gly–Gly motif in the substrate tunnel retained enough space to alleviate the obstruction through rotation or adjustment, so the C-terminus of Smt3 and the P1’ Ala were able to approach the catalytic pocket. Although, in the mutant Smt3
P substrates along with gradually-enlarged residues at the P1 and P2 position, such as Ala, Ser and Cys, the moving space in the substrate tunnel became limited, and the C-terminal tail was less flexible. Therefore, it was more difficult to decrease the steric hindrance of the P1’ position Ala from rotating. Based on this analysis, it can be speculated that the P1’ position Ala to Gly mutation may help expand Ulp1 inclusivity against the Smt3 residue at P1 and P2 positions because the side-chain-free Gly itself is highly-flexible, and P1 or P2 residues do not require more moving space to guarantee the high flexibility of the C-terminal tail.
To verify this speculation, Ulp1 specificity against the P1 and P2 sites of Smt3 when Smt3’s P1’ position was Ala or was mutated to Gly (Smt3
P–XX↓A/G) was simultaneously characterized. When Ala was at the P1’ position, the cleavage efficiency of Ulp1 against the substrates Smt3
P–GS↓A and Smt3
P–GT↓A was 46.0% and 2.3%, respectively (
Figure 5B). In comparison, when Gly was at the P1’ position, Ulp1 cleavage efficiency against the substrates Smt3
P–GS↓G and Smt3
P–GT↓G was increased to 99.1% and 94.88%, respectively. However, Ulp1 could not cleave the Smt3
P–GI↓G substrate, indicating that this is a synergistic effect that the P1 site could only accommodate for amino acids with side chains that are less bulky than Ile, even when P1’ is Gly (
Figure 5B). The specificity against the P2 position was also expanded. Compared to the fact that Ulp1 could only cleave substrates with a P2 residue smaller than Thr when Ala was in the P1’ position, the cleavage efficiency of Ulp1 against the Smt3
P–TG↓G and Smt3
P–WG↓G substrates was increased to 99.26% and 98.67%, respectively (
Figure 5B). Not surprisingly, Ulp1 became able to accept more dual-mutations at P1–P2 positions when the P1’ position was Gly. The cleavage efficiency against the Smt3
P–AA↓G substrate could be increased to 99.0% (
Figure 5B), and Ulp1 also presented a good cleavage efficiency of 54.32% and 44.46% against the dual-mutation substrates Smt3
P–AS↓G and Smt3
P–SA↓G, respectively, which were not able to be cleaved when the P1’ position was Ala (
Figure 5B). These results verified our speculation that the P1’ position Gly could expand Ulp1 inclusivity against the P1 and P2 positions of Smt3.
The effective cleavage of Ulp1 against bulky residues at the P1–P2 position, especially in the Smt3
P–
WG↓G substrates, was somewhat surprising, and the concern arose that the cleavage site might be shifted from Smt3
P–
WG↓GT to Smt3
P–
WGG↓T (the original P2’ residue of Smt3 is Thr). To validate whether the cleavage sites of Smt3
P substrates were shifted when P1’ was Gly, we mutated the original P2’ position Thr to Pro to generate the Smt3
P–
XX↓GP substrates. Pro was proven to be the blocking residue when it is at the P1’ position. If the cleavage site is not shifted, Ulp1 should show similar cleavage efficiency against both the Smt3
P–
XX↓GP and Smt3
P–XX↓GT substrates. Otherwise, Smt3
P–XXG↓P substrates would not be cleaved by Ulp1 at all. As shown in
Figure 5B,C, Ulp1 showed a similar high-cleavage efficiency of approximately 99% against the Smt3
P–GSGP, –GTGP and –AAGP substrates compared to that of the Smt3
P–GSGT, –GTGT and –AAGT substrates, indicating that the cleavage site was still between P2P1↓GP/T in these substrates. The Smt3
P–ASGT substrate showed a similar cleavage efficiency of 60% compared to that of the Smt3
P–ASGP substrate, concluding that the cleavage site was also unshifted. In contrast, the Smt3
P–TGGP and –WGGP substrates were almost unable to be cleaved while the Smt3
P–TGGT and –WGGT substrates were fully cleaved, indicating that the cleavage site shifted from XX↓GP/T to XXG↓P/T in these substrates. One interesting observation was that the cleavage efficiency of the Smt3
P–SAGP substrate had clearly decreased compared to that of the Smt3
P–SAGT substrate, indicating a possible wobbling cleavage site in the Smt3
P–SAGP substrate. Altogether, these results implied that a Gly at the P1’ position could expand the residue inclusivity of Ulp1 against the P1 position of Smt3
P from Gly > Ala > Ser > Cys to Gly = Ala = Ser = Cys = Thr. The expanded inclusivity of Ulp1 against huge residues in the P2 position of Smt3
P might be attributed to the shifted cleavage site from the original P1–P1’ to the P1’–P2’ position.
A previous work demonstrated that the SUMO P1’ position residue is involved in discriminating the activity of the human SUMO protease SENP1 on SUMO 1, SUMO 2 and SUMO 3 isoforms [
17]. Despite the fact that a detailed mechanism is unclear, the special significance of the P1’ position residue in the SUMO protein is worth being further explored. Here, our results revealed the essential role of the residue Ala at the P1’ position of Smt3 in maintaining Ulp1 specificity in the P1 and P2 sites of Smt3, that is, restricting only small amino acids with free space in the Ulp1 substrate tunnel can be flexibly rotated to decrease the steric hindrance of Ala in the active residues H514 and C580 and helping the P1–P1’ target peptide bond approach the nucleophilic residue C580 for cleavage. The P1, P2 and P1’ residues of Smt3 collectively conferred Ulp1’s unique specificity for the Smt3 precursor process. This unique mechanism, applied by the Ulp1–Smt3 complex, may help guarantee that all SUMO proteins are accurately cleaved behind the
GG↓ motif because the C-terminal fragment of the wild-type SUMO protein rarely contains the other small amino acids that we characterized here, such as Ala, Ser and Cys, which reflects the wisdom of the eukaryotes in evolving the SUMO system.