HIF-1α Negatively Regulates Irisin Expression Which Involves in Muscle Atrophy Induced by Hypoxia
Abstract
1. Introduction
2. Results
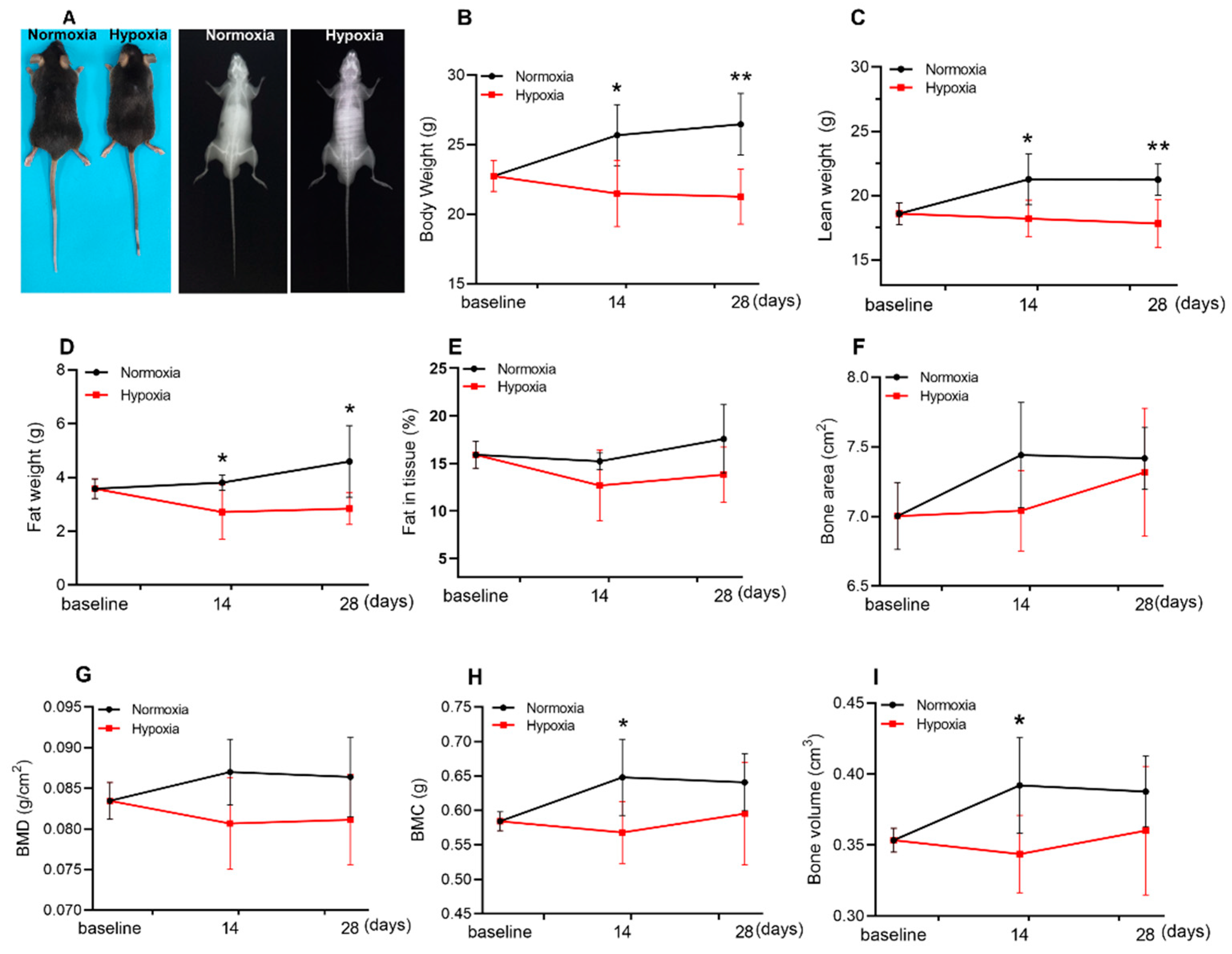
2.1. Hypoxia Reduced the Lean Weight and Fat Weight of Mice
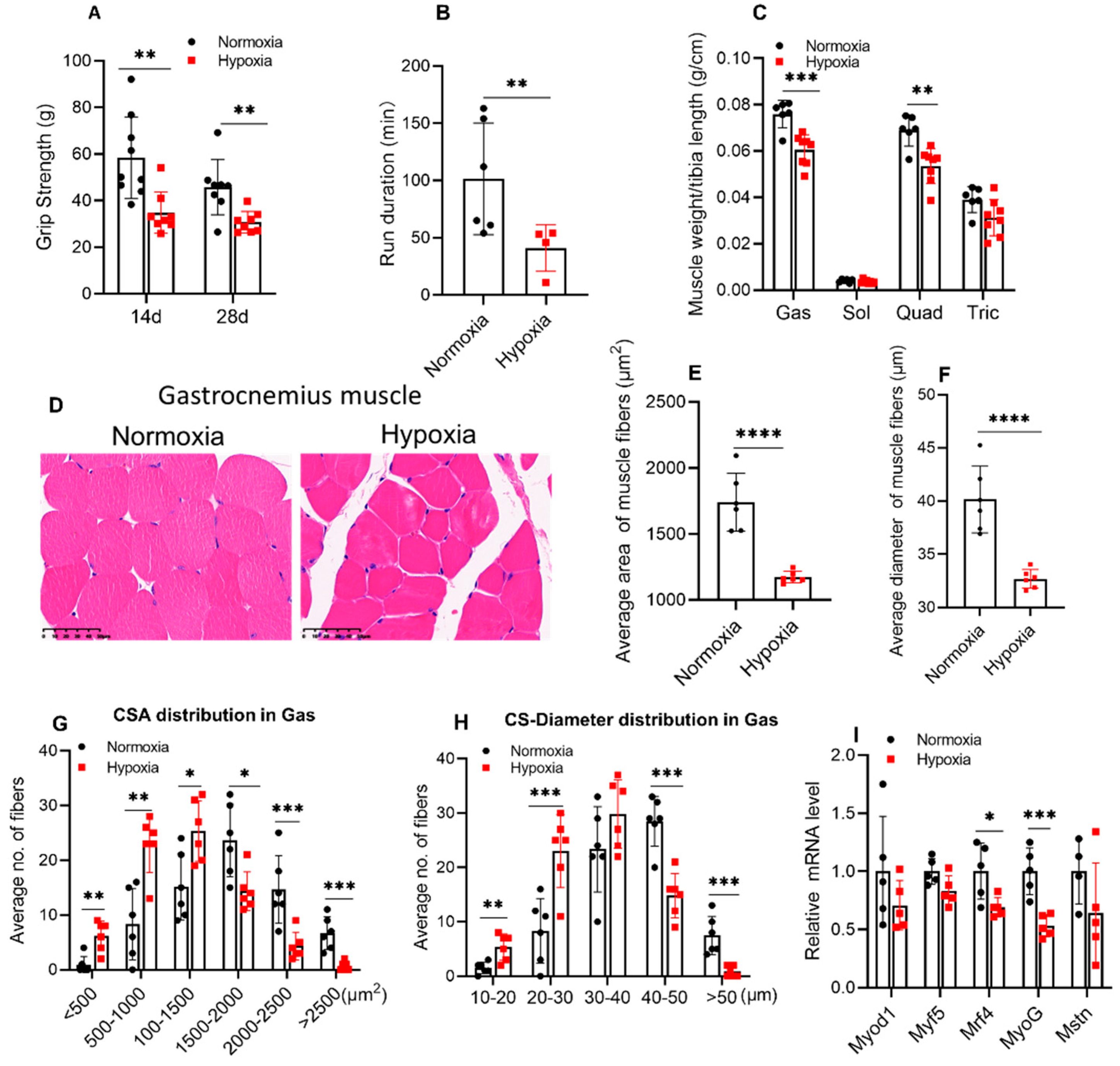
2.2. Four weeks of Hypoxic Exposure Induced Muscle Atrophy of Mice
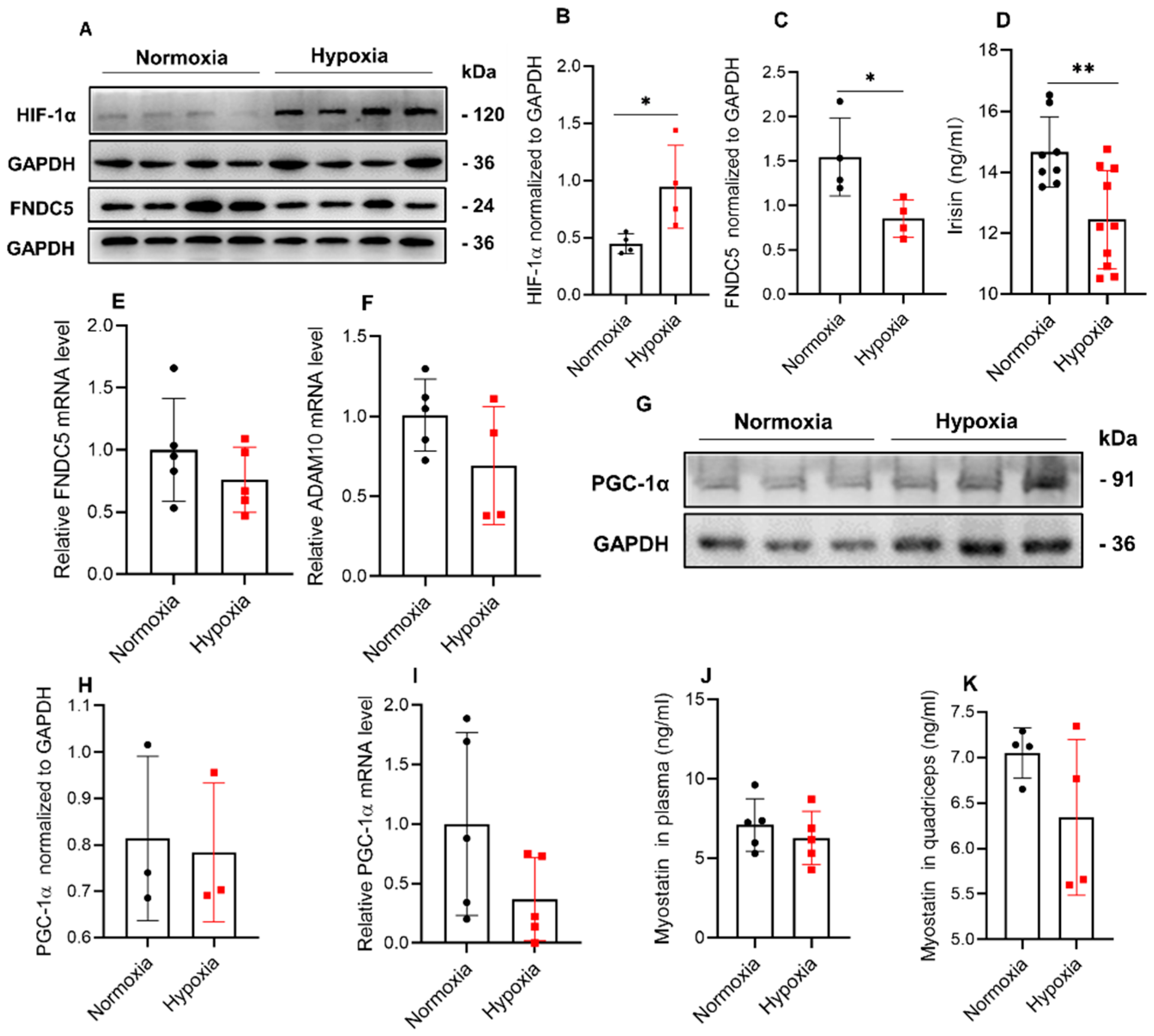
2.3. Hypoxia Significantly Reduced the Expression of FNDC5/Irisin in Mice
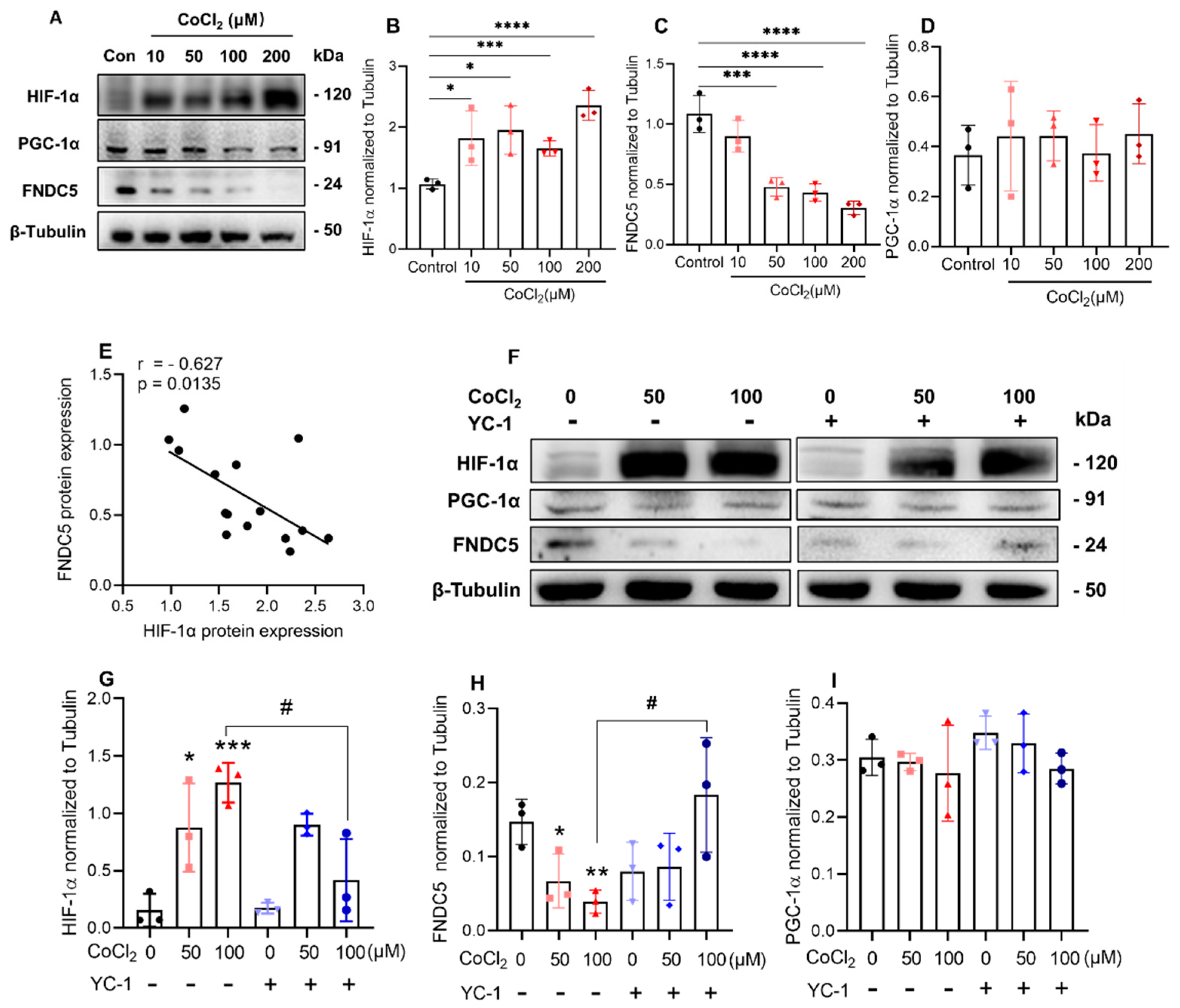
2.4. Inhibition of HIF-1α Induced by CoCl2 Increased FNDC5 Expression in C2C12 Cells
2.5. Inhibition of HIF-1α Induced by 1% O2 Ambient Hypoxia Increased FNDC5 Expression and Myotube Formation in C2C12 Cells
3. Discussion
4. Materials and Methods
4.1. Mice and Hypoxic Exposure
4.2. Body Composition and Grip Strength Measurement
4.3. Treadmill Experiment
4.4. ELISA for Determining Irisin and Myostatin Concentration in Plasma/Muscle Homogenate
4.5. Muscle Fiber Cross-Sectional Area (CSA) Measurement
4.6. Cell culture and Hypoxic Treatment
4.7. Cell Viability Assay
4.8. RNA Extraction and Real-Time Quantitative PCR (qPCR)
4.9. Western Blotting
4.10. Immunofluorescence Staining
4.11. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Frontera, W.R.; Ochala, J. Skeletal muscle: A brief review of structure and function. Calcif. Tissue Int. 2015, 96, 183–195. [Google Scholar] [CrossRef] [PubMed]
- D’Hulst, G.; Deldicque, L. Last Word on Viewpoint: Human skeletal muscle wasting in hypoxia: A matter of hypoxic dose? J. Appl. Physiol. 2017, 122, 412–413. [Google Scholar] [CrossRef]
- Pasiakos, S.M.; Berryman, C.E.; Carrigan, C.T.; Young, A.J.; Carbone, J.W. Muscle Protein Turnover and the Molecular Regulation of Muscle Mass during Hypoxia. Med. Sci. Sports Exerc. 2017, 49, 1340–1350. [Google Scholar] [CrossRef]
- Yin, H.; Li, D.; Wang, Y.; Zhao, X.; Liu, Y.; Yang, Z.; Zhu, Q. Myogenic regulatory factor (MRF) expression is affected by exercise in postnatal chicken skeletal muscles. Gene 2015, 561, 292–299. [Google Scholar] [CrossRef]
- Chen, R.; Jiang, T.; She, Y.; Xu, J.; Li, C.; Zhou, S.; Shen, H.; Shi, H.; Liu, S. Effects of Cobalt Chloride, a Hypoxia-Mimetic Agent, on Autophagy and Atrophy in Skeletal C2C12 Myotubes. BioMed Res. Int. 2017, 2017, 7097580. [Google Scholar] [CrossRef]
- Bensaid, S.; Fabre, C.; Fourneau, J.; Cieniewski-Bernard, C. Impact of different methods of induction of cellular hypoxia: Focus on protein homeostasis signaling pathways and morphology of C2C12 skeletal muscle cells differentiated into myotubes. J. Physiol. Biochem. 2019, 75, 367–377. [Google Scholar] [CrossRef]
- Pisani, D.F.; Dechesne, C.A. Skeletal muscle HIF-1alpha expression is dependent on muscle fiber type. J. Gen. Physiol. 2005, 126, 173–178. [Google Scholar] [CrossRef]
- Ono, Y.; Sensui, H.; Sakamoto, Y.; Nagatomi, R. Knockdown of hypoxia-inducible factor-1alpha by siRNA inhibits C2C12 myoblast differentiation. J. Cell. Biochem. 2006, 98, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Bostrom, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Bostrom, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Sathiakumar, D.; Lua, B.J.; Kukreti, H.; Lee, M.; McFarlane, C. Myostatin signals through miR-34a to regulate Fndc5 expression and browning of white adipocytes. Int. J. Obes. 2017, 41, 137–148. [Google Scholar] [CrossRef]
- Shan, T.; Liang, X.; Bi, P.; Kuang, S. Myostatin knockout drives browning of white adipose tissue through activating the AMPK-PGC1alpha-Fndc5 pathway in muscle. FASEB J. 2013, 27, 1981–1989. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Kou, W.; Xu, X.; Zhou, S.; Luan, P.; Xu, X.; Li, H.; Zhuang, J.; Wang, J.; Zhao, Y.; et al. FNDC5/Irisin inhibits pathological cardiac hypertrophy. Clin. Sci. 2019, 133, 611–627. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Kim, H.C.; Zhang, D.; Yeom, H.; Lim, S.K. The novel myokine irisin: Clinical implications and potential role as a biomarker for sarcopenia in postmenopausal women. Endocrine 2019, 64, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Jun, H.-S. Role of Myokines in Regulating Skeletal Muscle Mass and Function. Front. Physiol. 2019, 10, 42. [Google Scholar] [CrossRef]
- Reza, M.M.; Subramaniyam, N.; Sim, C.M.; Ge, X.; Sathiakumar, D.; McFarlane, C.; Sharma, M.; Kambadur, R. Irisin is a pro-myogenic factor that induces skeletal muscle hypertrophy and rescues denervation-induced atrophy. Nat. Commun. 2017, 8, 1104. [Google Scholar] [CrossRef]
- Colaianni, G.; Mongelli, T.; Cuscito, C.; Pignataro, P.; Lippo, L.; Spiro, G.; Notarnicola, A.; Severi, I.; Passeri, G.; Mori, G.; et al. Irisin prevents and restores bone loss and muscle atrophy in hind-limb suspended mice. Sci. Rep. 2017, 7, 2811. [Google Scholar] [CrossRef]
- Huh, J.Y.; Dincer, F.; Mesfum, E.; Mantzoros, C.S. Irisin stimulates muscle growth-related genes and regulates adipocyte differentiation and metabolism in humans. Int. J. Obes. 2014, 38, 1538–1544. [Google Scholar] [CrossRef]
- Moscoso, I.; Cebro-Márquez, M.; Rodríguez-Mañero, M.; González-Juanatey, J.R.; Lage, R. FNDC5/Irisin counteracts lipotoxic-induced apoptosis in hypoxic H9c2 cells. J. Mol. Endocrinol. 2019, 63, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Sliwicka, E.; Cison, T.; Kasprzak, Z.; Nowak, A.; Pilaczynska-Szczesniak, L. Serum irisin and myostatin levels after 2 weeks of high-altitude climbing. PLoS ONE 2017, 12, e0181259. [Google Scholar] [CrossRef]
- Sakurai, T.; Endo, S.; Hatano, D.; Ogasawara, J.; Kizaki, T.; Oh-ishi, S.; Izawa, T.; Ishida, H.; Ohno, H. Effects of exercise training on adipogenesis of stromal-vascular fraction cells in rat epididymal white adipose tissue. Acta Physiol. 2010, 200, 325–338. [Google Scholar] [CrossRef]
- Agrawal, A.; Rathor, R.; Kumar, R.; Suryakumar, G.; Ganju, L. Role of altered proteostasis network in chronic hypobaric hypoxia induced skeletal muscle atrophy. PLoS ONE 2018, 13, e0204283. [Google Scholar] [CrossRef]
- D’Hulst, G.; Ferri, A.; Naslain, D.; Bertrand, L.; Horman, S.; Francaux, M.; Bishop, D.J.; Deldicque, L. Fifteen days of 3200 m simulated hypoxia marginally regulates markers for protein synthesis and degradation in human skeletal muscle. Hypoxia 2016, 4, 1–14. [Google Scholar] [CrossRef] [PubMed][Green Version]
- De Theije, C.C.; Schols, A.M.W.J.; Lamers, W.H.; Neumann, D.; Köhler, S.E.; Langen, R.C.J. Hypoxia impairs adaptation of skeletal muscle protein turnover- and AMPK signaling during fasting-induced muscle atrophy. PLoS ONE 2018, 13, e0203630. [Google Scholar] [CrossRef] [PubMed]
- De Theije, C.C.; Schols, A.M.W.J.; Lamers, W.H.; Ceelen, J.J.M.; van Gorp, R.H.; Hermans, J.J.R.; Köhler, S.E.; Langen, R.C.J. Glucocorticoid Receptor Signaling Impairs Protein Turnover Regulation in Hypoxia-Induced Muscle Atrophy in Male Mice. Endocrinology 2018, 159, 519–534. [Google Scholar] [CrossRef] [PubMed]
- De Theije, C.C.; Langen, R.C.J.; Lamers, W.H.; Gosker, H.R.; Schols, A.M.W.J.; Köhler, S.E. Differential sensitivity of oxidative and glycolytic muscles to hypoxia-induced muscle atrophy. J. Appl. Physiol. 2015, 118, 200–211. [Google Scholar] [CrossRef]
- Chang, J.S.; Kim, T.H.; Nguyen, T.T.; Park, K.S.; Kim, N.; Kong, I.D. Circulating irisin levels as a predictive biomarker for sarcopenia: A cross-sectional community-based study. Geriatr. Gerontol. Int. 2017, 17, 2266–2273. [Google Scholar] [CrossRef]
- Zhao, M.; Zhou, X.; Yuan, C.; Li, R.; Ma, Y.; Tang, X. Association between serum irisin concentrations and sarcopenia in patients with liver cirrhosis: A cross-sectional study. Sci. Rep. 2020, 10, 16093. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, C.; Liu, S.; Zhang, S.; Mao, Y.; Fang, L. Eight Weeks of High-Intensity Interval Static Strength Training Improves Skeletal Muscle Atrophy and Motor Function in Aged Rats via the PGC-1alpha/FNDC5/UCP1 Pathway. Clin. Interv. Aging 2021, 16, 811–821. [Google Scholar] [CrossRef]
- Planella-Farrugia, C.; Comas, F.; Sabater-Masdeu, M.; Moreno, M.; Moreno-Navarrete, J.M.; Rovira, O.; Ricart, W.; Fernandez-Real, J.M. Circulating Irisin and Myostatin as Markers of Muscle Strength and Physical Condition in Elderly Subjects. Front. Physiol. 2019, 10, 871. [Google Scholar] [CrossRef]
- Chang, J.S.; Kong, I.D. Irisin prevents dexamethasone-induced atrophy in C2C12 myotubes. Pflug. Arch. 2020, 472, 495–502. [Google Scholar] [CrossRef]
- Barsoum, I.B.; Hamilton, T.K.; Li, X.; Cotechini, T.; Miles, E.A.; Siemens, D.R.; Graham, C.H. Hypoxia induces escape from innate immunity in cancer cells via increased expression of ADAM10: Role of nitric oxide. Cancer Res. 2011, 71, 7433–7441. [Google Scholar] [CrossRef]
- Guo, C.; Yang, Z.H.; Zhang, S.; Chai, R.; Xue, H.; Zhang, Y.H.; Li, J.Y.; Wang, Z.Y. Intranasal Lactoferrin Enhances alpha-Secretase-Dependent Amyloid Precursor Protein Processing via the ERK1/2-CREB and HIF-1alpha Pathways in an Alzheimer’s Disease Mouse Model. Neuropsychopharmacology 2017, 42, 2504–2515. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, L.; Zhou, Y.; Li, L.; Zhao, J.; Qin, W.; Jin, Z.; Liu, W. Increase in HDAC9 suppresses myoblast differentiation via epigenetic regulation of autophagy in hypoxia. Cell Death Dis. 2019, 10, 552. [Google Scholar] [CrossRef]
- Arany, Z.; Foo, S.-Y.; Ma, Y.; Ruas, J.L.; Bommi-Reddy, A.; Girnun, G.; Cooper, M.; Laznik, D.; Chinsomboon, J.; Rangwala, S.M.; et al. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1alpha. Nature 2008, 451, 1008–1012. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, K.F.; Beeson, G.C.; Beeson, C.C.; Baicu, C.F.; Zile, M.R.; McDermott, P.J. Estrogen-Related Receptor alpha (ERRalpha) is required for adaptive increases in PGC-1 isoform expression during electrically stimulated contraction of adult cardiomyocytes in sustained hypoxic conditions. Int. J. Cardiol. 2015, 187, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Levett, D.Z.; Radford, E.J.; Menassa, D.A.; Graber, E.F.; Morash, A.J.; Hoppeler, H.; Clarke, K.; Martin, D.S.; Ferguson-Smith, A.C.; Montgomery, H.E.; et al. Acclimatization of skeletal muscle mitochondria to high-altitude hypoxia during an ascent of Everest. FASEB J. 2012, 26, 1431–1441. [Google Scholar] [CrossRef]
- Chaudhary, P.; Suryakumar, G.; Prasad, R.; Singh, S.N.; Ali, S.; Ilavazhagan, G. Chronic hypobaric hypoxia mediated skeletal muscle atrophy: Role of ubiquitin-proteasome pathway and calpains. Mol. Cell. Biochem. 2012, 364, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Valle-Tenney, R.; Rebolledo, D.L.; Lipson, K.E.; Brandan, E. Role of hypoxia in skeletal muscle fibrosis: Synergism between hypoxia and TGF-beta signaling upregulates CCN2/CTGF expression specifically in muscle fibers. Matrix Biol. 2020, 87, 48–65. [Google Scholar] [CrossRef]

| Primers | Forward Primer Sequences | Reverse Primer Sequences |
|---|---|---|
| PGC-1α | 5′-CAACAATGAGCCTGCGAACA-3′ | 5′-CTTCATCCACGGGGAGACTG-3′ |
| FNDC5 | 5′-GGACCTGGAGGAGGACACAGAATA-3′ | 5′-CTGGCGGCAGAAGAGAGCTATAA-3′ |
| Mstn | 5′-GGATGGCAAGCCCAAATGTT-3′ | 5′-GATTCAGGCTGTTTGAGCCA-3′ |
| GAPDH | 5′-CGGTGCTGAGTATGTCGTGG-3′ | 5′-ATGAGCCCTTCCACAATGCC-3′ |
| ADAM10 | 5′-GGAAGCTTTAGTCATGGGTCTG-3′ | 5′-CTCCTTCCTCTACTCCAGTCAT-3′ |
| Myod1 | 5′-TACGACACCGCCTACTACAGTG-3′ | 5′-GTGGTGCATCTGCCAAAAG-3′ |
| Myf5 | 5′-CTGTCTGGTCCCGAAAGAAC -3′ | 5′-TGGAGAGAGGGAAGCTGTGT -3′ |
| MyoG | 5′-GCAATGCACTGGAGTTCG-3′ | 5′-ACGATGGACGTAAGGGAGTG-3′ |
| Mrf4 | 5′-TGCTAAGGAAGGAGGAGCAA-3′ | 5′-CCTGCTGGGTGAAGAATGTT-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Fu, P.; Ning, K.; Wang, R.; Yang, B.; Chen, J.; Xu, H. HIF-1α Negatively Regulates Irisin Expression Which Involves in Muscle Atrophy Induced by Hypoxia. Int. J. Mol. Sci. 2022, 23, 887. https://doi.org/10.3390/ijms23020887
Liu S, Fu P, Ning K, Wang R, Yang B, Chen J, Xu H. HIF-1α Negatively Regulates Irisin Expression Which Involves in Muscle Atrophy Induced by Hypoxia. International Journal of Molecular Sciences. 2022; 23(2):887. https://doi.org/10.3390/ijms23020887
Chicago/Turabian StyleLiu, Shiqiang, Pengyu Fu, Kaiting Ning, Rui Wang, Baoqiang Yang, Jiahui Chen, and Huiyun Xu. 2022. "HIF-1α Negatively Regulates Irisin Expression Which Involves in Muscle Atrophy Induced by Hypoxia" International Journal of Molecular Sciences 23, no. 2: 887. https://doi.org/10.3390/ijms23020887
APA StyleLiu, S., Fu, P., Ning, K., Wang, R., Yang, B., Chen, J., & Xu, H. (2022). HIF-1α Negatively Regulates Irisin Expression Which Involves in Muscle Atrophy Induced by Hypoxia. International Journal of Molecular Sciences, 23(2), 887. https://doi.org/10.3390/ijms23020887






