Abstract
Enzymatic oxidations of thiophenes, including thiophene-containing drugs, are important for biodesulfurization of crude oil and drug metabolism of mono- and poly-cyclic thiophenes. Thiophene oxidative dearomatization pathways involve reactive metabolites, whose detection is important in the pharmaceutical industry, and are catalyzed by monooxygenase (sulfoxidation, epoxidation) and dioxygenase (sulfoxidation, dihydroxylation) enzymes. Sulfoxide and epoxide metabolites of thiophene substrates are often unstable, and, while cis-dihydrodiol metabolites are more stable, significant challenges are presented by both types of metabolite. Prediction of the structure, relative and absolute configuration, and enantiopurity of chiral metabolites obtained from thiophene enzymatic oxidation depends on the substrate, type of oxygenase selected, and molecular docking results. The racemization and dimerization of sulfoxides, cis/trans epimerization of dihydrodiol metabolites, and aromatization of epoxides are all factors associated with the mono- and di-oxygenase-catalyzed metabolism of thiophenes and thiophene-containing drugs and their applications in chemoenzymatic synthesis and medicine.
1. Introduction
Although the link between aromaticity and resulting molecular stability is well established, enzymes have a remarkable capacity for dearomatization of many stable and recalcitrant arene and heteroarene substrates. Enzyme-catalyzed dearomatization reactions have been reported under both oxidative and reductive conditions. Examples of redox enzymatic dearomatization reactions of benzene rings A (R = H, Me, COSCoA) that give conjugated cyclohexadiene metabolites include: (i) monooxygenase (MO)-catalyzed epoxidation to yield an arene oxide B (R = H) [1], (ii) ring-hydroxylating dioxygenase (DO)-catalyzed cis dihydroxylation to yield a cis-dihydrodiol C (R = Me) [2], (iii) reductase (CoARed)-catalyzed reduction to give a dihydroarene D (R = COSCoA) (Scheme 1a) [3].
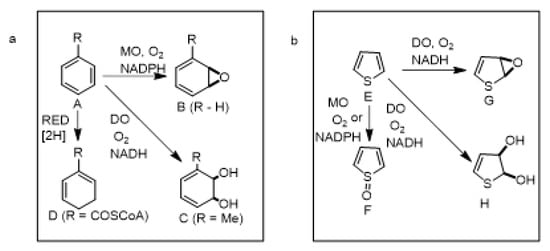
Scheme 1.
(a) Dearomatization of arenes (A). (b) Dearomatization of thiophene (E).
Attempted chemical approaches to the oxidative dearomatization reactions of carbocyclic arenes, shown in Scheme 1a, often result in further oxidation, or rearomatization of the initial products. Due to their instability, relatively few arene oxide metabolites B have been isolated from monooxygenase (MO)-catalyzed epoxidations of substituted benzene substrates A. Ring-hydroxylating dioxygenase (DO)-catalyzed cis-dihydroxylation of similar substrates A can, however, produce more stable cis-dihydrodiols C. Despite their limited stability, cis-dihydrodiol metabolites have been widely used in the chemoenzymatic synthesis of natural products and other metabolites [4,5,6,7,8,9].
The ability of similar oxidative dearomatization reactions of a thiophene substrate E to replicate MO-catalyzed epoxidation to yield epoxide G, DO-catalyzed cis-dihydroxylation to give cis-dihydrodiol H, and heteroatom oxidation to give sulfoxide F (Scheme 1b) using either type of oxygenase enzyme is examined in this review.
Aromaticity of the monocyclic six-membered arenes, e.g., benzene, and five-membered heteroarenes, e.g., pyrrole, thiophene, and furan, confers extra stability and depends on the descriptor used [10]. Resonance energies have been widely used as an indicator of decreasing aromaticity in a sequence, from the most stable benzene > thiophene > pyrrole > furan. Most other qualitative and quantitative descriptors of aromaticity also suggest that the sequence should be: benzene > thiophene > pyrrole > furan [11].
As acceptable thiophene substrates for ring-hydroxylating dioxygenases are generally smaller than for monooxygenase substrates, the topic of dioxygenase-catalyzed cis-dihydroxylation and sulfoxidation of thiophene substrates is initially considered. The only cis-dihydrodiol metabolites from the monocyclic five-membered heteroarenes, thiophene, pyrrole, and furan, of sufficient stability to be isolated and characterized are derived from thiophene substrates. Thus, the relatively stable cis-dihydrodiol metabolite H was obtained from toluene dioxygenase (TDO)-catalyzed oxidation of thiophene E along with the unstable thiophene-S-oxide F (Scheme 1b) [12].
Thiophenes and sulfur-containing polycyclic aromatic hydrocarbons (thiaarenes), present in the aromatic fraction of crude oil, include monocyclic thiophenes (e.g., dialkyl thiophenes), bicyclic thiaarenes (e.g., benzothiophenes), and tricyclic thiaarenes (e.g., dibenzothiophenes, naphthothiophenes) [13,14]. The requirement to remove these organosulfur constituents of crude oil stimulated interest in their oxidative biodegradation by Pseudomonas bacteria (biodesulfurization), as shown by the extensive studies of the Fedorak group [15,16,17,18,19,20,21,22,23,24,25]. Evidence of enzymatic dearomatization of thiophenes, to yield 2,3-dione, sulfoxide, sulfoxide dimer, and sulfone metabolites, was supplied by GC-MS and GC-FTIR analyses.
Early studies of the bacterial metabolism of thiaarenes also showed that dioxygenase-catalyzed cis-dihydroxylation of carbocyclic and heterocyclic rings could yield chiral cis-dihydrodiols as transient metabolites. Furthermore, isolation of these intermediates was possible when using P. putida mutant strains (or E. coli recombinant strains), expressing dioxygenases where an enzyme that is usually present in the metabolic pathway, a cis-dihydrodiol dehydrogenase was blocked (or absent) [26,27,28].
Biotransformations of monocyclic thiophene and polycyclic thiophene (thiaarene) substrates were conducted using wild-type, mutant, and recombinant bacterial strains expressing ring-hydroxylating dioxygenases with different active site capacities. The most widely used enzymes were toluene dioxygenase (TDO), naphthalene dioxygenase (NDO), and biphenyl dioxygenase (BPDO). The exact nature of the catalytic cycle involved in ring-hydroxylating dioxygenase-catalyzed cis-dihydroxylations remains unresolved, but possible mechanisms for producing arene metabolites are available [29,30].
This review deals with the stability, structure, stereochemistry, and mechanism involved during dioxygenase- and monooxygenase-catalyzed oxidative metabolism and dearomatization of mono- and poly-cyclic thiophenes via sulfoxidation, cis-dihydroxylation, and epoxidation. Ring-hydroxylating dioxygenase enzymes generally oxidize only relatively small (mono- to penta-cyclic) arene substrates compared with monooxygenase enzymes that can catalyze oxidation of much larger and more highly substituted substrates. Where common dioxygenase- and monooxygenase-catalyzed sulfoxidations occur, the results are linked by cross-references. The possibility of lessons learned from dioxygenase metabolism of thiophenes, being of value in monooxygenase metabolism, is explored.
2. Dioxygenase-Catalyzed Dearomatization of Thiophenes 1a–g
2.1. Enzymatic Oxidation of Thiophenes 1a–g to Yield cis-Dihydrodiols
The thienyl ring system is ubiquitous in the environment, with many monocyclic thiophenes being found as plant natural products and constituents of fossil fuels [13,31]. The bacterial biodegradation of thiophene 1a [12] and alkyl-substituted thiophenes, [22,25,32] present in bitumen and crude oils, was examined as a route to biodesulfurization.
Relatively few reports of dioxygenase-catalyzed cis-dihydroxylation of monocyclic thiophenes have been reported in the literature. BPDO-catalyzed dihydroxylation of 3-hexylthiophene yielded a dihydrodiol metabolite with an unknown stereoconfiguration and showed no evidence of sulfoxide bioproducts [32]. A study of the metabolites isolated from TDO- and NDO-catalyzed oxidations over a wider range of monocyclic thiophene substrates revealed cis-dihydrodiols, cis/trans-dihydrodiols, and sulfoxides as common chiral bioproducts (Section 2.1, Section 2.2, Section 2.3, Section 3.1 and Section 3.2) [12].
Enantiomeric excess (ee) values of thiophene cis-dihydrodiol metabolites were determined by chiral stationary phase HPLC and GC analyses [33,34]. An alternative method for enantiopure cis-dihydrodiol metabolites required NMR analysis of diMTPA esters formed by reactions of (+) and (−)-2-methoxy-2-(trifluoromethyl)phenylacetyl chloride (MTPA-Cl) [35] with 4-phenyl-1,2,4-triazoline-3,5-dione cycloadducts [36] or with hydrogenated cis-dihydrodiols [37].
NMR analyses of boronate diastereoisomers, formed by the reaction of thiophene cis-dihydrodiols with (+)- and (−)-[2-(-methoxyethyl)phenyl]boronic acid (MEPBA), provided both ee values and absolute configuration assignments [38,39,40]. Furthermore, X-ray crystallography of cis-dihydrodiols or their diMTPA derivatives [33,37,41], and comparison of electronic circular dichroism (ECD) spectra with density functional theory ECD spectra, gave unambiguous absolute configurations for thiophene cis-dihydrodiol metabolites [41,42,43].
Many enantiopure cis-dihydrodiol metabolites from arene and heteroarene substrates are used in the synthesis of natural products [4,5,6,7,8,9], including arene oxides [44]. The potential use of thiophene cis-dihydrodiols in the future chemoenzymatic synthesis of thiophene epoxide metabolites produced during monooxygenase-catalyzed oxidations is explored herein. The cis-dihydroxylation dearomatization of monocyclic thiophenes 1a,h–k, using TDO, (P.putida UV4) yielded the corresponding cis-diol metabolites 2a, 2h–k, along with the trans isomers 4a, h–k (Scheme 2, Table 1) [12]. The formation of trans-dihydrodiol 4a was unusual, since only cis-dihydrodiol metabolites were formed from monocyclic arenes with TDO [4,5,6,7,8,9]. trans-Dihydrodiol metabolites of carbocyclic arenes generally result from monooxygenase-catalyzed epoxidation followed by epoxide hydrolase catalyzed hydrolysis.
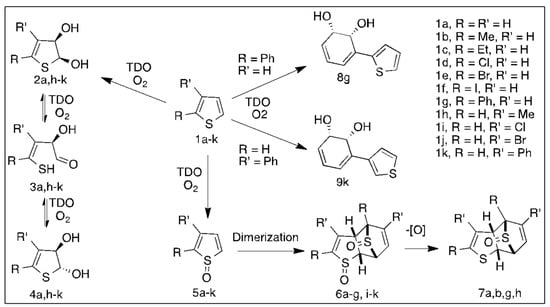
Scheme 2.
TDO-catalyzed metabolism of thiophenes 1a–k.

Table 1.
Relative yields of metabolites from thiophenes 1a–g [12].
The hemithioacetal cis-isomer 2a was the initially formed metabolite from thiophene 1a; it spontaneously epimerized with the trans isomer 4a via the undetected aldehyde intermediate 3a (Scheme 2) [12]. The equilibrium ratio was found to be solvent-dependent, favoring cis isomer 2a in CDCl3 (ca. 60%) and trans-isomer 4a in more polar solvents CD3OD and D2O (>90%). A similar type of epimerization process involving aldehyde intermediates occurs during the mutarotation of α- and β-glucose.
The cis-dihydrodiol metabolites, formed by TDO-catalyzed dihydroxylation, exclusively at the 2,3-bond of monosubstituted benzene substrates except for fluorobenzene, were enantiopure (>98% ee) [4,5,6,7,8,9]. Conversely, the isolated thiophene dihydrodiol metabolites were found to be mixtures of cis: trans isomers (2a:4a) and enantiomers (2a:4a, 44% ee) [12]. The lower ee values for dihydrodiols 2a and 4a could result from the reduced stereoselectivity due to weaker binding and more flexibility of the smaller heterocyclic ring 1a within the TDO active site. Alternatively, the low ee value of cis-dihydrodiol 2a could result from partial racemization of the aldehyde intermediate 3a. Phenyl ring cis-hydroxylation of the larger 2-phenyl thiophene 1g was also observed to yield 1R,2S-dihydrodiol 8g, (>98% ee) (Table 1).
Biotransformations of mono- or poly-cyclic arenes and azaarenes, using P. putida mutant and E. coli recombinant strains, yielded only carbocyclic ring cis-dihydrodiol metabolites [36,45,46,47]. However, dioxygenase-catalyzed oxidation of monocyclic thiophenes 1b–1g involved competing carbocyclic and heterocyclic ring cis-dihydroxylation and sulfoxidation pathways (Table 1). Replacement of phenylalanine 352 (Phe 352) by valine, within the NDO active site of E. coli-pDTG141, yielded E. coli-pF352V; this results in significant regio- and enantio-selectivity changes occurring during cis-dihydroxylation of polycyclic arenes [45,46]. A further marked change in regioselectivity is observed during NDO-catalyzed cis-dihydroxylation of 2-phenyl thiophene 1g. One strain (E. coli-pFDTG141) yielded only a phenyl ring cis-dihydrodiol 8g, while an alternative strain (E. coli-pF352V) gave thienyl ring cis:trans dihydrodiols (2g:4g) exclusively (Table 1). This shows the value of having different bacterial strains and dioxygenase enzymes available to produce preferred metabolites.
2.2. Oxidations of Thiophenes 1a–g to Yield Sulfoxide Metabolites
TDO- and NDO-catalyzed stereoselective sulfoxidations of alkylaryl sulfides using wild-type, mutant, recombinant strains and purified dioxygenase enzyme often yielded sulfoxide metabolites with high ee values [48,49,50]. Competition between cis-dihydroxylation and sulfoxidation of alkyl aryl sulfides and formation of cis-dihydrodiol sulfoxides was observed [51]. Results collected using recombinant strains expressing TDO and NDO provided supporting evidence that dioxygenases were also responsible for thiophene sulfoxidations, with both wild-type and mutant bacterial strains. A contest between sulfoxidation and epoxidation of monocyclic thiophenes and thiophene-containing drugs was also found using cytochrome P450 monooxygenases as biocatalysts (Section 6.2 and Section 6.3) [52,53,54,55,56,57,58].
Sulfoxidation of thiophenes 1a–g was the major metabolic route with the P. putida UV4 strain (Scheme 2, Table 1). The main metabolite isolated from thiophene 1a was sulfoxide dimer 6a (Table 1) [12]. In addition, traces of the unstable sulfoxide 5a were identified (GC-MS analysis) among other products. CYP-450 monooxygenase-catalyzed metabolism of thiophene 1a and peroxyacid oxidation of thiophene 1a yielded sulfoxide 5a, which rapidly dimerized to form compound 6a [12]
The monosulfoxide structure 5a was both that of a cyclic diene and a dienophile, resulting in a spontaneous dimerization by Diels Alder cycloaddition to give the dimer 6a (R = R′ = H). Further biotransformation via stereospecific deoxygenation yielded metabolite 7a (Table 1, Scheme 2) [12]. Some reductases were found to catalyze preferential deoxygenation of alkylaryl sulfoxides and also the sterically more accessible sulfoxide group of dimer 6a [59,60,61,62,63]. An unidentified sulfoxide reductase enzyme, expressed in P. putida UV4 [62] was particularly efficient. Methionine sulfoxide reductase-catalyzed deoxygenations of sulfoxides were also observed using Pseudomonas strains [59,60].
Sulfoxides formed by dioxygenase-catalyzed oxidation of alkylaryl sulfides [28,48,49,50] are configurationally stable, i.e., with inversion barriers (ΔG‡) > 23.0 kcal mol−1. Conversely, monocyclic thiophene sulfoxides (e.g., 1a and 1g) were predicted to have much lower barriers (ΔG≠ = 11–14 kcal mol−1), due to aromatic stabilization of their planar transition states [64,65,66]. Monocyclic thiophene sulfoxides, with bulky substituents, are more thermally stable. Thus 2,5-diphenylthiophene-1-oxide is sufficiently stable for X-ray crystallography studies [67]. Several racemic monocyclic thiophene sulfoxides, with bulky groups, were sufficiently stable to provide experimental confirmation of these relatively low barriers by NMR spectroscopic methods (ΔG≠ = 14.8; 15.9 kcal mol−1) [68,69]. Thiophene sulfoxide metabolites 5b–g are susceptible to rapid racemization and cycloaddition, thus yielding racemic sulfoxide dimers 6b–g (Scheme 2). Individual sulfoxide enantiomers can have different medical efficacy values, e.g., omeprazole, esomeprazole, modafinil, and armodafinil [70], but, with low sulfoxide inversion barriers for drugs containing monocyclic thiophenes, spontaneous racemization will occur.
The results in Table 1 show that TDO-catalyzed sulfoxidation of thiophenes 1a–g is the major dearomatization pathway, with cis-dihydroxylation of thienyl and phenyl rings, respectively, being minor pathways. CYP450-catalyzed oxidation of thiophenes 1a, 1b, and 1g yielded the corresponding sulfoxides 6a, 6b, and 6g, and their dimers 7a, 7b, and 7g, as major products, but deoxygenation of dimers was not reported (Scheme 2).
2.3. Molecular Docking of Thiophenes 1a and 1g at the TDO Active Site
The Autodock Vina program was used for docking toluene A (R = Me, Scheme 1a) and other arene and heteroarene substrates within the active site of toluene dioxygenase [71,72]. An X-ray crystal structure of the TDO showed the toluene substrate bound at the active site by proximate amino acids but without dioxygen complexed to Fe(III) [73]. An X-ray crystal structure of NDO displayed dioxygen coordinated with Fe(III) and indole substrate bonded at the active site [74]. The O2-Fe(III) complex of NDO was then inserted into the TDO active site employing the reported procedure [72]. The interaction between dioxygen and Fe(III) during the catalysis of the cis-dihydroxylation is considered to involve a hydroperoxide (Fe-OOH) [29]. Attractive interactions between substrates and proximate amino acids (Phe-216, His-222, Ile-276, Leu-272, Ile-324, Val-309, Leu-272, Figure 1A) allow the preferred binding orientation to be predicted; it matched both with the regio-and stereo-selectivity observed during TDO-catalyzed cis-dihydroxylation of toluene and other substituted benzene substrates to form cis-dihydrodiols (Scheme 1a) [72,74,75,76,77,78,79].
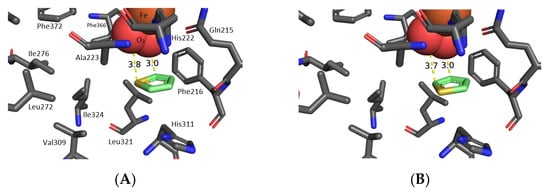
Figure 1.
Preferred orientation of thiophene 1a for TDO-catalyzed: sulfoxidation (A) and cis-dihydroxylation (B).
A similar in situ docking approach, applied earlier to other arene and heteroarene substrates [71,72], was used to determine the preferred binding orientations of thiophene 1a (Figure 1A,B) and 2-phenylthiophene 1g (Figure 2A,B). As expected for a smaller five-membered heteroarene substrate, binding energies (± 0.5 kcal mol−1) for thiophene 1a, Figure 1A (−3.63 kcal mol−1) and B (−3.3 kcal mol−1), were lower compared to a six-membered arene substrate, e.g., toluene A (R = Me, −5.0 kcal mol−1). The minimum distances between the nearest oxygen and sulfur atoms (proximity value, 3.8 Å, Figure 1A), or between proximate oxygen atoms and the 2,3-bond (3.0–3.7 Å, Figure 1B), are similar to those found between TDO and toluene A (R = Me). The favored orientation of thiophene 1a in Figure 1A is consistent with TDO-catalyzed sulfoxidation to yield sulfoxide 5a and derived bioproducts 6a and 7a [12]. The reduced substrate size, lower binding energy, but favorable proximity factor for compound 1a in Figure 1B could be factors in the reduced ee value (44%) and lower yield of the isolated heterocyclic cis/trans diols 2a/4a (Table 1).
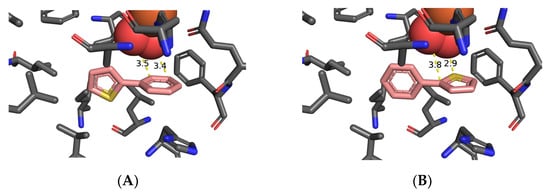
Figure 2.
Preferred orientation of 2-phenylthiophene 1g for TDO-catalyzed: cis-dihydroxylation (A) and sulfoxidation (B).
The preferred orientation of 2-phenylthiophene 1g at the TDO active site (Figure 2A) has a relatively high binding energy (−5.5 kcal mol−1) and proximity of the phenyl group to dioxygen (3.4–3.5 Å). The predicted regioselectivity and absolute configuration of cis-dihydrodiol 8g was found, experimentally, to be (1S,2R) (Table 1) [12].
The preferred orientation of substrate 1g, at the TDO active site, predicted from the binding energy (−5.3 kcal mol−1) and the distance of the sulfur to dioxygen (2.9 Å), should yield (1S)-sulfoxide 5g. However, the low inversion barrier, predicted for sulfoxide 5g ΔG = 11.2 kcal mol−1) [64], indicated that it would rapidly racemize, and this was confirmed by the isolation of racemic dimer 6g (Table 1). The docking of substrate 1g did not present an orientation that would lead to cis-dihydrodiol metabolite 2g, and none of the possible cis/trans-dihydrodiols 2b–g/4b–g were isolated (Table 1) [12].
3. Dioxygenase-Catalyzed Dearomatization of 3-Substituted Thiophenes 1h–k
3.1. Oxidations of Thiophenes 1h–k to Yield cis-Dihydrodiol and Sulfoxide Metabolites
Biotransformations (P. putida UV4) involving cis-dihydroxylations of 3-substituted thiophenes 1h–j yielded, mainly, cis-dihydrodiols 2h–j (Table 2) [12]. Spontaneous epimerization of metabolites 2h–j gave mixtures of cis/trans dihydrodiols and enantiomers 2h/4h (40–48% ee), 2i/4i (49% ee), and 2j/4j (44% ee). This very unusual observation merits rationalization since most cis-dihydrodiol metabolites from monosubstituted benzene substrates had very high ee values (>98%) [4,5,6,7,8,9]. It is proposed that the lower ee values found during TDO-catalyzed cis-dihydroxylation of thiophene 1a and 3-substituted thiophenes 1h–j could result from the reduced size of the substrates and increased flexibility within the TDO active site. TDO-catalyzed cis-dihydroxylation of the larger substrate 1k yielded dihydrodiols 2k/4k (>98% ee) and 9k (>98% ee, Table 2).

Table 2.
Relative yields of metabolites from 3-substituted thiophenes 1h–k [12].
Dioxygenase-catalyzed sulfoxidation was also an important dearomatization route for 3-substituted thiophenes 1h–k, yielding racemic sulfoxide dimers 6i–k from sulfoxide intermediates 5i–k (Table 2). Dimer 6h was undetected, as it was further metabolized via a stereoselective reductase-catalyzed partial deoxygenation to form monosulfoxide 7h (51% ee) [12].
The regioselectivity observed, during TDO-catalyzed cis-dihydroxylation of 3-substituted thiophenes 1h–k, involved exclusive attack at the unsubstituted 4,5-bond of thiophenes 1h–k to yield cis/trans-dihydrodiols 2h–k/4h–k (Scheme 3) [12].
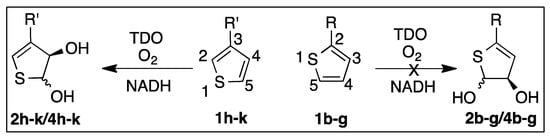
Scheme 3.
Contrasting TDO-catalyzed dihydroxylations for thiophenes 1h–k and 1b–g.
Molecular docking studies (Figure 2A,B and Figure 3A–C) provided supporting evidence for the preferred regioselectivity found in Scheme 3. TDO-catalyzed dihydroxylation, at the 4,5-bond of thiophenes 1h–k, is equivalent to cis-dihydroxylation exclusively at the 2,3-bond of monosubstituted benzene substrates (Scheme 1a).
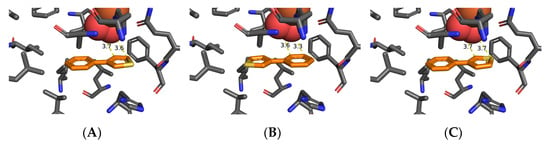
Figure 3.
Preferred orientation of 3-phenylthiophene 1k for TDO-catalyzed: thienyl cis-dihydroxylation (A), phenyl cis-dihydroxylation (B), and sulfoxidation (C).
A remarkable change in chemoselectivity was observed during NDO-catalyzed oxidation of 3-phenyl thiophene 1k; cis-hydroxylation of the phenyl ring gave diol 9k (98% E. coli DTG 141) while oxidation of the thiophene ring produced sufoxide dimer 6k (80% E. coli pF 353V, Table 2).
3.2. Molecular Docking of 3-Phenylthiophene 1k at the TDO Active Site
The preferred orientation of 3-phenyl thiophene 1k had a binding energy (−4.7 kcal mol−1) and proximity of dioxygen to the 4,5-bond of the thiophene ring (3.6–3.7 Å, Figure 3A). This would lead to cis-dihydroxylation and formation of cis-dihydrodiol 2k, prior to equilibration with trans-dihydrodiol 4k; it matched the experimental result (>98% ee, Table 2, Scheme 3) [12].
The orientation of substrate 1k (Figure 3B), with a binding energy (−4.5 kcal mol−1), and proximity of the 2,3-bond of the phenyl ring to dioxygen (3.3–3.6 Å), would also lead to cis-dihydroxylation and the (1S,2R) absolute configuration of the isolated dihydrodiol 9k (>98% ee, Table 2) [12]. The favored orientation of substrate 1k (Figure 3C), with binding energy (−4.7 kcal mol−1), and proximity of the S atom to the nearest dioxygen atom (3.7 Å), would result in sulfoxidation of substrate 1k to yield an excess of one enantiomer of 3-phenylthiophene-1-oxide 5k, prior to its rapid racemization and dimerization to give disulfoxide 6k (Table 2). Earlier GOLD and Autodock Vina molecular docking studies of mono- and poly-cyclic arene and heteroarene substrates within the TDO active site concluded that their preferred orientations were controlled by: (i) attractive edge-to-face (T-bond) interactions between orthogonal phenyl (Phe-216) groups of TDO and phenyl or pyridyl groups of substrates, (ii) van der Waals interactions with proximate hydrophobic amino acids (Ile-276, Leu-272, Ile-324, Val-309, Leu-272, and Phe-352, Figure 1A) [72,74,75,76,77,78,79]. Attractive edge-to-face interactions are also found between phenyl rings of TDO (Phe-216) and of substrates 1g (Figure 2A) and 1k (Figure 3B). Edge-to-face interactions, between phenyl and thienyl groups, were earlier identified by NMR and X-ray crystallographic studies of imines [80]. Similar novel edge-to-face interactions are observed between phenyl (Phe-216) and thienyl rings in substrates 1a (Figure 1A,B), 1g (Figure 2A,B), 1k (Figure 3A–C).
4. Dioxygenase-Catalyzed Dearomatization of Benzo[b]thiophenes 10a–d
Biodesulfurization of benzo[b]thiophene 10a and alkyl-substituted benzo[b]thiophenes, e.g., compounds 10b–d (Scheme 4), present in fossil fuels, was extensively investigated utilizing wild-type Pseudomonas strains (c.f. 1. Introduction) [15,16,17,18,19,20,21,22,23,24,25]. Metabolites identified during these studies included: (i) benzo[b]thiophene sulfoxides, derived sulfones and dimers, (ii) hydroxybenzo[b]thiophenes and dione metabolites, (iii) ring-opened bioproducts (Scheme 4). It was proposed that ring-hydroxylating dioxygenase enzymes (DO), present in wild-type strains of P. putida, were responsible for the initial steps in the metabolism of benzo[b]thiophene 10a, leading to cis-dihydrodiols, sulfoxides, and sulfoxide dimers [26,27]. Other enzymes, including cis-diol dehydrogenases, cis/trans isomerases, and catechol dioxygenases, catalyzed further metabolism, to yield catechols, diones, and ring-opened metabolites (Scheme 4). Cytochrome P450 and styrene monooxygenases also catalyzed sulfoxidation of benzo[b]thiophenes and benzo[b]thiophene-containing drugs (Section 6.3).
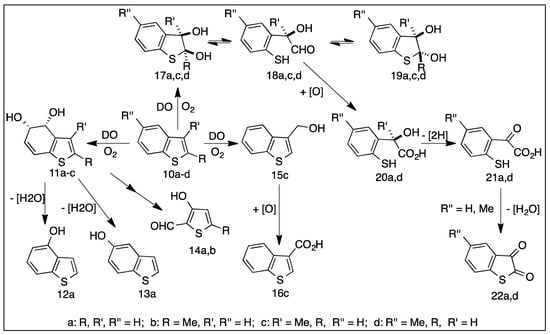
Scheme 4.
Metabolic pathways of benzo[b]thiophenes 10a–d using P. putida strains.
4.1. Biotransformations of Benzo[b]thiophenes 10a–d to Yield cis-Dihydrodiols 11a–c
Similar bacterial strains used for thiophene substrates 1a–k, expressing TDO or NDO, were again utilized for benzo[b]thiophenes 10a–d (Scheme 4 and Scheme 5), under similar biotransformation conditions. Toluene dioxygenase (P. putida UV4) and isopropylbenzene dioxygenase (P. putida RE213) oxidation studies of benzothiophene 10a indicated that 4R,5S-dihydrodiol 11a (>98% ee) was a relatively unstable minor product; partial dehydration occurred during the biotransformation, giving hydroxybenzo[b]thiophenes (12a and 13a, Scheme 4) [26,81,82,83].
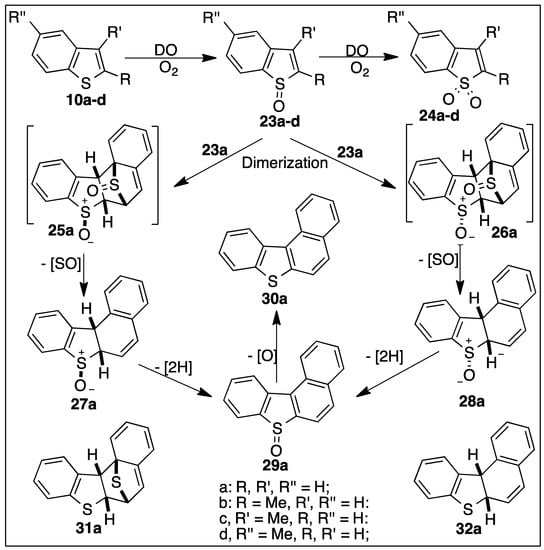
Scheme 5.
Proposed DO-catalyzed metabolic pathways of benzo[b]thiophenes 10a–d.
TDO-catalyzed cis-dihydroxylation of benzo[b]thiophene 10b and 10c similarly gave cis-dihydrodiols 4R,5S-11b (>98% ee) and 4R,5S-11c (>98% ee), but the presence of a methyl group at the C-5 position inhibited cis-dihydroxylation at the 4,5-bond of substrate 10d (Table 3).

Table 3.
Relative yields of metabolites from benzo[b]thiophenes 10a–d.
Using NDO-expressing P. putida strains (9816/11, NCIMB 8859, and RE204) a much wider range of metabolites from benzo[b]thiophenes 10a-c were obtained. Included were alcohol 15c [83], aldehydes 14a [26], 14b [83], carboxylic acid 16c [83], and cis-dihydrodiol 11b [81].
4.2. Biotransformations of Benzo[b]thiophenes 10a–d to Yield cis-Dihydrodiols 17a–d and trans-Dihydrodiols 19a, 19d
As found in TDO-catalyzed biotransformations (P. putida UV4) of monocyclic thiophenes 1h–k, metabolism of benzo[b]thiophenes 10a–d resulted in cis-dihydroxylation of the thiophene rings to yield thiohemiacetal cis-dihydrodiols 17a, 17b, and 17d (Scheme 4). Spontaneous epimerization of cis isomers 17a and 17d, via undetected aldehyde intermediates 18a and 18d, yielded isomeric mixtures with the trans-isomers (2S,3R)-17a/(2R,3R)-19a (62% ee) and (2S,3R)-17d/(2R,3R)-19d (>98% ee, Scheme 4, Table 2) [26,81,82,83]. Tentative evidence for mercaptoaldehyde 18a, being the intermediate formed during epimerization of diols 17a and 19a, was provided by HPLC and UV spectroscopic analyses [27]. 2-Methylbenzo[b]thiophene 10b yielded only cis-dihydrodiol (2S,3R)-17b (9% ee), which, as a thioacetal, was less susceptible to ring-opening isomerization and isomer 19b was not found. Dihydrodiols 17c was obtained as a very minor product but only from NDO-catalyzed oxidation (P. putida 9816/11, Table 3).
Isomeric mixtures of benzo[b]thiophene dihydrodiol metabolites 17a/19a and 17d/19d in very polar solvents showed mainly the trans isomer (98-100% in CD3OD and D2O), but, in a less polar solvent, the cis isomer was dominant (78-80% in CDCl3). Fractional recrystallization of mixtures of dihydrodiol isomers 19a/17a furnished pure samples (>98% ee) of isomers 19a (from MeOH) and 17a (from CH2Cl2-hexane) [81,82].
The thiophene ring cis-dihydrodiols (17a,c,d) and trans- dihydrodiol epimers (19a, 19d) are much more stable than the fused benzene ring cis-dihydrodiols (11a-c). This later led to the use of dihydrodiols 17a/19a in the first synthesis of a thiophene epoxide (Section 5.4) [82].
Benzo[b]thiophene 2,3-dione metabolites 22a and 22d from substrates 10a and 10d, were obtained from dihydrodiol intermediates 17a and 17d when using wild-type P. putida strains (Scheme 4) [16,20,27]. The metabolic sequence progressed via further intermediates (18a and 18d), → (20a and 20d) → (21a and 21d) and was also observed in other bacterial strains [84].
4.3. Biotransformations of Benzo[b]thiophenes 10a–d to Yield Sulfoxides 23a–d
The dioxygenase-catalyzed metabolism of the benzo[b]thiophene 10a results in sulfoxidation, dimerization, and formation of tetracyclic thiophenes (Scheme 5).
An authentic sample of the racemic monosulfoxide 23a was obtained by peroxyacid or dimethyldioxirane oxidation of benzo[b]thiophene 10a. It was found to be sufficiently stable for characterization in solution (CD3COCD3), but, on concentration, it disproportionated to form benzo[b]thiophene 10a and sulfone 24a [83]. As expected, the TDO-catalyzed (P. putida UV4) sulfoxidation of benzo[b]thiophene 10a yielded the unstable monosulfoxide 23a. Dimerization then yielded the undetected bis-sulfoxides 25a and 26a; further metabolism of these intermediates led to the isolation of minor tetracyclic bioproducts 27a–30a (Scheme 5) [81,83].
Several mechanisms were proposed for the formation of metabolites 27a–30a (Scheme 5) [19,83,85]. One mechanism involved spontaneous dimerization of monosulfoxide 23a to form transient bis-sulfoxide diastereoisomers 25a and 26a, followed by abiotic rearomatization to yield benzo[b]naphtho[1,2-d]thiophene 30a [19].
An alternative mechanism proposed dimerization of monosulfoxide 23a, yielding disulfoxide isomers 25a and 26a and extrusion of sulfur monoxide to produce tetracyclic diastereoisomers 27a and 28a (Table 3) [81,83]. Thermal extrusion of sulfur monoxide, from thiophene oxide cycloadducts, is a known reaction [86,87]. Direct TDO- and NDO-catalyzed desaturations could account for the formation of benzo[b]naphtho[1,2-d]thiophene sulfoxide 29a (3% ee, Table 3) from monosulfoxides 27a and 28a [88,89]. Reductase-catalyzed deoxygenation of metabolite 29a would yield benzo[b]naphtho[1,2-d]thiophene 30a [83]. Two additional compounds, 31a and 32a, formed when using a Sphingomonas strain, were also identified by GC-MS analysis (Scheme 5) [84]. Thermal deoxygenation of sulfoxides 25a–29a, during analysis, or sulfoxide reductase activity could account for products 31a and 32a. The metabolic sequence 10a → 23a → 25a, 26a → 27a, 28a. → 29a → 30a involves several dearomatization and rearomatization steps (Scheme 5).
The advantage of having different types of dioxygenase available is evident from the TDO-catalyzed (P. putida UV4) sulfoxidation of 2-methylbenzo[b]thiophene 10b to yield the stable (1R)-sulfoxide 23b (>98% ee); NDO-expressing strains (P. putida 9816/11 and E. coli pF352V) also produced sulfoxide 23b, but with P. putida 8859 an excess (56% ee) of the opposite (1S) enantiomer was found (Table 3) [83]. NDO-catalyzed oxidation of 3-methylbenzo[b]thiophene 10c, using the same strains, produced the stable sulfoxide 23c with P. putida 8859 yielding an excess of the (1R) enantiomer (41% ee) [83]. Absolute configurations of sulfoxides 23b and 23c were determined by ECD spectroscopy and ee values of all sulfoxides by chiral stationary phase HPLC analysis [83].
Ab initio computational and quantum chemistry studies predicted that enantiomers of benzo[b]thiophene sulfoxide 23a would undergo pyramidal inversion and racemization at ambient temperature with predicted inversion barriers of (ΔG≠ = 22.9-23.9 kcal mol−1) [64,65]. Experimental validation was finally provided by the isolation and observed racemization of the (1R)-benzo[b]thiophene sulfoxide 23a obtained by a styrene monoxygenase-catalyzed sulfoxidation of substrate 10a (Section 6.3). Further confirmation of predicted inversion barriers was provided by racemization of (1R)-enantiomers of sulfoxides 23b and 23c, at ambient temperature. This occurred at a slower rate over a 24h period, and kinetic studies provided pyramidal inversion barriers(ΔG≠) of 25.1 and 26.4 kcal mol−1, respectively, [83]. Similar inversion barriers are expected for sulfoxide metabolites formed from CYP450-catalyzed sulfoxidation of benzo[b]thiophene-containing drugs, e.g., zileuton 10e and brexpiprazole 10g (Section 6).
5. Dioxygenase-Catalyzed Dearomatization of Tri- and Tetra-Cyclic Thiaarenes
Larger thiaarenes, e.g., tricyclic (33) and tetracyclic (30a, 34) compounds (Figure 4), detected in crude oil, shale, coal, derived tars, and creosote, are generally more resistant to bacterial biodegradation compared with monocyclic and bicyclic thiophenes [15,16,17,18,19,20,21,22,23,24,25]. As observed during dioxygenase-catalyzed cis-dihydroxylation of polycyclic arenes, e.g., anthracene or phenanthrene [45,46], linear polycyclic thiophenes appear to be more recalcitrant than angular isomers [24].
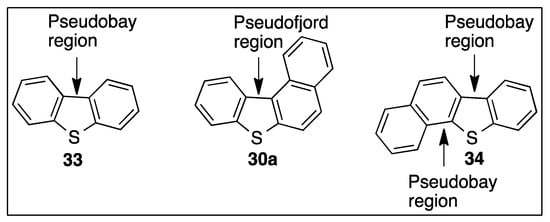
Figure 4.
Identification of pseudo-bay and pseudo-fjord regions of polycyclic thiophenes.
The angular junction, between three fused benzene rings found in phenanthrene or chrysene, is classified as a bay region. A strong preference for BPDO-catalyzed cis-dihydroxylation of bonds, proximate to a bay region, was found for tricyclic arenes, e.g., phenanthrene [45,46,90] and tetracyclic arenes, e.g., benz[a]anthracene or chrysene [91,92,93]. An angular region, between benzene rings fused with a thiophene ring, as in dibenzo[b,d]thiophene 33, or between a fused benzene, thiophene, and naphthalene ring, as in benzo[b]naphtho[2,1-d]thiophene 34, is designated a pseudo-bay region (Figure 4).
The angular junction between four fused benzene rings in benzo[c]phenanthrene is classified as a fjord region, and BPDO-catalyzed cis-dihydroxylation occurred, exclusively, at this region [94]. The region between fused benzene, thiophene, and naphthalene rings, found in benzo[b]naphtho[1,2-d]thiophene 30a, is assigned as a pseudo-fjord region.
Ring-hydroxylating dioxygenases with smaller active sites, e.g., TDO, normally only catalyze cis-dihydroxylation of mono- and bi-cyclic arenes [4,5,6,7,8,9]. The inability of its active site to accommodate many larger polycyclic thiophenes, e.g., tetracyclic substrates 30a and 34, allied to their limited aqueous solubility, are major factors in their resistance to metabolism. The larger active sites of NDO and BPDO are of similar structure (44% sequence identity) [95,96], and the wider entrance to the BPDO active site makes it is among the most successful dioxygenases for binding and biodegrading tri-, tetra-, and penta-cyclic arenes and thiaarenes.
5.1. Dioxygenase-Catalyzed Biotransformations of Dibenzo[b,d]thiophenes 33 and 38
Dibenzo[b,d]thiophene 33, one of the most common polycyclic thiophenes present in crude oil and other fossil fuels, was used as a model substrate for many biodegradation and biodesulfurization studies [97]. Dioxygenase-catalyzed biotransformations (P. putida, P. jianii, P. alcaligenes, P. stutzeri, Burkholdia, and Sphingomonas strains) of substrate 33 yielded sulfoxide 34. Ring-opened bioproducts like aldehyde 36 were derived from cis-dihydrodiol intermediate 35 (Scheme 6) [98,99]. NDO-catalyzed sulfoxidation of substrate 33 was also observed using the purified enzyme [100].
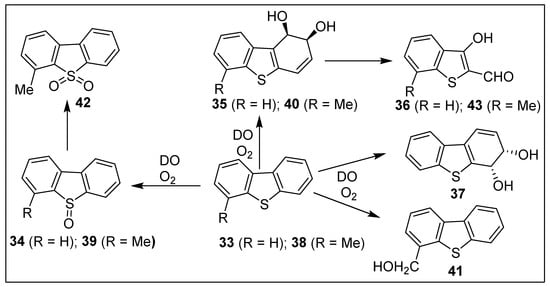
Scheme 6.
Dioxygenase-catalyzed oxidation of dibenzo[b,d]thiophene 33 and 4-methyldibenzo[b,d]thiophene 38.
Mutant (P. putida (9816/11) strain and a recombinant [E. coli JM109(DE3)(pDTG141)] strain expressing NDO [100], and mutant (Beijerinckia, B8/36) strain expressing BPDO [101], reclassified as Sphingomonas yanoikuyae B8/36 [102], were each utilized in the biotransformation of dibenzo[b,d]thiophene 33. In all cases, cis-dihydroxylation occurred at the favored pseudo-bay region to yield metabolite (1R,2S)-1,2-dihydrodiol 35 (>98% ee). The minor cis-3,4-dihydrodiol 37 was identified from NMR analysis of the unseparated isomeric mixture of cis-diols 35/37; it was also detected using other strains (P. putida 9816-11, P. fluorescens TTC1, and E. coli JM109(DE3)(pDTG141) (Scheme 6) [100,103].
Autodock Vina molecular docking studies of dibenzo[b,d]thiophene 33 as substrate led to the unexpected prediction that it could accommodate a tricyclic substrate within the relatively small active site of TDO [79]. Furthermore, it was predicted that the preferred in vitro orientation of dibenzo[b,d]thiophene 33 would result in the formation of (1R,2S)-dihydrodiol 35. This proposition was validated in vivo, when TDO-catalyzed (P. putida UV4) cis-dihydroxylation occurred within the pseudo-bay region, to give exclusively (1R,2S)-dihydrodiol 35 (>98% ee, 16% isolated yield) [79].
Dioxygenase-catalyzed oxidation of 4-methyldibenzo[b,d]thiophene 38, using wild- type Pseudomonas strains, yielded a wider range of metabolites including sulfoxide 39, benzylic alcohol 41, cis-dihydrodiol 40, sulfone 42, and aldehyde 43 (Scheme 6) [104]. A kinetic study of the racemization (130 °C) of an enantioenriched sample of sulfoxide 39 produced a higher inversion barrier (ΔG≠ = 33.0 kcal mol−1) compared with benzo[b]thiophene sulfoxides 23a and 23b; it was, however, very similar to the calculated value for sulfoxide 34, ΔG≠ = 32.3 kcal mol−1 [64].
5.2. Dioxygenase-Catalyzed Biotransformation of Benzo[b]naphtho[1,2-d]thiophene 30a and Tetrahydrobenzo[b]naphtho[1,2-d]thiophene 45
Benzo[b]naphtho[1,2-d]thiophene 30a and the corresponding sulfoxide 29a were isolated as metabolites from the biotransformations (P. putida strains, expressing TDO and NDO, and Sphingomonas XLDN2-5) of benzo[b]thiophene 10a (Scheme 5) [12,83,84,97]. No further TDO-catalyzed metabolites of compound 30a were detected due to its relatively large size. The larger active site of the BPDO (S. yanoikuyae B8/36) was able to accommodate and facilitate the metabolism of tetracyclic substrates 30a and 45 (Scheme 7). BPDO-catalyzed cis-dihydroxylation occurred exclusively within the fjord region of benzo[c]phenanthrene [94]. Under similar conditions, biotransformation of benzo[b]naphtho[1,2-d]thiophene 30a resulted in cis-dihydroxylation within the pseudo- fjord region to give (10S,11R)-dihydrodiol 44 exclusively (>98% ee; Scheme 7) [94].
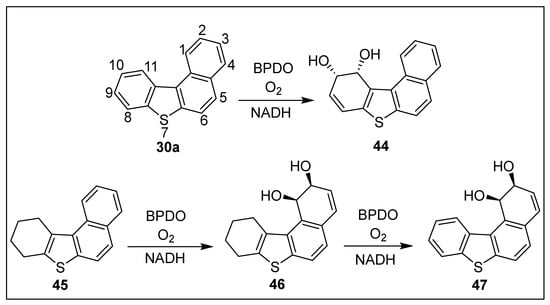
Scheme 7.
BPDO-catalyzed oxidation of benzo[b]naphtho[1,2-d]thiophene 30a and tetrahydrobenzo[b]naphtho[1,2-d]thiophene 45.
Biotransformation (BPDO) of tetrahydrobenzo[b]naphtho[1,2-d]thiophene 45 resulted in an unexpected (2:1) mixture of (1R,2S)- cis-dihydrodiols 46 and 47 and was separated by PLC (Scheme 7) [94]. Dioxygenase-catalyzed desaturations (TDO, NDO, or BPDO) are well documented reactions [105,106,107]. A BPDO-catalyzed stepwise double desaturation of metabolite 46, via a transient dihydrobenzo[b]naphtho[1,2-d]thiophene intermediate, could account for the minor (10S,11R) cis-dihydrodiol 47. This study demonstrates the importance of a pseudo-fjord region on the regiochemistry of BPDO-catalyzed cis-dihydroxylations.
5.3. Dioxygenase-Catalyzed Biotransformation of Benzo[b]naphtho[2,1-d]thiophene 34
Benzo[b]naphtho[2,1-d]thiophene 34 contains two pseudo-bay regions, and BPDO-catalyzed cis-dihydroxylation (S. yanoikuyae B8/36) occurred exclusively at one pseudo-bay region to form (1R,2S)-dihydrodiol 48 as the major metabolite (Scheme 8) [93].
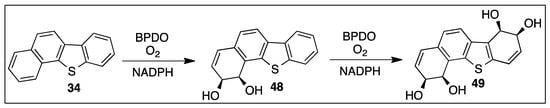
Scheme 8.
BPDO-catalyzed oxidation of benzo[b]naphtho[2,1-d]thiophene 34 to yield cis-dihydrodiol 48 and bis-cis-tetrahydrodiol 49.
A more polar minor metabolite, resulting from a further cis-dihydroxylation within the alternative pseudo-bay region, was identified as bis-cis-(1R,2S,7R,8S)-tetrahydrodiol 49. Confirmation for this metabolic sequence (Scheme 8) was obtained by using cis-dihydrodiol metabolite 48 as a substrate, and, further, cis-dihydroxylation yielded cis-tetraol 49. Metabolite 49 was the first identified member of the heteroarene bis-cis-dihydrodiol series but a similar type of BPDO-catalyzed tetrahydroxylation was observed when using chrysene as a substrate, which contained two bay regions [93].
Benzo[b]naphtho[2,1-d]thiophene 34 is a carcinogenic polycyclic thiaarene that has been identified as an environmental hazard [13]. It was tested as a substrate for CYP450 monooxygenase-catalyzed studies to identify the suspected metabolites responsible for its mutagenicity and carcinogenicity [108,109]. As found in CYP450 studies of monocyclic thiophenes [53,54,55,56], epoxidation, sulfoxidation, and hydroxylation of tetracyclic thiophene 34 was also reported (Scheme 14, Section 6.3) [108,109].
5.4. Application of Thiophene cis-Dihydrodiols in Thiophene Epoxide Synthesis
Enantiopure cis-dihydrodiols, derived from enzymatic cis-dihydroxylation of substituted arene substrates, have been widely used as precursors in the chemoenzymatic synthesis of many chiral natural products and other types of metabolites, including arene oxides and trans-dihydrodiols [4,5,6,7,8,9,44,71]. While few cis-dihydrodiol metabolites of heteroarene substrates have been utilized in synthesis, a small number of relatively stable bicyclic metabolites, derived from quinoline, 2-chloroquinoline, and 4-chloroquinoline, have proved to be useful chiral synthons. Thus, chemoenzymatic syntheses of chiral ligands [110,111], chiral metal organic frameworks [112], and arene oxide metabolites, resulting from CYP450-catalyzed oxidations [71], were achieved by the application of these metabolites.
The paucity of reports on the synthetic applications of thiophene sulfoxides and cis-dihydrodiols is possibly due to their unavailability and perceived instability. Monocyclic thiophene sulfoxides, although generally considered as unstable, can be stabilized by the presence of bulky substituents. More stable sulfoxides of this type have been used to determine sulfoxide inversion barriers by spectroscopic methods [68,69].
A further example of the synthetic application of thiophene sulfoxides is provided by the reaction of the relatively stable 3,4-di-tert-butylthiophene-1-oxide with dimethylacetylene dicarboxylate. This formed an unstable cycloadduct that decomposed spontaneously, providing a source of singlet sulfur monoxide [113]; its reaction with dienes and alkynes delivered a useful synthetic route to thiirane and thiirene oxides.
To test the potential application of thiophene cis-dihydrodiols in chemoenzymatic synthesis, it was important to select a relatively stable metabolite. The stability of cis- and trans-dihydrodiol metabolites 17a and 19a, formed by dioxygenase-catalyzed cis-dihydroxylation of benzo[b]thiophene 10a (Scheme 4), prompted a study of their use in the synthesis of a thiophene epoxide.
The three-step reaction sequence in Scheme 9 started from an isomeric mixture of metabolites 17a and 19a, proceeded via dioxoles 50, and trans-chloroacetate 51 as intermediates and yielded benzo[b]thiophene-2,3-oxide 52. A similar sequence was used in the synthesis of K-region arene oxides [114]. Epoxide 52 was identified by NMR spectroscopy (THF-d8) and MS analysis. On attempted chromatographic purification, it isomerized to a mixture of 3-hydroxybenzo[b]thiophene 53 and the keto tautomer 54 [82].
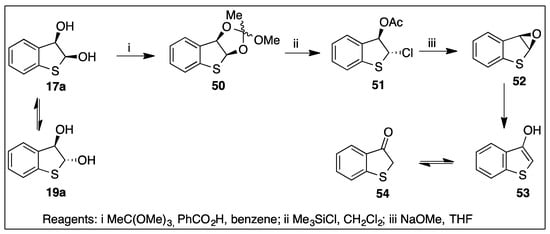
Scheme 9.
Synthesis of benzo[b]thiophene epoxide 52 from cis-diol metabolite 17a.
6. Monooxygenase-Catalyzed Sulfoxidation and Epoxidation of Thiophenes
6.1. CYP450-Catalyzed Sulfoxidation of Monocyclic Thiophenes
While thiophenes are considered as contaminants of fossil fuels [14,15,16,17,18,19,20,21,22,23,24,25], the thiophene ring is regarded as a useful bioisosteric building block for many important drugs (Figure 5) [57]. The results from monooxygenase (CYP450)-catalyzed sulfoxidation of thiophenes, in the context of metabolite identification, toxicity, and mechanism of drug metabolism, are rarely compared with sulfoxidations obtained using ring-hydroxylating dioxygenases.
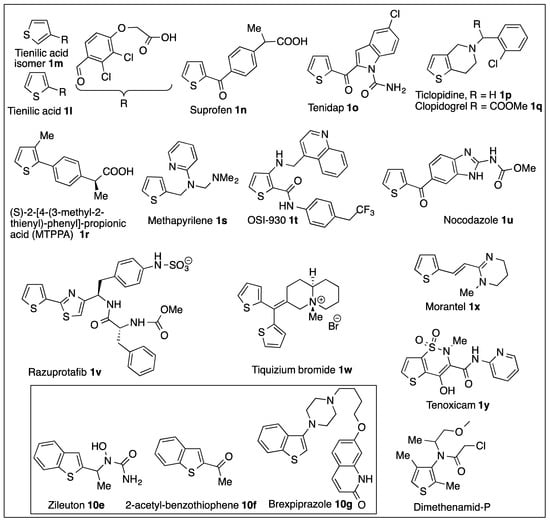
Figure 5.
Thiophene- and benzothiophene-containing drugs and analogs tested in metabolic studies.
Thiophenes were reported to be hepato- and nephro-toxic to rats [115,116], and these early reports proposed the formation of reactive intermediates, derived from thiophenes rings, which react with cellular nucleophiles; a thiophene sulfoxide N-acetylcysteine conjugate was isolated from urine of rats treated with thiophene [117].
In common with the dioxygenase-catalyzed results (Scheme 2), mammalian monooxygenase (CYP450)-catalyzed sulfoxidations of: (i) the model substrates, thiophene 1a, 2-phenyl-thiophene 1g, and 3-phenyl thiophene 1k and (ii) the thiophene-containing drugs analog, tienilic acid isomer 1m (Figure 5), was observed.
CYP450 isozymes in liver microsomes, and in recombinant pure form, catalyzed formation of the corresponding unstable sulfoxides as initial metabolites 5a,g,k,m (Scheme 10) [52,53,54,55,56,57]. TDO-catalyzed oxidations of thiophenes 1a,b,g,k also formed sulfoxides 5a,b,g,k. In both mono- and di-oxygenase studies, rapid dimerization occurred to give the corresponding dimers 6a,b,g,k, respectively (Scheme 2 and Scheme 10). Monocyclic thiophene sulfoxide metabolites 5a,m,p,q, s were also trapped as cycloadducts by other dienophiles, including N-methyl-maleimide (NMM), yielding cycloadducts 55a,m,p,q,s (Scheme 10) [53,118,119,120]. Michael addition of thiols, e.g., glutathione (GSH), N-acetylcysteine, or mercaptoethanol, proved to be a particularly useful method for trapping transient monocyclic sulfoxide metabolites 5a,g,k,m as adducts 56a,g,k,m and 57a,g,k,m (Scheme 10) [52,53,54,55,56,57,58,64,65]. A major advantage of this trapping method, for unstable thiophene sulfoxides, is that these thiols can also trap transient thiophene epoxide metabolites. If the rate of thiol addition is much faster than the rate of thiophene sulfoxide racemization [64,65,66], the configurationally stable thiol adducts could, in principle, provide indirect evidence of sulfoxide enantiomeric excess values.
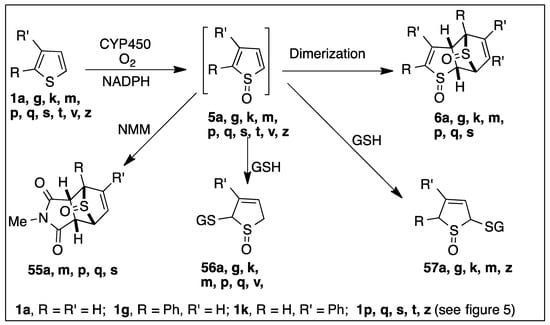
Scheme 10.
CYP450-catalyzed sulfoxidation of thiophenes 1a, g, k, m, p, q, s, t, v, z.
Cytochrome P450-catalyzed oxidation of the monosubstituted thiophene-containing drugs, tienilic acid isomer 1m, methapyrilene 1s, and the disubstituted thiophenes ticlopidine 1p, clopidogrel 1q, and OSI-930 1t was found to give transient sulfoxide metabolites 5m,s,p,q, and 5t, respectively, that rapidly dimerized to 6m,s,p,q,t (Scheme 10 and Figure 5) [57,121,122]. In the presence of NMM in the incubation, cyclo-adducts 55m,p,q, and 55s were obtained at the cost of the thiophene sulfoxide dimers [120]. Incubation of tri-substituted thiophene dimethenamid-P (Figure 5) herbicide with basidiomycete Irpex consors produced a stable thiophene sulfoxide and two isomeric 2-thiolenones [123].
6.2. CYP450-Catalyzed Epoxidation of Monocyclic Thiophenes
Both benzene oxide and benzene cis-dihydrodiol metabolites have been chemically synthesized and characterized. Scheme 1a illustrates that CYP450-catalyzed epoxidation of mono- and poly-cyclic arene substrates [124,125] can provide an alternative oxidative dearomatization pathway compared to dioxygenase-catalyzed cis-hydroxylation. Three major enzymatic oxidative dearomatization pathways (sulfoxidation, cis-dihydroxylation, and epoxidation) are possible for mono- and poly-cyclic thiophene substrates (Scheme 1b).
An important difference between cis-dihydroxylation and epoxidation of thiophenes is that thiophene cis-dihydrodiols have been isolated and fully characterized while, to date, thiophene epoxide metabolites have not. It is important to consider what evidence is available for the involvement of transient benzene oxide metabolites prior to their detection, isolation, and synthesis when attempting to identify transient thiophene epoxide metabolites.
Benzene oxides can: (i) aromatize to phenols, (ii) react with thiols and to yield trans-hydroxysulfides, and (iii) hydrolyze to yield trans-dihydrodiols, via epoxide hydrolase catalysis. Similarly, thiophene epoxide metabolites derived from thiophenes 1a,g,k,l, might be expected to aromatize to hydroxythiophenes [122], to form trans-hydroxysulfide adducts with thiols, and to hydrolyze, giving trans-dihydrodiols as shown in Scheme 11.
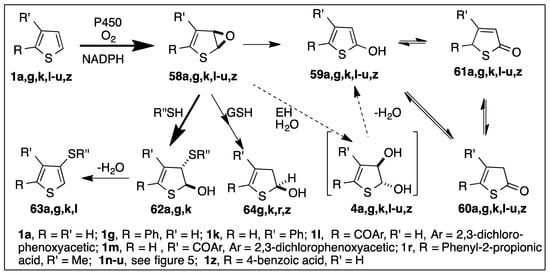
Scheme 11.
CYP450-catalyzed oxidation of thiophenes 1a,g,k,l–u,z.
The isolation of hydroxythiophene metabolites from monooxygenase-catalyzed model thiophene substrates and thiophene-containing drugs is consistent with thiophene epoxidation and isomerization [126,127]. Thus it was proposed that CYP450-catalyzed oxidation of monosubstituted thiophenes 1a,g,k and tienilic acid 1l could yield unstable thiophene epoxide intermediates 58a,g,k,l (Scheme 11); spontaneous isomerization of these epoxides could then account for the isolation of monohydroxylated thiophenes 59a,g,k,l and their corresponding keto tautomers 60a,g,k,l and 61a,g,kl [54,57,128]. Thus, 2-aroylthiophenic drugs tienilic acid 1l, suprofen 1n, tenidap 1o, and nocodazole 1u were 5-hydroxylated to produce hydroxythiophene 58l,n,o,u in equilibrium with the corresponding two thiolactones 60 and 61 having a strong chromophore around 390 nm [126,129,130,131]. Three of these 2-aroylthiophenic drugs were found to exhibit idiosyncratic toxicity and were withdrawn (tienilic acid 1l, liver toxicity; suprofen 1n, kidney toxicity), and use of tenidap 1o was stopped at the end of the clinical studies.
Other thiophene-containing drugs, tiquizium bromide 1w, morantel 1x, and tenoxicam 1y, were also oxidized in microsomal incubations in the thiophene ring, but the position of oxidation was not determined [132,133,134]. For two thiophene-containing drugs, duloxetine and eprosartan, and one benzothiophene-containing drug, raloxifene, metabolic oxidation of the thiophene ring was researched and not detected, other parts of the molecules being oxidized [57].
Following the report of an unusual rearrangement reaction of arenes during enzymatic aromatic hydroxylation, the NIH Shift, this observation was widely used as strong evidence for the intermediacy of transient arene oxides [125,135]. The NIH Shift requires migration of an atom (e.g., D or T) or group and retention at an adjacent site during aromatic hydroxylation of substituted arenes. A similar migration and retention of label was observed during TDO-catalyzed arene cis-dihydroxylation and dehydration of the D-labelled cis-dihydrodiol metabolites to give phenols [136]. In addition to these two mechanisms for the NIH Shift, other mechanisms have since been reported [137]. The NIH Shift was, however, not observed during CYP450-catalyzed oxidation of thiophenes 1a,g,k,l during formation of the corresponding hydroxythiophene metabolites 59a,g,k,l and, therefore, could not be used as evidence for epoxidation.
Arenes can be oxidized by dioxygen and CYP450s forming arene oxides that are then enzymatically hydrolyzed by microsomal epoxide hydrolase (mEH) to give stable trans-dihydrodiols. Arene oxides are relatively stable and have been produced by either chemical or enzymatic synthesis. Thiophene trans-dihydrodiols 4a,h–k have been isolated from TDO-catalyzed oxidations of thiophenes as epimers of the initially formed cis-dihydrodiols 2a,h–k [12]; they were relatively stable and did not readily dehydrate. Thus, it was expected that trans-dihydrodiols could be formed from thiophene epoxides 58a,g,k,l, during liver microsomal incubations, as presented in Scheme 11. Neither trans-dihydrodiols 4a,g,k,l, nor the corresponding cis epimers 2a,g,k,l, have been detected as metabolites during CYP450-catalyzed oxidation of any monocyclic thiophenes including compounds 1a,g,k,l [54,57,128].
Why were thiophene trans-dihydrodiols not found? One possibility would be that they are formed below the level of detection. Quantum chemical analysis results predicted that the energy barrier to direct hydrolysis of a thiophene epoxide by water was too high [122]. However, since arene oxide hydrolysis requires catalysis by mEH, a similar outcome might be expected for thiophene epoxides. The size of the active site of mEH is able to accommodate arene oxide sizes from one aromatic ring up to five fused rings and aliphatic epoxides and, thus, should accept small thiophene epoxides. The hydrophobicity of thiophene epoxides should be close to that of arene oxides of similar size. Maybe mEH is not the appropriate enzyme or the half-life of thiophene epoxides is too short for being transferred to the catalytic site of mEH. Despite some thiophenes and thiophene-containing drugs having similar types of substituents, no trans-dihydrodiols nor their cis epimers have been yet detected directly during microsomal incubations.
The stability of thiophene epoxides [122] could be increased using the same approach adopted for arene oxides [135] that were stabilized by the presence of bulky or electron-withdrawing substituents [137,138]. Despite some model thiophenes and thiophene-containing drugs having similar types of substituents, no epoxide metabolites have been detected directly.
The most convincing indirect evidence for thiophene epoxide intermediates being formed by CYP450-catalyzed oxidation of thiophenes comes from the formation of trans-hydroxysulfide adducts (Scheme 11). Nucleophilic attack of thiols, e.g., glutathione or mercaptoethanol, on thiophene epoxide metabolites, e.g., 58a,g,k, was found using liver microsomes and individual monooxygenase-expressing strains, e.g., recombinant CYP450 1A [55,56,57,121,128]. The initially formed trans-hydroxysulfides, e.g., 62a,g,k, were found to epimerize with the corresponding cis isomers. Dehydration of trans-hydroxysulfides 62a,g,k under acidic conditions formed alkylthienyl sulfides 63a,g,k
A substituted 2-phenyl thiophene, (S)-2-[4-(3-methyl-2-thienyl)phenyl]propionic acid 1r (MTPPA), as an anti-inflammatory agent, was metabolized to the 5-hydroxy-thiophene metabolite 59r and its thiolenone tautomers 60r and 61r in vivo and in vitro by CYP2C9 [139]. Incubation of thiophene 1r in the presence of glutathione did not yield adducts, but the detectable proportions of thiolenones decreased with increasing production of an unexpected metabolite. It was identified by NMR spectra and mass spectrometry as the thiophene hydrate 5-hydroxy-4,5-dihydro-3-methylthiophene 64r [140]. While stable at room temperature, metabolite 64r dehydrated in an acid medium, yielding thiophene 1r. Under similar conditions, 2-phenylthiophene 1g and 4-(2-thienyl)-benzoic acid 1z substrates also formed thiophene hydrates 64g and 64z, but the hydration mechanism was unclear [140].
Relatively few examples of hydrate formation during mono- or di-oxygenase-catalyzed oxidations of arenes or heteroarenes are available. Metabolites 64g, 64r, and 64z are rare examples of heteroarene hydrates formed through CYP450-catalyzed epoxidation of the corresponding thiophenes 1g,r, and 1z. [140]. Monohydroxylation (CYP450) at allylic or benzylic positions of dihydroarenes yielded polycyclic arene hydrates [141]. Dioxygenase-catalyzed cis-dihydroxylation of monosubstituted phenols also produced hydrates, and their keto tautomers, as minor metabolites [78]. The formation of thiophene hydrates and phenol hydrates, during oxidative biotransformations of the corresponding phenol and thiophene substrates, could be regarded as dearomatizations. However, in each case, several steps are involved rather than direct hydration reactions.
Monocyclic thiophene epoxides and sulfoxides have also been identified as biologically reactive intermediates, that react in situ with the enzyme responsible for their production. Several thiophene-containing drugs have been identified as mechanism-based inactivators of cytochrome P450s and to react with the apoenzyme [142,143,144,145,146].
No convincing evidence was found for epoxide hydrolase (EH)-catalyzed hydrolysis of thiophene epoxide metabolites, yielding trans-dihydrodiols from metabolism of thiophenes and thiophene-containing drugs (Scheme 11). It is, however, noteworthy that the bioactivation of thiazole-containing drugs 65 also involves CYP450-catalyzed formation of unstable thiazole epoxides 66 but is followed by hydrolysis yielding trans-dihydrodiols 67 during metabolism (Scheme 12) [147,148,149].
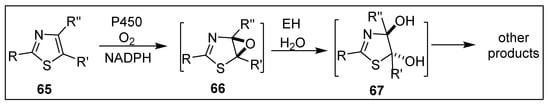
Scheme 12.
CYP450-catalyzed epoxidation of thiazoles 65.
6.3. Monooxygenase-Catalyzed Oxidation of Polycyclic Thiophenes
Benzo[b]thiophene sulfoxide 23a, obtained by the chemical oxidation of benzo[b]thiophene 10a (Section 4.3), and benzo[b]thiophene epoxide 52, synthesized from cis-dihydrodiol 17a (Scheme 9), were unstable in the neat state but were found to be more stable in solution. This prompted a reexamination of the metabolism of benzo[b]thiophene 10a by monooxygenases from rat liver microsomes (expressing CYP450 enzymes) and a bacterial recombinant strain [E. coli BL21 (DE3)] (expressing styrene monooxygenase, SMO) [52,83,150,151]. Following CYP450-catalyzed oxidation of benzo[b]thiophene 10a, benzo[b]thiophene sulfoxide 23a was identified as a major metabolite by HPLC analysis. In the presence of mercaptoethanol (pH 8.5), sulfoxide 23a was trapped as thiol adduct 68; no evidence of the epoxide intermediate 52 was obtained by trapping hydroxy sulfide 69 (Scheme 13) [52].
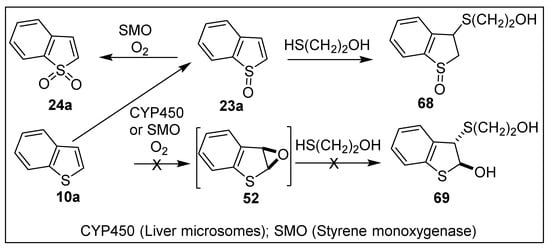
Scheme 13.
Monooxygenase-catalyzed oxidation of benzo[b]thiophene 10a.
The major metabolite, isolated from styrene monooxygenase-catalyzed [E. coli B121 (DE3)] sulfoxidation of benzo[b]thiophene 10a, was (1R)-benzo[b]thiophene-1-oxide 23a, [150]; benzo[b]thiophene-1,1-dioxide 24a was found as a very minor metabolite. Sulfoxide 23a, isolated at 0°C and identified by NMR analysis and slowly racemized over several hours in CDCl3 solution at ambient temperature, without decomposition [83].
SMO-catalyzed epoxidation of indene and indole was reported to yield the corresponding epoxides. Indene 1,2-oxide was formed using E. coli B121 (DE3) [151], while indirect evidence for indole 2,3-oxide was obtained using P. putida strains S12 and CA-3 [152]. Therefore, it was anticipated that SMO might catalyze epoxidation of the bioisosteric substrate benzo[b]thiophene 10a. Despite efforts to detect thiophene oxide 52 (Scheme 13) or to trap it as a hydroxysulfide adduct 69, epoxide 52 remains an undetected potential metabolite.
CYP450-catalyzed oxidation of the benzo[b]thiophene-containing drugs zileuton 10e, its analog 2-acetylbenzothiophene 10f, and brexpiprazole 10g (Figure 5) yielded thiophene sulfoxides without any evidence of epoxide metabolites [57,153,154]. Another benzo[b]thiophene-containing drug candidate, JNJ-26990990, was also converted to a sulfoxide[155].
Mono- and di-oxygenase-catalyzed oxidative metabolism of benzo[b]naphtho[2,1-d]thiophene 34 was studied, due to its mutagenicity, carcinogenicity, and presence in the environment [108,109]. While BPDO-catalyzed oxidation of thiophene 34 yielded cis-dihydrodiol 48 and cis-tetrahydrodiol 49 (Scheme 8, Section 5.3), an early CYP450-catalyzed metabolism study of substrate 34 yielded only sulfoxide 74 and sulfone 75 (Scheme 14) [156].
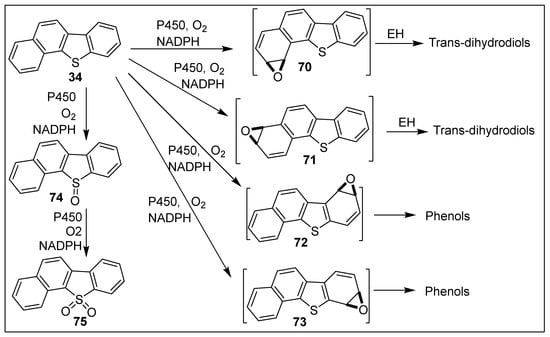
Scheme 14.
CYP450-catalyzed oxidation of benzo[b]naphtho[2,1-d]thiophene 34.
Later studies of CYP450-catalyzed metabolism of tetracyclic thiophene 34 also identified sulfoxide 74 and sulfone 75; in addition, two trans-dihydrodiols, derived from the transient arene oxides 70 and 71 and two phenols, resulting from undetected arene oxides 72 and 73, were identified (Scheme 14) [109]. The proposed CYP450-catalyzed oxidations shown in Scheme 14 involve five enzymatic dearomatization reactions during production of one sulfoxide 74 and four arene oxide intermediates 70–73.
6.4. Monooxygenase-Catalyzed Thiophene Ring Oxidation of Thienopyridine Prodrugs
Some important antiplatelet and antiaggregant prodrugs, with a tetrahydro-thienopyridine structure 76 (Ticlopidine 1p, Clopidogrel 1q, Figure 5 and Scheme 15), are metabolized in mammals by a series of reactions on the thiophene ring, leading to a thiol acid derivative that inhibits the G-protein ADP receptor P2Y12 [157]. This family of compounds was discovered in 1978 [158], the receptor was located in 2000 and the complex formation of the active metabolite was deciphered during 2009–2013 [158].
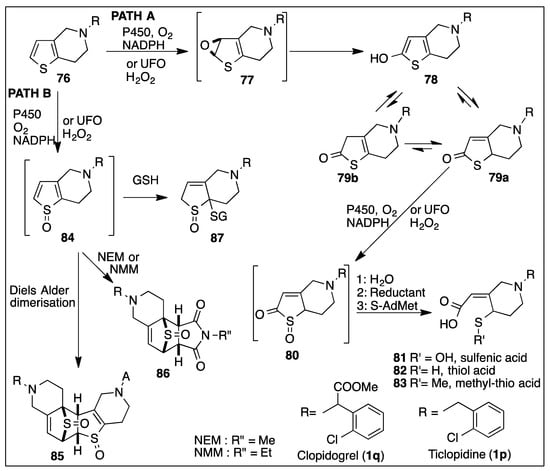
Scheme 15.
CYP450- or UFO-catalyzed oxidative metabolism of thienopyridine antiplatelet compounds 76 (ticlopidine 1p and clopidogrel 1q).
The important role played by CYP450 and unspecific fungal peroxygenase (UFO)-catalyzed epoxidation and sulfoxidation is exemplified by the thienopyridine drug metabolism route in Scheme 15. Further reactions of the unstable epoxide and sulfoxide metabolites led to the formation of many other products, including sulfoxide dimers, hydroxythiophenes, thiolactones, sulfenic acids, and thiols.
One of the first steps in the metabolism of thienopyridines 76, clopidogrel 1q, and ticlopidine 1p was oxidation of the thiophene ring by a CYP450 monooxygenase (or UFO peroxygenase) to yield an unstable thiophene epoxide 77. This intermediate isomerized into a 2-hydroxythiophene 78 and its thiolactone tautomers 79a and 79b (Path A, Scheme 15) [159]. Further CYP450- or peroxygenase-catalyzed oxidation of tautomers 78/79a/79b led to a reactive thiolactone S-oxide 80 that hydrolyzed to a sulfenic acid 81 and was trapped using dimedone [120,160,161,162,163,164]. The sulfenic acid metabolite 81 was reduced, in vivo, to thiol acid diastereoisomers 82, only one being active with the receptor P2Y12. Reducing species, like thiols, ascorbic acid, and tricarboxyethyl-phosphine, were used in vitro [120,160,161,162,163,164,165]. Thiol acid metabolites 82 were eliminated in vivo [166,167] and also as S-methylated adducts 83. The dialkyl sulfides 83 were also obtained in vitro after methylation of 82 by S-adenosine-methionine dependent S-methyl transferase [168,169].
Some metabolites of the 2-substituted thiophene razuprotafib 1v, including a methylthioether, may also be formed by Path A with Path B being also involved in formation of other metabolites [170,171]. Metabolic reactions of thiophenes catalyzed by cytochrome P450 were reviewed recently [172].
During the metabolism of ticlopidine 1p and clopidogrel 1q, a competitive CYP450 monooxygenation pathway (Path B, Scheme 15) led to thiophene-S-oxide 84 and S-oxide dimers 85. The unstable S-oxide 84 was trapped by dienophiles, e.g., N-methyl- (NMM) and N-ethyl-maleimide (NEM) [119,120] to give product 86, or thiols, e.g., glutathione (GSH) to give adducts 87 [173]. The oxidation reactions (Path A and Path B) were also catalyzed by unspecific fungal peroxygenases (UFO) in the presence of ascorbic acid and hydrogen peroxide [159].
7. Conclusions
A major emphasis of this review has been on the complementary nature of monooxygenase and dioxygenase enzyme activities, in the context of oxidative dearomatization of mono- and poly-cyclic thiophenes. Monooxygenase enzymes, expressed by mammalian, fungal, and bacterial cells, were used in the oxidative aromatization of a wider range of types and sizes of thiophenes compared with ring-hydroxylating dioxygenases.
Wild-type bacteria, expressing ring hydroxylating dioxygenase enzymes, have been employed to metabolize and remove thiophenes from fossil fuels. Mutant and recombinant bacterial strains, expressing these enzymes, were utilized to intercept and scale up the production of thiophene sulfoxide and dihydrodiol metabolites from relatively small thiophene substrates. Factors influencing chemo-, regio-, and stereo-selectivity, stability and mechanisms of dioxygenase-catalyzed oxidations of thiophenes are discussed.
Enantioenriched thiophene sulfoxide metabolites were used to determine inversion barriers and compare with predictions from calculated values. These barriers are of potential importance within the context of individual sulfoxide enantiomers from drugs having different efficacies. A range of inversion barriers were predicted for sulfoxide metabolites obtained during CYP450-catalyzed oxidation of thiophene-containing drugs. The application of a thiophene cis-dihydrodiol metabolite in the first synthesis a thiophene epoxide suggests that more stable thiophene epoxides could be obtained using this method.
Lessons can be learned from comparisons of mono- and di-oxygenase-catalyzed oxidation of arenes with thiophenes where metabolite instability is often a major problem. Factors to address this include the use of bulky or electron-withdrawing substituents or benzo-fusion to stabilize thiophene sulfoxides and epoxides. Factors found to influence the thermal stability and dynamic stereochemistry (racemization, cis/trans isomerization) of metabolites, isolated from dioxygenase-catalyzed oxidation of thiophenes, are also applicable to those derived from monooxygenases. Metabolism of carbocyclic arenes by dioxygenases can yield cis-dihydrodiols while transient arene oxides produced by monooxygenases hydrolyze to isolable trans-dihydrodiols. Possible reasons are discussed for the almost reverse scenario, where dioxygenases catalyze formation of thiophene trans-dihydrodiols as major metablites under aqueous condations while monooxygenases do not.
Further research using suitably substituted thiophene substrates with appropriate dioxygenases could produce cis-dihydrodiol metabolites for use in the synthesis of more stable thiophene epoxides and sulfoxides. Monooxygenase-catalyzed production of transient thiophene epoxide metabolites in the presence of a range of epoxide hydrolases might finally lead to trans-dihydrodiol production. The question of why model benzo[b]thiophenes and benzo[b]thiophene-containing drugs and monooxygenases do not appear to produce either epoxides or trans-dihydrodiols remains unanswered.
Author Contributions
D.R.B. and P.M.D. wrote the manuscript, N.D.S. edited, P.J.S., P.H. and C.C.R.A. commented on the paper, and all authors participated in a number of the cited papers. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Does not apply.
Data Availability Statement
Does not apply.
Acknowledgments
This research was supported in part by the C.N.R.S (PMD) and by the Leverhulme Trust (CCRA and PH).
Conflicts of Interest
The authors declare no conflict of interest.
Dedication
This paper is dedicated to our mentors, Donald M. Jerina and John W. Daly.
References
- Monks, T.J.; Butterworth, M.; Lau, S.S. The fate of benzene-oxide. Chem. Biol. Interact. 2010, 184, 201–206. [Google Scholar] [CrossRef]
- Ziffer, H.; Jerina, D.M.; Gibson, D.T.; Kobal, V.M. Absolute stereochemistry of the (+)-cis-1,2-dihydroxy-3-methylcyclohexa-3,5-diene produced from toluene by Pseudomonas putida. J. Am. Chem. Soc. 1973, 95, 4048–4049. [Google Scholar] [CrossRef]
- Heider, J.; Fuchs, G. Anaerobic Metabolism of Aromatic Compounds. Eur. J. Biochem. 1997, 243, 577–596. [Google Scholar] [CrossRef] [PubMed]
- Boyd, D.R.; Sheldrake, G.N. The dioxygenase-catalysed formation of vicinal cis-diols. Nat. Prod. Rep. 1998, 15, 309–324. [Google Scholar] [CrossRef]
- Hudlicky, T.; Gonzalez, D.; Gibson, D.T. Enzymatic dihydroxylation of aromatics in enantioselective synthesis: Expanding asymmetric methodology. Aldrichim. Acta 1999, 32, 35–62. [Google Scholar] [CrossRef]
- Johnson, R.A. Microbial Arene Oxidations. Org. React. 2004, 63, 117–264. [Google Scholar] [CrossRef]
- Lewis, S.E. Asymmetric Dearomatization Under Enzymatic Conditions. In Asymmetric Dearomatization Reactions; Wiley-VHF Verlag GMBH: Weinheim, Germany, 2016; pp. 279–346. [Google Scholar] [CrossRef]
- Hudlicky, T. Benefits of Unconventional Methods in the Total Synthesis of Natural Products. ACS Omega 2018, 3, 17326–17340. [Google Scholar] [CrossRef] [PubMed]
- Taher, E.S.; Banwell, M.; Buckler, J.; Yan, Q.; Lan, P. The Exploitation of Enzymatically-Derivedcis-1,2-Dihydrocatechols and Related Compounds in the Synthesis of Biologically Active Natural Products. Chem. Rec. 2017, 18, 239–264. [Google Scholar] [CrossRef]
- Solà, M. Why Aromaticity Is a Suspicious Concept? Why? Front. Chem. 2017, 5, 22. [Google Scholar] [CrossRef]
- Horner, K.E.; Karadakov, P.B. Chemical Bonding and Aromaticity in Furan, Pyrrole, and Thiophene: A Magnetic Shielding Study. J. Org. Chem. 2013, 78, 8037–8043. [Google Scholar] [CrossRef]
- Boyd, D.R.; Sharma, N.D.; Gunaratne, N.; Haughey, S.A.; Kennedy, M.A.; Malone, J.F.; Allen, C.C.R.; Dalton, H. Dioxygenase-catalysed oxidation of monosubstituted thiophenes: Sulfoxidation versus dihydrodiol formation. Org. Biomol. Chem. 2003, 1, 984–994. [Google Scholar] [CrossRef]
- Jacob, J. Sulfur Analogues of Polycyclic Aromatic Hydrocarbons (Thiaarenes); Cambridge University Press: Cambridge, UK, 1990; pp. 41–54. [Google Scholar]
- Boshagh, F.; Rahmani, M.; Rostami, K.; Yousefifar, M. Key Factors Affecting the Development of Oxidative Desulfurization of Liquid Fuels: A Critical Review. Energy Fuels 2021, 36, 98–132. [Google Scholar] [CrossRef]
- Fedorak, P. Microbial metabolism of organosulfur compounds in petroleum. In Geochemistry of Sulfur in Fossil Fuels; Orr, W., White, C.M., Eds.; American Chemical Society: Washington, DC, USA, 1990; Volume 429, pp. 93–112. [Google Scholar] [CrossRef]
- Fedorak, P.M.; Grbić-Galić, D. Aerobic Microbial Cometabolism of Benzothiophene and 3-Methylbenzothiophene. Appl. Environ. Microbiol. 1991, 57, 932–940. [Google Scholar] [CrossRef]
- Saftic, S.; Fedorak, P.M.; Andersson, J.T. Diones, sulfoxides, and sulfones from the aerobic cometabolism of methylbenzothiophenes by Pseudomonas strain BT1. Environ. Sci. Technol. 1992, 26, 1759–1764. [Google Scholar] [CrossRef]
- Fedorak, P.M.; Peakman, T.M. Aerobic microbial metabolism of some alkylthiophenes found in petroleum. Biodegradation 1992, 2, 223–236. [Google Scholar] [CrossRef]
- Kropp, K.G.; Gonçalves, J.A.; Andersson, J.T.; Fedorak, P.M. Microbially Mediated Formation of Benzonaphthothiophenes from Benzo[ b ]thiophenes. Appl. Environ. Microbiol. 1994, 60, 3624–3631. [Google Scholar] [CrossRef]
- Kropp, K.G.; Goncalves, J.A.; Andersson, J.T.; Fedorak, P.M. Bacterial Transformations of Benzothiophene and Methylbenzothiophenes. Environ. Sci. Technol. 1994, 28, 1348–1356. [Google Scholar] [CrossRef] [PubMed]
- Kropp, K.G.; Saftic, S.; Andersson, J.T.; Fedorak, P.M. Transformations of six isomers of dimethylbenzothiophene by three Pseudomonas strains. Biodegradation 1996, 7, 203–221. [Google Scholar] [CrossRef] [PubMed]
- Fedorak, P.M.; Coy, D.L.; Peakman, T.M. Microbial metabolism of some 2,5-substituted thiophenes. Biodegradation 1996, 7, 313–327. [Google Scholar] [CrossRef]
- Kropp, K.G.; Andersson, J.T.; Fedorak, P.M. Bacterial transformations of 1,2,3,4-tetrahydrodibenzothiophene and dibenzothiophene. Appl. Environ. Microbiol. 1997, 63, 3032–3042. [Google Scholar] [CrossRef]
- Kropp, K.G.; Andersson, J.T.; Fedorak, P.M. Bacterial transformations of naphthothiophenes. Appl. Environ. Microbiol. 1997, 63, 3463–3473. [Google Scholar] [CrossRef]
- Bressler, D.C.; Norman, J.A.; Fedorak, P.M. Ring cleavage of sulfur heterocycles: How does it happen? Biodegradation 1997, 8, 297–311. [Google Scholar] [CrossRef]
- Boyd, D.R.; Sharma, N.D.; Boyle, R.; McMurray, B.T.; Evans, T.A.; Malone, J.F.; Dalton, H.; Chima, J.; Sheldrake, G.N. Biotransformation of unsaturated heterocyclic rings by Pseudomonas putida to yield cis-diols. J. Chem. Soc. Chem. Commun. 1993, 49–51. [Google Scholar] [CrossRef]
- Eaton, R.W.; Nitterauer, J.D. Biotransformation of benzothiophene by isopropylbenzene-degrading bacteria. J. Bacteriol. 1994, 176, 3992–4002. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Brand, J.; Gibson, D. Stereospecific Sulfoxidation by Toluene and Naphthalene Dioxygenases. Biochem. Biophys. Res. Commun. 1995, 212, 9–15. [Google Scholar] [CrossRef]
- Bugg, T.D.; Ramaswamy, S. Non-heme iron-dependent dioxygenases: Unravelling catalytic mechanisms for complex enzymatic oxidations. Curr. Opin. Chem. Biol. 2008, 12, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Barry, S.M.; Challis, G.L. Mechanism and Catalytic Diversity of Rieske Non-Heme Iron-Dependent Oxygenases. ACS Catal. 2013, 3, 2362–2370. [Google Scholar] [CrossRef]
- Ibrahim, S.R.M.; Abdallah, H.M.; El-Halawany, A.M.; Mohamed, G.A. Naturally occurring thiophenes: Isolation, purification, structural elucidation, and evaluation of bioactivities. Phytochem. Rev. 2016, 15, 197–220. [Google Scholar] [CrossRef]
- Misawa, N.; Shindo, K.; Takahashi, H.; Suenaga, H.; Iguchi, K.; Okazaki, H.; Harayama, S.; Furukawa, K. Hydroxylation of various molecules including heterocyclic aromatics using recombinant Escherichia coli cells expressing modified biphenyl dioxygenase genes. Tetrahedron 2002, 58, 9605–9612. [Google Scholar] [CrossRef]
- Boyd, D.R.; Sharma, N.D.; Hand, M.V.; Groocock, M.R.; Kerley, N.A.; Dalton, H.; Chima, J.; Sheldrake, G.N. Stereodirecting substituent effects during enzyme-catalysed synthesis of cis-dihydrodiol metabolites of 1,4-disubstituted benzene substrates. J. Chem. Soc. Chem. Commun. 1993, 974–976. [Google Scholar] [CrossRef]
- Yildirim, S.; Franco, T.T.; Wohlgemuth, R.; Kohler, H.-P.E.; Witholt, B.; Schmid, A. Recombinant Chlorobenzene Dioxygenase fromPseudomonas sp. P51: A Biocatalyst for Regioselective Oxidation of Aromatic Nitriles. Adv. Synth. Catal. 2005, 347, 1060–1072. [Google Scholar] [CrossRef]
- Hoye, T.R.; Jeffrey, C.S.; Shao, F. Mosher ester analysis for the determination of absolute configuration of stereogenic (chiral) carbinol carbons. Nat. Protoc. 2007, 2, 2451–2458. [Google Scholar] [CrossRef] [PubMed]
- Boyd, D.R.; Dorrity, M.R.J.; Hand, M.V.; Malone, J.F.; Sharma, N.D.; Dalton, H.; Gray, D.J.; Sheldrake, G.N. Enantiomeric excess and absolute configuration determination of cis-dihydrodiols from bacterial metabolism of monocyclic arenes. J. Am. Chem. Soc. 1991, 113, 666–667. [Google Scholar] [CrossRef]
- Boyd, D.R.; Sharma, N.D.; Boyle, R.; McMordie, R.S.; Chima, J.; Dalton, H. A H-nmr method for the determination of enantiomeric excess and absolute configuration of cis-dihydrodiol metabolites of polycyclic arenes and heteroarenes. Tetrahedron Lett. 1992, 33, 1241–1244. [Google Scholar] [CrossRef]
- Burgess, K.; Porte, A.M. A Reagent for Determining Optical Purities of Diols by Formation of Diastereomeric Arylboronate Esters. Angew. Chem. Int. Ed. 1994, 33, 1182–1184. [Google Scholar] [CrossRef]
- Resnick, S.M.; Torok, D.S.; Lee, K.; Brand, J.M.; Gibson, D.T. Regiospecific and streoselective hydroxylation pf 1-indanone and 2-indanone by naphthalene dioxygenase and toluene dioxygenase. Appl. Environ. Microbiol. 1995, 61, 847. [Google Scholar] [CrossRef] [PubMed]
- Boyd, D.R.; Sharma, N.D.; Goodrich, P.A.; Malone, J.F.; McConville, G.; Harrison, J.S.; Stevenson, P.J.; Allen, C.C. Enantiopurity and absolute configuration determination of arenecis-dihydrodiol metabolites and derivatives using chiral boronic acids. Chirality 2017, 30, 5–18. [Google Scholar] [CrossRef]
- Gawronski, J.K.; Kwit, M.; Boyd, D.R.; Sharma, N.D.; Malone, A.J.F.; Drake, A.F. Absolute Configuration, Conformation, and Circular Dichroism of Monocyclic Arene Dihydrodiol Metabolites: It is All Due to the Heteroatom Substituents. J. Am. Chem. Soc. 2005, 127, 4308–4319. [Google Scholar] [CrossRef]
- Kwit, M.; Gawroński, J.; Boyd, D.R.; Sharma, N.D.; Kaik, M.; More O’Ferrall, R.A.; Kudavalli, J.S. Toluene Dioxygenase-Catalyzed Synthesis of cis-Dihydrodiol Metabolites from 2-Substituted Naphthalene Substrates: Assignments of Absolute Configurations and Conformations from Circular Dichroism and Optical Rotation Measurements. Chem.—Eur. J. 2008, 14, 11500–11511. [Google Scholar] [CrossRef]
- Kwit, M.; Gawronski, J.; Sbircea, L.; Sharma, N.D.; Kaik, M.; Boyd, D.R. Circular dichroism spectra, optical rotations and absolute configurations ofcis-dihydrodiol metabolites of quinoline and derivatives: The role of the nitrogen atom. Chirality 2009, 21, E37–E47. [Google Scholar] [CrossRef]
- Boyd, D.R.; Sharma, N.D.; Ljubez, V.; McGeehin, P.K.M.; Stevenson, P.J.; Blain, M.; Allen, C.C.R. Chemoenzymatic synthesis of monocyclic arene oxides and arene hydrates from substituted benzene substrates. Org. Biomol. Chem. 2013, 11, 3020–3029. [Google Scholar] [CrossRef] [PubMed]
- Jerina, D.M.; Selander, H.; Yagi, H.; Wells, M.C.; Davey, J.F.; Mahadevan, V.; Gibson, D.T. Dihydrodiols from anthracene and phenanthrene. J. Am. Chem. Soc. 1976, 98, 5988–5996. [Google Scholar] [CrossRef] [PubMed]
- Parales, R.E.; Resnick, S.M.; Yu, C.-L.; Boyd, D.R.; Sharma, N.D.; Gibson, D.T. Regioselectivity and Enantioselectivity of Naphthalene Dioxygenase during Arene cis -Dihydroxylation: Control by Phenylalanine 352 in the α Subunit. J. Bacteriol. 2000, 182, 5495–5504. [Google Scholar] [CrossRef] [PubMed]
- Boyd, D.R.; Sharma, N.D.; Coen, G.P.; Hempenstall, F.; Ljubez, V.; Malone, J.F.; Allen, C.C.R.; Hamilton, J.T.G. Regioselectivity and stereoselectivity of dioxygenase catalysed cis-dihydroxylation of mono- and tri-cyclic azaarene substrates. Org. Biomol. Chem. 2008, 6, 3957–3966. [Google Scholar] [CrossRef]
- Alien, C.C.R.; Boyd, D.R.; Dalton, H.; Sharma, N.D.; Haughey, S.A.; McMordie, R.A.S.; McMurray, B.T.; Sheldrake, G.N.; Sproule, K. Sulfoxides of high enantiopurity from bacterial dioxygenase-catalysed oxidation. J. Chem. Soc. Chem. Commun. 1995, 119–120. [Google Scholar] [CrossRef]
- Boyd, D.R.; Sharma, N.D.; Haughey, S.A.; Kennedy, M.A.; McMurray, B.T.; Sheldrake, G.N.; Allen, C.C.R.; Dalton, H.; Sproule, K. Toluene and naphthalene dioxygenase-catalysed sulfoxidation of alkyl aryl sulfides. J. Chem. Soc. Perkin Trans. 1998, 1929–1934. [Google Scholar] [CrossRef]
- Kerridge, A.; Willetts, A.; Holland, H. Stereoselective oxidation of sulfides by cloned naphthalene dioxygenase. J. Mol. Catal. B Enzym. 1999, 6, 59–65. [Google Scholar] [CrossRef]
- Boyd, D.R.; Sharma, N.D.; Byrne, B.E.; Haughey, S.A.; Kennedy, M.A.; Allen, C.C.R. Dioxygenase-catalysed oxidation of alkylaryl sulfides: Sulfoxidation versus cis-dihydrodiol formation. Org. Biomol. Chem. 2004, 2, 2530–2537. [Google Scholar] [CrossRef]
- Mansuy, D.; Valadon, P.; Erdelmeier, I.; Lopez-Garcia, P.; Amar, C.; Girault, J.P.; Dansette, P.M. Thiophene S-oxides as new reactive metabolites: Formation by cytochrome P-450 dependent oxidation and reaction with nucleophiles. J. Am. Chem. Soc. 1991, 113, 7825–7826. [Google Scholar] [CrossRef]
- Treiber, A.; Dansette, P.M.; El Amri, H.; Girault, J.-P.; Ginderow, D.; Mornon, A.J.-P.; Mansuy, D. Chemical and Biological Oxidation of Thiophene: Preparation and Complete Characterization of Thiophene S-Oxide Dimers and Evidence for Thiophene S-Oxide as an Intermediate in Thiophene Metabolism In Vivo and In Vitro. J. Am. Chem. Soc. 1997, 119, 1565–1571. [Google Scholar] [CrossRef]
- Dansette, P.M.; Bertho, G.; Mansuy, D. First evidence that cytochrome P450 may catalyze both S-oxidation and epoxidation of thiophene derivatives. Biochem. Biophys. Res. Commun. 2005, 338, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Medower, C.; Wen, L.; Johnson, W.W. Cytochrome P450 Oxidation of the Thiophene-Containing Anticancer Drug 3-[(Quinolin-4-ylmethyl)-amino]-thiophene-2-carboxylic Acid (4-Trifluoromethoxy-phenyl)-amide to an Electrophilic Intermediate. Chem. Res. Toxicol. 2008, 21, 1570–1577. [Google Scholar] [CrossRef]
- Rademacher, P.M.; Woods, C.M.; Huang, Q.; Szklarz, G.D.; Nelson, S.D. Differential Oxidation of Two Thiophene-Containing Regioisomers to Reactive Metabolites by Cytochrome P450 2C9. Chem. Res. Toxicol. 2012, 25, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Gramec, D.; Mašič, L.P.; Dolenc, M.S. Bioactivation Potential of Thiophene-Containing Drugs. Chem. Res. Toxicol. 2014, 27, 1344–1358. [Google Scholar] [CrossRef] [PubMed]
- Heine, T.; Scholtissek, A.; Hofmann, S.; Koch, R.; Tischler, D. Accessing Enantiopure Epoxides and Sulfoxides: Related Flavin-Dependent Monooxygenases Provide Reversed Enantioselectivity. ChemCatChem 2019, 12, 199–209. [Google Scholar] [CrossRef]
- Yang, J.; Yuan, Z.; Zhou, Y.; Zhao, J.; Yang, M.; Cheng, X.; Ou, G.; Chen, Y. Asymmetric reductive resolution of racemic sulfoxides by recombinant methionine sulfoxide reductase from a pseudomonas monteilii strain. J. Mol. Catal. B Enzym. 2016, 133, S588–S592. [Google Scholar] [CrossRef]
- Yang, J.; Wen, Y.; Peng, L.; Chen, Y.; Cheng, X.; Chen, Y.-Z. Identification of MsrA homologues for the preparation of (R)-sulfoxides at high substrate concentrations. Org. Biomol. Chem. 2019, 17, 3381–3388. [Google Scholar] [CrossRef]
- Luckarift, H.R.; Dalton, H.; Sharma, N.D.; Boyd, D.R.; Holt, R.A. Isolation and characterisation of bacterial strains containing enantioselective DMSO reductase activity: Application to the kinetic resolution of racemic sulfoxides. Appl. Microbiol. Biotechnol. 2004, 65, 678–685. [Google Scholar] [CrossRef]
- Boyd, D.R.; Sharma, N.D.; King, A.W.T.; Shepherd, S.D.; Allen, C.C.R.; Holt, R.A.; Luckarift, H.R.; Dalton, H. Stereoselective reductase-catalysed deoxygenation of sulfoxides in aerobic and anaerobic bacteria. Org. Biomol. Chem. 2004, 2, 554–561. [Google Scholar] [CrossRef]
- Anselmi, S.; Aggarwal, N.; Moody, T.S.; Castagnolo, D. Unconventional Biocatalytic Approaches to the Synthesis of Chiral Sulfoxides. ChemBioChem 2020, 22, 298–307. [Google Scholar] [CrossRef]
- Verlinden, S.; Woller, T.; De Proft, F.; Verniest, G.; Alonso, M. Towards the Design of Optically Active Thiophene S-Oxides using Quantum Chemistry. Chem.—Eur. J. 2019, 25, 2840–2851. [Google Scholar] [CrossRef]
- Jenks, W.S.; Matsunaga, N.; Gordon, M. Effects of Conjugation and Aromaticity on the Sulfoxide Bond. J. Org. Chem. 1996, 61, 1275–1283. [Google Scholar] [CrossRef]
- Bongini, A.; Arbizzani, C.; Barbarella, G.; Zambianchi, M.; Mastragostino, M. Thiophene S-oxides: Orbital energies and electrochemical properties. Chem. Commun. 2000, 439–440. [Google Scholar] [CrossRef]
- Pouzet, P.; Erdelmeier, I.; Ginderow, D.; Mornon, J.-P.; Dansette, P.; Mansuy, D. Thiophene S-oxides: Convenient preparation, first complete structural characterization and unexpected dimerization of one of them, 2,5-diphenylthiophene-1-oxide. J. Chem. Soc. Chem. Commun. 1995, 473–474. [Google Scholar] [CrossRef]
- Otani, T.; Miyoshi, M.; Shibata, T.; Matsuo, T.; Hashizume, D.; Tamao, K. Thermally Stable Monosubstituted Thiophene 1-Oxide and 1-Imides Stabilized by a Bulky Rind Group at Their 3-Position: Synthesis, Structure, and Inversion Barriers on the Sulfur Atom. Bull. Chem. Soc. Jpn. 2017, 90, 697–705. [Google Scholar] [CrossRef]
- Mock, W.L. Stable thiophene sulfoxides. J. Am. Chem. Soc. 1970, 92, 7610–7612. [Google Scholar] [CrossRef]
- Xiong, F.; Yang, B.-B.; Zhang, J.; Li, L. Enantioseparation, Stereochemical Assignment and Chiral Recognition Mechanism of Sulfoxide-Containing Drugs. Molecules 2018, 23, 2680. [Google Scholar] [CrossRef]
- Boyd, D.R.; Sharma, N.D.; Loke, P.L.; Carroll, J.G.; Stevenson, P.J.; Hoering, P.; Allen, C.C.R. Toluene Dioxygenase-Catalyzed cis-Dihydroxylation of Quinolines: A Molecular Docking Study and Chemoenzymatic Synthesis of Quinoline Arene Oxides. Front. Bioeng. Biotechnol. 2021, 8. [Google Scholar] [CrossRef]
- Höring, P.; Rothschild-Mancinelli, K.; Sharma, N.D.; Boyd, D.R.; Allen, C.C.R. Oxidative biotransformations of phenol substrates catalysed by toluene dioxygenase: A molecular docking study. J. Mol. Catal. B Enzym. 2016, 134, 396–406. [Google Scholar] [CrossRef][Green Version]
- Friemann, R.; Lee, K.; Brown, E.; Gibson, D.T.; Eklund, H.; Ramaswamy, S. Structures of the multicomponent Rieske non-heme iron toluene 2,3-dioxygenase enzyme system. Acta Crystallogr. Sect. D Biol. Crystallogr. 2008, 65, 24–33. [Google Scholar] [CrossRef]
- Karlsson, A.; Parales, J.V.; Parales, R.E.; Gibson, D.T.; Eklund, H.; Ramaswamy, S. Crystal Structure of Naphthalene Dioxygenase: Side-on Binding of Dioxygen to Iron. Science 2003, 299, 1039–1042. [Google Scholar] [CrossRef]
- Vila, M.A.; Pazos, M.; Iglesias, C.; Veiga, N.; Seoane, G.; Carrera, I. Toluene Dioxygenase-Catalysed Oxidation of Benzyl Azide to Benzonitrile: Mechanistic Insights for an Unprecedented Enzymatic Transformation. ChemBioChem 2016, 17, 291–295. [Google Scholar] [CrossRef]
- Vila, M.A.; Umpiérrez, D.; Seoane, G.; Rodríguez, S.; Carrera, I.; Veiga, N. Computational insights into the oxidation of mono- and 1,4 disubstituted arenes by the Toluene Dioxygenase enzymatic complex. J. Mol. Catal. B Enzym. 2016, 133, S410–S419. [Google Scholar] [CrossRef]
- Vila, M.A.; Umpiérrez, D.; Veiga, N.; Seoane, G.; Carrera, I.; Giordano, S.R. Site-Directed Mutagenesis Studies on the Toluene Dioxygenase Enzymatic System: Role of Phenylalanine 366, Threonine 365 and Isoleucine 324 in the Chemo-, Regio-, and Stereoselectivity. Adv. Synth. Catal. 2017, 359, 2149–2157. [Google Scholar] [CrossRef]
- Boyd, D.R.; Sharma, N.D.; McIntyre, P.B.A.; Stevenson, P.J.; McRoberts, W.C.; Gohil, A.; Hoering, P.; Allen, C. Enzyme-Catalysed Synthesis of Cyclohex-2-en-1-one cis-Diols from Substituted Phenols, Anilines and Derived 4-Hydroxycyclohex-2-en-1-ones. Adv. Synth. Catal. 2017, 359, 4002–4014. [Google Scholar] [CrossRef]
- Boyd, D.R.; Sharma, N.D.; Brannigan, I.N.; McGivern, C.J.; Nockemann, P.; Stevenson, P.J.; McRoberts, C.; Hoering, P.; Allen, C.C.R. Cis-Dihydroxylation of Tricyclic Arenes and Heteroarenes Catalyzed by Toluene Dioxygenase: A Molecular Docking Study and Experimental Validation. Adv. Synth. Catal. 2019, 361, 2526–2537. [Google Scholar] [CrossRef]
- González-Rosende, M.E.; Castillo, E.; Jennings, W.B.; Malone, J.F. Stereodynamics and edge-to-face CH–π aromatic interactions in imino compounds containing heterocyclic rings. Org. Biomol. Chem. 2017, 15, 1484–1494. [Google Scholar] [CrossRef] [PubMed]
- Boyd, D.R.; Sharma, N.D.; Brannigan, I.N.; Haughey, S.A.; Malone, J.F.; Clarke, D.A.; Dalton, H. Dioxygenase-catalysed formation of cis/trans-dihydrodiol metabolites of mono- and bi-cyclic heteroarenes. Chem. Commun. 1996, 2361–2362. [Google Scholar] [CrossRef]
- Boyd, D.R.; Sharma, N.D.; Brannigan, I.N.; Evans, T.A.; Haughey, S.A.; McMurray, B.T.; Malone, J.F.; McIntyre, P.B.A.; Stevenson, P.J.; Allen, C.C.R. Toluene dioxygenase-catalyzed cis-dihydroxylation of benzo[b]thiophenes and benzo[b]furans: Synthesis of benzo[b]thiophene 2,3-oxide. Org. Biomol. Chem. 2012, 10, 7292–7304. [Google Scholar] [CrossRef]
- Boyd, D.R.; Sharma, N.D.; McMurray, B.; Haughey, S.A.; Allen, C.C.R.; Hamilton, J.T.G.; McRoberts, W.C.; More O’Ferrall, R.A.; Nikodinovic-Runic, J.; Coulombel, L.A.; et al. Bacterial dioxygenase- and monooxygenase-catalysed sulfoxidation of benzo[b]thiophenes. Org. Biomol. Chem. 2011, 10, 782–790. [Google Scholar] [CrossRef]
- Gai, Z.H.; Yu, B.; Wang, X.Y.; Deng, Z.X.; Xu, P. Microbial transformation of benzothiophenes, with carbazole as the auxiliary substrate, by Sphingomonas sp strain XLDN2–5. Microbiology 2008, 154, 3804–3812. [Google Scholar] [CrossRef]
- Boyd, D.R.; Sharma, N.D.; Haughey, S.A.; Malone, J.F.; McMurray, B.T.; Sheldrake, G.N.; Allen, C.C.R.; Dalton, H. Enantioselective dioxygenase-catalysed formation and thermal racemisation of chiral thiophene sulfoxides. Chem. Commun. 1996, 2363–2364. [Google Scholar] [CrossRef]
- Thiemann, T.; Fujii, H.; Ohira, D.; Arima, K.; Li, Y.; Mataka, S. Cycloaddition of thiophene S-oxides to allenes, alkynes and to benzyne. New J. Chem. 2003, 27, 1377–1384. [Google Scholar] [CrossRef]
- Joost, M.; Nava, M.; Transue, W.J.; Martin-Drumel, M.-A.; McCarthy, M.C.; Patterson, D.; Cummins, C.C. Sulfur monoxide thermal release from an anthracene-based precursor, spectroscopic identification, and transfer reactivity. Proc. Natl. Acad. Sci. USA 2018, 115, 5866–5871. [Google Scholar] [CrossRef]
- Torok, D.S.; Resnick, S.M.; Brand, J.M.; Cruden, D.L.; Gibson, D.T. Desaturation and oxygenation of 1,2-dihydronaphthalene by toluene dioxygenase and naphthalene dioxygenase. J. Bacteriol. 1995, 177, 5799–5805. [Google Scholar] [CrossRef]
- Boyd, D.R.; Sharma, N.D.; Kerley, N.A.; McMordie, R.A.S.; Sheldrake, G.N.; Williams, P.; Dalton, H. Dioxygenase-catalysed oxidation of dihydronaphthalenes to yield arene hydrate and cis-dihydro naphthalenediols. J. Chem. Soc. Perkin Trans. 1996, 67–74. [Google Scholar] [CrossRef]
- Koreeda, M.; Akhtar, M.N.; Boyd, D.R.; Neill, J.D.; Gibson, D.T.; Jerina, D.M. Absolute stereochemistry of cis-1,2-, trans-1,2-, and cis-3,4-dihydrodiol metabolites of phenanthrene. J. Org. Chem. 1978, 43, 1023–1027. [Google Scholar] [CrossRef]
- Jerina, D.M.; Van Bladeren, P.J.; Yagi, H.; Gibson, D.T.; Mahadevan, V.; Neese, A.S.; Koreeda, M.; Sharma, N.D.; Boyd, D.R. Synthesis and absolute configuration of the bacterial cis-1,2-, cis-8,9-, and cis-10,11-dihydro diol metabolites of benz[a]anthracene formed by strain of Beijerinckia. J. Org. Chem. 1984, 49, 3621–3628. [Google Scholar] [CrossRef]
- Boyd, D.R.; Sharma, N.D.; Agarwal, R.; Resnick, S.M.; Schocken, M.J.; Gibson, D.T.; Sayer, J.M.; Yagi, H.; Jerina, D.M. Bacterial dioxygenase-catalysed dihydroxylation and chemical resolution routes to enantiopure cis-dihydrodiols of chrysene. J. Chem. Soc. Perkin Trans. 1997, 1715–1724. [Google Scholar] [CrossRef]
- Boyd, D.R.; Sharma, N.D.; Hempenstall, F.; Kennedy, M.A.; Malone, J.F.; Allen, C.C.R.; Resnick, S.M.; Gibson, D.T. bis-cis-Dihydrodiols: A New Class of Metabolites Resulting from Biphenyl Dioxygenase-Catalyzed Sequential Asymmetriccis-Dihydroxylation of Polycyclic Arenes and Heteroarenes. J. Org. Chem. 1999, 64, 4005–4011. [Google Scholar] [CrossRef]
- Boyd, D.R.; Sharma, N.D.; Harrison, J.S.; Kennedy, M.A.; Allen, C.C.R.; Gibson, D.T. Regio- and stereo-selective dioxygenase-catalysed cis-dihydroxylation of fjord-region polycyclic arenes. J. Chem. Soc. Perkin Trans. 2001, 1264–1269. [Google Scholar] [CrossRef]
- Ferraro, D.J.; Brown, E.N.; Yu, C.L.; Parales, R.E.; Gibson, D.T.; Ramaswamy, S. Structural investigations of the ferredoxin and terminal oxygenase components of the biphenyl 2,3-dioxygenase from Sphingobium yanoikuyae B1. BMC Struct. Biol. 2007, 7, 10. [Google Scholar] [CrossRef]
- Ferraro, D.J.; Okerlund, A.; Brown, E.; Ramaswamy, S. One enzyme, many reactions: Structural basis for the various reactions catalyzed by naphthalene 1,2-dioxygenase. IUCrJ 2017, 4, 648–656. [Google Scholar] [CrossRef] [PubMed]
- Gai, Z.; Yu, B.; Li, L.; Wang, Y.; Ma, C.; Feng, J.; Deng, Z.; Xu, P. Cometabolic Degradation of Dibenzofuran and Dibenzothiophene by a Newly Isolated Carbazole-Degrading Sphingomonas sp. Strain. Appl. Environ. Microbiol. 2007, 73, 2832–2838. [Google Scholar] [CrossRef] [PubMed]
- Kodama, K.; Umehara, K.; Shimizu, K.; Nakatani, S.; Minoda, Y.; Yamada, K. Identification of Microbial Products from Dibenzothiophene and Its Proposed Oxidation Pathway. Agric. Biol. Chem. 1973, 37, 45–50. [Google Scholar] [CrossRef]
- Kodama, K.; Nakatani, S.; Umehara, K.; Shimizu, K.; Minoda, Y.; Yamada, K. Microbial Conversion of Petro-sulfur Compounds. Agric. Biol. Chem. 1970, 34, 1320–1324. [Google Scholar] [CrossRef]
- Resnick, S.M.; Gibson, D.T. Regio- and stereospecific oxidation of fluorene, dibenzofuran, and dibenzothiophene by naphthalene dioxygenase from Pseudomonas sp strain NCIB 9816–4. Appl. Environ. Microbiol. 1996, 62, 4073–4080. [Google Scholar] [CrossRef]
- Laborde, A.L.; Gibson, D.T. Metabolism of dibenzothiophene by a Beijerinckia species. Appl. Environ. Microbiol. 1977, 34, 783–790. [Google Scholar] [CrossRef]
- Khan, A.A.; Wang, R.F.; Cao, W.W.; Franklin, W.; Cerniglia, C.E. Reclassification of a polycyclic aromatic hydrocarbon-metabolizing bacterium, Beijerinckia sp strain B1, as Sphingomonas yanoikuyae by fatty acid analysis, protein pattern analysis, DNA-DNA hybridization, and 16S ribosomal DNA sequencing. Int. J. System. Bacteriol. 1996, 46, 466–469. [Google Scholar] [CrossRef]
- Bianchi, D.; Bosetti, A.; Cidaria, D.; Bernardi, A.; Gagliardi, I.; D’Amico, P. Oxidation of polycyclic aromatic heterocycles by Pseudomonas fluorescens TTC1. Appl. Microbiol. Biotechnol. 1997, 47, 596–599. [Google Scholar] [CrossRef]
- Saftic, S.; Fedorak, P.M.; Andersson, J.T. Transformations of methyldibenzothiophenes by three Pseudomonas isolates. Environ. Sci. Technol. 1993, 27, 2577–2584. [Google Scholar] [CrossRef]
- Gibson, D.T.; Resnick, S.M.; Lee, K.; Brand, J.M.; Torok, D.S.; Wackett, L.P.; Schocken, M.J.; Haigler, B.E. Desaturation, dioxygenation, and monooxygenation reactions catalyzed by naphthalene dioxygenase from Pseudomonas SP strain-9816–4. J. Bacteriol. 1995, 177, 2615–2621. [Google Scholar] [CrossRef]
- Resnick, S.; Lee, K.; Gibson, D.T. Diverse reactions catalyzed by naphthalene dioxygenase fromPseudomonas sp strain NCIB 9816. J. Ind. Microbiol. Biotechnol. 1996, 17, 438–457. [Google Scholar] [CrossRef]
- Eaton, S.L.; Resnick, S.M.; Gibson, D.T. Initial reactions in the oxidation of 1,2-dihydronaphthalene by Sphingomonas yanoikuyae strains. Appl. Environ. Microbiol. 1996, 62, 4388–4394. [Google Scholar] [CrossRef] [PubMed]
- Misra, B.; Amin, S. Synthesis and mutagenicity of trans-dihydrodiol metabolites of benzo[b]naphtho[2,1-d]thiophene. Chem. Res. Toxicol. 1990, 3, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.E.; Amin, S.; Coletta, K.; Hoffmann, D. Rat liver metabolism of benzo[b]naphtho[2,1-d]thiophene. Chem. Res. Toxicol. 1992, 5, 491–495. [Google Scholar] [CrossRef]
- Boyd, D.R.; Sharma, N.D.; Sbircea, L.; Murphy, D.; Belhocine, T.; Malone, J.F.; James, S.L.; Allen, C.C.R.; Hamilton, J.T.G. Azaarene cis-dihydrodiol-derived 2,2′-bipyridine ligands for asymmetric allylic oxidation and cyclopropanation. Chem. Commun. 2008, 5535–5537. [Google Scholar] [CrossRef] [PubMed]
- Boyd, D.R.; Sharma, N.D.; Sbircea, L.; Murphy, D.; Malone, J.F.; James, S.L.; Allen, C.C.R.; Hamilton, J.T.G. Chemoenzymatic synthesis of chiral 2,2′-bipyridine ligands and their N-oxide derivatives: Applications in the asymmetric aminolysis of epoxides and asymmetric allylation of aldehydes. Org. Biomol. Chem. 2010, 8, 1081–1090. [Google Scholar] [CrossRef]
- Sbircea, L.; Sharma, N.D.; Clegg, W.; Harrington, R.W.; Horton, P.N.; Hursthouse, M.B.; Apperley, D.C.; Boyd, D.R.; James, S.L. Chemoenzymatic synthesis of chiral 4,4′-bipyridyls and their metal–organic frameworks. Chem. Commun. 2008, 5538–5540. [Google Scholar] [CrossRef]
- Nakayama, J.; Tajima, Y.; Xue-Hua, P.; Sugihara, Y. [1 + 2] Cycloadditions of Sulfur Monoxide (SO) to Alkenes and Alkynes and [1 + 4] Cycloadditions to Dienes (Polyenes). Generation and Reactions of Singlet SO? J. Am. Chem. Soc. 2007, 129, 7250–7251. [Google Scholar] [CrossRef]
- Dansette, P.; Jerina, D.M. Facile synthesis of arene oxides at the K regions of polycyclic hydrocarbons. J. Am. Chem. Soc. 1974, 96, 1224–1225. [Google Scholar] [CrossRef]
- Mitchell, J.R.; Jollows, D.J. Metabolic Activation of Drugs to Toxic Substances. Gastroenterology 1975, 68, 392–410. [Google Scholar] [CrossRef]
- McMurtry, R.; Mitchell, J. Renal and hepatic necrosis after metabolic activation of 2-substituted furans and thiophenes, including furosemide and cephaloridine. Toxicol. Appl. Pharmacol. 1977, 42, 285–300. [Google Scholar] [CrossRef]
- Dansette, P.M.; Thang, D.C.; ElAmri, H.; Mansuy, D. Evidence for thiophene-S-oxide as primary reactive metabolite of thiophene in vivo- formation of a dihydrothiophene sulfoxide mercapturic acid. Biochem. Biophys. Res. Commun. 1992, 186, 1624–1630. [Google Scholar] [CrossRef]
- Li, Y.; Thiemann, T.; Sawada, T.; Mataka, S.; Tashiro, M. Lewis Acid Catalysis in the Oxidative Cycloaddition of Thiophenes1. J. Org. Chem. 1997, 62, 7926–7936. [Google Scholar] [CrossRef]
- Sodhi, J.K.; Delarosa, E.M.; Halladay, J.S.; Driscoll, J.P.; Mulder, T.; Dansette, P.M.; Khojasteh, S.C. Inhibitory Effects of Trapping Agents of Sulfur Drug Reactive Intermediates against Major Human Cytochrome P450 Isoforms. Int. J. Mol. Sci. 2017, 18, 1553. [Google Scholar] [CrossRef] [PubMed]
- Dansette, P.M.; Thebault, S.; Durand-Gasselin, L.; Bertho, G.; Mansuy, D. Reactive metabolites of thiophenic compounds: A new trapping method for thiophene sulfoxides. Drug Metab. Rev. 2010, 42, 233–234. [Google Scholar]
- Du, F.; Ruan, Q.; Zhu, M.; Xing, J. Detection and characterization of ticlopidine conjugates in rat bile using high-resolution mass spectrometry: Applications of various data acquisition and processing tools. J. Mass Spectrom. 2013, 48, 413–422. [Google Scholar] [CrossRef]
- Jaladanki, C.K.; Taxak, N.; Varikoti, R.A.; Bharatam, P.V. Toxicity Originating from Thiophene Containing Drugs: Exploring the Mechanism using Quantum Chemical Methods. Chem. Res. Toxicol. 2015, 28, 2364–2376. [Google Scholar] [CrossRef] [PubMed]
- Imami, A.; Herold, N.; Spielmeyer, A.; Hausmann, H.; Dötzer, R.; Behnken, H.N.; Leonhardt, S.; Weil, A.; Schoof, S.; Zorn, H. Biotransformation of Dimethenamid-P by the basidiomycete Irpex consors. Chemosphere 2016, 165, 59–66. [Google Scholar] [CrossRef]
- Jerina, D.M.; Daly, J.W.; Witkop, B.; Zaltzman-Nirenberg, P.; Udenfriend, S. The role of arene oxide-oxepin systems in the metabolism of aromatic substrates. III. Formation of 1,2-naphthalene oxide from naphthalene by liver microsomes. J. Am. Chem. Soc. 1968, 90, 6525–6527. [Google Scholar] [CrossRef]
- Jerina, D.M.; Daly, J.W. Arene Oxides: A New Aspect of Drug Metabolism. Science 1974, 185, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Mansuy, D.; Dansette, P.M.; Foures, C.; Jaouen, M.; Moinet, G.; Bayer, N. Metabolic hydroxylation of the thiophene ring: Isolation of 5-hydroxy-tienilic acid as the major urinary metabolite of tienilic acid in man and rat. Biochem. Pharmacol. 1984, 33, 1429–1435. [Google Scholar] [CrossRef]
- Dalvie, D.K.; Kalgutkar, A.S.; Khojasteh-Bakht, S.C.; Obach, R.S.; O’Donnell, J.P. Biotransformation Reactions of Five-Membered Aromatic Heterocyclic Rings. Chem. Res. Toxicol. 2002, 15, 269–299. [Google Scholar] [CrossRef]
- Belghazi, M.; Jean, P.; Poli, S.; Schmitter, J.M.; Mansuy, D.; Dansette, P.M. Use of isotopes and LC-MS-ESI-TOF for mechanistic studies of Tienilic Acid metabolic activation. In Biological Reactive Intermediates VI; Dansette, P.M., Snyder, R., Delaforge, M., Gibson, G.G., Greim, H., Jollow, D.J., Monks, T.J., Sipes, I.G., Eds.; Kluwer Academic/Plenum Publishers: New York, NY, USA; Dordrecht, The Netherlands; London, UK; Moscow, Russia, 2001; Volume 500, pp. 139–144. [Google Scholar] [CrossRef]
- Mori, Y.; Sakai, Y.; Kuroda, N.; Yokoya, F.; Toyoshi, K.; Horie, M.; Baba, S. Further structural analysis of urinary metabolites of suprofen in the rat. Drug Metab. Dispos. 1984, 12, 767–771. [Google Scholar]
- Fouda, H.G.; Avery, M.J.; Dalvie, D.; Falkner, F.C.; Melvin, L.S.; Ronfeld, R.A. Disposition and metabolism of tenidap in the rat. Drug Metab. Dispos. 1997, 25, 140–148. [Google Scholar]
- Neau, E.; Dansette, P.; Andronik, V.; Mansuy, D. Hydroxylation of the thiophene ring by hepatic monooxygenases: Evidence for 5-hydroxylation of 2-aroylthiophenes as a general metabolic pathway using a simple UV-visible assay. Biochem. Pharmacol. 1990, 39, 1101–1107. [Google Scholar] [CrossRef]
- Nishikawa, T.; Nagata, O.; Tanbo, K.; Yamada, T.; Takahara, Y.; Kato, H.; Yamamoto, Y. Absorption, excretion and metabolism of tiquizium bromide in dogs, and relationship between pharmacological effect and plasma levels of unchanged drug. Xenobiotica 1985, 15, 1053–1060. [Google Scholar] [CrossRef]
- Lynch, M.J.; Mosher, F.R.; Levesque, W.R.; Newby, T.J. The In Vitro and In Vivo Metabolism of Morantel in Cattle and Toxicology Species. Drug Metab. Rev. 1987, 18, 253–288. [Google Scholar] [CrossRef] [PubMed]
- Ichihara, S.; Tsuyuki, Y.; Tomisawa, H.; Fukazawa, H.; Nakayama, N.; Tateishi, M.; Joly, R. Metabolism of tenoxicam in rats. Xenobiotica 1984, 14, 727–739. [Google Scholar] [CrossRef]
- Boyd, D.R.; Sharma, N.D. The changing face of arene oxide–oxepine chemistry. Chem. Soc. Rev. 1996, 25, 289–296. [Google Scholar] [CrossRef]
- Barr, S.A.; Bowers, N.; Boyd, D.R.; Sharma, N.D.; Hamilton, L.; Austin, R.; McMordie, R.A.S.; Dalton, H. The potential role of cis-dihydrodiol intermediates in bacterial aromatic hydroxylation and the NIH Shift. J. Chem. Soc. Perkin Trans. 1998, 3443–3452. [Google Scholar] [CrossRef]
- Stok, J.E.; Chow, S.; Krenske, E.H.; Soto, C.F.; Matyas, C.; Poirier, R.A.; Williams, C.M.; De Voss, J.J. Direct Observation of an Oxepin from a Bacterial Cytochrome P450-Catalyzed Oxidation. Chem.—Eur. J. 2016, 22, 4408–4412. [Google Scholar] [CrossRef] [PubMed]
- Boyd, D.R.; Sharma, N.D.; Harrison, J.S.; Malone, J.F.; McRoberts, W.C.; Hamilton, J.T.G.; Harper, D.B. Enzyme-catalysed synthesis and reactions of benzene oxide/oxepine derivatives of methyl benzoates. Org. Biomol. Chem. 2008, 6, 1251–1259. [Google Scholar] [CrossRef]
- Taguchi, K.; Konishi, T.; Nishikawa, H.; Kitamura, S. Identification of human cytochrome P450 isoforms involved in the metabolism ofS-2-[4-(3-methyl-2-thienyl)phenyl]propionic acid. Xenobiotica 1999, 29, 899–907. [Google Scholar] [CrossRef]
- Dansette, P.M.; Kitamura, S.; Bertho, G.; Mansuy, D. A new thiophene metabolite: NADPH, O2 and thiol dependent formation of a thiophene hydrate from (S) MTPPA (methyl-thienyl-phenyl-propionic acid) by CYP2C9. Drug Metab. Rev. 2005, 37, 34. [Google Scholar]
- Boyd, D.R.; Sharma, N.D.; Agarwal, R.; McMordie, R.A.S.; Bessems, J.; van Ommen, B.; van Bladeren, P.J. Biotransformation of 1,2-dihydronaphthalene and 1,2-dihydroanthracene by rat liver microsomes and purified cytochromes P-450. Formation of arene hydrates of naphthalene and anthracene. Chem. Res. Toxicol. 1993, 6, 808–812. [Google Scholar] [CrossRef]
- Lopez-Garcia, M.P.; Dansette, P.M.; Mansuy, D. Thiophene derivatives as new mechanism-based inhibitors of cytochromes P-450: Inactivation of yeast-expressed human liver cytochrome P-450 2C9 by tienilic acid. Biochemistry 1994, 33, 166–175. [Google Scholar] [CrossRef]
- Ha-Duong, N.-T.; Dijols, S.; Macherey, A.-C.; Goldstein, J.A.; Dansette, A.P.M.; Mansuy, D. Ticlopidine as a Selective Mechanism-Based Inhibitor of Human Cytochrome P450 2C19. Biochemistry 2001, 40, 12112–12122. [Google Scholar] [CrossRef] [PubMed]
- Richter, T.; Mürdter, T.E.; Heinkele, G.; Pleiss, J.; Tatzel, S.; Schwab, M.; Eichelbaum, M.; Zanger, U.M. Potent Mechanism-Based Inhibition of Human CYP2B6 by Clopidogrel and Ticlopidine. J. Pharmacol. Exp. Ther. 2003, 308, 189–197. [Google Scholar] [CrossRef]
- Hutzler, J.M.; Balogh, L.M.; Zientek, M.; Kumar, V.; Tracy, T.S. Mechanism-Based Inactivation of Cytochrome P450 2C9 by Tienilic Acid and (±)-Suprofen: A Comparison of Kinetics and Probe Substrate Selection. Drug Metab. Dispos. 2008, 37, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-L.; Zhang, H.; Medower, C.; Hollenberg, P.F.; Johnson, W.W. Inactivation of Cytochrome P450 (P450) 3A4 but not P450 3A5 by OSI-930, a Thiophene-Containing Anticancer Drug. Drug Metab. Dispos. 2010, 39, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Kalgutkar, A.S.; Driscoll, J.; Zhao, S.X.; Walker, G.S.; Shepard, R.M.; Soglia, J.R.; Atherton, J.; Yu, L.; Mutlib, A.E.; Munchhof, M.J.; et al. A Rational Chemical Intervention Strategy To Circumvent Bioactivation Liabilities Associated with a Nonpeptidyl Thrombopoietin Receptor Agonist Containing a 2-Amino-4-arylthiazole Motif. Chem. Res. Toxicol. 2007, 20, 1954–1965. [Google Scholar] [CrossRef] [PubMed]
- Barnette, D.; Schleiff, M.; Osborn, L.R.; Flynn, N.; Matlock, M.; Swamidass, S.J.; Miller, G.P. Dual mechanisms suppress meloxicam bioactivation relative to sudoxicam. Toxicology 2020, 440, 152478. [Google Scholar] [CrossRef]
- Obach, R.S.; Kalgutkar, A.S.; Ryder, T.F.; Walker, G.S. In Vitro Metabolism and Covalent Binding of Enol-Carboxamide Derivatives and Anti-Inflammatory Agents Sudoxicam and Meloxicam: Insights into the Hepatotoxicity of Sudoxicam. Chem. Res. Toxicol. 2008, 21, 1890–1899. [Google Scholar] [CrossRef] [PubMed]
- Nikodinovic-Runic, J.; Coulombel, L.; Francuski, D.; Sharma, N.D.; Boyd, D.R.; More O’Ferrall, R.A.; O’Connor, K.E. The oxidation of alkylaryl sulfides and benzo[b]thiophenes by Escherichia coli cells expressing wild-type and engineered styrene monooxygenase from Pseudomonas putida CA-3. Appl. Microbiol. Biotechnol. 2013, 97, 4849–4858. [Google Scholar] [CrossRef]
- Gursky, L.J.; Nikodinović-Runić, J.; Feenstra, K.A.; O’Connor, K.E. In vitro evolution of styrene monooxygenase from Pseudomonas putida CA-3 for improved epoxide synthesis. Appl. Microbiol. Biotechnol. 2010, 85, 995–1004. [Google Scholar] [CrossRef]
- O’Connor, K.E.; Dobson, A.D.; Hartmans, S. Indigo formation by microorganisms expressing styrene monooxygenase activity. Appl. Environ. Microbiol. 1997, 63, 4287–4291. [Google Scholar] [CrossRef]
- Chen, B.; Zhang, X.-D.; Wen, J.; Zhang, B.; Chen, D.; Wang, S.; Cai, J.-P.; Hu, G.-X. Effects of 26 Recombinant CYP3A4 Variants on Brexpiprazole Metabolism. Chem. Res. Toxicol. 2020, 33, 172–180. [Google Scholar] [CrossRef]
- Joshi, E.M.; Heasley, B.H.; Chordia, M.; Macdonald, T.L. In Vitro Metabolism of 2-Acetylbenzothiophene: Relevance to Zileuton Hepatotoxicity. Chem. Res. Toxicol. 2004, 17, 137–143. [Google Scholar] [CrossRef]
- Parker, M.H.; Smith-Swintosky, V.L.; McComsey, D.F.; Huang, Y.; Brenneman, D.; Klein, B.; Malatynska, E.; White, H.S.; Milewski, M.E.; Herb, M.; et al. Novel, Broad-Spectrum Anticonvulsants Containing a Sulfamide Group: Advancement of N-((Benzo[b]thien-3-yl)methyl)sulfamide (JNJ-26990990) into Human Clinical Studies. J. Med. Chem. 2009, 52, 7528–7536. [Google Scholar] [CrossRef]
- Jacob, J.; Schmoldt, A.; Grimmer, G. The predominant role of S-oxidation in rat liver metabolism of thiaarenes. Cancer Lett. 1986, 32, 107–116. [Google Scholar] [CrossRef]
- Hollopeter, G.; Jantzen, H.-M.; Vincent, D.; Li, G.; England, L.; Ramakrishnan, V.; Yang, R.-B.; Nurden, P.; Nurden, A.; Julius, D.; et al. Identification of the platelet ADP receptor targeted by antithrombotic drugs. Nature 2001, 409, 202–207. [Google Scholar] [CrossRef]
- Maffrand, J.-P. The story of clopidogrel and its predecessor, ticlopidine: Could these major antiplatelet and antithrombotic drugs be discovered and developed today? Comptes Rendus Chim. 2012, 15, 737–743. [Google Scholar] [CrossRef]
- Kiebist, J.; Schmidtke, K.-U.; Schramm, M.; König, R.; Quint, S.; Kohlmann, J.; Zuhse, R.; Ullrich, R.; Hofrichter, M.; Scheibner, K. Biocatalytic Syntheses of Antiplatelet Metabolites of the Thienopyridines Clopidogrel and Prasugrel Using Fungal Peroxygenases. J. Fungi 2021, 7, 752. [Google Scholar] [CrossRef]
- Dansette, P.M.; Libraire, J.; Bertho, G.; Mansuy, D. Metabolic Oxidative Cleavage of Thioesters: Evidence for the Formation of Sulfenic Acid Intermediates in the Bioactivation of the Antithrombotic Prodrugs Ticlopidine and Clopidogrel. Chem. Res. Toxicol. 2009, 22, 369–373. [Google Scholar] [CrossRef]
- Dansette, P.M.; Thébault, S.; Bertho, G.; Mansuy, D. Formation and Fate of a Sulfenic Acid Intermediate in the Metabolic Activation of the Antithrombotic Prodrug Prasugrel. Chem. Res. Toxicol. 2010, 23, 1268–1274. [Google Scholar] [CrossRef] [PubMed]
- Dansette, P.M.; Rosi, J.; Bertho, G.; Mansuy, D. Paraoxonase-1 and clopidogrel efficacy. Nat. Med. 2011, 17, 1040–1041. [Google Scholar] [CrossRef] [PubMed]
- Dansette, P.M.; Rosi, J.; Bertho, G.; Mansuy, D. Cytochromes P450 Catalyze Both Steps of the Major Pathway of Clopidogrel Bioactivation, whereas Paraoxonase Catalyzes the Formation of a Minor Thiol Metabolite Isomer. Chem. Res. Toxicol. 2012, 25, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Dansette, P.M.; Levent, D.; Hessani, A.; Bertho, G.; Mansuy, D. Thiolactone Sulfoxides as New Reactive Metabolites Acting as Bis-Electrophiles: Implication in Clopidogrel and Prasugrel Bioactivation. Chem. Res. Toxicol. 2013, 26, 794–802. [Google Scholar] [CrossRef]
- Zhang, H.; Lau, W.C.; Hollenberg, P.F. Formation of the thiol conjugates and active metabolite of clopidogrel by human liver microsomes. Mol. Pharmacol. 2012, 82, 302–309. [Google Scholar] [CrossRef]
- Tuong, A.; Bouyssou, A.; Paret, J.; Cuong, T.G. Metabolism of triclopidine in rats: Identification and quantitative determination of some its metabolites in plasma, urine and bile. Eur. J. Drug Metab. Pharmacokinet. 1981, 6, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Farid, N.A.; Smith, R.L.; Gillespie, T.A.; Rash, T.J.; Blair, P.E.; Kurihara, A.; Goldberg, M.J. The Disposition of Prasugrel, a Novel Thienopyridine, in Humans. Drug Metab. Dispos. 2007, 35, 1096–1104. [Google Scholar] [CrossRef]
- Liu, C.; Chen, Z.; Zhong, K.; Li, L.; Zhu, W.; Chen, X.; Zhong, D. Human Liver Cytochrome P450 Enzymes and Microsomal Thiol Methyltransferase Are Involved in the Stereoselective Formation and Methylation of the Pharmacologically Active Metabolite of Clopidogrel. Drug Metab. Dispos. 2015, 43, 1632–1641. [Google Scholar] [CrossRef] [PubMed]
- Kazui, M.; Hagihara, K.; Izumi, T.; Ikeda, T.; Kurihara, A. Hepatic Microsomal Thiol Methyltransferase Is Involved in Stereoselective Methylation of Pharmacologically Active Metabolite of Prasugrel. Drug Metab. Dispos. 2014, 42, 1138–1145. [Google Scholar] [CrossRef]
- Camilleri, P.; Soldo, B.; Buch, A.; Janusz, J. Oxidative metabolism of razuprotafib (AKB-9778), a sulfamic acid phosphatase inhibitor, in human microsomes and recombinant human CYP2C8 enzyme. Xenobiotica 2021, 51, 1110–1121. [Google Scholar] [CrossRef]
- Soldo, B.L.; Camilleri, P.; Buch, A.; Janusz, J. The in vivo disposition of subcutaneous injected 14C-razuprotafib (14C-AKB-9778), a sulphamic acid phosphatase inhibitor, in nonclinical species and human. Xenobiotica 2021, 51, 1132–1145. [Google Scholar] [CrossRef] [PubMed]
- Guengerich, F.P. A history of the roles of cytochrome P450 enzymes in the toxicity of drugs. Toxicol. Res. 2020, 37, 1–23. [Google Scholar] [CrossRef]
- Shimizu, S.; Atsumi, R.; Nakazawa, T.; Fujimaki, Y.; Sudo, K.; Okazaki, O. Metabolism of Ticlopidine in Rats: Identification of the Main Biliary Metabolite as a Glutathione Conjugate of Ticlopidine S-Oxide. Drug Metab. Dispos. 2009, 37, 1904–1915. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).