Effects of Isorhamnetin on Diabetes and Its Associated Complications: A Review of In Vitro and In Vivo Studies and a Post Hoc Transcriptome Analysis of Involved Molecular Pathways
Abstract
1. Introduction
2. General Overview on Bioactive Molecules in Particular Polyphenols and Flavonoids
2.1. Oxidative Stress as an Origin of Bioactive Molecules in Plants
2.2. Classification of Natural Antioxidants
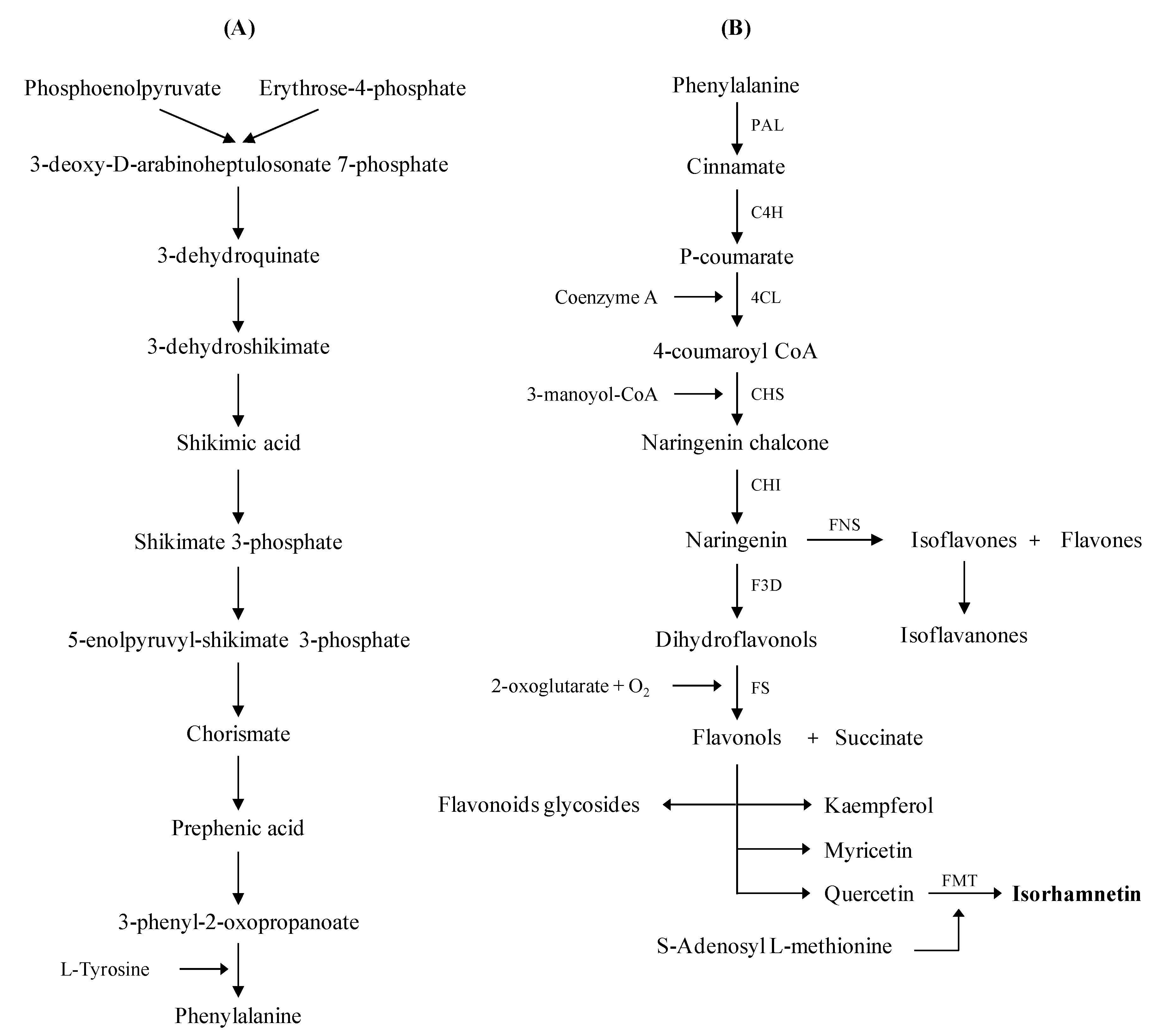
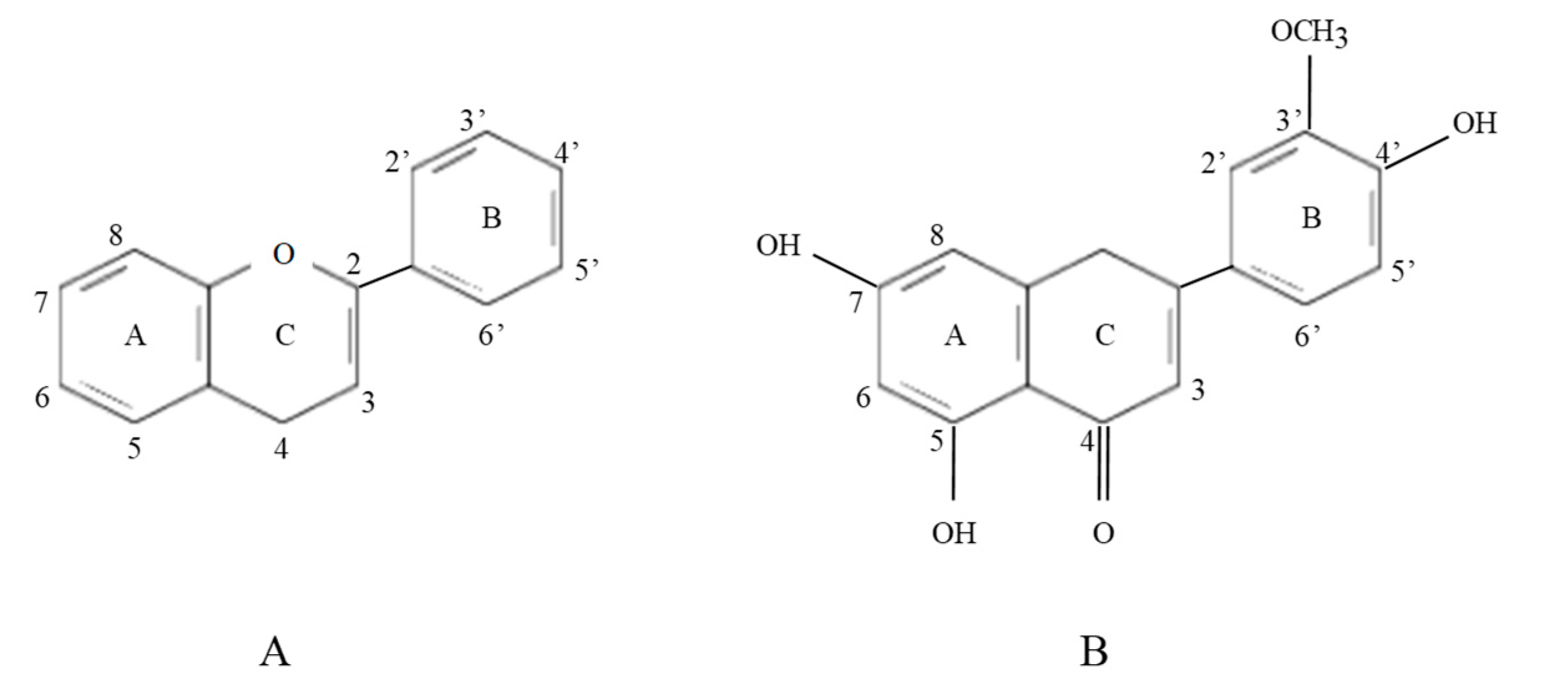
2.3. Origins and Biochemical Structure of Flavonoids, in Particular, Isorhamnetin
2.4. Isolation and Analyses of Isorhamnetin Originated from Medicinal Plants
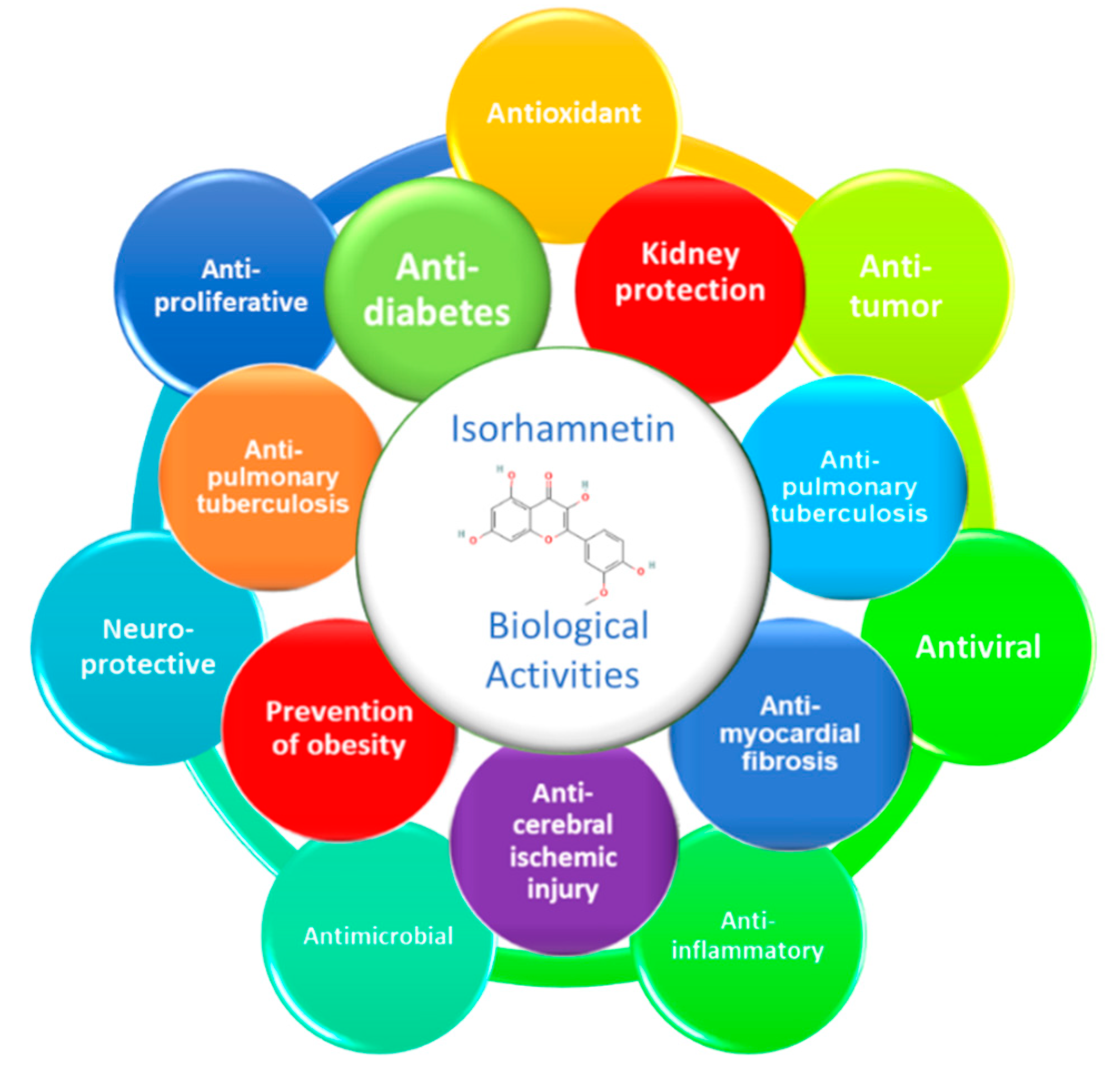
3. General Overview of Biological Activities of Isorhamnetin
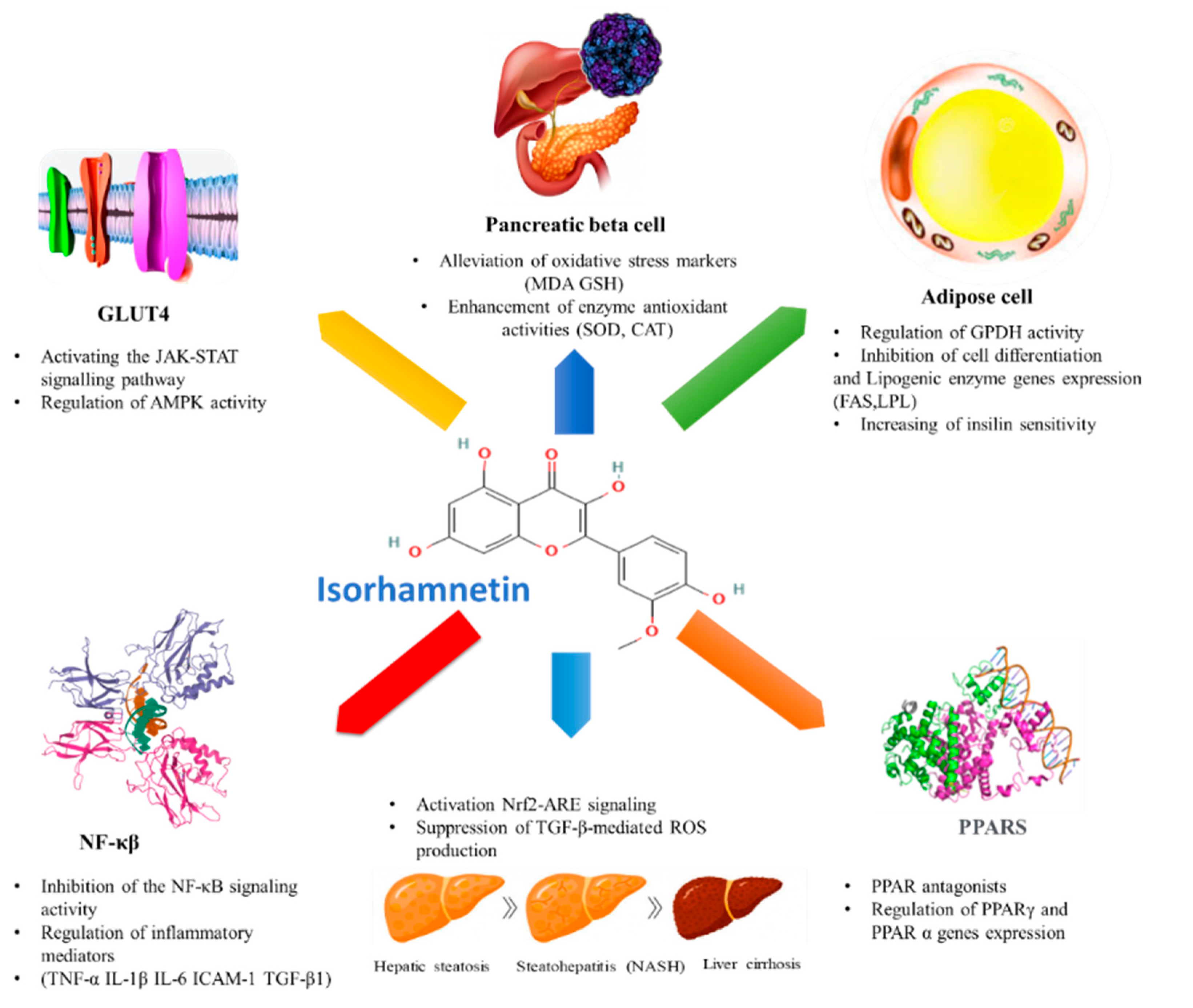
4. Anti-Diabetic Effect of Isorhamnetin
4.1. General Overview on Diabetes and Its Link with Metabolic Syndrome
4.2. Effect of Isorhamnetin on Associated Metabolic Pathways
4.2.1. Effect of Isorhamnetin on Glucose Transporters
4.2.2. Effect of Isorhamnetin on Peroxisome Proliferator-Activated Receptors (PPARs)
4.2.3. Effect of Isorhamnetin on Hepatic Enzymes
Non-Alcoholic Steatohepatitis
Hepatic Fibrosis
Hepatocellular Carcinoma
4.2.4. Effect of Isorhamnetin on Pancreatic β-Cell Dysfunction
4.2.5. Effect of Isorhamnetin on NF-κB
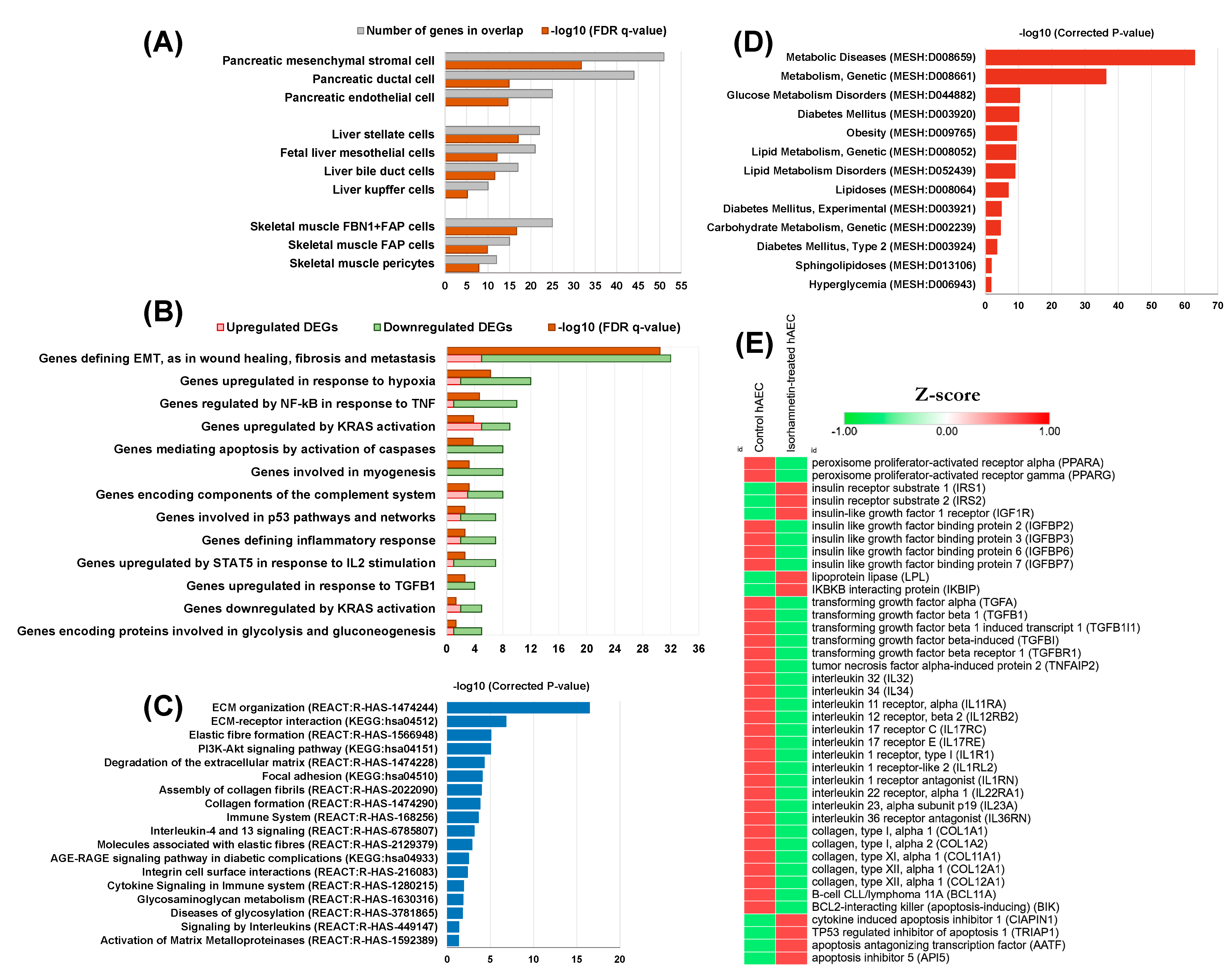
5. A Post Hoc Transcriptome Analysis Predicts the Potential Effect of the Isorhamnetin on Diabetes in a Stem Cell-Based Tool
5.1. Cell Type Signature Gene Sets
5.2. Significantly Enriched Hallmark Gene Sets
5.3. Significantly Enriched Pathways
5.4. Significantly Enriched Metabolic Diseases and Related Gene Expressions
6. Bioavailability and Intestinal Absorption of Isorhamnetin Aglycone and Its Glycosylated Derivatives
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Corpas, F.J.; Gupta, D.K.; Palma, J.M. Production sites of reactive oxygen species (ROS) in organelles from plant cells. In Reactive Oxygen Species and Oxidative Damage in Plants under Stress; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–22. [Google Scholar]
- Falleh, H.; Oueslati, S.; Guyot, S.; Dali, A.B.; Magné, C.; Abdelly, C.; Ksouri, R. LC/ESI-MS/MS characterisation of procyanidins and propelargonidins responsible for the strong antioxidant activity of the edible halophyte Mesembryanthemum edule L. Food Chem. 2011, 127, 1732–1738. [Google Scholar] [CrossRef]
- Trabelsi, N.; Oueslati, S.; Falleh, H.; Waffo-Téguo, P.; Papastamoulis, Y.; Mérillon, J.-M.; Abdelly, C.; Ksouri, R. Isolation of powerful antioxidants from the medicinal halophyte Limoniastrum guyonianum. Food Chem. 2012, 135, 1419–1424. [Google Scholar] [CrossRef] [PubMed]
- Boulaaba, M.; Mkadmini, K.; Tsolmon, S.; Han, J.; Smaoui, A.; Kawada, K.; Ksouri, R.; Isoda, H.; Abdelly, C. In vitro antiproliferative effect of Arthrocnemum indicum extracts on Caco-2 cancer cells through cell cycle control and related phenol LC-TOF-MS identification. Evid.-Based Complement. Altern. Med. 2013, 2013, 529375. [Google Scholar] [CrossRef]
- Karker, M.; De Tommasi, N.; Smaoui, A.; Abdelly, C.; Ksouri, R.; Braca, A. New sulphated flavonoids from Tamarix africana and biological activities of its polar extract. Planta Med. 2016, 82, 1374–1380. [Google Scholar] [CrossRef]
- Bourgou, S.; Rebey, I.B.; Mkadmini, K.; Isoda, H.; Ksouri, R.; Ksouri, W.M. LC-ESI-TOF-MS and GC-MS profiling of Artemisia herba-alba and evaluation of its bioactive properties. Food Res. Int. 2017, 99, 702–712. [Google Scholar] [CrossRef]
- Boulaaba, M.; Medini, F.; Hajlaoui, H.; Mkadmini, K.; Falleh, H.; Ksouri, R.; Isoda, H.; Smaoui, A.; Abdelly, C. Biological activities and phytochemical analysis of phenolic extracts from Salsola kali L. Role of endogenous factors in the selection of the best plant extracts. S. Afr. J. Bot. 2019, 123, 193–199. [Google Scholar] [CrossRef]
- Najjaa, H.; Abdelkarim, B.A.; Doria, E.; Boubakri, A.; Trabelsi, N.; Falleh, H.; Tlili, H.; Neffati, M. Phenolic composition of some Tunisian medicinal plants associated with anti-proliferative effect on human breast cancer MCF-7 cells. EuroBiotechnol. J. 2020, 4, 104–112. [Google Scholar] [CrossRef]
- Bourgou, S.; Bettaieb Rebey, I.; Ben Kaab, S.; Hammami, M.; Dakhlaoui, S.; Sawsen, S.; Msaada, K.; Isoda, H.; Ksouri, R.; Fauconnier, M.-L. Green Solvent to Substitute Hexane for Bioactive Lipids Extraction from Black Cumin and Basil Seeds. Foods 2021, 10, 1493. [Google Scholar] [CrossRef]
- Oueslati, S.; Ksouri, R.; Falleh, H.; Pichette, A.; Abdelly, C.; Legault, J. Phenolic content, antioxidant, anti-inflammatory and anticancer activities of the edible halophyte Suaeda fruticosa Forssk. Food Chem. 2012, 132, 943–947. [Google Scholar] [CrossRef]
- Boulaaba, M.; Tsolmon, S.; Ksouri, R.; Han, J.; Kawada, K.; Smaoui, A.; Abdelly, C.; Isoda, H. Anticancer effect of Tamarix gallica extracts on human colon cancer cells involves Erk1/2 and p38 action on G 2/M cell cycle arrest. Cytotechnology 2013, 65, 927–936. [Google Scholar] [CrossRef] [PubMed]
- Boulaaba, M.; Zar Kalai, F.; Dakhlaoui, S.; Ezzine, Y.; Selmi, S.; Bourgou, S.; Smaoui, A.; Isoda, H.; Ksouri, R. Antioxidant, antiproliferative and anti-inflammatory effects of Glaucium flavum fractions enriched in phenolic compounds. Med. Chem. Res. 2019, 28, 1995–2001. [Google Scholar] [CrossRef]
- Medini, F.; Fellah, H.; Ksouri, R.; Abdelly, C. Total phenolic, flavonoid and tannin contents and antioxidant and antimicrobial activities of organic extracts of shoots of the plant Limonium delicatulum. J. Taibah Univ. Sci. 2014, 8, 216–224. [Google Scholar] [CrossRef]
- Bouraoui, N.; Oueslati, S.; Falleh, H.; Harbaoui, F.; Ksouri, R.; Legault, J.; Lachaâl, M. Antioxidant, antimicrobial, anti-inflammatory and anticancer activities of Carthamus tinctorius flowers. Planta Med. 2011, 77, PM136. [Google Scholar] [CrossRef]
- Boulaaba, M.; Snoussi, M.; Saada, M.; Mkadmini, K.; Smaoui, A.; Abdelly, C.; Ksouri, R. Antimicrobial activities and phytochemical analysis of Tamarix gallica extracts. Ind. Crops Prod. 2015, 76, 1114–1122. [Google Scholar] [CrossRef]
- Kalai, F.Z.; Han, J.; Ksouri, R.; Abdelly, C.; Isoda, H. Oral administration of Nitraria retusa ethanolic extract enhances hepatic lipid metabolism in db/db mice model ‘BKS. Cg-Dock7m+/+ Leprdb/J’through the modulation of lipogenesis–lipolysis balance. Food Chem. Toxicol. 2014, 72, 247–256. [Google Scholar] [CrossRef]
- Zar Kalai, F.; Han, J.; Ksouri, R.; El Omri, A.; Abdelly, C.; Isoda, H. Antiobesity effects of an edible halophyte Nitraria retusa Forssk in 3T3-L1 preadipocyte differentiation and in C57B6J/L mice fed a high fat diet-induced obesity. Evid.-Based Complement. Altern. Med. 2013, 2013, 368658. [Google Scholar] [CrossRef]
- Ganbold, M.; Owada, Y.; Ozawa, Y.; Shimamoto, Y.; Ferdousi, F.; Tominaga, K.; Zheng, Y.-W.; Ohkohchi, N.; Isoda, H. Isorhamnetin alleviates steatosis and fibrosis in mice with nonalcoholic steatohepatitis. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Moraes, M.L.L.; da Silva, H.D.T.; Blanes, L.; Doble, P.; Tavares, M.F.M. Optimization of chemometric approaches for the extraction of isorhamnetin-3-O-rutinoside from Calendula officinalis L. J. Pharm. Biomed. Anal. 2016, 125, 408–414. [Google Scholar] [CrossRef]
- Seo, K.; Yang, J.H.; Kim, S.C.; Ku, S.K.; Ki, S.H.; Shin, S.M. The antioxidant effects of isorhamnetin contribute to inhibit COX-2 expression in response to inflammation: A potential role of HO-1. Inflammation 2014, 37, 712–722. [Google Scholar] [CrossRef]
- Li, C.; Yang, X.; Hu, J.-B.; Liao, J.-Z. Isorhamnetin suppresses the growth of gefitinib resistant human lung cancer PC9 cells. Her. Med. 2012, 31, 831–834. [Google Scholar]
- Chauhan, A.K.; Kim, J.; Lee, Y.; Balasubramanian, P.K.; Kim, Y. Isorhamnetin Has Potential for the Treatment of Escherichia coli-Induced Sepsis. Molecules 2019, 24, 3984. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Ta, W.; Tang, W.; Hua, R.; Wang, J.; Wang, C.; Lu, W. Potential antiviral activity of isorhamnetin against SARS-CoV-2 spike pseudotyped virus in vitro. Drug Dev. Res. 2021, 82, 1124–1130. [Google Scholar] [CrossRef] [PubMed]
- Gong, G.; Guan, Y.-Y.; Zhang, Z.-L.; Rahman, K.; Wang, S.-J.; Zhou, S.; Luan, X.; Zhang, H. Isorhamnetin: A review of pharmacological effects. Biomed. Pharmacother. 2020, 128, 110301. [Google Scholar] [CrossRef] [PubMed]
- Antunes-Ricardo, M.; Gutiérrez-Uribe, J.A.; Martínez-Vitela, C.; Serna-Saldívar, S.O. Topical anti-inflammatory effects of isorhamnetin glycosides isolated from Opuntia ficus-indica. BioMed Res. Int. 2015, 2015, 847320. [Google Scholar] [CrossRef]
- Farias-Pereira, R.; Savarese, J.; Yue, Y.; Lee, S.-H.; Park, Y. Fat-lowering effects of isorhamnetin are via NHR-49-dependent pathway in Caenorhabditis elegans. Curr. Res. Food Sci. 2020, 2, 70–76. [Google Scholar] [CrossRef]
- Kim, B.; Choi, Y.E.; Kim, H.S. Eruca sativa and its flavonoid components, quercetin and isorhamnetin, improve skin barrier function by activation of peroxisome proliferator-activated receptor (PPAR)-α and suppression of inflammatory cytokines. Phytother. Res. 2014, 28, 1359–1366. [Google Scholar] [CrossRef]
- Lee, J.; Jung, E.; Lee, J.; Kim, S.; Huh, S.; Kim, Y.; Kim, Y.; Byun, S.Y.; Kim, Y.S.; Park, D. Isorhamnetin represses adipogenesis in 3T3-L1 cells. Obesity 2009, 17, 226–232. [Google Scholar] [CrossRef]
- Lee, J.; Lee, J.; Jung, E.; Hwang, W.; Kim, Y.-S.; Park, D. Isorhamnetin-induced anti-adipogenesis is mediated by stabilization of β-catenin protein. Life Sci. 2010, 86, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Sun, G.; Zhang, Y.; Li, X.; Wang, R. Involvement of the NF-κB signaling pathway in the renoprotective effects of isorhamnetin in a type 2 diabetic rat model. Biomed. Rep. 2016, 4, 628–634. [Google Scholar] [CrossRef]
- Yang, B.; Li, X.-P.; Ni, Y.-F.; Du, H.-Y.; Wang, R.; Li, M.-J.; Wang, W.-C.; Li, M.-M.; Wang, X.-H.; Li, L. Protective effect of isorhamnetin on lipopolysaccharide-induced acute lung injury in mice. Inflammation 2016, 39, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gu, M.; Cai, W.; Yu, L.; Feng, L.; Zhang, L.; Zang, Q.; Wang, Y.; Wang, D.; Chen, H. Dietary component isorhamnetin is a PPARγ antagonist and ameliorates metabolic disorders induced by diet or leptin deficiency. Sci. Rep. 2016, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, D.; Singh, S.; Kumar, V.; Romero, R.; Prasad, R.; Singh, J. Antioxidant enzymes regulation in plants in reference to reactive oxygen species (ROS) and reactive nitrogen species (RNS). Plant Gene 2019, 19, 100182. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, A.; Kumar, A.; Kaur, N. ROS and oxidative burst: Roots in plant development. Plant Divers. 2020, 42, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.-T.; Wan, Z.-Y.; Li, S.; Zhang, Y. Spatiotemporal production of reactive oxygen species by NADPH oxidase is critical for tapetal programmed cell death and pollen development in Arabidopsis. Plant Cell 2014, 26, 2007–2023. [Google Scholar] [CrossRef]
- Jallali, I.; Megdiche, W.; M’Hamdi, B.; Oueslati, S.; Smaoui, A.; Abdelly, C.; Ksouri, R. Changes in phenolic composition and antioxidant activities of the edible halophyte Crithmum maritimum L. with physiological stage and extraction method. Acta Physiol. Plant 2012, 34, 1451–1459. [Google Scholar] [CrossRef]
- Medini, F.; Ksouri, R.; Falleh, H.; Megdiche, W.; Trabelsi, N.; Abdelly, C. Effects of physiological stage and solvent on polyphenol composition, antioxidant and antimicrobial activities of Limonium densiflorum. J. Med. Plants Res. 2011, 5, 6719–6730. [Google Scholar]
- Fico, G.; Bilia, A.R.; Morelli, I.; Tome, F. Flavonoid distribution in Pyracantha coccinea plants at different growth phases. Biochem. Syst. Ecol. 2000, 6, 673–678. [Google Scholar] [CrossRef]
- Feten, Z.K.; Mondher, B.; Najla, T.; Pierre, W.T.; Michel, M.J.; Abderrazak, S.; Chedly, A.; Riadh, K. Phenolic content and biological activities of Limonium densiflorum crude extract and its methanolic fraction: Influence of genotype and fractionation. Int. J. Med. Plants 2013, 105, 16. [Google Scholar]
- Akyol, T.Y.; Yilmaz, O.; Uzilday, B.; Uzilday, R.Ö.; Türkan, İ. Plant response to salinity: An analysis of ROS formation, signaling, and antioxidant defense. Tuk. J. Bot. 2020, 44, 13. [Google Scholar]
- Li, L.; Yi, H. Effect of sulfur dioxide on ROS production, gene expression and antioxidant enzyme activity in Arabidopsis plants. Plant Physiol. Biochem. 2012, 58, 46–53. [Google Scholar] [CrossRef]
- Shang, S.; Tang, Y.; Dai, J.; Wu, C.; Yan, Y.; Tie, W.; Li, M.; Yang, J.; Zeng, J.; Chen, M.; et al. Genomic Analysis of the Principal Members of Antioxidant Enzymes in Simulated Stresses Response and Postharvest Physiological Deterioration in Cassava. Trop. Plant Biol. 2021, 14, 419–428. [Google Scholar] [CrossRef]
- Karou, D.; Dicko, M.H.; Simpore, J.; Traore, A.S. Antioxidant and antibacterial activities of polyphenols from ethnomedicinal plants of Burkina Faso. Afr. J. Biotechnol. 2005, 4, 823–828. [Google Scholar]
- Cheynier, V.; Comte, G.; Davies, K.M.; Lattanzio, V.; Martens, S.J. Plant phenolics: Recent advances on their biosynthesis, genetics, and ecophysiology. Plant Physiol. Biochem. 2013, 72, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Ghedira, K. Les flavonoïdes: Structure, propriétés biologiques, rôle prophylactique et emplois en thérapeutique. Phytotherapy 2005, 3, 162–169. [Google Scholar] [CrossRef]
- Tohge, T.; Watanabe, M.; Hoefgen, R.; Fernie, A.R. Shikimate and Phenylalanine Biosynthesis in the Green Lineage. Front. Plant Sci. 2013, 4, 62. [Google Scholar] [CrossRef] [PubMed]
- Niessen, W.M.A. Liquid Chromatography Mass Spectrometry, 3rd ed.; Cazes, J., Ed.; Taylor & Francis Group: Abingdon, UK, 2006; Volume 97. [Google Scholar]
- Kougan, G.B.; Tabopda, T.; Kuete, V.; Verpoorte, R. Simple Phenols, Phenolic Acids, and Related Esters from the Medicinal Plants of Africa. In Medicinal Plant Research in Africa; Elsevier: Amsterdam, The Netherlands, 2013; pp. 225–249. [Google Scholar]
- Kim, B.-G.; Sung, S.H.; Jung, N.R.; Chong, Y.; Ahn, J.-H. Biological synthesis of isorhamnetin 3-O-glucoside using engineered glucosyltransferase. J. Mol. Catal. B Enzym. 2010, 63, 194–199. [Google Scholar] [CrossRef]
- Bannour, M.; Fellah, B.; Rocchetti, G.; Ashi-Smiti, S.; Lachenmeier, D.W.; Lucini, L.; Khadhri, A. Phenolic profiling and antioxidant capacity of Calligonum azel Maire, a Tunisian desert plant. Food Res. Int. 2017, 101, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Rokbeni, N.; M’rabet, Y.; Cluzet, S.; Richard, T.; Krisa, S.; Boussaid, M.; Boulila, A. Determination of phenolic composition and antioxidant activities of Pancratium maritimum L. from Tunisia. Ind. Crops Prod. 2016, 94, 505–513. [Google Scholar] [CrossRef]
- Bettaib, J.; Talarmin, H.; Kalai, F.Z.; Giroux-Metges, M.-A.; Ksouri, R. Limoniastrum guyonianum prevents H2O2-induced oxidative damage in IEC-6 cells by enhancing enzyamtic defense, reducing glutathione depletion and JNK phosphorylation. Biomed. Pharmacother. 2017, 95, 1404–1411. [Google Scholar] [CrossRef]
- Serreli, G.; Deiana, M. Biological relevance of extra virgin olive oil polyphenols metabolites. Antioxidants 2018, 7, 170. [Google Scholar] [CrossRef]
- Dehaghani, Z.A.; Asghari, G.; Dinani, M.S. Isolation and Identification of Nicotiflorin and Narcissin from the Aerial Parts of Peucedanum aucheri Boiss. J. Agric. Sci. Technol. A 2017, 7, 45–51. [Google Scholar]
- Santos, S.A.O.; Villaverde, J.J.; Silva, C.M.; Neto, C.P.; Silvestre, A.J.D. Supercritical fluid extraction of phenolic compounds from Eucalyptus globulus Labill bark. J. Supercrit. Fluids 2012, 71, 71–79. [Google Scholar] [CrossRef]
- Schieber, A.; Keller, P.; Streker, P.; Klaiber, I.; Carle, R. Detection of isorhamnetin glycosides in extracts of apples (Malus domestica cv. Brettacher) by HPLC-PDA and HPLC-APCI-MS/MS. Phytochem. Anal. 2002, 13, 87–94. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative stress in cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef]
- Wei, W.; Liu, Q.; Tan, Y.; Liu, L.; Li, X.; Cai, L. Oxidative stress, diabetes, and diabetic complications. Hemoglobin 2009, 33, 370–377. [Google Scholar] [CrossRef]
- Madamanchi, N.R.; Vendrov, A.; Runge, M.S. Oxidative stress and vascular disease. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 29–38. [Google Scholar] [CrossRef]
- Barnham, K.J.; Masters, C.L.; Bush, A.I. Neurodegenerative diseases and oxidative stress. Nat. Rev. Drug Discov. 2004, 3, 205–214. [Google Scholar] [CrossRef]
- De Souza Farias, S.A.N.; da Costa, K.S.; Martins, J.o.B. Analysis of Conformational, Structural, Magnetic, and Electronic Properties Related to Antioxidant Activity: Revisiting Flavan, Anthocyanidin, Flavanone, Flavonol, Isoflavone, Flavone, and Flavan-3-ol. ACS Omega 2021, 6, 8908–8918. [Google Scholar] [CrossRef] [PubMed]
- Ronsisvalle, S.; Panarello, F.; Longhitano, G.; Siciliano, E.A.; Montenegro, L.; Panico, A. Natural flavones and flavonols: Relationships among antioxidant activity, glycation, and metalloproteinase inhibition. Cosmetics 2020, 7, 71. [Google Scholar] [CrossRef]
- Seo, S.; Seo, K.; Ki, S.H.; Shin, S.M. Isorhamnetin inhibits reactive oxygen species-dependent hypoxia inducible factor (HIF)-1α accumulation. Biol. Pharm. Bull. 2016, 39, 1830–1838. [Google Scholar] [CrossRef] [PubMed]
- Pengfei, L.; Tiansheng, D.; Xianglin, H.; Jianguo, W. Antioxidant properties of isolated isorhamnetin from the sea buckthorn marc. Plant Foods Hum. Nutr. 2009, 64, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Zuo, A.; Yanying, Y.; Li, J.; Binbin, X.; Xiongying, Y.; Yan, Q.; Shuwen, C. Study on the relation of structure and antioxidant activity of isorhamnetin, quercetin, phloretin, silybin and phloretin isonicotinyl hydrazone. Free Radic. Antioxid. 2011, 1, 39–47. [Google Scholar] [CrossRef]
- Wu, Q.; Kroon, P.A.; Shao, H.; Needs, P.W.; Yang, X. Differential effects of quercetin and two of its derivatives, isorhamnetin and isorhamnetin-3-glucuronide, in inhibiting the proliferation of human breast-cancer MCF-7 cells. J. Agric. Food Chem. 2018, 66, 7181–7189. [Google Scholar] [CrossRef]
- Wei, J.; Su, H.; Bi, Y.; Li, J.; Feng, L.; Sheng, W. Anti-proliferative effect of isorhamnetin on HeLa cells through inducing G2/M cell cycle arrest. Exp. Ther. Med. 2018, 15, 3917–3923. [Google Scholar] [CrossRef]
- Aonuma, K.; Ferdousi, F.; Xu, D.; Tominaga, K.; Isoda, H. Effects of isorhamnetin in human amniotic epithelial stem cells in vitro and its cardioprotective effects in vivo. Front. Cell Dev. Biol. 2020, 8, 578197. [Google Scholar] [CrossRef]
- Zhao, J.-J.; Song, J.-Q.; Pan, S.-Y.; Wang, K. Treatment with isorhamnetin protects the brain against ischemic injury in mice. Neurochem. Res. 2016, 41, 1939–1948. [Google Scholar] [CrossRef]
- Ishola, I.O.; Osele, M.O.; Chijioke, M.C.; Adeyemi, O.O. Isorhamnetin enhanced cortico-hippocampal learning and memory capability in mice with scopolamine-induced amnesia: Role of antioxidant defense, cholinergic and BDNF signaling. Brain Res. 2019, 1712, 188–196. [Google Scholar] [CrossRef]
- Yang, J.H.; Kim, S.C.; Kim, K.M.; Jang, C.H.; Cho, S.S.; Kim, S.J.; Ku, S.K.; Cho, I.J.; Ki, S.H. Isorhamnetin attenuates liver fibrosis by inhibiting TGF-β/Smad signaling and relieving oxidative stress. Eur. J. Pharmacol. 2016, 783, 92–102. [Google Scholar] [CrossRef]
- Dou, W.; Zhang, J.; Li, H.; Kortagere, S.; Sun, K.; Ding, L.; Ren, G.; Wang, Z.; Mani, S. Plant flavonol isorhamnetin attenuates chemically induced inflammatory bowel disease via a PXR-dependent pathway. J. Nutr. Biochem. 2014, 25, 923–933. [Google Scholar] [CrossRef]
- Jnawali, H.N.; Jeon, D.; Jeong, M.-C.; Lee, E.; Jin, B.; Ryoo, S.; Yoo, J.; Jung, I.D.; Lee, S.J.; Park, Y.-M. Antituberculosis activity of a naturally occurring flavonoid, isorhamnetin. J. Nat. Prod. 2016, 79, 961–969. [Google Scholar] [CrossRef]
- Ibarra, M.; Pérez-Vizcaíno, F.; Cogolludo, A.; Duarte, J.; Zaragozá-Arnáez, F.; López-López, J.G.; Tamargo, J. Cardiovascular effects of isorhamnetin and quercetin in isolated rat and porcine vascular smooth muscle and isolated rat atria. Planta Med. 2002, 68, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Chang, Z.; Wang, J.l.; Jing, Z.c.; Ma, P.; Xu, Q.b.; Na, J.r.; Tian, J.; Ma, X.; Zhou, W.; Zhou, R. Protective effects of isorhamnetin on pulmonary arterial hypertension: In vivo and in vitro studies. Phytother. Res. 2020, 34, 2730–2744. [Google Scholar] [CrossRef]
- Yang, L.; Chen, Q.; Wang, F.; Zhang, G. Antiosteoporotic compounds from seeds of Cuscuta chinensis. J. Ethnopharmacol. 2011, 135, 553–560. [Google Scholar] [CrossRef]
- Rodríguez, L.; Badimon, L.; Méndez, D.; Padró, T.; Vilahur, G.; Peña, E.; Carrasco, B.; Vogel, H.; Palomo, I.; Fuentes, E. Antiplatelet Activity of Isorhamnetin via Mitochondrial Regulation. Antioxidants 2021, 10, 666. [Google Scholar] [CrossRef]
- Dong, G.-Z.; Lee, J.-H.; Ki, S.H.; Yang, J.H.; Cho, I.J.; Kang, S.H.; Zhao, R.J.; Kim, S.C.; Kim, Y.W. AMPK activation by isorhamnetin protects hepatocytes against oxidative stress and mitochondrial dysfunction. Eur. J. Pharmacol. 2014, 740, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Lei, J.; Hong, E.-K.; Lu, D.; Yuan, W.; Yang, Z.; Ming, C. Anti-hypoxia effects of the ethanol extract of Oxytropis ochrocephala. Legume Res.-Int. J. 2016, 39, 914–920. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Q.; Cheng, M.L.; Ma, L.; Meng, Q.Z.; Duan, L.; Chen, Y.; Tan, J.W.; Chen, M.; Liang, T.T. Effect of the Miaoyao Fanggan sachet-derived isorhamnetin on TLR2/4 and NKp46 expression in mice. J. Ethnopharmacol. 2012, 144, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Rigano, D.; Formisano, C.; Basile, A.; Lavitola, A.; Senatore, F.; Rosselli, S.; Bruno, M. Antibacterial activity of flavonoids and phenylpropanoids from Marrubium globosum ssp. libanoticum. Phytother. Res. 2007, 21, 395–397. [Google Scholar] [CrossRef]
- Ren, X.; Bao, Y.; Zhu, Y.; Liu, S.; Peng, Z.; Zhang, Y.; Zhou, G. Isorhamnetin, hispidulin, and cirsimaritin identified in Tamarix ramosissima barks from southern Xinjiang and their antioxidant and antimicrobial activities. Molecules 2019, 24, 390. [Google Scholar] [CrossRef]
- Bhattacharya, D.; Ghosh, D.; Bhattacharya, S.; Sarkar, S.; Karmakar, P.; Koley, H.; Gachhui, R. Antibacterial activity of polyphenolic fraction of Kombucha against Vibrio cholerae: Targeting cell membrane. Lett. Appl. Microbiol. 2018, 66, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Dayem, A.A.; Choi, H.Y.; Kim, Y.B.; Cho, S.-G. Antiviral effect of methylated flavonol isorhamnetin against influenza. PLoS ONE 2015, 10, e0121610. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-K.; Kang, B.-J.; Park, K.-J.; Ko, B.-S.; Whang, W.-K. Anti-Herpes Simplex Virus type I (HSV-1) Effect of Isorhamnetin 3-0-beta-D-Glucopyranoside Isolated from Brassica rapa. Yakhak Hoeji 1998, 42, 607–612. [Google Scholar]
- Matboli, M.; Saad, M.; Hasanin, A.H.; Saleh, L.A.; Baher, W.; Bekhet, M.M.; Eissa, S. New insight into the role of isorhamnetin as a regulator of insulin signaling pathway in type 2 diabetes mellitus rat model: Molecular and computational approach. Biomed. Pharmacother. 2021, 135, 111176. [Google Scholar] [CrossRef] [PubMed]
- Kylin, E. Studien ueber das Hypertonie-Hyperglyka “mie-Hyperurika” miesyndrom. Zent. Inn. Med. 1923, 44, 105–127. [Google Scholar]
- Reaven, G.M. Role of insulin resistance in human disease. Diabetes 1988, 37, 1595–1607. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.Y.; Wong, J.L.M.; Sim, Y.J.; Wong, S.S.; Elhassan, S.A.M.; Tan, S.H.; Lim, G.P.L.; Tay, N.W.R.; Annan, N.C.; Bhattamisra, S.K. Type 1 and 2 diabetes mellitus: A review on current treatment approach and gene therapy as potential intervention. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 364–372. [Google Scholar] [CrossRef]
- Bruce, K.D.; Hanson, M.A. The developmental origins, mechanisms, and implications of metabolic syndrome. J. Nutr. Biochem. 2010, 140, 648–652. [Google Scholar] [CrossRef]
- Oboh, G.; Ademiluyi, A.O.; Akinyemi, A.J.; Henle, T.; Saliu, J.A.; Schwarzenbolz, U. Inhibitory effect of polyphenol-rich extracts of jute leaf (Corchorus olitorius) on key enzyme linked to type 2 diabetes (α-amylase and α-glucosidase) and hypertension (angiotensin I converting) in vitro. J. Funct. Foods 2012, 4, 450–458. [Google Scholar] [CrossRef]
- Jiang, H.; Yamashita, Y.; Nakamura, A.; Croft, K.; Ashida, H. Quercetin and its metabolite isorhamnetin promote glucose uptake through different signalling pathways in myotubes. Sci. Rep. 2019, 9, 2690. [Google Scholar] [CrossRef]
- Lee, D.; Park, J.Y.; Lee, S.; Kang, K.S. In Vitro Studies to Assess the α-Glucosidase Inhibitory Activity and Insulin Secretion Effect of Isorhamnetin 3-O-Glucoside and Quercetin 3-O-Glucoside Isolated from Salicornia herbacea. Processes 2021, 9, 483. [Google Scholar] [CrossRef]
- Manach, C.; Texier, O.; Régérat, F.; Agullo, G.; Demigné, C.; Rémésy, C. Dietary quercetin is recovered in rat plasma as conjugated derivatives of isorhamnetin and quercetin. J. Nutr. Biochem. 1996, 7, 375–380. [Google Scholar] [CrossRef]
- Eid, H.M.; Martineau, L.C.; Saleem, A.; Muhammad, A.; Vallerand, D.; Benhaddou-Andaloussi, A.; Nistor, L.; Afshar, A.; Arnason, J.T.; Haddad, P.S. Stimulation of AMP-activated protein kinase and enhancement of basal glucose uptake in muscle cells by quercetin and quercetin glycosides, active principles of the antidiabetic medicinal plant Vaccinium vitis-idaea. Mol. Nutr. Food Res. 2010, 54, 991–1003. [Google Scholar] [CrossRef]
- Lee, M.-K.; Yang, H.-K.; Ha, N.-R.; Sung, S.-H.; Kim, Y.-C. Isorhamnetin from Oenanthe javanica attenuates fibrosis in rat hepatic stellate cells via inhibition of ERK signaling pathway. Nat. Prod. Sci. 2008, 14, 81–85. [Google Scholar]
- Heim, M. The Jak–STAT pathway: Specific signal transduction from the cell membrane to the nucleus. Eur. J. Clin. Investig. 1996, 26, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, J. Identification of new antidiabetic agents targeting GLUT4 protein using in silico analysis. Int. J. Green Pharm. 2019, 12, S876. [Google Scholar] [CrossRef]
- Eid, H.M.; Nachar, A.; Thong, F.; Sweeney, G.; Haddad, P.S. The molecular basis of the antidiabetic action of quercetin in cultured skeletal muscle cells and hepatocytes. Pharmacogn. Mag. 2015, 11, 74. [Google Scholar] [PubMed]
- Xu, M.; Hu, J.; Zhao, W.; Gao, X.; Jiang, C.; Liu, K.; Liu, B.; Huang, F. Quercetin differently regulates insulin-mediated glucose transporter 4 translocation under basal and inflammatory conditions in adipocytes. Mol. Nutr. Food Res. 2014, 58, 931–941. [Google Scholar] [CrossRef]
- Calkin, A.C.; Thomas, M.C. PPAR agonists and cardiovascular disease in diabetes. PPAR Res. 2008, 2008, 245410. [Google Scholar] [CrossRef]
- Holm, L.J.; Mønsted, M.Ø.; Haupt-Jorgensen, M.; Buschard, K. PPARs and the development of type 1 diabetes. PPAR Res. 2020, 2020, 6198628. [Google Scholar] [CrossRef]
- Lamichane, S.; Dahal Lamichane, B.; Kwon, S.-M. Pivotal roles of peroxisome proliferator-activated receptors (PPARs) and their signal cascade for cellular and whole-body energy homeostasis. Int. J. Mol. Sci. 2018, 19, 949. [Google Scholar] [CrossRef]
- Varga, T.; Czimmerer, Z.; Nagy, L. PPARs are a unique set of fatty acid regulated transcription factors controlling both lipid metabolism and inflammation. Biochim. Biophys. Acta -Mol. Basis Dis. 2011, 1812, 1007–1022. [Google Scholar] [CrossRef] [PubMed]
- Krishnapuram, R.; Dhurandhar, E.J.; Dubuisson, O.; Hegde, V.; Dhurandhar, N.V. Doxycycline-regulated 3T3-L1 preadipocyte cell line with inducible, stable expression of adenoviral E4orf1 gene: A cell model to study insulin-independent glucose disposal. PLoS ONE 2013, 8, e60651. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Ojeda, F.J.; Rupérez, A.I.; Gomez-Llorente, C.; Gil, A.; Aguilera, C.M. Cell models and their application for studying adipogenic differentiation in relation to obesity: A review. Int. J. Mol. Sci. 2016, 17, 1040. [Google Scholar] [CrossRef] [PubMed]
- Sherwin, R.S. Role of liver in glucose homeostasis. Diabetes Care 1980, 3, 261–265. [Google Scholar] [CrossRef]
- Roden, M. Mechanisms of disease: Hepatic steatosis in type 2 diabetes—Pathogenesis and clinical relevance. Nat. Clin. Pract. Endocrinol. Metab. 2006, 2, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Koyama, Y.; Brenner, D.A. Liver inflammation and fibrosis. J. Clin. Investig. 2017, 127, 55–64. [Google Scholar] [CrossRef]
- Marks, J.B.; Raskin, P. Cardiovascular risk in diabetes: A brief review. J. Diabetes Its Complicat. 2000, 14, 108–115. [Google Scholar] [CrossRef]
- Marra, F.; Gastaldelli, A.; Baroni, G.S.; Tell, G.; Tiribelli, C. Molecular basis and mechanisms of progression of non-alcoholic steatohepatitis. Trends Mol. Med. 2008, 14, 72–81. [Google Scholar] [CrossRef]
- Ahmad, A.; Ahmad, R. Understanding the mechanism of hepatic fibrosis and potential therapeutic approaches. Saudi J. Gastroenterol. 2012, 18, 155. [Google Scholar] [CrossRef]
- Sánchez-Valle, V.; Chavez-Tapia, N.C.; Uribe, M.; Méndez-Sánchez, N. Role of oxidative stress and molecular changes in liver fibrosis: A review. Curr. Med. Chem. 2012, 19, 4850–4860. [Google Scholar] [CrossRef]
- Shek, F.W.; Benyon, R.C. How can transforming growth factor beta be targeted usefully to combat liver fibrosis? Eur. J. Gastroenterol. Hepatol. 2004, 16, 123–126. [Google Scholar] [CrossRef]
- Liu, X.; Hu, H.; Yin, J.Q. Therapeutic strategies against TGF-β signaling pathway in hepatic fibrosis. Liver Int. 2006, 26, 8–22. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Feng, J.; Lu, X.; Yao, Z.; Liu, Q.; Lv, Y.; Han, Y.; Deng, J.; Zhou, Y. Isorhamnetin inhibits liver fibrosis by reducing autophagy and inhibiting extracellular matrix formation via the TGF-β1/Smad3 and TGF-β1/p38 MAPK pathways. Mediat. Inflamm. 2019, 2019, 6175091. [Google Scholar] [CrossRef] [PubMed]
- Katyal, S.; Oliver, J.H., III; Peterson, M.S.; Ferris, J.V.; Carr, B.S.; Baron, R.L. Extrahepatic metastases of hepatocellular carcinoma. Radiology 2000, 216, 698–703. [Google Scholar] [CrossRef]
- Singh, M.K.; Das, B.K.; Choudhary, S.; Gupta, D.; Patil, U.K. Diabetes and hepatocellular carcinoma: A pathophysiological link and pharmacological management. Biomed. Pharmacother. 2018, 106, 991–1002. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.D.; Ahmed, F.; Mara, K.C.; Addissie, B.D.; Allen, A.M.; Gores, G.J.; Roberts, L.R. Diabetes is associated with increased risk of hepatocellular carcinoma in patients with cirrhosis from nonalcoholic fatty liver disease. Hepatology 2020, 71, 907–916. [Google Scholar] [CrossRef]
- Teng, B.-s.; Lu, Y.-H.; Wang, Z.-T.; Tao, X.-Y.; Wei, D.-Z. In vitro anti-tumor activity of isorhamnetin isolated from Hippophae rhamnoides L. against BEL-7402 cells. Pharmacol. Res. 2006, 54, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Youness, R.; Assal, R.; Gad, M.; Motaal, A.A. Hijacking hepatocellular carcinoma (HCC) tumour progression through restoring TP53/miR-15a/miR-16 tumour suppressor axis by a novel quercetin glycoside. ESMO Open 2018, 3, A160. [Google Scholar] [CrossRef]
- Burganova, G.; Bridges, C.; Thorn, P.; Landsman, L. The Role of Vascular Cells in Pancreatic Beta-Cell Function. Front. Endocrinol. 2021, 12, 442. [Google Scholar] [CrossRef]
- Marchetti, P.; Ferrannini, E. International Textbook of Diabetes Mellitus; Wiley-Blackwell: Hoboken, NJ, USA, 2015; Volume 2, p. 1240. [Google Scholar]
- Marchetti, P.; Bugliani, M.; De Tata, V.; Suleiman, M.; Marselli, L. Pancreatic beta cell identity in humans and the role of type 2 diabetes. Front. Cell Dev. Biol. 2017, 5, 55. [Google Scholar] [CrossRef] [PubMed]
- Cerf, M.E. Beta cell dysfunction and insulin resistance. Front. Endocrinol. 2013, 4, 37. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-L.; Quan, Q.; Ji, R.; Guo, X.-Y.; Zhang, J.-M.; Li, X.; Liu, Y.-G. Isorhamnetin suppresses PANC-1 pancreatic cancer cell proliferation through S phase arrest. Biomed. Pharmacother. 2018, 108, 925–933. [Google Scholar] [CrossRef] [PubMed]
- Grdović, N.; Dinić, S.; Arambašić, J.; Mihailović, M.; Uskoković, A.; Marković, J.; Poznanović, G.; Vidović, S.; Zeković, Z.; Mujić, A. The protective effect of a mix of Lactarius deterrimus and Castanea sativa extracts on streptozotocin-induced oxidative stress and pancreatic β-cell death. Br. J. Nutr. 2012, 108, 1163–1176. [Google Scholar] [CrossRef]
- Vessal, M.; Hemmati, M.; Vasei, M. Antidiabetic effects of quercetin in streptozocin-induced diabetic rats. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2003, 135, 357–364. [Google Scholar] [CrossRef]
- Coskun, O.; Kanter, M.; Korkmaz, A.; Oter, S. Quercetin, a flavonoid antioxidant, prevents and protects streptozotocin-induced oxidative stress and β-cell damage in rat pancreas. Pharmacol. Res. 2005, 51, 117–123. [Google Scholar] [CrossRef]
- Hii, C.; Howell, S. Effects of flavonoids on insulin secretion and 45Ca2+ handling in rat islets of Langerhans. J. Endocrinol. 1985, 107, 1–8. [Google Scholar] [CrossRef]
- He, Z.; King, G.L. Microvascular complications of diabetes. Endocrinol. Metab. Clin. 2004, 33, 215–238. [Google Scholar] [CrossRef]
- Patel, S.; Santani, D. Role of NF-κB in the pathogenesis of diabetes and its associated complications. Pharmacol. Rep. 2009, 61, 595–603. [Google Scholar] [CrossRef]
- Suryavanshi, S.; Kulkarni, Y. NF-κβ: A potential target in the management of vascular complications of diabetes. Front. Pharmacol. 2017, 8, 798. [Google Scholar] [CrossRef]
- Singh, V.P.; Bali, A.; Singh, N.; Jaggi, A.S. Advanced glycation end products and diabetic complications. Korean J. Physiol. Pharmacol. 2014, 18, 1–14. [Google Scholar] [CrossRef]
- Leo, C.-H.; Woodman, O.L. Flavonols in the prevention of diabetes-induced vascular dysfunction. J. Cardiovasc. Pharmacol. 2015, 65, 532. [Google Scholar] [CrossRef] [PubMed]
- Hämäläinen, M.; Nieminen, R.; Vuorela, P.; Heinonen, M.; Moilanen, E. Anti-inflammatory effects of flavonoids: Genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and NF-κB activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only NF-κB activation along with their inhibitory effect on iNOS expression and NO production in activated macrophages. Mediat. Inflamm. 2007, 2007, 045673. [Google Scholar]
- Bejaoui, M.; Ferdousi, F.; Zheng, Y.-W.; Oda, T.; Isoda, H. Regulating cell fate of human amnion epithelial cells using natural compounds: An example of enhanced neural and pigment differentiation by 3,4,5-tri-O-caffeoylquinic acid. Cell Commun. Signal. 2021, 19, 26. [Google Scholar] [CrossRef] [PubMed]
- Ferdousi, F.; Kondo, S.; Sasaki, K.; Uchida, Y.; Ohkohchi, N.; Zheng, Y.-W.; Isoda, H. Microarray analysis of verbenalin-treated human amniotic epithelial cells reveals therapeutic potential for Alzheimer’s Disease. Aging 2020, 12, 5516. [Google Scholar] [CrossRef]
- Ferdousi, F.; Sasaki, K.; Ohkohchi, N.; Zheng, Y.-W.; Isoda, H. Exploring the Potential Role of Rosmarinic Acid in Neuronal Differentiation of Human Amnion Epithelial Cells by Microarray Gene Expression Profiling. Front. Neurosci. 2019, 13, 779. [Google Scholar] [CrossRef]
- Takahashi, S.; Ferdousi, F.; Zheng, Y.-W.; Oda, T.; Isoda, H. Human Amniotic Epithelial Cells as a Tool to Investigate the Effects of Cyanidin 3-O-Glucoside on Cell Differentiation. Int. J. Mol. Sci. 2021, 22, 3768. [Google Scholar] [CrossRef]
- Ferdousi, F.; Furuya, K.; Sasaki, K.; Zheng, Y.-W.; Oda, T.; Isoda, H. DNA Microarray-Based Global Gene Expression Profiling in Human Amniotic Epithelial Cells Predicts the Potential of Microalgae-Derived Squalene for the Nervous System and Metabolic Health. Biomedicines 2022, 10, 48. [Google Scholar] [CrossRef]
- Grskovic, M.; Javaherian, A.; Strulovici, B.; Daley, G.Q. Induced pluripotent stem cells—Opportunities for disease modelling and drug discovery. Nat. Rev. Drug Discov. 2011, 10, 915–929. [Google Scholar] [CrossRef] [PubMed]
- Laustriat, D.; Gide, J.; Peschanski, M. Human Pluripotent Stem Cells in Drug Discovery and Predictive Toxicology; Portland Press Ltd.: London, UK, 2010. [Google Scholar]
- Thomson, H. Bioprocessing of embryonic stem cells for drug discovery. Trends Biotechnol. 2007, 25, 224–230. [Google Scholar] [CrossRef]
- Gottipamula, S.; Sridhar, K.N. Large-scale Isolation, Expansion and Characterization of Human Amniotic Epithelial Cells. Int. J. Stem Cells 2018, 11, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Miki, T.; Lehmann, T.; Cai, H.; Stolz, D.B.; Strom, S.C. Stem cell characteristics of amniotic epithelial cells. Stem Cells 2005, 23, 1549–1559. [Google Scholar] [CrossRef]
- Miki, T.; Marongiu, F.; Dorko, K.; Ellis, E.C.; Strom, S.C. Isolation of amniotic epithelial stem cells. Curr. Protoc. Stem Cell Biol. 2010, 12, 1E.3.1–1E.3.10. [Google Scholar]
- Murphy, S.; Rosli, S.; Acharya, R.; Mathias, L.; Lim, R.; Wallace, E.; Jenkin, G. Amnion epithelial cell isolation and characterization for clinical use. Curr. Protoc. Stem Cell Biol. 2010, 13, 1E.6.1–1E.6.25. [Google Scholar] [CrossRef]
- Díaz-Prado, S.; Muiños-López, E.; Hermida-Gómez, T.; Rendal-Vázquez, M.E.; Fuentes-Boquete, I.; de Toro, F.J.; Blanco, F.J. Multilineage differentiation potential of cells isolated from the human amniotic membrane. J. Cell. Biochem. 2010, 111, 846–857. [Google Scholar] [CrossRef] [PubMed]
- Furuya, K.; Zheng, Y.-W.; Sako, D.; Iwasaki, K.; Zheng, D.-X.; Ge, J.-Y.; Liu, L.-P.; Furuta, T.; Akimoto, K.; Yagi, H. Enhanced hepatic differentiation in the subpopulation of human amniotic stem cells under 3D multicellular microenvironment. World J. Stem Cells 2019, 11, 705. [Google Scholar] [CrossRef]
- Marongiu, F.; Gramignoli, R.; Dorko, K.; Miki, T.; Ranade, A.R.; Paola Serra, M.; Doratiotto, S.; Sini, M.; Sharma, S.; Mitamura, K. Hepatic differentiation of amniotic epithelial cells. Hepatology 2011, 53, 1719–1729. [Google Scholar] [CrossRef]
- Maymó, J.L.; Riedel, R.; Pérez-Pérez, A.; Magatti, M.; Maskin, B.; Dueñas, J.L.; Parolini, O.; Sánchez-Margalet, V.; Varone, C.L. Proliferation and survival of human amniotic epithelial cells during their hepatic differentiation. PLoS ONE 2018, 13, e0191489. [Google Scholar] [CrossRef]
- Moritoki, Y.; Ueno, Y.; Kanno, N.; Yamagiwa, Y.; Fukushima, K.; Gershwin, M.E.; Shimosegawa, T. Amniotic epithelial cell–derived cholangiocytes in experimental cholestatic ductal hyperplasia. Hepatol. Res. 2007, 37, 286–294. [Google Scholar] [CrossRef]
- Okere, B.; Alviano, F.; Costa, R.; Quaglino, D.; Ricci, F.; Dominici, M.; Paolucci, P.; Bonsi, L.; Iughetti, L. In vitro differentiation of human amniotic epithelial cells into insulin-producing 3D spheroids. Int. J. Immunopathol. Pharmacol. 2015, 28, 390–402. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-J.; Fang, N.; Chen, D.-X.; Yu, L.-M.; Yu, H.-F.; Zhao, C.-H. Study on inducing differentiation of human amniotic epithelial cells into insulin secreting cells in vitro. Zhongguo Ying Yong Sheng Li Xue Za Zhi Zhongguo Yingyong Shenglixue Zazhi Chin. J. Appl. Physiol. 2012, 28, 139–143. [Google Scholar]
- Hou, Y.; Huang, Q.; Liu, T.; Guo, L. Human amnion epithelial cells can be induced to differentiate into functional insulin-producing cells. Acta Biochim. Biophys. Sin. 2008, 40, 830–839. [Google Scholar] [CrossRef][Green Version]
- Szukiewicz, D.; Pyzlak, M.; Stangret, A.; Rongies, W.; Maslinska, D. Decrease in expression of histamine H 2 receptors by human amniotic epithelial cells during differentiation into pancreatic beta-like cells. Inflamm. Res. 2010, 59, 205–207. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.P.; Zhang, T.S.; Kawa, S.; Aizawa, T.; Ota, M.; Akaike, T.; Kato, K.; Konishi, I.; Nikaido, T. Human amnion-isolated cells normalize blood glucose in streptozotocin-induced diabetic mice. Cell Transplant. 2003, 12, 545–552. [Google Scholar] [CrossRef]
- Zheng, Y.; Zheng, S.; Fan, X.; Li, L.; Xiao, Y.; Luo, P.; Liu, Y.; Wang, L.; Cui, Z.; He, F. Amniotic epithelial cells accelerate diabetic wound healing by modulating inflammation and promoting neovascularization. Stem Cells Int. 2018, 2018, 1082076. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.; Zhong, C.; Yang, X.; Shu, F.; Xiao, S.; Gong, T.; Luo, P.; Li, L.; Chen, Z.; Zheng, Y. Exosomes derived from human amniotic epithelial cells accelerate diabetic wound healing via PI3K-AKT-mTOR-mediated promotion in angiogenesis and fibroblast function. Burn. Trauma 2020, 8, tkaa020. [Google Scholar] [CrossRef]
- Lebreton, F.; Lavallard, V.; Bellofatto, K.; Bonnet, R.; Wassmer, C.H.; Perez, L.; Kalandadze, V.; Follenzi, A.; Boulvain, M.; Kerr-Conte, J. Insulin-producing organoids engineered from islet and amniotic epithelial cells to treat diabetes. Nat. Commun. 2019, 10, 4491. [Google Scholar] [CrossRef]
- Lebreton, F.; Bellofatto, K.; Wassmer, C.H.; Perez, L.; Lavallard, V.; Parnaud, G.; Cottet-Dumoulin, D.; Kerr-Conte, J.; Pattou, F.; Bosco, D. Shielding islets with human amniotic epithelial cells enhances islet engraftment and revascularization in a murine diabetes model. Am. J. Transplant. 2020, 20, 1551–1561. [Google Scholar] [CrossRef]
- Lim, R.; Hodge, A.; Moore, G.; Wallace, E.M.; Sievert, W. A pilot study evaluating the safety of intravenously administered human amnion epithelial cells for the treatment of hepatic fibrosis. Front. Pharmacol. 2017, 8, 549. [Google Scholar] [CrossRef] [PubMed]
- Manuelpillai, U.; Lourensz, D.; Vaghjiani, V.; Tchongue, J.; Lacey, D.; Tee, J.-Y.; Murthi, P.; Chan, J.; Hodge, A.; Sievert, W. Human amniotic epithelial cell transplantation induces markers of alternative macrophage activation and reduces established hepatic fibrosis. PLoS ONE 2012, 7, e38631. [Google Scholar] [CrossRef]
- Lin, J.S.; Zhou, L.; Sagayaraj, A.; Jumat, N.H.B.; Choolani, M.; Chan, J.K.Y.; Biswas, A.; Wong, P.C.; Lim, S.G.; Dan, Y.Y. Hepatic differentiation of human amniotic epithelial cells and in vivo therapeutic effect on animal model of cirrhosis. J. Gastroenterol. Hepatol. 2015, 30, 1673–1682. [Google Scholar] [CrossRef]
- Liu, Q.-W.; Liu, Q.-Y.; Li, J.-Y.; Wei, L.; Ren, K.-K.; Zhang, X.-C.; Ding, T.; Xiao, L.; Zhang, W.-J.; Wu, H.-Y. Therapeutic efficiency of human amniotic epithelial stem cell-derived functional hepatocyte-like cells in mice with acute hepatic failure. Stem Cell Res. Ther. 2018, 9, 321. [Google Scholar] [CrossRef] [PubMed]
- Uchida, Y.; Ferdousi, F.; Zheng, Y.-W.; Oda, T.; Isoda, H. Global Gene Expression Profiling Reveals Isorhamnetin Induces Hepatic-Lineage Specific Differentiation in Human Amniotic Epithelial Cells. Front. Cell Dev. Biol. 2020, 8, 578036. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef]
- Muraro, M.J.; Dharmadhikari, G.; Grün, D.; Groen, N.; Dielen, T.; Jansen, E.; Van Gurp, L.; Engelse, M.A.; Carlotti, F.; De Koning, E.J. A single-cell transcriptome atlas of the human pancreas. Cell Syst. 2016, 3, 385–394.e3. [Google Scholar] [CrossRef] [PubMed]
- Aizarani, N.; Saviano, A.; Mailly, L.; Durand, S.; Herman, J.S.; Pessaux, P.; Baumert, T.F.; Grün, D. A human liver cell atlas reveals heterogeneity and epithelial progenitors. Nature 2019, 572, 199–204. [Google Scholar] [CrossRef]
- Cao, J.; O’Day, D.R.; Pliner, H.A.; Kingsley, P.D.; Deng, M.; Daza, R.M.; Zager, M.A.; Aldinger, K.A.; Blecher-Gonen, R.; Zhang, F. A human cell atlas of fetal gene expression. Science 2020, 370, eaba7612. [Google Scholar] [CrossRef]
- Rubenstein, A.B.; Smith, G.R.; Raue, U.; Begue, G.; Minchev, K.; Ruf-Zamojski, F.; Nair, V.D.; Wang, X.; Zhou, L.; Zaslavsky, E. Single-cell transcriptional profiles in human skeletal muscle. Sci. Rep. 2020, 10, 229. [Google Scholar] [CrossRef]
- Liberzon, A.; Birger, C.; Thorvaldsdóttir, H.; Ghandi, M.; Mesirov, J.P.; Tamayo, P. The molecular signatures database hallmark gene set collection. Cell Syst. 2015, 1, 417–425. [Google Scholar] [CrossRef]
- Hu, C.-M.; Tien, S.-C.; Hsieh, P.-K.; Jeng, Y.-M.; Chang, M.-C.; Chang, Y.-T.; Chen, Y.-J.; Chen, Y.-J.; Eva, Y.-H.L.; Lee, W.-H. High glucose triggers nucleotide imbalance through O-GlcNAcylation of key enzymes and induces KRAS mutation in pancreatic cells. Cell Metab. 2019, 29, 1334–1349.e10. [Google Scholar] [CrossRef]
- Menini, S.; Iacobini, C.; De Latouliere, L.; Manni, I.; Vitale, M.; Pilozzi, E.; Pesce, C.; Cappello, P.; Novelli, F.; Piaggio, G. Diabetes promotes invasive pancreatic cancer by increasing systemic and tumour carbonyl stress in Kras G12D/+ mice. J. Exp. Clin. Cancer Res. 2020, 39, 152. [Google Scholar] [CrossRef]
- Al-Qahtani, W.S.; Al-Olayan, E.; Albani, F.G.; Suliman, R.S.; Aljarba, N.H.; Al-Humaidhi, E.; Almurshedi, A.S.; Domiaty, D.M.; Alduwish, M.A.; Al-Otaibi, A.M. Utility of KRAS Gene and Clinicopathological Features in the Assessment of the Risk of Type 2 Diabetes in the Etiology of Colon Cancer. Glob. Med. Genet. 2020, 7, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.P.; Grondin, C.J.; Johnson, R.J.; Sciaky, D.; Wiegers, J.; Wiegers, T.C.; Mattingly, C.J. Comparative toxicogenomics database (CTD): Update 2021. Nucleic Acids Res. 2021, 49, D1138–D1143. [Google Scholar] [CrossRef]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef]
- Soman, S.; Raju, R.; Sandhya, V.K.; Advani, J.; Khan, A.A.; Harsha, H.; Prasad, T.K.; Sudhakaran, P.; Pandey, A.; Adishesha, P.K. A multicellular signal transduction network of AGE/RAGE signaling. J. Cell Commun. Signal. 2013, 7, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Stitt, A.W.; Li, Y.M.; Gardiner, T.A.; Bucala, R.; Archer, D.B.; Vlassara, H. Advanced glycation end products (AGEs) co-localize with AGE receptors in the retinal vasculature of diabetic and of AGE-infused rats. Am. J. Pathol. 1997, 150, 523. [Google Scholar] [PubMed]
- Tanikawa, T.; Okada, Y.; Tanikawa, R.; Tanaka, Y. Advanced glycation end products induce calcification of vascular smooth muscle cells through RAGE/p38 MAPK. J. Vasc. Res. 2009, 46, 572–580. [Google Scholar] [CrossRef]
- Prasain, J.K.; Wang, C.-C.; Barnes, S. Mass spectrometric methods for the determination of flavonoids in biological samples. Free Radic. Biol. Med. 2004, 37, 1324–1350. [Google Scholar] [CrossRef] [PubMed]
- Mullen, W.; Boitier, A.; Stewart, A.; Crozier, A. Flavonoid metabolites in human plasma and urine after the consumption of red onions: Analysis by liquid chromatography with photodiode array and full scan tandem mass spectrometric detection. J. Chromatogr. A 2004, 1058, 163–168. [Google Scholar] [CrossRef]
- Choi, M.S.; Kim, J.-K.; Kim, D.-H.; Yoo, H.H. Effects of Gut Microbiota on the Bioavailability of Bioactive Compounds from Ginkgo Leaf Extracts. Metabolites 2019, 9, 132. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Yu, Y.; Zheng, X.; Wu, J.; Li, Y.; Wu, X.; Du, Q.; Yin, X. Comparative investigation of in vitro biotransformation of 14 components in Ginkgo biloba extract in normal, diabetes and diabetic nephropathy rat intestinal bacteria matrix. J. Pharm. Biomed. Anal. 2014, 100, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Walle, T. Absorption and metabolism of flavonoids. Free Radic. Biol. Med. 2004, 36, 829–837. [Google Scholar] [CrossRef]
- Duan, J.; Xie, Y.; Luo, H.; Li, G.; Wu, T.; Zhang, T. Transport characteristics of isorhamnetin across intestinal Caco-2 cell monolayers and the effects of transporters on it. Food Chem. Toxicol. 2014, 66, 313–320. [Google Scholar] [CrossRef] [PubMed]
| Plant Sources | Extraction Methods | Action Modes | References |
|---|---|---|---|
| Vaccinium vitis-idaea | Fractionation guided 80% ethanol > ethyl acetate > water | Enhances muscle cell glucose uptake | [98] |
| Nitraria retusa | Maceration in water-ethanol solution | Modulation of lipogenesis–lipolysis balance | [17] |
| Nitraria retusa | Maceration in water-ethanol solution | 3T3-L1 preadipocyte differentiation regulation | [18] |
| Corchorus olitorius | Soxhlet extraction with methanol, the residue from the above soluble extraction was hydrolyzed directly with 200 mL of 4 M NaOH solution. Then, the mixture was adjusted to pH 2 with concentrated HCl and the bound phytochemicals were extracted with ethyl acetate. | - α-Amylase inhibition - α-Glucosidase inhibition - Angiotensin I converting enzyme (ACE) inhibition - Degradation of deoxyribose | [95] |
| Salicornia herbacea | Fractionation guided ethanol under reflux > ethyl acetate ethyl acetate fraction was eluted on a silica gel chromatography by gradient elution with chloroform and methanol | - α-Glucosidase - Regulating the expression of ERK, PI3K, AKT, IRS-2, and PDX-1 | [96] |
| Eruca sativa | Extraction with ethanol by the accelerated solvent extractor, at 50 °C, 1500 psi for 20 min | Activation of PPAR-α and suppression of inflammatory cytokines | [28] |
| Oenanthe javanica | Isorhamnetin was isolated from the aerial part of O. javanica and its purity was higher than 95.0% | Attenuation of fibrosis in rat hepatic stellate cells via inhibition of ERK signaling pathway | [99] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalai, F.Z.; Boulaaba, M.; Ferdousi, F.; Isoda, H. Effects of Isorhamnetin on Diabetes and Its Associated Complications: A Review of In Vitro and In Vivo Studies and a Post Hoc Transcriptome Analysis of Involved Molecular Pathways. Int. J. Mol. Sci. 2022, 23, 704. https://doi.org/10.3390/ijms23020704
Kalai FZ, Boulaaba M, Ferdousi F, Isoda H. Effects of Isorhamnetin on Diabetes and Its Associated Complications: A Review of In Vitro and In Vivo Studies and a Post Hoc Transcriptome Analysis of Involved Molecular Pathways. International Journal of Molecular Sciences. 2022; 23(2):704. https://doi.org/10.3390/ijms23020704
Chicago/Turabian StyleKalai, Feten Zar, Mondher Boulaaba, Farhana Ferdousi, and Hiroko Isoda. 2022. "Effects of Isorhamnetin on Diabetes and Its Associated Complications: A Review of In Vitro and In Vivo Studies and a Post Hoc Transcriptome Analysis of Involved Molecular Pathways" International Journal of Molecular Sciences 23, no. 2: 704. https://doi.org/10.3390/ijms23020704
APA StyleKalai, F. Z., Boulaaba, M., Ferdousi, F., & Isoda, H. (2022). Effects of Isorhamnetin on Diabetes and Its Associated Complications: A Review of In Vitro and In Vivo Studies and a Post Hoc Transcriptome Analysis of Involved Molecular Pathways. International Journal of Molecular Sciences, 23(2), 704. https://doi.org/10.3390/ijms23020704







