Development of the Ontogenetic Self-Regulation Clock
Abstract
1. Introduction
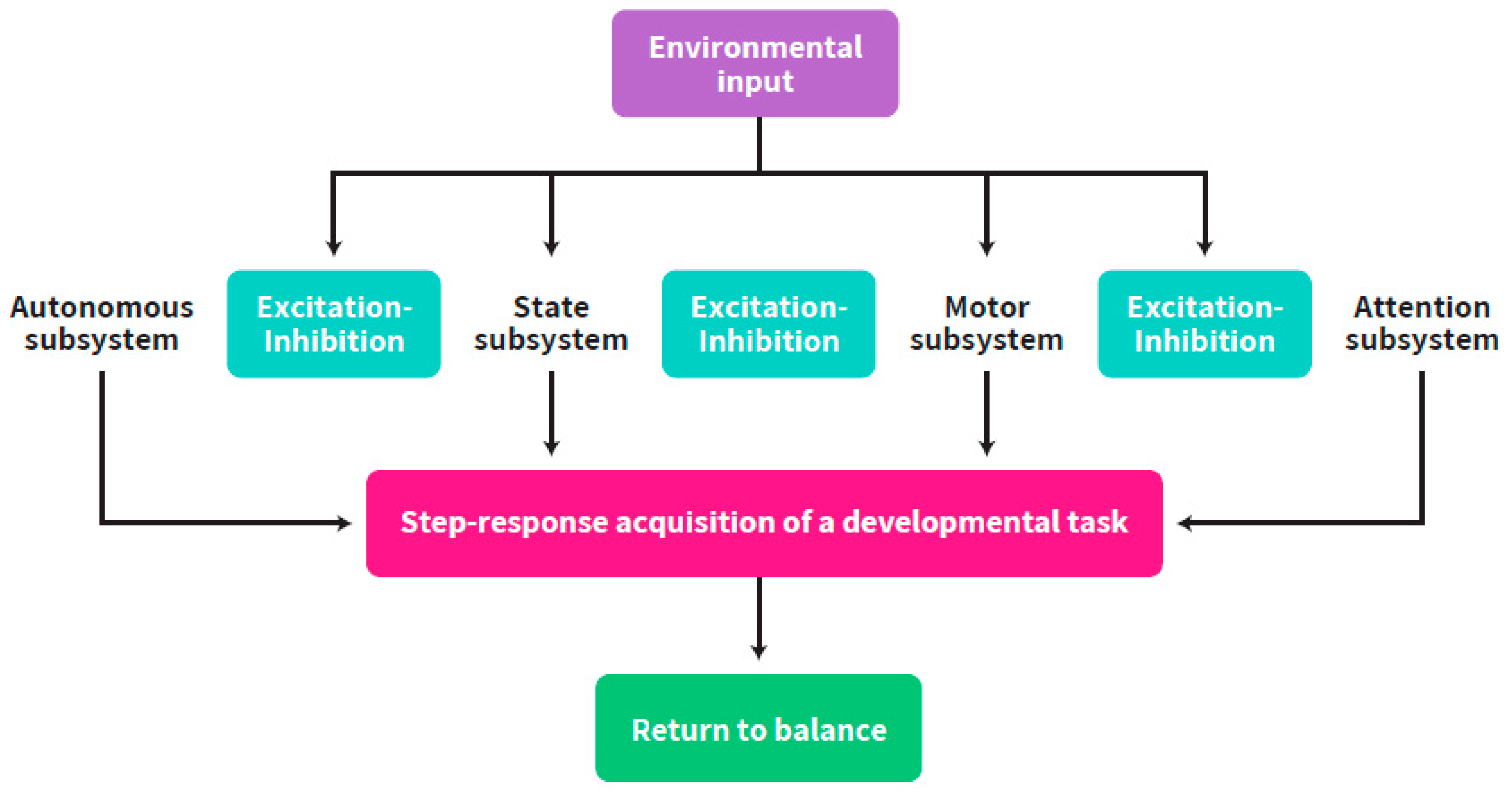
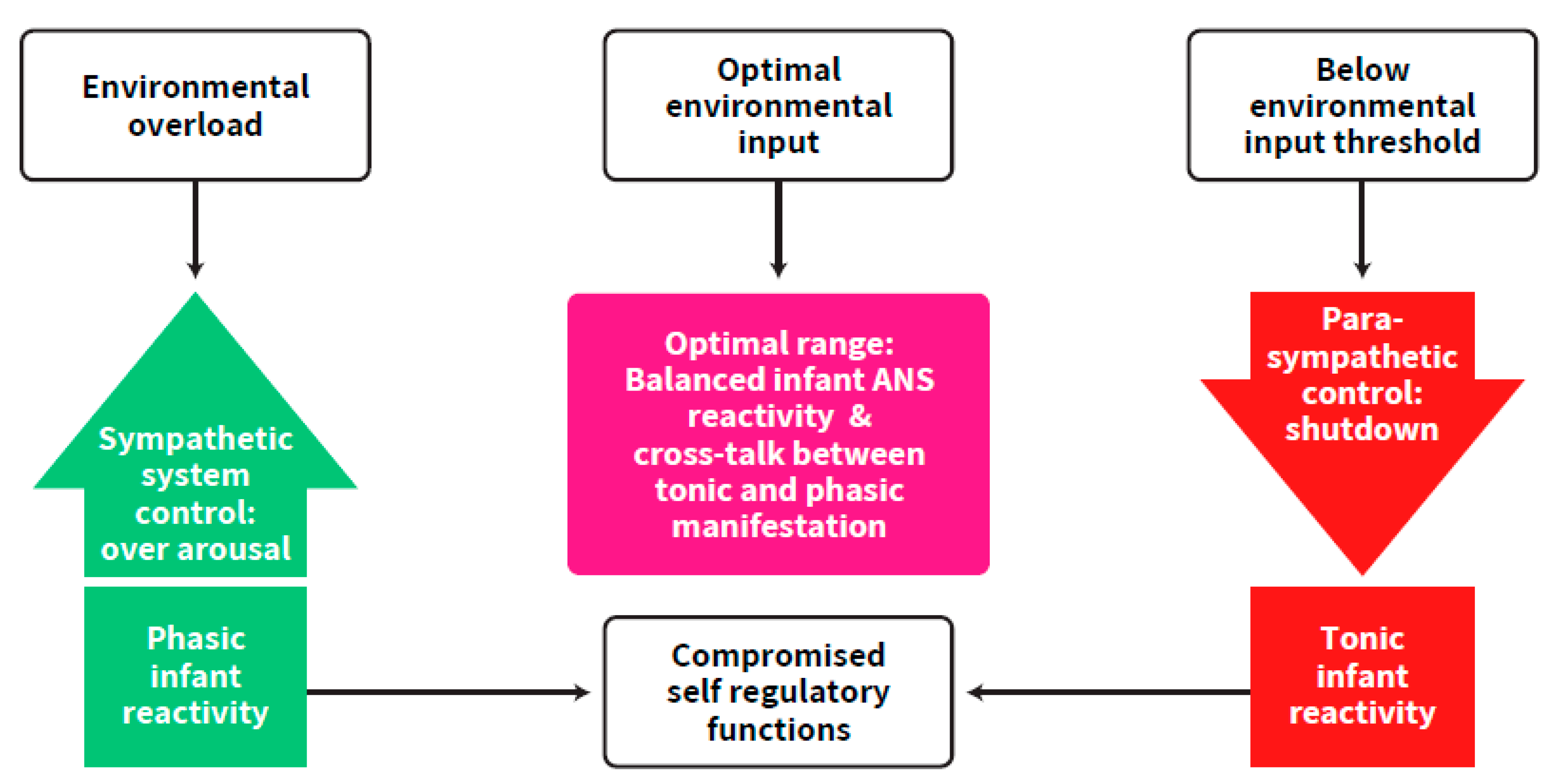
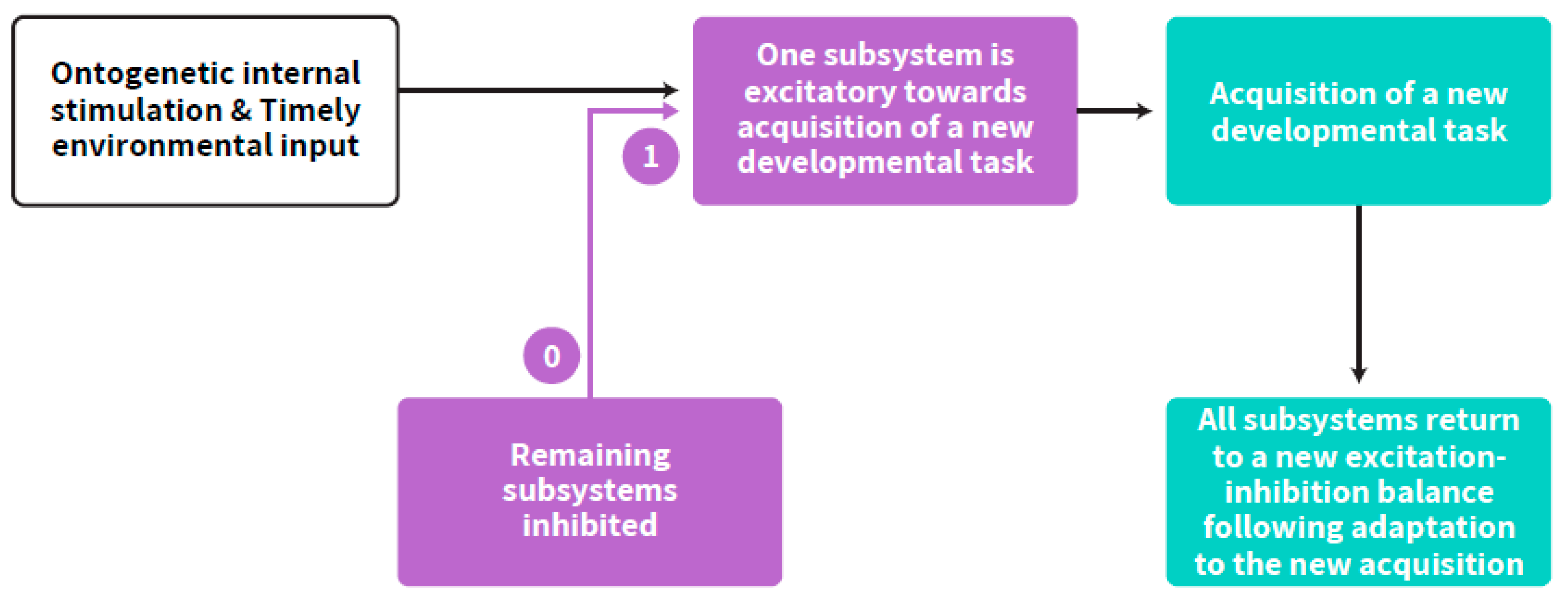
2. Self-Regulation: An Intra-Individual Perspective
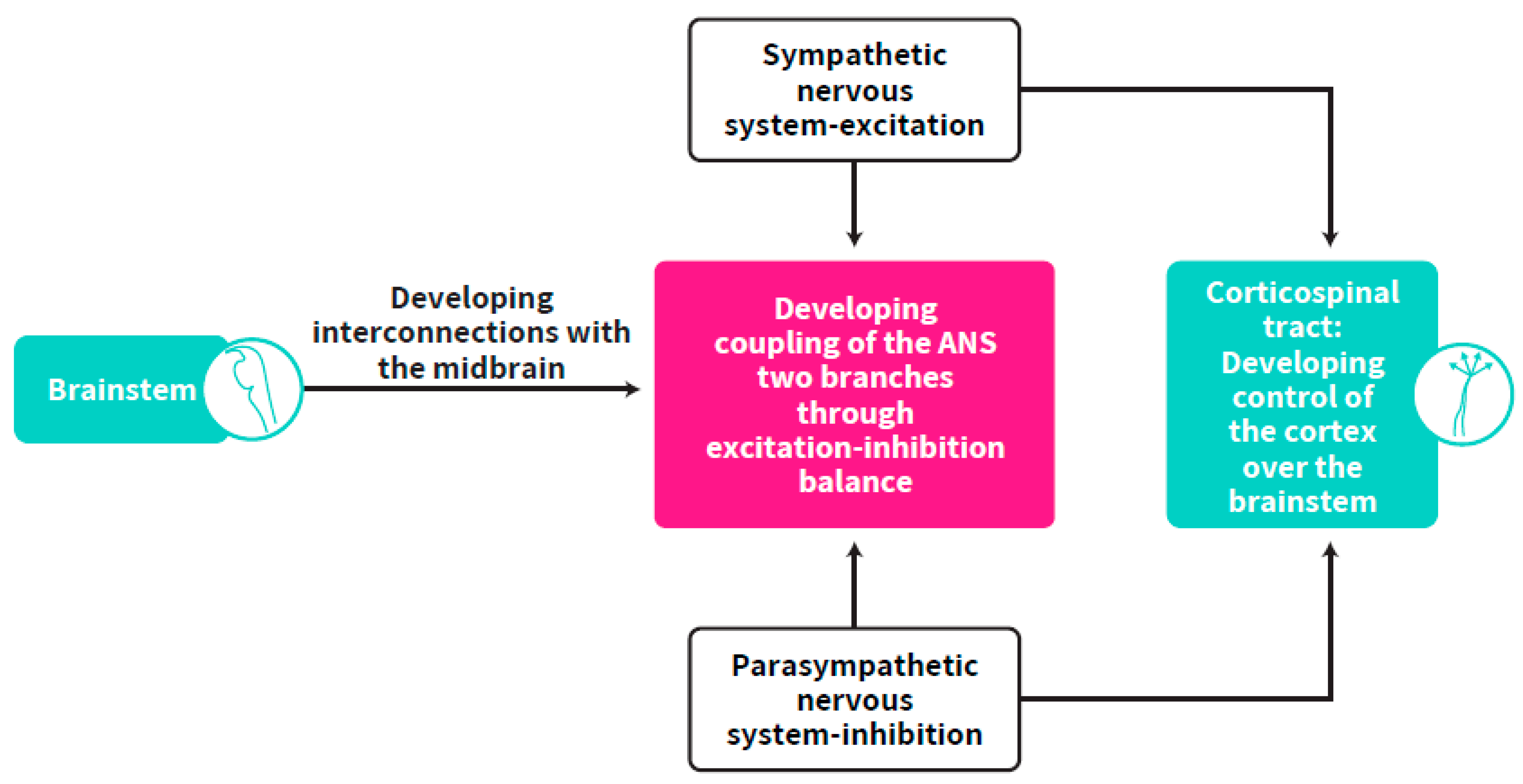
3. The Crucial Milestones of ANS Synchrony Development and the Multi-Level Consolidation of the Self-Regulation Clock
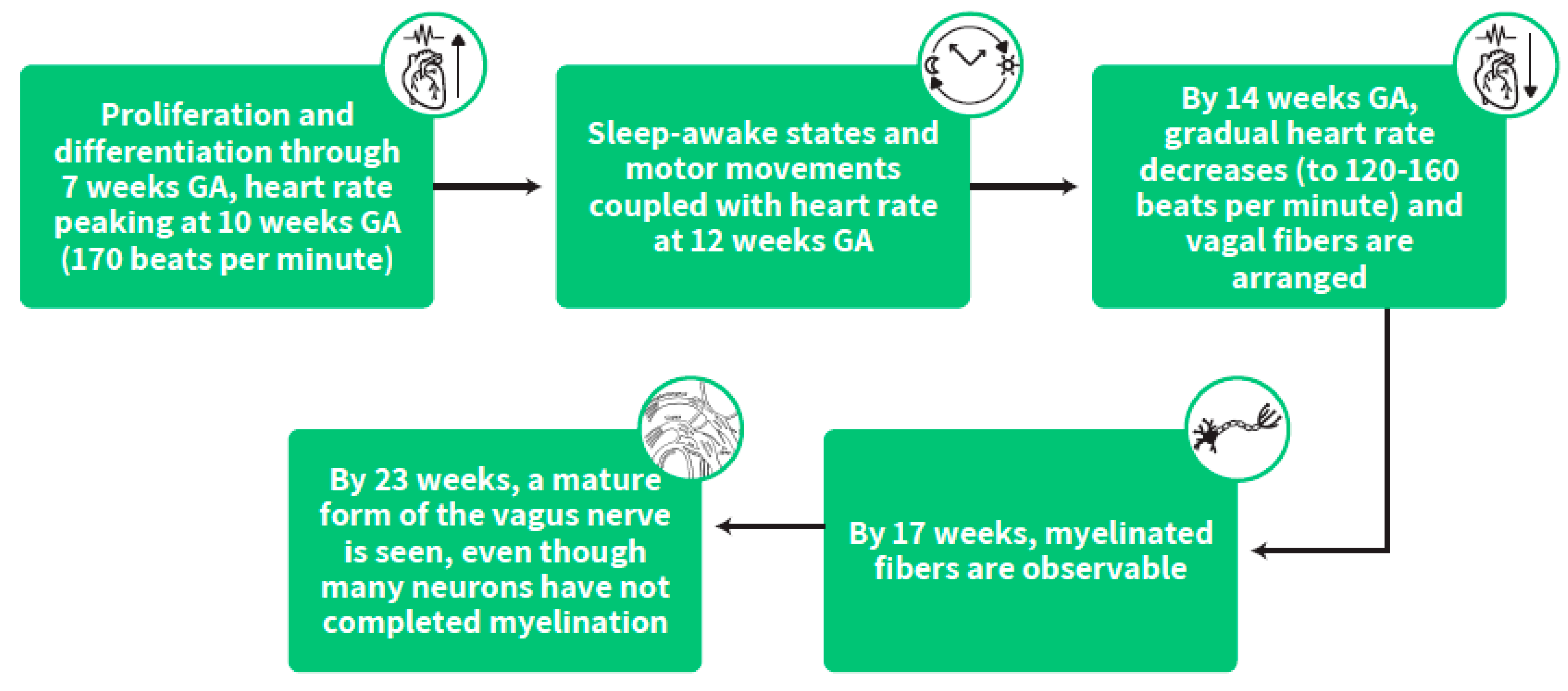
3.1. Early Gestation
3.2. Mid- to Late-Gestation
3.3. Birth and Postnatal Period
4. Brainstem Regulation of Cortical Development
5. The Self-Regulation Clock
5.1. Location of the Self-Regulation Clock
5.2. Rhythms of the Clock
6. For Further Research
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- de Zambotti, M.; Trinder, J.; Silvani, A.; Colrain, I.M.; Baker, F.C. Dynamic coupling between the central and autonomic nervous systems during sleep: A review. Neurosci. Biobehav. Rev. 2018, 90, 84–103. [Google Scholar] [CrossRef] [PubMed]
- Kostovic´(, I.; Kostovic´(, K.; Hr), I.; Judaš, M. The development of the subplate and thalamocortical connections in the human foetal brain. Acta Pædiatrica 2010, 99, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Lagercrantz, H. Infant Brain Development: Formation of the Mind and the Emergence of Consciousness; Springer International Publishing: Cham, Switzerland, 2016; ISBN 9783319448459. [Google Scholar]
- Hüppi, P.S.; Schuknecht, B.; Boesch, C.; Bossi, E.; Felblinger, J.; Fusch, C.; Herschkowitz, N. Structural and neurobehavioral delay in postnatal brain development of preterm infants. Pediatr. Res. 1996, 39, 895–901. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Als, H.; Duffy, F.H.; McAnulty, G.B. Behavioral differences between preterm and full-term newborns as measured with the APIB system scores: I. Infant Behav. Dev. 1988, 11, 305–318. [Google Scholar] [CrossRef]
- Mouradian, L.E.; Als, H.; Coster, W.J. Neurobehavioral functioning of healthy preterm infants of varying gestational ages. J. Dev. Behav. Pediatr. 2000, 21, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.; Bilms, J.; Suveg, C. In sync and in control: A meta-analysis of parent-child positive behavioral synchrony and youth self-regulation. Fam. Process 2017, 56, 962–980. [Google Scholar] [CrossRef]
- Als, H. Reading the premature infant. In Developmental Interventions in the Neonatal Intensive Care Nursery; Goldson, E., Ed.; Oxford University Press: New York, NY, USA, 1999. [Google Scholar]
- Als, H.; Duffy, F.U.; McAnulty, G.B. The APIB, an assessment of functional competence in preterm and full-term newborns regardless of gestational age at birth: II. Infant Behav. Dev. 1988, 11, 319–331. [Google Scholar] [CrossRef]
- Mouradian, L.E.; Als, H. The influence of neonatal intensive care unit caregiving practices on motor functioning of preterm infants. Am. J. Occup. Ther. Off. Publ. Am. Occup. Ther. Assoc. 1994, 48, 527–533. [Google Scholar] [CrossRef][Green Version]
- Als, H.; Butler, S.; Kosta, S.; McAnulty, G. The Assessment of Preterm Infants’ Behavior (APIB): Furthering the understanding and measurement of neurodevelopmental competence in preterm and full-term infants. Ment. Retard. Dev. Disabil. Res. Rev. 2005, 11, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Raffaelli, M.; Crockett, L.J.; Shen, Y.L. Developmental stability and change in self-regulation from childhood to adolescence. J. Genet. Psychol. 2005, 166, 54–76. [Google Scholar] [CrossRef]
- Kopp, C.B. Emotion-focused coping in young children: Self and self-regulatory processes. New Dir. Child Adolesc. Dev. 2009, 2009, 33–46. [Google Scholar] [CrossRef]
- Swingler, M.M.; Perry, N.B.; Calkins, S.D. Neural plasticity and the development of attention: Intrinsic and extrinsic influences. Dev. Psychopathol. 2015, 27, 443–457. [Google Scholar] [CrossRef]
- Taylor, H.G.; Clark, C.A.C. Executive function in children born preterm: Risk factors and implications for outcome. Semin. Perinatol. 2016, 40, 520–529. [Google Scholar] [CrossRef]
- Ferber, S.G.S.; Laudon, M.; Kuint, J.; Weller, A.; Zisapel, N. Massage therapy by mothers enhances the adjustment of circadian rhythms to the nocturnal period in full-term infants. Dev. Behav. Pediatr. 2002, 23, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Ferber, S.G. Biofeedback mechanisms between shapeable endogen structures and contingent social complexes: The nature of determination for developmental paths. Behav. Brain Sci. 2009, 32, 392–393. [Google Scholar] [CrossRef]
- Ferber, S.G. The concept of coregulation between neurobehavioral subsystems: The logic interplay between excitatory and inhibitory ends. Behav. Brain Sci. 2008, 31, 337–338. [Google Scholar] [CrossRef]
- Ferber, S.G.; Makhoul, I.R. The effect of skin-to-skin contact (Kangaroo Care) ahortly after birth on the neurobehavioral responses of the term newborn: A randomized, controlled trial. Pediatrics 2004, 113, 858–865. [Google Scholar] [CrossRef] [PubMed]
- Als, H. A synactive model of neonatal behavioral organization: Framework for the assessment of neurobehavioral development in the premature infant and for support of infants and parents in the neonatal intensive care environment. Phys. Occup. Ther. Pediatr. 1986, 6, 3–53. [Google Scholar] [CrossRef]
- Als, H. Toward a synactive theory of development: Promise for the assessment and support of infant individuality. Infant Ment. Health J. 1982, 3, 229–243. [Google Scholar] [CrossRef]
- Prager, E.M.; Bergstrom, H.C.; Wynn, G.H.; Braga, M.F.M. The basolateral amygdala γ-aminobutyric acidergic system in health and disease. J. Neurosci. Res. 2016, 94, 548–567. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, M.K.; Tronick, E.Z.; Cohn, J.F.; Olson, K.L. Gender differences in emotional expressivity and self-regulation during early infancy. Dev. Psychol. 1999, 35, 175–188. [Google Scholar] [CrossRef]
- Kim, S.; Kochanska, G. Child temperament moderates effects of parent-child mutuality on self-regulation: A relationship-based path for emotionally negative infants. Child Dev. 2012, 83, 1275–1289. [Google Scholar] [CrossRef]
- Rothbart, M.K.; Sheese, B.E.; Rueda, M.R.; Posner, M.I. Developing mechanisms of self-regulation in early life. Emot. Rev. 2011, 3, 207–213. [Google Scholar] [CrossRef]
- Herlenius, E.; Lagercrantz, H. Development of neurotransmitter systems during critical periods. Exp. Neurol. 2004, 190, 8. [Google Scholar] [CrossRef] [PubMed]
- Ferber, S.G.; Als, H.; McAnulty, G.; Peretz, H.; Zisapel, N. Melatonin and mental capacities in newborn infants. J. Pediatr. 2011, 159, 99–103.e1. [Google Scholar] [CrossRef]
- Ferber, S.G.; Makhoul, I.R. Neurobehavioural assessment of skin-to-skin effects on reaction to pain in preterm infants: A randomized, controlled within-subject trial. Acta Paediatr. Int. J. Paediatr. 2008, 97, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Antsaklis, P.; Gao, Z. Control system design. In The Electronics Engineers’ Handbook, 5th ed.; McGraw-Hill: New York, NY, USA, 2005; pp. 19.1–19.30. [Google Scholar]
- Kinney, H.C.; Panigrahy, A.; Rava, L.A.; White, W.F. Three-dimensional distribution of [3H] quinuclidinyl benzilate binding to muscarinic cholinergic receptors in the developing human brainstem. J. Comp. Neurol. 1995, 362, 350–367. [Google Scholar] [CrossRef]
- Fields, R.D.; Dutta, D.J.; Belgrad, J.; Robnett, M. Cholinergic signaling in myelination. Glia 2017, 65, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Ritter, L.M.; Unis, A.S.; Meador-Woodruff, J.H. Ontogeny of ionotropic glutamate receptor expression in human fetal brain. Dev. Brain Res. 2001, 127, 123–133. [Google Scholar] [CrossRef]
- Brade, T.; Pane, L.S.; Moretti, A.; Chien, K.R.; Laugwitz, K.L. Embryonic Heart Progenitors and Cardiogenesis. Cold Spring Harb. Perspect. Med. 2013, 3, a013847. [Google Scholar] [CrossRef]
- Burton, G.J.; Jauniaux, E. Development of the human placenta and fetal heart: Synergic or independent? Front. Physiol. 2018, 9, 373. [Google Scholar] [CrossRef] [PubMed]
- Wozniak, W.; O’Rahilly, R. Fine structure and myelination of the developing human vagus nerve. Acta Anat. 1981, 109, 218–230. [Google Scholar] [CrossRef]
- Zizzo, A.R.; Kirkegaard, I.; Hansen, J.; Uldbjerg, N.; Mølgaard, H. Fetal Heart Rate Variability Is Affected by Fetal Movements: A Systematic Review. Front. Physiol. 2020, 11, 1177. [Google Scholar] [CrossRef] [PubMed]
- Dalton, K.J.; Dawes, G.S.; Patrick, J.E. The autonomic nervous system and fetal heart rate variability. Am. J. Obstet. Gynecol. 1983, 146, 456–462. [Google Scholar] [CrossRef]
- Sachis, P.N.; Armstrong, D.L.; Becker, L.E.; Bryan, A.C. Myelination of the human vagus nerve from 24 weeks oostconceptional age to adolescence. J. Neuropathol. Exp. Neurol. 1982, 41, 466–472. [Google Scholar] [CrossRef]
- DiPietro, J.A.; Costigan, K.A.; Voegtline, K.M. Studies in Fetal Behavior: Revisited, Renewed, and Reimagined. Monogr. Soc. Res. Child Dev. 2015, 80, vii. [Google Scholar] [CrossRef]
- Longin, E.; Gerstner, T.; Schaible, T.; Lenz, T.; König, S. Maturation of the autonomic nervous system: Differences in heart rate variability in premature vs. term infants. J. Perinat. Med. 2006, 34, 303–308. [Google Scholar] [CrossRef]
- Mulkey, S.B.; Kota, S.; Swisher, C.B.; Hitchings, L.; Metzler, M.; Wang, Y.; Maxwell, G.L.; Baker, R.; du Plessis, A.J.; Govindan, R. Autonomic nervous system depression at term in neurologically normal premature infants. Early Hum. Dev. 2018, 123, 11–16. [Google Scholar] [CrossRef]
- Mao, C.; Lv, J.; Li, H.; Chen, Y.; Wu, J.; Xu, Z. Development of fetal nicotine and muscarinic receptors in utero. Braz. J. Med. Biol. Res. 2007, 40, 735–741. [Google Scholar] [CrossRef]
- Baghdoyan, H.A.; Rodrigo-Angulo, M.L.; McCarley, R.W.; Allan Hobson, J. A neuroanatomical gradient in the pontine tegmentum for the cholinoceptive induction of desynchronized sleep signs. Brain Res. 1987, 414, 245–261. [Google Scholar] [CrossRef]
- Velazquez-Moctezuma, J.; Shiromani, P.J.; Christian Gillin, J. Acetylcholine and acetylcholine receptor subtypes in REM sleep generation. Prog. Brain Res. 1990, 84, 407–413. [Google Scholar] [CrossRef]
- Simchovitz, A.; Heneka, M.T.; Soreq, H. Personalized genetics of the cholinergic blockade of neuroinflammation. J. Neurochem. 2017, 142, 178–187. [Google Scholar] [CrossRef]
- Miyoshi, R.; Kito, S.; Shimizu, M.; Matsubayashi, H. Ontogeny of muscarinic receptors in the rat brain with emphasis on the differentiation of M1- and M2-subtypes- semi-quantitative in vitro autoradiography. Brain Res. 1987, 420, 302–312. [Google Scholar] [CrossRef]
- Owens, D.F.; Kriegstein, A.R. Is there more to GABA than synaptic inhibition? Nat. Rev. Neurosci. 2002, 3, 715–727. [Google Scholar] [CrossRef] [PubMed]
- Owens, D.F.; Kriegstein, A.R. Developmental neurotransmitters? Neuron 2002, 36, 989–991. [Google Scholar] [CrossRef][Green Version]
- Ben-Ari, Y. Excitatory actions of GABA during development: The nature of the nurture. Nat. Rev. Neurosci. 2002, 3, 728–739. [Google Scholar] [CrossRef]
- Northington, F.J.; Traystman, R.J.; Koehler, R.C.; Martin, L.J. GLT1, glial glutamate transporter, is transiently expressed in neurons and develops astrocyte specificity only after midgestation in the ovine fetal brain. J. Neurobiol. 1999, 39, 515–526. [Google Scholar] [CrossRef]
- Liu, Q.; Wong-Riley, M.T.T. Postnatal developmental expressions of neurotransmitters and receptors in various brain stem nuclei of rats. J. Appl. Physiol. 2005, 98, 1442–1457. [Google Scholar] [CrossRef][Green Version]
- Northington, F.J.; Traystman, R.J.; Koehler, R.C.; Rothstein, J.D.; Martin, L.J. Regional and cellular expression of glial (GLT1) and neuronal (EAAC1) glutamate transporter proteins in ovine fetal brain. Neuroscience 1998, 85, 1183–1193. [Google Scholar] [CrossRef]
- Xu, G.; Broadbelt, K.G.; Haynes, R.L.; Folkerth, R.D.; Borenstein, N.S.; Belliveau, R.A.; Trachtenberg, F.L.; Volpe, J.J.; Kinney, H.C. Late development of the GABAergic system in the human cerebral cortex and white matter. J. Neuropathol. Exp. Neurol. 2011, 70, 841–858. [Google Scholar] [CrossRef] [PubMed]
- Volpe, J.J. Dysmaturation of Premature Brain: Importance, Cellular Mechanisms, and Potential Interventions. Pediatr. Neurol. 2019, 95, 42–66. [Google Scholar] [CrossRef]
- Hamrick, S.E.G.; Miller, S.P.; Leonard, C.; Glidden, D.V.; Goldstein, R.; Ramaswamy, V.; Piecuch, R.; Ferriero, D.M. Trends in severe brain injury and neurodevelopmental outcome in premature newborn infants: The role of cystic periventricular leukomalacia. J. Pediatr. 2004, 145, 593–599. [Google Scholar] [CrossRef]
- Back, S.A. White matter injury in the preterm infant: Pathology and mechanisms. Acta Neuropathol. 2017, 134, 331–349. [Google Scholar] [CrossRef]
- Dehorter, N.; Vinay, L.; Hammond, C.; Ben-Ari, Y. Timing of developmental sequences in different brain structures: Physiological and pathological implications. Eur. J. Neurosci. 2012, 35, 1846–1856. [Google Scholar] [CrossRef] [PubMed]
- Hartley, C.I.; Farmer, S.I.; Berthouze, L.I. Temporal ordering of input modulates connectivity formation in a developmental neuronal network model of the cortex. PLoS ONE 2020, 15, e0226772. [Google Scholar] [CrossRef]
- Fischi-Gómez, E.; Vasung, L.; Meskaldji, D.E.; Lazeyras, F.; Borradori-Tolsa, C.; Hagmann, P.; Barisnikov, K.; Thiran, J.-P.; Hüppi, P.S. Structural Brain Connectivity in School-Age Preterm Infants Provides Evidence for Impaired Networks Relevant for Higher Order Cognitive Skills and Social Cognition. Cereb. Cortex 2015, 25, 2793–2805. [Google Scholar] [CrossRef]
- Jaimes, C.; Machado-Rivas, F.; Afacan, O.; Khan, S.; Marami, B.; Ortinau, C.M.; Rollins, C.K.; Velasco-Annis, C.; Warfield, S.K.; Gholipour, A. In vivo characterization of emerging white matter microstructure in the fetal brain in the third trimester. Hum. Brain Mapp. 2020, 41, 3177–3185. [Google Scholar] [CrossRef] [PubMed]
- Tau, G.Z.; Peterson, B.S. Normal Development of Brain Circuits. Neuropsychopharmacology 2010, 35, 147–168. [Google Scholar] [CrossRef]
- De Asis-Cruz, J.; Andersen, N.; Kapse, K.; Khrisnamurthy, D.; Quistorff, J.; Lopez, C.; Vezina, G.; Limperopoulos, C. Global Network Organization of the Fetal Functional Connectome. Cereb. Cortex 2021, 31, 3034–3046. [Google Scholar] [CrossRef]
- Ferradal, S.L.; Gagoski, B.; Jaimes, C.; Yi, F.; Carruthers, C.; Vu, C.; Litt, J.S.; Larsen, R.; Sutton, B.; Grant, P.E.; et al. System-specific patterns of thalamocortical connectivity in early brain development as revealed by structural and functional MRI. Cereb. Cortex 2019, 29, 1218–1229. [Google Scholar] [CrossRef] [PubMed]
- Hooker, J.D.; Khan, M.A.; Farkas, A.B.; Lirette, S.T.; Joyner, D.A.; Gordy, D.P.; Storrs, J.M.; Roda, M.S.; Bofill, J.A.; Smith, A.D.; et al. Third-trimester in utero fetal brain diffusion tensor imaging fiber tractography: A prospective longitudinal characterization of normal white matter tract development. Pediatr. Radiol. 2020, 50, 973–983. [Google Scholar] [CrossRef] [PubMed]
- Nijhuis, I.J.M.; Ten Hof, J. Development of fetal heart rate and behavior: Indirect measures to assess the fetal nervous system. Eur. J. Obstet. Gynecol. Reprod. Biol. 1999, 87, 1–2. [Google Scholar] [CrossRef]
- DiPietro, J.A. Neurobehavioral assessment before birth. Ment. Retard. Dev. Disabil. Res. Rev. 2005, 11, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Mulkey, S.B.; Hitchings, L.; Persaud, R.; Kota, S.; Maxwell, G.L.; Baker, R.; du Plessis, A.; Govindan, R. Cerebral cortical autonomic connectivity in low-risk term newborns. Clin. Auton. Res. 2021, 31, 415–424. [Google Scholar] [CrossRef]
- Shuffrey, L.C.; Myers, M.M.; Odendaal, H.J.; Elliott, A.J.; du Plessis, C.; Groenewald, C.; Burd, L.; Angal, J.; Nugent, J.D.; Isler, J.R.; et al. Fetal heart rate, heart rate variability, and heart rate/movement coupling in the Safe Passage Study. J. Perinatol. 2019, 39, 608–618. [Google Scholar] [CrossRef]
- DiPietro, J.A.; Hodgson, D.M.; Costigan, K.A.; Hilton, S.C.; Johnson, T.R.B. Development of fetal movement—fetal heart rate coupling from 20 weeks through term. Early Hum. Dev. 1996, 44, 139–151. [Google Scholar] [CrossRef]
- Brändle, J.; Preissl, H.; Draganova, R.; Ortiz, E.; Kagan, K.O.; Abele, H.; Brucker, S.Y.; Kiefer-Schmidt, I. Heart rate variability parameters and fetal movement complement fetal behavioral states detection via magnetography to monitor neurovegetative development. Front. Hum. Neurosci. 2015, 9, 147. [Google Scholar] [CrossRef]
- Pereyra, P.M.; Zhang, W.; Schmidt, M.; Becker, L.E. Development of myelinated and unmyelinated fibers of human vagus nerve during the first year of life. J. Neurol. Sci. 1992, 110, 107–113. [Google Scholar] [CrossRef]
- Doussard-Roosevelt, J.; McClenny, B.; Porges, S. Neonatal cardiac vagal tone and school-age developmental outcome in very low birth weight infants-PubMed. Dev. Psychobiol. 2001, 38, 56–66. [Google Scholar] [CrossRef]
- Myers, M.M.; Fifer, W.P.; Grose-Fifer, J.J.; Sahni, R.; Stark, R.I.; Schulze, K.F. A novel quantitative measure of Tracé-Alternant EEG activity and its association with sleep states of preterm infants. Dev. Psychobiol. 1997, 31, 167–174. [Google Scholar] [CrossRef]
- Hrachovy, R.A.; Mizrahi, E.M. Atlas of Neonatal Electroencephalography; Springer Publishing Company: Cham, Switzerland, 2015. [Google Scholar]
- Sawaguchi, H.; Ogawa, T.; Takano, T.; Sato, K. Developmental changes in electroencephalogram for term and preterm infants using an autoregressive model. Acta Paediatr. Jpn. Overseas Ed. 1996, 38, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Pavlidis, E.; Lloyd, R.O.; Mathieson, S.; Boylan, G.B. A review of important electroencephalogram features for the assessment of brain maturation in premature infants. Acta Paediatr. Int. J. Paediatr. 2017, 106, 1394–1408. [Google Scholar] [CrossRef]
- Witte, H.; Putsche, P.; Schwab, K.; Eiselt, M.; Helbig, M.; Suesse, T. On the spatio-temporal organisation of quadratic phase-couplings in “tracé alternant” EEG pattern in full-term newborns. Clin. Neurophysiol. 2004, 115, 2308–2315. [Google Scholar] [CrossRef] [PubMed]
- Riekkinen, P.; Buzsaki, G.; Riekkinen, P.; Soininen, H.; Partanen, J. The cholinergic system and EEG slow waves. Electroencephalogr. Clin. Neurophysiol. 1991, 78, 89–96. [Google Scholar] [CrossRef]
- Brown, R.E.; Basheer, R.; McKenna, J.T.; Strecker, R.E.; McCarley, R.W. Control of sleep and wakefulness. Physiol. Rev. 2012, 92, 1087–1187. [Google Scholar] [CrossRef]
- Japaridze, N.; Muthuraman, M.; Reinicke, C.; Moeller, F.; Anwar, A.R.; Mideksa, K.G.; Pressler, R.; Deuschl, G.; Stephani, U.; Siniatchkin, M. Neuronal networks during burst suppression as revealed by source analysis. PLoS ONE 2015, 10, e0123807. [Google Scholar] [CrossRef]
- Miles, R. Neurobiology: A homeostatic switch. Nature 1999, 397, 215–216. [Google Scholar] [CrossRef]
- Rivera, C.; Voipio, J.; Payne, J.A.; Ruusuvuori, E.; Lahtinen, H.; Lamsa, K.; Pirvola, U.; Saarma, M.; Kaila, K. The K+/Cl- co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature 1999, 397, 251–255. [Google Scholar] [CrossRef]
- Weinstein, M.; Marom, R.; Berger, I.; Ben Bashat, D.; Gross-Tsur, V.; Ben-Sira, L.; Artzi, M.; Uliel, S.; Leitner, Y.; Geva, R. Neonatal neuropsychology: Emerging relations of neonatal sensory-motor responses to white matter integrity. Neuropsychologia 2014, 62, 209–219. [Google Scholar] [CrossRef]
- Darwin, C. On the Origin of Species; PF Collier & Son: New York, NY, USA, 1909. [Google Scholar]
- Silvani, A.; Calandra-Buonaura, G.; Dampney, R.A.L.; Cortelli, P. Brain-heart interactions: Physiology and clinical implications. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2016, 374, 20150181. [Google Scholar] [CrossRef]
- Scammell, T.E.; Arrigoni, E.; Lipton, J.O. Neural circuitry of wakefulness and sleep. Neuron 2017, 93, 747–765. [Google Scholar] [CrossRef] [PubMed]
- Karin, J.; Hirsch, M.; Akselrod, S. An estimate of fetal autonomic state by spectral analysis of fetal heart rate fluctuations. Pediatr. Res. 1993, 34, 134–138. [Google Scholar] [CrossRef]
- Porges, S.W.; Furman, S.A. The early development of the autonomic nervous system provides a neural platform for social behaviour: A polyvagal perspective. Infant Child Dev. 2011, 20, 106–118. [Google Scholar] [CrossRef]
- Ernst, G. Heart-Rate Variability—More than Heart Beats? Front. Public Health 2017, 5, 1. [Google Scholar] [CrossRef]
- Shaffer, F.; McCraty, R.; Zerr, C.L. A healthy heart is not a metronome: An integrative review of the heart’s anatomy and heart rate variability. Front. Psychol. 2014, 5, 1040. [Google Scholar] [CrossRef] [PubMed]
- Porges, S.W. The polyvagal perspective. Biol. Psychol. 2007, 74, 116–143. [Google Scholar] [CrossRef] [PubMed]
- Steriade, M.; McCarley, R.W.; Steriade, M.; McCarley, R.W. REM Sleep as a Biological Rhythm. In Brainstem Control of Wakefulness and Sleep; Springer: New York, NY, USA, 1990; pp. 363–393. [Google Scholar]
- Santarelli, S.; Lesuis, S.L.; Wang, X.D.; Wagner, K.V.; Hartmann, J.; Labermaier, C.; Scharf, S.H.; Müller, M.B.; Holsboer, F.; Schmidt, M.V. Evidence supporting the match/mismatch hypothesis of psychiatric disorders. Eur. Neuropsychopharmacol. 2014, 24, 907–918. [Google Scholar] [CrossRef]
- Conradt, E.; Adkins, D.E.; Crowell, S.E.; Raby, K.L.; Diamond, L.M.; Ellis, B. Incorporating epigenetic mechanisms to advance fetal programming theories. Dev. Psychopathol. 2018, 30, 807–824. [Google Scholar] [CrossRef]
- McAnulty, G.; Duffy, F.H.; Kosta, S.; Weisenfeld, N.I.; Warfield, S.K.; Butler, S.C.; Alidoost, M.; Bernstein, J.H.; Robertson, R.; Zurakowski, D.; et al. School-age effects of the newborn individualized developmental care and assessment program for preterm infants with intrauterine growth restriction: Preliminary findings. BMC Pediatr. 2013, 13, 1–21. [Google Scholar] [CrossRef]
- McAnulty, G.; Duffy, F.; Kosta, S.; Butler, S.; Bernstein, J.; Als, H.; Weisenfeld, N.; Warfield, S.; Zurakowski, D. School age effects of the Newborn Individualized Developmental Care and Assessment Program for medically low-risk preterm infants: Preliminary findings. J. Clin. Neonatol. 2012, 1, 184. [Google Scholar] [CrossRef]
- Ferreira, R.d.C.; Alves, C.R.L.; Guimarães, M.A.P.; Menezes, K.K.P.d.; Magalhães, L.d.C. Effects of early family-centered interventions on the development of children born preterm and/or at social risk: A meta-analysis. J. Pediatr. (Rio. J). 2019. [Google Scholar] [CrossRef]
- Lester, B.M.; Hawes, K.; Abar, B.; Sullivan, M.; Miller, R.; Bigsby, R.; Laptook, A.; Salisbury, A.; Taub, M.; Lagasse, L.L.; et al. Single-family room care and neurobehavioral and medical outcomes in preterm infants. Pediatrics 2014, 134, 754–760. [Google Scholar] [CrossRef]
- Anderson, D.E.; Patel, A.D. Infants born preterm, stress, and neurodevelopment in the neonatal intensive care unit: Might music have an impact? Dev. Med. Child Neurol. 2018, 60, 256–266. [Google Scholar] [CrossRef]
- Bastani, F.; Rajai, N.; Farsi, Z.; Als, H. The effects of kangaroo care on the sleep and wake states of preterm infants. J. Nurs. Res. 2017, 25, 231–239. [Google Scholar] [CrossRef]
- Butruille, L.; Blouin, A.; De Jonckheere, J.; Mur, S.; Margez, T.; Rakza, T.; Storme, L. Impact of skin-to-skin contact on the autonomic nervous system in the preterm infant and his mother. Infant Behav. Dev. 2017, 49, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Moody, C.; Callahan, T.J.; Aldrich, H.; Gance-Cleveland, B.; Sables-Baus, S. Early Initiation of Newborn Individualized Developmental Care and Assessment Program (NIDCAP) Reduces Length of Stay: A Quality Improvement Project. J. Pediatr. Nurs. 2017, 32, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Westrup, B. Newborn Individualized Developmental Care and Assessment Program (NIDCAP)—Family-centered developmentally supportive care. Early Hum. Dev. 2007, 83, 443–449. [Google Scholar] [CrossRef]
- Als, H.; Duffy, F.H.; McAnulty, G.B.; Rivkin, M.J.; Vajapeyam, S.; Mulkern, R.V.; Warfield, S.K.; Huppi, P.S.; Butler, S.C.; Conneman, N.; et al. Early experience alters brain function and structure. Pediatrics 2004, 113, 846–857. [Google Scholar] [CrossRef]
- Eyre, J.A. Corticospinal tract development and its plasticity after perinatal injury. Neurosci. Biobehav. Rev. 2007, 31, 1136–1149. [Google Scholar] [CrossRef] [PubMed]
- Braga, R.M.; Roze, E.; Ball, G.; Merchant, N.; Tusor, N.; Arichi, T.; Edwards, D.; Rueckert, D.; Counsell, S.J. Development of the corticospinal and callosal tracts from extremely premature birth up to 2 years of age. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.H. The corticospinal system: From development to motor control. Neuroscientist 2005, 11, 161–173. [Google Scholar] [CrossRef]
- Eyre, J.; Miller, S.; Clowry, G.; Conway, E.; Watts, C. Functional corticospinal projections are established prenatally in the human foetus permitting involvement in the development of spinal motor centres. Brain 2000, 123, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Armand, J. The Origin, Course and Terminations of Corticospinal Fibers in Various Mammals. Prog. Brain Res. 1982, 57, 329–360. [Google Scholar] [CrossRef]
- Welniarz, Q.; Dusart, I.; Roze, E. The corticospinal tract: Evolution, development, and human disorders. Dev. Neurobiol. 2017, 77, 810–829. [Google Scholar] [CrossRef] [PubMed]
- Eyre, J.A. Development and plasticity of the corticospinal system in man. Neural Plast. 2003, 10, 93–106. [Google Scholar] [CrossRef]
- Mürner-Lavanchy, I.M.; Kelly, C.E.; Reidy, N.; Doyle, L.W.; Lee, K.J.; Inder, T.; Thompson, D.K.; Morgan, A.T.; Anderson, P.J. White matter microstructure is associated with language in children born very preterm. NeuroImage Clin. 2018, 20, 808–822. [Google Scholar] [CrossRef]
- Travis, K.E.; Ben-Shachar, M.; Myall, N.J.; Feldman, H.M. Variations in the neurobiology of reading in children and adolescents born full term and preterm. NeuroImage Clin. 2016, 11, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Niogi, S.N.; McCandliss, B.D. Left lateralized white matter microstructure accounts for individual differences in reading ability and disability. Neuropsychologia 2006, 44, 2178–2188. [Google Scholar] [CrossRef]
- Lu, Q.; Kim, J.Y. Mammalian circadian networks mediated by the suprachiasmatic nucleus. FEBS J. 2021. [Google Scholar] [CrossRef]
- Honma, S. Development of the mammalian circadian clock. Eur. J. Neurosci. 2020, 51, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Carmona-Alcocer, V.; Abel, J.H.; Sun, T.C.; Petzold, L.R.; Doyle, F.J.; Simms, C.L.; Herzog, E.D. Ontogeny of Circadian Rhythms and Synchrony in the Suprachiasmatic Nucleus. J. Neurosci. 2018, 38, 1326–1334. [Google Scholar] [CrossRef]
- Fatima, G.; Sharma, V.P.; Shukla, R. Circadian rhythm in patients with spinal cord injuries. Ann. Neurosci. 2010, 16, 31–33. [Google Scholar] [CrossRef]
- Lee, S.-J.; Kim, J.-S.; Song, I.-U.; An, J.-Y.; Kim, Y.-I.; Lee, K.-S. Poststroke restless legs syndrome and lesion location: Anatomical considerations. Mov. Disord. 2009, 24, 77–84. [Google Scholar] [CrossRef]
- Kowalski, J.L.; Nemanich, S.T.; Nawshin, T.; Chen, M.; Peyton, C.; Zorn, E.; Hickey, M.; Rao, R.; Georgieff, M.; Rudser, K.; et al. Motor evoked potentials as potential biomarkers of early atypical corticospinal tract development in infants with perinatal stroke. J. Clin. Med. 2019, 8, 1208. [Google Scholar] [CrossRef] [PubMed]
- Dowman, R.; Wolpaw, J.R. Diurnal rhythms in primate spinal reflexes and accompanying cortical somatosensory evoked potentials. Electroencephalogr. Clin. Neurophysiol. 1989, 72, 69–80. [Google Scholar] [CrossRef]
- Chen, X.Y.; Chen, L.; Wolpaw, J.R.; Jakeman, L.B. Corticospinal tract transection reduces H-reflex circadian rhythm in rats. Brain Res. 2002, 942, 101–108. [Google Scholar] [CrossRef]
- Babik, I.; Galloway, J.C.; Lobo, M.A. infants born preterm demonstrate impaired exploration of their bodies and surfaces throughout the first 2 years of life. Phys. Ther. 2017, 97, 915–925. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, B.D.; Kumar, A.; Deepshikha; Prakash, U. Effect of prematurity and intrauterine growth restriction on H-reflex recovery cycle in neonates. Neurosci. Lett. 2011, 488, 107–111. [Google Scholar] [CrossRef]
- Marschik, P.B.; Prechtl, H.F.R.; Prayer, D.; Peyton, C.; Einspieler, C. An antecedent of later developing communicative functions: The fetal index finger. BMJ 2013, 347, f7232. [Google Scholar] [CrossRef]
- Morley, P.; Hogan, M.J.; Hakim, A.M. Calcium-mediated mechanisms of ischemic injury and protection. Brain Pathol. 1994, 4, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Jänig, W. Sympathetic nervous system and inflammation: A conceptual view. Auton. Neurosci. 2014, 182, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.R.; Leite, P.E.C. The Involvement of Parasympathetic and Sympathetic Nerve in the Inflammatory Reflex. J. Cell. Physiol. 2016, 231, 1862–1869. [Google Scholar] [CrossRef] [PubMed]
- Goldstein Ferber, S.; Als, H.; McAnulty, G.; Klinger, G.; Weller, A. Multi-level hypothalamic neuromodulation of self-regulation and cognition in preterm infants: Towards a control systems model. Compr. Psychoneuroendocrinology, 2022, in press.

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goldstein Ferber, S.; Weller, A.; Ben-Shachar, M.; Klinger, G.; Geva, R. Development of the Ontogenetic Self-Regulation Clock. Int. J. Mol. Sci. 2022, 23, 993. https://doi.org/10.3390/ijms23020993
Goldstein Ferber S, Weller A, Ben-Shachar M, Klinger G, Geva R. Development of the Ontogenetic Self-Regulation Clock. International Journal of Molecular Sciences. 2022; 23(2):993. https://doi.org/10.3390/ijms23020993
Chicago/Turabian StyleGoldstein Ferber, Sari, Aron Weller, Michal Ben-Shachar, Gil Klinger, and Ronny Geva. 2022. "Development of the Ontogenetic Self-Regulation Clock" International Journal of Molecular Sciences 23, no. 2: 993. https://doi.org/10.3390/ijms23020993
APA StyleGoldstein Ferber, S., Weller, A., Ben-Shachar, M., Klinger, G., & Geva, R. (2022). Development of the Ontogenetic Self-Regulation Clock. International Journal of Molecular Sciences, 23(2), 993. https://doi.org/10.3390/ijms23020993






