Upregulation of miR145 and miR126 in EVs from Renal Cells Undergoing EMT and Urine of Diabetic Nephropathy Patients
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
2.1. uEV Characterization
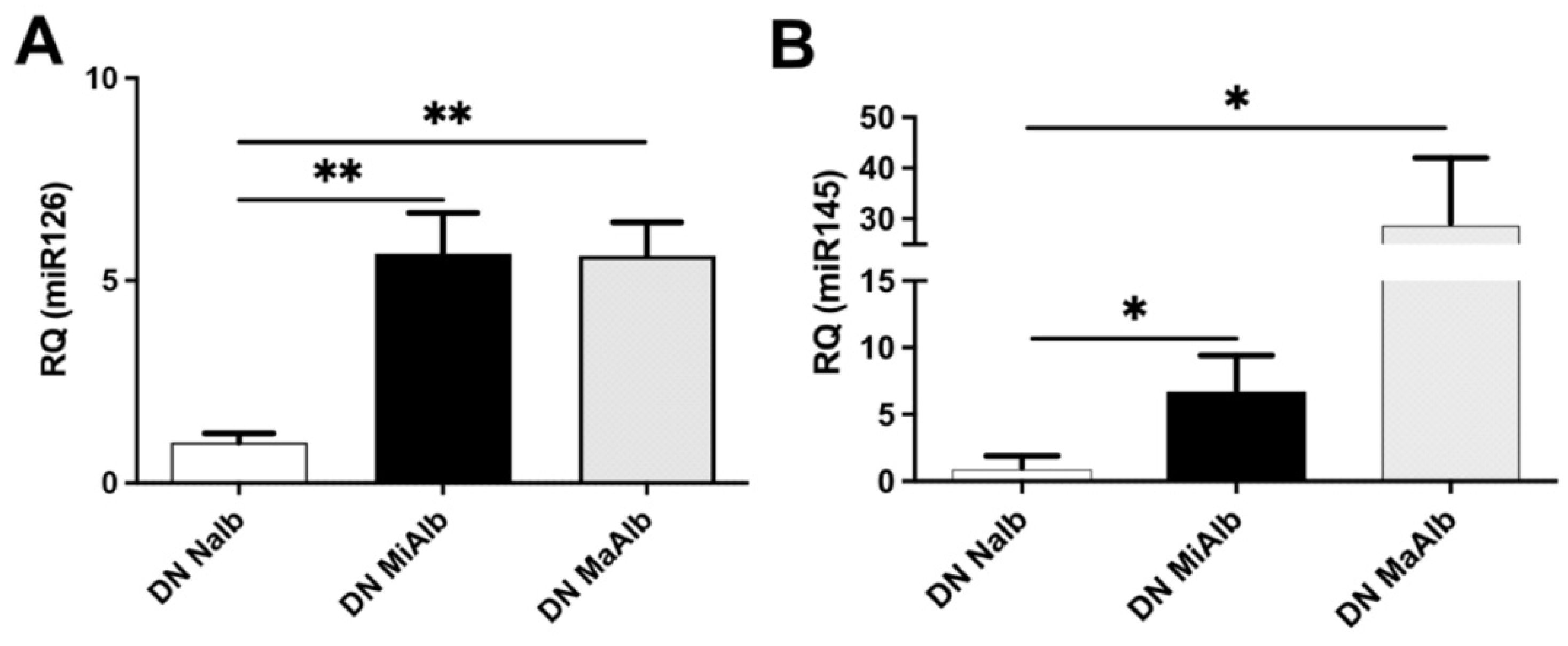
2.2. miRNA Modulation in uEVs from Diabetic Patients
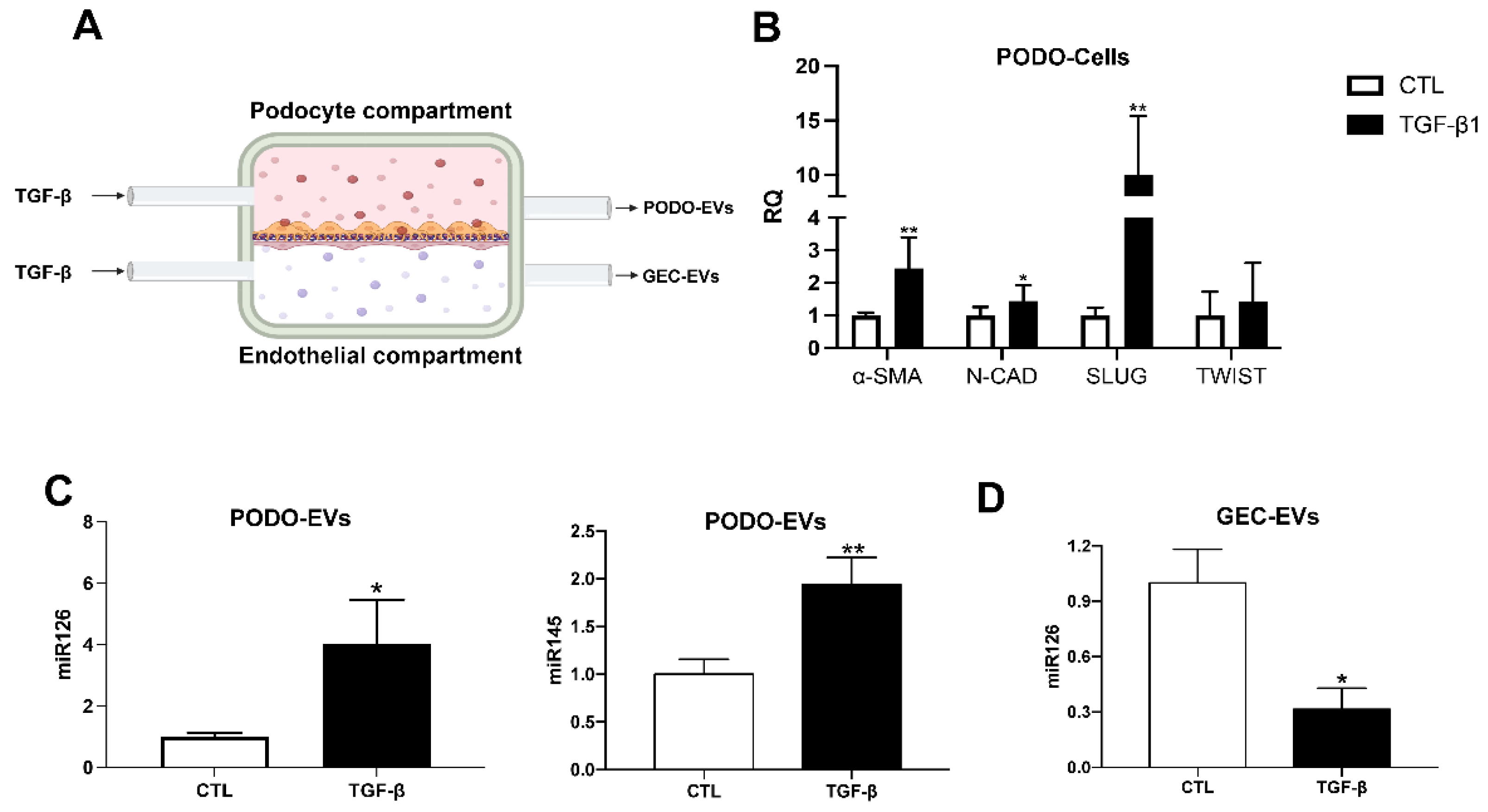
2.3. miRNA Modulation in EVs Released by Glomerular Cells in an In Vitro Diabetic Model of Nephropathy
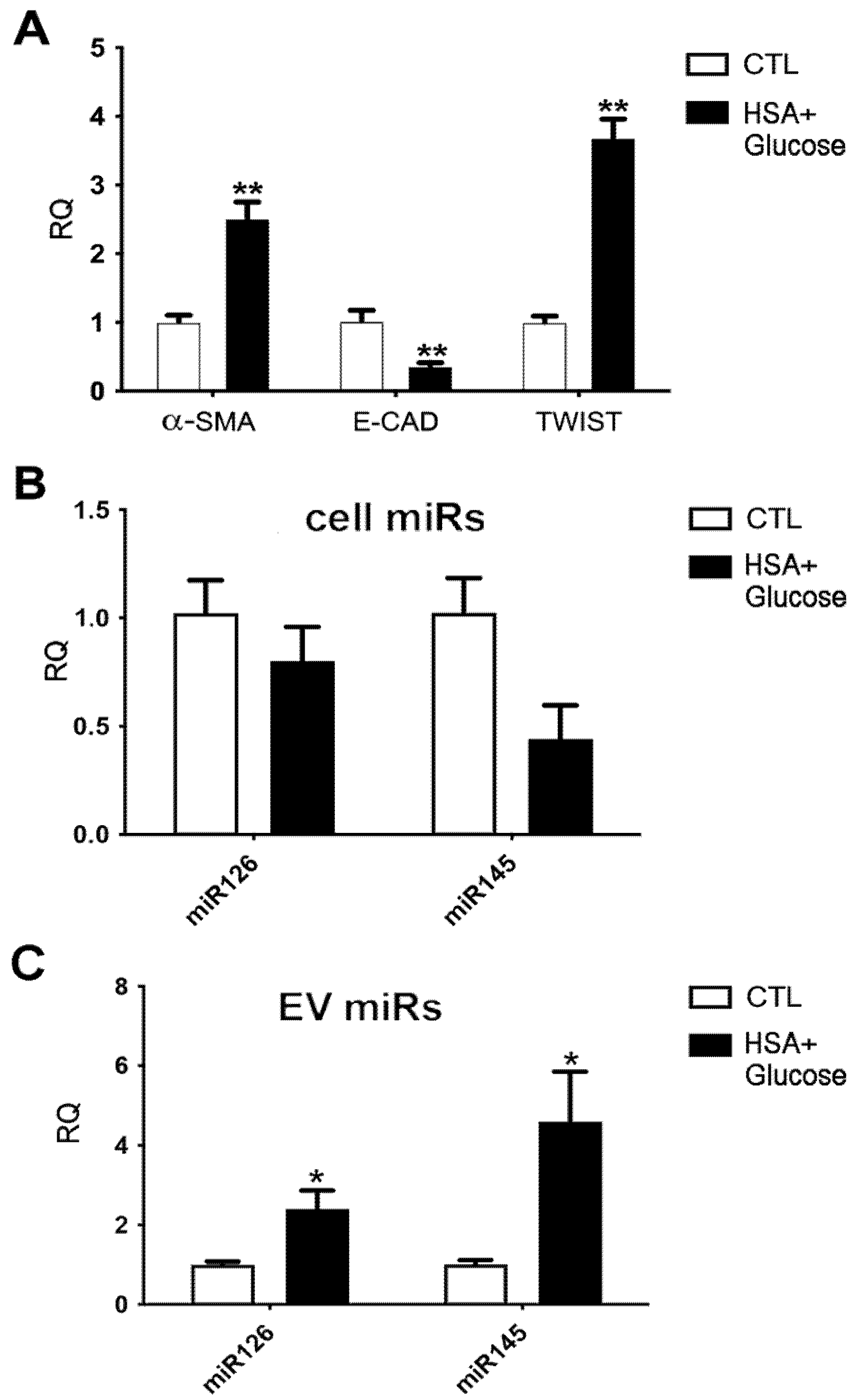
2.4. miRNA Modulation in EVs Released by Tubular Cells in an In Vitro Model of Diabetic Nephropathy
3. Discussion
4. Materials and Methods
4.1. Study Groups
4.2. Patients’ Urine Collection and uEV Isolation
4.3. Cell Lines
4.4. In Vitro Diabetes-Mimicking Models
4.5. EV Isolation from Cell Supernatant
4.6. Transmission Electron Microscopy
4.7. Nanoparticle Tracking Analysis
4.8. Super-Resolution Microscopy
4.9. MACSPlex Exosome Kit
4.10. Total RNA Extraction
4.11. RNA and miRNA Analysis
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sulaiman, M.K. Diabetic nephropathy: Recent advances in pathophysiology and challenges in dietary management. Diabetol. Metab. Syndr. 2019, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, G.; Penno, G.; Natali, A.; Barutta, F.; Di Paolo, S.; Reboldi, G.; Gesualdo, L.; De Nicola, L. Diabetic kidney disease: New clinical and therapeutic issues. Joint position statement of the Italian Diabetes Society and the Italian Society of Nephrology on “The natural history of diabetic kidney disease and treatment of hyperglycemia in patients with type 2 diabetes and impaired renal function”. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 1127–1150. [Google Scholar] [PubMed]
- Rico-Fontalvo, J.; Aroca, G.; Cabrales, J.; Daza-Arnedo, R.; Yánez-Rodríguez, T.; Martínez-Ávila, M.C.; Uparella-Gulfo, I.; Raad-Sarabia, M. Molecular Mechanisms of Diabetic Kidney Disease. Int. J. Mol. Sci. 2022, 23, 8668. [Google Scholar] [CrossRef]
- De Nicola, L.; Gabbai, F.B.; Liberti, M.E.; Sagliocca, A.; Conte, G.; Minutolo, R. Sodium/glucose cotransporter 2 inhibitors and prevention of diabetic nephropathy: Targeting the renal tubule in diabetes. Am. J. Kidney Dis. 2014, 64, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Provenzano, M.; Maritati, F.; Abenavoli, C.; Bini, C.; Corradetti, V.; La Manna, G.; Comai, G. Precision Nephrology in Patients with Diabetes and Chronic Kidney Disease. Int. J. Mol. Sci. 2022, 23, 5719. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Ly, N.D.K.; Tesch, G.H.; Poronnik, P.; Nikolic-Paterson, D.J. Reduced tubular degradation of glomerular filtered plasma albumin is a common feature in acute and chronic kidney disease. Clin. Exp. Pharmacol. Physiol. 2018, 45, 241–249. [Google Scholar] [CrossRef]
- Oshima, M.; Shimizu, M.; Yamanouchi, M.; Toyama, T.; Hara, A.; Furuichi, K.; Wada, T. Trajectories of kidney function in diabetes: A clinicopathological update. Nat. Rev. Nephrol. 2021, 17, 740–750. [Google Scholar] [CrossRef]
- Grange, C.; Bussolati, B. Extracellular vesicles in kidney disease. Nat. Rev. Nephrol. 2022, 18, 499–513. [Google Scholar] [CrossRef]
- Erdbrügger, U.; Blijdorp, C.J.; Bijnsdorp, I.V.; Borràs, F.E.; Burger, D.; Bussolati, B.; Byrd, J.B.; Clayton, A.; Dear, J.W.; Falcón-Pérez, J.M.; et al. Urinary extracellular vesicles: A position paper by the Urine Task Force of the International Society for Extracellular Vesicles. J. Extracell. Vesicles. 2021, 10, e12093. [Google Scholar] [CrossRef]
- Kaur, P.; Kotru, S.; Singh, S.; Munshi, A. miRNA signatures in diabetic retinopathy and nephropathy: Delineating underlying mechanisms. J. Physiol. Biochem. 2022, 78, 19–37. [Google Scholar] [CrossRef]
- Sun, I.O.; Lerman, L.O. Urinary microRNA in kidney disease: Utility and roles. Am. J. Physiol. Renal Physiol. 2019, 316, F785–F793. [Google Scholar] [CrossRef] [PubMed]
- Zang, J.; Maxwell, A.P.; Simpson, D.A.; McKay, G.J. Differential Expression of Urinary Exosomal MicroRNAs miR-21-5p and miR-30b-5p in Individuals with Diabetic Kidney Disease. Sci. Rep. 2019, 9, 10900. [Google Scholar] [CrossRef] [PubMed]
- Delić, D.; Eisele, C.; Schmid, R.; Baum, P.; Wiech, F.; Gerl, M.; Zimdahl, H.; Pullen, S.S.; Urquhart, R. Urinary exosomal miRNA signature in type II diabetic nephropathy patients. PLoS ONE 2016, 11, e0150154. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Hu, X.; Lee, S.H.; Chen, F.; Jiang, K.; Tu, Z.; Liu, Z.; Du, J.; Wang, L.; Yin, C.; et al. Diabetes Exacerbates Myocardial Ischemia/Reperfusion Injury by Down-Regulation of MicroRNA and Up-Regulation of O-GlcNAcylation. JACC Basic Transl. Sci. 2018, 3, 350–362. [Google Scholar] [CrossRef]
- Liu, H.N.; Li, X.; Wu, N.; Tong, M.M.; Chen, S.; Zhu, S.S.; Qian, W.; Chen, X.L. Serum microRNA-221 as a biomarker for diabetic retinopathy in patients associated with type 2 diabetes. Int. J. Ophthalmol. 2018, 11, 1889–1894. [Google Scholar]
- Chen, S. circ_000166/miR-296 Aggravates the Process of Diabetic Renal Fibrosis by Regulating the SGLT2 Signaling Pathway in Renal Tubular Epithelial Cells. Dis. Markers 2022, 2022, 6103086. [Google Scholar] [CrossRef]
- Jansen, F.; Wang, H.; Przybilla, D.; Franklin, B.S.; Dolf, A.; Pfeifer, P.; Schmitz, T.; Flender, A.; Endl, E.; Nickenig, G.; et al. Vascular endothelial microparticles-incorporated microRNAs are altered in patients with diabetes mellitus. Cardiovasc. Diabetol. 2016, 15, 49. [Google Scholar] [CrossRef]
- Ortega, F.J.; Mercader, J.M.; Moreno-Navarrete, J.M.; Rovira, O.; Guerra, E.; Esteve, E.; Xifra, G.; Martínez, C.; Ricart, W.; Rieusset, J.; et al. Profiling of circulating microRNAs reveals common microRNAs linked to type 2 diabetes that change with insulin sensitization. Diabetes Care 2014, 37, 1375–1383. [Google Scholar] [CrossRef]
- Barutta, F.; Tricarico, M.; Corbelli, A.; Annaratone, L.; Pinach, S.; Grimaldi, S.; Bruno, G.; Cimino, D.; Taverna, D.; Deregibus, M.C.; et al. Urinary exosomal MicroRNAs in incipient diabetic nephropathy. PLoS ONE 2013, 8, e73798. [Google Scholar] [CrossRef]
- Beltrami, C.; Simpson, K.; Jesky, M.; Wonnacott, A.; Carrington, C.; Holmans, P.; Newbury, L.; Jenkins, R.; Ashdown, T.; Dayan, C.; et al. Association of Elevated Urinary miR-126. miR-155. and miR-29b with Diabetic Kidney Disease. Am. J. Pathol. 2018, 188, 1982–1992. [Google Scholar] [CrossRef]
- Dimuccio, V.; Ranghino, A.; Praticò Barbato, L.; Fop, F.; Biancone, L.; Camussi, G.; Bussolati, B. Urinary CD133+ extracellular vesicles are decreased in kidney transplanted patients with slow graft function and vascular damage. PLoS ONE 2014, 9, e104490. [Google Scholar] [CrossRef] [PubMed]
- Bellucci, L.; Montini, G.; Collino, F.; Bussolati, B. Mesenchymal Stromal Cell-Derived Extracellular Vesicles Pass through the Filtration Barrier and Protect Podocytes in a 3D Glomerular Model under Continuous Perfusion. Tissue Eng. Regen. Med. 2021, 18, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Słomka, A.; Kornek, M.; Cho, W.C. Small Extracellular Vesicles and Their Involvement in Cancer Resistance: An Up-to-Date Review. Cells 2022, 11, 2913. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.C.S. Extracellular Vesicles: Biology and Potentials in Cancer Therapeutics. Int. J. Mol. Sci. 2021, 22, 9586. [Google Scholar] [CrossRef]
- Backes, C.; Meese, E.; Keller, A. Specific miRNA Disease Biomarkers in Blood. Serum and Plasma: Challenges and Prospects. Mol. Diagn. Ther. 2016, 20, 509–518. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Orekhov, A.N.; Bobryshev, Y.V. The role of miR-126 in embryonic angiogenesis adult vascular homeostasis. and vascular repair and its alterations in atherosclerotic disease. J. Mol. Cell. Cardiol. 2016, 97, 47–55. [Google Scholar] [CrossRef]
- Alique, M.; Bodega, G.; Giannarelli, C.; Carracedo, J.; Ramírez, R. MicroRNA-126 regulates Hypoxia-Inducible Factor-1α which inhibited migration. proliferation. and angiogenesis in replicative endothelial senescence. Sci. Rep. 2019, 9, 7381. [Google Scholar] [CrossRef]
- Fujimoto, S.; Fujita, Y.; Kadota, T.; Araya, J.; Kuwano, K. Intercellular Communication by Vascular Endothelial Cell-Derived Extracellular Vesicles and Their MicroRNAs in Respiratory Diseases. Front. Mol. Biosci. 2021, 29, 619697. [Google Scholar] [CrossRef]
- Feng, X.; Tan, W.; Cheng, S.; Wang, H.; Ye, S.; Yu, C.; He, Y.; Zeng, J.; Cen, J.; Hu, J.; et al. Upregulation of microRNA-126 in hepatic stellate cells may affect pathogenesis of liver fibrosis through the NF-κB pathway. DNA Cell Biol. 2015, 34, 470–480. [Google Scholar] [CrossRef]
- Liakouli, V.; Cipriani, P.; Di Benedetto, P.; Panzera, N.; Ruscitti, P.; Pantano, I.; Berardicurti, O.; Carubbi, F.; Esteves, F.; Mavria, G.; et al. Epidermal Growth Factor Like-domain 7 and miR-126 are abnormally expressed in diffuse Systemic Sclerosis fibroblasts. Sci. Rep. 2019, 9, 4589. [Google Scholar] [CrossRef]
- Wu, J.; Huang, Q.; Li, P.; Wang, Y.; Zheng, C.; Lei, X.; Li, S.; Gong, W.; Yin, B.; Luo, C.; et al. MicroRNA-145 promotes the epithelial-mesenchymal transition in peritoneal dialysis-associated fibrosis by suppressing fibroblast growth factor 10. J. Biol. Chem. 2019, 294, 15052–15067. [Google Scholar] [CrossRef] [PubMed]
- Manvati, S.; Mangalhara, K.C.; Kalaiarasan, P.; Chopra, R.; Agarwal, G.; Kumar, R.; Saini, S.K.; Kaushik, M.; Arora, A.; Kumari, U. miR-145 supports cancer cell survival and shows association with DDR genes. methylation pattern and epithelial to mesenchymal transition. Cancer Cell Int. 2019, 19, 230. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, J.; Yan, B.; Tu, H.; Huang, C.; Costa, M. Loss of MEG3 and upregulation of miR-145 play an important role in the invasion and migration of Cr(VI)-transformed cells. Heliyon 2022, 8, e10086. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Han, Y.; Zhu, D.; Li, Z.; Shan, S.; Jin, W.; Lu, Q.; Ren, T. miR-145 and miR-497 suppress TGF-β-induced epithelial–mesenchymal transition of non-small cell lung cancer by targeting MTDH. Cancer Cell Int. 2018, 18, 105. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, J.; Zhu, X.; Song, H.; Ren, L.; Tang, Q.; Xu, X.; Liu, C.; Zhang, J.; Hu, W.; et al. A novel approach to identify the mechanism of miR-145-5p toxicity to podocytes based on the essential genes targeting analysis. Mol. Ther. Nucleic Acids 2021, 26, 749–759. [Google Scholar] [CrossRef]
- Díaz-López, A.; Moreno-Bueno, G.; Cano, A. Role of microRNA in epithelial to mesenchymal transition and metastasis and clinical perspectives. Cancer Manag. Res. 2014, 6, 205–216. [Google Scholar]
- Bruno, S.; Chiabotto, G.; Cedrino, M.; Ceccotti, E.; Pasquino, C.; De Rosa, S.; Grange, C.; Tritta, S.; Camussi, G. Extracellular Vesicles Derived from Human Liver Stem Cells Attenuate Chronic Kidney Disease Development in an In Vivo Experimental Model of Renal Ischemia and Reperfusion Injury. Int. J. Mol. Sci. 2022, 23, 1485. [Google Scholar] [CrossRef]
- Skovronova, R.; Grange, C.; Dimuccio, V.; Deregibus, M.C.; Camussi, G.; Bussolati, B. Surface Marker Expression in Small and Medium/Large Mesenchymal Stromal Cell-Derived Extracellular Vesicles in Naive or Apoptotic Condition Using Orthogonal Techniques. Cells 2021, 10, 2948. [Google Scholar] [CrossRef]

| Patient Group | N. | Sex (Male/Female) | Age (Years) | Creatinine (mg/dL) | eGFR (mL/min) | Albuminuria (mg/dL) | HbAlc. (mmol/mol) | EV Mean and Mode Size (nm) |
|---|---|---|---|---|---|---|---|---|
| NAlb DN | 17 | 11/6 | 68 (63–77) | 1.16 ± 0.12 | 72.4± 6.6 | 0.1 ± 0.1 | 74.1 ± 6.2 | Mean: 264.9 ± 8.2 Mode: 207.7 ± 8.6 |
| MiAlb DN | 15 | 10/5 | 79 (72–85) | 1.40 ± 0.24 a | 62.4± 6.4 | 22.4 ± 2.2 c | 58.7 ± 4.4 | Mean: 251.1 ± 3.2 Mode: 198.3 ± 7.5 |
| MaAlb DN | 14 | 8/6 | 82 (70–87) | 1.57 ± 0.22 a | 45.1± 7.1 b | 129.3 ± 27.2 d | 58.8 ± 3.1 | Mean: 250.2 ± 6.7 Mode: 195.9 ± 9.9 |
| Gene | Forward Sequence | Reverse Sequence |
|---|---|---|
| GAPDH | F-GTCTCCTCTGACTTCAACAGCG | R-ACCACCCTGTTGCTGTAGCCAA |
| N-cadherin | F-CCTCCAGAGTTTACTGCCATGAC | R-GTAGGATCTCCGCCACTGATTC |
| E-cadherin | F-GCCTCCTGAAAAGAGAGTGGAAG | R-TGGCAGTGTCTCTCCAAATCCG |
| Twist family bHLH transcription factor 1(TWIST1) | F-GCCAGGTACATCGACTTCCTCT | R-TCCATCCTCCAGACCGAGAAGG |
| α-smooth muscle cell actin (α-SMA) | F-CTATGCCTCTGGACGCACAACT | R-CAGATCCAGACGCATGATGGCA |
| Snail family transcriptional repressor 2 (SLUG) | F-AAACTACAGCGAACTGGACACACA | R-GAGCAGCGGTAGTCCACACAG |
| RNU6B | F-TGCGGCTGCGCAAGGATGA | |
| miR126 | F-CATTATTACTTTTGGTACGC | |
| miR145 | F-TGCGGTGAGATGAAGCACT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dimuccio, V.; Bellucci, L.; Genta, M.; Grange, C.; Brizzi, M.F.; Gili, M.; Gallo, S.; Centomo, M.L.; Collino, F.; Bussolati, B. Upregulation of miR145 and miR126 in EVs from Renal Cells Undergoing EMT and Urine of Diabetic Nephropathy Patients. Int. J. Mol. Sci. 2022, 23, 12098. https://doi.org/10.3390/ijms232012098
Dimuccio V, Bellucci L, Genta M, Grange C, Brizzi MF, Gili M, Gallo S, Centomo ML, Collino F, Bussolati B. Upregulation of miR145 and miR126 in EVs from Renal Cells Undergoing EMT and Urine of Diabetic Nephropathy Patients. International Journal of Molecular Sciences. 2022; 23(20):12098. https://doi.org/10.3390/ijms232012098
Chicago/Turabian StyleDimuccio, Veronica, Linda Bellucci, Marianna Genta, Cristina Grange, Maria Felice Brizzi, Maddalena Gili, Sara Gallo, Maria Laura Centomo, Federica Collino, and Benedetta Bussolati. 2022. "Upregulation of miR145 and miR126 in EVs from Renal Cells Undergoing EMT and Urine of Diabetic Nephropathy Patients" International Journal of Molecular Sciences 23, no. 20: 12098. https://doi.org/10.3390/ijms232012098
APA StyleDimuccio, V., Bellucci, L., Genta, M., Grange, C., Brizzi, M. F., Gili, M., Gallo, S., Centomo, M. L., Collino, F., & Bussolati, B. (2022). Upregulation of miR145 and miR126 in EVs from Renal Cells Undergoing EMT and Urine of Diabetic Nephropathy Patients. International Journal of Molecular Sciences, 23(20), 12098. https://doi.org/10.3390/ijms232012098









