Self-Assembling Lectin Nano-Block Oligomers Enhance Binding Avidity to Glycans
Abstract
:1. Introduction
2. Results
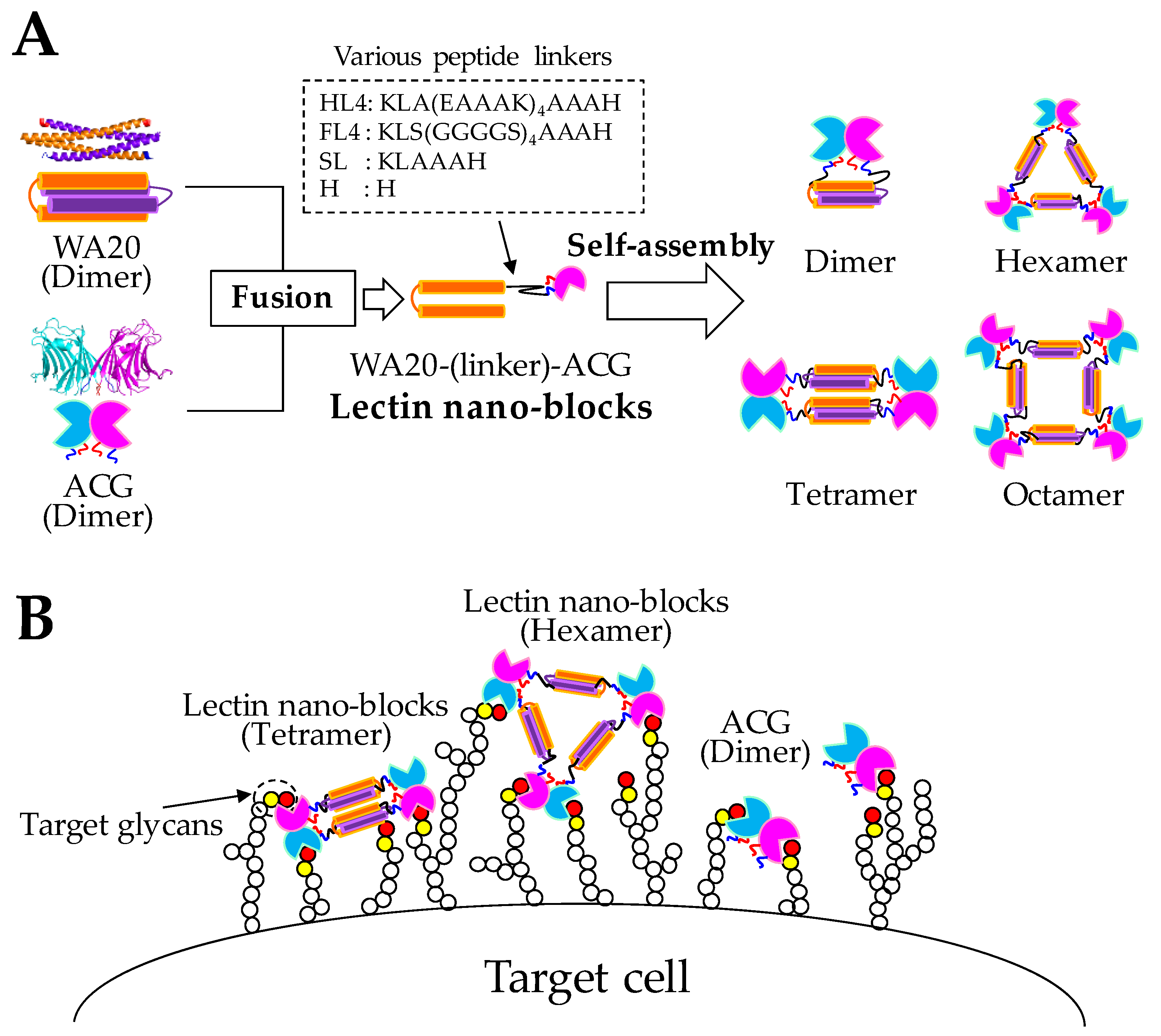
2.1. Design and Construction of Lectin Nano-Blocks
2.2. Structural Characterization of Lectin Nano-Blocks
2.2.1. Size Exclusion Chromatography–Multi-Angle Light Scattering (SEC–MALS) Analysis
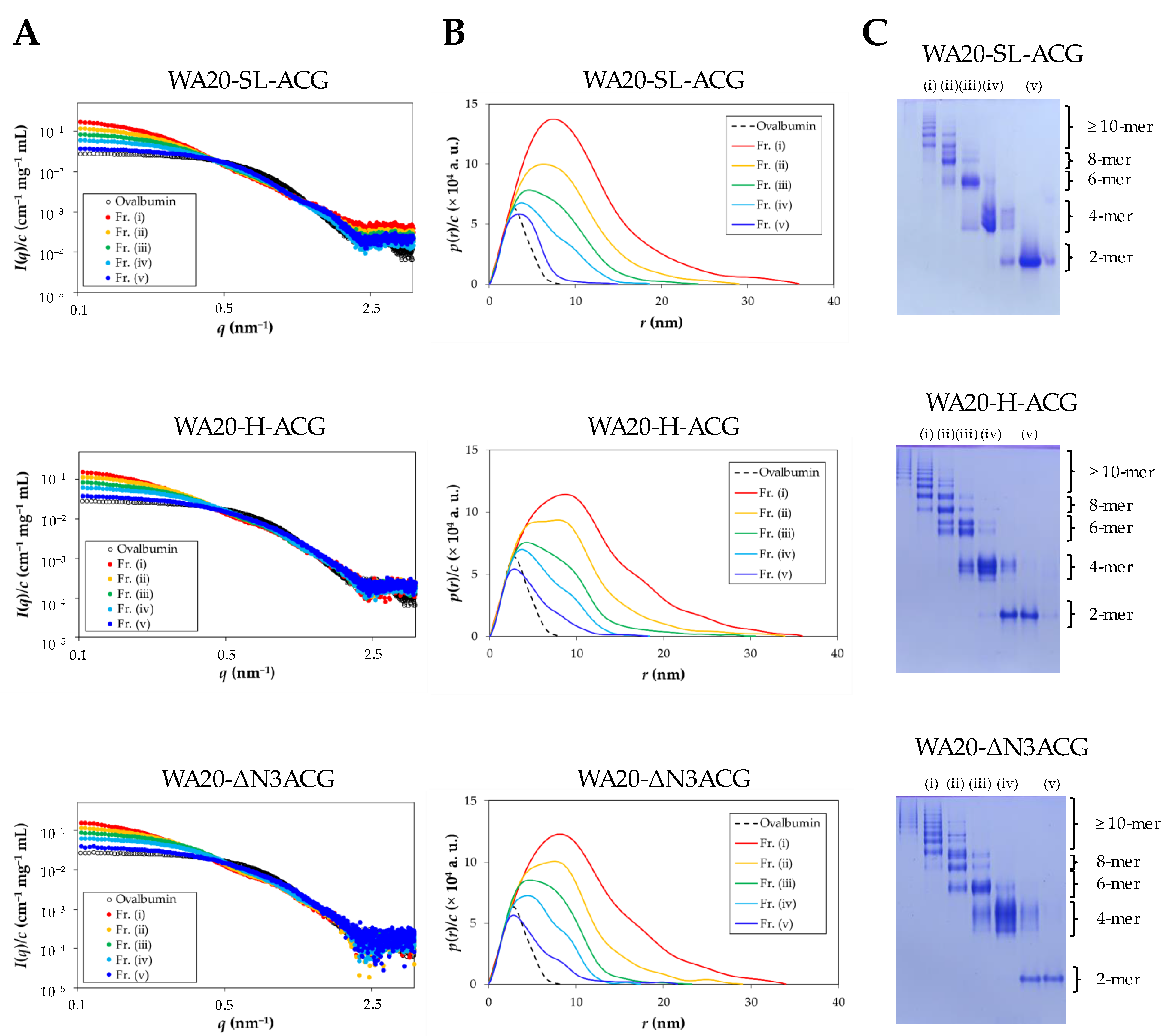
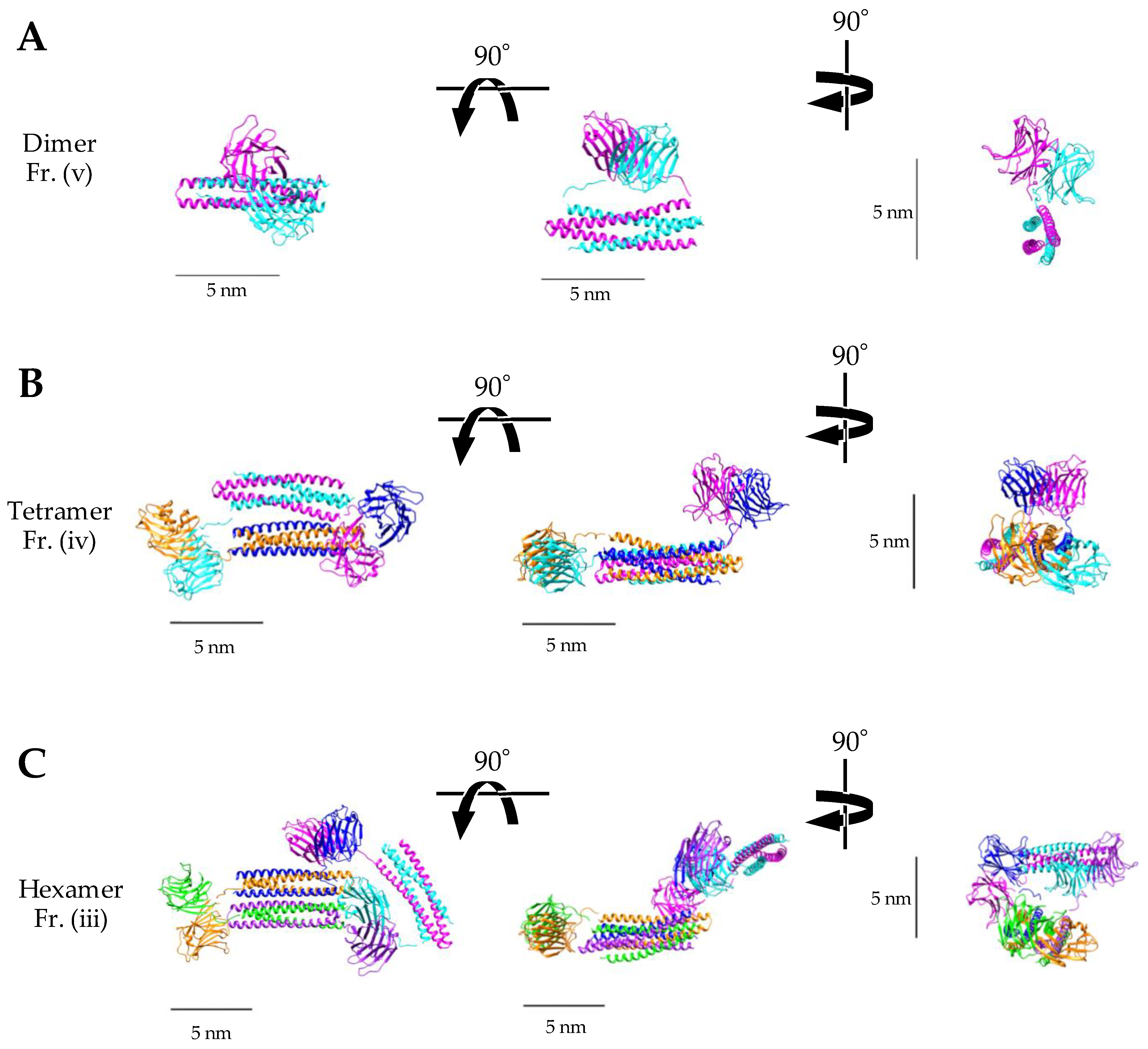
2.2.2. Small-Angle X-ray Scattering (SAXS) Analysis
2.3. Functional Characterization of Lectin Nano-Blocks
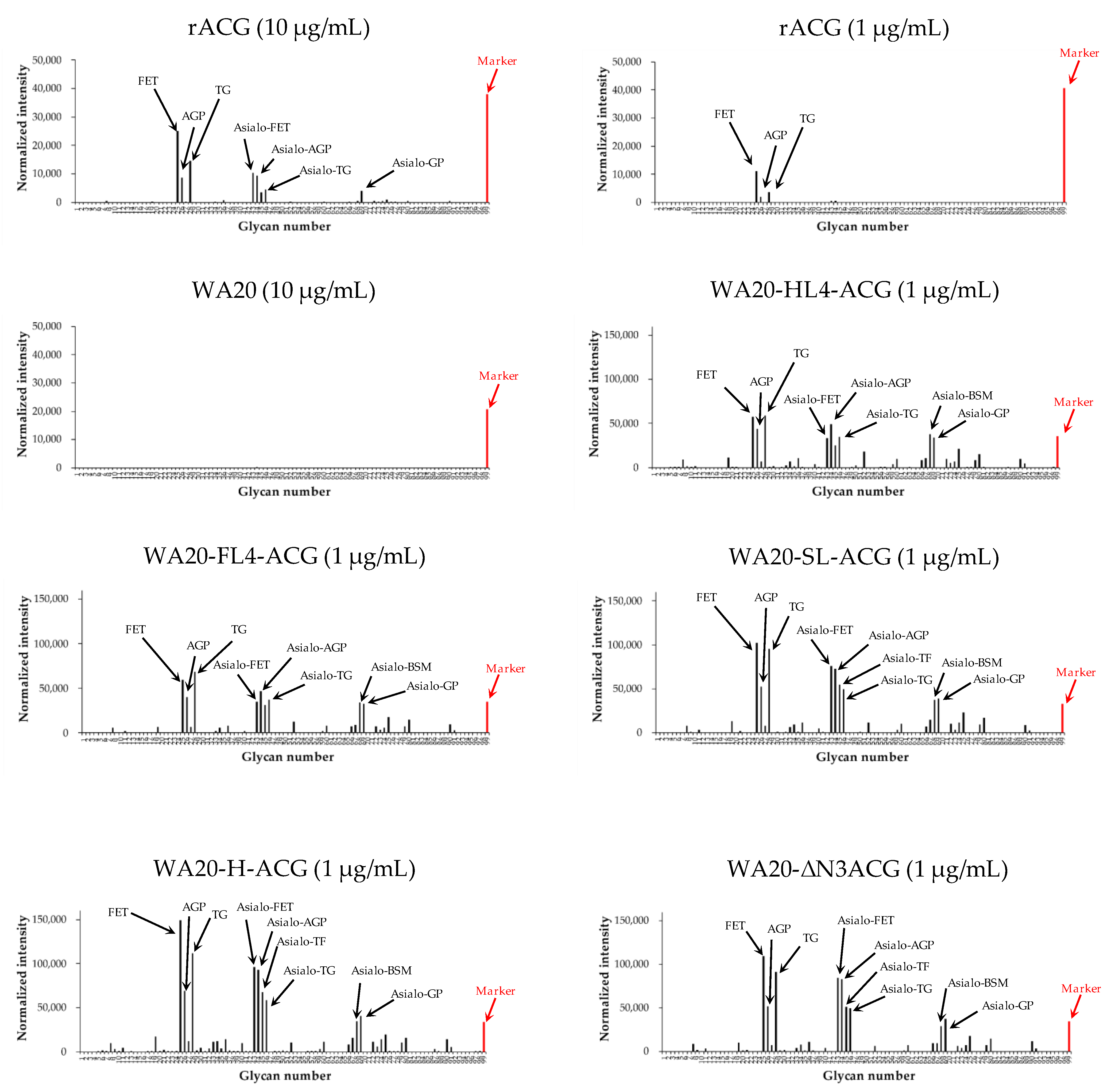
2.3.1. Glycoconjugate Microarray Analysis
2.3.2. Hemagglutinating Activity of Lectin Nano-Blocks
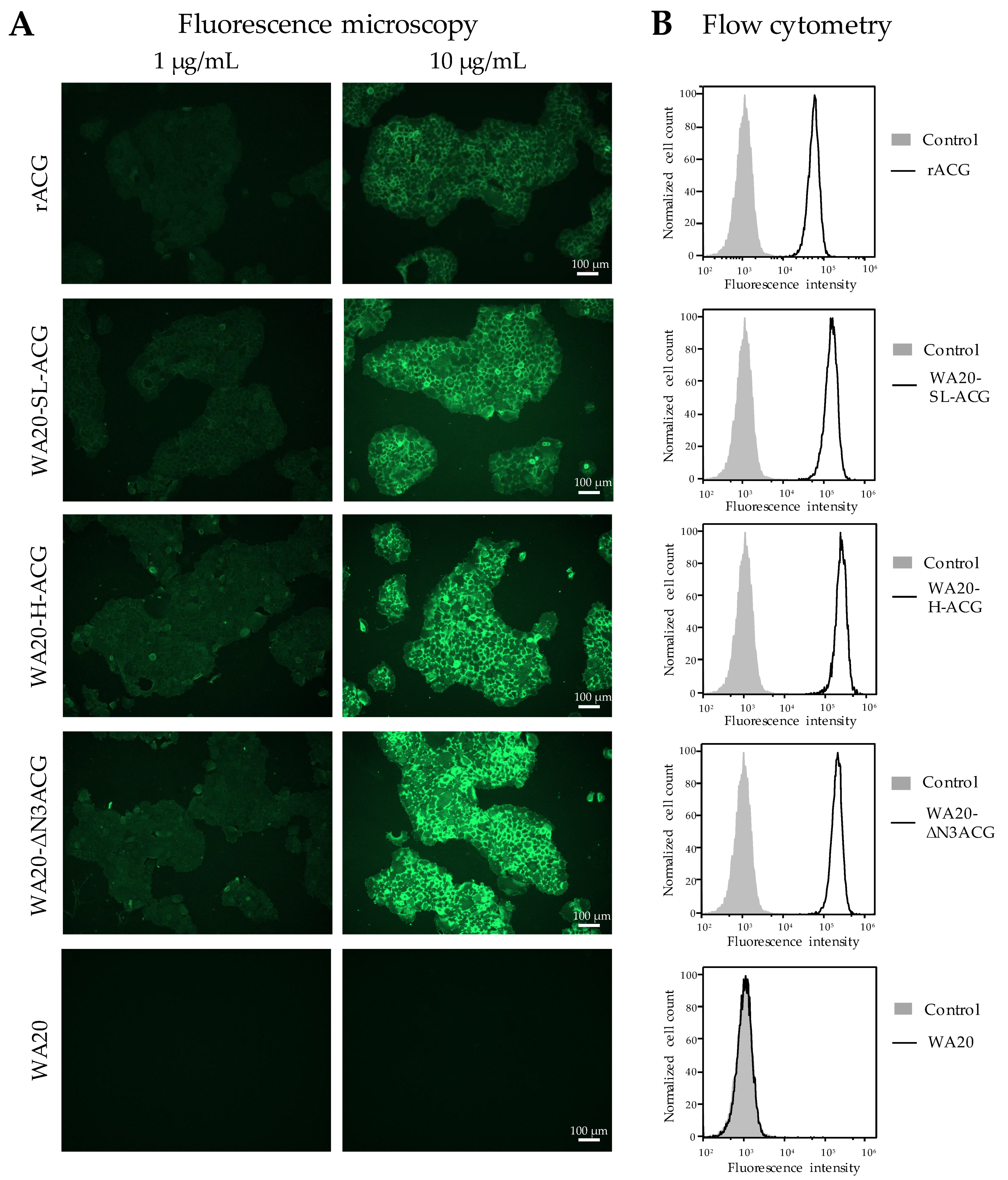
2.3.3. Cell Staining Experiments with Lectin Nano-Blocks
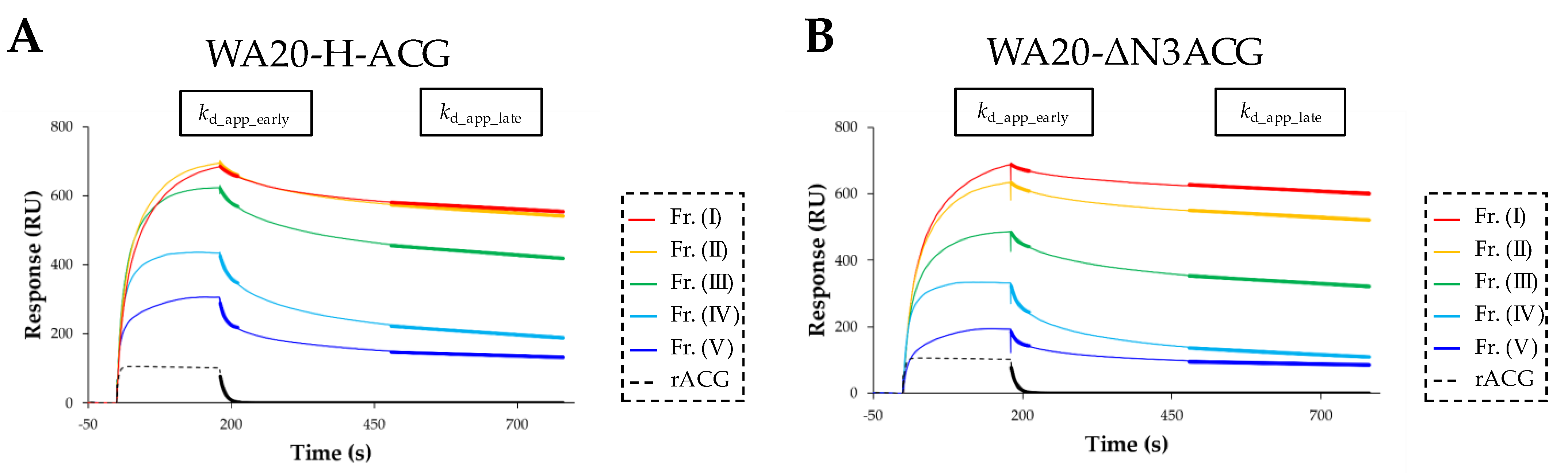
2.3.4. Surface Plasmon Resonance (SPR) Analysis of Lectin Nano-Block Oligomers
3. Discussion
4. Materials and Methods
4.1. Construction of WA20-ACG Protein Expression Plasmids
4.2. Protein Expression and Purification
4.3. Size Exclusion Chromatography–Multi Angle Light Scattering (SEC–MALS)
4.4. Small-Angle X-ray Scattering (SAXS)
4.5. Glycoconjugate Microarray
4.6. Hemagglutination Assay
4.7. Cell Staining Experiment
4.8. Flow Cytometry
4.9. Surface Plasmon Resonance (SPR) Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goodsell, D.S.; Olson, A.J. Structural symmetry and protein function. Annu. Rev. Biophys. Biomol. Struct. 2000, 29, 105–153. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.S.; Boyken, S.E.; Baker, D. The coming of age of de novo protein design. Nature 2016, 537, 320–327. [Google Scholar] [CrossRef]
- Arai, R. Hierarchical design of artificial proteins and complexes toward synthetic structural biology. Biophys. Rev. 2018, 10, 391–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeates, T.O.; Liu, Y.; Laniado, J. The design of symmetric protein nanomaterials comes of age in theory and practice. Curr. Opin. Struct. Biol. 2016, 39, 134–143. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, N.; Arai, R. Design and construction of self-assembling supramolecular protein complexes using artificial and fusion proteins as nanoscale building blocks. Curr. Opin. Biotech. 2017, 46, 57–65. [Google Scholar] [CrossRef]
- Hansen, W.A.; Khare, S.D. Recent progress in designing protein-based supramolecular assemblies. Curr. Opin. Struct. Biol. 2020, 63, 106–114. [Google Scholar] [CrossRef]
- Hecht, M.H.; Das, A.; Go, A.; Bradley, L.H.; Wei, Y. De novo proteins from designed combinatorial libraries. Protein Sci. 2004, 13, 1711–1723. [Google Scholar] [CrossRef] [Green Version]
- Patel, S.C.; Bradley, L.H.; Jinadasa, S.P.; Hecht, M.H. Cofactor binding and enzymatic activity in an unevolved superfamily of de novo designed 4-helix bundle proteins. Protein Sci. 2009, 18, 1388–1400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arai, R.; Kobayashi, N.; Kimura, A.; Sato, T.; Matsuo, K.; Wang, A.F.; Platt, J.M.; Bradley, L.H.; Hecht, M.H. Domain-swapped dimeric structure of a stable and functional de novo four-helix bundle protein, WA20. J. Phys. Chem. B 2012, 116, 6789–6797. [Google Scholar] [CrossRef]
- Kimura, N.; Mochizuki, K.; Umezawa, K.; Hecht, M.H.; Arai, R. Hyperstable de novo protein with a dimeric bisecting topology. ACS Synth. Biol. 2020, 9, 254–259. [Google Scholar] [CrossRef]
- Irumagawa, S.; Kobayashi, K.; Saito, Y.; Miyata, T.; Umetsu, M.; Kameda, T.; Arai, R. Rational thermostabilisation of four-helix bundle dimeric de novo proteins. Sci. Rep. 2021, 11, 7526. [Google Scholar] [CrossRef]
- Kobayashi, N.; Yanase, K.; Sato, T.; Unzai, S.; Hecht, M.H.; Arai, R. Self-assembling nano-architectures created from a protein nano-building block using an intermolecularly folded dimeric de novo protein. J. Am. Chem. Soc. 2015, 137, 11285–11293. [Google Scholar] [CrossRef]
- Kobayashi, N.; Inano, K.; Sasahara, K.; Sato, T.; Miyazawa, K.; Fukuma, T.; Hecht, M.H.; Song, C.; Murata, K.; Arai, R. Self-assembling supramolecular nanostructures constructed from de novo extender protein nanobuilding blocks. ACS Synth. Biol. 2018, 7, 1381–1394. [Google Scholar] [CrossRef] [PubMed]
- Bonnardel, F.; Mariethoz, J.; Salentin, S.; Robin, X.; Schroeder, M.; Perez, S.; Lisacek, F.; Imberty, A. UniLectin3D, a database of carbohydrate binding proteins with curated information on 3D structures and interacting ligands. Nucleic Acids Res. 2019, 47, D1236–D1244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ward, E.M.; Kizer, M.E.; Imperiali, B. Strategies and tactics for the development of selective glycan-binding proteins. ACS Chem. Biol. 2021, 16, 1795–1813. [Google Scholar] [CrossRef] [PubMed]
- Varki, A. Biological roles of glycans. Glycobiology 2017, 27, 3–49. [Google Scholar] [CrossRef] [Green Version]
- Arnaud, J.; Audfray, A.; Imberty, A. Binding sugars: From natural lectins to synthetic receptors and engineered neolectins. Chem. Soc. Rev. 2013, 42, 4798–4813. [Google Scholar] [CrossRef] [PubMed]
- Hirabayashi, J.; Arai, R. Lectin engineering: The possible and the actual. Interface Focus 2019, 9, 20180068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribeiro, J.P.; Villringer, S.; Goyard, D.; Coche-Guerente, L.; Hoferlin, M.; Renaudet, O.; Romer, W.; Imberty, A. Tailor-made Janus lectin with dual avidity assembles glycoconjugate multilayers and crosslinks protocells. Chem. Sci. 2018, 9, 7634–7641. [Google Scholar] [CrossRef] [Green Version]
- Kitaguchi, D.; Oda, T.; Enomoto, T.; Ohara, Y.; Owada, Y.; Akashi, Y.; Furuta, T.; Yu, Y.; Kimura, S.; Kuroda, Y.; et al. Lectin drug conjugate therapy for colorectal cancer. Cancer Sci. 2020, 111, 4548–4557. [Google Scholar] [CrossRef]
- Coves-Datson, E.M.; King, S.R.; Legendre, M.; Gupta, A.; Chan, S.M.; Gitlin, E.; Kulkarni, V.V.; Garcia, J.P.; Smee, D.F.; Lipka, E.; et al. A molecularly engineered antiviral banana lectin inhibits fusion and is efficacious against influenza virus infection in vivo. Proc. Natl. Acad. Sci. USA 2020, 117, 2122–2132. [Google Scholar] [CrossRef] [Green Version]
- Meany, D.L.; Zhang, Z.; Sokoll, L.J.; Zhang, H.; Chan, D.W. Glycoproteomics for prostate cancer detection: Changes in serum PSA glycosylation patterns. J. Proteome Res. 2009, 8, 613–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weis, W.I.; Drickamer, K. Structural basis of lectin-carbohydrate recognition. Annu. Rev. Biochem. 1996, 65, 441–473. [Google Scholar] [CrossRef] [PubMed]
- Notova, S.; Bonnardel, F.; Lisacek, F.; Varrot, A.; Imberty, A. Structure and engineering of tandem repeat lectins. Curr. Opin. Struct. Biol. 2020, 62, 39–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yabe, R.; Itakura, Y.; Nakamura-Tsuruta, S.; Iwaki, J.; Kuno, A.; Hirabayashi, J. Engineering a versatile tandem repeat-type alpha2-6sialic acid-binding lectin. Biochem. Biophys. Res. Commun. 2009, 384, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, S.; Ramya, T.N.C. Nature-inspired engineering of an F-type lectin for increased binding strength. Glycobiology 2018, 28, 933–948. [Google Scholar] [CrossRef]
- Hamorsky, K.T.; Kouokam, J.C.; Dent, M.W.; Grooms, T.N.; Husk, A.S.; Hume, S.D.; Rogers, K.A.; Villinger, F.; Morris, M.K.; Hanson, C.V.; et al. Engineering of a lectibody targeting high-mannose-type glycans of the HIV envelope. Mol. Ther. 2019, 27, 2038–2052. [Google Scholar] [CrossRef] [Green Version]
- Yagi, F.; Miyamoto, M.; Abe, T.; Minami, Y.; Tadera, K.; Goldstein, I.J. Purification and carbohydrate-binding specificity of Agrocybe cylindracea lectin. Glycoconj. J. 1997, 14, 281–288. [Google Scholar] [CrossRef]
- Yagi, F.; Hiroyama, H.; Kodama, S. Agrocybe cylindracea lectin is a member of the galectin family. Glycoconj. J. 2001, 18, 745–749. [Google Scholar] [CrossRef]
- Ban, M.; Yoon, H.J.; Demirkan, E.; Utsumi, S.; Mikami, B.; Yagi, F. Structural basis of a fungal galectin from Agrocybe cylindracea for recognizing sialoconjugate. J. Mol. Biol. 2005, 351, 695–706. [Google Scholar] [CrossRef]
- Imamura, K.; Takeuchi, H.; Yabe, R.; Tateno, H.; Hirabayashi, J. Engineering of the glycan-binding specificity of Agrocybe cylindracea galectin towards α(2,3)-linked sialic acid by saturation mutagenesis. J. Biochem. 2011, 150, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Tateno, H.; Hirabayashi, J. Lectin engineering, a molecular evolutionary approach to expanding the lectin utilities. Molecules 2015, 20, 7637–7656. [Google Scholar] [CrossRef] [Green Version]
- Hu, D.; Tateno, H.; Sato, T.; Narimatsu, H.; Hirabayashi, J. Tailoring GalNAcα1-3Galβ-specific lectins from a multi-specific fungal galectin: Dramatic change of carbohydrate specificity by a single amino-acid substitution. Biochem. J. 2013, 453, 261–270. [Google Scholar] [CrossRef]
- Hu, D.; Huang, H.; Tateno, H.; Nakakita, S.-i.; Sato, T.; Narimatsu, H.; Yao, X.; Hirabayashi, J. Engineering of a 3′-sulpho-Galβ1-4GlcNAc-specific probe by a single amino acid substitution of a fungal galectin. J. Biochem. 2015, 157, 197–200. [Google Scholar] [CrossRef]
- Hirabayashi, J.; Hu, D.; Tateno, H.; Kuwabara, N.; Kato, R.; Yagi, F. Carbohydrate recognition mechanism of the mushroom galectin ACG. Trends Glycosci. Glycotech. 2018, 30, Se75–Se88. [Google Scholar] [CrossRef] [Green Version]
- Arai, R. Design of helical linkers for fusion proteins and protein-based nanostructures. Methods Enzymol. 2021, 647, 209–230. [Google Scholar] [CrossRef] [PubMed]
- Arai, R.; Ueda, H.; Kitayama, A.; Kamiya, N.; Nagamune, T. Design of the linkers which effectively separate domains of a bifunctional fusion protein. Protein Eng. 2001, 14, 529–532. [Google Scholar] [CrossRef]
- Arai, R.; Wriggers, W.; Nishikawa, Y.; Nagamune, T.; Fujisawa, T. Conformations of variably linked chimeric proteins evaluated by synchrotron X-ray small-angle scattering. Proteins 2004, 57, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Petoukhov, M.V.; Franke, D.; Shkumatov, A.V.; Tria, G.; Kikhney, A.G.; Gajda, M.; Gorba, C.; Mertens, H.D.; Konarev, P.V.; Svergun, D.I. New developments in the ATSAS program package for small-angle scattering data analysis. J. Appl. Crystallogr. 2012, 45, 342–350. [Google Scholar] [CrossRef] [Green Version]
- Tateno, H.; Mori, A.; Uchiyama, N.; Yabe, R.; Iwaki, J.; Shikanai, T.; Angata, T.; Narimatsu, H.; Hirabayashi, J. Glycoconjugate microarray based on an evanescent-field fluorescence-assisted detection principle for investigation of glycan-binding proteins. Glycobiology 2008, 18, 789–798. [Google Scholar] [CrossRef] [Green Version]
- Munkley, J. The glycosylation landscape of pancreatic cancer. Oncol. Lett. 2019, 17, 2569–2575. [Google Scholar] [CrossRef] [Green Version]
- Munoz, E.M.; Correa, J.; Riguera, R.; Fernandez-Megia, E. Real-time evaluation of binding mechanisms in multivalent interactions: A surface plasmon resonance kinetic approach. J. Am. Chem. Soc. 2013, 135, 5966–5969. [Google Scholar] [CrossRef] [PubMed]
- Erijman, A.; Dantes, A.; Bernheim, R.; Shifman, J.M.; Peleg, Y. Transfer-PCR (TPCR): A highway for DNA cloning and protein engineering. J. Struct. Biol. 2011, 175, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Pace, C.N.; Vajdos, F.; Fee, L.; Grimsley, G.; Gray, T. How to measure and predict the molar absorption coefficient of a protein. Protein Sci. 1995, 4, 2411–2423. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, I.; Mizuno, Y.; Seno, N. Activation of Sepharose with epichlorohydrin and subsequent immobilization of ligand for affinity adsorbent. J. Biochem. 1979, 85, 1091–1098. [Google Scholar] [CrossRef]
- Wyatt, P.J. Light-scattering and the absolute characterization of macromolecules. Anal. Chim. Acta 1993, 272, 1–40. [Google Scholar] [CrossRef]
- Shimizu, N.; Mori, T.; Nagatani, Y.; Ohta, H.; Saijo, S.; Takagi, H.; Takahashi, M.; Yatabe, K.; Kosuge, T.; Igarashi, N. BL-10C, the small-angle x-ray scattering beamline at the photon factory. AIP Conf. Proc. 2019, 2054, 060041. [Google Scholar] [CrossRef]
- Shimizu, N.; Yatabe, K.; Nagatani, Y.; Saijyo, S.; Kosuge, T.; Igarashi, N. Software development for analysis of small-angle X-ray scattering data. AIP Conf. Proc. 2016, 1741, 050017. [Google Scholar] [CrossRef] [Green Version]
- Svergun, D.I. Determination of the regularization parameter in indirect-transform methods using perceptual criteria. J. Appl. Crystallogr. 1992, 25, 495–503. [Google Scholar] [CrossRef]
- Franke, D.; Petoukhov, M.V.; Konarev, P.V.; Panjkovich, A.; Tuukkanen, A.; Mertens, H.D.T.; Kikhney, A.G.; Hajizadeh, N.R.; Franklin, J.M.; Jeffries, C.M.; et al. ATSAS 2.8: A comprehensive data analysis suite for small-angle scattering from macromolecular solutions. J. Appl. Crystallogr. 2017, 50, 1212–1225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glatter, O.; Kratky, O. Small-Angle X-Ray Scattering; Academic Press: New York, NY, USA, 1982. [Google Scholar]
- Franke, D.; Svergun, D.I. DAMMIF, a program for rapid ab-initio shape determination in small-angle scattering. J. Appl. Crystallogr. 2009, 42, 342–346. [Google Scholar] [CrossRef] [Green Version]
- Volkov, V.V.; Svergun, D.I. Uniqueness of ab initio shape determination in small-angle scattering. J. Appl. Crystallogr. 2003, 36, 860–864. [Google Scholar] [CrossRef] [Green Version]
- Svergun, D.I. Restoring low resolution structure of biological macromolecules from solution scattering using simulated annealing. Biophys. J. 1999, 76, 2879–2886. [Google Scholar] [CrossRef] [Green Version]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kikhney, A.G.; Borges, C.R.; Molodenskiy, D.S.; Jeffries, C.M.; Svergun, D.I. SASBDB: Towards an automatically curated and validated repository for biological scattering data. Protein Sci. 2020, 29, 66–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
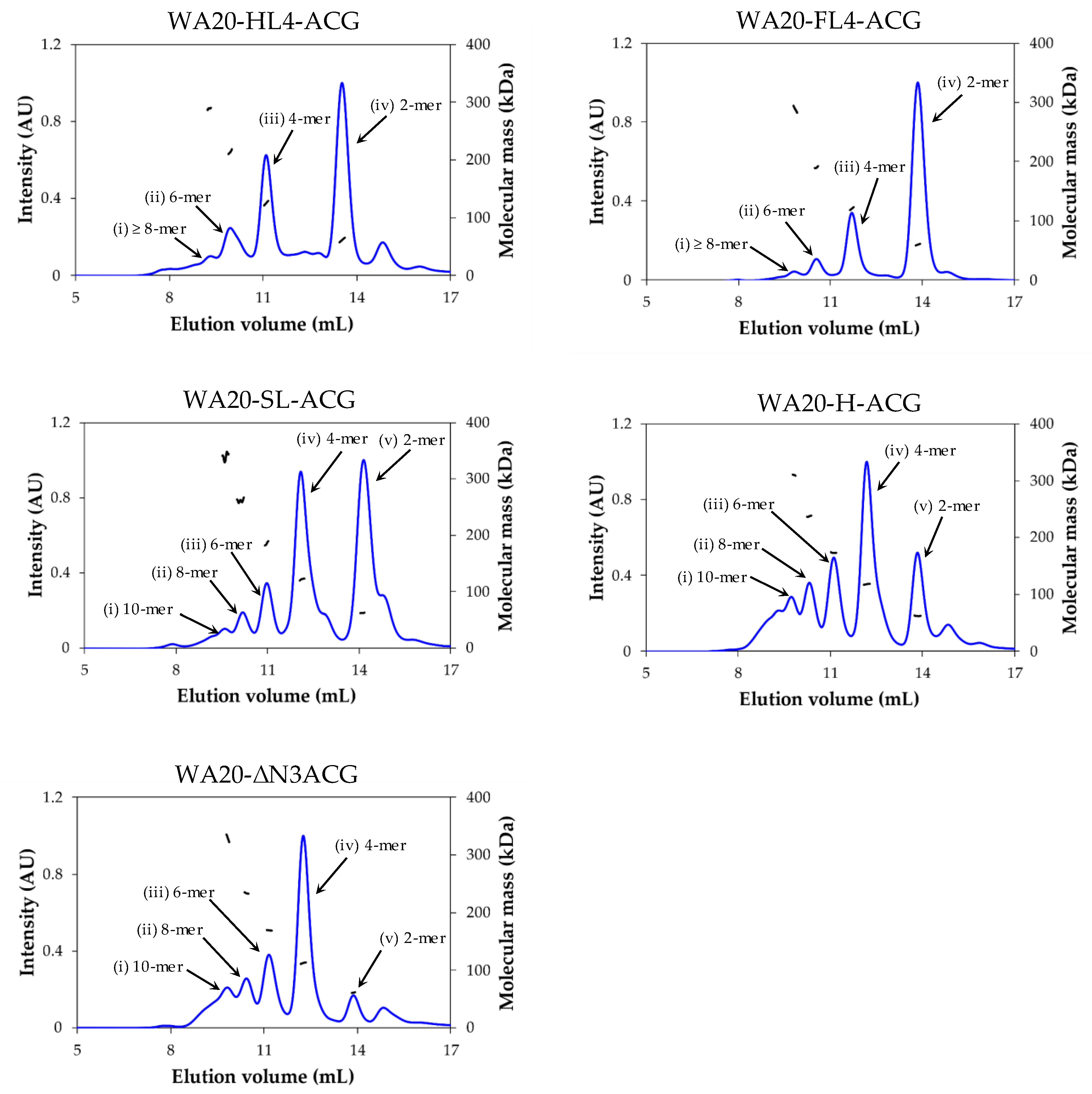
| Sample (Peak) | Mass Fraction (%) | Molecular Mass (Mw) (kDa) | Mw/Theoretical m of a Monomer | Oligomeric State (mer) |
|---|---|---|---|---|
| WA20-HL4-ACG (i) | 5.9 | 289 | 9.0 | 8, 10 |
| WA20-HL4-ACG (ii) | 16.3 | 214 | 6.7 | 6 |
| WA20-HL4-ACG (iii) | 30.4 | 125 | 3.9 | 4 |
| WA20-HL4-ACG (iv) | 47.3 | 61.6 | 1.9 | 2 |
| WA20-FL4-ACG (i) | 3.5 | 288 | 9.1 | 8, 10 |
| WA20-FL4-ACG (ii) | 7.0 | 190 | 6.0 | 6 |
| WA20-FL4-ACG (iii) | 22.7 | 121 | 3.8 | 4 |
| WA20-FL4-ACG (iv) | 66.8 | 59.8 | 1.9 | 2 |
| WA20-SL-ACG (i) | 5.3 | 339 | 11.3 | 10, 12 |
| WA20-SL-ACG (ii) | 6.5 | 261 | 8.7 | 8, 10 |
| WA20-SL-ACG (iii) | 11.8 | 185 | 6.1 | 6 |
| WA20-SL-ACG (iv) | 37.2 | 122 | 4.0 | 4 |
| WA20-SL-ACG (v) | 39.2 | 62.0 | 2.1 | 2 |
| WA20-H-ACG (i) | 19.1 | 310 | 10.4 | 10 |
| WA20-H-ACG (ii) | 11.6 | 237 | 8.0 | 8 |
| WA20-H-ACG (iii) | 16.7 | 173 | 5.8 | 6 |
| WA20-H-ACG (iv) | 36.1 | 118 | 4.0 | 4 |
| WA20-H-ACG (v) | 16.5 | 62.4 | 2.1 | 2 |
| WA20-ΔN3ACG (i) | 16.7 | 329 | 11.3 | 10, 12 |
| WA20-ΔN3ACG (ii) | 11.9 | 234 | 8.0 | 8 |
| WA20-ΔN3ACG (iii) | 19.0 | 169 | 5.8 | 6 |
| WA20-ΔN3ACG (iv) | 44.8 | 113 | 3.9 | 4 |
| WA20-ΔN3ACG (v) | 7.5 | 61.1 | 2.1 | 2 |
| Sample (Fraction) | I(q→0)/c (cm−1 mg−1 mL) | Dmax (nm) | Rg (nm) | Mw (kDa) |
|---|---|---|---|---|
| WA20-SL-ACG (i) | 0.2125 | 36.0 | 7.6 | 340 |
| WA20-SL-ACG (ii) | 0.1397 | 29.0 | 6.6 | 223 |
| WA20-SL-ACG (iii) | 0.0936 | 24.2 | 5.4 | 150 |
| WA20-SL-ACG (iv) | 0.0650 | 18.6 | 4.7 | 104 |
| WA20-SL-ACG (v) | 0.0378 | 14.9 | 3.4 | 60.5 |
| WA20-H-ACG (i) | 0.2003 | 36.0 | 8.3 | 320 |
| WA20-H-ACG (ii) | 0.1362 | 34.0 | 6.5 | 218 |
| WA20-H-ACG (iii) | 0.0971 | 30.6 | 6.3 | 155 |
| WA20-H-ACG (iv) | 0.0669 | 18.4 | 4.8 | 107 |
| WA20-H-ACG (v) | 0.0394 | 18.1 | 3.9 | 63.0 |
| WA20-ΔN3ACG (i) | 0.2046 | 34.0 | 8.1 | 327 |
| WA20-ΔN3ACG (ii) | 0.1370 | 29.0 | 6.5 | 219 |
| WA20-ΔN3ACG (iii) | 0.0996 | 23.2 | 5.5 | 159 |
| WA20-ΔN3ACG (iv) | 0.0689 | 17.4 | 4.5 | 110 |
| WA20-ΔN3ACG (v) | 0.0416 | 21.6 | 4.1 | 66.5 |
| rACG | 0.0199 | 6.8 | 2.2 | 31.8 |
| WA20_H86K | 0.0157 | 11.0 | 2.7 | 25.1 |
| Ovalbumin * | 0.0277 | 8.2 | 2.3 | 44.3 |
| Sample | MCA |
|---|---|
| WA20 | No agglutination |
| rACG | 42 nM |
| WA20-HL4-ACG | 5.2 nM |
| WA20-FL4-ACG | 1.3 nM |
| WA20-SL-ACG | 1.3 nM |
| WA20-H-ACG | 1.3 nM |
| WA20-ΔN3ACG | 2.6 nM |
| Sample (Fraction) | Rmax_app (RU) | KD_app (M) | kd_app_early (s−1) (181–211 s) | kd_app_late (s−1) (480–780 s) |
|---|---|---|---|---|
| WA20-H-ACG (I) (≥ decamer) | 889.8 | 3.07 × 10−7 | 4.46 × 10−2 | 1.57 × 10−4 |
| WA20-H-ACG (II) (octamer) | 875.9 | 2.55 × 10−7 | 3.89 × 10−2 | 1.92 × 10−4 |
| WA20-H-ACG (III) (hexamer) | 799.4 | 2.84 × 10−7 | 5.23 × 10−2 | 2.83 × 10−4 |
| WA20-H-ACG (IV) (tetramer) | 675.5 | 7.40 × 10−7 | 6.69 × 10−2 | 5.49 × 10−4 |
| WA20-H-ACG (V) (dimer) | 556.8 | 1.36 × 10−6 | 1.02 × 10−1 | 3.62 × 10−4 |
| WA20-ΔN3ACG (I) (≥ decamer) | 937.7 | 3.75 × 10−7 | 4.49 × 10−2 | 1.46 × 10−4 |
| WA20-ΔN3ACG (II) (octamer) | 870.0 | 3.89 × 10−7 | 3.60 × 10−2 | 1.80 × 10−4 |
| WA20-ΔN3ACG (III) (hexamer) | 733.6 | 6.08 × 10−7 | 6.01 × 10−2 | 3.13 × 10−4 |
| WA20-ΔN3ACG (IV) (tetramer) | 613.5 | 1.12 × 10−6 | 7.80 × 10−2 | 7.27 × 10−4 |
| WA20-ΔN3ACG (V)(dimer) | 519.3 | 1.99 × 10−6 | 9.13 × 10−2 | 3.51 × 10−4 |
| rACG (dimer) | 490.6 | 1.97 × 10−5 | 1.14 × 10−1 (kd_app) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Irumagawa, S.; Hiemori, K.; Saito, S.; Tateno, H.; Arai, R. Self-Assembling Lectin Nano-Block Oligomers Enhance Binding Avidity to Glycans. Int. J. Mol. Sci. 2022, 23, 676. https://doi.org/10.3390/ijms23020676
Irumagawa S, Hiemori K, Saito S, Tateno H, Arai R. Self-Assembling Lectin Nano-Block Oligomers Enhance Binding Avidity to Glycans. International Journal of Molecular Sciences. 2022; 23(2):676. https://doi.org/10.3390/ijms23020676
Chicago/Turabian StyleIrumagawa, Shin, Keiko Hiemori, Sayoko Saito, Hiroaki Tateno, and Ryoichi Arai. 2022. "Self-Assembling Lectin Nano-Block Oligomers Enhance Binding Avidity to Glycans" International Journal of Molecular Sciences 23, no. 2: 676. https://doi.org/10.3390/ijms23020676
APA StyleIrumagawa, S., Hiemori, K., Saito, S., Tateno, H., & Arai, R. (2022). Self-Assembling Lectin Nano-Block Oligomers Enhance Binding Avidity to Glycans. International Journal of Molecular Sciences, 23(2), 676. https://doi.org/10.3390/ijms23020676







