Diagnostic Utility of Genetic and Immunohistochemical H3-3A Mutation Analysis in Giant Cell Tumour of Bone
Abstract
1. Introduction
2. Results
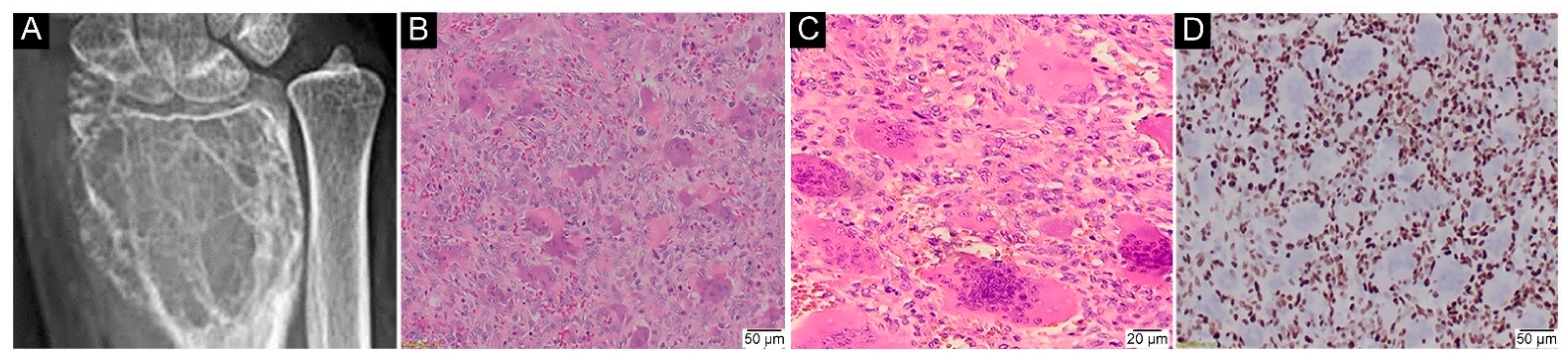
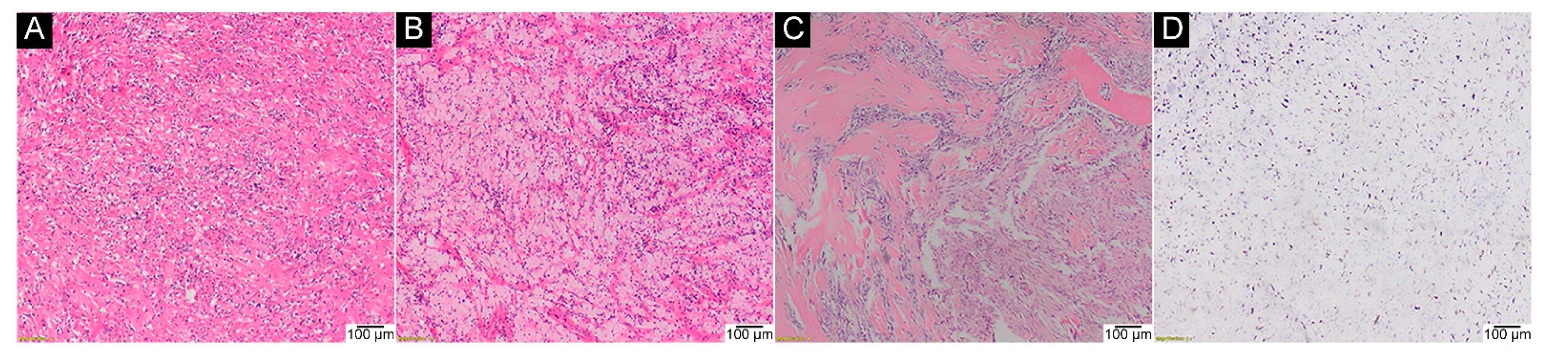
2.1. General Characteristics of the GCTB Cohort
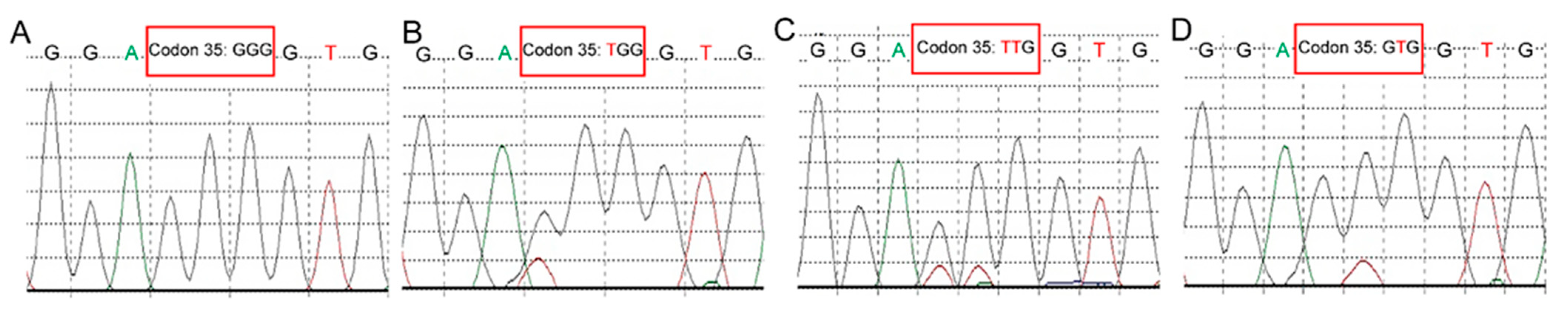
2.2. Molecular Analysis
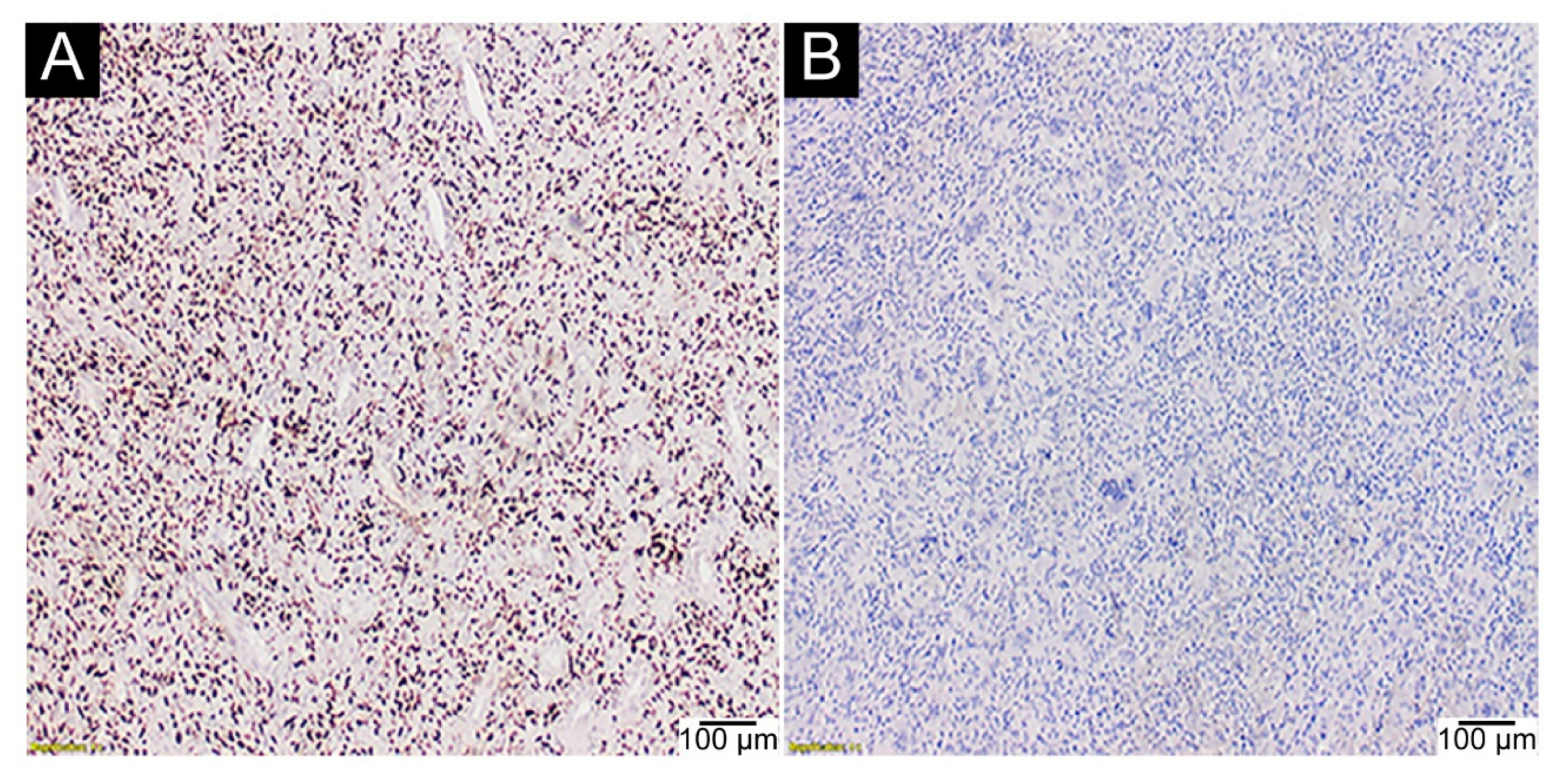
2.3. IHC Assays
2.4. H3-3A Mutation Analysis in GCTB after Denosumab Treatment
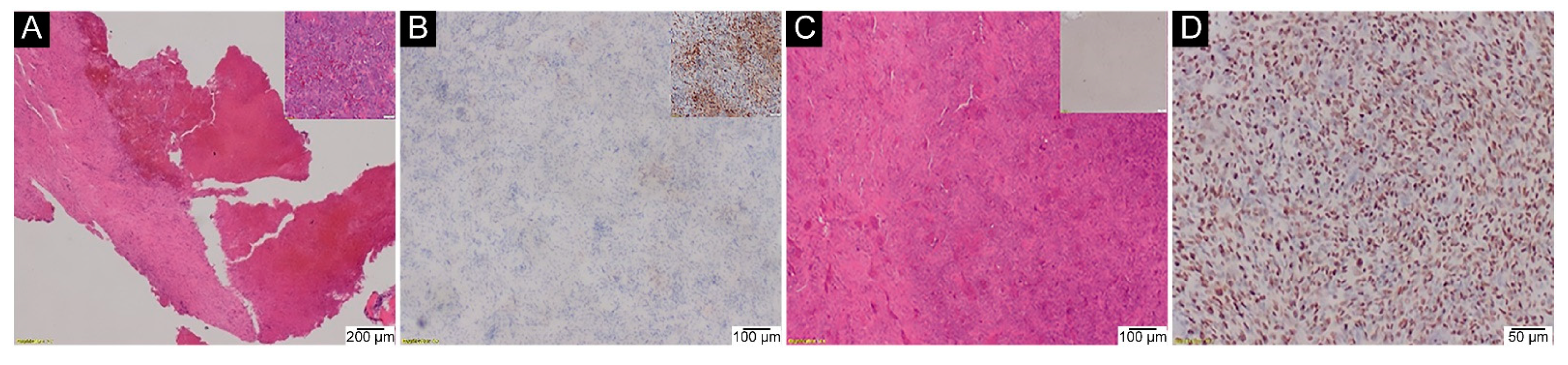
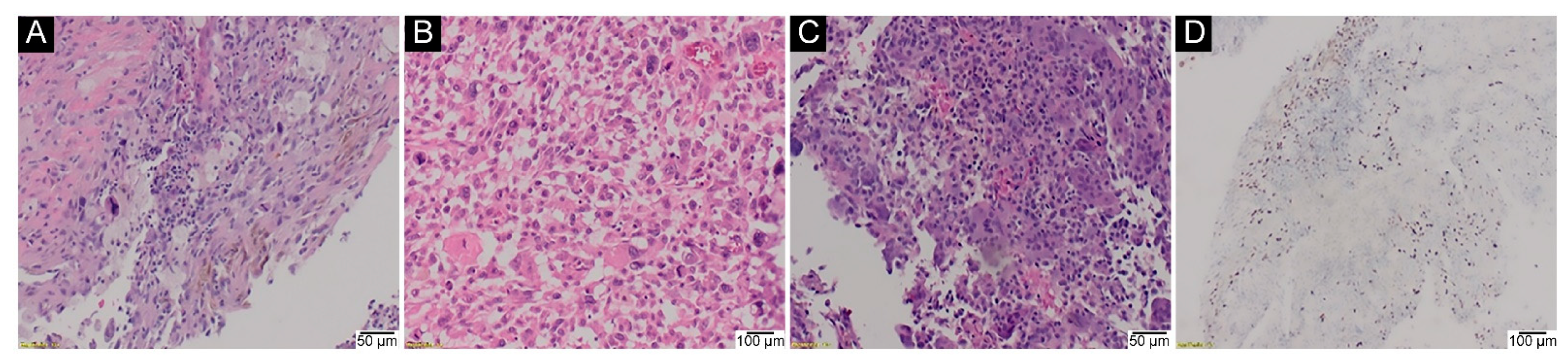
2.5. H3-3A Mutation Analysis in Malignant GCTB
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Flanagan, A.M.; Larousserie, F.; O’Donnell, P.G.; Yoshida, A. Giant cell tumour of bone. In WHO Classification of Tumours. Soft Tissue and Bone Tumours, 5th ed.; International Agency for Research on Cancer: Lyon, France, 2020; pp. 440–446. [Google Scholar]
- Liede, A.; Bach, B.A.; Stryker, S.; Hernandez, R.K.; Sobocki, P.; Bennett, B.; Wong, S.S. Regional Variation and Challenges in Estimating the Incidence of Giant Cell Tumor of Bone. J. Bone Jt. Surg. 2014, 96, 1999–2007. [Google Scholar] [CrossRef]
- Unni, K.K. Tumors of the bones and joints. In AFIP Atlas of Tumor Pathology; American Registry of Pathology; Armed Forces Institute of Pathology: Washington, DC, USA, 2005. [Google Scholar]
- Schajowicz, F. Tumors and Tumorlike Lesions of Bone: Pathology, Radiology, and Treatment; Springer: Berlin/Heidelberg, Germany, 1994. [Google Scholar]
- Chakarun, C.J.; Forrester, D.M.; Gottsegen, C.; Patel, D.B.; White, E.A.; Matcuk, G. Giant Cell Tumor of Bone: Review, Mimics, and New Developments in Treatment. Radiographics 2013, 33, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Balke, M.; Henrichs, M.P.; Gosheger, G.; Ahrens, H.; Streitbuerger, A.; Koehler, M.; Bullmann, V.; Hardes, J. Giant Cell Tumors of the Axial Skeleton. Sarcoma 2012, 2012, 1–10. [Google Scholar] [CrossRef][Green Version]
- Lee, M.; Sallomi, D.; Munk, P.; Janzen, D.; Connell, D.; O’Connell, J.; Logan, P.; Masri, B. Pictorial review: Giant cell tumours of bone. Clin. Radiol. 1998, 53, 481–489. [Google Scholar] [CrossRef]
- Lodwick, G.S.; Wilson, A.J.; Farrell, C.; Virtama, P.; Dittrich, F. Determining growth rates of focal lesions of bone from radiographs. Radiology 1980, 134, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Murphey, M.D.; Nomikos, G.C.; Flemming, D.J.; Gannon, F.H.; Temple, H.T.; Kransdorf, M.J. Imaging of Giant Cell Tumor and Giant Cell Reparative Granuloma of Bone: Radiologic-Pathologic Correlation. Radiographics 2001, 21, 1283–1309. [Google Scholar] [CrossRef] [PubMed]
- Behjati, S.; Tarpey, P.S.; Presneau, N.; Scheipl, S.; Pillay, N.; Van Loo, P.; Wedge, D.; Cooke, S.L.; Gundem, G.; Davies, H.; et al. Distinct H3F3A and H3F3B driver mutations define chondroblastoma and giant cell tumor of bone. Nat. Genet. 2013, 45, 1479–1482. [Google Scholar] [CrossRef] [PubMed]
- Presneau, N.; Baumhoer, D.; Behjati, S.; Pillay, N.; Tarpey, P.; Campbell, P.J.; Jundt, G.; Hamoudi, R.; Wedge, D.C.; Van Loo, P.; et al. Diagnostic value of H3F3A mutations in giant cell tumour of bone compared to osteoclast-rich mimics. J. Pathol. Clin. Res. 2015, 1, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Cleven, A.H.; Höcker, S.; Bruijn, I.B.-D.; Szuhai, K.; Cleton-Jansen, A.-M.; Bovée, J.V. Mutation Analysis of H3F3A and H3F3B as a Diagnostic Tool for Giant Cell Tumor of Bone and Chondroblastoma. Am. J. Surg. Pathol. 2015, 39, 1576–1583. [Google Scholar] [CrossRef]
- Wu, R.S.; Tsai, S.; Bonner, W.M. Patterns of histone variant synthesis can distinguish go from G1 cells. Cell 1982, 31, 367–374. [Google Scholar] [CrossRef]
- Hake, S.B.; Garcia, B.A.; Duncan, E.M.; Kauer, M.; Dellaire, G.; Shabanowitz, J.; Bazett-Jones, D.P.; Allis, C.D.; Hunt, D.F. Expression Patterns and Post-translational Modifications Associated with Mammalian Histone H3 Variants. J. Biol. Chem. 2006, 281, 559–568. [Google Scholar] [CrossRef]
- Mariño-Ramírez, L.; Kann, M.G.; Shoemaker, B.A.; Landsman, D. Histone structure and nucleosome stability. Expert Rev. Proteom. 2005, 2, 719–729. [Google Scholar] [CrossRef]
- Davey, C.A.; Sargent, D.F.; Luger, K.; Maeder, A.W.; Richmond, T.J. Solvent Mediated Interactions in the Structure of the Nucleosome Core Particle at 1.9Å Resolution. J. Mol. Biol. 2002, 319, 1097–1113. [Google Scholar] [CrossRef]
- Griffiths, A.J.F.; Miller, J.H.; Suzuki, D.T.; Lewontin, R.C.; Gelbart, W.M. Three-dimensional structure of chromosomes. In An Introduction to Genetic Analysis, 7th ed.; W. H. Freeman: New York, NY, USA, 2000. [Google Scholar]
- Felsenfeld, G.; Boyes, J.; Chung, J.; Clark, D.; Studitsky, V. Chromatin structure and gene expression. Proc. Natl. Acad. Sci. USA 1996, 93, 9384–9388. [Google Scholar] [CrossRef]
- Toledo, R.; Qin, Y.; Cheng, Z.-M.; Gao, Q.; Iwata, S.; Silva, G.M.; Prasad, M.L.; Ocal, I.T.; Rao, S.; Aronin, N.; et al. Recurrent Mutations of Chromatin-Remodeling Genes and Kinase Receptors in Pheochromocytomas and Paragangliomas. Clin. Cancer Res. 2016, 22, 2301–2310. [Google Scholar] [CrossRef]
- Toledo, R.A. Genetics of pheochromocytomas and paragangliomas: An overview on the recently implicated genes MERTK, MET, fibroblast growth factor receptor 1, and H3F3A. Endocrinol. Metab. Clin. North Am. 2017, 46, 459–489. [Google Scholar] [CrossRef]
- Amary, F.; Berisha, F.; Ye, H.; Gupta, M.; Gutteridge, A.; Baumhoer, D.; Gibbons, R.; Tirabosco, R.; O’Donnell, P.; Flanagan, A.M. H3F3A (Histone 3.3) G34W immunohistochemistry: A reliable marker defining benign and malignant giant cell tumor of bone. Am. J. Surg. Pathol. 2017, 41, 1059–1068. [Google Scholar] [CrossRef] [PubMed]
- Righi, A.; Mancini, I.; Gambarotti, M.; Picci, P.; Gamberi, G.; Marraccini, C.; Tos, A.P.D.; Simi, L.; Pinzani, P.; Franchi, A. Histone 3.3 mutations in giant cell tumor and giant cell–rich sarcomas of bone. Hum. Pathol. 2017, 68, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Koelsche, C.; Schrimpf, D.; Tharun, L.; Roth, E.; Sturm, D.; Jones, D.T.W.; Renker, E.-K.; Sill, M.; Baude, A.; Sahm, F.; et al. Histone 3.3 hotspot mutations in conventional osteosarcomas: A comprehensive clinical and molecular characterization of six H3F3A mutated cases. Clin. Sarcoma Res. 2017, 7, 9. [Google Scholar] [CrossRef]
- Yamamoto, H.; Iwasaki, T.; Yamada, Y.; Matsumoto, Y.; Otsuka, H.; Yoshimoto, M.; Kohashi, K.; Taguchi, K.; Yokoyama, R.; Nakashima, Y.; et al. Diagnostic utility of histone H3.3 G34W, G34R, and G34V mutant-specific antibodies for giant cell tumors of bone. Hum. Pathol. 2018, 73, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.-I.; Nakano, Y.; Honda-Kitahara, M.; Wakai, S.; Motoi, T.; Ogura, K.; Sano, N.; Shibata, T.; Okuma, T.; Iwata, S.; et al. Absence of H3F3A mutation in a subset of malignant giant cell tumor of bone. Mod. Pathol. 2019, 32, 1751–1761. [Google Scholar] [CrossRef] [PubMed]
- Girolami, I.; Mancini, I.; Simoni, A.; Baldi, G.G.; Simi, L.; Campanacci, D.; Beltrami, G.; Scoccianti, G.; D’Arienzo, A.; Capanna, R.; et al. Denosumab treated giant cell tumour of bone: A morphological, immunohistochemical and molecular analysis of a series. J. Clin. Pathol. 2016, 69, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.H.; Zhang, W.; Sun, X.Q.; Zhang, M.; Ding, Y. DNA sequencing of H3F3A mutations in H3.3 immunohistochemistry-negative giant cell tumors of bone. Zhonghua Bing Li Xue Za Zhi 2021, 50, 190–193. [Google Scholar]
- Kervarrec, T.; Collin, C.; Larousserie, F.; Bouvier, C.; Aubert, S.; Gomez-Brouchet, A.; Marie, B.; Miquelestorena-Standley, E.; Le Nail, L.R.; Avril, P.; et al. H3F3 mutation status of giant cell tumors of the bone, chondroblastomas and their mimics: A combined high resolution melting and pyrosequencing approach. Mod. Pathol. 2017, 30, 393–406. [Google Scholar] [CrossRef]
- Gong, L.; Bui, M.M.; Zhang, W.; Sun, X.; Zhang, M.; Yi, D. H3F3A G34 mutation DNA sequencing and G34W immunohistochemistry analysis in 366 cases of giant cell tumors of bone and other bone tumors. Histol. Histopathol. 2021, 36, 61–68. [Google Scholar]
- Kato, I.; Furuya, M.; Matsuo, K.; Kawabata, Y.; Tanaka, R.; Ohashi, K. Giant cell tumours of bone treated with denosumab: Histological, immunohistochemical and H3F3A mutation analyses. Histopathology 2018, 72, 914–922. [Google Scholar] [CrossRef]
- Ogura, K.; Hosoda, F.; Nakamura, H.; Hama, N.; Totoki, Y.; Yoshida, A.; Ohashi, S.; Rokutan, H.; Takai, E.; Yachida, S.; et al. Highly recurrent H3F3A mutations with additional epigenetic regulator alterations in giant cell tumor of bone. Genes Chromosom. Cancer 2017, 56, 711–718. [Google Scholar] [CrossRef]
- Grizzle, W.E. Special symposium: Fixation and tissue processing models. Biotech. Histochem. 2009, 84, 185–193. [Google Scholar] [CrossRef]
- Qureshi, A.; Pervez, S. Allred scoring for ER reporting and its impact in clearly distinguishing ER negative from ER positive breast cancers. J. Pak. Med. Assoc. 2010, 60, 350–353. [Google Scholar] [PubMed]
| N = 119 | % | ||
|---|---|---|---|
| Sex | male | 58 | 48.7 |
| female | 61 | 51.3 | |
| Site | left | 55 | 46.2 |
| right | 49 | 41.2 | |
| axial | 15 | 12.6 | |
| Campanacci grade | latent (I) | 12 | 10.0 |
| active (II) | 69 | 58.0 | |
| aggressive (III) | 38 | 32.0 | |
| Clinical progression | no progression | 93 | 78.2 |
| (recurrence or distal deposits) | progressive GCTB | 26 | 21.8 |
| Study Cohorts | All | p-Value | |||
|---|---|---|---|---|---|
| GCTB | Non-GCTB | ||||
| p.Gly35 mutation | yes | 120 (100.0%) | 0 (0.0%) | 120 (56.1%) | <0.00001 |
| no | 0 (0.0%) | 94 (100.0%) | 94 (43.9%) | ||
| all | 120 (100.0%) | 94 (100.0%) | 214 (100.0%) | ||
| Study Cohorts | All | p-Value | |||
|---|---|---|---|---|---|
| GCTB | Non-GCTB | ||||
| Intensity | >0 | 83 (94.3%) | 0 (0.0%) | 83 (56.5%) | <0.00001 |
| 0 | 5 (5.7%) | 59 (100.0%) | 64 (43.5%) | ||
| All | 88 (100.0%) | 59 (100.0%) | 147 (100.0%) | ||
| Percentage | >0 | 83 (94.3%) | 0 (0.0%) | 83 (56.5%) | <0.00001 |
| 0 | 5 (5.7%) | 59 (100.0%) | 64 (43.5%) | ||
| All | 88 (100.0%) | 59 (100.0%) | 147 (100.0%) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wągrodzki, M.; Tysarowski, A.; Seliga, K.; Wojnowska, A.; Stepaniuk, M.; Castañeda Wysocka, P.; Makuła, D.; Pieńkowski, A.; Szostakowski, B.; Zub, R.; et al. Diagnostic Utility of Genetic and Immunohistochemical H3-3A Mutation Analysis in Giant Cell Tumour of Bone. Int. J. Mol. Sci. 2022, 23, 969. https://doi.org/10.3390/ijms23020969
Wągrodzki M, Tysarowski A, Seliga K, Wojnowska A, Stepaniuk M, Castañeda Wysocka P, Makuła D, Pieńkowski A, Szostakowski B, Zub R, et al. Diagnostic Utility of Genetic and Immunohistochemical H3-3A Mutation Analysis in Giant Cell Tumour of Bone. International Journal of Molecular Sciences. 2022; 23(2):969. https://doi.org/10.3390/ijms23020969
Chicago/Turabian StyleWągrodzki, Michał, Andrzej Tysarowski, Katarzyna Seliga, Aneta Wojnowska, Maria Stepaniuk, Patrycja Castañeda Wysocka, Donata Makuła, Andrzej Pieńkowski, Bartłomiej Szostakowski, Renata Zub, and et al. 2022. "Diagnostic Utility of Genetic and Immunohistochemical H3-3A Mutation Analysis in Giant Cell Tumour of Bone" International Journal of Molecular Sciences 23, no. 2: 969. https://doi.org/10.3390/ijms23020969
APA StyleWągrodzki, M., Tysarowski, A., Seliga, K., Wojnowska, A., Stepaniuk, M., Castañeda Wysocka, P., Makuła, D., Pieńkowski, A., Szostakowski, B., Zub, R., & Rutkowski, P. (2022). Diagnostic Utility of Genetic and Immunohistochemical H3-3A Mutation Analysis in Giant Cell Tumour of Bone. International Journal of Molecular Sciences, 23(2), 969. https://doi.org/10.3390/ijms23020969







