The Roles of IL-22 and Its Receptor in the Regulation of Inflammatory Responses in the Brain
Abstract
:1. Introduction
2. Results
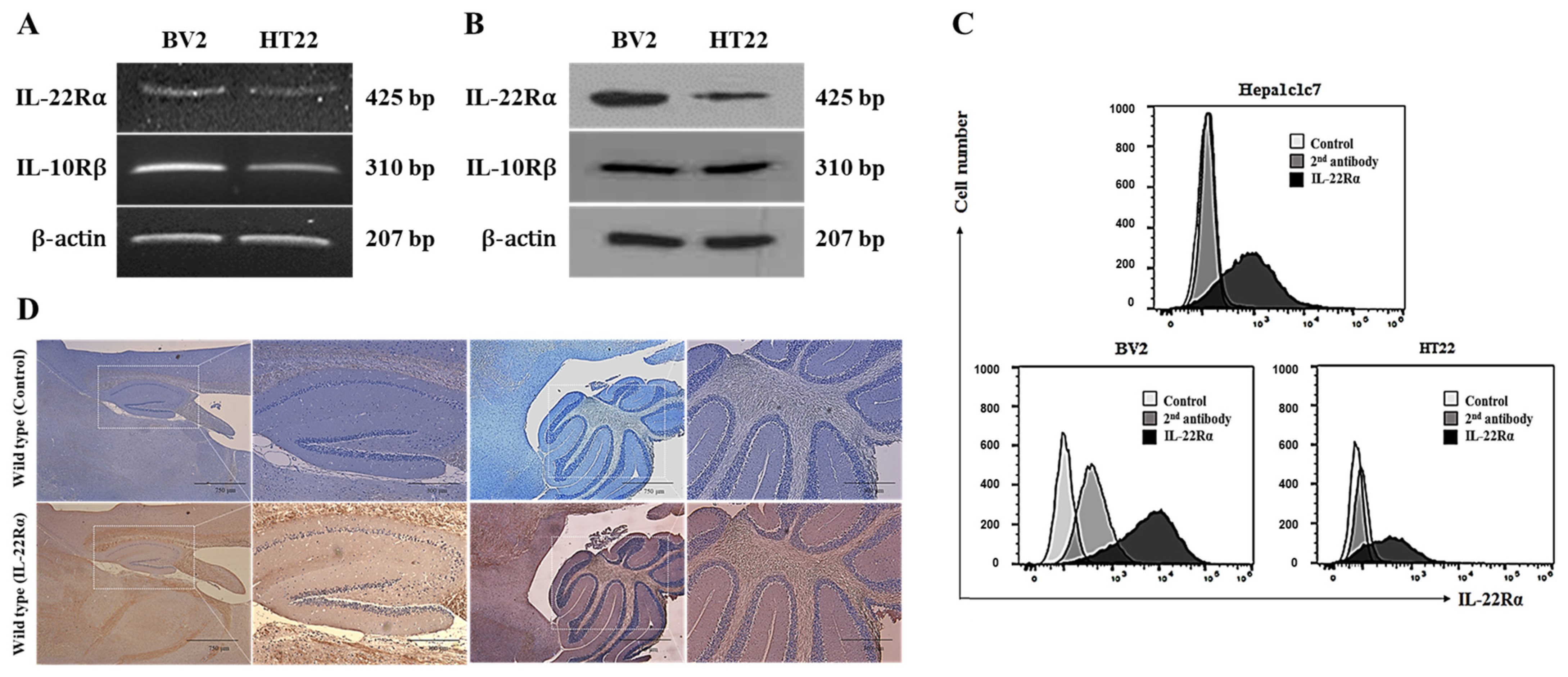
2.1. IL-22Rα Is Constitutively Expressed in BV2 Murine Microglial Cells, HT22 Hippocampal Neuronal Cells, and Mouse Brain Tissue
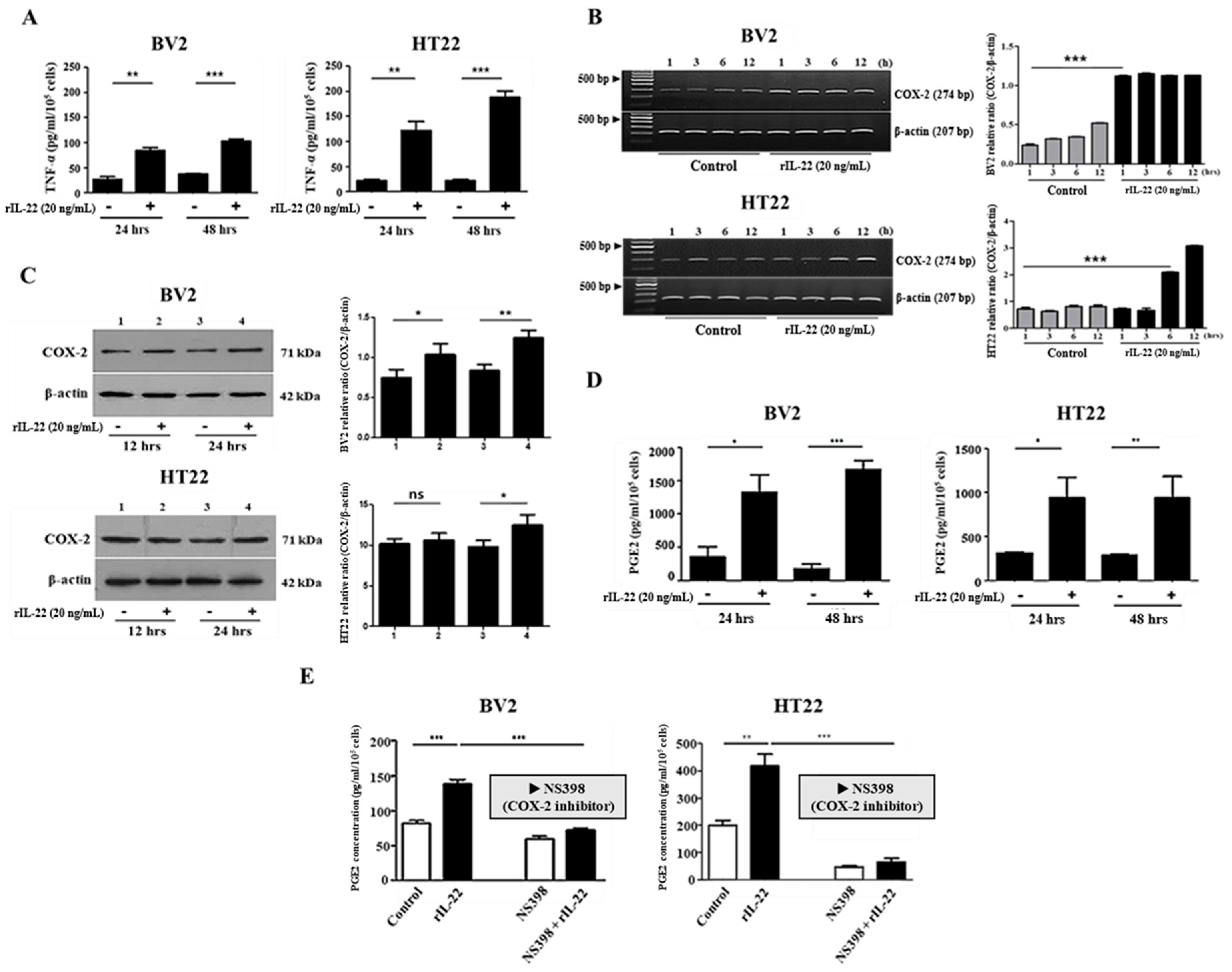
2.2. The Interaction of IL-22 with IL-22Rα Induces Proinflammatory Cytokine Production in BV2 and HT22 Cells
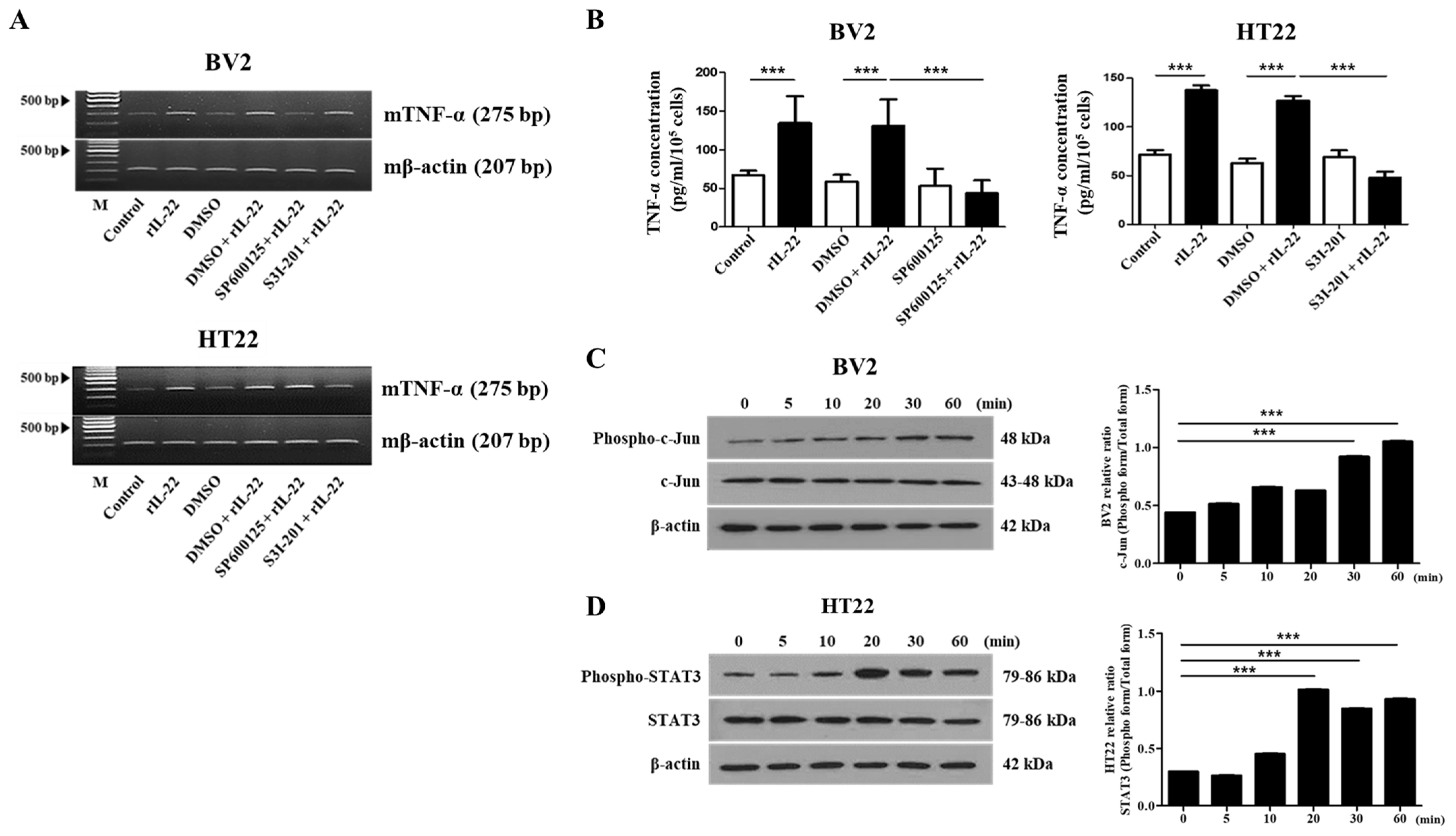
2.3. The JNK and STAT3 Signaling Pathways Play an Important Role in IL-22-Induced Proinflammatory Cytokine Production in BV2 and HT22 Cells, Respectively
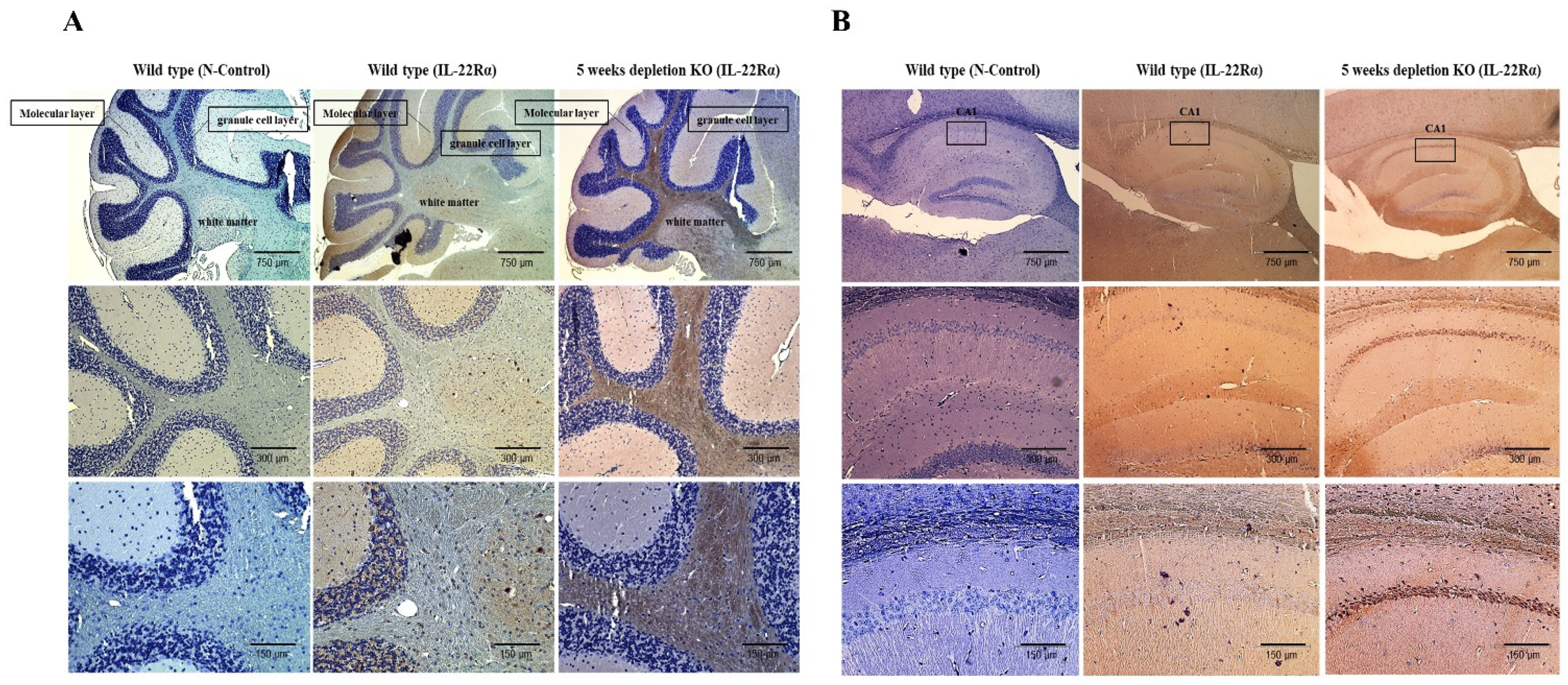
2.4. IL-22Rα Expression Is Increased in the Gulo (-/-) Mouse Brain upon Inflammation
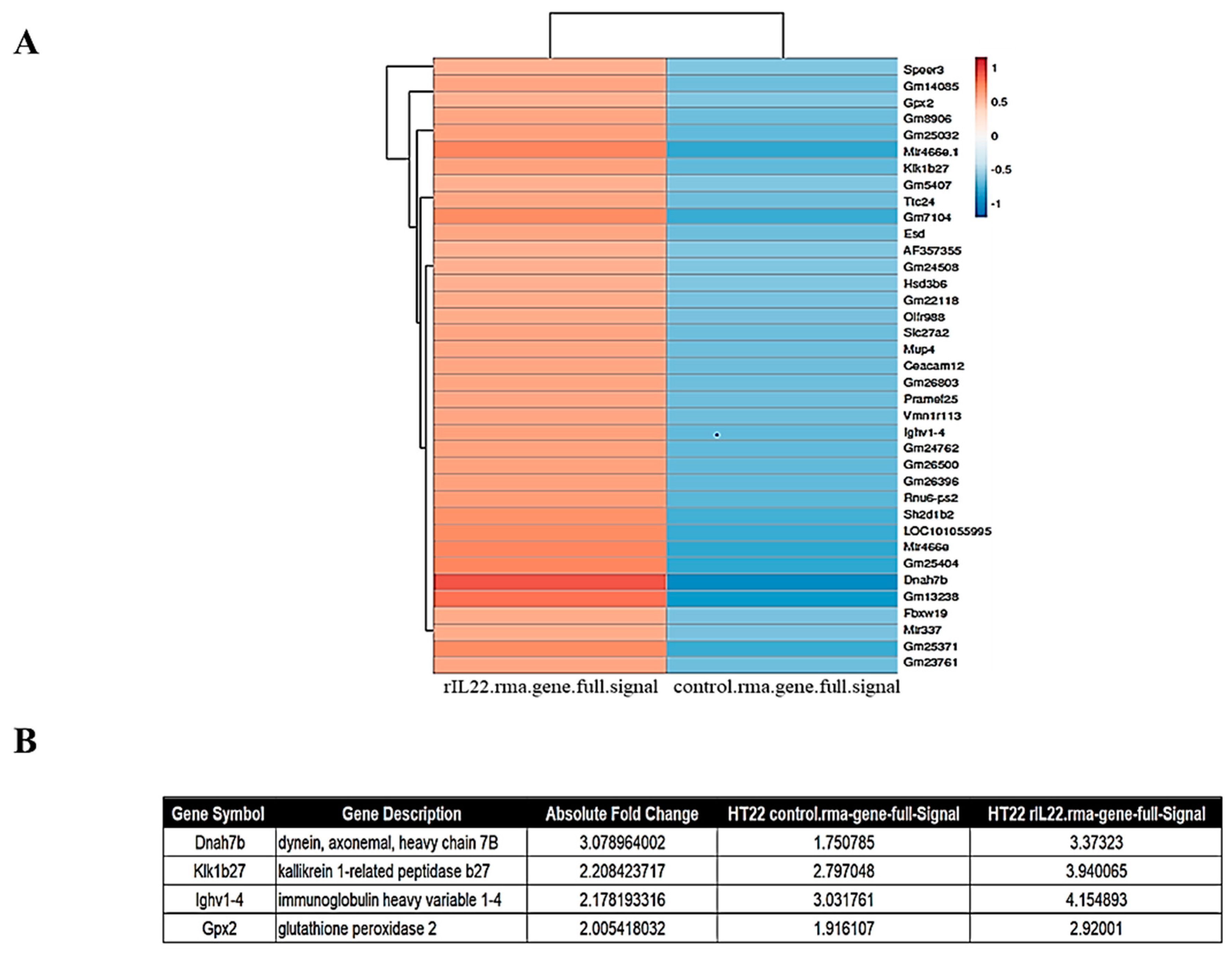
2.5. Inflammatory Genes Are Upregulated by IL-22 Treatment of HT22 Cells
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Culture Conditions
4.2. Animals
4.3. Immunohistochemistry
4.4. Flow Cytometry
4.5. Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
4.6. Western Blotting
4.7. Enzyme-Linked Immunosorbent Assay (ELISA)
4.8. Gene Expression Profiling
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, E.; Cen, Y.; Lu, D.; Luo, W.; Jiang, H. IL-22 inactivates hepatic stellate cells via downregulation of the TGF-beta1/Notch signaling pathway. Mol. Med. Rep. 2018, 17, 5449–5453. [Google Scholar]
- Mihi, B.; Gong, Q.; Nolan, L.S.; Gale, S.E.; Goree, M.; Hu, E.; Lanik, W.E.; Rimer, J.M.; Liu, V.; Parks, O.B.; et al. Interleukin-22 signaling attenuates necrotizing enterocolitis by promoting epithelial cell regeneration. Cell Rep. Med. 2021, 2, 100320. [Google Scholar] [CrossRef] [PubMed]
- Sakemi, R.; Mitsuyama, K.; Morita, M.; Yoshioka, S.; Kuwaki, K.; Tokuyasu, H.; Fukunaga, S.; Mori, A.; Araki, T.; Yoshimura, T.; et al. Altered serum profile of the interleukin-22 system in inflammatory bowel disease. Cytokine 2020, 136, 155264. [Google Scholar] [CrossRef]
- Kong, X.; Feng, D.; Wang, H.; Hong, F.; Bertola, A.; Wang, F.-S.; Gao, B. Interleukin-22 induces hepatic stellate cell senescence and restricts liver fibrosis in mice. Hepatology 2012, 56, 1150–1159. [Google Scholar] [CrossRef] [PubMed]
- Mossner, S.; Kuchner, M.; Modares, N.F.; Knebel, B.; Al-Hasani, H.; Floss, D.M.; Scheller, J. Synthetic interleukin 22 (IL-22) signaling reveals biological activity of homodimeric IL-10 receptor 2 and functional cross-talk with the IL-6 receptor gp130. J. Biol. Chem. 2020, 295, 12378–12397. [Google Scholar] [CrossRef]
- Akil, H.; Abbaci, A.; Lalloué, F.; Bessette, B.; Costes, L.M.; Domballe, L.; Lecron, J.C.; Bernard, F.X.; Morel, F.; Tapon, K.; et al. IL22/IL-22R pathway induces cell survival in human glioblastoma cells. PLoS ONE 2015, 10, e0119872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keir, M.E.; Yi, T.; Lu, T.T.; Ghilardi, N. The role of IL-22 in intestinal health and disease. J. Exp. Med. 2020, 217, e20192195. [Google Scholar] [CrossRef] [PubMed]
- Stallhofer, J.; Friedrich, M.; Konrad-Zerna, A.; Wetzke, M.; Lohse, P.; Glas, J.; Tillack-Schreiber, C.; Schnitzler, F.; Beigel, F.; Brand, S. Lipocalin-2 Is a Disease Activity Marker in Inflammatory Bowel Disease Regulated by IL-17A, IL-22, and TNF-α and Modulated by IL23R Genotype Status. Inflamm. Bowel Dis. 2015, 21, 2327–2340. [Google Scholar] [CrossRef]
- Nograles, K.E.; Zaba, L.C.; Shemer, A.; Fuentes-Duculan, J.; Cardinale, I.; Kikuchi, T.; Guttman-Yassky, E.; Bergman, R.; Krueger, J.; Yassky, E.G.; et al. IL-22-producing “T22” T cells account for upregulated IL-22 in atopic dermatitis despite reduced IL-17-producing TH17 T cells. J. Allergy Clin. Immunol. 2009, 123, 1244.e2–1252 e2. [Google Scholar] [CrossRef] [Green Version]
- Liang, S.C.; Nickerson-Nutter, C.; Pittman, D.D.; Carrier, Y.; Goodwin, D.G.; Shields, K.M.; Lambert, A.-J.; Schelling, S.H.; Medley, Q.G.; Ma, H.-L.; et al. IL-22 Induces an Acute-Phase Response. J. Immunol. 2010, 185, 5531–5538. [Google Scholar] [CrossRef] [Green Version]
- Trifari, S.; Spits, H. IL-22-producing CD4+ T cells: Middle-men between the immune system and its environment. Eur. J. Immunol. 2010, 40, 2369–2371. [Google Scholar] [CrossRef]
- Das, S.; Croix, C.S.; Good, M.; Chen, J.; Zhao, J.; Hu, S.; Ross, M.; Myerburg, M.M.; Pilewski, J.M.; Williams, J.; et al. Interleukin-22 Inhibits Respiratory Syncytial Virus Production by Blocking Virus-Mediated Subversion of Cellular Autophagy. iScience 2020, 23, 101256. [Google Scholar] [CrossRef]
- Patnaude, L.; Mayo, M.; Mario, R.; Wu, X.; Knight, H.; Creamer, K.; Wilson, S.; Pivorunas, V.; Karman, J.; Phillips, L.; et al. Mechanisms and regulation of IL-22-mediated intestinal epithelial homeostasis and repair. Life Sci. 2021, 271, 119195. [Google Scholar] [CrossRef] [PubMed]
- Nikoopour, E.; Bellemore, S.M.; Singh, B. IL-22, cell regeneration and autoimmunity. Cytokine 2015, 74, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Pickert, G.; Neufert, C.; Leppkes, M.; Zheng, Y.; Wittkopf, N.; Warntjen, M.; Lehr, H.-A.; Hirth, S.; Weigmann, B.; Wirtz, S.; et al. STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J. Exp. Med. 2009, 206, 1465–1472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitra, A.; Raychaudhuri, S.K.; Raychaudhuri, S.P. IL-22 induced cell proliferation is regulated by PI3K/Akt/mTOR signaling cascade. Cytokine 2012, 60, 38–42. [Google Scholar] [CrossRef]
- Yu, L.Z.; Wang, H.Y.; Yang, S.P.; Yuan, Z.P.; Xu, F.Y.; Sun, C.; Shi, R.H. Expression of interleukin-22/STAT3 signaling pathway in ulcerative colitis and related carcinogenesis. World J. Gastroenterol. 2013, 19, 2638–2649. [Google Scholar] [CrossRef]
- Park, G.-H.; Noh, H.; Shao, Z.; Ni, P.; Qin, Y.; Liu, D.; Beaudreault, C.P.; Park, J.S.; Abani, C.P.; Park, J.M.; et al. Activated microglia cause metabolic disruptions in developmental cortical interneurons that persist in interneurons from individuals with schizophrenia. Nat. Neurosci. 2020, 23, 1352–1364. [Google Scholar] [CrossRef]
- Rodríguez-Gómez, J.A.; Kavanagh, E.; Engskog-Vlachos, P.; Engskog, M.K.; Herrera, A.J.; Espinosa-Oliva, A.M.; Joseph, B.; Hajji, N.; Venero, J.L.; Burguillos, M.A. Microglia: Agents of the CNS Pro-Inflammatory Response. Cells 2020, 9, 1717. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.L.; Yim, A.K.Y.; Kim, K.-W.; Avey, D.; Czepielewski, R.S.; Colonna, M.; Milbrandt, J.; Randolph, G.J. Peripheral nerve resident macrophages share tissue-specific programming and features of activated microglia. Nat. Commun. 2020, 11, 2552. [Google Scholar] [CrossRef]
- Pinto, B.; Morelli, G.; Rastogi, M.; Savardi, A.; Fumagalli, A.; Petretto, A.; Bartolucci, M.; Varea, E.; Catelani, T.; Contestabile, A.; et al. Rescuing Over-activated Microglia Restores Cognitive Performance in Juvenile Animals of the Dp(16) Mouse Model of Down Syndrome. Neuron 2020, 108, 887.e12–904.e12. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, J.; Nutma, E.; Van Der Valk, P.; Amor, S. Inflammation in CNS neurodegenerative diseases. Immunology 2018, 154, 204–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urban, S.L.; Jensen, I.J.; Shan, Q.; Pewe, L.L.; Xue, H.-H.; Badovinac, V.; Harty, J.T. Peripherally induced brain tissue–resident memory CD8+ T cells mediate protection against CNS infection. Nat. Immunol. 2020, 21, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Seidler, R.D.; Bernard, J.A.; Burutolu, T.B.; Fling, B.W.; Gordon, M.T.; Gwin, J.T.; Kwak, Y.; Lipps, D.B. Motor control and aging: Links to age-related brain structural, functional, and biochemical effects. Neurosci. Biobehav. Rev. 2010, 34, 721–733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, J.H.; Kim, B.H.; Chung, S.J.; Yoo, H.S.; Lee, Y.H.; Baik, K.; Lee, P.H. Motor Cerebellar Connectivity and Future Development of Freezing of Gait in De Novo Parkinson’s Disease. Mov. Disord. 2020, 35, 2240–2249. [Google Scholar] [CrossRef]
- Kosuge, Y.; Nango, H.; Kasai, H.; Yanagi, T.; Mawatari, T.; Nishiyama, K.; Miyagishi, H.; Ishige, K.; Ito, Y. Generation of Cellular Reactive Oxygen Species by Activation of the EP2 Receptor Contributes to Prostaglandin E2-Induced Cytotoxicity in Motor Neuron-Like NSC-34 Cells. Oxidative Med. Cell. Longev. 2020, 2020, 1–14. [Google Scholar] [CrossRef]
- Fransen, N.L.; Hsiao, C.-C.; Van Der Poel, M.; Engelenburg, H.J.; Verdaasdonk, K.; Vincenten, M.C.J.; Remmerswaal, E.B.M.; Kuhlmann, T.; Mason, M.R.J.; Hamann, J.; et al. Tissue-resident memory T cells invade the brain parenchyma in multiple sclerosis white matter lesions. Brain 2020, 143, 1714–1730. [Google Scholar] [CrossRef]
- Grzanna, M.W.; Au, R.Y.; Au, A.Y.; Rashmir, A.M.; Frondoza, C.G. Avocado/Soybean Unsaponifiables, Glucosamine and Chondroitin Sulfate Combination Inhibits Proinflammatory COX-2 Expression and Prostaglandin E2 Production in Tendon-Derived Cells. J. Med. Food 2020, 23, 139–146. [Google Scholar] [CrossRef]
- Zhu, X.; Yao, Y.; Yang, J.; Zhengxie, J.; Li, X.; Hu, S.; Zhang, A.; Dong, J.; Zhang, C.; Gan, G. COX-2-PGE2 signaling pathway contributes to hippocampal neuronal injury and cognitive impairment in PTZ-kindled epilepsy mice. Int. Immunopharmacol. 2020, 87, 106801. [Google Scholar] [CrossRef]
- Yu, Y.; Jiang, J. COX-2/PGE2 axis regulates hippocampal BDNF/TrkB signaling via EP2 receptor after prolonged seizures. Epilepsia Open. 2020, 5, 418–431. [Google Scholar] [CrossRef]
- Alvarez, A.M.; DeOcesano-Pereira, C.; Teixeira, C.; Moreira, V. IL-1beta and TNF-alpha Modulation of Proliferated and Committed Myoblasts: IL-6 and COX-2-Derived Prostaglandins as Key Actors in the Mechanisms Involved. Cells 2020, 9, 2005. [Google Scholar] [CrossRef]
- Kim, H.; Kim, Y.; Bae, S.; Lim, S.H.; Jang, M.; Choi, J.; Lee, W.J. Vitamin C Deficiency Causes Severe Defects in the Development of the Neonatal Cerebellum and in the Motor Behaviors of Gulo(-/-) Mice. Antioxidants Redox Signal. 2015, 23, 1270–1283. [Google Scholar] [CrossRef]
- Lee, H.K.; Widmayer, S.J.; Huang, M.-N.; Aylor, D.L.; Marchuk, D.A. Novel Neuroprotective Loci Modulating Ischemic Stroke Volume in Wild-Derived Inbred Mouse Strains. Genetics 2019, 213, 1079–1092. [Google Scholar] [CrossRef]
- McConaha, M.; Eckstrum, K.; An, J.; Steinle, J.J.; Bany, B.M. Microarray assessment of the influence of the conceptus on gene expression in the mouse uterus during decidualization. Reproduction 2011, 141, 511–527. [Google Scholar] [CrossRef] [Green Version]
- Dubey, S.; Heinen, S.; Krantic, S.; McLaurin, J.; Branch, D.R.; Hynynen, K.; Aubert, I. Clinically approved IVIg delivered to the hippocampus with focused ultrasound promotes neurogenesis in a model of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2020, 117, 32691–32700. [Google Scholar] [CrossRef] [PubMed]
- Buckley, N.A.; Baskaya, M.K.; Darsie, M.E. Intravenous Immunoglobulin (IVIG) in Severe Heparin-Induced Thrombocytopenia (HIT) in a Traumatic Brain Injury (TBI) Patient with Cerebral Venous Sinus Thrombosis (CVST). Neurocrit. Care 2020, 34, 1103–1107. [Google Scholar] [CrossRef]
- Brigelius-Flohé, R.; Kipp, A. Physiological functions of GPx2 and its role in inflammation-triggered carcinogenesis. Ann. N. Y. Acad. Sci. 2012, 1259, 19–25. [Google Scholar] [CrossRef]
- Bennett, F.; Bennett, M.; Yaqoob, F.; Mulinyawe, S.B.; Grant, G.A.; Gephart, M.H.; Plowey, E.D.; Barres, B.A. A Combination of Ontogeny and CNS Environment Establishes Microglial Identity. Neuron 2018, 98, 1170–1183.e8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutmann, D.H.; Kettenmann, H. Microglia/Brain Macrophages as Central Drivers of Brain Tumor Pathobiology. Neuron 2019, 104, 442–449. [Google Scholar] [CrossRef]
- Walker, D.G.; Tang, T.M.; Mendsaikhan, A.; Tooyama, I.; Serrano, G.E.; Sue, L.I.; Beach, T.G.; Lue, L.-F. Patterns of Expression of Purinergic Receptor P2RY12, a Putative Marker for Non-Activated Microglia, in Aged and Alzheimer’s Disease Brains. Int. J. Mol. Sci. 2020, 21, 678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leppkes, M.; Neurath, M.F. Cytokines in inflammatory bowel diseases—Update 2020. Pharmacol. Res. 2020, 158, 104835. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-Y.; Wang, X.-J.; Su, Y.-L.; Wang, Q.; Huang, S.-W.; Pan, Z.-F.; Chen, Y.-P.; Liang, J.-J.; Zhang, M.-L.; Xie, X.-Q.; et al. Baicalein ameliorates ulcerative colitis by improving intestinal epithelial barrier via AhR/IL-22 pathway in ILC3s. Acta Pharmacol. Sin. 2021, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gao, S.; Zhang, J.; Li, C.; Li, H.; Lin, J. Interleukin-22 attenuates allergic airway inflammation in ovalbumin-induced asthma mouse model. BMC Pulm. Med. 2021, 21, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bar, M. The proactive brain: Memory for predictions. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1235–1243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masuda, T.; Amann, L.; Sankowski, R.; Staszewski, O.; Lenz, M.; Snaidero, N.; Prinz, M. Novel Hexb-based tools for studying microglia in the CNS. Nat. Immunol. 2020, 21, 802–815. [Google Scholar] [CrossRef]
- Ressler, R.L.; Goode, T.D.; Kim, S.; Ramanathan, K.R.; Maren, S. Covert capture and attenuation of a hippocampus-dependent fear memory. Nat. Neurosci. 2021, 24, 677–684. [Google Scholar] [CrossRef]
- De Zeeuw, C.I.; Lisberger, S.G.; Raymond, J.L. Diversity and dynamism in the cerebellum. Nat. Neurosci. 2021, 24, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Welniarz, Q.; Worbe, Y.; Gallea, C. The Forward Model: A Unifying Theory for the Role of the Cerebellum in Motor Control and Sense of Agency. Front. Syst. Neurosci. 2021, 15. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, J. Parkinson’s disease: Clinical features and diagnosis. J. Neurol. Neurosurg. Psychiatry 2008, 79, 368–376. [Google Scholar] [CrossRef] [Green Version]
- Zhao, N.; Yu, M.J.; Xu, J.; Wang, H.Y.; Liang, B.; Ding, L.; Leng, B.L. MicroRNA-29b mediates Th17/Treg imbalance in chronic obstructive pulmonary disease by targeting IL-22. J. Biol. Regul. Homeost. Agents 2021, 35, 987–999. [Google Scholar] [PubMed]
- Min, H.K.; Won, J.Y.; Kim, B.M.; Lee, K.A.; Lee, S.J.; Lee, S.H.; Kim, K.W. Interleukin (IL)-25 suppresses IL-22-induced osteoclastogenesis in rheumatoid arthritis via STAT3 and p38 MAPK/IkappaBalpha pathway. Arthritis Res. Ther. 2020, 22, 222. [Google Scholar] [CrossRef]
- Meng, J.; Chen, F.-R.; Yan, W.-J.; Lin, Y.-K. MiR-15a-5p targets FOSL1 to inhibit proliferation and promote apoptosis of keratinocytes via MAPK/ERK pathway. J. Tissue Viability 2021, 30, 544–551. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, M.; Liu, Y.; Dong, X.; Zhang, C.; Jiang, H.; Chen, X. Paroxetine combined with fluorouracil plays a therapeutic role in mouse models of colorectal cancer with depression through inhibiting IL-22 expression to regulate the MAPK signaling pathway. Exp. Ther. Med. 2020, 20, 240. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Suzuki, S.; Ishii, Y.; Okamura, M.; Matsubara, T.; Fujita, H.; Nozawa, N.; Kobayashi, S.; Hirata, K. Plasma prostaglandin D2 synthase levels in sleep and neurological diseases. J. Neurol. Sci. 2020, 411, 116692. [Google Scholar] [CrossRef] [PubMed]
- López, D.E.; Ballaz, S.J. The Role of Brain Cyclooxygenase-2 (Cox-2) Beyond Neuroinflammation: Neuronal Homeostasis in Memory and Anxiety. Mol. Neurobiol. 2020, 57, 5167–5176. [Google Scholar] [CrossRef]
- Cervellati, C.; Trentini, A.; Pecorelli, A.; Valacchi, G. Inflammation in Neurological Disorders: The Thin Boundary Between Brain and Periphery. Antioxidants Redox Signal. 2020, 33, 191–210. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, H.; Wang, C.; Broekman, B.F.P.; Chong, Y.-S.; Shek, L.P.; Gluckman, P.D.; Meaney, M.J.; Fortier, M.V.; Qiu, A. Inflammatory modulation of the associations between prenatal maternal depression and neonatal brain. Neuropsychopharmacology 2020, 46, 470–477. [Google Scholar] [CrossRef]
- Ferrucci, V.; Asadzadeh, F.; Collina, F.; Siciliano, R.; Boccia, A.; Marrone, L.; Spano, D.; Carotenuto, M.; Chiarolla, C.M.; De Martino, D.; et al. Prune-1 drives polarization of tumor-associated macrophages (TAMs) within the lung metastatic niche in triple-negative breast cancer. iScience 2020, 24, 101938. [Google Scholar] [CrossRef] [PubMed]
- Magee, J.C.; Grienberger, C. Synaptic Plasticity Forms and Functions. Annu. Rev. Neurosci. 2020, 43, 95–117. [Google Scholar] [CrossRef] [Green Version]
- Freedman, A.; Jacobsen, E. Follicular lymphoma: 2020 update on diagnosis and management. Am. J. Hematol. 2019, 95, 316–327. [Google Scholar] [CrossRef] [Green Version]
- Canizal-García, M.; Olmos-Orizaba, B.E.; Moreno-Jiménez, M.; Calderón-Cortés, E.; Saavedra-Molina, A.; Cortés-Rojo, C. Glutathione peroxidase 2 (Gpx2) preserves mitochondrial function and decreases ROS levels in chronologically aged yeast. Free Radic. Res. 2021, 55, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Ennerfelt, H.E.; Lukens, J.R. The role of innate immunity in Alzheimer’s disease. Immunol. Rev. 2020, 297, 225–246. [Google Scholar] [CrossRef] [PubMed]
- Daily, J.W.; Kang, S.; Park, S. Protection against Alzheimer’s disease by luteolin: Role of brain glucose regulation, anti-inflammatory activity, and the gut microbiota-liver-brain axis. Biofactors 2021, 47, 218–231. [Google Scholar] [CrossRef] [PubMed]
- Shahdadi, S.H.; Jebali, A.; Iman, M. Dual function of interleukin-23 Aptamer to suppress brain inflammation via attachment to macrophage stimulating 1 kinase and interleukin-23. Colloids Surf. B Biointerfaces 2020, 185, 110619. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.; Jo, H.; Go, C.; Jang, Y.; Chu, N.; Bae, S.; Kang, D.; Kim, Y.; Kang, J.S. The Roles of IL-22 and Its Receptor in the Regulation of Inflammatory Responses in the Brain. Int. J. Mol. Sci. 2022, 23, 757. https://doi.org/10.3390/ijms23020757
Lee D, Jo H, Go C, Jang Y, Chu N, Bae S, Kang D, Kim Y, Kang JS. The Roles of IL-22 and Its Receptor in the Regulation of Inflammatory Responses in the Brain. International Journal of Molecular Sciences. 2022; 23(2):757. https://doi.org/10.3390/ijms23020757
Chicago/Turabian StyleLee, Dahae, Hyejung Jo, Cheolhyeon Go, Yoojin Jang, Naghyung Chu, Suhyun Bae, Dongmin Kang, Yejin Kim, and Jae Seung Kang. 2022. "The Roles of IL-22 and Its Receptor in the Regulation of Inflammatory Responses in the Brain" International Journal of Molecular Sciences 23, no. 2: 757. https://doi.org/10.3390/ijms23020757
APA StyleLee, D., Jo, H., Go, C., Jang, Y., Chu, N., Bae, S., Kang, D., Kim, Y., & Kang, J. S. (2022). The Roles of IL-22 and Its Receptor in the Regulation of Inflammatory Responses in the Brain. International Journal of Molecular Sciences, 23(2), 757. https://doi.org/10.3390/ijms23020757






