Obesity and Male Reproduction: Do Sirtuins Play a Role?
Abstract
:1. Introduction
1.1. Obesity: A Global Health Problem
1.2. Male Infertility: A Problem of Epidemic Dimensions
1.3. The Relationship between Obesity and Male Reproduction
2. Sirtuins, Obesity and Male Fertility: Is There a Relationship?
2.1. What Are Sirtuins?
2.2. Sirtuins and Obesity
2.2.1. In Vitro Studies
In Vitro Studies: SIRTs and Lipid Metabolism
In Vitro Studies: SIRTs and Adipogenesis
In Vitro Studies: SIRTs and Glucose Metabolism
2.2.2. In Vivo Studies
2.3. Sirtuins and the Male Reproductive System
2.3.1. SIRT1
2.3.2. SIRT2
2.3.3. SIRT3
2.3.4. SIRT4
2.3.5. SIRT5
2.3.6. SIRT6
2.3.7. SIRT7
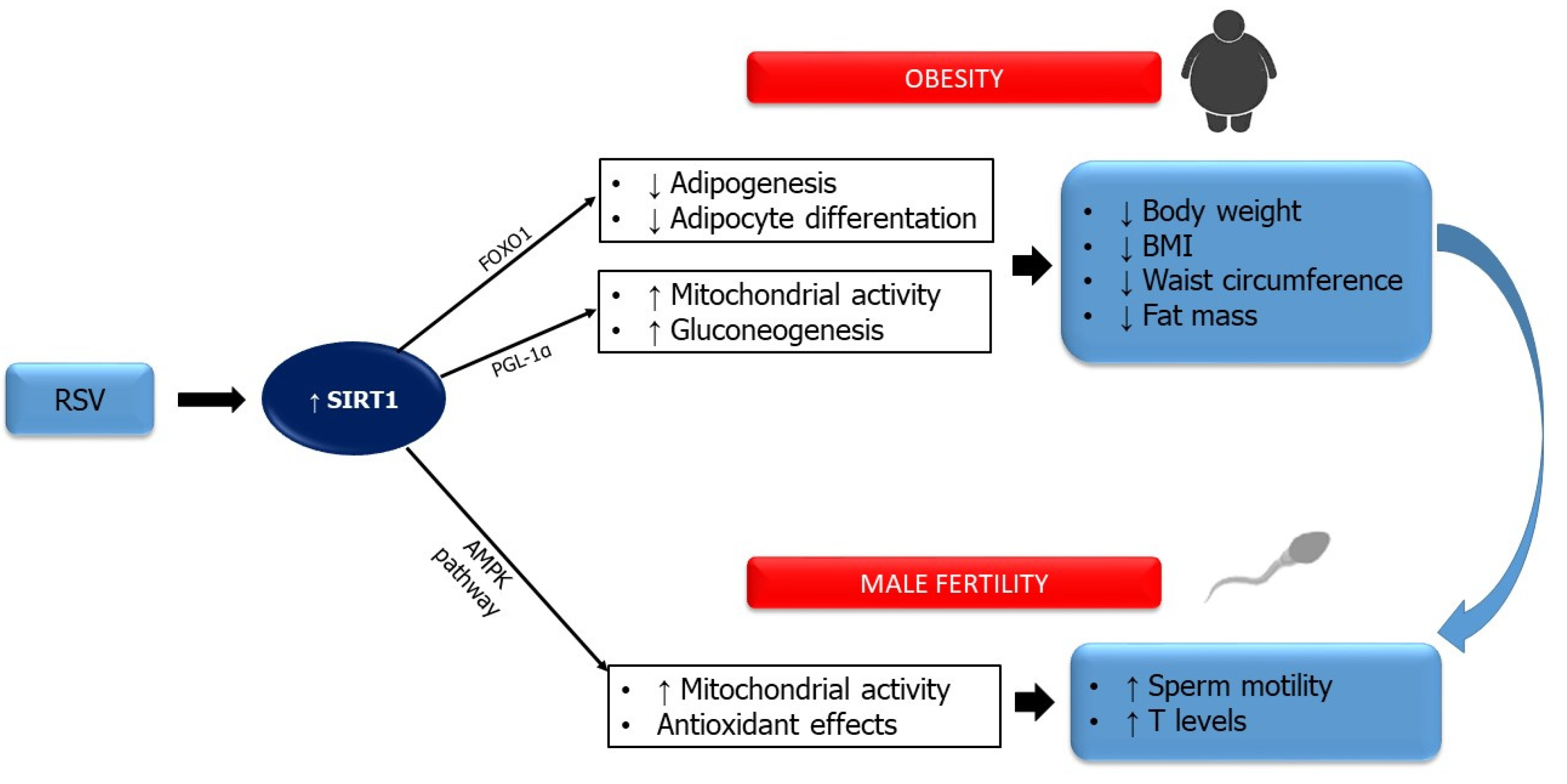
3. Sirtuins: Possible Molecular Targets for the Treatment of Obesity-Induced Male Infertility
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. Obesity and Overweight. FactSheet N°311 January 2015. Available online: http://www.who.int/mediacentre/factsheets/fs311/en/ (accessed on 30 November 2021).
- De Lorenzo, A.; Gratteri, S.; Gualtieri, P.; Cammarano, A.; Bertucci, P.; Di Renzo, L. Why primary obesity is a disease? J. Transl. Med. 2019, 17, 169. [Google Scholar] [CrossRef] [Green Version]
- Jastrboff, A.M.; Kotz, C.M.; Kahan, S.; Kelly, A.S.; Heymsfield, S.B. Obesity as a disease: The Obesity Society 2018 Position Statement. Obesity 2019, 27, 7–9. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Report of the Meeting on the Prevention of Infertility at the Primary Health Care Levels; WHO: Geneva, Switzerland, 1983. [Google Scholar]
- Agarwal, A.; Mulgund, A.; Hamada, A.; Chyatte, M.R. A unique view on male infertility around the globe. Reprod Biol. Endocrinol. 2015, 13, 37–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valenti, D.; La Vignera, S.; Condorelli, R.A.; Rago, R.; Barone, N.; Vicari, E.; Calogero, A.E. Follicle-stimulating hormone treatment in normogonadotropic infertile men. Nat. Rev. Urol. 2013, 10, 55–62. [Google Scholar] [CrossRef]
- Davidson, L.M.; Millar, K.; Jones, C.; Fatum, M.; Coward, K. Deleterious effects of obesity upon the hormonal and molecular mechanisms controlling spermatogenesis and male fertility. Hum. Fertil. 2015, 18, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Cannarella, R.; Condorelli, R.A.; Gusmano, C.; Barone, N.; Burrello, N.; Aversa, A.; Calogero, A.E.; LaVignera, S. Temporal Trend of Conventional Sperm Parameters in a Sicilian Population in the Decade 2011–2020. J. Clin. Med. 2021, 10, 993. [Google Scholar] [CrossRef]
- Condorelli, R.A.; La Vignera, S.; Giacone, F.; Iacoviello, L.; Vicari, E.; Mongioi’, L.; Calogero, A.E. In vitro effects of nicotine on sperm motility and bio-functional flow cytometry sperm parameters. Int. J. Immunopathol. Pharmacol. 2013, 26, 739–746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbagallo, F.; Condorelli, R.A.; Mongioì, L.M.; Cannarella, R.; Cimino, L.; Magagnini, M.C.; Crafa, A.; La Vignera, S.; Calogero, A.E. Molecular Mechanisms Underlying the Relationship between Obesity and Male Infertility. Metabolites. 2021, 11, 840. [Google Scholar] [CrossRef]
- Barbagallo, F.; Condorelli, R.A.; Mongioì, L.M.; Cannarella, R.; Aversa, A.; Calogero, A.E.; La Vignera, S. Effects of Bisphenols on TesticularSteroidogenesis. Front Endocrinol. 2020, 11, 373. [Google Scholar] [CrossRef]
- Ješeta, M.; Navrátilová, J.; Franzová, K.; Fialková, S.; Kempisty, B.; Ventruba, P.; Žáková, J.; Crha, I. Overview of the Mechanisms of Action of Selected Bisphenols and Perfluoroalkyl Chemicals on the Male Reproductive Axes. Front Genet. 2021, 12, 692897. [Google Scholar] [CrossRef]
- López-Botella, A.; Velasco, I.; Acién, M.; Sáez-Espinosa, P.; Todolí-Torró, J.L.; Sánchez-Romero, R.; Gómez-Torres, M.J. Impact of Heavy Metals on Human Male Fertility-An Overview. Antioxidants 2021, 10, 1473. [Google Scholar] [CrossRef]
- Calogero, A.E.; Fiore, M.; Giacone, F.; Altomare, M.; Asero, P.; Ledda, C.; Romeo, G.; Mongioì, L.M.; Copat, C.; Giuffrida, M.; et al. Exposure to multiple metals/metalloids and human semen quality: A cross-sectional study. Ecotoxicol. Env. Saf. 2021, 215, 112165. [Google Scholar] [CrossRef]
- Leisegang, K.; Dutta, S. Do lifestyle practices impede male fertility? Andrologia. 2021, 53, e13595. [Google Scholar] [CrossRef] [PubMed]
- Chambers, T.J.; Richard, R.A. Theimpactofobesityonmalefertility. Hormones 2015, 14, 563–568. [Google Scholar] [PubMed]
- Campbell, J.M.; Lane, M.; Owens, J.A.; Bakos, H.W. Paternal obesity negatively affects male fertility and assisted reproduction outcomes:A systematic review and meta-analysis. Reprod. Biomed. Online 2015, 31, 593–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mushtaq, R.; Pundir, J.; Achilli, C.; Naji, O.; Khalaf, Y.; ElToukhy, T. Effect of male body mass index on assisted reproduction treatment outcome:An updated systematic review and meta-analysis. Reprod. Biomed. Online 2018, 36, 459–471. [Google Scholar] [CrossRef] [Green Version]
- Jensen, T.K.; Andersson, A.M.; Jorgensen, N.; Andersen, A.G.; Carlsen, E.; Petersen, J.H.; Skakkebaek, N.E. Body mass index in relation to semen quality and reproductive hormones among 1558 danish men. Fertil. Steril. 2004, 82, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Hammoud, A.O.; Wilde, N.; Gibson, M.; Parks, A.; Carrell, D.T.; Meikle, A.W. Male obesity and alteration in sperm parameters. Fertil. Steril. 2008, 90, 2222–2225. [Google Scholar] [CrossRef]
- Martini, A.C.; Tissera, A.; Estofán, D.; Molina, R.I.; Mangeaud, A.; de Cuneo, M.F.; Ruiz, R.D. Overweight and seminal quality: A study of 794 patients. Fertil. Steril. 2010, 94, 1739–1743. [Google Scholar] [CrossRef]
- LaVignera, S.; Condorelli, R.A.; Vicari, E.; Calogero, A.E. Negative effect of increased body weight on sperm conventional and nonconventional flow cytometric sperm parameters. J. Androl. 2012, 33, 53–58. [Google Scholar] [CrossRef]
- Sermodade, N.; Faure, C.; Fezeu, L.; Shayeb, A.G.; Bonde, J.P.; Jensen, T.K.; VanWely, M.; Cao, J.; Martini, A.C.; Eskandar, M.; et al. BMI in relation to sperm count:An updated systematic review and collaborative meta-analysis. Hum. Reprod. Update 2013, 19, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Kort, H.I.; Massey, J.B.; Elsner, C.W.; Mitchell-Leef, D.; Shapiro, D.B.; Witt, M.A.; Roudebush, W.E. Impact of body mass index values on sperm quantity and quality. J. Androl. 2006, 27, 450–452. [Google Scholar] [CrossRef] [PubMed]
- Chavarro, J.E.; Toth, T.L.; Wright, D.L.; Meeker, J.; Hauser, R. Body mass index in relation to semen quality, sperm DNA integrity, and serum reproductive hormone levels among men attending an infertility clinic. Fertil. Steril. 2010, 93, 2222–2231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramlau-Hansen, C.H.; Hansen, M.; Jensen, C.R.; Olsen, J.; Bonde, J.P.; Thulstrup, A.M. Semen quality and reproductive hormones according to birthweight and body mass index in childhood and adult life: Two decades of follow-up. Fertil. Steril. 2010, 94, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Kerr, J.B.; Millar, M.; Maddocks, S.; Sharpe, R.M. Stage-dependent changes in spermatogenesis and Sertoli cells in relation to the onset of spermatogenic failure following withdrawal of testosterone. Anat. Rec. 1993, 235, 547–559. [Google Scholar] [CrossRef]
- Soubry, A.; Guo, L.; Huang, Z.; Hoyo, C.; Romanus, S.; Price, T.; Murphy, S.K. Obesity-related DNA methylation at imprinted genes in human sperm: Results from the TIEGER study. Clin. Epigenet. 2016, 8, 51. [Google Scholar] [CrossRef] [Green Version]
- Hu, Q.; Lu, Y.; Hu, F.; He, S.; Xu, X.; Niu, Y.; Zhang, H.; Li, X.; Su, Q. Resistant dextrin reduces obesity and attenuates adipose tissue inflammation in high-fat diet-fed mice. Int. J. Med. Sci. 2020, 17, 2611–2621. [Google Scholar] [CrossRef]
- Tremellen, K. Oxidative stress and male infertility—A clinical perspective. Hum. Reprod. Update 2008, 14, 243–258. [Google Scholar] [CrossRef]
- La Vignera, S.; Cannarella, R.; Galvano, F.; Grillo, A.; Aversa, A.; Cimino, L.; Magagnini, C.M.; Mongioì, L.M.; Condorelli, R.A.; Calogero, A.E. The ketogenic diet corrects metabolic hypogonadism and preserves pancreatic ß-cell function in overweight/obese men: A single-arm uncontrolled study. Endocrine 2021, 72, 392–399. [Google Scholar] [CrossRef]
- Shoba, B.; Lwin, Z.M.; Ling, L.S.; Bay, B.-H.; Yip, G.W.; Kumar, S.D. Function of Sirtuins in Biological Tissues. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2009, 292, 536–543. [Google Scholar] [CrossRef]
- Wang, Y.; He, J.; Liao, M.; Hu, M.; Li, W.; Ouyang, H.; Wang, X.; Ye, T.; Zhang, Y.; Ouyang, L. An overview of Sirtuins as potential therapeutic target: Structure, function and modulators. Eur. J. Med. Chem. 2018, 161, 48–77. [Google Scholar] [CrossRef]
- Loganathan, C.; Kannan, A.; Panneerselvam, A.; Mariajoseph-Antony, L.F.; Kumar, S.A.; Anbarasu, K.; Prahalathan, C. The possible role of sirtuins in male reproduction. Mol. Cell. Biochem. 2021, 476, 2857–2867. [Google Scholar] [CrossRef]
- Kurylowicz, A. In Search of New Therapeutic Targets in Obesity Treatment: Sirtuins. Int. J. Mol. Sci. 2016, 17, 572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kupis, W.; Pałyga, J.; Tomal, E.; Niewiadomska, E. The role of sirtuins in cellular homeostasis. J. Physiol. Biochem. 2016, 72, 371–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tatone, C.; Di Emidio, G.; Barbonetti, A.; Carta, G.; Luciano, A.M.; Falone, S.; Amicarelli, F. Sirtuins in gamete biology and reproductive physiology: Emerging roles and therapeutic potential in female and male infertility. Hum. Reprod. Updat. 2018, 24, 267–289. [Google Scholar] [CrossRef] [PubMed]
- Guarente, L. Sirtuins, aging, and metabolism. In Cold Spring Harbor Symposia on Quantitative Biology; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2011; Volume 76, pp. 81–90. [Google Scholar]
- Finkel, T.; Deng, C.-X.; Mostoslavsky, R. Recent progress in the biology and physiology of sirtuins. Nature 2009, 460, 587–591. [Google Scholar] [CrossRef] [Green Version]
- Nijhawan, P.; Behl, T. Role of sirtuins in obesity. Obes. Med. 2019, 17, 100156. [Google Scholar] [CrossRef]
- Ren, Y.; Du, C.; Shi, Y.; Wei, J.; Wu, H.; Cui, H. The Sirt1 activator, SRT1720, attenuates renal fibrosis by inhibiting CTGF and oxidative stress. Int. J. Mol. Med. 2017, 39, 1317–1324. [Google Scholar] [CrossRef] [Green Version]
- Samuel, V.T.; Shulman, G.I. Mechanisms for Insulin Resistance: Common Threads and Missing Links. Cell 2012, 148, 852–871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maiese, K. New Insights for Oxidative Stress and Diabetes Mellitus. Oxidative Med. Cell. Longev. 2015, 2015, 875961. [Google Scholar] [CrossRef] [Green Version]
- Kitada, M.; Koya, D. SIRT1 in Type 2 Diabetes: Mechanisms and Therapeutic Potential. Diabetes Metab. J. 2013, 37, 315–325. [Google Scholar] [CrossRef]
- Picard, F.; Kurtev, M.; Chung, N.; Topark-Ngarm, A.; Senawong, T.; Machado De Oliveira, R.; Leid, M.; McBurney, M.W.; Guarente, L. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature 2004, 429, 771–776. [Google Scholar] [CrossRef]
- Wang, F.; Tong, Q. SIRT2 suppresses adipocyte differentiation by deacetylating FOXO1 and enhancing FOXO1’s repressive interaction with PPARgamma. Mol. Biol. Cell 2009, 20, 801–808. [Google Scholar] [CrossRef] [Green Version]
- Jing, E.; Gesta, S.; Kahn, C.R. SIRT2 Regulates Adipocyte Differentiation through FoxO1 Acetylation/Deacetylation. Cell Metab. 2007, 6, 105–114. [Google Scholar] [CrossRef] [Green Version]
- Shin, J.; He, M.; Liu, Y.; Paredes, S.; Villanova, L.; Brown, K.; Qiu, X.; Nabavi, N.; Mohrin, M.; Wojnoonski, K.; et al. SIRT7 Represses Myc Activity to Suppress ER Stress and Prevent Fatty Liver Disease. Cell Rep. 2013, 5, 654–665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Jiménez, V.; Cortez-Espinosa, N.; Rodríguez-Varela, E.; Vega-Cárdenas, M.; Briones-Espinoza, M.; Ruíz-Rodríguez, V.M.; López-López, N.; Briseño-Medina, A.; Turiján-Espinoza, E.; Portales-Pérez, D.P. Altered levels of sirtuin genes (SIRT1, SIRT2, SIRT3 and SIRT6) and their target genes in adipose tissue from individual with obesity. Diabetes Metab. Syndr. Clin. Res. Rev. 2018, 13, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Bordone, L.; Motta, M.C.; Picard, F.; Robinson, A.; Jhala, U.S.; Apfeld, J.; McDonagh, T.; Lemieux, M.; McBurney, M.; Szilvasi, A.; et al. Sirt1 regulates insulin secretion by repressing UCP2 in pancreatic beta cells. PLoS Biol. 2006, 4, e31. [Google Scholar] [CrossRef] [Green Version]
- Nogueiras, R.; Habegger, K.M.; Chaudhary, N.; Finan, B.; Banks, A.S.; Dietrich, M.O.; Horvath, T.L.; Sinclair, D.A.; Pfluger, P.T.; Tschöop, M.H. Sirtuin 1 and Sirtuin 3: Physiological Modulators of Metabolism. Physiol. Rev. 2012, 92, 1479–1514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahuja, N.; Schwer, B.; Carobbio, S.; Waltregny, D.; North, B.J.; Castronovo, V.; Maechler, P.; Verdin, E. Regulation of insu-lin secretion by SIRT4, a mitochondrial ADP-ribosyltransferase. J. Biol. Chem. 2007, 282, 33583–33592. [Google Scholar] [CrossRef] [Green Version]
- Lang, A.; Grether-Beck, S.; Singh, M.; Kuck, F.; Jakob, S.; Kefalas, A.; Altinoluk-Hambüchen, S.; Graffmann, N.; Schneider, M.; Lindecke, A.; et al. MicroRNA-15b regulates mitochondrial ROS production and the senescence-associated secre-tory phenotype through sirtuin 4/SIRT4. Aging 2016, 8, 484–505. [Google Scholar] [CrossRef] [Green Version]
- Zhong, L.; Durso, A.; Toiber, D.; Sebastian, C.; Henry, R.E.; Vadysirisack, D.D.; Guimaraes, A.; Marinelli, B.; Wikstrom, J.; Nir, T.; et al. The Histone Deacetylase Sirt6 Regulates Glucose Homeostasis via Hif1α. Cell 2010, 140, 280–293. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Hernando, C.; Suárez, Y.; Rayner, K.; Moore, K. MicroRNAs in lipid metabolism. Curr. Opin. Lipidol. 2011, 22, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Purushotham, A.; Schug, T.T.; Xu, Q.; Surapureddi, S.; Guo, X.; Li, X. Hepatocyte-Specific Deletion of SIRT1 Alters Fatty Acid Metabolism and Results in Hepatic Steatosis and Inflammation. Cell Metab. 2009, 9, 327–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, R.-H.; Li, C.; Deng, C.-X. Liver Steatosis and Increased ChREBP Expression in Mice Carrying a Liver Specific SIRT1 Null Mutation under a Normal Feeding Condition. Int. J. Biol. Sci. 2010, 6, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Pfluger, P.T.; Herranz, D.; Velasco-Miguel, S.; Serrano, M.; Tschöp, M.H. Sirt1 Protects against High-Fat Diet-Induced Meta-bolic Damage. Proc. Natl. Acad. Sci USA 2008, 105, 9793–9798. [Google Scholar] [CrossRef] [Green Version]
- Mariani, S.; Di Giorgio, M.R.; Martini, P.; Persichetti, A.; Barbaro, G.; Basciani, S.; Contini, S.; Poggiogalle, E.; Sarnicola, A.; Genco, A.; et al. Inverse Association of Circulating SIRT1 and Adipos-ity: A Study on Underweight, Normal Weight, and Obese Patients. Front. Endocrinol. 2018, 9, 449. [Google Scholar] [CrossRef]
- Coussens, M.; Maresh, J.G.; Yanagimachi, R.; Maeda, G.; Allsopp, R. Sirt1 Deficiency Attenuates Spermatogenesis and Germ Cell Function. PLoS ONE 2008, 3, e1571. [Google Scholar] [CrossRef] [Green Version]
- Kolthur-Seetharam, U.; Teerds, K.; de Rooij, D.G.; Wendling, O.; McBurney, M.; Sassone-Corsi, P.; Davidson, I. The Histone Deacetylase SIRT1 Controls Male Fertility in Mice Through Regulation of Hypothalamic-Pituitary Gonadotropin Signaling1. Biol. Reprod. 2009, 80, 384–391. [Google Scholar] [CrossRef] [Green Version]
- Seifert, E.L.; Caron, A.Z.; Morin, K.; Coulombe, J.; He, X.H.; Jardine, K.; Dewar-Darch, D.; Boekelheide, K.; Harper, M.; McBurney, M.W. SirT1 catalytic activity is required for male fertility and metabolic homeostasis in mice. FASEB J. 2011, 26, 555–566. [Google Scholar] [CrossRef]
- Mostafa, T.; Nabil, N.; Rashed, L.; Makeen, K.; El-Kasas, M.A.; Mohamaed, H.A. Seminal SIRT1 expression in infertile oligo-asthenoteratozoospermic men with varicocoele. Andrology 2018, 6, 301–305. [Google Scholar] [CrossRef] [Green Version]
- Alam, F.; Syed, H.; Amjad, S.; Baig, M.; Khan, T.A.; Rehman, R. Interplay between oxidative stress, SIRT1, reproductive and metabolic functions. Curr. Res. Physiol. 2021, 4, 119–124. [Google Scholar] [CrossRef]
- Mostafa, T.; Nabil, N.; Rashed, L.; Abo-Sief, A.F.; Eissa, H.H. Seminal SIRT1-oxidative stress relationship in infertile oligo-asthenoteratozoospermic men with varicocele after its surgical repair. Andrologia 2020, 52, e13456. [Google Scholar] [CrossRef]
- Liu, C.; Song, Z.; Wang, L.; Yu, H.; Liu, W.; Shang, Y.; Xu, Z.; Zhao, H.; Gao, F.; Wen, J.; et al. Sirt1 regulates acrosome biogenesis by modulating autophagic flux during spermiogenesis in mice. Development 2017, 144, 441–451. [Google Scholar]
- North, B.J.; Marshall, B.L.; Borra, M.T.; Denu, J.M.; Verdin, E. The human Sir2 ortholog, SIRT2, is an NAD+-dependent tubu-lin deacetylase. Mol. Cell. 2003, 11, 437–444. [Google Scholar] [CrossRef]
- Parab, S.; Shetty, O.; Gaonkar, R.; Balasinor, N.; Khole, V.; Parte, P. HDAC6 deacetylates alpha tubulin in sperm and modu-lates sperm motility in Holtzman rat. Cell Tissue Res. 2015, 359, 665–678. [Google Scholar] [CrossRef] [PubMed]
- Di Emidio, G.; Falone, S.; Artini, P.G.; Amicarelli, F.; D’Alessandro, A.M.; Tatone, C. MitochondrialSirtuins in Reproduction. Antioxidants 2021, 10, 1047. [Google Scholar] [CrossRef] [PubMed]
- Ahn, B.-H.; Kim, H.-S.; Song, S.; Lee, I.H.; Liu, J.; Vassilopoulos, A.; Deng, C.-X.; Finkel, T. A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc. Natl. Acad. Sci. USA 2008, 105, 14447–14452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasiri, A.; Vaisi-Raygani, A.; Rahimi, Z.; Bakhtiari, M.; Bahrehmand, F.; Kiani, A.; Mozafari, H.; Pourmotabbed, T. Evalua-tion of The Relationship among The Levels of SIRT1 and SIRT3 with Oxidative Stress and DNA Fragmentation in Asthe-noteratozoospermic Men. Int. J. FertilSteril. 2021, 15, 135–140. [Google Scholar]
- Someya, S.; Yu, W.; Hallows, W.C.; Xu, J.; Vann, J.M.; Leeuwenburgh, C.; Tanokura, M.; Denu, J.M.; Prolla, T.A. Sirt3 medi-ates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell 2010, 143, 802–812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramatchandirin, B.; Sadasivam, M.; Kannan, A.; Prahalathan, C. Sirtuin 4 Regulates Lipopolysaccharide Mediated Leydig Cell Dysfunction. J. Cell. Biochem. 2015, 117, 904–916. [Google Scholar] [CrossRef]
- Barbagallo, F.; La Vignera, S.; Cannarella, R.; Aversa, A.; Calogero, A.E.; Condorelli, R.A. Evaluation of Sperm Mitochon-drial Function: A Key Organelle for Sperm Motility. J. Clin. Med. 2020, 9, 363. [Google Scholar] [CrossRef] [Green Version]
- Vertika, S.; Singh, K.K.; Rajender, S. Mitochondria, spermatogenesis, and male infertility—An update. Mitochondrion 2020, 54, 26–40. [Google Scholar] [CrossRef] [PubMed]
- Bello, J.H.; Khan, M.J.; Amir, S.; Kakakhel, H.G.; Tahir, F.; Sultan, S.; Raza, S.Q.; Mamoulakis, C.; Zachariou, A.; Tsatsakis, A.; et al. Dysregulation of mitochondrial sirtuin genes is associated with human male infertility. Andrologia 2021, 54, e14274. [Google Scholar] [CrossRef] [PubMed]
- Palmer, N.O.; Fullston, T.; Mitchell, M.; Setchell, B.P.; Lane, M. SIRT6 in mouse spermatogenesis is modulated by di-et-induced obesity. ReprodFertil Dev. 2011, 23, 929–939. [Google Scholar]
- Mao, Z.; Hine, C.; Tian, X.; Van Meter, M.; Au, M.; Vaidya, A.; Seluanov, A.; Gorbunova, V. SIRT6 promotes DNA repair under stress by activating PARP1. Science 2011, 332, 1443–1446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vazquez, B.N.; Thackray, J.; Simonet, N.; Kane-Goldsmith, N.; Martinez-Redondo, P.; Nguyen, T.; Bunting, S.; Vaquero, A.; Tischfield, J.; Serrano, L. SIRT 7 promotes genome integrity and modulates non-homologous end joining DNA repair. EMBO J. 2016, 35, 1488–1503. [Google Scholar] [CrossRef]
- Ford, E.; Voit, R.; Liszt, G.; Magin, C.; Grummt, I.; Guarente, L. Mammalian Sir2 homolog SIRT7 is an activator of RNA polymerase I transcription. Genes Dev. 2006, 20, 1075–1080. [Google Scholar] [CrossRef] [Green Version]
- Banks, A.S.; Kon, N.; Knight, C.; Matsumoto, M.; Gutiérrez-Juárez, R.; Rossetti, L.; Gu, W.; Accili, D. SirT1 Gain of Function Increases Energy Efficiency and Prevents Diabetes in Mice. Cell Metab. 2008, 8, 333–341. [Google Scholar] [CrossRef] [Green Version]
- Rato, L.P.; Alves, M.G.; Silva, B.M.; Sousa, M.; Oliveira, P.F. Sirtuins: Novel Players in Male Reproductive Health. Curr. Med. Chem. 2016, 23, 1084–1099. [Google Scholar] [CrossRef]
- Bhat, K.P.L.; Kosmeder, J.W., 2nd; Pezzuto, J.M. Biological Effects of Resveratrol. Antioxid. Redox Signal. 2001, 3, 1041–1064. [Google Scholar] [CrossRef]
- Alamo, A.; Condorelli, R.A.; Mongioì, L.M.; Cannarella, R.; Giacone, F.; Calabrese, V.; La Vignera, S.; Calogero, A.E. Envi-ronment and Male Fertility: Effects of Benzo-α-Pyrene and Resveratrol on Human Sperm Function In Vitro. J. Clin. Med. 2019, 8, 561. [Google Scholar] [CrossRef] [Green Version]
- Camont, L.; Cottart, C.H.; Rhayem, Y.; Nivet-Antoine, V.; Djelidi, R.; Collin, F.; Beaudeux, J.L.; Bonnefont-Rousselot, D. Simple spectrophotometric assessment of the trans-/cis-resveratrol ratio in aqueous solutions. Anal. Chim. Acta. 2009, 634, 121–128. [Google Scholar] [CrossRef]
- Jang, M.; Cai, L.; Udeani, G.O.; Slowing, K.V.; Thomas, C.F.; Beecher, C.W.W.; Fong, H.H.S.; Farnsworth, N.R.; Kinghorn, A.D.; Mehta, R.G.; et al. Cancer Chemopreventive Activity of Resveratrol, a Natural Product Derived from Grapes. Science 1997, 275, 218–220. [Google Scholar] [CrossRef] [Green Version]
- Mongioì, L.; La Vignera, S.; Cannarella, R.; Cimino, L.; Compagnone, M.; Condorelli, R.; Calogero, A. The Role of Resveratrol Administration in Human Obesity. Int. J. Mol. Sci. 2021, 22, 4362. [Google Scholar] [CrossRef]
- Tabrizi, R.; Tamtaji, O.R.; Lankarani, K.B.; Akbari, M.; Dadgostar, E.; Dabbaghmanesh, M.H.; Kolahdooz, F.; Shamshirian, A.; Momen-Heravi, M.; Asemi, Z. The effects of resveratrol intake on weight loss: A systematic review and meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2018, 60, 375–390. [Google Scholar] [CrossRef] [PubMed]
- Lagouge, M.; Argmann, C.; Gerhart-Hines, Z.; Meziane, H.; Lerin, C.; Daussin, F.; Messadeq, N.; Milne, J.; Lambert, P.; Elliott, P.; et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1α. Cell 2006, 127, 1109–1122. [Google Scholar] [CrossRef] [PubMed]
- Kang, N.E.; Ha, A.W.; Kim, J.Y.; Kim, W.K. Resveratrol inhibits the protein expression of transcription factors related adi-pocyte differentiation and the activity of matrix metalloproteinase in mouse fibroblast 3T3-L1 preadipocytes. Nutr. Res. Pr. 2012, 6, 499–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bitterman, J.L.; Chung, J.H. Metabolic effects of resveratrol: Addressing the controversies. Cell. Mol. Life Sci. 2014, 72, 1473–1488. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-J.; Wang, Q.; Lv, Z.-M.; Wang, C.-L.; Li, C.-P.; Rong, Y.-L. Resveratrol appears to protect against oxidative stress and steroidogenesis collapse in mice fed high-calorie and high-cholesterol diet. Andrologia 2014, 47, 59–65. [Google Scholar] [CrossRef]
- Gregorio, B.M.; A De Oliveira, F.; Costa, W.S.; Sampaio, F.J.B. Resveratrol attenuates metabolic, sperm, and testicular changes in adult Wistar rats fed a diet rich in lipids and simple carbohydrates. Asian J. Androl. 2019, 21, 201–207. [Google Scholar] [CrossRef]
- Lv, Z.-M.; Ling, M.-Y.; Chen, C. Comparative proteomics reveals protective effect of resveratrol on a high-fat diet-induced damage to mice testis. Syst. Biol. Reprod. Med. 2020, 66, 37–49. [Google Scholar] [CrossRef] [Green Version]
- Cui, X.; Jing, X.; Wu, X.; Yan, M. Protective effect of resveratrol on spermatozoa function in male infertility induced by ex-cess weight and obesity. Mol. Med. Rep. 2016, 14, 4659–4665. [Google Scholar] [CrossRef] [Green Version]
- Shpakov, A.O. Improvement Effect of Metformin on Female and Male Reproduction in Endocrine Pathologies and Its Mechanisms. Pharmaceuticals 2021, 14, 42. [Google Scholar] [CrossRef]
- Morgante, G.; Tosti, C.; Orvieto, R.; Musacchio, M.C.; Piomboni, P.; De Leo, V. Metformin improves semen characteristics of oligo-terato-asthenozoospermic men with metabolic syndrome. Fertil. Steril. 2011, 95, 2150–2152. [Google Scholar] [CrossRef]
- Liu, C.Y.; Chang, T.C.; Lin, S.H.; Wu, S.T.; Cha, T.L.; Tsao, C.W. Metformin Ameliorates Testicular Function and Spermato-genesis in Male Mice with High-Fat and High-Cholesterol Diet-Induced Obesity. Nutrients 2020, 12, 1932. [Google Scholar] [CrossRef] [PubMed]
- Bertoldo, M.J.; Faure, M.; Dupont, J.; Froment, P. AMPK: A master energy regulator for gonadal function. Front. Neurosci. 2015, 9, 235. [Google Scholar] [CrossRef] [Green Version]
- Decara, J.; Arrabal, S.; Beiroa, D.; Rivera, P.; Vargas, A.; Serrano, A.; Pavon, F.J.; Ballesteros, J.; Dieguez, C.; Nogueiras, R.; et al. Antiobesity efficacy of GLP-1 receptor agonist liraglutide is associated with peripheral tissue-specific modulation of lipid metabolic regulators. BioFactors 2016, 42, 600–611. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.; Zhang, X.; Ge, S.Q.; Zhang, E.H.; Zhang, B. Expression of SIRT1 in the ovaries of rats with polycystic ovary syn-drome before and after therapeutic intervention with exenatide. Int. J. Clin. Exp. Pathol. 2015, 8, 8276–8283. [Google Scholar]
- Kamalipour, F.; Jalali, H.; Azarnia, M. Comparison the Effect of Metformin and Clomiphene Citrate on Sirtuin3 gene Ex-pression in the Oocytes of Mice with Polycystic Ovary Syndrome. Iran J. Pharm. Res. 2020, 19, 160–168. [Google Scholar] [PubMed]
- Abd El-Hakim, Y.M.; Abdel-Rahman Mohamed, A.; Khater, S.I.; Hamed Arisha, A.; Metwally, M.M.M.; Nassan, M.A.; Hassan, M.E. Chitosan-Stabilized Selenium Nanoparticles and Metformin Synergistically Rescue Testicular Oxidative Damage and Steroidogenesis-Related Genes Dysregulation in High-Fat Diet/Streptozotocin-Induced Diabetic Rats. Antioxidants 2020, 10, 17. [Google Scholar] [CrossRef]
- Lei, X.; Huo, P.; Wang, Y.; Xie, Y.; Shi, Q.; Tu, H.; Yao, J.; Mo, Z.; Zhang, S. Lycium barbarum Polysaccharides Improve Tes-ticular Spermatogenic Function in Streptozotocin-Induced Diabetic Rats. Front Endocrinol. 2020, 11, 164. [Google Scholar] [CrossRef] [PubMed]
- Mihanfar, A.; Nouri, M.; Roshangar, L.; Khadem-Ansari, M.H. Therapeutic potential of quercetin in an animal model of PCOS: Possible involvement of AMPK/SIRT-1 axis. Eur. J. Pharmacol. 2021, 900, 174062. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, D.; Kuang, H.; Feng, X.; Ai, W.; Wang, Y.; Shi, S.; Chen, J.; Fan, R. Berberine increases glucose uptake and intracel-lular ROS levels by promoting Sirtuin 3 ubiquitination. Biomed Pharmacother. 2020, 121, 109563. [Google Scholar] [CrossRef]
- Dittenhafer-Reed, K.E.; Feldman, J.L. Catalysis and mechanistic insights into sirtuin activation. Chembiochem 2011, 12, 281–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mongioì, L.M.; Cimino, L.; Greco, E.; Cannarella, R.; Condorelli, R.A.; La Vignera, S.; Calogero, A.E. Very-low-calorie ke-togenic diet: An alternative to a pharmacological approach to improve glycometabolic and gonadal profile in men with obesity. Curr. Opin. Pharmacol. 2021, 60, 72–82. [Google Scholar] [CrossRef]
- Mongioì, L.M.; Cimino, L.; Condorelli, R.A.; Magagnini, M.C.; Barbagallo, F.; Cannarella, R.; La Vignera, S.; Calogero, A.E. Effectiveness of a Very Low Calorie Ketogenic Diet on Testicular Function in Overweight/Obese Men. Nutrients 2020, 12, 2967. [Google Scholar] [CrossRef] [PubMed]
- Abduraman, M.A.; Azizan, N.A.; Teoh, S.H.; Tan, M.L. Ketogenesis and SIRT1 as a tool in managing obesity. Obes. Res. Clin. Pract. 2020, 15, 10–18. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbagallo, F.; La Vignera, S.; Cannarella, R.; Mongioì, L.M.; Garofalo, V.; Leanza, C.; Marino, M.; Calogero, A.E.; Condorelli, R.A. Obesity and Male Reproduction: Do Sirtuins Play a Role? Int. J. Mol. Sci. 2022, 23, 973. https://doi.org/10.3390/ijms23020973
Barbagallo F, La Vignera S, Cannarella R, Mongioì LM, Garofalo V, Leanza C, Marino M, Calogero AE, Condorelli RA. Obesity and Male Reproduction: Do Sirtuins Play a Role? International Journal of Molecular Sciences. 2022; 23(2):973. https://doi.org/10.3390/ijms23020973
Chicago/Turabian StyleBarbagallo, Federica, Sandro La Vignera, Rossella Cannarella, Laura M. Mongioì, Vincenzo Garofalo, Claudia Leanza, Marta Marino, Aldo E. Calogero, and Rosita A. Condorelli. 2022. "Obesity and Male Reproduction: Do Sirtuins Play a Role?" International Journal of Molecular Sciences 23, no. 2: 973. https://doi.org/10.3390/ijms23020973
APA StyleBarbagallo, F., La Vignera, S., Cannarella, R., Mongioì, L. M., Garofalo, V., Leanza, C., Marino, M., Calogero, A. E., & Condorelli, R. A. (2022). Obesity and Male Reproduction: Do Sirtuins Play a Role? International Journal of Molecular Sciences, 23(2), 973. https://doi.org/10.3390/ijms23020973









