Narrow Genetic Diversity of Wolbachia Symbionts in Acrididae Grasshopper Hosts (Insecta, Orthoptera)
Abstract
1. Introduction
2. Results
2.1. Wolbachia Occurrence in Acrididae
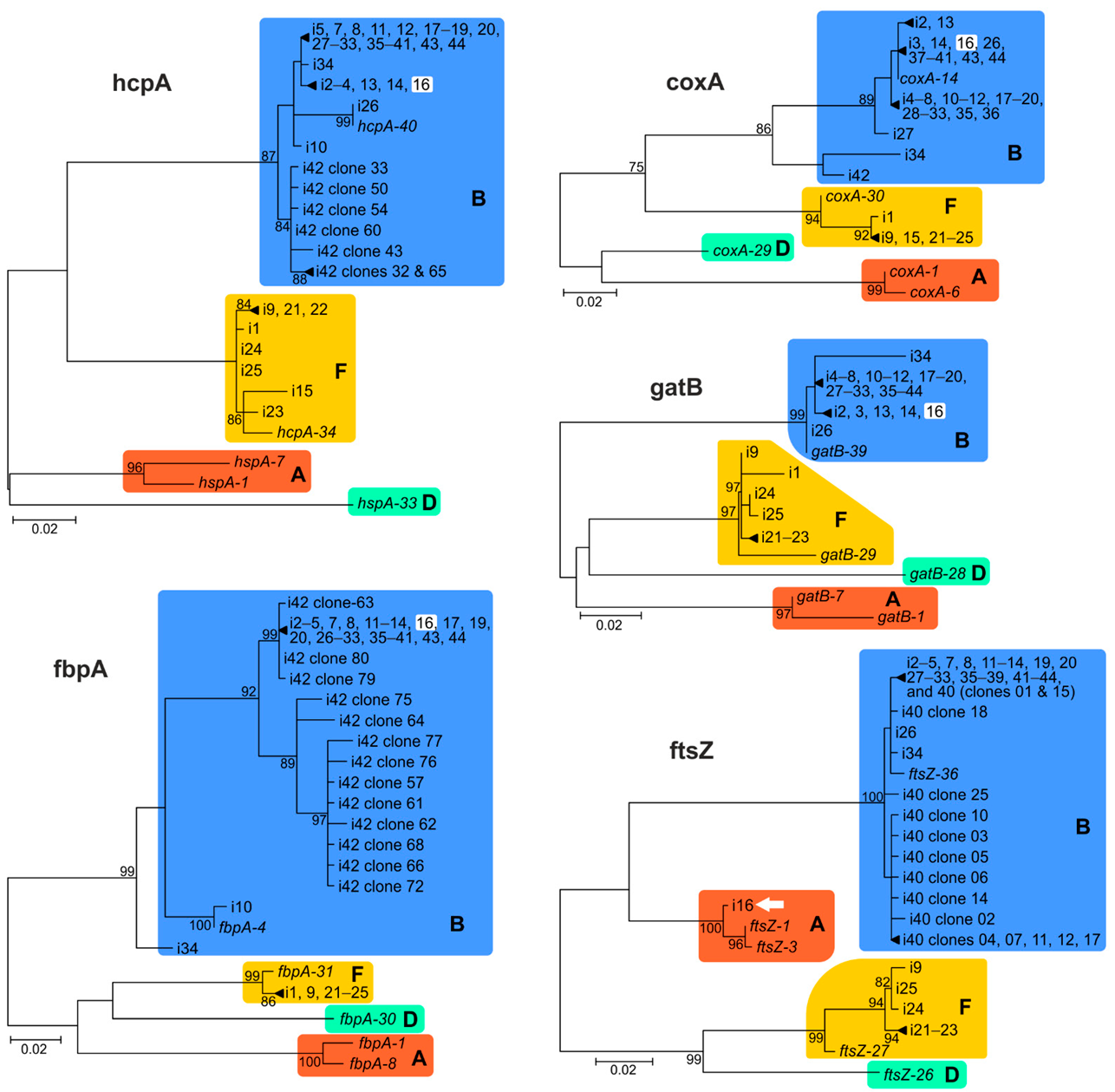
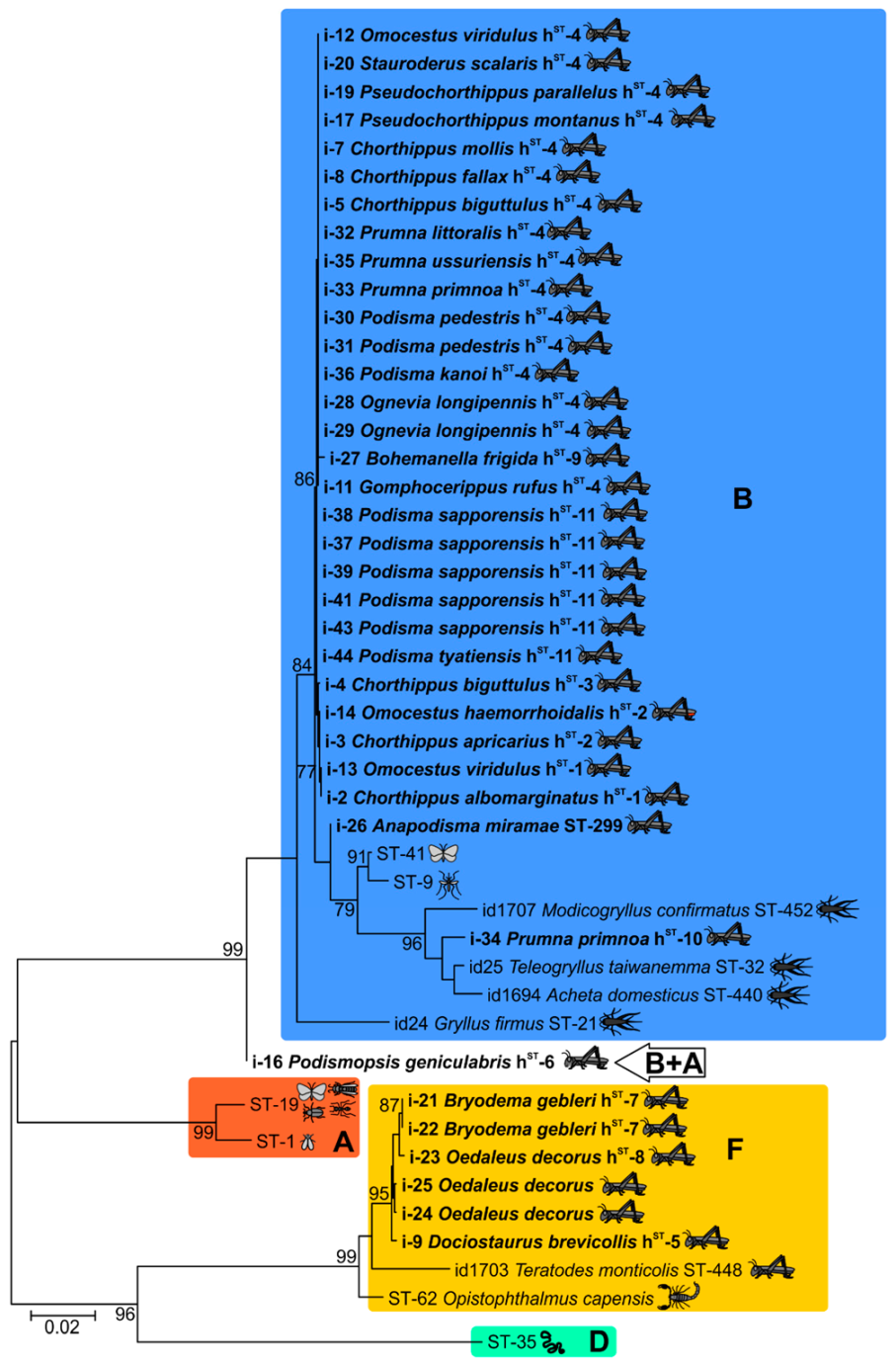
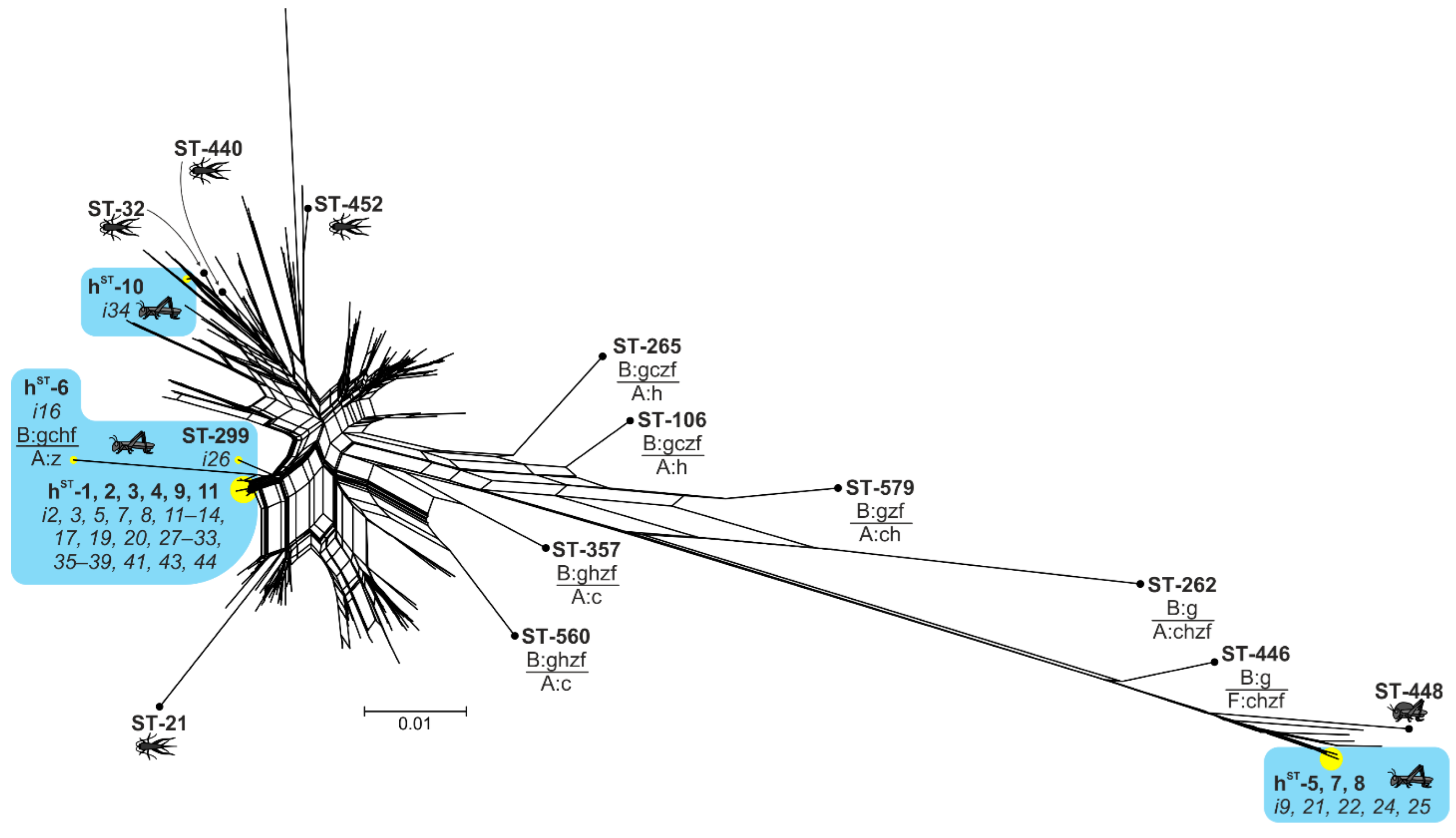
2.2. Genetic Diversity of Wolbachia Isolates
2.3. Incomplete MLST Profiles
3. Discussion
4. Materials and Methods
4.1. Collection of Specimens
4.2. Screening and Sequencing
4.3. Evolutionary Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gerth, M.; Gansauge, M.-T.; Weigert, A.; Bleidorn, C. Phylogenomic analyses uncover origin and spread of the Wolbachia pandemic. Nat. Commun. 2014, 5, 5117. [Google Scholar] [CrossRef]
- Werren, J.H. Biology of Wolbachia. Annu. Rev. Entomol. 1997, 42, 587–609. [Google Scholar] [CrossRef] [PubMed]
- Zug, R.; Hammerstein, P. Bad guys turned nice? A critical assessment of Wolbachia mutualisms in arthropod hosts. Biol. Rev. 2015, 90, 89–111. [Google Scholar] [CrossRef]
- Werren, J.H.; Windsor, D.; Guo, L.R. Distribution of Wolbachia among neotropical arthropods. Proc. R. Soc. Lond. Ser. B Biol. Sci. 1995, 262, 197–204. [Google Scholar] [CrossRef]
- Glowska, E.; Dragun-Damian, A.; Dabert, M.; Gerth, M. New Wolbachia supergroups detected in quill mites (Acari: Syringophilidae). Infect. Genet. Evol. 2015, 30, 140–146. [Google Scholar] [CrossRef]
- Laidoudi, Y.; Levasseur, A.; Medkour, H.; Maaloum, M.; Ben Khedher, M.; Sambou, M.; Bassene, H.; Davoust, B.; Fenollar, F.; Raoult, D.; et al. An Earliest Endosymbiont, Wolbachia massiliensis sp. nov., Strain PL13 from the Bed Bug (Cimex hemipterus), Type Strain of a New Supergroup T. Int. J. Mol. Sci. 2020, 21, 8064. [Google Scholar] [CrossRef] [PubMed]
- Ferri, E.; Bain, O.; Barbuto, M.; Martin, C.; Lo, N.; Uni, S.; Landmann, F.; Baccei, S.G.; Guerrero, R.; Lima, S.D.S.; et al. New Insights into the Evolution of Wolbachia Infections in Filarial Nematodes Inferred from a Large Range of Screened Species. PLoS ONE 2011, 6, e20843. [Google Scholar] [CrossRef]
- Campbell, B.C.; Bragg, T.S.; Turner, C.E. Phylogeny of symbiotic bacteria of four weevil species (coleoptera: Curculionidae) based on analysis of 16S ribosomal DNA. Insect Biochem. Mol. Biol. 1992, 22, 415–421. [Google Scholar] [CrossRef]
- Lo, N.; Casiraghi, M.; Salati, E.; Bazzocchi, C.; Bandi, C. How Many Wolbachia Supergroups Exist? Mol. Biol. Evol. 2002, 19, 341–346. [Google Scholar] [CrossRef]
- Sontowski, R.; Bernhard, D.; Bleidorn, C.; Schlegel, M.; Gerth, M. Wolbachia distribution in selected beetle taxa characterized by PCR screens and MLST data. Ecol. Evol. 2015, 5, 4345–4353. [Google Scholar] [CrossRef]
- Liu, Y.; He, B.; Li, F.; Li, K.; Zhang, L.; Li, X.; Zhao, L. Molecular Identification of Bartonella melophagi and Wolbachia Supergroup F from Sheep Keds in Xinjiang, China. Korean J. Parasitol. 2018, 56, 365–370. [Google Scholar] [CrossRef]
- Baldo, L.; Hotopp, J.D.; Jolley, K.A.; Bordenstein, S.R.; Biber, S.A.; Choudhury, R.R.; Hayashi, C.; Maiden, M.C.J.; Tettelin, H.; Werren, J.H. Multilocus Sequence Typing System for the Endosymbiont Wolbachia pipientis. Appl. Environ. Microbiol. 2006, 72, 7098–7110. [Google Scholar] [CrossRef] [PubMed]
- Jäckel, R.; Mora, D.; Dobler, S. Evidence for selective sweeps byWolbachiainfections: Phylogeny ofAlticaleaf beetles and their reproductive parasites. Mol. Ecol. 2013, 22, 4241–4255. [Google Scholar] [CrossRef] [PubMed]
- Ramalho, M.O.; Moreau, C.S. The Evolution and Biogeography of Wolbachia in Ants (Hymenoptera: Formicidae). Diversity 2020, 12, 426. [Google Scholar] [CrossRef]
- Zimmermann, B.L.; Cardoso, G.M.; Bouchon, D.; Pezzi, P.H.; Palaoro, A.V.; Araujo, P.B. Supergroup F Wolbachia in terrestrial isopods: Horizontal transmission from termites? Evol. Ecol. 2021, 35, 165–182. [Google Scholar] [CrossRef]
- Lorenzo-Carballa, M.O.; Torres-Cambas, Y.; Heaton, K.; Hurst, G.D.D.; Charlat, S.; Sherratt, T.N.; Van Gossum, H.; Cordero-Rivera, A.; Beatty, C.D. Widespread Wolbachia infection in an insular radiation of damselflies (Odonata, Coenagrionidae). Sci. Rep. 2019, 9, 11933. [Google Scholar] [CrossRef]
- Baldo, L.; Prendini, L.; Corthals, A.; Werren, J.H. Wolbachia Are Present in Southern African Scorpions and Cluster with Supergroup F. Curr. Microbiol. 2007, 55, 367–373. [Google Scholar] [CrossRef][Green Version]
- Niehuis, O.; Hartig, G.; Grath, S.; Pohl, H.; Lehmann, J.; Tafer, H.; Donath, A.; Krauss, V.; Eisenhardt, C.; Hertel, J.; et al. Genomic and Morphological Evidence Converge to Resolve the Enigma of Strepsiptera. Curr. Biol. 2012, 22, 1309–1313. [Google Scholar] [CrossRef]
- Casiraghi, M.; Bordenstein, S.R.; Baldo, L.; Lo, N.; Beninati, T.; Wernegreen, J.J.; Werren, J.; Bandi, C. Phylogeny of Wolbachia pipientis based on gltA, groEL and ftsZ gene sequences: Clustering of arthropod and nematode symbionts in the F supergroup, and evidence for further diversity in the Wolbachia tree. Microbiology 2005, 151, 4015–4022. [Google Scholar] [CrossRef] [PubMed]
- Lo, N.; Evans, T.A. Phylogenetic diversity of the intracellular symbiont Wolbachia in termites. Mol. Phylogenet. Evol. 2007, 44, 461–466. [Google Scholar] [CrossRef]
- Hellemans, S.; Kaczmarek, N.; Marynowska, M.; Calusinska, M.; Roisin, Y.; Fournier, D. Bacteriome-associated Wolbachia of the parthenogenetic termite Cavitermes tuberosus. FEMS Microbiol. Ecol. 2019, 95, fiy235. [Google Scholar] [CrossRef]
- Vavre, F.; Fleury, F.; Lepetit, D.; Fouillet, P.; Bouletreau, M. Phylogenetic evidence for horizontal transmission of Wolbachia in host-parasitoid associations. Mol. Biol. Evol. 1999, 16, 1711–1723. [Google Scholar] [CrossRef] [PubMed]
- Kittayapong, P.; Jamnongluk, W.; Thipaksorn, A.; Milne, J.R.; Sindhusake, C. Wolbachia infection complexity among insects in the tropical rice-field community. Mol. Ecol. 2003, 12, 1049–1060. [Google Scholar] [CrossRef]
- Haine, E.R.; Pickup, N.J.; Cook, J.M. Horizontal transmission of Wolbachia in a Drosophila community. Ecol. Entomol. 2005, 30, 464–472. [Google Scholar] [CrossRef]
- Sintupachee, S.; Milne, J.R.; Poonchaisri, S.; Baimai, V.; Kittayapong, P. Closely related Wolbachia strains within the pumpkin arthropod community and the potential for horizontal transmission via the plant. Microb. Ecol. 2006, 51, 294–301. [Google Scholar] [CrossRef]
- Le Clec’H, W.; Chevalier, F.; Genty, L.; Bertaux, J.; Bouchon, D.; Sicard, M. Cannibalism and Predation as Paths for Horizontal Passage of Wolbachia between Terrestrial Isopods. PLoS ONE 2013, 8, e60232. [Google Scholar] [CrossRef]
- Yang, X.-H.; Zhu, D.-H.; Liu, Z.; Zhao, L.; Su, C.-Y. High Levels of Multiple Infections, Recombination and Horizontal Transmission of Wolbachia in the Andricus mukaigawae (Hymenoptera; Cynipidae) Communities. PLoS ONE 2013, 8, e78970. [Google Scholar] [CrossRef]
- Ahmed, M.Z.; Li, S.-J.; Xue, X.; Yin, X.-J.; Ren, S.-X.; Jiggins, F.M.; Greeff, J.; Qiu, B.-L. The Intracellular Bacterium Wolbachia Uses Parasitoid Wasps as Phoretic Vectors for Efficient Horizontal Transmission. PLoS Pathog. 2015, 11, e1004672. [Google Scholar] [CrossRef]
- Li, S.J.; Muhammad, Z.A.; Lv, N.; Shi, P.Q.; Wang, X.M.; Huang, J.L.; Qiu, B.L. Plant–mediated horizontal transmission of Wolbachia between whiteflies. Int. Soc. Microb. Ecol. J. 2017, 11, 1019–1028. [Google Scholar]
- Johannesen, J. Tracing the history and ecological context of Wolbachia double infection in a specialist host (Urophora cardui)-parasitoid (Eurytoma serratulae) system. Ecol. Evol. 2017, 7, 986–996. [Google Scholar] [CrossRef]
- Hou, H.Q.; Zhao, G.Z.; Su, C.Y.; Zhu, D.H. Wolbachia prevalence patterns: Horizontal transmission, recombination, and multiple infections in chestnut gall wasp-parasitoid communities. Entomol. Exp. Appl. 2020, 168, 752–765. [Google Scholar] [CrossRef]
- Martínez-Rodríguez, P.; Bella, J.L.; Nichols, R.A. Wolbachia Infection in Podisma Pedestris Hybrid Zone. Conference SPECIATION2013, Poster. 2013. Available online: https://www.researchgate.net/publication/236591475_Wolbachia_infection_in_Podisma_pedestris (accessed on 12 December 2021).
- Bugrov, A.; Ilinsky, Y.Y.; Strunov, A.; Zhukova, M.; Kiseleva, E.; Akimoto, S.; Tatsuta, H. First evidence of Wolbachia infection in populations of grasshopper Podisma sapporensis (Orthoptera: Acrididae). Èntomol. Sci. 2016, 19, 296–300. [Google Scholar] [CrossRef]
- Werren, J.H.; Windsor, D.M. Wolbachia infection frequencies in insects: Evidence of a global equilibrium? Proc. R. Soc. Lond. Ser. B Biol. Sci. 2000, 267, 1277–1285. [Google Scholar] [CrossRef]
- Weeks, A.R.; Velten, R.; Stouthamer, R. Incidence of a new sex–ratio–distorting endosymbiotic bacterium among arthropods. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2003, 270, 1857–1865. [Google Scholar] [CrossRef]
- Duron, O.; Bouchon, D.; Boutin, S.; Bellamy, L.; Zhou, L.; Engelstädter, J.; Hurst, G.D. The diversity of reproductive parasites among arthropods: Wolbachia do not walk alone. BMC Biol. 2008, 6, 27. [Google Scholar] [CrossRef] [PubMed]
- Kumalawati, D.A.; Supriyati, E.; Rachman, M.P.; Oktriani, R.; Kurniasari, I.; Candrasari, D.S.; Hidayati, L.; Handayaningsih, A.E.; Probowati, V.C.; Arianto, B. Wolbachia infection prevalence as common insects’ endosymbiont in the rural area of Yogyakarta, Indonesia. Biodivers. J. Biol. Divers. 2020, 21, 5608–5614. [Google Scholar] [CrossRef]
- Dillon, R.; Webster, G.; Weightman, A.; Dillon, V.; Blanford, S.; Charnley, A. Composition of Acridid gut bacterial communities as revealed by 16S rRNA gene analysis. J. Invertebr. Pathol. 2008, 97, 265–272. [Google Scholar] [CrossRef]
- Martinez, P.; Del Castillo, P.; Bella, J. Cytological detection of Wolbachia in squashed and paraffin embedded insect tissues. Biotech. Histochem. 2010, 84, 347–353. [Google Scholar] [CrossRef]
- Zabal-Aguirre, M.; Arroyo, F.; Bella, J.L. Distribution of Wolbachia infection in Chorthippus parallelus populations within and beyond a Pyrenean hybrid zone. Heredity 2010, 104, 174–184. [Google Scholar] [CrossRef]
- Sarasa, J.; Bernal, A.; Fernandez-Calvin, B.; Bella, J. WolbachiaInduced Cytogenetical Effects as Evidenced in Chorthippus parallelus (Orthoptera). Cytogenet. Genome Res. 2013, 139, 36–43. [Google Scholar] [CrossRef]
- Funkhouser-Jones, L.J.; Sehnert, S.R.; Martínez-Rodríguez, P.; Toribio-Fernández, R.; Pita, M.; Bella, J.L.; Bordenstein, S.R. Wolbachia co-infection in a hybrid zone: Discovery of horizontal gene transfers from two Wolbachia supergroups into an animal genome. PeerJ 2015, 3, e1479. [Google Scholar] [CrossRef]
- Toribio-Fernández, R.; Bella, J.L.; Martínez-Rodríguez, P.; Funkhouser-Jones, L.J.; Bordenstein, S.R.; Pita, M. Chromosomal localization of Wolbachia inserts in the genomes of two subspecies of Chorthippus parallelus forming a Pyrenean hybrid zone. Chromosom. Res. 2017, 25, 215–225. [Google Scholar] [CrossRef]
- Martínez-Rodríguez, P.; Bella, J.L. Chorthippus parallelus and Wolbachia: Overlapping Orthopteroid and Bacterial Hybrid Zones. Front. Genet. 2018, 9, 604. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Rodríguez, P.; Rolán-Alvarez, E.; del Mar Pérez-Ruiz, M.; Arroyo-Yebras, F.; Carpena-Catoira, C.; Carvajal-Rodríguez, A.; Bella, J.L. Geographic and Temporal Variation of Distinct Intracellular Endosymbiont Strains of Wolbachia sp. in the Grasshopper Chorthippus parallelus: A Frequency-Dependent Mechanism? Microb. Ecol. 2019, 77, 1036–1047. [Google Scholar] [CrossRef]
- Ilinsky, Y.; Kosterin, O.E. Molecular diversity of Wolbachia in Lepidoptera: Prevalent allelic content and high recombination of MLST genes. Mol. Phylogenet. Evol. 2017, 109, 164–179. [Google Scholar] [CrossRef]
- Tseng, S.-P.; Hsu, P.-W.; Lee, C.-C.; Wetterer, J.K.; Hugel, S.; Wu, L.-H.; Lee, C.-Y.; Yoshimura, T.; Yang, C.-C.S. Evidence for Common Horizontal Transmission of Wolbachia among Ants and Ant Crickets: Kleptoparasitism Added to the List. Microorganisms 2020, 8, 805. [Google Scholar] [CrossRef] [PubMed]
- Klasson, L.; Kumar, N.; Bromley, R.; Sieber, K.; Flowers, M.; Ott, S.H.; Tallon, L.J.; Andersson, S.G.E.; Hotopp, J.C.D. Extensive duplication of the Wolbachia DNA in chromosome four of Drosophila ananassae. BMC Genom. 2014, 15, 1097. [Google Scholar] [CrossRef]
- Koutsovoulos, G.; Makepeace, B.; Tanya, V.N.; Blaxter, M. Palaeosymbiosis Revealed by Genomic Fossils of Wolbachia in a Strongyloidean Nematode. PLoS Genet. 2014, 10, e1004397. [Google Scholar] [CrossRef]
- Brelsfoard, C.; Tsiamis, G.; Falchetto, M.; Gomulski, L.M.; Telleria, E.; Alam, U.; Doudoumis, V.; Scolari, F.; Benoit, J.B.; Swain, M.; et al. Presence of Extensive Wolbachia Symbiont Insertions Discovered in the Genome of Its Host Glossina morsitans morsitans. PLoS Negl. Trop. Dis. 2014, 8, e2728. [Google Scholar] [CrossRef]
- Conner, W.R.; Blaxter, M.L.; Anfora, G.; Ometto, L.; Rota-Stabelli, O.; Turelli, M. Genome comparisons indicate recent transfer of wR i-like Wolbachia between sister species Drosophila suzukii and D. subpulchrella. Ecol. Evol. 2017, 7, 9391–9404. [Google Scholar] [CrossRef]
- Cordaux, R.; Gilbert, C. Evolutionary Significance of Wolbachia-to-Animal Horizontal Gene Transfer: Female Sex Determination and the f Element in the Isopod Armadillidium vulgare. Genes 2017, 8, 186. [Google Scholar] [CrossRef] [PubMed]
- Rosselló-Mora, R.; Amann, R. The species concept for prokaryotes. FEMS Microbiol. Rev. 2001, 25, 39–67. [Google Scholar] [CrossRef]
- Cohan, F.M. Bacterial species and speciation. Syst. Biol. 2001, 50, 513–524. [Google Scholar] [CrossRef] [PubMed]
- Wiedenbeck, J.; Cohan, F.M. Origins of bacterial diversity through horizontal genetic transfer and adaptation to new ecological niches. FEMS Microbial. Rev. 2011, 35, 957–976. [Google Scholar] [CrossRef]
- Pfarr, K.; Foster, J.; Slatko, B.; Hoerauf, A.; Eisen, J.A. On the taxonomic status of the intracellular bacterium Wolbachia pipientis: Should this species name include the intracellular bacteria of filarial nematodes? Int. J. Syst. Evol. Microbiol. 2007, 57 Pt 8, 1677. [Google Scholar] [CrossRef]
- Ellegaard, K.M.; Klasson, L.; Näslund, K.; Bourtzis, K.; Andersson, S.G. Comparative genomics of Wolbachia and the bacterial species concept. PLoS Genet. 2013, 9, e1003381. [Google Scholar] [CrossRef]
- Ramírez-Puebla, S.T.; Servín-Garcidueñas, L.E.; Ormeño-Orrillo, E.; de León, A.V.P.; Rosenblueth, M.; Delaye, L.; Martínez, J.; Martínez-Romero, E. Species in Wolbachia? Proposal for the designation of ‘Candidatus Wolbachia bourtzisii’,‘Candidatus Wolbachia onchocercicola’,‘Candidatus Wolbachia blaxteri’,‘Candidatus Wolbachia brugii’,‘Candidatus Wolbachia taylori’,‘Candidatus Wolbachia collembolicola’and ‘Candidatus Wolbachia multihospitum’ for the different species within Wolbachia supergroups. Syst. Appl. Microbiol. 2015, 38, 390–399. [Google Scholar]
- Lindsey, A.R.; Bordenstein, S.R.; Newton, I.L.; Rasgon, J.L. Wolbachia pipientis should not be split into multiple species: A response to Ramírez-Puebla et al., “Species in Wolbachia? Proposal for the designation of ‘Candidatus Wolbachia bourtzisii’,‘Candidatus Wolbachia onchocercicola’,‘Candidatus Wolbachia blaxteri’,‘Candidatus Wolbachia brugii’,‘Candidatus Wolbachia taylori’,‘Candidatus Wolbachia collembolicola’and ‘Candidatus Wolbachia multihospitum’for the different species within Wolbachia supergroups”. Syst. Appl. Microbiol. 2016, 39, 220–222. [Google Scholar]
- Baldo, L.; Bordenstein, S.; Wernegreen, J.J.; Werren, J.H. Widespread recombination throughout Wolbachia genomes. Mol. Biol. Evol. 2006, 23, 437–449. [Google Scholar] [CrossRef]
- Baldo, L.; Werren, J.H. Revisiting Wolbachia supergroup typing based on WSP: Spurious lineages and discordance with MLST. Curr. Microbiol. 2007, 55, 81–87. [Google Scholar] [CrossRef]
- Shaikevich, E.; Bogacheva, A.; Rakova, V.; Ganushkina, L.; Ilinsky, Y. Wolbachia symbionts in mosquitoes: Intra-and intersupergroup recombinations, horizontal transmission and evolution. Mol. Phylogenetics Evol. 2019, 134, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xiong, X.; Cao, W.; Zhang, C.; Werren, J.H.; Wang, X. Phylogenomic Analysis of Wolbachia Strains Reveals Patterns of Genome Evolution and Recombination. Genome Biol. Evol. 2020, 12, 2508–2520. [Google Scholar] [CrossRef]
- Scholz, M.; Albanese, D.; Tuohy, K.; Donati, C.; Segata, N.; Rota-Stabelli, O. Large scale genome reconstructions illuminate Wolbachia evolution. Nat. Commun. 2020, 11, 5235. [Google Scholar] [CrossRef]
- Badawi, M.; Giraud, I.; Vavre, F.; Grève, P.; Cordaux, R. Signs of Neutralization in a Redundant Gene Involved in Homologous Recombination in Wolbachia Endosymbionts. Genome Biol. Evol. 2014, 6, 2654–2664. [Google Scholar] [CrossRef][Green Version]
- Nice, C.C.; Gompert, Z.; Forister, M.L.; Fordyce, J.A. An unseen foe in arthropod conservation efforts: The case of Wolbachia infections in the Karner blue butterfly. Biol. Conserv. 2009, 142, 3137–3146. [Google Scholar] [CrossRef]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar] [PubMed]
- Bykov, R.; Kerchev, I.; Demenkova, M.; Ryabinin, A.; Ilinsky, Y. Sex-Specific Wolbachia Infection Patterns in Populations of Polygraphus proximus Blandford (Coleoptera; Curculionidae: Scolytinae). Insects 2020, 11, 547. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. MUSCLE: A multiple sequence alignment method with reduced time and space complexity. BMC Bioinform. 2004, 5, 113. [Google Scholar] [CrossRef] [PubMed]
- Jolley, K.A.; Bray, J.E.; Maiden, M.C.J. Open-access bacterial population genomics: BIGSdb software, the PubMLST.org website and their applications. Wellcome Open Res. 2018, 3, 124. [Google Scholar] [CrossRef]
- Huson, D.H.; Bryant, D. Application of Phylogenetic Networks in Evolutionary Studies. Mol. Biol. Evol. 2006, 23, 254–267. [Google Scholar] [CrossRef]
- Bryant, D.; Moulton, V. Neighbor-net: An Agglomerative Method for the Construction of Planar Phylogenetic Networks. Mol. Biol. Evol. 2004, 21, 255–265. [Google Scholar] [CrossRef] [PubMed]

| Subfamily | Species | Region and Year of Collection | No. of Infected Specimens/Total |
|---|---|---|---|
| Gomphocerinae | Arcyptera (Arcyptera) fusca (Pallas, 1773) | Russia, Altai Mts, 2017 | 2/2 |
| Arcyptera (Pararcyptera) microptera (Fischer von Waldheim, 1833) | Russia, Altai Mts, 2017 | 0/5 | |
| Chorthippus (Chorthippus) albomarginatus (De Geer, 1773) | Russia, Irkutsk region, 2016 | 10/10 | |
| Chorthippus (Glyptobothrus) apricarius (Linnaeus, 1758) | Russia, Irkutsk region, 2016 | 7/7 | |
| Chorthippus (Glyptobothrus) biguttulus (Linnaeus, 1758) | East Kazakhstan, 2007 | 0/6 | |
| -‘’- | Russia, Novosibirsk region, 2017 | 174/198 | |
| -‘’- | Russia, Irkutsk region | 4/6 | |
| -‘’- | Russia, Altai Mts, 2015 | 5/5 | |
| Chorthippus (Altichorthippus) intermedius (Bey-Bienko, 1926) | Russia, Altai Mts, 2003 | 0/8 | |
| Chorthippus (Glyptobothrus) mollis (Charpentier, 1825) | Russia, Altai Mts, 2003 | 2/5 | |
| -‘’- | Turkey, 2003 | 0/9 | |
| Chorthippus fallax (Zubovski, 1900) | Russia, Novosibirsk region, 2017 | 16/17 | |
| Chorthippus hammarstroemi (Miram, 1907) | Russia, Altai Mts, 2003 | 0/5 | |
| Dociostaurus (Kazakia) brevicollis (Eversmann, 1848) | East Kazakhstan, 2007 | 3/7 | |
| Dociostaurus (Kazakia) tartarus (Stshelkanovtzev, 1921) | East Kazakhstan, 2007 | 0/5 | |
| Eclipophleps glacialis (Bey-Bienko, 1933) | Russia, Altai Mts, 2003 | 2/8 | |
| Eremippus simplex (Eversmann, 1859) | East Kazakhstan, 2007 | 0/2 | |
| Euthystira brachyptera (Ocskay, 1826) | Russia, Altai Mts, 2003 | 0/2 | |
| Gomphocerippus rufus (Linnaeus, 1758) | Russia, Novosibirsk region, 2017 | 1/7 | |
| Megaulacobothrus aethalinus (Zubovski, 1899) | Russia, Altai Mts, 2003 | 0/6 | |
| Omocestus (Omocestus) viridulus (Linnaeus, 1758) | Russia, Novosibirsk region, 2009 | 3/4 | |
| -‘’- | Russia, Altai Mts, 2017 | 1/1 | |
| Omocestus (Omocestus) haemorrhoidalis (Charpentier, 1825) | Russia, Altai Mts, 2017 | 1/2 | |
| Podismopsis altaica (Zubovski, 1900) | Russia, Altai Mts, 2003 | 3/4 | |
| Podismopsis genicularibus (Shiraki, 1910) | Russia, Sakhalin Is., 2010 | 2/3 | |
| Pseudochorthippus montanus (Charpentier, 1825) | Russia, Novosibirsk region, 2017 | 40/50 | |
| Pseudochorthippus parallelus (Zetterstedt, 1821) | Russia, Novosibirsk region, 2017 | 5/5 | |
| Stauroderus scalaris (Fischer von Waldheim, 1846) | Russia, Altai Mts, 2017 | 8/8 | |
| Stenobothrus eurasius (Zubovski, 1898) | Russia, Altai Mts, 2003 | 0/2 | |
| Oedipodinae | Bryodema gebleri (Fischer von Waldheim, 1836) | Russia, Altai Mts, 2003 | 2/3 |
| -‘’- | Russia, Altai Mts, 2017 | 2/2 | |
| Bryodema tuberculata (Fabricius, 1775) | Russia, Altai Mts, 2003 | 0/3 | |
| Locusta migratoria (Linnaeus, 1758) | Central Kazakhstan, 2007 | 0/4 | |
| Oedaleus decorus (Germar, 1825) | Russia, Altai Mts, 2017 | 1/1 | |
| -‘’- | East Kazakhstan, 2007 | 3/4 | |
| -‘’- | Tadzhikistan, 2009 | 1/1 | |
| Psophus stridulus (Linnaeus, 1758) | Russia, Altai Mts, 2003 | 0/1 | |
| Pyrgodera armata (Fischer von Waldheim, 1846) | East Kazakhstan, 2007 | 0/4 | |
| Podisminae | Anapodisma miramae (Dovnar-Zapolskij, 1932) | Russia, Maritima region of Far East, 2008 | 1/1 |
| Bohemanella frigida (Boheman, 1846) | Russia, Altai Mts, 2003 | 1/3 | |
| Ognevia longipennis (Shiraki, 1910) | Japan, Hokkaido, 2005 | 4/4 | |
| -‘’- | Russia, Altai Mts, Edigan, 2003 | 5/5 | |
| Podisma pedestris (Linnaeus, 1758) | Russia, Altai Mts, 2003 | 5/5 | |
| -‘’- | Russia, Altai Mts, 2016 | 1/4 | |
| Podisma kanoi (Storozhenko, 1994) | Japan, Honshu, 2005 | 1/1 | |
| Podisma sapporensis (Shiraki, 1910) | Japan, Hokkaido Is, Tanno town vicinities, 2005 | 5/5 | |
| -‘’- | Japan, Hokkaido Akan town vicinities, 2005 | 5/5 | |
| -‘’- | Japan, Hokkaido, Yotei Mt., 2005 | 5/5 | |
| -‘’- | Japan, Hokkaido, Disengen Mt., 2005 | 5/5 | |
| -‘’- | Japan, Hokkaido, Naganuma town vicinities, 2005 | 5/5 | |
| -‘’- | Japan, Hokkaido, Teine Mt., 2005 | 10/10 | |
| -‘’- | Japan, Japan, Hokkaido, Shimokawa town vicinities, 2005 | 3/3 | |
| Podisma tyatiensis (Bugrov & Sergeev, 1997) | Russia, Kuril Arch., Kunashir Is, 2001 | 1/1 | |
| Prumna littoralis (Tarbinsky, 1932) | Russia, Maritima region of Far East, 2008 | 1/1 | |
| Prumna primnoa (Motschulsky, 1846) | Russia, Sakhalin Is, 2010 | 5/5 | |
| Prumna ussuriensis (Tarbinsky, 1930) | Russia, Maritima region of Far East, 2008 | 1/5 | |
| Sinopodisma punctata (Mishchenko, 1954) | Japan, Ryukyu Arch., Ishigaki Is, 2005 | 0/3 |
| Isolate | Species (Region, Year) | Supergroup | gatB | coxA | hcpA | ftsZ | fbpA | Sequ-Ence Type * |
|---|---|---|---|---|---|---|---|---|
| i-1 | Arcyptera fusca (Altai, 2017) | F | ~73 ** (MZ816480) | ~63 (MZ816523) | ~261 (MZ816567) | No *** | 410 (MZ816669) | not full |
| i-2 | Chorthippus albomarginatus (Irkutsk, 2016) | B | 134 (MZ816445) | 168 (MZ816488) | ~6 (MZ816532) | 106 (MZ816581) | 197 (MZ816634) | hST-1 |
| i-3 | Ch. Apricarius (Irkutsk, 2016) | B | 134 (MZ816446) | 14 (MZ816489) | ~6 (MZ816533) | 106 (MZ816582) | 197 (MZ816635) | hST-2 |
| i-4 | Ch. Biguttulus (Irkutsk, 2016) | B | 9 (MZ816447) | 133 (MZ816490) | ~6 (MZ816534) | 106 (MZ816583) | 197 (MZ816636) | hST-3 |
| i-5 | Ch. Biguttulus (Novosibirsk, 2017) | B | 9 (MZ816448) | 133 (MZ816491) | 6 (MZ816535) | 106 (MZ816584) | 197 (MZ816637) | hST-4 |
| i-6 | Ch. Biguttulus (Altai, 2015) | B | 9 (MZ816481) | 133 (MZ816524) | ? **** | ? | ? | not full |
| i-7 | Ch. Mollis (Altai, 2003) | B | 9 (MZ816449) | 133 (MZ816492) | 6 (MZ816536) | 106 (MZ816585) | 197 (MZ816638) | hST-4 |
| i-8 | Ch. Fallax (Novosibirsk, 2017) | B | 9 (MZ816450) | 133 (MZ816493) | 6 (MZ816537) | 106 (MZ816586) | 197 (MZ816639) | hST-4 |
| i-9 | Dociostaurus brevicollis (Kazakhstan, 2007) | F | ~73 (MZ816451) | ~63 (MZ816494) | ~261 (MZ816538) | ~269 (MZ816587) | 410 (MZ816640) | hST-5 |
| i-10 | Eclipophleps glacialis (G, Altai, 2003) | B | 9 (MZ816482) | 133 (MZ816525) | ~6R (MZ816568) | No | ~4 (MZ816670) | not full |
| i-11 | Gomphocerippus rufus (G, Novosibirsk, 2017) | B | 9 (MZ816452) | 133 (MZ816495) | 6 (MZ816539) | 106 (MZ816588) | 197 (MZ816641) | hST-4 |
| i-12 | Omocestus viridulus (Novosibirsk, 2009) | B | 9 (MZ816453) | 133 (MZ816496) | 6 (MZ816540) | 106 (MZ816589) | 197 (MZ816642) | hST-4 |
| i-13 | Om. Viridulus (Altai, 2017) | B | 134 (MZ816454) | 168 (MZ816497) | ~6 (MZ816541) | 106 (MZ816590) | 197 (MZ816643) | hST-1 |
| i-14 | Om. Haemorrhoidalis (Altai, 2017) | B | 134 (MZ816455) | 14 (MZ816498) | ~6 (MZ816542) | 106 (MZ816591) | 197 (MZ816644) | hST-2 |
| i-15 | Podismopsis altaica (Altai, 2003) | F | No | ~63 (MZ816526) | ~325 (MZ816569) | ? | ? | not full |
| i-16 | Podismopsis genicularibus (Sakhalin Is., 2010) | B–A | 134 (MZ816456) | 14 (MZ816499) | ~6 (MZ816543) | 226 (MZ816592) | 197 (MZ816645) | hST-6 |
| i-17 | Pseudochorthippus montanus (Novosibirsk, 2017) | B | 9 (MZ816457) | 133 (MZ816500) | 6 (MZ816544) | 106 (MZ816593) | 197 (MZ816646) | hST-4 |
| i-18 | Ps. Montanus (Novosibirsk, 2017) | B | 9 (MZ816483) | 133 (MZ816527) | 6 (MZ816570) | ? | ? | not full |
| i-19 | Ps. Parallelus (Novosibirsk, 2017) | B | 9 (MZ816458) | 133 (MZ816501) | 6 (MZ816545) | 106 (MZ816594) | 197 (MZ816647) | hST-4 |
| i-20 | Stauroderus scalaris (Altai, 2017) | B | 9 (MZ816459) | 133 (MZ816502) | 6 (MZ816546) | 106 (MZ816595) | 197 (MZ816648) | hST-4 |
| i-21 | Bryodema gebleri (Altai, 2003) | F | ~73 (MZ816460) | ~63 (MZ816503) | ~261 (MZ816547) | ~205 (MZ816596) | 410 (MZ816649) | hST-7 |
| i-22 | Bryodema gebleri (Altai, 2017) | F | ~73 (MZ816461) | ~63 (MZ816504) | ~261 (MZ816548) | ~205 (MZ816597) | 410 (MZ816650) | hST-7 |
| i-23 | Oedaleus decorus (Altai, 2017) | F | ~73 (MZ816462) | ~63 (MZ816505) | ~35 (MZ816549) | ~205 (MZ816598) | 410 (MZ816651) | hST-8 |
| i-24 | Oe. Decorus (Kazakhstan, 2006) | F | ~243 (MZ816484) | ~63RK (MZ816528) | ~261 (MZ816571) | ~205R (MZ816616) | 410 (MZ816671) | N, full |
| i-25 | Oe. Decorus (Tajikistan, 2009) | F | ~243Y (MZ816485) | ~30YR (MZ816529) | ~261Y (MZ816572) | ~205 (MZ816617) | 410 (MZ816672) | N, full |
| i-26 | Anapodisma miramae (Far East, Russia, 2008 ) | B | 39 (MZ816463) | 14 (MZ816506) | 40 (MZ816550) | 7 (MZ816599) | 197 (MZ816652) | ST299 |
| i-27 | Bohemanella frigida (Altai 2003) | B | 9 (MZ816464) | 9 (MZ816507) | 6 (MZ816551) | 106 (MZ816600) | 197 (MZ816653) | hST-9 |
| i-28 | Ognevia longipennis (Japan, 2005) | B | 9 (MZ816465) | 133 (MZ816508) | 6 (MZ816552) | 106 (MZ816601) | 197 (MZ816654) | hST-4 |
| i-29 | Og. Longipennis (Altai, 2003) | B | 9 (MZ816466) | 133 (MZ816509) | 6 (MZ816553) | 106 (MZ816602) | 197 (MZ816655) | hST-4 |
| i-30 | Podisma pedestris (Altai, 2003) | B | 9 (MZ816467) | 133 (MZ816510) | 6 (MZ816554) | 106 (MZ816603) | 197 (MZ816656) | hST-4 |
| i-31 | P. pedestris (Altai, 2016) | B | 9 (MZ816468) | 133 (MZ816511) | 6 (MZ816555) | 106 (MZ816604) | 197 (MZ816657) | hST-4 |
| i-32 | Prumna littoralis (Far East, Russia, 2008) | B | 9 (MZ816469) | 133 (MZ816512) | 6 (MZ816556) | 106 (MZ816605) | 197 (MZ816658) | hST-4 |
| i-33 | Pr. Primnoa (Sakhalin, 2010) | B | 9 (MZ816470) | 133 (MZ816513) | 6 (MZ816557) | 106 (MZ816606) | 197 (MZ816659) | hST-4 |
| i-34 | Pr. Primnoa (Sakhalin Is., 2010) | B | 188 (MZ816471) | 224 (MZ816514) | ~6 (MZ816558) | 20 (MZ816607) | 25 (MZ816660) | hST-10 |
| i-35 | Pr. Ussuriensis (Far East, Russia, 2008) | B | 9 (MZ816472) | 133 (MZ816515) | 6 (MZ816559) | 106 (MZ816608) | 197 (MZ816661) | hST-4 |
| i-36 | Podisma kanoi (Honshu, Japan, 2005) | B | 9 (MZ816473) | 133 (MZ816516) | 6 (MZ816560) | 106 (MZ816609) | 197 (MZ816662) | hST-4 |
| i-37 | P. sapporensis (Japan, Tanno, 2005) | B | 9 (MZ816474) | 14 (MZ816517) | 6 (MZ816561) | 106 (MZ816610) | 197 (MZ816663) | hST-11 |
| i-38 | P. sapporensis (Japan, Akan, 2005) | B | 9 (MZ816475) | 14 (MZ816518) | 6 (MZ816562) | 106 (MZ816611) | 197 (MZ816664) | hST-11 |
| i-39 | P. sapporensis (Japan, Yotei, 2005) | B | 9 (MZ816476) | 14 (MZ816519) | 6 (MZ816563) | 106 (MZ816612) | 197 (MZ816665) | hST-11 |
| i-40 | P. sapporensis (Japan, Disengen, 2005) | B | 9 (MZ816486) | 14 (MZ816530) | 6 (MZ816573) | Mix ***** (MZ816618-MZ816632) | 197 (MZ816673) | Mix |
| i-41 | P. sapporensis (Japan, Naganuma, 2005) | B | 9 (MZ816477) | 14 (MZ816520) | 6 (MZ816564) | 106 (MZ816613) | 197 (MZ816666) | hST-11 |
| i-42 | P. sapporensis (Japan, Teine, 2005) | B | 9 (MZ816487) | 73 (MZ816531) | Mix (MZ816574-MZ816580) | 106 (MZ816633) | Mix (MZ816674-MZ816686) | Mix |
| i-43 | P. sapporensis (Japan, Shimokawa, 2005) | B | 9 (MZ816478) | 14 (MZ816521) | 6 (MZ816565) | 106 (MZ816614) | 197 (MZ816667) | hST-11 |
| i-44 | P. tyatiensis (Kunashir Is., Russia, 2001) | B | 9 (MZ816479) | 14 (MZ816522) | 6 (MZ816566) | 106 (MZ816615) | 197 (MZ816668) | hST-11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilinsky, Y.; Demenkova, M.; Bykov, R.; Bugrov, A. Narrow Genetic Diversity of Wolbachia Symbionts in Acrididae Grasshopper Hosts (Insecta, Orthoptera). Int. J. Mol. Sci. 2022, 23, 853. https://doi.org/10.3390/ijms23020853
Ilinsky Y, Demenkova M, Bykov R, Bugrov A. Narrow Genetic Diversity of Wolbachia Symbionts in Acrididae Grasshopper Hosts (Insecta, Orthoptera). International Journal of Molecular Sciences. 2022; 23(2):853. https://doi.org/10.3390/ijms23020853
Chicago/Turabian StyleIlinsky, Yury, Mary Demenkova, Roman Bykov, and Alexander Bugrov. 2022. "Narrow Genetic Diversity of Wolbachia Symbionts in Acrididae Grasshopper Hosts (Insecta, Orthoptera)" International Journal of Molecular Sciences 23, no. 2: 853. https://doi.org/10.3390/ijms23020853
APA StyleIlinsky, Y., Demenkova, M., Bykov, R., & Bugrov, A. (2022). Narrow Genetic Diversity of Wolbachia Symbionts in Acrididae Grasshopper Hosts (Insecta, Orthoptera). International Journal of Molecular Sciences, 23(2), 853. https://doi.org/10.3390/ijms23020853







