Synergetic Fermentation of Glucose and Glycerol for High-Yield N-Acetylglucosamine Production in Escherichia coli
Abstract
:1. Introduction
2. Results
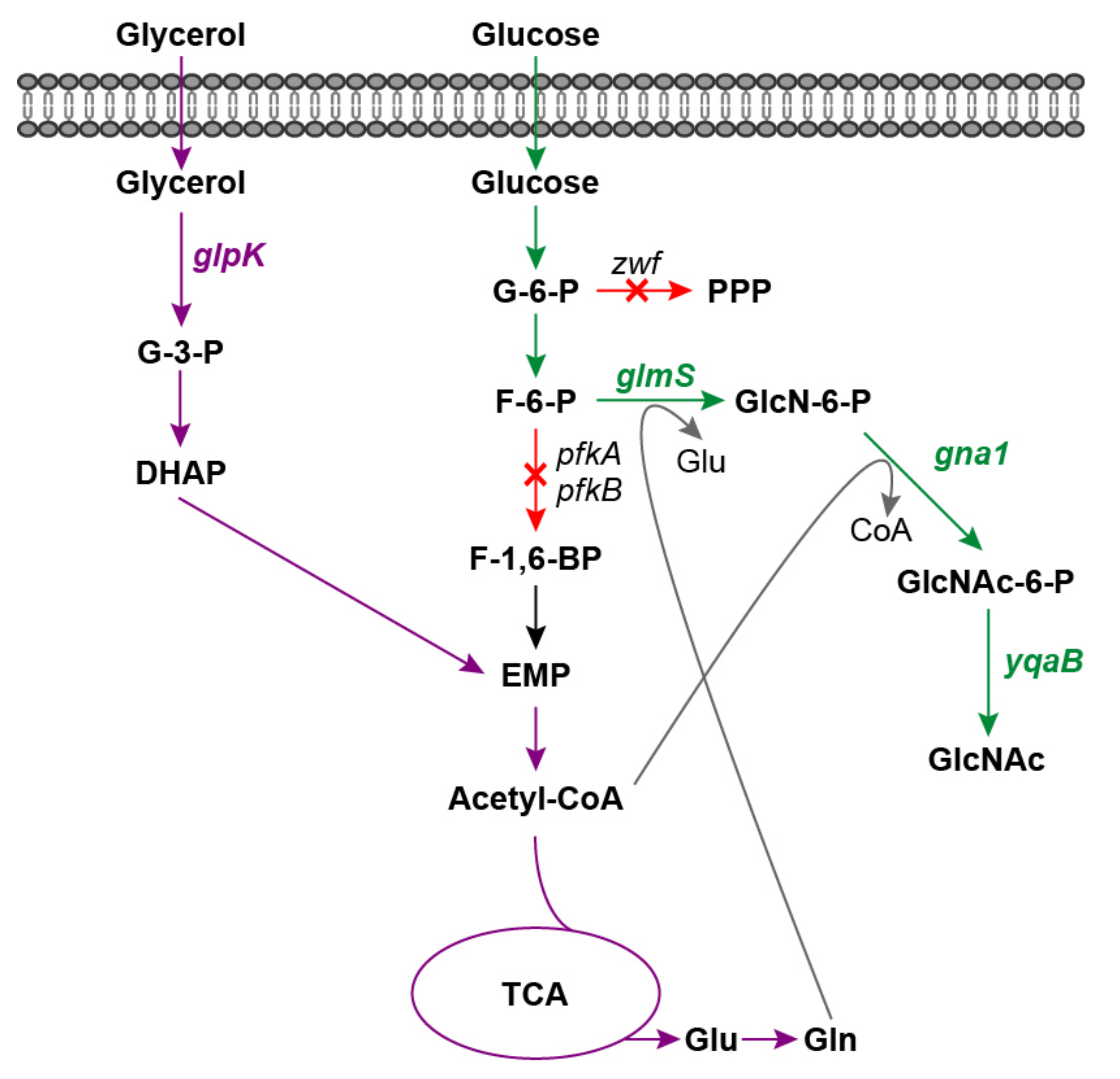
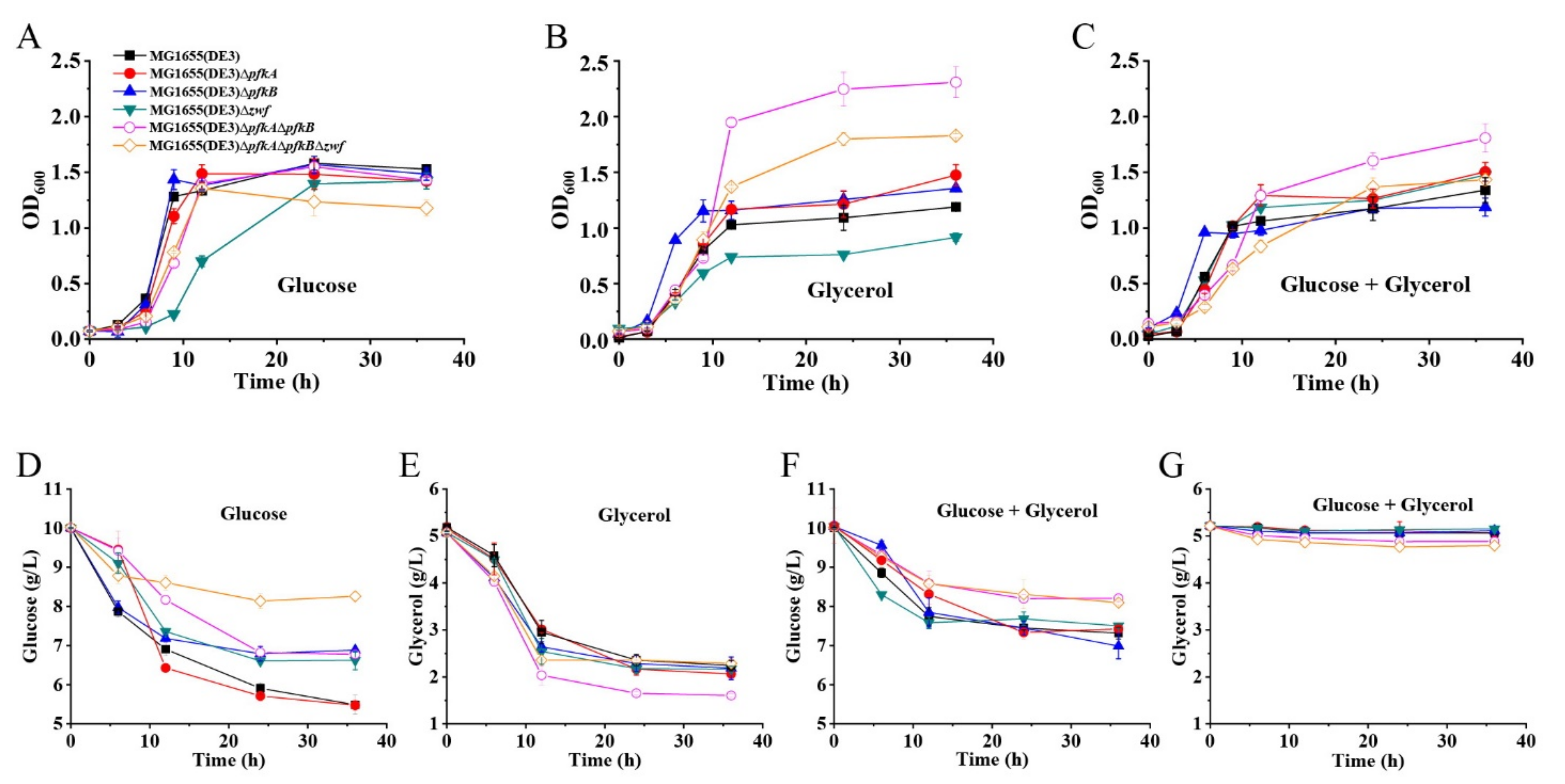
2.1. Construction and Characterization of an E. coli Platform Strain with High F-6-P Supply
2.2. GlcNAc Production by Synergetic Utilization of Glucose and Glycerol
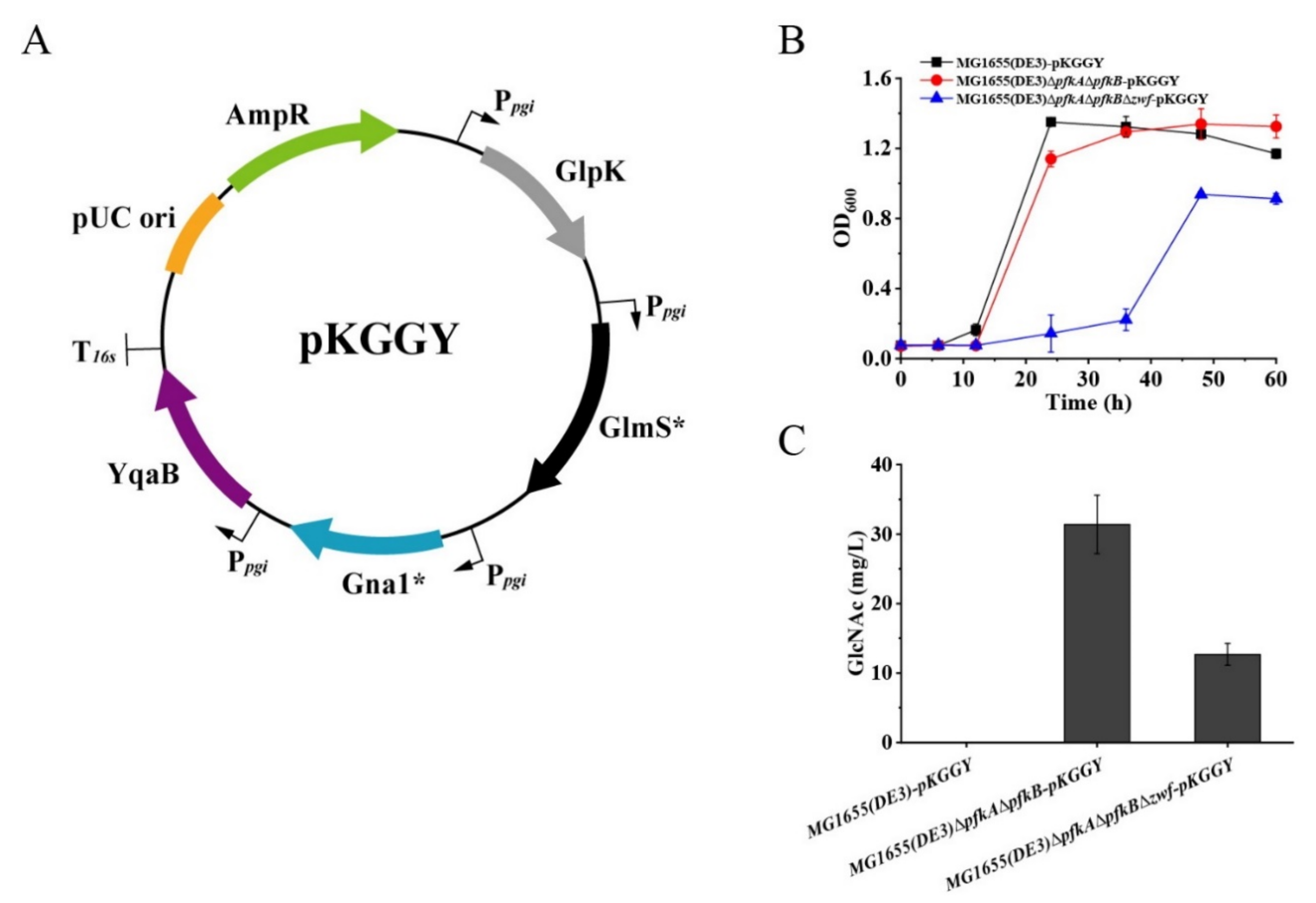
2.3. Medium Optimization for Enhanced Growth of MG1655(DE3)∆pfkA∆pfkB∆zwf-pKGGY
2.4. GlcNAc Production Using the Optimized Fermentation Medium
3. Discussion
4. Materials and Methods
4.1. Strains and Culture Media
4.2. Plasmid Construction
4.3. Mutant Screening
4.4. Batch Fermentation
4.5. Analytical Methods
4.6. RT-qPCR Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmad, S.I.; Ahmad, R.; Khan, M.S.; Kant, R.; Shahid, S.; Gautam, L.; Hasan, G.M.; Hassan, M.I. Chitin and its derivatives: Structural properties and biomedical applications. Int. J. Biol. Macromol. 2020, 164, 526–539. [Google Scholar] [CrossRef] [PubMed]
- Henrotin, Y.; Mobasheri, A. Natural products for promoting joint health and managing osteoarthritis. Curr. Rheumatol. Rep. 2018, 20, 72. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.C.; Lall, R.; Srivastava, A.; Sinha, A. Hyaluronic acid: Molecular mechanisms and therapeutic trajectory. Front. Vet. Sci. 2019, 6, 192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.-K.; Shen, C.-R.; Liu, C.-L. N-acetylglucosamine: Production and applications. Mar. Drugs 2010, 8, 2493–2516. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Liu, Y.; Shin, H.-d.; Chen, R.; Li, J.; Du, G.; Chen, J. Microbial production of glucosamine and N-acetylglucosamine: Advances and perspectives. Appl. Microbiol. Biotechnol. 2013, 97, 6149–6158. [Google Scholar] [CrossRef]
- Zhang, A.; Wang, C.; Chen, J.; Wei, G.; Zhou, N.; Li, G.; Chen, K.; Ouyang, P. Efficient enzymatic hydrolysis of chitin into N-acetyl glucosamine using alkali as a recyclable pretreatment reagent. Green Chem. 2021, 23, 3081–3089. [Google Scholar] [CrossRef]
- Subramanian, K.; Sadaiappan, B.; Aruni, W.; Kumarappan, A.; Thirunavukarasu, R.; Srinivasan, G.P.; Bharathi, S.; Nainangu, P.; Renuga, P.S.; Elamaran, A.; et al. Bioconversion of chitin and concomitant production of chitinase and N-acetylglucosamine by novel Achromobacter xylosoxidans isolated from shrimp waste disposal area. Sci. Rep. 2020, 10, 11898. [Google Scholar] [CrossRef]
- Deng, M.-D.; Severson, D.K.; Grund, A.D.; Wassink, S.L.; Burlingame, R.P.; Berry, A.; Running, J.A.; Kunesh, C.A.; Song, L.; Jerrell, T.A.; et al. Metabolic engineering of Escherichia coli for industrial production of glucosamine and N-acetylglucosamine. Metab. Eng. 2005, 7, 201–214. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, L.; Shin, H.-d.; Chen, R.R.; Li, J.; Du, G.; Chen, J. Pathway engineering of Bacillus subtilis for microbial production of N-acetylglucosamine. Metab. Eng. 2013, 19, 107–115. [Google Scholar] [CrossRef]
- Lee, S.W.; Oh, M.K. Improved production of N-acetylglucosamine in Saccharomyces cerevisiae by reducing glycolytic flux. Biotechnol. Bioeng. 2016, 113, 2524–2528. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Li, J.; Du, G.; Chen, J. Improved glucosamine and N-acetylglucosamine production by an engineered Escherichia coli via step-wise regulation of dissolved oxygen level. Bioresour. Technol. 2012, 110, 534–538. [Google Scholar] [CrossRef]
- Niu, T.; Lv, X.; Liu, Z.; Li, J.; Du, G.; Liu, L. Synergetic engineering of central carbon and nitrogen metabolism for the production of N-acetylglucosamine in Bacillus subtilis. Biotechnol. Appl. Biochem. 2020, 67, 123–132. [Google Scholar] [CrossRef]
- Lee, S.-W.; Lee, B.-Y.; Oh, M.-K. Combination of three methods to reduce glucose metabolic rate for improving N-acetylglucosamine production in Saccharomyces cerevisiae. J. Agric. Food Chem. 2018, 66, 13191–13198. [Google Scholar] [CrossRef]
- Zhou, D.; Jiang, Z.; Pang, Q.; Zhu, Y.; Wang, Q.; Qi, Q. CRISPR/Cas9-assisted seamless genome editing in Lactobacillus plantarum and its application in acetylglucosamine production. Appl. Environ. Microbiol. 2019, 85, e01367-19. [Google Scholar] [CrossRef]
- Deng, C.; Lv, X.; Li, J.; Zhang, H.; Liu, Y.; Du, G.; Amaro, R.L.; Liu, L. Synergistic improvement of N-acetylglucosamine production by engineering transcription factors and balancing redox cofactors. Metab. Eng. 2021, 67, 330–346. [Google Scholar] [CrossRef]
- Niu, T.; Lv, X.; Liu, Y.; Li, J.; Du, G.; Ledesma-Amaro, R.; Liu, L. The elucidation of phosphosugar stress response in Bacillus subtilis guides strain engineering for high N-acetylglucosamine production. Biotechnol. Bioeng. 2021, 118, 383–396. [Google Scholar] [CrossRef]
- Lee, S.-W.; Oh, M.-K. A synthetic suicide riboswitch for the high-throughput screening of metabolite production in Saccharomyces cerevisiae. Metab. Eng. 2015, 28, 143–150. [Google Scholar] [CrossRef]
- You, R.; Wang, L.; Shi, C.; Chen, H.; Zhang, S.; Hu, M.; Tao, Y. Efficient production of myo-inositol in Escherichia coli through metabolic engineering. Microb. Cell Fact. 2020, 19, 109. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Lee, J.-H.; Kim, N.-H.; Yeom, S.-J.; Kim, S.-W.; Oh, D.-K. Increase of lycopene production by supplementing auxiliary carbon sources in metabolically engineered Escherichia coli. Appl. Microbiol. Biotechnol. 2011, 90, 489–497. [Google Scholar] [CrossRef]
- Park, J.O.; Liu, N.; Holinski, K.M.; Emerson, D.F.; Qiao, K.; Woolston, B.M.; Xu, J.; Lazar, Z.; Islam, M.A.; Vidoudez, C.; et al. Synergistic substrate cofeeding stimulates reductive metabolism. Nat. Metab. 2019, 1, 643–651. [Google Scholar] [CrossRef]
- Zhao, D.; Yuan, S.; Xiong, B.; Sun, H.; Ye, L.; Li, J.; Zhang, X.; Bi, C. Development of a fast and easy method for Escherichia coli genome editing with CRISPR/Cas9. Microb. Cell Fact. 2016, 15, 205. [Google Scholar] [CrossRef] [Green Version]
- Shiue, E.; Brockman, I.M.; Prather, K.L.J. Improving product yields on D-glucose in Escherichia coli via knockout of pgi and zwf and feeding of supplemental carbon sources. Biotechnol. Bioeng. 2015, 112, 579–587. [Google Scholar] [CrossRef] [Green Version]
- Diaz, C.A.C.; Bennett, R.K.; Papoutsakis, E.T.; Antoniewicz, M.R. Deletion of four genes in Escherichia coli enables preferential consumption of xylose and secretion of glucose. Metab. Eng. 2019, 52, 168–177. [Google Scholar] [CrossRef]
- Gleizer, S.; Ben-Nissan, R.; Bar-On, Y.M.; Antonovsky, N.; Noor, E.; Zohar, Y.; Jona, G.; Krieger, E.; Shamshoum, M.; Bar-Even, A.; et al. Conversion of Escherichia coli to generate all biomass carbon from CO2. Cell 2019, 179, 1255–1263.e1212. [Google Scholar] [CrossRef] [Green Version]
- Satanowski, A.; Dronsella, B.; Noor, E.; Vögeli, B.; He, H.; Wichmann, P.; Erb, T.J.; Lindner, S.N.; Bar-Even, A. Awakening a latent carbon fixation cycle in Escherichia coli. Nat. Commun. 2020, 11, 5812. [Google Scholar] [CrossRef]
- Guitart Font, E.; Sprenger, G.A. Opening a novel biosynthetic pathway to dihydroxyacetone and glycerol in Escherichia coli mutants through expression of a gene variant (fsaAA129S) for fructose 6-phosphate aldolase. Int. J. Mol. Sci. 2020, 21, 9625. [Google Scholar] [CrossRef]
- Kuznetsova, E.; Proudfoot, M.; Gonzalez, C.F.; Brown, G.; Omelchenko, M.V.; Borozan, I.; Carmel, L.; Wolf, Y.I.; Mori, H.; Savchenko, A.V.; et al. Genome-wide analysis of substrate specificities of the Escherichia coli haloacid dehalogenase-like phosphatase family. J. Biol. Chem. 2006, 281, 36149–36161. [Google Scholar] [CrossRef] [Green Version]
- Niyas, A.M.M.; Eiteman, M.A. Phosphatases and phosphate affect the formation of glucose from pentoses in Escherichia coli. Eng. Life Sci. 2017, 17, 579–584. [Google Scholar] [CrossRef]
- Deng, M.D.; Grund, A.D.; Wassink, S.L.; Peng, S.S.; Nielsen, K.L.; Huckins, B.D.; Burlingame, R.P. Directed evolution and characterization of Escherichia coli glucosamine synthase. Biochimie 2006, 88, 419–429. [Google Scholar] [CrossRef]
- Ma, W.; Liu, Y.; Lv, X.; Li, J.; Du, G.; Liu, L. Combinatorial pathway enzyme engineering and host engineering overcomes pyruvate overflow and enhances overproduction of N-acetylglucosamine in Bacillus subtilis. Microb. Cell Fact. 2019, 18, 1. [Google Scholar] [CrossRef] [Green Version]
- Kumari, S.; Beatty, C.M.; Browning, D.F.; Busby, S.J.W.; Simel, E.J.; Hovel-Miner, G.; Wolfe, A.J. Regulation of acetyl coenzyme A synthetase in Escherichia coli. J. Bacteriol. 2000, 182, 4173–4179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valgepea, K.; Adamberg, K.; Nahku, R.; Lahtvee, P.-J.; Arike, L.; Vilu, R. Systems biology approach reveals that overflow metabolism of acetate in Escherichia coli is triggered by carbon catabolite repression of acetyl-CoA synthetase. BMC Syst. Biol. 2010, 4, 166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, K.; Lim, H.C.; Hong, J. Acetic acid formation in Escherichia coli fermentation. Biotechnol. Bioeng. 1992, 39, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.G.; Rhee, J.S.; Lebeault, J.M. Physiological constraints in increasing biomass concentration of Escherichia coli B in fed-batch culture. Biotechnol. Lett. 1987, 9, 89–94. [Google Scholar] [CrossRef]
- Vijayendran, C.; Polen, T.; Wendisch, V.F.; Friehs, K.; Niehaus, K.; Flaschel, E. The plasticity of global proteome and genome expression analyzed in closely related W3110 and MG1655 strains of a well-studied model organism, Escherichia coli-K12. J. Biotechnol. 2007, 128, 747–761. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, B.; Duan, C.; Sun, B.; Yang, J.; Yang, S. Multigene editing in the Escherichia coli genome via the CRISPR-Cas9 system. Appl. Environ. Microbiol. 2015, 81, 2506–2514. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Struewing, I.; Wymer, L.; Tettenhorst, D.R.; Shoemaker, J.; Allen, J. Use of qPCR and RT-qPCR for monitoring variations of microcystin producers and as an early warning system to predict toxin production in an Ohio inland lake. Water Res. 2020, 170, 115262. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]



| Strains/Plasmids | Relevant Characteristics | Sources |
|---|---|---|
| Strains | ||
| E. coli | ||
| Top 10 | F−, mcrA, Δ(mrr-hsdRMS-mcrBC), φ80, lacZΔM15, ΔlacX74, nupG, recA1, araD139, Δ(ara-leu)7697, galE15, galK16, rpsL(StrR), endA1, λ− | Invitrogen |
| MG1655(DE3) | K-12 F–, λ(DE3), ilvG–, rfb-50, rph-1, wild-type strain | [35] |
| MG1655(DE3)∆pfkA | MG1655(DE3), pfkA deletion mutant | This work |
| MG1655(DE3)∆pfkB | MG1655(DE3), pfkB deletion mutant | This work |
| MG1655(DE3)∆pfkA∆pfkB | MG1655(DE3), pfkA and pfkB double-deletion mutant | This work |
| MG1655(DE3)∆zwf | MG1655(DE3), zwf deletion mutant | This work |
| MG1655(DE3)∆pfkA∆pfkB∆zwf | MG1655(DE3), pfkA, pfkB and zwf triple-deletion mutant | This work |
| MG1655(DE3)-pKGGY | MG1655(DE3), harboring plasmid pKGGY | This work |
| MG1655(DE3)∆pfkA∆pfkB-pKGGY | MG1655(DE3)∆pfkA∆pfkB, harboring plasmid pKGGY | This work |
| MG1655(DE3)∆pfkA∆pfkB∆zwf-pKGGY | MG1655(DE3)∆pfkA∆pfkB∆zwf, harboring plasmid pKGGY | This work |
| Plasmids | ||
| pRed_Cas9_recA_∆poxb300 | Exo, bet, gam, recA, arabinose operon, Cas9, gRNA and homologous arms for poxb deletion | [21] |
| pRed_Cas9_recA | Derived from pRed_Cas9_recA_∆poxb300, Exo, bet, gam, recA, arabinose operon and Cas9 | This work |
| p∆pfkA | pEASY-T3, gRNA and homologous arms for pfkA deletion | This work |
| p∆pfkB | pEASY-T3, gRNA and homologous arms for pfkB deletion | This work |
| p∆zwf | pEASY-T3, gRNA and homologous arms for zwf deletion | This work |
| pKGGY | pEASY-T3, harboring glpK, glmS* (glmS*72, a mutated form of glucosamine-6-phosphate synthase), gna1* (CeGAN1-Q155V/C158G, a mutated form of glucosamine-6-phosphate N-acetyltransferase) and yqaB expression cassettes | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; Wang, X.; Luo, H.; Wang, Y.; Wang, Y.; Tu, T.; Qin, X.; Bai, Y.; Huang, H.; Yao, B.; et al. Synergetic Fermentation of Glucose and Glycerol for High-Yield N-Acetylglucosamine Production in Escherichia coli. Int. J. Mol. Sci. 2022, 23, 773. https://doi.org/10.3390/ijms23020773
Wang K, Wang X, Luo H, Wang Y, Wang Y, Tu T, Qin X, Bai Y, Huang H, Yao B, et al. Synergetic Fermentation of Glucose and Glycerol for High-Yield N-Acetylglucosamine Production in Escherichia coli. International Journal of Molecular Sciences. 2022; 23(2):773. https://doi.org/10.3390/ijms23020773
Chicago/Turabian StyleWang, Kaikai, Xiaolu Wang, Huiying Luo, Yaru Wang, Yuan Wang, Tao Tu, Xing Qin, Yingguo Bai, Huoqing Huang, Bin Yao, and et al. 2022. "Synergetic Fermentation of Glucose and Glycerol for High-Yield N-Acetylglucosamine Production in Escherichia coli" International Journal of Molecular Sciences 23, no. 2: 773. https://doi.org/10.3390/ijms23020773
APA StyleWang, K., Wang, X., Luo, H., Wang, Y., Wang, Y., Tu, T., Qin, X., Bai, Y., Huang, H., Yao, B., Su, X., & Zhang, J. (2022). Synergetic Fermentation of Glucose and Glycerol for High-Yield N-Acetylglucosamine Production in Escherichia coli. International Journal of Molecular Sciences, 23(2), 773. https://doi.org/10.3390/ijms23020773






