Secretome from Human Mesenchymal Stem Cells-Derived Endothelial Cells Promotes Wound Healing in a Type-2 Diabetes Mouse Model
Abstract
1. Introduction
2. Results
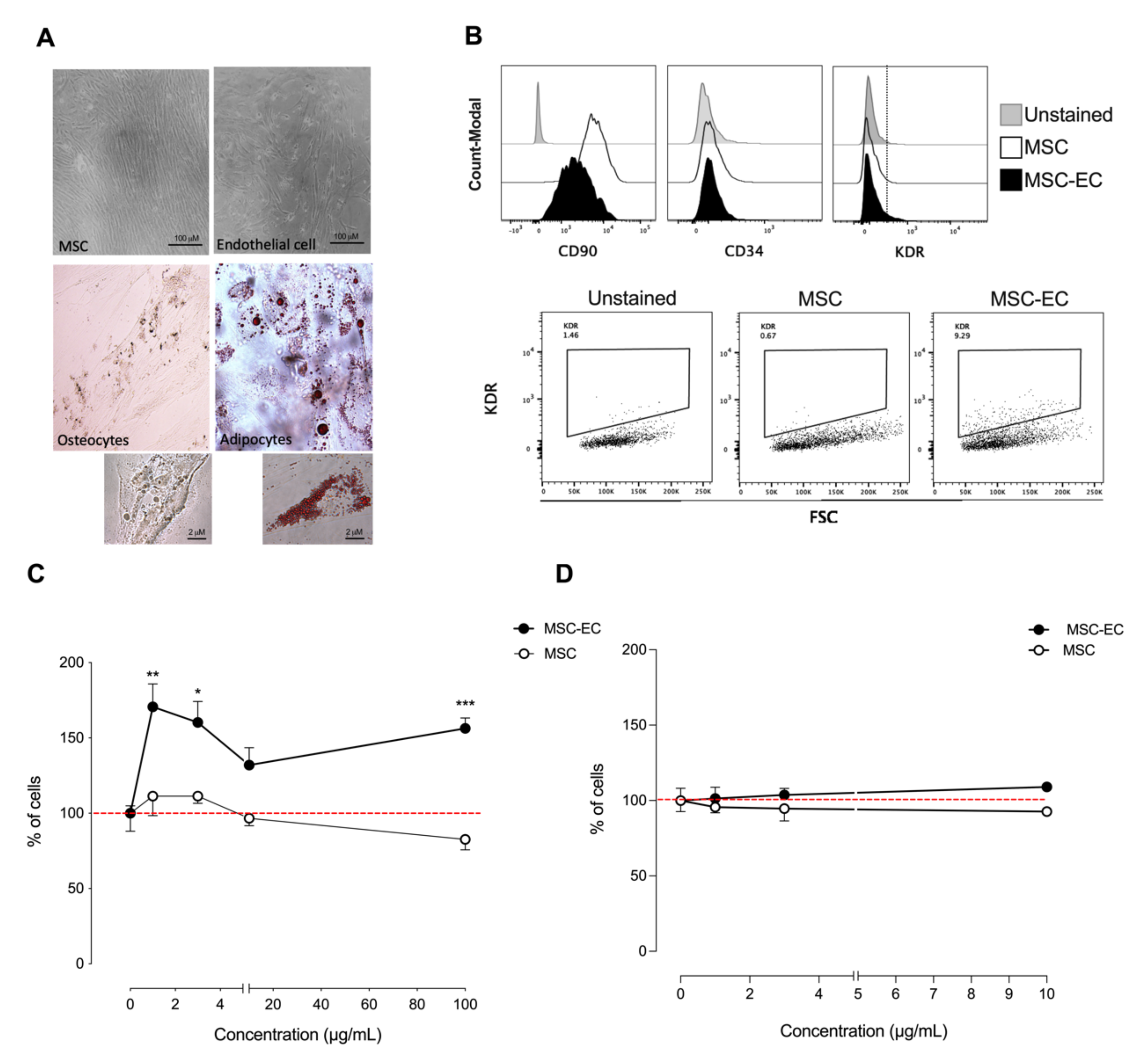
2.1. hMSC-EC Secretome Promotes Endothelial-like Cells Growth In Vitro
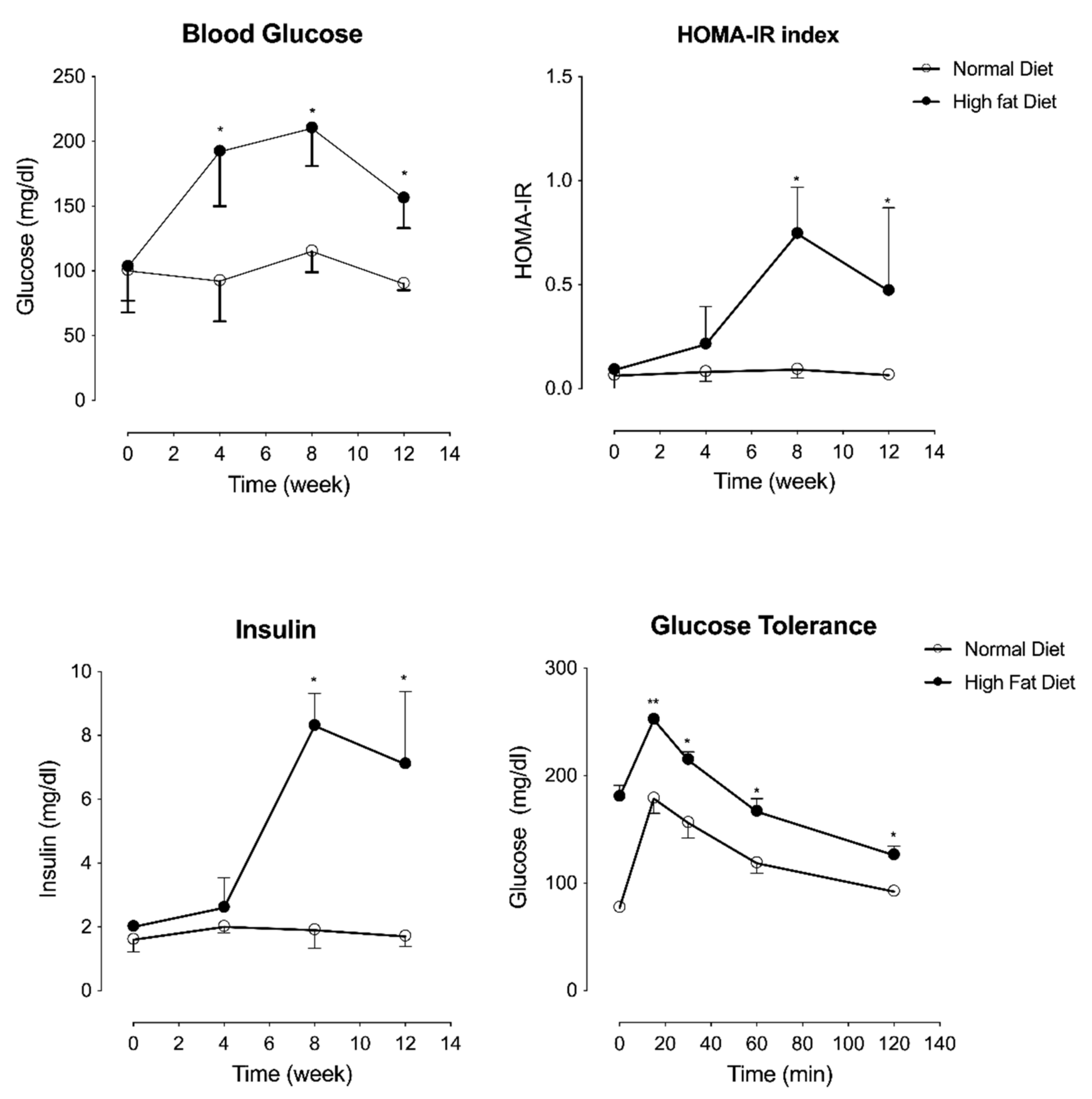
2.2. High Fat Diet-Induced Metabolic Disturbance Associated with Type-2 Diabetes in C57Bl/6J Mice
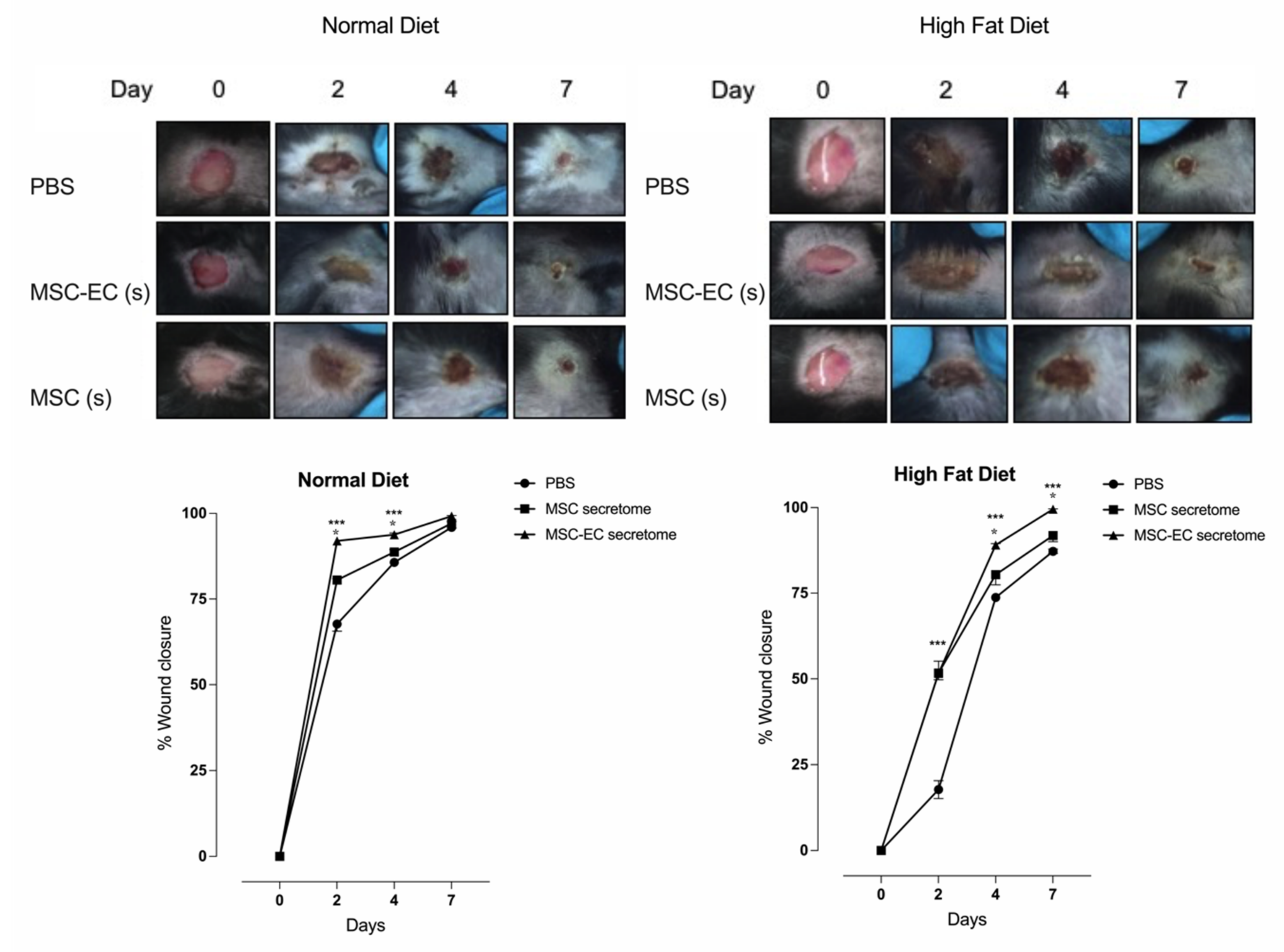
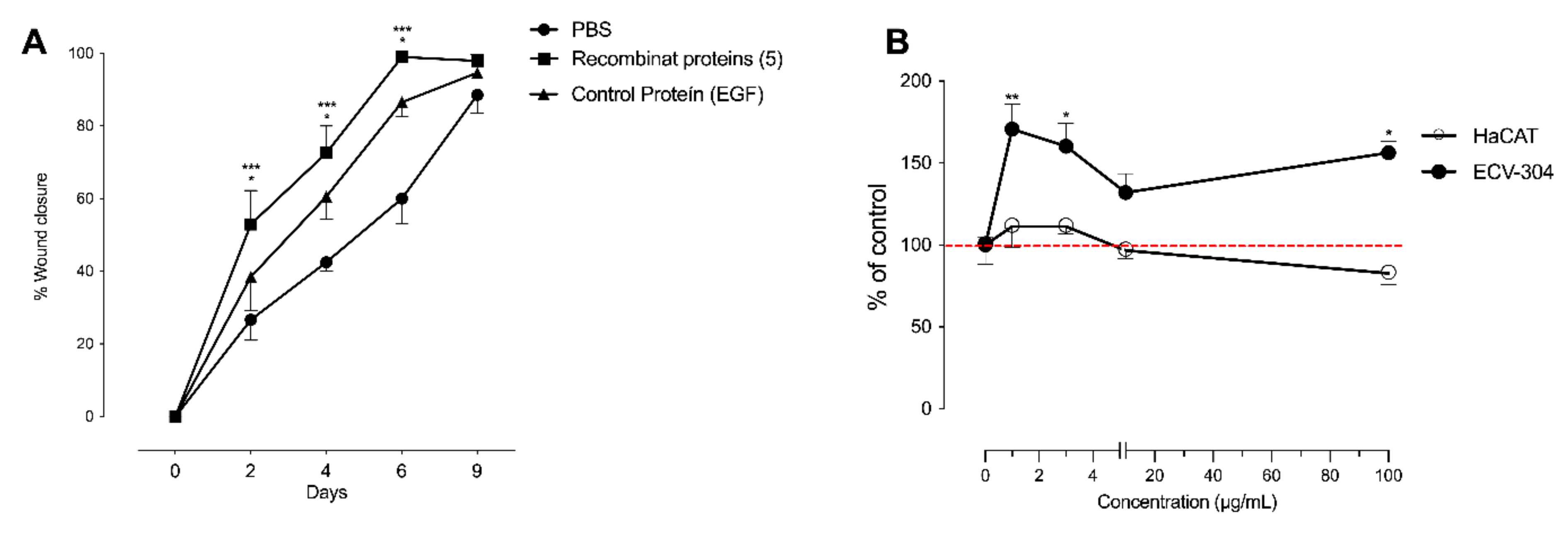
2.3. hMSC-EC Secretome Improved the Rate of Wound Closure In Vivo
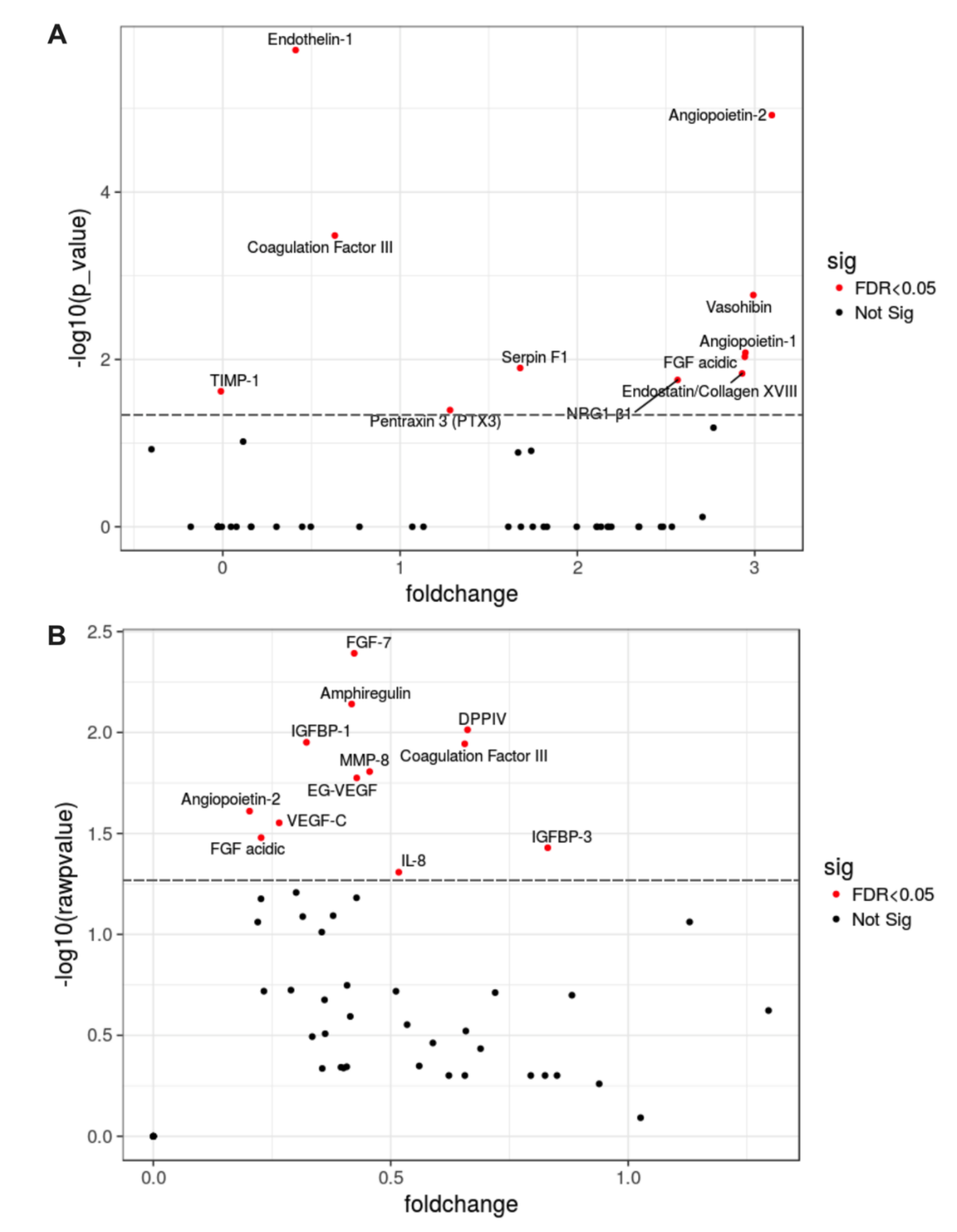
2.4. Hallmark in the Proteomic Analysis of hMSC-EC Secretome
2.5. Significantly Enriched Proteins in MSC-EC Secretome Promoted Wound Healing in a Type-2 Diabetes Mouse Model
3. Discussion
3.1. Mesenchymal Stem Cells and Wound Healing
3.2. Paracrine Function of MSC
4. Materials and Methods
4.1. Mice Models
4.2. Isolation and Culture of hMSCs
4.3. Human Mesenchymal Stem Cells (hMSCs) Differentiation
4.4. hMSCs Differentiation to Endothelial Cell (hMSC-EC)
4.5. Secretoma Collection
4.6. Cell Density/Cell Proliferation Assays Using Secretome of hMSC-EC
4.7. Protein Analysis by Protein Array
4.8. Wound Healing Model and hMSC Transplantation
4.9. Metabolic Analysis
4.10. Histologic Examination
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pastar, I.; Stojadinovic, O.; Yin, N.C.; Ramirez, H.; Nusbaum, A.G.; Sawaya, A.; Patel, S.B.; Khalid, L.; Isseroff, R.R.; Tomic-Canic, M. Epithelialization in Wound Healing: A Comprehensive Review. Adv. Wound Care 2014, 3, 445–464. [Google Scholar] [CrossRef] [PubMed]
- Werdin, F.; Tennenhaus, M.; Schaller, H.E.; Rennekampff, H.O. Evidence-based management strategies for treatment of chronic wounds. Eplasty 2009, 9, e19. [Google Scholar]
- Brem, H.; Tomic-Canic, M. Cellular and molecular basis of wound healing in diabetes. J. Clin. Investig. 2007, 117, 1219–1222. [Google Scholar] [CrossRef] [PubMed]
- Blair, M. Diabetes Mellitus Review. Urol. Nurs. 2016, 36, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Gaetani, M.; Chinnici, C.M.; Carreca, A.P.; Di Pasquale, C.; Amico, G.; Conaldi, P.G. Unbiased and quantitative proteomics reveals highly increased angiogenesis induction by the secretome of mesenchymal stromal cells isolated from fetal rather than adult skin. J. Tissue Eng. Regen. Med. 2018, 12, e949–e961. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Katagiri, W.; Osugi, M.; Sugimura, Y.; Hibi, H.; Ueda, M. Secretomes from bone marrow-derived mesenchymal stromal cells enhance periodontal tissue regeneration. Cytotherapy 2015, 17, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Osugi, M.; Katagiri, W.; Yoshimi, R.; Inukai, T.; Hibi, H.; Ueda, M. Conditioned media from mesenchymal stem cells enhanced bone regeneration in rat calvarial bone defects. Tissue Eng. Part. A 2012, 18, 1479–1489. [Google Scholar] [CrossRef]
- Michaels, J.; Dobryansky, M.; Galiano, R.D.; Bhatt, K.A.; Ashinoff, R.; Ceradini, D.J.; Gurtner, G.C. Topical vascular endothelial growth factor reverses delayed wound healing secondary to angiogenesis inhibitor administration. Wound Repair. Regen. 2005, 13, 506–512. [Google Scholar] [CrossRef]
- Zarei, F.; Soleimaninejad, M. Role of growth factors and biomaterials in wound healing. Artif. Cells Nanomed. Biotechnol. 2018, 46, 906–911. [Google Scholar] [CrossRef]
- Bakhshayesh, M.; Soleimani, M.; Mehdizadeh, M.; Katebi, M. Effects of TGF-beta and b-FGF on the potential of peripheral blood-borne stem cells and bone marrow-derived stem cells in wound healing in a murine model. Inflammation 2012, 35, 138–142. [Google Scholar] [CrossRef]
- Aguilera, V.; Briceno, L.; Contreras, H.; Lamperti, L.; Sepulveda, E.; Diaz-Perez, F.; Leon, M.; Veas, C.; Maura, R.; Toledo, J.R.; et al. Endothelium trans differentiated from Wharton’s jelly mesenchymal cells promote tissue regeneration: Potential role of soluble pro-angiogenic factors. PLoS ONE 2014, 9, e111025. [Google Scholar] [CrossRef]
- Tao, X.; Sun, M.; Chen, M.; Ying, R.; Su, W.; Zhang, J.; Xie, X.; Wei, W.; Meng, X. HMGB1-modified mesenchymal stem cells attenuate radiation-induced vascular injury possibly via their high motility and facilitation of endothelial differentiation. Stem Cell Res. Ther. 2019, 10, 92. [Google Scholar] [CrossRef]
- Liao, J.; Chen, X.; Li, Y.; Ge, Z.; Duan, H.; Zou, Y.; Ge, J. Transfer of bone-marrow-derived mesenchymal stem cells influences vascular remodeling and calcification after balloon injury in hyperlipidemic rats. J. Biomed. Biotechnol. 2012, 2012, 165296. [Google Scholar] [CrossRef]
- Tigges, U.; Komatsu, M.; Stallcup, W.B. Adventitial pericyte progenitor/mesenchymal stem cells participate in the restenotic response to arterial injury. J. Vasc. Res. 2013, 50, 134–144. [Google Scholar] [CrossRef]
- Alaminos, M.; Pérez-Köhler, B.; Garzón, I.; García-Honduvilla, N.; Romero, B.; Campos, A.; Buján, J. Transdifferentiation Potentiality of Human Wharton’s Jelly Stem Cells Towards Vascular Endothelial Cells. J. Cell Physiol. 2013, 223, 640–647. [Google Scholar] [CrossRef]
- Oswald, J.; Boxberger, S.; Jørgensen, B.; Feldmann, S.; Ehninger, G.; Bornhäuser, M.; Werner, C. Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem Cells. 2013, 22, 377–384. [Google Scholar] [CrossRef]
- Liu, J.W.; Dunoyer-Geindre, S.; Serre-Beinier, V.; Mai, G.; Lambert, J.F.; Fish, R.J.; Pernod, G.; Buehler, L.; Bounameaux, H.; Kruithof, E.K. Characterization of endothelial-like cells derived from human mesenchymal stem cells. J. Thromb. Haemost. 2007, 5, 826–883. [Google Scholar] [CrossRef]
- Kato, J.; Kamiya, H.; Himeno, T.; Shibata, T.; Kondo, M.; Okawa, T.; Fujiya, A.; Fukami, A.; Uenishi, E.; Seino, Y.; et al. Mesenchymal stem cells ameliorate impaired wound healing through enhancing keratinocyte functions in diabetic foot ulcerations on the plantar skin of rats. J. Diabetes Complications 2014, 28, 588–595. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, L.; Scott, P.G.; Tredget, E.E. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells 2007, 25, 2648–2659. [Google Scholar] [CrossRef]
- Fong, C.Y.; Gauthaman, K.; Cheyyatraivendran, S.; Lin, H.D.; Biswas, A.; Bongso, A. Human umbilical cord Wharton’s jelly stem cells and its conditioned medium support hematopoietic stem cell expansion ex vivo. J. Cell Biochem. 2012, 11, 658–668. [Google Scholar] [CrossRef]
- Joseph, A.; Baiju, I.; Bhat, I.A.; Pandey, S.; Bharti, M.; Verma, M.; Pratap Singh, A.; Ansari, M.M.; Chandra, V.; Saikumar, G.; et al. Mesenchymal stem cell-conditioned media: A novel alternative of stem cell therapy for quality wound healing. J. Cell Physiol. 2020, 235, 5555–5569. [Google Scholar] [CrossRef] [PubMed]
- Schlosser, S.; Dennler, C.; Schweizer, R.; Eberli, D.; Stein, J.V.; Enzmann, V.; Giovanoli, P.; Erni, D.; Plock, J.A. Paracrine effects of mesenchymal stem cells enhance vascular regeneration in ischemic murine skin. Microvasc. Res. 2012, 83, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Tredget, E.E.; Wu, P.Y.; Wu, Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS ONE 2008, 3, e1886. [Google Scholar] [CrossRef] [PubMed]
- Kinnaird, T.; Stabile, E.; Burnett, M.S.; Epstein, S.E. Bone-marrow-derived cells for enhancing collateral development: Mechanisms, animal data, and initial clinical experiences. Circ. Res. 2004, 95, 354–363. [Google Scholar] [CrossRef]
- Kinnaird, T.; Stabile, E.; Burnett, M.; Lee, C.; Barr, S.; Fuchs, S.; Epstein, S. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ. Res. 2004, 94, 678–685. [Google Scholar] [CrossRef]
- Beckermann, B.M.; Kallifatidis, G.; Groth, A.; Frommhold, D.; Apel, A.; Mattern, J.; Salnikov, A.V.; Moldenhauer, G.; Wagner, W.; Diehlmann, A.; et al. VEGF expression by mesenchymal stem cells contributes to angiogenesis in pancreatic carcinoma. Br. J. Cancer 2008, 99, 622–631. [Google Scholar] [CrossRef]
- Wang, C.; Li, Y.; Yang, M.; Zou, Y.; Liu, H.; Liang, Z.; Yin, Y.; Niu, G.; Yan, Z.; Zhang, B. Efficient Differentiation of Bone Marrow Mesenchymal Stem Cells into Endothelial Cells in Vitro. Eur. J. Vasc. Endovasc. Surg. 2018, 55, 257–265. [Google Scholar] [CrossRef]
- Khaki, M.; Salmanian, A.H.; Abtahi, H.; Ganji, A.; Mosayebi, G. Mesenchymal Stem Cells Differentiate to Endothelial Cells Using Recombinant Vascular Endothelial Growth Factor—A. Rep. Biochem. Mol. Biol. 2018, 6, 144–150. [Google Scholar]
- Kuchroo, P.; Dave, V.; Vijayan, A.; Viswanathan, C.; Ghosh, D. Paracrine factors secreted by umbilical cord-derived mesenchymal stem cells induce angiogenesis in vitro by a VEGF-independent pathway. Stem Cells Dev. 2015, 24, 437–450. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, H.; Cho, H.; Bae, Y.; Suh, K.; Jung, J. Direct comparison of human mesenchymal stem cells derived from adipose tissues and bone marrow in mediating neovascularization in response to vascular ischemia. Cell Physiol. Biochem. 2007, 20, 867–876. [Google Scholar] [CrossRef]
- Tuo, Q.H.; Zeng, H.; Stinnett, A.; Yu, H.; Aschner, J.L.; Liao, D.F.; Chen, J.X. Critical role of angiopoietins/Tie-2 in hyperglycemic exacerbation of myocardial infarction and impaired angiogenesis. Am. J. Physiol Heart Circ. Physiol. 2008, 294, H2547–H2557. [Google Scholar] [CrossRef][Green Version]
- Cannella, V.; Piccione, G.; Altomare, R.; Marino, A.; Di Marco, P.; Russotto, L.; Di Bella, S.; Purpari, G.; Gucciardi, F.; Cassata, G.; et al. Differentiation and characterization of rat adipose tissue mesenchymal stem cells into endothelial-like cells. Anat. Histol. Embryol. 2018, 47, 11–20. [Google Scholar] [CrossRef]
- Feraud, O.; Mallet, C.; Vilgrain, I. Expressional regulation of the angiopoietin-1 and -2 and the endothelial-specific receptor tyrosine kinase Tie2 in adrenal atrophy: A study of adrenocorticotropin-induced repair. Endocrinology 2003, 144, 4607–4615. [Google Scholar] [CrossRef][Green Version]
- Skalnikova, H.K. Proteomic techniques for characterisation of mesenchymal stem cell secretome. Biochimie 2013, 95, 2196–2211. [Google Scholar] [CrossRef]
- Zimmerlin, L.; Park, T.S.; Zambidis, E.T.; Donnenberg, V.S.; Donnenberg, A.D. Mesenchymal stem cell secretome and regenerative therapy after cancer. Biochimie 2013, 95, 2235–2245. [Google Scholar] [CrossRef]
- Asahara, T.; Bauters, C.; Zheng, L.P.; Takeshita, S.; Bunting, S.; Ferrara, N.; Symes, J.F.; Isner, J.M. Synergistic effect of vascular endothelial growth factor and basic fibroblast growth factor on angiogenesis in vivo. Circulation 1995, 92, II365–II371. [Google Scholar] [CrossRef]
- Cao, R.; Bråkenhielm, E.; Pawliuk, R.; Wariaro, D.; Post, M.J.; Wahlberg, E.; Leboulch, P.; Cao, Y. Angiogenic synergism, vascular stability and improvement of hind-limb ischemia by a combination of PDGF-BB and FGF-2. Nat. Med. 2003, 9, 604–613. [Google Scholar] [CrossRef]
- Lopes, L.; Setia, O.; Aurshina, A.; Liu, S.; Hu, H.; Isaji, T.; Liu, H.; Wang, T.; Ono, S.; Guo, X.; et al. Stem cell therapy for diabetic foot ulcers: A review of preclinical and clinical research. Stem. Cell Res. Ther. 2018, 9, 188. [Google Scholar] [CrossRef]
- Vizoso, F.J.; Eiro, N.; Cid, S.; Schneider, J.; Perez-Fernandez, R. Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. Int. J. Mol. Sci. 2017, 18, 1852. [Google Scholar] [CrossRef]
- Cooper, D.R.; Wang, C.; Patel, R.; Trujillo, A.; Patel, N.A.; Prather, J.; Gould, L.J.; Wu, M.H. Human Adipose-Derived Stem Cell Conditioned Media and Exosomes Containing MALAT1 Promote Human Dermal Fibroblast Migration and Ischemic Wound Healing. Adv. Wound Care 2018, 7, 299–308. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ormazabal, V.; Nova-Lampeti, E.; Rojas, D.; Zúñiga, F.A.; Escudero, C.; Lagos, P.; Moreno, A.; Pavez, Y.; Reyes, C.; Yáñez, M.; et al. Secretome from Human Mesenchymal Stem Cells-Derived Endothelial Cells Promotes Wound Healing in a Type-2 Diabetes Mouse Model. Int. J. Mol. Sci. 2022, 23, 941. https://doi.org/10.3390/ijms23020941
Ormazabal V, Nova-Lampeti E, Rojas D, Zúñiga FA, Escudero C, Lagos P, Moreno A, Pavez Y, Reyes C, Yáñez M, et al. Secretome from Human Mesenchymal Stem Cells-Derived Endothelial Cells Promotes Wound Healing in a Type-2 Diabetes Mouse Model. International Journal of Molecular Sciences. 2022; 23(2):941. https://doi.org/10.3390/ijms23020941
Chicago/Turabian StyleOrmazabal, Valeska, Estefanía Nova-Lampeti, Daniela Rojas, Felipe A. Zúñiga, Carlos Escudero, Paola Lagos, Alexa Moreno, Yanara Pavez, Camila Reyes, Milly Yáñez, and et al. 2022. "Secretome from Human Mesenchymal Stem Cells-Derived Endothelial Cells Promotes Wound Healing in a Type-2 Diabetes Mouse Model" International Journal of Molecular Sciences 23, no. 2: 941. https://doi.org/10.3390/ijms23020941
APA StyleOrmazabal, V., Nova-Lampeti, E., Rojas, D., Zúñiga, F. A., Escudero, C., Lagos, P., Moreno, A., Pavez, Y., Reyes, C., Yáñez, M., Vidal, M., Cabrera-Vives, G., Oporto, K., & Aguayo, C. (2022). Secretome from Human Mesenchymal Stem Cells-Derived Endothelial Cells Promotes Wound Healing in a Type-2 Diabetes Mouse Model. International Journal of Molecular Sciences, 23(2), 941. https://doi.org/10.3390/ijms23020941







