Design of Cyclic Peptide-Based Nanospheres and the Delivery of siRNA
Abstract
1. Introduction
2. Results
2.1. Design of (CP)6NS
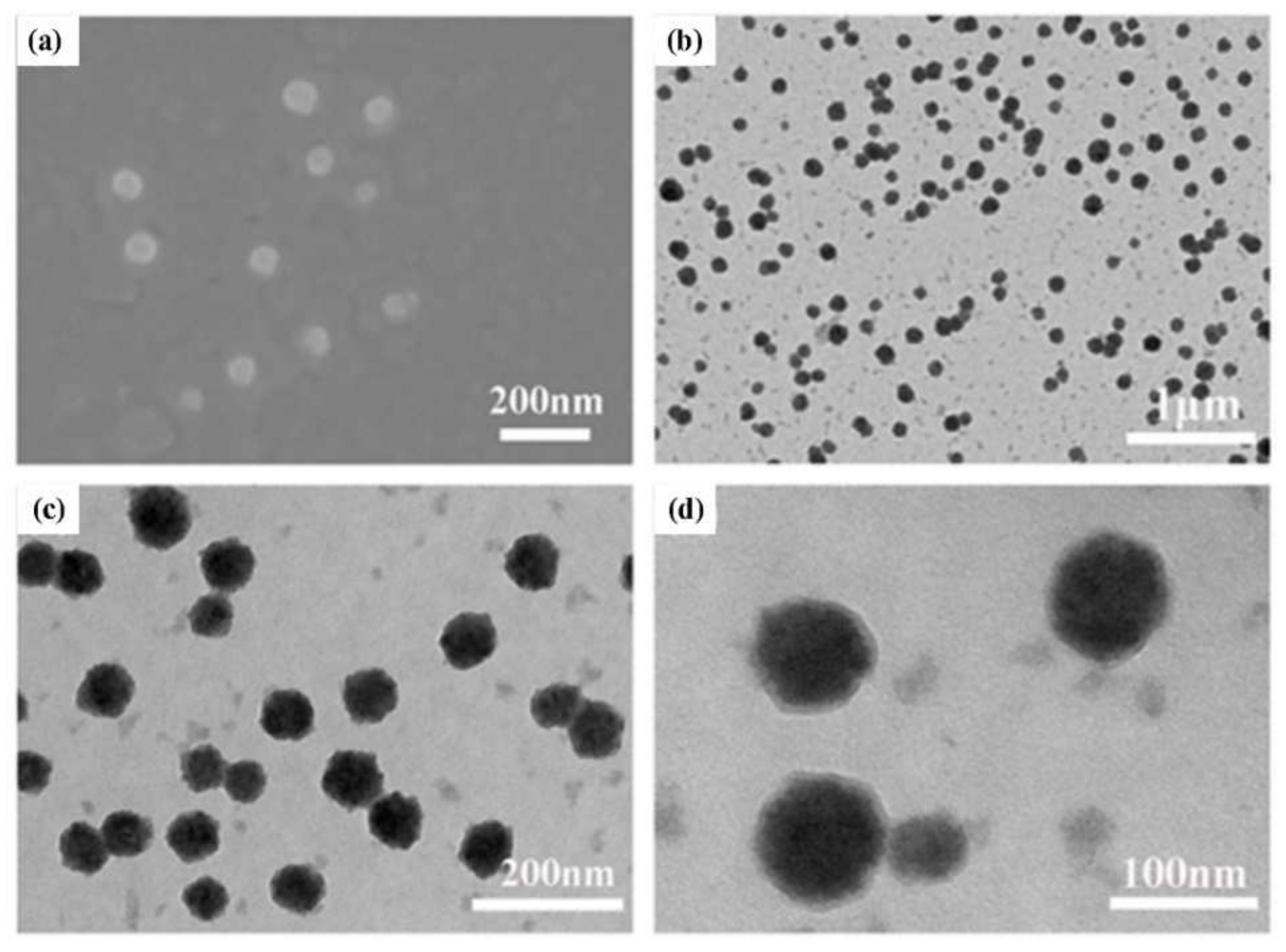
2.2. Characterization of (CP)6NS
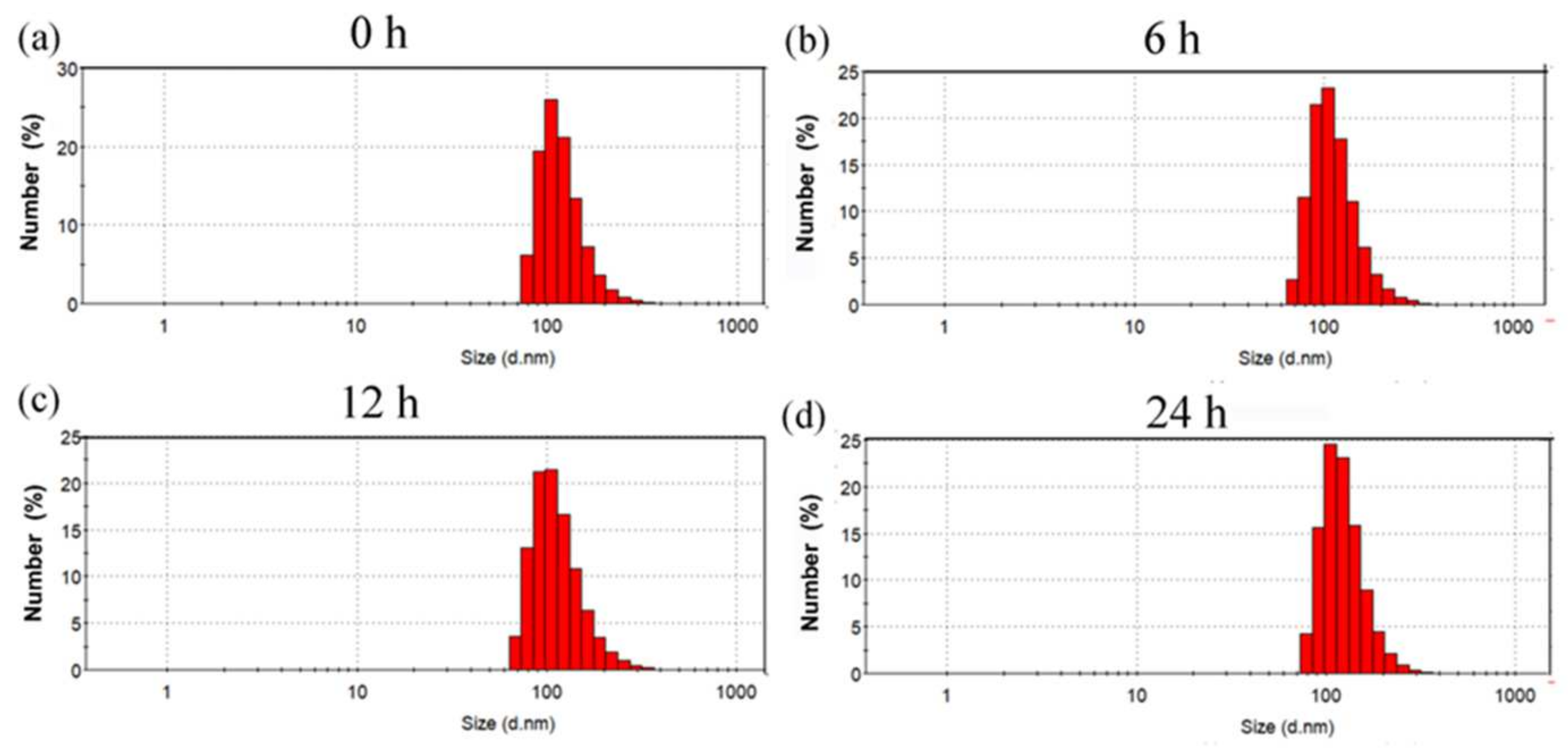
2.3. Stability of (CP)6NS
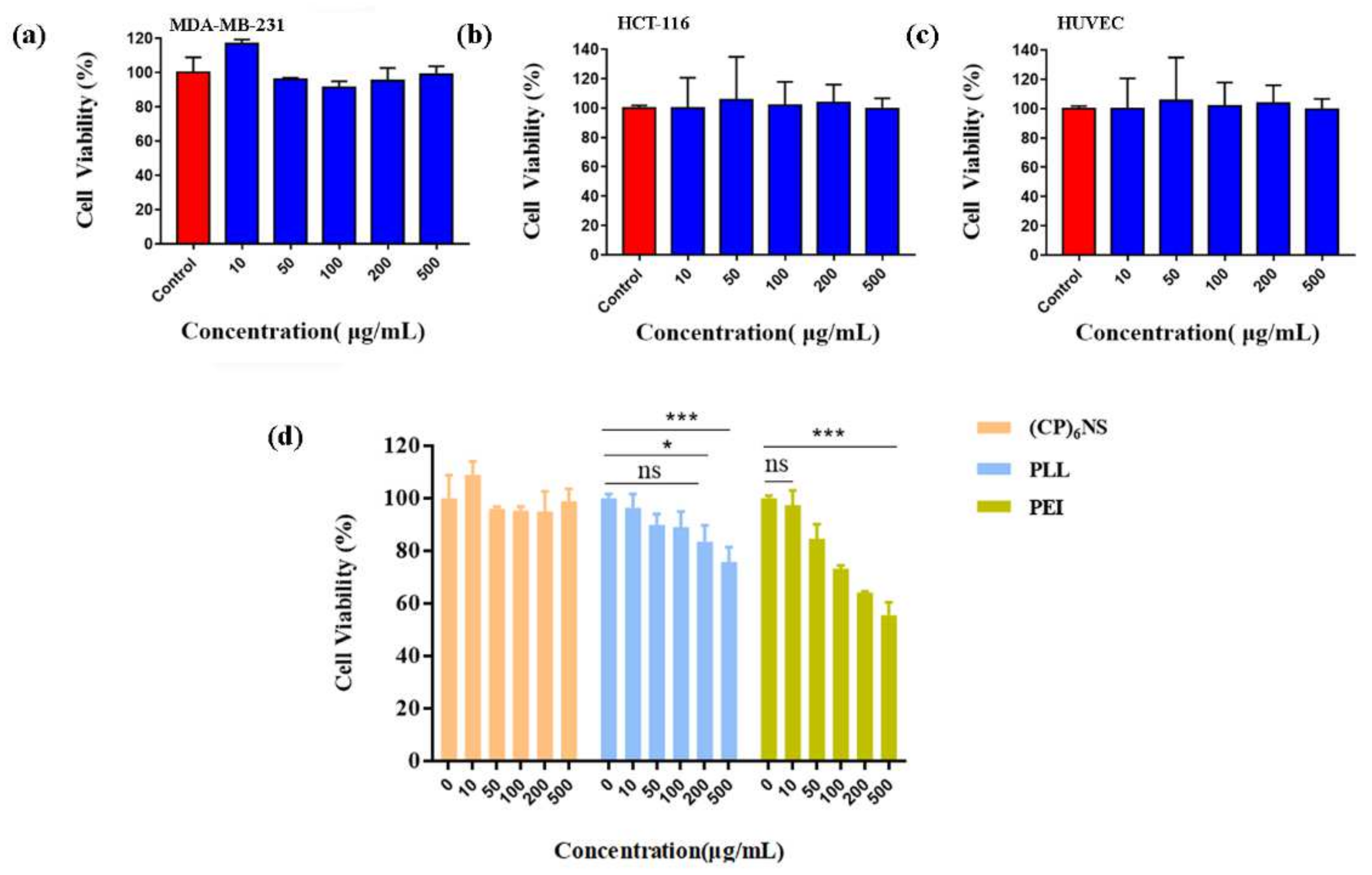
2.4. Effect of (CP)6NS on Cell Viability
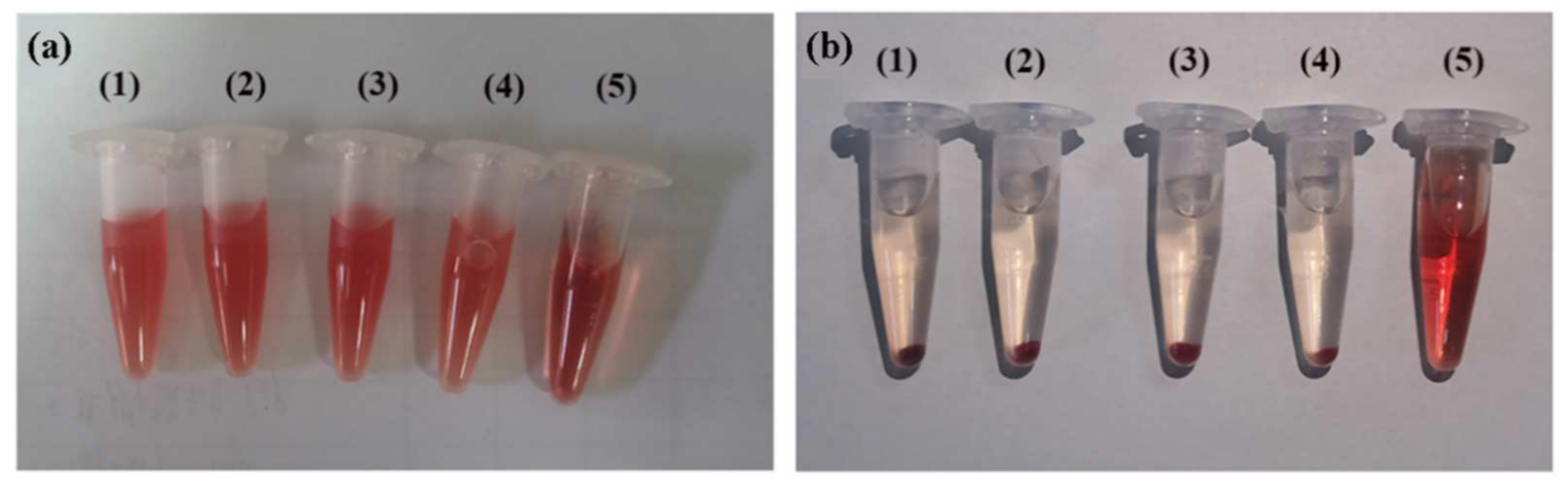
2.5. Hemocompatibility of (CP)6NS
2.6. The Ability of (CP)6NS to Load and Deliver siRNA
2.7. Anti-Tumor Effects In Vitro
3. Discussion
4. Materials and Methods
4.1. Reagents and Antibodies
4.2. Preparation of Nanospheres by Reaction of Cyclo-(DP)3 and 1,6-Hexanediamine
4.3. Preparation of Nanospheres by Reaction of Cyclo-(DP)3 and Cyclo-(RR)
4.4. Construction of (CP)6NS
4.5. Characterization of the Assembly Structure
4.6. Cell Culture and MTT Assays
4.7. Hemolysis Test
4.8. (CP)6NS and siRNA Binding Ability Assay
4.9. Detection of (CP)6NS Ability to Deliver FAM-siRNA
4.10. Quantitative Real-Time-PCR and Western Blot Assays
4.11. Cell Apoptosis
4.12. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Claro, B.; Peon, A.; Gonzalez-Freire, E.; Goormaghtigh, E.; Amorin, M.; Granja, J.R.; Garcia-Fandino, R.; Bastos, M. Macromolecular assembly and membrane activity of antimicrobial D,L-alpha-Cyclic peptides. Colloids Surf. B Biointerfaces 2021, 208, 112086. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Chen, Y.; Zhu, D.; Shi, K.; Ma, C.; Zhang, W.; Rocchi, P.; Jiang, L.; Liu, X. Self-assembly of amphiphilic phospholipid peptide dendrimer-based nanovectors for effective delivery of siRNA therapeutics in prostate cancer therapy. J. Control. Release Off. J. Control. Release Soc. 2020, 322, 416–425. [Google Scholar] [CrossRef]
- Li, F.; Wang, M.; Guan, S.; Huang, Z.; Liu, S.; Li, X.; Jiang, X.; Luo, Q.; Xu, J.; Liu, J. Cucurbit[8]uril-based supramolecular polymer nanocapsules as an effective siRNA delivery platform for gene therapy. Polym. Chem. 2019, 10, 5659–5664. [Google Scholar] [CrossRef]
- Nikam, R.R.; Gore, K.R. Journey of siRNA: Clinical Developments and Targeted Delivery. Nucleic Acid Ther. 2018, 28, 209–224. [Google Scholar] [CrossRef]
- Carthew, R.W. Gene silencing by double-stranded RNA. Curr. Opin. Cell Biol. 2001, 13, 244–248. [Google Scholar] [CrossRef]
- Devi, G.R. siRNA-based approaches in cancer therapy. Cancer Gene Ther. 2006, 13, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.D. The potential of RNA interference-based therapies for viral infections. Sci. HIV Med. 2008, 5, 33–39. [Google Scholar] [CrossRef]
- Wittrup, A.; Lieberman, J. Knocking down disease: A progress report on siRNA therapeutics. Nat. Rev. Genet. 2015, 16, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Li, Y.; Ding, S.W. Small RNA-based antimicrobial immunity. Nat. Rev. Immunol. 2019, 19, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Dowdy, S.F. Overcoming cellular barriers for RNA therapeutics. Nat. Biotechnol. 2017, 35, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Abe, K.; Fujiyoshi, Y. Cryo-electron microscopy for structure analyses of membrane proteins in the lipid bilayer. Curr. Opin. Struct. Biol. 2016, 39, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Juliano, R.L. The delivery of therapeutic oligonucleotides. Nucleic Acids Res. 2016, 44, 6518–6548. [Google Scholar] [CrossRef] [PubMed]
- Dominska, M.; Dykxhoorn, D.M. Breaking down the barriers: siRNA delivery and endosome escape. J. Cell Sci. 2010, 123, 1183–1189. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Yuan, Y.; Li, H.; Fu, Z.; Wang, M.; Guan, S.; Wang, L. Design and anti-tumor activity of self-loaded nanocarriers of siRNA. Colloids Surf. B Biointerfaces 2019, 183, 110385. [Google Scholar] [CrossRef]
- Chang, Y.; Yang, K.; Wei, P.; Huang, S.; Pei, Y.; Zhao, W.; Pei, Z. Cationic Vesicles Based on Amphiphilic Pillar[5]arene Capped with Ferrocenium: A Redox-Responsive System for Drug/siRNA Co-Delivery. Angew. Chem. Int. Ed. 2014, 53, 13126–13130. [Google Scholar] [CrossRef]
- Saw, P.E.; Yao, H.; Lin, C.; Tao, W.; Farokhzad, O.C.; Xu, X. Stimuli-Responsive Polymer–Prodrug Hybrid Nanoplatform for Multistage siRNA Delivery and Combination Cancer Therapy. Nano Lett. 2019, 19, 5967–5974. [Google Scholar] [CrossRef]
- Wang, S.; Liu, X.; Chen, S.; Liu, Z.; Zhang, X.; Liang, X.-J.; Li, L. Regulation of Ca2+ Signaling for Drug-Resistant Breast Cancer Therapy with Mesoporous Silica Nanocapsule Encapsulated Doxorubicin/siRNA Cocktail. ACS Nano 2018, 13, 274–283. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, C.J.; Liu, B. A Photoactivatable AIE Polymer for Light-Controlled Gene Delivery: Concurrent Endo/Lysosomal Escape and DNA Unpacking. Angew. Chem. Int. Ed. 2015, 54, 11419–11423. [Google Scholar] [CrossRef]
- Yin, F.; Hu, K.; Chen, Y.; Yu, M.; Wang, D.; Wang, Q.; Yong, K.T.; Lu, F.; Liang, Y.; Li, Z. SiRNA Delivery with PEGylated Graphene Oxide Nanosheets for Combined Photothermal and Genetherapy for Pancreatic Cancer. Theranostics 2017, 7, 1133–1148. [Google Scholar] [CrossRef]
- Yang, T.; Chen, Y.; Zhao, P.; Xue, H.; You, J.; Li, B.; Liu, Y.; He, C.; Zhang, X.; Fan, L.; et al. Enhancing the therapeutic effect via elimination of hepatocellular carcinoma stem cells using Bmi1 siRNA delivered by cationic cisplatin nanocapsules. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 2009–2021. [Google Scholar] [CrossRef]
- Xu, X.; Wu, J.; Liu, Y.; Saw, P.E.; Tao, W.; Yu, M.; Zope, H.; Si, M.; Victorious, A.; Rasmussen, J.; et al. Multifunctional Envelope-Type siRNA Delivery Nanoparticle Platform for Prostate Cancer Therapy. ACS Nano 2017, 11, 2618–2627. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.-Y.; Chu, Y.-F.; Tao, L.; Xu, S.-S.; Li, Z.-Y.; Zhuo, R.-X.; Zhang, X.-Z. Controllable micro/nanostructures via hierarchical self-assembly of cyclopeptides. Soft Matter 2011, 7, 8635–8641. [Google Scholar] [CrossRef]
- Park, W.M.; Champion, J.A. Thermally Triggered Self-Assembly of Folded Proteins into Vesicles. J. Am. Chem. Soc. 2014, 136, 17906–17909. [Google Scholar] [CrossRef]
- Tan, X.; Lu, X.; Jia, F.; Liu, X.; Sun, Y.; Logan, J.K.; Zhang, K. Blurring the Role of Oligonucleotides: Spherical Nucleic Acids as a Drug Delivery Vehicle. J. Am. Chem. Soc. 2016, 138, 10834–10837. [Google Scholar] [CrossRef] [PubMed]
- Pi, F.; Binzel, D.W.; Lee, T.J.; Li, Z.; Sun, M.; Rychahou, P.; Li, H.; Haque, F.; Wang, S.; Croce, C.M.; et al. Nanoparticle orientation to control RNA loading and ligand display on extracellular vesicles for cancer regression. Nat. Nanotechnol. 2017, 13, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Shuai, Q.; Cai, Y.; Zhao, G.; Sun, X. Cell-Penetrating Peptide Modified PEG-PLA Micelles for Efficient PTX Delivery. Int. J. Mol. Sci. 2020, 21, 1856. [Google Scholar] [CrossRef]
- Zhang, L.; Jing, D.; Jiang, N.; Rojalin, T.; Baehr, C.M.; Zhang, D.; Xiao, W.; Wu, Y.; Cong, Z.; Li, J.J.; et al. Transformable peptide nanoparticles arrest HER2 signalling and cause cancer cell death in vivo. Nat. Nanotechnol. 2020, 15, 145–153. [Google Scholar] [CrossRef]
- Zhang, L.; Qi, Y.; Min, H.; Ni, C.; Wang, F.; Wang, B.; Qin, H.; Zhang, Y.; Liu, G.; Qin, Y.; et al. Cooperatively Responsive Peptide Nanotherapeutic that Regulates Angiopoietin Receptor Tie2 Activity in Tumor Microenvironment to Prevent Breast Tumor Relapse after Chemotherapy. ACS Nano 2019, 13, 5091–5102. [Google Scholar] [CrossRef]
- Nazeer, N.; Simmons, J.R.; Rainey, J.K.; Rodriguez-Lecompte, J.C.; Ahmed, M. Antibacterial activities of physiologically stable, self-assembled peptide nanoparticles. J. Mater. Chem. B 2021, 9, 9041–9054. [Google Scholar] [CrossRef]
- Han, H.; Yin, Q.; Tang, X.; Yu, X.; Gao, Q.; Tang, Y.; Grzybowski, A.; Yao, K.; Ji, J.; Shentu, X. Development of mucoadhesive cationic polypeptide micelles for sustained cabozantinib release and inhibition of corneal neovascularization. J. Mater. Chem. B 2020, 8, 5143–5154. [Google Scholar] [CrossRef]
- Mandal, D.; Tiwari, R.K.; Shirazi, A.N.; Oh, D.; Ye, G.F.; Banerjee, A.; Yadav, A.; Parang, K. Self-assembled surfactant cyclic peptide nanostructures as stabilizing agents. Soft Matter 2013, 9, 9465–9475. [Google Scholar] [CrossRef] [PubMed]
- Panigrahi, B.; Singh, R.K.; Mishra, S.; Mandal, D. Cyclic peptide-based nanostructures as efficient siRNA carriers. Artif. Cells Nanomed. Biotechnol. 2018, 46, S763–S773. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Guan, X.W.; Hu, Y.Y.; Tian, H.Y.; Chen, X.S. Peptide-Based and Polypeptide-Based Gene Delivery Systems. Top. Curr. Chem. 2017, 375, 28. [Google Scholar] [CrossRef]
- Liu, C.X.; Liu, F.X.; Feng, L.X.; Li, M.; Zhang, J.; Zhang, N. The targeted co-delivery of DNA and doxorubicin to tumor cells via multifunctional PEI-PEG based nanoparticles. Biomaterials 2013, 34, 2547–2564. [Google Scholar] [CrossRef]
- Zhang, K.; Li, Y.; Liu, W.; Gao, X.; Zhang, K. Silencing survivin expression inhibits the tumor growth of non-small-cell lung cancer cells in vitro and in vivo. Mol. Med. Rep. 2015, 11, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Croci, D.O.; Cogno, I.S.; Vittar, N.B.; Salvatierra, E.; Trajtenberg, F.; Podhajcer, O.L.; Osinaga, E.; Rabinovich, G.A.; Rivarola, V.A. Silencing survivin gene expression promotes apoptosis of human breast cancer cells through a caspase-independent pathway. J. Cell. Biochem. 2008, 105, 381–390. [Google Scholar] [CrossRef]
- Hao, Y.; Bai, X.; Liu, X.; Kang, S.; Zhang, X.; Liu, C.; Li, Z. Downregulation of survivin by adenovirus-mediated shRNA promotes apoptosis in skin cancer cells. OncoTargets Ther. 2019, 12, 2921–2930. [Google Scholar] [CrossRef]
- Chen, X.; Duan, N.; Zhang, C.; Zhang, W. Survivin and Tumorigenesis: Molecular Mechanisms and Therapeutic Strategies. J. Cancer 2016, 7, 314–323. [Google Scholar] [CrossRef]
- Li, M.; Ehlers, M.; Schlesiger, S.; Zellermann, E.; Knauer, S.K.; Schmuck, C. Incorporation of a Non-Natural Arginine Analogue into a Cyclic Peptide Leads to Formation of Positively Charged Nanofibers Capable of Gene Transfection. Angew. Chem. Int. Ed. 2016, 55, 598–601. [Google Scholar] [CrossRef]
- Murugan, K.; Choonara, Y.E.; Kumar, P.; Bijukumar, D.; du Toit, L.C.; Pillay, V. Parameters and characteristics governing cellular internalization and trans-barrier trafficking of nanostructures. Int. J. Nanomed. 2015, 10, 2191–2206. [Google Scholar] [CrossRef]
- Chen, L.; Zheng, J.; Du, J.L.; Yu, S.P.; Yang, Y.Z.; Liu, X.G. Folic acid-conjugated magnetic ordered mesoporous carbon nanospheres for doxorubicin targeting delivery. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 104, 109939. [Google Scholar] [CrossRef] [PubMed]
- Qiu, M.; Ouyang, J.; Wei, Y.; Zhang, J.; Lan, Q.; Deng, C.; Zhong, Z. Selective Cell Penetrating Peptide-Functionalized Envelope-Type Chimeric Lipopepsomes Boost Systemic RNAi Therapy for Lung Tumors. Adv. Healthc. Mater. 2019, 8, e1900500. [Google Scholar] [CrossRef]
- Turner, R.A.; Oliver, A.G.; Lokey, R.S. Click chemistry as a macrocyclization tool in the solid-phase synthesis of small cyclic peptides. Org. Lett. 2007, 9, 5011–5014. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Song, J.I.; Song, Q.; Rho, J.Y.; Mansfield, E.D.H.; Hall, S.C.L.; Sambrook, M.; Huang, F.H.; Perrier, S. Hierarchical Self-Assembled Photo-Responsive Tubisomes from a Cyclic Peptide-Bridged Amphiphilic Block Copolymer. Angew. Chem. Int. Ed. 2020, 59, 8860–8863. [Google Scholar] [CrossRef]
- Claro, B.; Gonzalez-Freire, E.; Calvelo, M.; Bessa, L.J.; Goormaghtigh, E.; Amorin, M.; Granja, J.R.; Garcia-Fandino, R.; Bastos, M. Membrane targeting antimicrobial cyclic peptide nanotubes—An experimental and computational study. Colloid Surf. B-Biointerfaces 2020, 196, 111349. [Google Scholar] [CrossRef]
- Kim, D.; Kim, E.; Lee, J.; Hong, S.; Sung, W.; Lim, N.; Park, C.G.; Kim, K. Direct Synthesis of Polymer Nanocapsules: Self-Assembly of Polymer Hollow Spheres through Irreversible Covalent Bond Formation. J. Am. Chem. Soc. 2010, 132, 9908–9919. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Zhang, Y.; Guan, S.W.; Huang, Q.X.; Wang, R.B.; Tian, R.Z.; Zang, M.S.; Qiao, S.P.; Zhang, X.; Liu, S.D.; et al. Reductive-Responsive, Single-Molecular-Layer Polymer Nanocapsules Prepared by Lateral-Functionalized Pillar 5 arenes for Targeting Anticancer Drug Delivery. ACS Appl. Mater. Interfaces 2018, 10, 14281–14286. [Google Scholar] [CrossRef]
- Baek, K.; Yun, G.; Kim, Y.; Kim, D.; Hota, R.; Hwang, I.; Xu, D.; Ko, Y.H.; Gu, G.H.; Suh, J.H.; et al. Free-Standing, Single-Monomer-Thick Two-Dimensional Polymers through Covalent Self-Assembly in Solution. J. Am. Chem. Soc. 2013, 135, 6523–6528. [Google Scholar] [CrossRef]
- Roy, I.; Shetty, D.; Hota, R.; Baek, K.; Kim, J.; Kim, C.; Kappert, S.; Kim, K. A Multifunctional Subphthalocyanine Nanosphere for Targeting, Labeling, and Killing of Antibiotic-Resistant Bacteria. Angew. Chem. Int. Ed. 2015, 54, 15152–15155. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ke, J.; Zhang, J.; Li, J.; Liu, J.; Guan, S. Design of Cyclic Peptide-Based Nanospheres and the Delivery of siRNA. Int. J. Mol. Sci. 2022, 23, 12071. https://doi.org/10.3390/ijms232012071
Ke J, Zhang J, Li J, Liu J, Guan S. Design of Cyclic Peptide-Based Nanospheres and the Delivery of siRNA. International Journal of Molecular Sciences. 2022; 23(20):12071. https://doi.org/10.3390/ijms232012071
Chicago/Turabian StyleKe, Junfeng, Jingli Zhang, Junyang Li, Junqiu Liu, and Shuwen Guan. 2022. "Design of Cyclic Peptide-Based Nanospheres and the Delivery of siRNA" International Journal of Molecular Sciences 23, no. 20: 12071. https://doi.org/10.3390/ijms232012071
APA StyleKe, J., Zhang, J., Li, J., Liu, J., & Guan, S. (2022). Design of Cyclic Peptide-Based Nanospheres and the Delivery of siRNA. International Journal of Molecular Sciences, 23(20), 12071. https://doi.org/10.3390/ijms232012071





