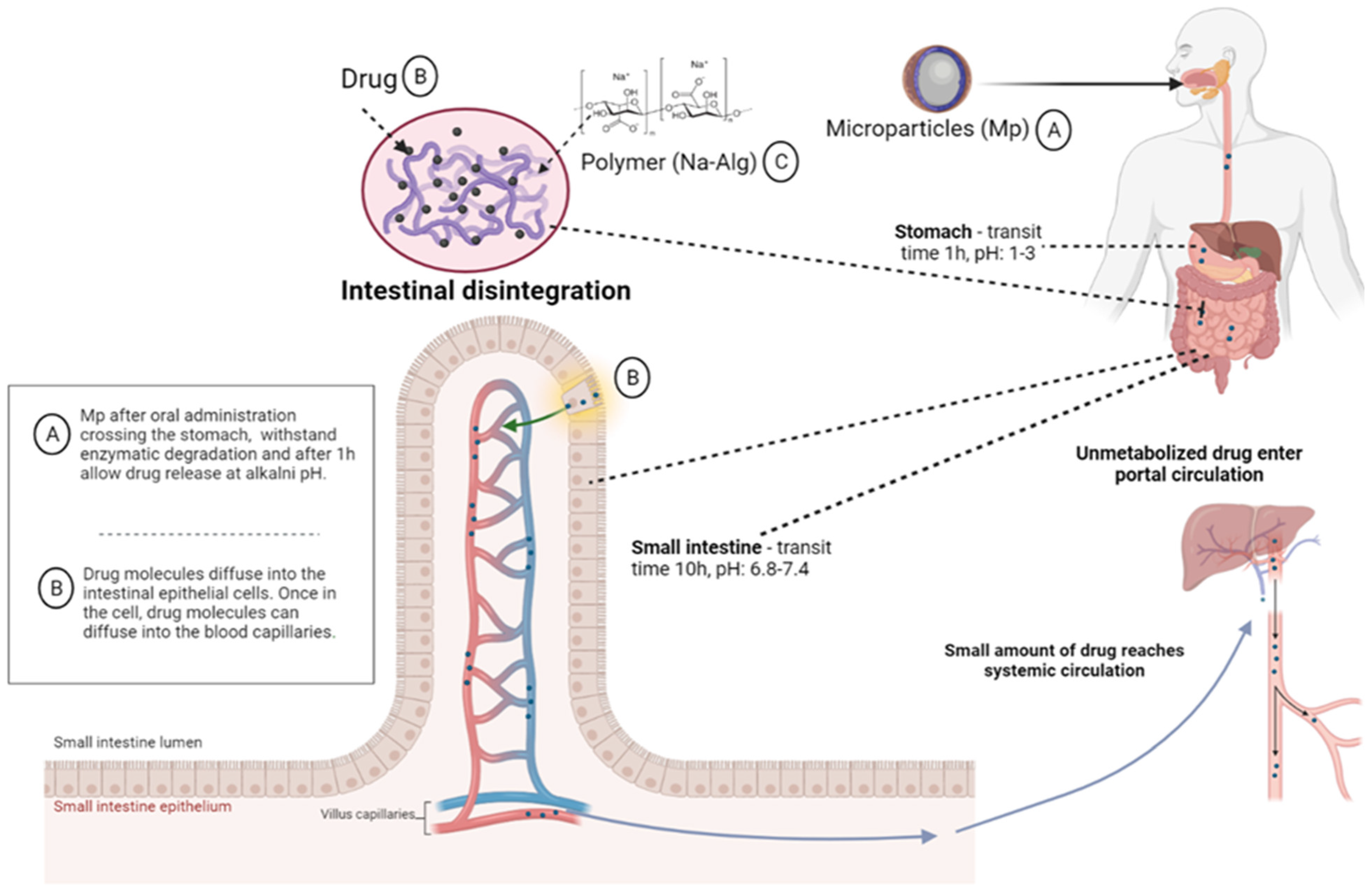
Sodium Alginate—Natural Microencapsulation Material of Polymeric Microparticles
Abstract
1. Introduction
2. Chemical Structure of Alginate
3. Physico-Chemical Properties of Sodium Alginate
3.1. Physico-Chemical Properties
3.1.1. Molecular Weight
3.1.2. Solubility
3.1.3. Stability
3.2. Mechanical Properties
3.2.1. Viscosity
3.2.2. Mucoadhesion
3.3. Biological Properties
Biocompatibility, Toxicity, Immunogenicity and Biodegradation
3.4. Other Properties
3.4.1. Ionic Reticular Capacity of Alginate with Ca2+ Ions
3.4.2. Complex Coacervation Capacity of Alginate with Chitosan
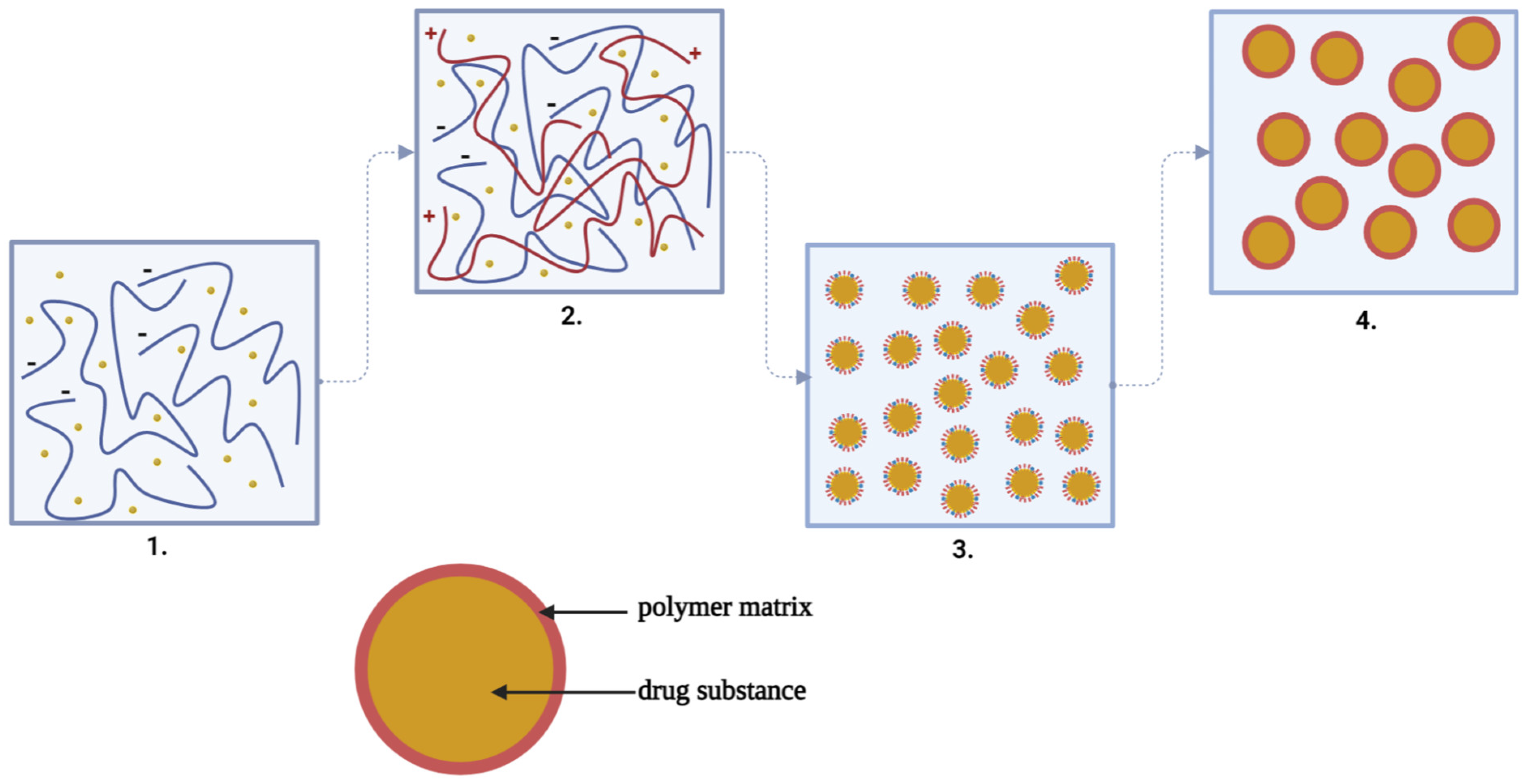
| Obtained PF | Polymers Used | DS | Advantages of the Method of Complex Coacervation | Ref. |
|---|---|---|---|---|
| MPs | Ch, CMC | Indomethacin | Modified-release PF with few adverse effects were obtained | [62] |
| Ms | Ch, Gelatin B | Tramadol | Reducing the frequency of dosages | [63] |
| NPs | Ch, Na-Alg | Insulin | The possibility of directing the manifestation of the effect to a specific target such as the colon | [67] |
| Mc | Ch, Na-Alg | Amoxicillin | Increased patient compliance | [68] |
| NPs | Ch, Na-Alg | Nifedipine | Obtaining PF with a size appropriate to absorption at GI level | [69] |
| Ms | Na-Alg, Ch | Selenium | Allows one to obtain fast-release PF in phosphate buffer solution (pH = 7.4) | [70] |
| Ms | Ch, Na-Alg | Quercetin | Allows the encapsulation in the PF of some hydrophobic DS | [11] |
| Ms | Ch, Gelatin B | Ketorolac tromethamine | The low degree of crystallinity is an advantage for controlled release | [71] |
| Ms | Na-Alg, Ch | Isoniazid | The type of polymers included in the matrix can extend the duration of release of the DS | [72] |
| Ms | Na-Alg, Gelatin B | Buryti oil | By using this encapsulation method, certain DS of polyphenolic type or volatile oils are protected from attacks of environmental factors | [73] |
| Ms | Na-Alg, Ch | Prednisolone | Rough PF can be obtained, with a similar appearance, wrinkled/smooth at the surface, with a compact structure and large number of folds, stable from temperature, and can be used at normal physiological temperature as delivery systems of the drug | [74] |
| Ms | Na-Alg, Ch | Prednisolone | Avoids the use of toxic reticular chemical agents | [75] |
| Mc | Na-Alg, Gelatin A | Astaxanthin oleoresin | Allows the obtaining of Ms with a high degree of entrapping and release of the embedded ingredients | [76] |
| Mc | Na-Alg, Ch | Triamcinolone | The use of Ch with high molecular weight together with Na-Alg has been observed to lead to Ms of lower sizes, mucoadhesive with better release rates | [77] |
| Mc | Na-Alg, Ch | Nitrofurantoin | Limitation of the occurrence of GI side effects manifested by nausea and vomiting given by certain DS (nitrofurantoin) following oral administration | [78] |
| MPs | Na-Alg, Gelatin B | Ginger volatile oil | Allows one to obtain PF with high stability to light, heat and oxygen | [79] |
| MPs | Gelatin, gum arabic | Lutein | Obtained particle have good stability at light, heat and oxygen | [80] |
| Mc | Gelatin, Na-Alg | Eugenol | If one of the polymers of the matrix is Na-Alg, it can potentiate the antioxidant effect of MPs | [81] |
| Mc | Gelatin B, corn oil, acacia BP 1993, bloom strength 225 | Vit.A palmitate | Allow the incorporation of large amounts of lipophilic drugs | [82] |
| Mc | Ch, karaya gum, paraffin oil, formaldehyde | Diclofenac sodium | It favors the sustained release of the active ingredient from the particulate system | [83] |
| Mc | Na-Alg, HACC | Tea tree | Obtained PF with spherical shape and antimicrobial effect | [84] |
| Nc | Acacia, gelatin | Capsaicin | Obtained spherical and stabile particulate system | [85] |
| Mps | Ch, Na-Alg, CMC | Tanic acid | Could be used in formulations for dental abscess and superficial tissue treating wounds | [86,87] |
| Mps | I-carrageenan, Ch, gellan | Curcumin | These PF can be destined for oral administration with the colon as the therapeutic target for the controlled drug release | [88,89] |
| Ms | Na-Alg | Stellaria media | Such microspheres can be destined for oral administration. | [90] |
4. Factors That Can Influence the Process of Microparticles Formation by Complex Coacervation
4.1. Polymer Concentration, Nature and Properties
4.2. Medicinal Substance
4.3. Stirring Speed
4.4. Stirring Time
4.5. Release Time
4.6. Release Capacity of the Medicinal Product
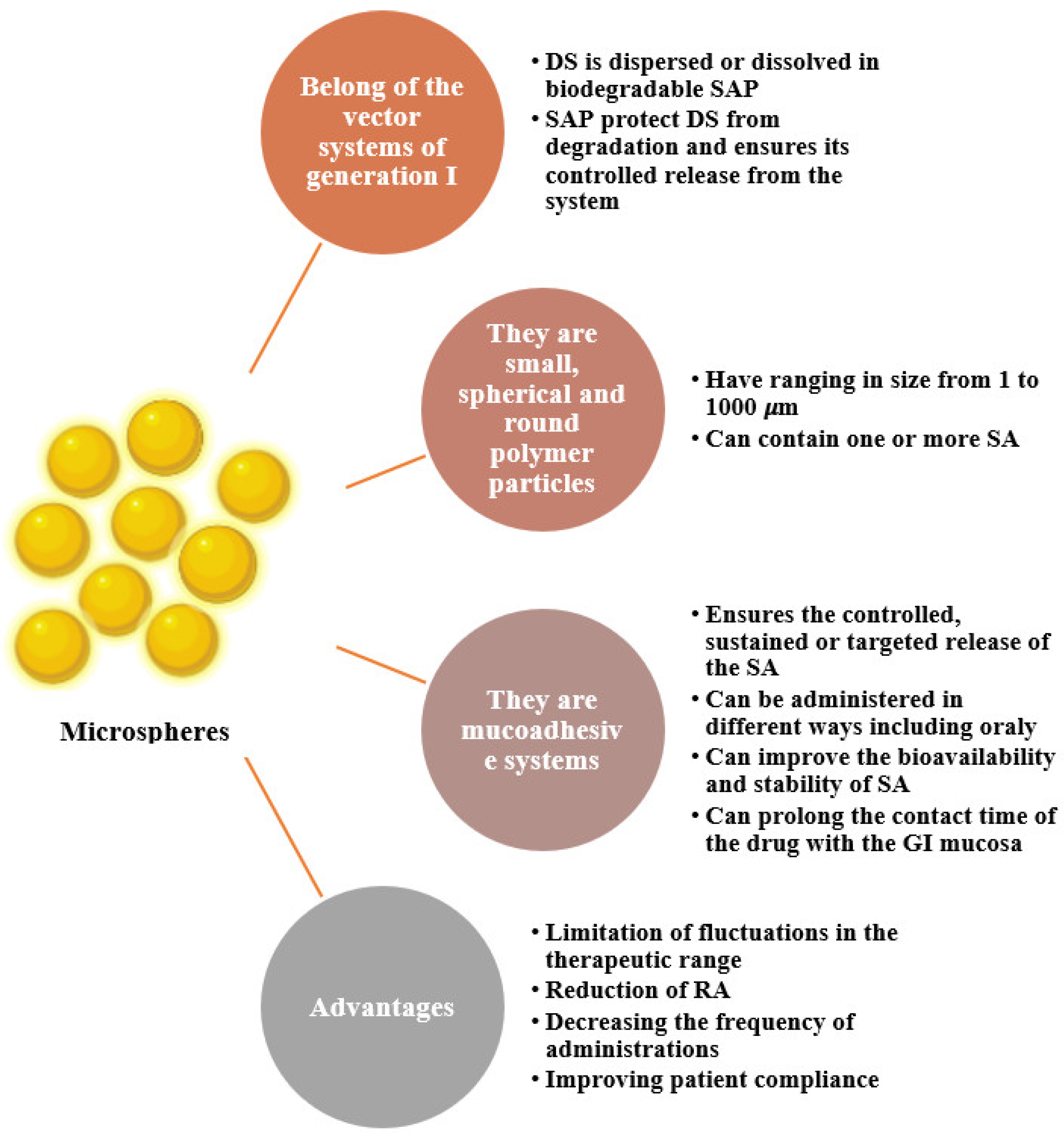
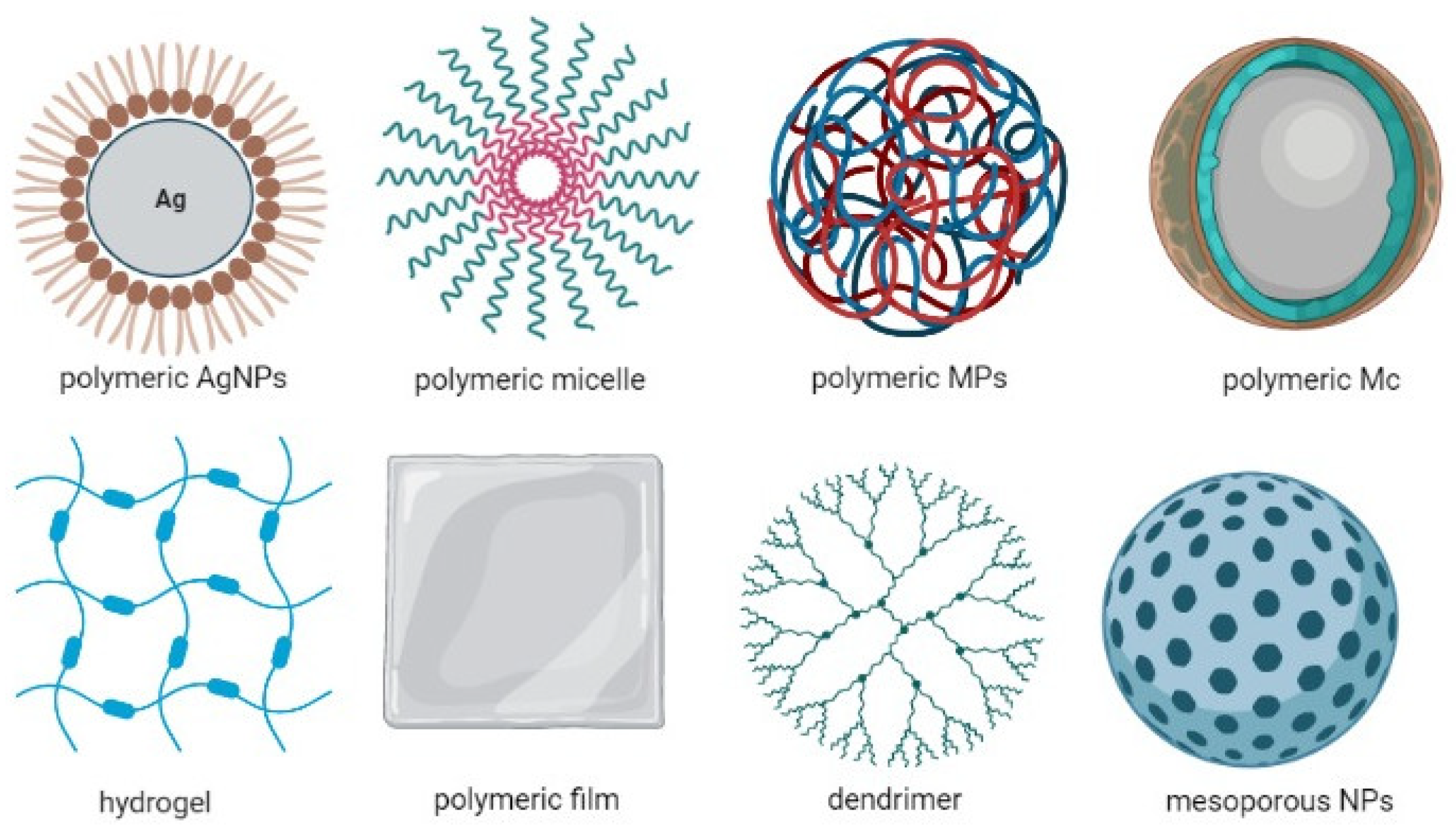
5. The Use of Alginate as a Microencapsulation Material
| Preparation Technique | Advantages | Active Substance | Potential Apps | Reference |
|---|---|---|---|---|
| Emulsification with pectin, Na caseinate and whey protein | Development of effective MPs, with a diameter between 45–70 μm, with high swelling and release rates of extract | Olive leaf extract | MPs | [130] |
| Coacervation with mucina | Oral administration of microencapsulated and enteric-coated insulin can control blood sugar effectively | Insulin | MPs | [131] |
| Multiple U/A/U emulsification using sunflower oil and Span 80 | Good antioxidant and antimicrobial properties, and in vitro studies have shown an initial release in the form of an explosion followed by slow release | Essential oil of Satureja hortensis | MPs | [132] |
| Emulsification with HPMC using tween 85 and CaCl2 dihydrate | Administration of chemotherapeutic agents by inhalation route, directly into the lungs, in the therapy of cancer | Paclitaxel | MPs | [133] |
| Spray-drying and ionotropic gelling with Ch and CaCI2 | Potential administration to the colon for the treatment of IBD | 5-aminosalicylic acid | MPs | [134] |
| Extruders with denatured whey protein | Carrier of promising drugs to improve oral administration of insulin | Insulin | MPs | [135] |
| Emulsification with paraffin oil and tween 80 | Simple and economical encapsulation method that allowed the controlled release of the drug from FF | Diclofenac sodium | MPs | [136] |
| Coacervation with Ch | Alternative to treat tuberculosis | Rifampicin | MPs | [137] |
| Microfluidic method with gelatin and CaCI2 dihydrate | Ensures the intestinal release of MS | Ketoprofen | MPs | [138] |
| Extruders with CaCI2 and Ch | Directing the action of MS in the lower parts of the GI tract and EE >75% | Naproxen | MPs | [139] |
| Spray-drying with Ch and CaCI2 and enteric coating with Eudragit S100 | Local treatment of IBD | Budesonide | MPs | [140] |
| Reticulation with CaCI2 and Ch by spray-drying | Increased BD MS at the tumor site for a longer period of time and provides a specific release into the lymphatic system | Tamoxifen | MPs | [141] |
| Spray-drying with Ch | According to in vitro release studies complexation with Ch controlled the release of MS from MPs and increased their BD | Metoclopramide | MPs | [142] |
| Method of ionic gelling of Ch with Na-TPP and coverage with Na-Alg | Covering Ch-MPs with a layer of Na-Alg increases Ms’s resistance to gastric degradation and prolongs the release of MS from FF at the intestinal level | Metoprolol succinate | MPs | [143] |
| Spray-drying with CaCI2 | Spray-drying can achieve Mucoadhesive Ms with high EE and high production yield | Metformin | MPs | [144] |
| The emulsification/external gelation method with CaCI2, isopropanol, tween 80, paraffin oil and bis-(1,3-dibutylbarbituric acid) trimethine oxonol | The development of these FF has significantly reduced some of the adverse effects of amphotericin B | Amphotericin B | MPs | [145] |
| The coacervation technique with Ch | It allows obtaining controlled-release MPs, which have a rough surface from which MS are released through the diffusion process | Vancomycin chloride | MPs | [99] |
| The emulsion-cross-linking method with liquid paraffin, Span 80, methanol, sopropyl alcohol and CaCI2 as cross-linker | Obtaining enteric release Ms due to MS release in alkaline pH medium, with high entrapment EE 91% of bioactive hydrophilic compounds | Isoniazid | Ms | [146] |
| The emulsification–cross-linking method cu HPMC folosind hexane, Span 80, CaCI2, isopropyl alcohol | Development of FF for intranasal administration of MS | Metoprolol tartrate | Ms | [147] |
| The extrusion technique using CaCI2 | Possibility of incorporating probiotics into the microencapsulated FF matrix | Lactobacillus acidophilus. | MPs | [148] |
| The emulsification method with magnesium stearate using liquid paraffin, Span 80, calcium chloride, isopropyl alcohol | Getting Ms with sustained release | Ibuprofen | Ms | [115] |
| The spray-drying technique with CaCI2 | Possibility of proteic MS encapsulation in microparticulate FF for oral administration | Insulin | MPs | [149] |
| The spray-drying technique with CaCI2 | The release of FF at the intestinal level where following the process of swelling and then erosion is released MS | Caffeine | MPs | [150] |
| The ionotropic-gelation technique using polysaccharide extracted from seeds of Tamarindus indica L. and CaCl2 as cross-linker | Preparation of intestinal-release FF for 10 h with EE of 94.86 ± 3.92% SM | Metformin HCl | MPs | [151] |
| The aerosolization technique using CaCl2 as cross-linker and maltodextrin as lyoprotectant | Obtaining high FF entrapping of the drug due to the high concentration of Na-Alg used, of spherical shape and smooth surface due to the use of maltodextrin | Metformin HCI | Ms | [152] |
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fertah, M.; Belfkira, A.; Dahmane, E.-m.; Taourirte, M.; Brouillette, F. Extraction and characterization of sodium alginate from Moroccan Laminaria digitata brown seaweed. Arab. J. Chem. 2017, 10, S3707–S3714. [Google Scholar] [CrossRef]
- Goh, C.H.; Heng, P.W.S.; Chan, L.W. Alginates as a useful natural polymer for microencapsulation and therapeutic applications. Carbohydr. Polym. 2012, 88, 1–12. [Google Scholar] [CrossRef]
- Phillips, G.O.; Williams, P.A. Handbook of Hydrocolloids; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar] [CrossRef]
- Sachan, N.K.; Pushkar, S.; Jha, A.; Bhattcharya, A. Sodium alginate: The wonder polymer for controlled drug delivery. J. Pharm. Res. 2009, 2, 1191–1199. [Google Scholar]
- Sellimi, S.; Younes, I.; Ben Ayed, H.; Maalej, H.; Montero, V.; Rinaudo, M.; Dahia, M.; Mechichi, T.; Hajji, M.; Nasri, M. Structural, physicochemical and antioxidant properties of sodium alginate isolated from a Tunisian brown seaweed. Int. J. Biol. Macromol. 2015, 72, 1358–1367. [Google Scholar] [CrossRef] [PubMed]
- Agüero, L.; Zaldivar-Silva, D.; Peña, L.; Dias, M.L. Alginate microparticles as oral colon drug delivery device: A review. Carbohydr. Polym. 2017, 168, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Fernando, I.P.S.; Lee, W.; Han, E.J.; Ahn, G. Alginate-based nanomaterials: Fabrication techniques, properties, and applications. Chem. Eng. J. 2010, 391, 123823. [Google Scholar] [CrossRef]
- Fiset, J.-F.; Blais, J.-F.; Riveros, P.A. Review on the Removal of Metal Ions from Effluents Using Seaweeds, Alginate Derivatives and Other Sorbents. Rev. Sci. L’eau 2008, 21, 283–308. [Google Scholar] [CrossRef][Green Version]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Engineer, C.; Parikh, J.; Raval, A. Review on hydrolytic degradation behavior of biodegradable polymers from controlled drug delivery system. Trends Biomater. Artif. Organs 2011, 25, 79–85. [Google Scholar]
- Frenț, O.D.; Duteanu, N.; Teusdea, A.C.; Ciocan, S.; Vicaș, L.; Jurca, T.; Muresan, M.; Pallag, A.; Ianasi, P.; Marian, E. Preparation and Characterization of Chitosan-Alginate Microspheres Loaded with Quercetin. Polymers 2022, 14, 490. [Google Scholar] [CrossRef]
- Uyen, N.T.T.; Hamid, Z.A.A.; Tram, N.X.T.; Ahmad, N. Fabrication of alginate microspheres for drug delivery: A review. Int. J. Biol. Macromol. 2020, 153, 1035–1046. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Guo, C.; Hao, J.; Zhao, Z.; Long, H.; Li, M. Adsorption of heavy metal ions by sodium alginate based adsorbent-a review and new perspectives. Int. J. Biol. Macromol. 2020, 164, 4423–4434. [Google Scholar] [CrossRef] [PubMed]
- Buranachai, T.; Praphairaksit, N.; Muangsin, N. Chitosan/Polyethylene Glycol Beads Crosslinked with Tripolyphosphate and Glutaraldehyde for Gastrointestinal Drug Delivery. AAPS PharmSciTech 2010, 11, 1128–1137. [Google Scholar] [CrossRef]
- Ghimire, K.N.; Inoue, K.; Ohto, K.; Hayashida, T. Adsorption study of metal ions onto crosslinked seaweed Laminaria japonica. Bioresour. Technol. 2008, 99, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Paudyal, H.; Pangeni, B.; Inoue, K.; Kawakita, H.; Ohto, K.; Ghimire, K.N.; Alam, S. Preparation of novel alginate based anion exchanger from Ulva japonica and its application for the removal of trace concentrations of fluoride from water. Bioresour. Technol. 2013, 148, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Zahoor, A.; Sharma, S.; Khuller, G.K. Inhalable alginate nanoparticles as antitubercular drug carriers against experimental tuberculosis. Int. J. Antimicrob. Agents 2005, 26, 298–303. [Google Scholar] [CrossRef]
- Ramdhan, T.; Ching, S.H.; Prakash, S.; Bhandari, B. Physical and mechanical properties of alginate based composite gels. Trends Food Sci. Technol. 2020, 106, 150–159. [Google Scholar] [CrossRef]
- Hariyadi, D.M.; Islam, N. Current Status of Alginate in Drug Delivery. Adv. Pharmacol. Pharm. Sci. 2020, 2020, 8886095. [Google Scholar] [CrossRef]
- Simó, G.; Fernández-Fernández, E.; Vila-Crespo, J.; Ruipérez, V.; Rodríguez-Nogales, J.M. Research progress in coating techniques of alginate gel polymer for cell encapsulation. Carbohydr. Polym. 2017, 170, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Lu, W.; Mata, A.; Nishinari, K.; Fang, Y. Egg-box model-based gelation of alginate and pectin: A review. Carbohydr. Polym. 2020, 242, 116389. [Google Scholar] [CrossRef]
- Chaturvedi, K.; Ganguly, K.; More, U.A.; Reddy, K.R.; Dugge, T.; Naik, B.; Aminabhavi, T.M.; Noolvi, M.N. Sodium alginate in drug delivery and biomedical areas. In Natural Polysaccharides in Drug Delivery and Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 59–100. [Google Scholar]
- Kong, H.J.; Smith, M.K.; Mooney, D.J. Designing alginate hydrogels to maintain viability of immobilized cells. Biomaterials 2003, 24, 4023–4029. [Google Scholar] [CrossRef]
- Batista, P.S.P.; de Morais, A.M.M.B.; Pintado, M.M.E.; de Morais, R.M.S.C. Alginate: Pharmaceutical and Medical Applications. In Extracellular Sugar-Based Biopolymers Matrices; Springer: Berlin/Heidelberg, Germany, 2019; pp. 649–691. [Google Scholar] [CrossRef]
- Guo, X.; Wang, Y.; Qin, Y.M.; Shen, P.L.; Peng, Q. Structures, properties and application of alginic acid: A review. Int. J. Biol. Macromol. 2020, 162, 618–628. [Google Scholar] [CrossRef] [PubMed]
- Huq, T.; Fraschini, C.; Khan, A.; Riedl, B.; Bouchard, J.; Lacroix, M. Alginate based nanocomposite for microencapsulation of probiotic: Effect of cellulose nanocrystal (CNC) and lecithin. Carbohydr. Polym. 2017, 168, 61–69. [Google Scholar] [CrossRef] [PubMed]
- George, M.; Abraham, T.E. Polyionic hydrocolloids for the intestinal delivery of protein drugs: Alginate and chitosan—A review. J. Control. Release 2006, 114, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Agulhon, P.; Markova, V.; Robitzer, M.; Quignard, F.; Mineva, T. Structure of Alginate Gels: Interaction of Diuronate Units with Divalent Cations from Density Functional Calculations. Biomacromolecules 2012, 13, 1899–1907. [Google Scholar] [CrossRef] [PubMed]
- Bennacef, C.; Desobry-Banon, S.; Probst, L.; Desobry, S. Advances on alginate use for spherification to encapsulate biomolecules. Food Hydrocoll. 2021, 118, 106782. [Google Scholar] [CrossRef]
- Ahmed, S.; Ikram, S. Chitosan & its derivatives: A review in recent innovations. Int. J. Pharm. Sci. Res. 2015, 6, 14. [Google Scholar]
- Ćirić, A.; Krajišnik, D.; Čalija, B.; Đekić, L. Biocompatible non-covalent complexes of chitosan and different polymers: Characteristics and application in drug delivery. Arh. Farm. 2020, 70, 173–197. [Google Scholar] [CrossRef]
- Markovic, D.; Zarubica, A.; Stojkovic, N.; Vasic, M.; Cakic, M.; Nikolic, G.; Dragana, M.; Aleksandra, Z.; Nikola, S.; Marija, V.; et al. Alginates and similar exopolysaccharides in biomedical application and pharmacy: Controled delivery of drugs. Adv. Technol. 2016, 5, 39–52. [Google Scholar] [CrossRef]
- Matricardi, P.; Di Meo, C.; Coviello, T.; Alhaique, F. Recent advances and perspectives on coated alginate microspheres for modified drug delivery. Expert Opin. Drug Deliv. 2008, 5, 417–425. [Google Scholar] [CrossRef]
- Panos, I.; Acosta, N.; Heras, A. New Drug Delivery Systems Based on Chitosan. Curr. Drug Discov. Technol. 2008, 5, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.A.; AbouGhaly, M.H.; Schryer-Praga, J.V.; Chadwick, K. The effect of ionotropic gelation residence time on alginate cross-linking and properties. Carbohydr. Polym. 2017, 155, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Mohamadnia, Z.; Zohuriaan-Mehr, M.J.; Kabiri, K.; Jamshidi, A.; Mobedi, H. Ionically cross-linked carrageenan-alginate hydrogel beads. J. Biomater. Sci. Polym. Ed. 2008, 19, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Duan, B.; Zhang, L. Fabrication and characterization of novel macroporous cellulose–alginate hydrogels. Polymer 2009, 50, 5467–5473. [Google Scholar] [CrossRef]
- Rezvanian, M.; Ahmad, N.; Amin, M.C.I.M.; Ng, S.-F. Optimization, characterization, and in vitro assessment of alginate-pectin ionic cross-linked hydrogel film for wound dressing applications. Int. J. Biol. Macromol. 2017, 97, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Chopra, M.; Bernela, M.; Kaur, P.; Manuja, A.; Kumar, B.; Thakur, R. Alginate/gum acacia bipolymeric nanohydrogels—Promising carrier for Zinc oxide nanoparticles. Int. J. Biol. Macromol. 2015, 72, 827–833. [Google Scholar] [CrossRef] [PubMed]
- De Santis, S.; Diociaiuti, M.; Cametti, C.; Masci, G. Hyaluronic acid and alginate covalent nanogels by template cross-linking in polyion complex micelle nanoreactors. Carbohydr. Polym. 2014, 101, 96–103. [Google Scholar] [CrossRef]
- Hall, K.; Asfura, K.G.; Stabler, C. Microencapsulation of islets within alginate/poly(ethylene glycol) gels cross-linked via Staudinger ligation. Acta Biomater. 2011, 7, 614–624. [Google Scholar] [CrossRef]
- Yang, C.H.; Wang, M.X.; Haider, H.; Yang, J.; Sun, J.-Y.; Chen, Y.M.; Zhou, J.; Suo, Z. Strengthening Alginate/Polyacrylamide Hydrogels Using Various Multivalent Cations. ACS Appl. Mater. Interfaces 2013, 5, 10418–10422. [Google Scholar] [CrossRef]
- Zhou, Q.; Kang, H.; Bielec, M.; Wu, X.; Cheng, Q.; Wei, W.; Dai, H. Influence of different divalent ions cross-linking sodium alginate-polyacrylamide hydrogels on antibacterial properties and wound healing. Carbohydr. Polym. 2018, 197, 292–304. [Google Scholar] [CrossRef]
- Hariyadi, D.M.; Hendradi, E.; Purwanti, T.; Fadil, F.D.G.P.; Ramadani, C.N. Effect of cross linking agent and polymer on the characteristics of ovalbumin loaded alginate microspheres. Int. J. Pharm. Pharm. Sci. 2014, 6, 469–474. [Google Scholar]
- Yan, S.; Wang, T.; Feng, L.; Zhu, J.; Zhang, K.; Chen, X.; Cui, L.; Yin, J. Injectable In Situ Self-Cross-Linking Hydrogels Based on Poly(l-glutamic acid) and Alginate for Cartilage Tissue Engineering. Biomacromolecules 2014, 15, 4495–4508. [Google Scholar] [CrossRef] [PubMed]
- da Silva, M.A.; Bierhalz, A.C.K.; Kieckbusch, T.G. Alginate and pectin composite films crosslinked with Ca2+ ions: Effect of the plasticizer concentration. Carbohydr. Polym. 2009, 77, 736–742. [Google Scholar] [CrossRef]
- Moxon, S.R.; Corbett, N.J.; Fisher, K.; Potjewyd, G.; Domingos, M.; Hooper, N.M. Blended alginate/collagen hydrogels promote neurogenesis and neuronal maturation. Mater. Sci. Eng. C 2019, 104, 109904. [Google Scholar] [CrossRef]
- Pires, A.L.R.; Motta, L.D.A.; Dias, A.M.; de Sousa, H.C.; Moraes, M.; Braga, M.E. Towards wound dressings with improved properties: Effects of poly(dimethylsiloxane) on chitosan-alginate films loaded with thymol and beta-carotene. Mater. Sci. Eng. C 2018, 93, 595–605. [Google Scholar] [CrossRef]
- Babu, V.R.; Sairam, M.; Hosamani, K.M.; Aminabhavi, T.M. Preparation of sodium alginate–methylcellulose blend microspheres for controlled release of nifedipine. Carbohydr. Polym. 2007, 69, 241–250. [Google Scholar] [CrossRef]
- Agnihotri, S.A.; Mallikarjuna, N.N.; Aminabhavi, T.M. Recent advances on chitosan-based micro- and nanoparticles in drug delivery. J. Control. Release 2004, 100, 5–28. [Google Scholar] [CrossRef]
- Black, K.A.; Priftis, D.; Perry, S.L.; Yip, J.; Byun, W.Y.; Tirrell, M. Protein Encapsulation via Polypeptide Complex Coacervation. ACS Macro Lett. 2014, 3, 1088–1091. [Google Scholar] [CrossRef]
- Frenț, O.D.; Vicaș, L.; Jurca, T.; Ciocan, S.; Duteanu, N.; Pallag, A.; Muresan, M.; Marian, E.; Negrea, A.; Micle, O. A Review: Uses of Chitosan in Pharmaceutical Forms. In Reviews of Physiology, Biochemistry and Pharmacology; Springer: Berlin/Heidelberg, Germany, 2021; pp. 1–37. [Google Scholar] [CrossRef]
- Popovici, I.; Lupuleasa, D. The influence of different polymers on the pharmaco-technological characteristics of propiconazole nitrate bioadhesive oromucosal tablets. Tehn. Farm. 2017, 3, 689–728. [Google Scholar]
- Timilsena, Y.P.; Akanbi, T.O.; Khalid, N.; Adhikari, B.; Barrow, C.J. Complex coacervation: Principles, mechanisms and applications in microencapsulation. Int. J. Biol. Macromol. 2019, 121, 1276–1286. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.-Q.; Sun, Y.-T.; Xiao, J.-X.; Yang, J. Complex coacervation of soybean protein isolate and chitosan. Food Chem. 2012, 135, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Neamtu, B.; Tita, O.; Neamtu, M.; Tita, M.; Hila, M.; Maniu, I. Identification of Probiotic Strains from Human Milk in Breastfed Infants with Respiratory Infections. Acta Univ. Cibiniensis. Ser. E Food Technol. 2014, 18, 73–84. [Google Scholar] [CrossRef]
- Espinosa-Andrews, H.; Báez-González, J.G.; Cruz-Sosa, F.; Vernon-Carter, E.J. Gum arabic−chitosan complex coacervation. Biomacromolecules 2007, 8, 1313–1318. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.A.; Syeda, J.T.M.; Wasan, K.M.; Wasan, E.K. An Overview of Chitosan Nanoparticles and Its Application in Non-Parenteral Drug Delivery. Pharmaceutics 2017, 9, 53. [Google Scholar] [CrossRef]
- Sahil, K.; Akanksha, M.; Premjeet, S.; Bilandi, A.; Kapoor, B. Microsphere: A review. Int. J. Res. Pharm. Chem. 2011, 1, 1184–1198. [Google Scholar]
- Chang, L.-W. Sequence Control of Complex Coacervation. Ph.D. Thesis, University of Massachusetts Amherst, Amherst, MA, USA, 2020. [Google Scholar]
- Dong, Z.-J.; Xia, S.-Q.; Hua, S.; Hayat, K.; Zhang, X.-M.; Xu, S.-Y. Optimization of cross-linking parameters during production of transglutaminase-hardened spherical multinuclear microcapsules by complex coacervation. Colloids Surf. B Biointerfaces 2008, 63, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Tiyaboonchai, W.; Ritthidej, G.C. Development of indomethacin sustained release microcapsules using chitosan-carboxymethyl-cellulose complex coacervation. Development 2003, 25, 246. [Google Scholar]
- Basu, S.K.; Kavitha, K.; Rupeshkumar, M. Evaluation of Ionotropic Cross-Linked Chitosan/Gelatin B Microspheres of Tramadol Hydrochloride. AAPS PharmSciTech 2011, 12, 28–34. [Google Scholar] [CrossRef] [PubMed]
- El-Leithy, E.S.; Shaker, D.S.; Ghorab, M.K.; Abdel-Rashid, R.S. Evaluation of Mucoadhesive Hydrogels Loaded with Diclofenac Sodium–Chitosan Microspheres for Rectal Administration. AAPS PharmSciTech 2010, 11, 1695–1702. [Google Scholar] [CrossRef]
- Biswas, S.; Chattopadhyay, M.; Sen, K.K.; Saha, M.K. Development and characterization of alginate coated low molecular weight chitosan nanoparticles as new carriers for oral vaccine delivery in mice. Carbohydr. Polym. 2015, 121, 403–410. [Google Scholar] [CrossRef]
- Bagre, A.P.; Jain, K.; Jain, N.K. Alginate coated chitosan core shell nanoparticles for oral delivery of enoxaparin: In vitro and in vivo assessment. Int. J. Pharm. 2013, 456, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Sarmento, B.; Ribeiro, A.; Veiga, F.; Sampaio, P.; Neufeld, R.J.; Ferreira, D. Alginate/Chitosan Nanoparticles are Effective for Oral Insulin Delivery. Pharm. Res. 2007, 24, 2198–2206. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Budhiraja, R.D. Chitosan-alginate microcapsules of amoxicillin for gastric stability and mucoadhesion. J. Adv. Pharm. Technol. Res. 2012, 3, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Dai, Y.-N.; Zhang, J.-P.; Wang, A.-Q.; Wei, Q. Chitosan-Alginate Nanoparticles as a Novel Drug Delivery System for Nifedipine. Int. J. Biomed. Sci. 2008, 4, 221–228. [Google Scholar] [PubMed]
- Cavalu, S.; Prokisch, J.; Laslo, V.; Vicas, S. Preparation, structural characterisation and release study of novel hybrid microspheres entrapping nanoselenium, produced by green synthesis. IET Nanobiotechnol. 2017, 11, 426–432. [Google Scholar] [CrossRef]
- Basu, S.K.; Kavitha, K.; Rupeshkumar, M. Evaluation of Ketorolac Tromethamine Microspheres by Chitosan/Gelatin B Complex Coacervation. Sci. Pharm. 2010, 78, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Lucinda-Silva, R.M.; Evangelista, R.C. Microspheres of alginate-chitosan containing isoniazid. J. Microencapsul. 2003, 20, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Lemos, Y.P.; Marfil, P.H.M.; Nicoletti, V.R. Particle size characteristics of buriti oil microcapsules produced by gelatin-sodium alginate complex coacervation: Effect of stirring speed. Int. J. Food Prop. 2017, 20, 1439–1447. [Google Scholar] [CrossRef]
- Honary, S.; Maleki, M.; Karami, M. The effect of chitosan molecular weight on the properties of alginate/ chitosan microparticles containing prednisolone. Trop. J. Pharm. Res. 2009, 8, 53–61. [Google Scholar] [CrossRef]
- Wittaya-Areekul, S.; Kruenate, J.; Prahsarn, C. Preparation and in vitro evaluation of mucoadhesive properties of alginate/chitosan microparticles containing prednisolone. Int. J. Pharm. 2006, 312, 113–118. [Google Scholar] [CrossRef]
- Li, R.; Chen, R.; Liu, W.; Qin, C.; Han, J. Preparation of enteric-coated microcapsules of astaxanthin oleoresin by complex coacervation. Pharm. Dev. Technol. 2018, 23, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Lucinda-Silva, R.M.; Salgado, H.; Evangelista, R.C. Alginate–chitosan systems: In vitro controlled release of triamcinolone and in vivo gastrointestinal transit. Carbohydr. Polym. 2010, 81, 260–268. [Google Scholar] [CrossRef]
- Hari, P.R.; Chandy, T.; Sharma, C.P. Chitosan/calcium alginate microcapsules for intestinal delivery of nitrofurantoin. J. Microencapsul. 1996, 13, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.X.; Yang, S.W.; Cao, J.L.; Zhao, S.H.; Wang, W.W. Microencapsulation of Ginger Volatile Oil Based on Gelatin/Sodium Alginate Polyelectrolyte Complex. Chem. Pharm. Bull. 2016, 64, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Qv, X.-Y.; Zeng, Z.-P.; Jiang, J.-G. Preparation of lutein microencapsulation by complex coacervation method and its physicochemical properties and stability. Food Hydrocoll. 2011, 25, 1596–1603. [Google Scholar] [CrossRef]
- Shinde, U.; Nagarsenker, M. Microencapsulation of eugenol by gelatin-sodium alginate complex coacervation. Indian J. Pharm. Sci. 2011, 73, 311. [Google Scholar] [PubMed]
- Junyaprasert, V.B.; Mitrevej, A.; Sinchaipanid, N.; Boonme, P.; Wurster, D.E. Effect of Process Variables on the Microencapsulation of Vitamin A Palmitate by Gelatin-Acacia Coacervation. Drug Dev. Ind. Pharm. 2001, 27, 561–566. [Google Scholar] [CrossRef]
- Babu, G.M.M.; Himasankar, K.; Narayan, C.P.; Murthy, K.R. Controlled Release of Dicolfenac Sodium by Gum Karaya-Chitosan Complex Coacervate: In Vivo Evaluation. Indian J. Pharm. Sci. 2001, 63, 408. [Google Scholar]
- Chen, M.; Hu, Y.; Zhou, J.; Xie, Y.; Wu, H.; Yuan, T.; Yang, Z. Facile fabrication of tea tree oil-loaded antibacterial microcapsules by complex coacervation of sodium alginate/quaternary ammonium salt of chitosan. RSC Adv. 2016, 6, 13032–13039. [Google Scholar] [CrossRef]
- Jincheng, W.; Xiaoyu, Z.; Sihao, C. Preparation and Properties of Nanocapsulated Capsaicin by Complex Coacervation Method. Chem. Eng. Commun. 2010, 197, 919–933. [Google Scholar] [CrossRef]
- Aelenei, N.; Popa, M.I.; Novac, O.; Lisa, G.; Balaita, L. Tannic acid incorporation in chitosan-based microparticles and in vitro controlled release. J. Mater. Sci. Mater. Med. 2009, 20, 1095–1102. [Google Scholar] [CrossRef] [PubMed]
- Iurciuc-Tincu, C.-E.; Atanase, L.I.; Ochiuz, L.; Jérôme, C.; Sol, V.; Martin, P.; Popa, M. Curcumin-loaded polysaccharides-based complex particles obtained by polyelectrolyte complexation and ionic gelation. I-Particles obtaining and characterization. Int. J. Biol. Macromol. 2020, 147, 629–642. [Google Scholar] [CrossRef] [PubMed]
- Kamal, M.A.H.M.; Ahmed, M.; Wahed, M.I.I.; Amran, M.S.; Shaheen, S.M.; Rashid, M.; Anwar-Ul-Islam, M. Development of indomethacin sustained release microcapsules using ethyl cellulose and hydroxy propyl methyl cellulose phthalate by O/W emulsification. Dhaka Univ. J. Pharm. Sci. 2008, 7, 83–88. [Google Scholar] [CrossRef]
- Cavalu, S.; Bisboaca, S.; Mates, I.M.; Pasca, P.M.; Laslo, V.; Costea, T.; Fritea, L.; Vicas, S. Novel Formulation Based on Chitosan-Arabic Gum Nanoparticles Entrapping Propolis Extract Production, physico-chemical and structural characterization. Rev. Chim. 2018, 69, 3756–3760. [Google Scholar] [CrossRef]
- Miere, F.; Teusdea, A.C.; Laslo, V.; Fritea, L.; Moldovan, L.; Costea, T.; Uivaroșan, D.; Vicas, S.I.; Pallag, A. Natural Polymeric Beads for Encapsulation of Stellaria media Extract with Antioxidant Properties. Mater. Plast 2019, 56, 671–679. [Google Scholar] [CrossRef]
- Tiyaboonchai, W. Chitosan nanoparticles: A promising system for drug delivery. Naresuan Univ. J. Sci. Technol. 2013, 11, 51–66. [Google Scholar]
- Becherán-Marón, L.; Peniche, C.; Argüelles-Monal, W. Study of the interpolyelectrolyte reaction between chitosan and alginate: Influence of alginate composition and chitosan molecular weight. Int. J. Biol. Macromol. 2004, 34, 127–133. [Google Scholar] [CrossRef]
- Dubin, P.; Stewart, R.J. Complex coacervation. Soft Matter 2018, 14, 329–330. [Google Scholar] [CrossRef]
- Priftis, D.; Tirrell, M. Phase behaviour and complex coacervation of aqueous polypeptide solutions. Soft Matter 2012, 8, 9396–9405. [Google Scholar] [CrossRef]
- Butstraen, C.; Salaün, F. Preparation of microcapsules by complex coacervation of gum Arabic and chitosan. Carbohydr. Polym. 2014, 99, 608–616. [Google Scholar] [CrossRef]
- Yang, Y.-Y.; Chung, T.-S.; Bai, X.-L.; Chan, W.-K. Effect of preparation conditions on morphology and release profiles of biodegradable polymeric microspheres containing protein fabricated by double-emulsion method. Chem. Eng. Sci. 2000, 55, 2223–2236. [Google Scholar] [CrossRef]
- Freiberg, S.; Zhu, X.X. Polymer microspheres for controlled drug release. Int. J. Pharm. 2004, 282, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Eghbal, N.; Choudhary, R. Complex coacervation: Encapsulation and controlled release of active agents in food systems. LWT 2018, 90, 254–264. [Google Scholar] [CrossRef]
- Unagolla, J.M.; Jayasuriya, A.C. Drug transport mechanisms and in vitro release kinetics of vancomycin encapsulated chitosan-alginate polyelectrolyte microparticles as a controlled drug delivery system. Eur. J. Pharm. Sci. 2018, 114, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Liang, Y.; Liu, L.; Yin, M.; Wang, A.; Sun, K.; Li, Y.; Shi, Y. Research on the fate of polymeric nanoparticles in the process of the intestinal absorption based on model nanoparticles with various characteristics: Size, surface charge and pro-hydrophobics. J. Nanobiotechnol. 2021, 19, 32. [Google Scholar] [CrossRef] [PubMed]
- Du, X.-J.; Wang, J.-L.; Iqbal, S.; Li, H.-J.; Cao, Z.-T.; Wang, Y.-C.; Du, J.-Z.; Wang, J. The effect of surface charge on oral absorption of polymeric nanoparticles. Biomater. Sci. 2018, 6, 642–650. [Google Scholar] [CrossRef] [PubMed]
- De Anda-Flores, Y.; Carvajal-Millan, E.; Campa-Mada, A.; Lizardi-Mendoza, J.; Rascon-Chu, A.; Tanori-Cordova, J.; Martínez-López, A. Polysaccharide-Based Nanoparticles for Colon-Targeted Drug Delivery Systems. Polysaccharides 2021, 2, 626–647. [Google Scholar] [CrossRef]
- Jafari, S.M.; Katouzian, I.; Akhavan, S. Safety and regulatory issues of nanocapsules. In Nanoencapsulation Technologies for the Food and Nutraceutical Industries; Elsevier: Amsterdam, The Netherlands, 2017; pp. 545–590. [Google Scholar]
- Swarbrick, J. Encyclopedia of Pharmaceutical Technology; CRC Press: Boca Raton, FL, USA, 2013; Volume 6. [Google Scholar]
- Dhamecha, D.; Movsas, R.; Sano, U.; Menon, J.U. Applications of alginate microspheres in therapeutics delivery and cell culture: Past, present and future. Int. J. Pharm. 2019, 569, 118627. [Google Scholar] [CrossRef]
- Finch, C.A.; Bodmeier, R. Microencapsulation; Wiley: Weinheim, Germany, 2000. [Google Scholar]
- Ghosh, S.K. Functional Coatings: By Polymer Microencapsulation; Wiley: Weinheim, Germany, 2006. [Google Scholar]
- Jyothi, S.S.; Seethadevi, A.; Prabha, K.S.; Muthuprasanna, P.; Pavitra, P. Microencapsulation: A review. Int. J. Pharm. Biol. Sci 2012, 3, 509–531. [Google Scholar]
- Lengyel, M.; Kállai-Szabó, N.; Antal, V.; Laki, A.J.; Antal, I. Microparticles, Microspheres, and Microcapsules for Advanced Drug Delivery. Sci. Pharm. 2019, 87, 20. [Google Scholar] [CrossRef]
- Nordstierna, L.; Abdalla, A.A.; Nordin, M.; Nydén, M. Comparison of release behaviour from microcapsules and microspheres. Prog. Org. Coat. 2010, 69, 49–51. [Google Scholar] [CrossRef]
- Hamed, I.; Özogul, F.; Regenstein, J.M. Industrial applications of crustacean by-products (chitin, chitosan, and chitooligosaccharides): A review. Trends Food Sci. Technol. 2016, 48, 40–50. [Google Scholar] [CrossRef]
- Puvvada, Y.S.; Vankayalapati, S.; Sukhavasi, S. Extraction of chitin from chitosan from exoskeleton of shrimp for application in the pharmaceutical industry. Int. Curr. Pharm. J. 2012, 1, 258–263. [Google Scholar] [CrossRef]
- Yadav, P.R.; Pattanayek, S.K. Modulation of Physicochemical Properties of Polymers for Effective Insulin Delivery Systems. In Biointerface Engineering: Prospects in Medical Diagnostics and Drug Delivery; Springer: Berlin/Heidelberg, Germany, 2020; pp. 123–148. [Google Scholar] [CrossRef]
- Rahaman, S.T.; Mukherjee, J. A review on mucoadhesive microspheres as an efficient drug delivery system. Indo. Am. J. Pharm. Sci. 2020, 10, 573–579. [Google Scholar]
- Nagpal, M.; Maheshwari, D.; Rakha, P.; Dureja, H.; Goyal, S.; Dhingra, G. Formulation Development and Evaluation of Alginate Microspheres of Ibuprofen. J. Young Pharm. 2012, 4, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Varma, K.; Gopi, S. Biopolymers and their role in medicinal and pharmaceutical applications. In Biopolymers and their Industrial Applications; Elsevier: Amsterdam, The Netherlands, 2021; pp. 175–191. [Google Scholar] [CrossRef]
- Augst, A.D.; Kong, H.J.; Mooney, D.J. Alginate Hydrogels as Biomaterials. Macromol. Biosci. 2006, 6, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Gudimalla, A.; Jose, J.; Rajendran, J.V.; Gurram, G.; Thomas, S. Synthesis of silver nanoparticles by plant extract, incorporated into alginate films and their characterizations. Chem. Pap. 2022, 76, 1031–1043. [Google Scholar] [CrossRef]
- Lecaros, R.L.G.; Ho, S.-Y.; Tsai, H.-A.; Hung, W.-S.; Hu, C.-C.; Huang, S.-H.; Lee, K.-R.; Lai, J.-Y. Ionically cross-linked sodium alginate and polyamidoamine dendrimers for ethanol/water separation through pervaporation. Sep. Purif. Technol. 2021, 275, 119125. [Google Scholar] [CrossRef]
- Liu, C.; Jiang, F.; Xing, Z.; Fan, L.; Li, Y.; Wang, S.; Ling, J.; Ouyang, X.-K. Efficient Delivery of Curcumin by Alginate Oligosaccharide Coated Aminated Mesoporous Silica Nanoparticles and In Vitro Anticancer Activity against Colon Cancer Cells. Pharmaceutics 2022, 14, 1166. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Madry, H.; Cucchiarini, M. Application of Alginate Hydrogels for Next-Generation Articular Cartilage Regeneration. Int. J. Mol. Sci. 2022, 23, 1147. [Google Scholar] [CrossRef]
- Manna, K.; Patra, P.; Roy, A.; Roy, R.K.; Sunka, K.C.; Dhara, S.; Patra, N.; Pal, S. Amino Acid Inspired Alginate-Based pH Sensitive Polymeric Micelles via Reversible Addition–Fragmentation Chain Transfer Polymerization. ACS Appl. Polym. Mater. 2022, 4, 4432–4444. [Google Scholar] [CrossRef]
- Ning, H.; Lu, L.; Xu, J.; Lu, L.; Pan, L.; Lin, Z. Development of sodium alginate-based antioxidant and antibacterial bioactive films added with IRMOF-3/Carvacrol. Carbohydr. Polym. 2022, 292, 119682. [Google Scholar] [CrossRef] [PubMed]
- Pawar, S.N.; Edgar, K.J. Alginate derivatization: A review of chemistry, properties and applications. Biomaterials 2012, 33, 3279–3305. [Google Scholar] [CrossRef] [PubMed]
- Koland, M.; Anchan, R.B.; Mukund, S.G.; Mulleria, S.S. Design and Investigation of Alginate Coated Solid Lipid Nanoparticles for Oral Insulin Delivery. Indian J. Pharm. Educ. Res. 2021, 55, 383–394. [Google Scholar] [CrossRef]
- Reis, C.P.; Ribeiro, A.J.; Neufeld, R.J.; Veiga, F. Alginate microparticles as novel carrier for oral insulin delivery. Biotechnol. Bioeng. 2007, 96, 977–989. [Google Scholar] [CrossRef]
- Krisanti, E.A.; Lazuardi, D.; Kiresya, K.K.; Mulia, K. Tablet Formulation Containing Chitosan-Alginate Microparticles: Characterization and Release Profile of Xanthones. Int. J. Technol. 2020, 11, 900. [Google Scholar] [CrossRef]
- Yang, D.; Gao, K.; Bai, Y.; Lei, L.; Jia, T.; Yang, K.; Xue, C. Microfluidic synthesis of chitosan-coated magnetic alginate microparticles for controlled and sustained drug delivery. Int. J. Biol. Macromol. 2021, 182, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Samani, S.M.; Karimaddini, S.; Sobhani, Z.; Ahmadi, F. Preparation and evaluation of an oral mucoadhesive gel containing nystatin-loaded alginate microparticles. Eur. Pharm. J. 2020, 67, 15–21. [Google Scholar] [CrossRef]
- Flamminii, F.; Di Mattia, C.D.; Nardella, M.; Chiarini, M.; Valbonetti, L.; Neri, L.; Difonzo, G.; Pittia, P. Structuring alginate beads with different biopolymers for the development of functional ingredients loaded with olive leaves phenolic extract. Food Hydrocoll. 2020, 108, 105849. [Google Scholar] [CrossRef]
- Builders, P.F.; Kunle, O.O.; Okpaku, L.C.; Builders, M.I.; Attama, A.A.; Adikwu, M.U. Preparation and evaluation of mucinated sodium alginate microparticles for oral delivery of insulin. Eur. J. Pharm. Biopharm. 2008, 70, 777–783. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Hosseini, H.; Mohammadifar, M.A.; Mortazavian, A.M.; Mohammadi, A.; Khosravi-Darani, K.; Shojaee-Alibadi, S.; Dehghan, S.; Khaksar, R. Incorporation of essential oil in alginate microparticles by multiple emulsion/ionic gelation process. Int. J. Biol. Macromol. 2013, 62, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Alipour, S.; Montaseri, H.; Tafaghodi, M. Preparation and characterization of biodegradable paclitaxel loaded alginate microparticles for pulmonary delivery. Colloids Surf. B Biointerfaces 2010, 81, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Mladenovska, K.; Cruaud, O.; Richomme, P.; Belamie, E.; Raicki, R.S.; Venier-Julienne, M.-C.; Popovski, E.; Benoit, J.P.; Goracinova, K. 5-ASA loaded chitosan–Ca–alginate microparticles: Preparation and physicochemical characterization. Int. J. Pharm. 2007, 345, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Déat-Lainé, E.; Hoffart, V.; Cardot, J.-M.; Subirade, M.; Beyssac, E. Development and in vitro characterization of insulin loaded whey protein and alginate microparticles. Int. J. Pharm. 2012, 439, 136–144. [Google Scholar] [CrossRef]
- Ahmed, M.M.; Abd El-Rasoul, S.; Auda, S.H.; Ibrahim, M.A. Emulsification/internal gelation as a method for preparation of diclofenac sodium-sodium alginate microparticles. Saudi Pharm. J. 2013, 21, 61–69. [Google Scholar] [CrossRef]
- Lacerda, L.; Parize, A.L.; Favere, V.; Laranjeira, M.C.M.; Stulzer, H.K. Development and evaluation of pH-sensitive sodium alginate/chitosan microparticles containing the antituberculosis drug rifampicin. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 39, 161–167. [Google Scholar] [CrossRef]
- Rajesh, K.; Khanrah, A.; Biswanath, S. Release of ketoprofen from alginate microparticles containing film forming polymers. J. Sci. Ind. Res. 2003, 62, 985–989. [Google Scholar]
- Čalija, B.; Cekic, N.; Savić, S.; Krajisnik, D.; Daniels, R.; Milic, J. An investigation of formulation factors affecting feasibility of alginate-chitosan microparticles for oral delivery of naproxen. Arch. Pharmacal Res. 2011, 34, 919–929. [Google Scholar] [CrossRef]
- Crcarevska, M.S.; Dodov, M.G.; Goracinova, K. Chitosan coated Ca–alginate microparticles loaded with budesonide for delivery to the inflamed colonic mucosa. Eur. J. Pharm. Biopharm. 2008, 68, 565–578. [Google Scholar] [CrossRef]
- Coppi, G.; Iannuccelli, V. Alginate/chitosan microparticles for tamoxifen delivery to the lymphatic system. Int. J. Pharm. 2009, 367, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Gavini, E.; Rassu, G.; Sanna, V.; Cossu, M.; Giunchedi, P. Mucoadhesive microspheres for nasal administration of an antiemetic drug, metoclopramide: In-vitro/ex-vivo studies. J. Pharm. Pharmacol. 2005, 57, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Dhawan, N.; Sharma, H.; Patwal, P.S.; Vaidya, S.; Vaidya, B. Bilayer mucoadhesive microparticles for the delivery of metoprolol succinate: Formulation and evaluation. Artif. Cells Nanomed. Biotechnol. 2015, 43, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Szekalska, M.; Sosnowska, K.; Czajkowska-Kośnik, A.; Winnicka, K. Calcium Chloride Modified Alginate Microparticles Formulated by the Spray Drying Process: A Strategy to Prolong the Release of Freely Soluble Drugs. Materials 2018, 11, 1522. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Berrios, M.P.; Aponte-Reyes, L.M.; Diaz-Figueroa, L.; Vivero-Escoto, J.; Johnston, A.; Sanchez-Rodriguez, D. Preparation and In Vitro Evaluation of Alginate Microparticles Containing Amphotericin B for the Treatment of Candida Infections. Int. J. Biomater. 2020, 2020, 2514387. [Google Scholar] [CrossRef]
- Rastogi, R.; Sultana, Y.; Aqil, M.; Ali, A.; Kumar, S.; Chuttani, K.; Mishra, A. Alginate microspheres of isoniazid for oral sustained drug delivery. Int. J. Pharm. 2007, 334, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Rajinikanth, P.S.; Sankar, C.; Mishra, B. Sodium Alginate Microspheres of Metoprolol Tartrate for Intranasal Systemic Delivery: Development and Evaluation. Drug Deliv. 2003, 10, 21–28. [Google Scholar] [CrossRef]
- Lotfipour, F.; Mirzaeei, S.; Maghsoodi, M. Evaluation of the effect of CaCl2 and alginate concentrations and hardening time on the characteristics of Lactobacillus acidophilus loaded alginate beads using response surface analysis. Adv. Pharm. Bull. 2012, 2, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Bowey, K.; Swift, B.E.; Flynn, L.E.; Neufeld, R.J. Characterization of biologically active insulin-loaded alginate microparticles prepared by spray drying. Drug Dev. Ind. Pharm. 2013, 39, 457–465. [Google Scholar] [CrossRef]
- Bagheri, L.; Madadlou, A.; Yarmand, M.; Mousavi, M.E. Spray-dried alginate microparticles carrying caffeine-loaded and potentially bioactive nanoparticles. Food Res. Int. 2014, 62, 1113–1119. [Google Scholar] [CrossRef]
- Nayak, A.K.; Pal, D.; Santra, K. Swelling and drug release behavior of metformin HCl-loaded tamarind seed polysaccharide-alginate beads. Int. J. Biol. Macromol. 2016, 82, 1023–1027. [Google Scholar] [CrossRef]
- Hariyadi, D.M.; Hendradi, E.; Erawati, T.; Jannah, E.N.; Febrina, W. Influence of drug-polymer ratio on physical characteristics and release of metformin hydrochloride from metforminalginate microspheres. Trop. J. Pharm. Res. 2018, 17, 7. [Google Scholar] [CrossRef]
- Benavides, S.; Cortés, P.; Parada, J.; Franco, W. Development of alginate microspheres containing thyme essential oil using ionic gelation. Food Chem. 2016, 204, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Paques, J.P.; Sagis, L.M.; van Rijn, C.J.; van der Linden, E. Nanospheres of alginate prepared through w/o emulsification and internal gelation with nanoparticles of CaCO3. Food Hydrocoll. 2014, 40, 182–188. [Google Scholar] [CrossRef]
- de Spadari, C.C.; Lopes, L.B.; Ishida, K. Potential use of alginate-based carriers as antifungal delivery system. Front. Microbiol. 2017, 8, 97. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frent, O.D.; Vicas, L.G.; Duteanu, N.; Morgovan, C.M.; Jurca, T.; Pallag, A.; Muresan, M.E.; Filip, S.M.; Lucaciu, R.-L.; Marian, E. Sodium Alginate—Natural Microencapsulation Material of Polymeric Microparticles. Int. J. Mol. Sci. 2022, 23, 12108. https://doi.org/10.3390/ijms232012108
Frent OD, Vicas LG, Duteanu N, Morgovan CM, Jurca T, Pallag A, Muresan ME, Filip SM, Lucaciu R-L, Marian E. Sodium Alginate—Natural Microencapsulation Material of Polymeric Microparticles. International Journal of Molecular Sciences. 2022; 23(20):12108. https://doi.org/10.3390/ijms232012108
Chicago/Turabian StyleFrent, Olimpia Daniela, Laura Gratiela Vicas, Narcis Duteanu, Claudia Mona Morgovan, Tunde Jurca, Annamaria Pallag, Mariana Eugenia Muresan, Sanda Monica Filip, Roxana-Liana Lucaciu, and Eleonora Marian. 2022. "Sodium Alginate—Natural Microencapsulation Material of Polymeric Microparticles" International Journal of Molecular Sciences 23, no. 20: 12108. https://doi.org/10.3390/ijms232012108
APA StyleFrent, O. D., Vicas, L. G., Duteanu, N., Morgovan, C. M., Jurca, T., Pallag, A., Muresan, M. E., Filip, S. M., Lucaciu, R.-L., & Marian, E. (2022). Sodium Alginate—Natural Microencapsulation Material of Polymeric Microparticles. International Journal of Molecular Sciences, 23(20), 12108. https://doi.org/10.3390/ijms232012108







.jpg)