Diet Prevents Social Stress-Induced Maladaptive Neurobehavioural and Gut Microbiota Changes in a Histamine-Dependent Manner
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Ethical Approval
2.2. Chronic Social Defeat Stress
2.3. Social Interaction Test
2.4. Novel Object Recognition Test
2.5. Novel Object Location Test
2.6. Open Field Test
2.7. Western Blot Analysis
2.8. Long-Term Potentiation
2.9. Real-Time PCR of Gene Expression in the Hippocampus
2.10. Oxylipins Analysis
2.11. Fatty Acid Analysis
2.12. Microbiome Analysis
2.13. Quantification and Statistical Analysis
2.14. Microbiome Statistical Analysis
3. Results
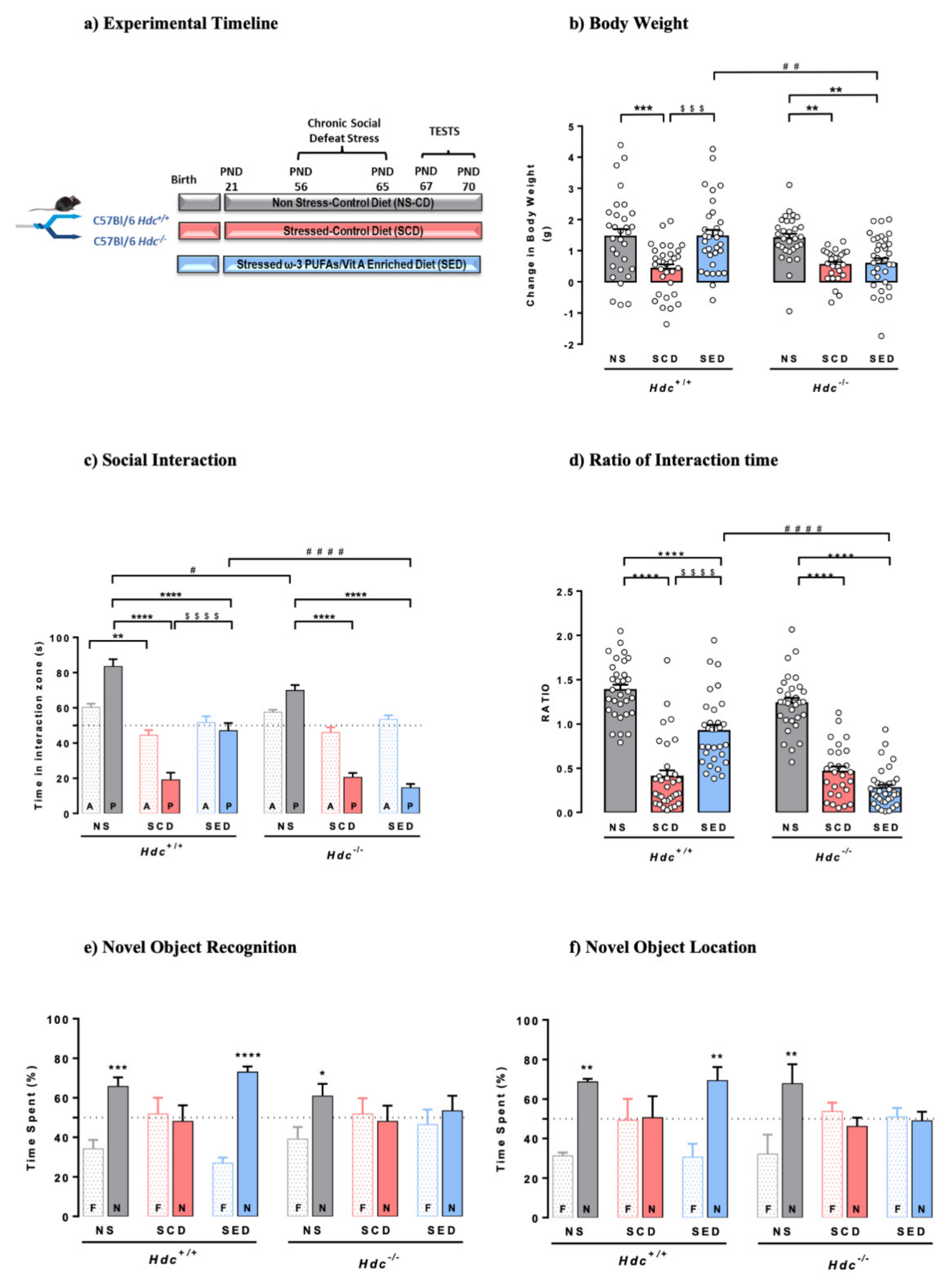
3.1. Effect of Stress and the Enriched Diet on Body Weight and Food Consumption
3.2. The Enriched Diet Prevents the Development of Social Avoidance of Hdc+/+ but Not of Hdc−/− Mice
3.3. Locomotor Activity and Anxiety-like Related Behaviours of Hdc+/+ and Hdc−/− Mice
3.4. The Enriched Diet Prevents Stress-Induced Memory Impairment of Hdc+/+, but Not That of Hdc−/− Mice
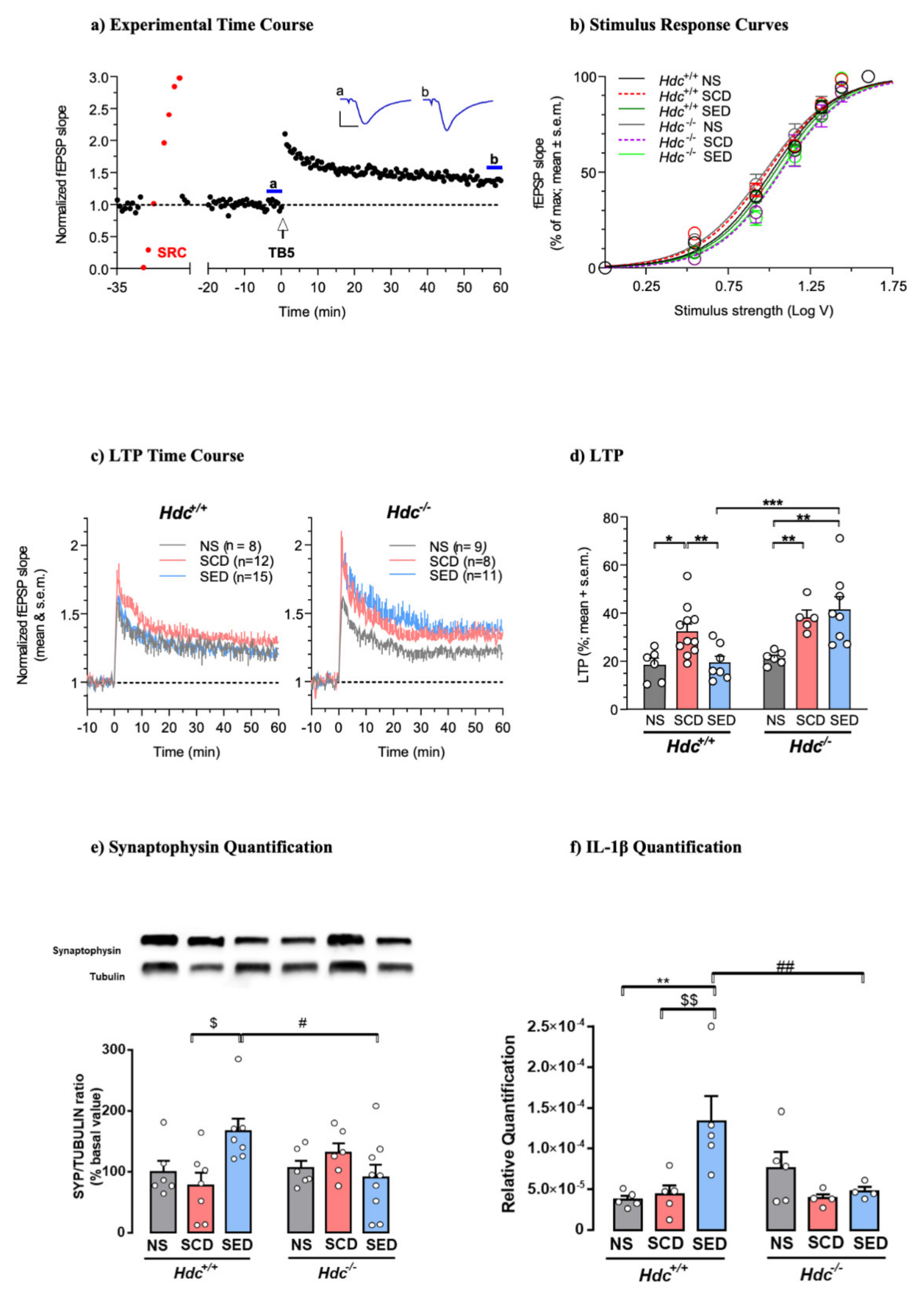
3.5. Effects of the Enriched Diet on Stress-Induced Changes of Hippocampal Synaptic Plasticity
3.6. Diet Affects the Expression of Cytokines in Hdc+/+ Mice Hippocampus
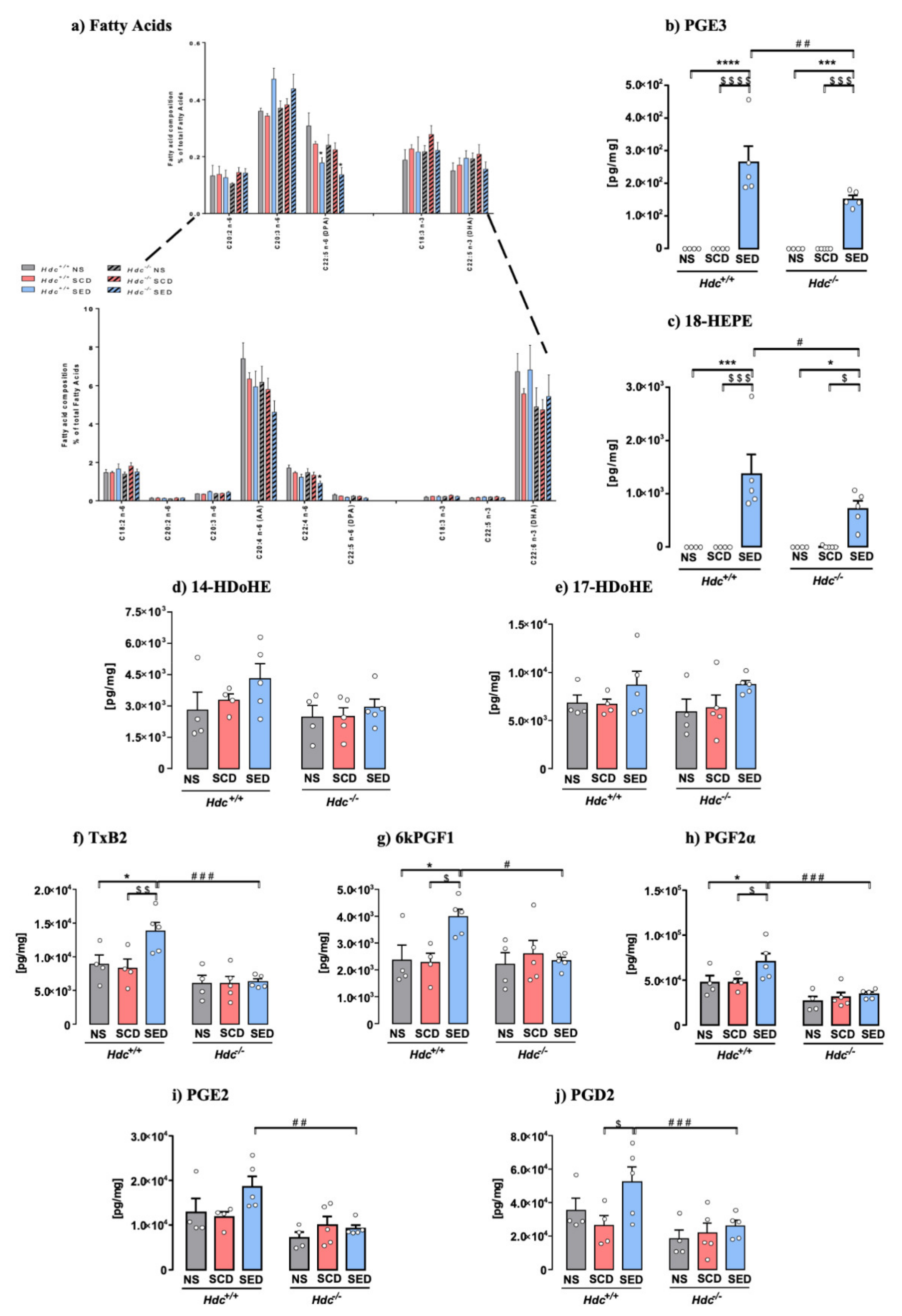
3.7. Effect of Stress and Diet on Hippocampal Fatty Acid and Oxylipins Composition
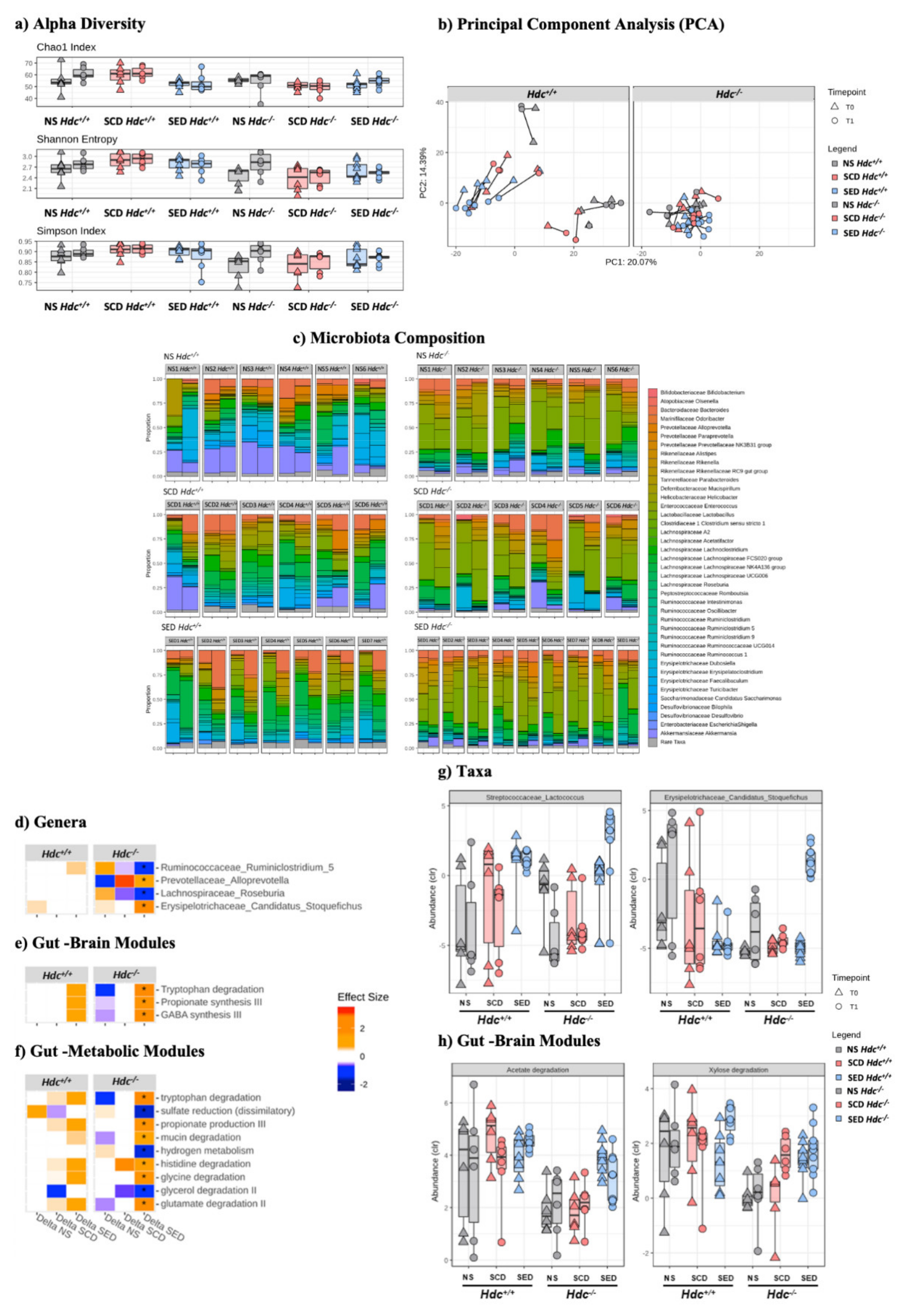
3.8. Stress and Diet Modify Microbiota Composition Differently in Hdc+/+ and Hdc−/− Mice
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McEwen, B.S.; Bowles, N.P.; Gray, J.D.; Hill, M.N.; Hunter, R.G.; Karatsoreos, I.N.; Nasca, C. Mechanisms of stress in the brain. Nat. Neurosci. 2015, 18, 1353–1363. [Google Scholar] [CrossRef]
- Kennedy, D.P.; Adolphs, R. The social brain in psychiatric and neurological disorders. Trends Cogn. Sci. 2012, 16, 559–572. [Google Scholar] [CrossRef] [Green Version]
- Meltzer, H.; Ford, T.; Goodman, R.; Vostanis, P. The Burden of Caring for Children with Emotional or Conduct Disorders. Int. J. Fam. Med. 2011, 2011, 1–8. [Google Scholar] [CrossRef]
- Delpech, J.-C.; Madore, C.; Nadjar, A.; Joffre, C.; Wohleb, E.S.; Layé, S. Microglia in neuronal plasticity: Influence of stress. Neuropharmacology 2015, 96, 19–28. [Google Scholar] [CrossRef]
- Tzanoulinou, S.; Sandi, C. The Programming of the Social Brain by Stress During Childhood and Adolescence: From Rodents to Humans. Curr. Top. Behav. Neurosci. 2017, 30, 411–429. [Google Scholar] [CrossRef] [PubMed]
- Sandi, C.; Haller, J. Stress and the social brain: Behavioural effects and neurobiological mechanisms. Nat. Rev. Neurosci. 2015, 16, 290–304. [Google Scholar] [CrossRef] [Green Version]
- Rani, B.; Santangelo, A.; Romano, A.; Koczwara, J.B.; Friuli, M.; Provensi, G.; Blandina, P.; Casarrubea, M.; Gaetani, S.; Passani, M.B.; et al. Brain histamine and oleoylethanolamide restore behavioral deficits induced by chronic social defeat stress in mice. Neurobiol. Stress 2021, 14, 100317. [Google Scholar] [CrossRef]
- Christoffel, D.; A Golden, S.; Walsh, J.; Guise, K.G.; Heshmati, M.; Friedman, A.K.; Dey, A.; Smith, M.; Rebusi, N.; Pfau, M.; et al. Excitatory transmission at thalamo-striatal synapses mediates susceptibility to social stress. Nat. Neurosci. 2015, 18, 962–964. [Google Scholar] [CrossRef]
- Weber, M.D.; McKim, D.B.; Niraula, A.; Witcher, K.G.; Yin, W.; Sobol, C.G.; Wang, Y.; Sawicki, C.M.; Sheridan, J.F.; Godbout, J.P. The Influence of Microglial Elimination and Repopulation on Stress Sensitization Induced by Repeated Social Defeat. Biol. Psychiatry 2019, 85, 667–678. [Google Scholar] [CrossRef] [PubMed]
- Van der Kooij, M.A.; Jene, T.; Treccani, G.; Miederer, I.; Hasch, A.; Voelxen, N.; Walenta, S.; Müller, M.B. Chronic social stress-induced hyperglycemia in mice couples individual stress susceptibility to impaired spatial memory. Proc. Natl. Acad. Sci. USA 2018, 115, E10187–E10196. [Google Scholar] [CrossRef] [Green Version]
- Venzala, E.; García-García, A.; Elizalde, N.; Tordera, R. Social vs. environmental stress models of depression from a behavioural and neurochemical approach. Eur. Neuropsychopharmacol. 2013, 23, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Provensi, G.; Schmidt, S.D.; Boehme, M.; Bastiaanssen, T.F.S.; Rani, B.; Costa, A.; Busca, K.; Fouhy, F.; Strain, C.; Stanton, C.; et al. Preventing adolescent stress-induced cognitive and microbiome changes by diet. Proc. Natl. Acad. Sci. USA 2019, 116, 9644–9651. [Google Scholar] [CrossRef] [PubMed]
- Rey, C.; Delpech, J.; Madore, C.; Nadjar, A.; Greenhalgh, A.; Amadieu, C.; Aubert, A.; Pallet, V.; Vaysse, C.; Layé, S.; et al. Dietary n-3 long chain PUFA supplementation promotes a pro-resolving oxylipin profile in the brain. Brain Behav. Immun. 2019, 76, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Bazinet, R.P.; Layé, S. Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat. Rev. Neurosci. 2014, 15, 771–785. [Google Scholar] [CrossRef] [PubMed]
- Biyong, E.F.; Alfos, S.; Dumetz, F.; Helbling, J.-C.; Aubert, A.; Brossaud, J.; Foury, A.; Moisan, M.-P.; Layé, S.; Richard, E.; et al. Dietary vitamin A supplementation prevents early obesogenic diet-induced microbiota, neuronal and cognitive alterations. Int. J. Obes. 2021, 45, 588–598. [Google Scholar] [CrossRef]
- Provensi, G.; Costa, A.; Rani, B.; Blandina, P.; Passani, M.B. A Duet Between Histamine and Oleoylethanolamide in the Control of Homeostatic and Cognitive Processes. Curr. Top. Behav. Neurosci. 2021, 1–22. [Google Scholar] [CrossRef]
- Gotoh, K.; Fukagawa, K.; Fukagawa, T.; Noguchi, H.; Kakuma, T.; Sakata, T.; Yoshimatsu, H. Glucagon-like peptide-1, corticotropin-releasing hormone, and hypothalamic neuronal histamine interact in the leptin-signaling pathway to regulate feeding behavior. FASEB J. 2005, 19, 1131–1133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benetti, F.; Furini, C.; Myskiw, J.D.C.; Provensi, G.; Passani, B.; Baldi, E.; Bucherelli, C.; Munari, L.; Izquierdo, I.; Blandina, P. Histamine in the basolateral amygdala promotes inhibitory avoidance learning independently of hippocampus. Proc. Natl. Acad. Sci. USA 2015, 112, E2536–E2542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fabbri, R.; Furini, C.; Passani, M.B.; Provensi, G.; Baldi, E.; Bucherelli, C.; Izquierdo, I.; Myskiw, J.D.C.; Blandina, P. Memory retrieval of inhibitory avoidance requires histamine H1 receptor activation in the hippocampus. Proc. Natl. Acad. Sci. USA 2016, 113, E2714–E2720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miklos, I.H.; Kovacs, K.J. Functional heterogeneity of the responses of histaminergic neuron subpopulations to various stress challenges. Eur. J. Neurosci. 2003, 18, 3069–3079. [Google Scholar] [CrossRef]
- Endou, M.; Yanai, K.; Sakurai, E.; Fukudo, S.; Hongo, M.; Watanabe, T. Food-deprived activity stress decreased the activity of the histaminergic neuron system in rats. Brain Res. 2001, 891, 32–41. [Google Scholar] [CrossRef]
- Blandina, P.; Munari, L.; Provensi, G.; Passani, M.B. Histamine neurons in the tuberomamillary nucleus: A whole center or distinct subpopulations? Front. Syst. Neurosci. 2012, 6, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bosch-Bouju, C.; Larrieu, T.; Linders, L.; Manzoni, O.J.; Layé, S. Endocannabinoid-Mediated Plasticity in Nucleus Accumbens Controls Vulnerability to Anxiety after Social Defeat Stress. Cell Rep. 2016, 16, 1237–1242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kesner, R.P.; Lee, I.; Gilbert, P. A Behavioral Assessment of Hippocampal Function Based on a Subregional Analysis. Rev. Neurosci. 2004, 15, 333–352. [Google Scholar] [CrossRef]
- Calcia, M.; Bonsall, D.R.; Bloomfield, P.S.; Selvaraj, S.; Barichello, T.; Howes, O.D. Stress and neuroinflammation: A systematic review of the effects of stress on microglia and the implications for mental illness. Psychopharmacology 2016, 233, 1637–1650. [Google Scholar] [CrossRef] [Green Version]
- Grippo, A.J.; Scotti, M.A. Stress and neuroinflammation. Mod. Trends Pharmacopsychiatry 2013, 28, 20–32. [Google Scholar]
- Kiecolt-Glaser, J.K.; Belury, M.A.; Andridge, R.; Malarkey, W.B.; Glaser, R. Omega-3 supplementation lowers inflammation and anxiety in medical students: A randomized controlled trial. Brain Behav. Immun. 2011, 25, 1725–1734. [Google Scholar] [CrossRef] [Green Version]
- Lonergan, P.; Martin, D.S.D.; Horrobin, D.F.; Lynch, M.A. Neuroprotective actions of eicosapentaenoic acid on lipopolysaccharide-induced dysfunction in rat hippocampus. J. Neurochem. 2004, 91, 20–29. [Google Scholar] [CrossRef]
- Fourrier, C.; Remus-Borel, J.; Greenhalgh, A.D.; Guichardant, M.; Bernoud-Hubac, N.; Lagarde, M.; Joffre, C.; Layé, S. Docosahexaenoic acid-containing choline phospholipid modulates LPS-induced neuroinflammation in vivo and in microglia in vitro. J. Neuroinflamm. 2017, 14, 1–13. [Google Scholar] [CrossRef]
- Madore, C.; Leyrolle, Q.; Morel, L.; Rossitto, M.; Greenhalgh, A.D.; Delpech, J.C.; Martinat, M.; Bosch-Bouju, C.; Bourel, J.; Rani, B.; et al. Essential omega-3 fatty acids tune microglial phagocytosis of synaptic elements in the mouse developing brain. Nat. Commun. 2020, 11, 6133. [Google Scholar] [CrossRef]
- McKim, D.B.; Niraula, A.; Tarr, A.J.; Wohleb, E.S.; Sheridan, J.F.; Godbout, J.P. Neuroinflammatory Dynamics Underlie Memory Impairments after Repeated Social Defeat. J. Neurosci. 2016, 36, 2590–2604. [Google Scholar] [CrossRef] [PubMed]
- Robertson, R.C.; Oriach, C.S.; Murphy, K.; Moloney, G.; Cryan, J.F.; Dinan, T.; Ross, R.; Stanton, C. Omega-3 polyunsaturated fatty acids critically regulate behaviour and gut microbiota development in adolescence and adulthood. Brain Behav. Immun. 2017, 59, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Bastiaanssen, T.F.; Gururajan, A.; van de Wouw, M.; Moloney, G.M.; Ritz, N.L.; Long-Smith, C.M.; Wiley, N.C.; Murphy, A.B.; Lyte, J.M.; Fouhy, F.; et al. Volatility as a Concept to Understand the Impact of Stress on the Microbiome. Psychoneuroendocrinology 2021, 124, 105047. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef]
- Golden, S.A.; Covington, H.E.; Berton, O.; Russo, S.J. A standardized protocol for repeated social defeat stress in mice. Nat. Protoc. 2011, 6, 1183–1191. [Google Scholar] [CrossRef] [PubMed]
- Bartolomucci, A.; Palanza, P.; Gaspani, L.; Limiroli, E.; E Panerai, A.; Ceresini, G.; Poli, M.D.; Parmigiani, S. Social status in mice: Behavioral, endocrine and immune changes are context dependent. Physiol. Behav. 2001, 73, 401–410. [Google Scholar] [CrossRef]
- Finger, B.C.; Dinan, T.G.; Cryan, J.F. The temporal impact of chronic intermittent psychosocial stress on high-fat diet-induced alterations in body weight. Psychoneuroendocrinology 2012, 37, 729–741. [Google Scholar] [CrossRef]
- Mlinar, B.; Mascalchi, S.; Mannaioni, G.; Morini, R.; Corradetti, R. 5-HT4 receptor activation induces long-lasting EPSP-spike potentiation in CA1 pyramidal neurons. Eur. J. Neurosci. 2006, 24, 719–731. [Google Scholar] [CrossRef]
- Mlinar, B.; Mascalchi, S.; Morini, R.; Giachi, F.; Corradetti, R. MDMA induces EPSP-Spike potentiation in rat ventral hippocampus in vitro via serotonin and noradrenaline release and coactivation of 5-HT4 and beta1 receptors. Neuropsychopharmacology 2008, 33, 1464–1475. [Google Scholar] [CrossRef]
- Morini, R.; Mlinar, B.; Baccini, G.; Corradetti, R. Enhanced hippocampal long-term potentiation following repeated MDMA treatment in Dark–Agouti rats. Eur. Neuropsychopharmacol. 2011, 21, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Anderson, W.W.; Collingridge, G.L. The LTP Program: A data acquisition program for on-line analysis of long-term potentiation and other synaptic events. J. Neurosci. Methods 2001, 108, 71–83. [Google Scholar] [CrossRef]
- Mingam, R.; Moranis, A.; Bluthé, R.-M.; De Smedt-Peyrusse, V.; Kelley, K.W.; Guesnet, P.; Lavialle, M.; Dantzer, R.; Layé, S. Uncoupling of interleukin-6 from its signalling pathway by dietary n-3-polyunsaturated fatty acid deprivation alters sickness behaviour in mice. Eur. J. Neurosci. 2008, 28, 1877–1886. [Google Scholar] [CrossRef] [Green Version]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Morrison, W.R.; Smith, L.M. Preparation of fatty acid methylesters and dimethylacetals from lipids with boron fluoride-methanol. J. Lipid Res. 1964, 5, 600–608. [Google Scholar] [CrossRef]
- Fouhy, F.; Stanton, C.; Cotter, P.; Hill, C.; Walsh, F. Proteomics as the final step in the functional metagenomics study of antimicrobial resistance. Front. Microbiol. 2015, 6, 172. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; Mcmurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandes, A.D.; Reid, J.N.; Macklaim, J.M.; McMurrough, T.A.; Edgell, D.R.; Gloor, G.B. Unifying the analysis of high-throughput sequencing datasets: Characterizing RNA-seq, 16S rRNA gene sequencing and selective growth experiments by compositional data analysis. Microbiome 2014, 2, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gloor, G.B.; Macklaim, J.M.; Pawlowsky-Glahn, V.; Egozcue, J.J. Microbiome Datasets Are Compositional: And This Is Not Optional. Front. Microbiol. 2017, 8, 2224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsieh, T.C.; Ma, K.H.; Chao, A. iNEXT: An R package for rarefaction and extrapolation of species diversity (Hill numbers). Methods Ecol. Evol. 2016, 7, 1451–1456. [Google Scholar] [CrossRef]
- Iwai, S.; Weinmaier, T.; Schmidt, B.L.; Albertson, D.G.; Poloso, N.J.; Dabbagh, K.; DeSantis, T.Z. Piphillin: Improved Prediction of Metagenomic Content by Direct Inference from Human Microbiomes. PLoS ONE 2016, 11, e0166104. [Google Scholar] [CrossRef] [Green Version]
- Valles-Colomer, M.; Falony, G.; Darzi, Y.; Tigchelaar, E.F.; Wang, J.; Tito, R.Y.; Schiweck, C.; Kurilshikov, A.; Joossens, M.; Wijmenga, C.; et al. The neuroactive potential of the human gut microbiota in quality of life and depression. Nat. Microbiol. 2019, 4, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Coccurello, R.; Romano, A.; Giacovazzo, G.; Tempesta, B.; Fiore, M.; Giudetti, A.M.; Marrocco, I.; Altieri, F.; Moles, A.; Gaetani, S. Increased intake of energy-dense diet and negative energy balance in a mouse model of chronic psychosocial defeat. Eur. J. Nutr. 2018, 57, 1485–1498. [Google Scholar] [CrossRef] [PubMed]
- Ulrich-Lai, Y.M.; Fulton, S.; Wilson, M.; Petrovich, G.; Rinaman, L. Stress exposure, food intake and emotional state. Stress 2015, 18, 381–399. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, V.; Han, M.-H.; Graham, D.L.; Berton, O.; Renthal, W.; Russo, S.J.; LaPlant, Q.; Graham, A.; Lutter, M.; Lagace, D.C.; et al. Molecular Adaptations Underlying Susceptibility and Resistance to Social Defeat in Brain Reward Regions. Cell 2007, 131, 391–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsankova, N.M.; Berton, O.; Renthal, W.; Kumar, A.; Neve, R.L.; Nestler, E.J. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat. Neurosci. 2006, 9, 519–525. [Google Scholar] [CrossRef]
- Torrisi, S.A.; Lavanco, G.; Maurel, O.M.; Gulisano, W.; Laudani, S.; Geraci, F.; Grasso, M.; Barbagallo, C.; Caraci, F.; Bucolo, C.; et al. A novel arousal-based individual screening reveals susceptibility and resilience to PTSD-like phenotypes in mice. Neurobiol. Stress 2021, 14, 100286. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S.; Nasca, C.; Gray, J.D. Stress Effects on Neuronal Structure: Hippocampus, Amygdala, and Prefrontal Cortex. Neuropsychopharmacology 2016, 41, 3–23. [Google Scholar] [CrossRef] [Green Version]
- Larson, J.; Munkácsy, E. Theta-burst LTP. Brain Res. 2015, 1621, 38–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCormick, C.M.; Thomas, C.M.; Sheridan, C.S.; Nixon, F.; Flynn, J.A.; Mathews, I.Z. Social instability stress in adolescent male rats alters hippocampal neurogenesis and produces deficits in spatial location memory in adulthood. Hippocampus 2012, 22, 1300–1312. [Google Scholar] [CrossRef]
- Venna, V.R.; Deplanque, D.; Allet, C.; Belarbi, K.; Hamdane, M.; Bordet, R. PUFA induce antidepressant-like effects in parallel to structural and molecular changes in the hippocampus. Psychoneuroendocrinology 2009, 34, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Arisi, G.M. Nervous and immune systems signals and connections: Cytokines in hippocampus physiology and pathology. Epilepsy Behav. 2014, 38, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Balschun, D.; Wetzel, W.; Del Rey, A.; Pitossi, F.; Schneider, H.; Zuschratter, W.; Besedovsky, H.O. Interleukin-6: A cytokine to forget. FASEB J. 2004, 18, 1788–1790. [Google Scholar] [CrossRef] [Green Version]
- Schneider, H.; Pitossi, F.; Balschun, D.; Wagner, A.; del Rey, A.; Besedovsky, H.O. A neuromodulatory role of interleukin-1beta in the hippocampus. Proc. Natl. Acad. Sci. USA 1998, 95, 7778–7783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chepkova, A.; Yanovsky, E.; Parmentier, R.; Ohtsu, H.; Haas, H.L.; Lin, J.S.; Sergeeva, O.A. Histamine receptor expression, hippocampal plasticity and ammonia in histidine decarboxylase knockout mice. Cell Mol. Neurobiol. 2012, 32, 17–25. [Google Scholar] [CrossRef]
- Waider, J.; Popp, S.; Mlinar, B.; Montalbano, A.; Bonfiglio, F.; Aboagye, B.; Thuy, E.; Kern, R.; Thiel, C.; Araragi, N.; et al. Serotonin Deficiency Increases Context-Dependent Fear Learning Through Modulation of Hippocampal Activity. Front. Neurosci. 2019, 13, 245. [Google Scholar] [CrossRef] [PubMed]
- Diamond, D.M.; Park, C.R.; Woodson, J.C. Stress generates emotional memories and retrograde amnesia by inducing an endogenous form of hippocampal LTP. Hippocampus 2004, 14, 281–291. [Google Scholar] [CrossRef]
- Diamond, D.M.; Park, C.R.; Campbell, A.M.; Woodson, J.C. Competitive interactions between endogenous LTD and LTP in the hippocampus underlie the storage of emotional memories and stress-induced amnesia. Hippocampus 2005, 15, 1006–1025. [Google Scholar] [CrossRef]
- Pérez, M. Ángel; Peñaloza-Sancho, V.; Ahumada, J.; Fuenzalida, M.; Dagnino-Subiabre, A. n-3 Polyunsaturated fatty acid supplementation restored impaired memory and GABAergic synaptic efficacy in the hippocampus of stressed rats. Nutr. Neurosci. 2018, 21, 1–14. [Google Scholar] [CrossRef]
- Turk, H.F.; Chapkin, R.S. Membrane lipid raft organization is uniquely modified by n-3 polyunsaturated fatty acids. Prostaglandins Leukot. Essent. Fat. Acids 2013, 88, 43–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamon-Fava, S.; So, J.; Mischoulon, D.; Ziegler, T.R.; Dunlop, B.W.; Kinkead, B.; Schettler, P.J.; Nierenberg, A.A.; Felger, J.C.; Maddipati, K.R.; et al. Dose- and time-dependent increase in circulating anti-inflammatory and pro-resolving lipid mediators following eicosapentaenoic acid supplementation in patients with major depressive disorder and chronic inflammation. Prostaglandins Leukot. Essent. Fat. Acids 2020, 164, 102219. [Google Scholar] [CrossRef] [PubMed]
- Deyama, S.; Shimoda, K.; Ikeda, H.; Fukuda, H.; Shuto, S.; Minami, M. Resolvin E3 attenuates lipopolysaccharide-induced depression-like behavior in mice. J. Pharmacol. Sci. 2018, 138, 86–88. [Google Scholar] [CrossRef] [PubMed]
- Di Miceli, M.; Bosch-Bouju, C.; Layé, S. PUFA and their derivatives in neurotransmission and synapses: A new hallmark of synaptopathies. Proc. Nutr. Soc. 2020, 79, 1–16. [Google Scholar] [CrossRef]
- Kaelberer, M.M.; Buchanan, K.L.; Klein, M.E.; Barth, B.B.; Montoya, M.M.; Shen, X.; Bohórquez, D.V. A gut-brain neural circuit for nutrient sensory transduction. Science 2018, 361, 361. [Google Scholar] [CrossRef] [Green Version]
- Goldstein, N.; McKnight, A.D.; Carty, J.R.; Arnold, M.; Betley, J.N.; Alhadeff, A.L. Hypothalamic detection of macronutrients via multiple gut-brain pathways. Cell Metab. 2021, 33, 676–687.e5. [Google Scholar] [CrossRef] [PubMed]
- Romano, A.; Coccurello, R.; Giacovazzo, G.; Bedse, G.; Moles, A.; Gaetani, S. Oleoylethanolamide: A Novel Potential Pharmacological Alternative to Cannabinoid Antagonists for the Control of Appetite. BioMed Res. Int. 2014, 2014, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Schink, M.; Konturek, P.C.; Tietz, E.; Dieterich, W.; Pinzer, T.C.; Wirtz, S.; Neurath, M.F.; Zopf, Y. Microbial patterns in patients with histamine intolerance. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2018, 69, 579–593. [Google Scholar]
- Buffington, S.A.; Dooling, S.W.; Sgritta, M.; Noecker, C.; Murillo, O.D.; Felice, D.F.; Turnbaugh, P.J.; Costa-Mattioli, M. Dissecting the contribution of host genetics and the microbiome in complex behaviors. Cell 2021, 184, 1740–1756.e16. [Google Scholar] [CrossRef]
- Eng, A.; Borenstein, E. Microbial community design: Methods, applications, and opportunities. Curr. Opin. Biotechnol. 2019, 58, 117–128. [Google Scholar] [CrossRef]
- Narayan, N.R.; Weinmaier, T.; Laserna-Mendieta, E.J.; Claesson, M.J.; Shanahan, F.; Dabbagh, K.; Iwai, S.; DeSantis, T.Z. Piphillin predicts metagenomic composition and dynamics from DADA2-corrected 16S rDNA sequences. BMC Genom. 2020, 21, 56. [Google Scholar]
- Misto, A.; Provensi, G.; Vozella, V.; Passani, M.B.; Piomelli, D. Mast Cell-Derived Histamine Regulates Liver Ketogenesis via Oleoylethanolamide Signaling. Cell Metab. 2019, 29, 91–102.e5. [Google Scholar] [CrossRef] [Green Version]
- Shan, L.; Bao, A.-M.; Swaab, D.F. The human histaminergic system in neuropsychiatric disorders. Trends Neurosci. 2015, 38, 167–177. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, A.; Rani, B.; Bastiaanssen, T.F.S.; Bonfiglio, F.; Gunnigle, E.; Provensi, G.; Rossitto, M.; Boehme, M.; Strain, C.; Martínez, C.S.; et al. Diet Prevents Social Stress-Induced Maladaptive Neurobehavioural and Gut Microbiota Changes in a Histamine-Dependent Manner. Int. J. Mol. Sci. 2022, 23, 862. https://doi.org/10.3390/ijms23020862
Costa A, Rani B, Bastiaanssen TFS, Bonfiglio F, Gunnigle E, Provensi G, Rossitto M, Boehme M, Strain C, Martínez CS, et al. Diet Prevents Social Stress-Induced Maladaptive Neurobehavioural and Gut Microbiota Changes in a Histamine-Dependent Manner. International Journal of Molecular Sciences. 2022; 23(2):862. https://doi.org/10.3390/ijms23020862
Chicago/Turabian StyleCosta, Alessia, Barbara Rani, Thomaz F. S. Bastiaanssen, Francesco Bonfiglio, Eoin Gunnigle, Gustavo Provensi, Moira Rossitto, Marcus Boehme, Conall Strain, Clara S. Martínez, and et al. 2022. "Diet Prevents Social Stress-Induced Maladaptive Neurobehavioural and Gut Microbiota Changes in a Histamine-Dependent Manner" International Journal of Molecular Sciences 23, no. 2: 862. https://doi.org/10.3390/ijms23020862
APA StyleCosta, A., Rani, B., Bastiaanssen, T. F. S., Bonfiglio, F., Gunnigle, E., Provensi, G., Rossitto, M., Boehme, M., Strain, C., Martínez, C. S., Blandina, P., Cryan, J. F., Layé, S., Corradetti, R., & Passani, M. B. (2022). Diet Prevents Social Stress-Induced Maladaptive Neurobehavioural and Gut Microbiota Changes in a Histamine-Dependent Manner. International Journal of Molecular Sciences, 23(2), 862. https://doi.org/10.3390/ijms23020862





