Genome-Wide Analysis of the Peroxidase Gene Family and Verification of Lignin Synthesis-Related Genes in Watermelon
Abstract
1. Introduction
2. Results
2.1. Identification of ClPRX Gene Family Members
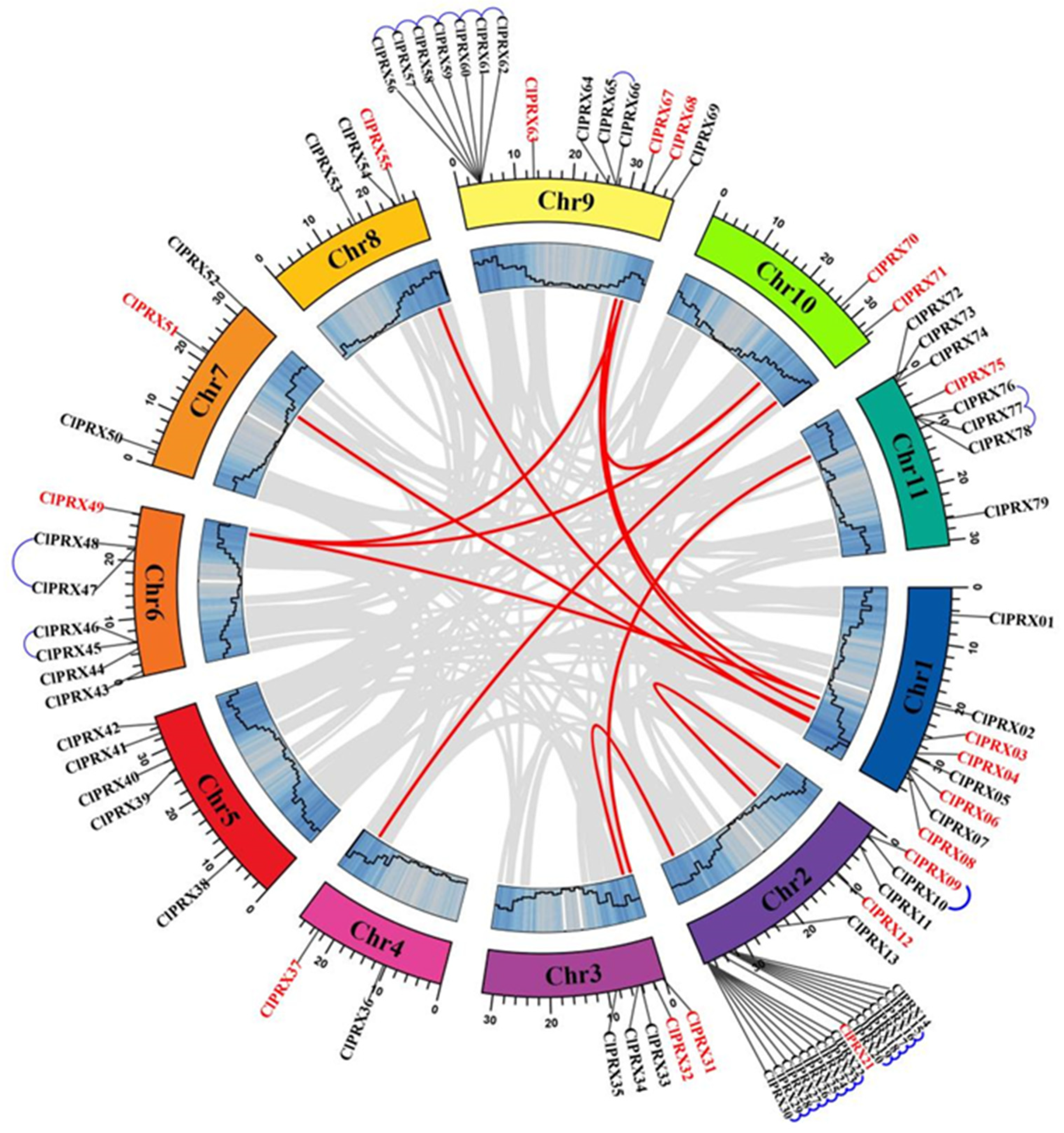
2.2. Chromosomal Localization and Gene Replication Relationship of ClPRXs
2.3. Interspecific Collinearity of PRXs
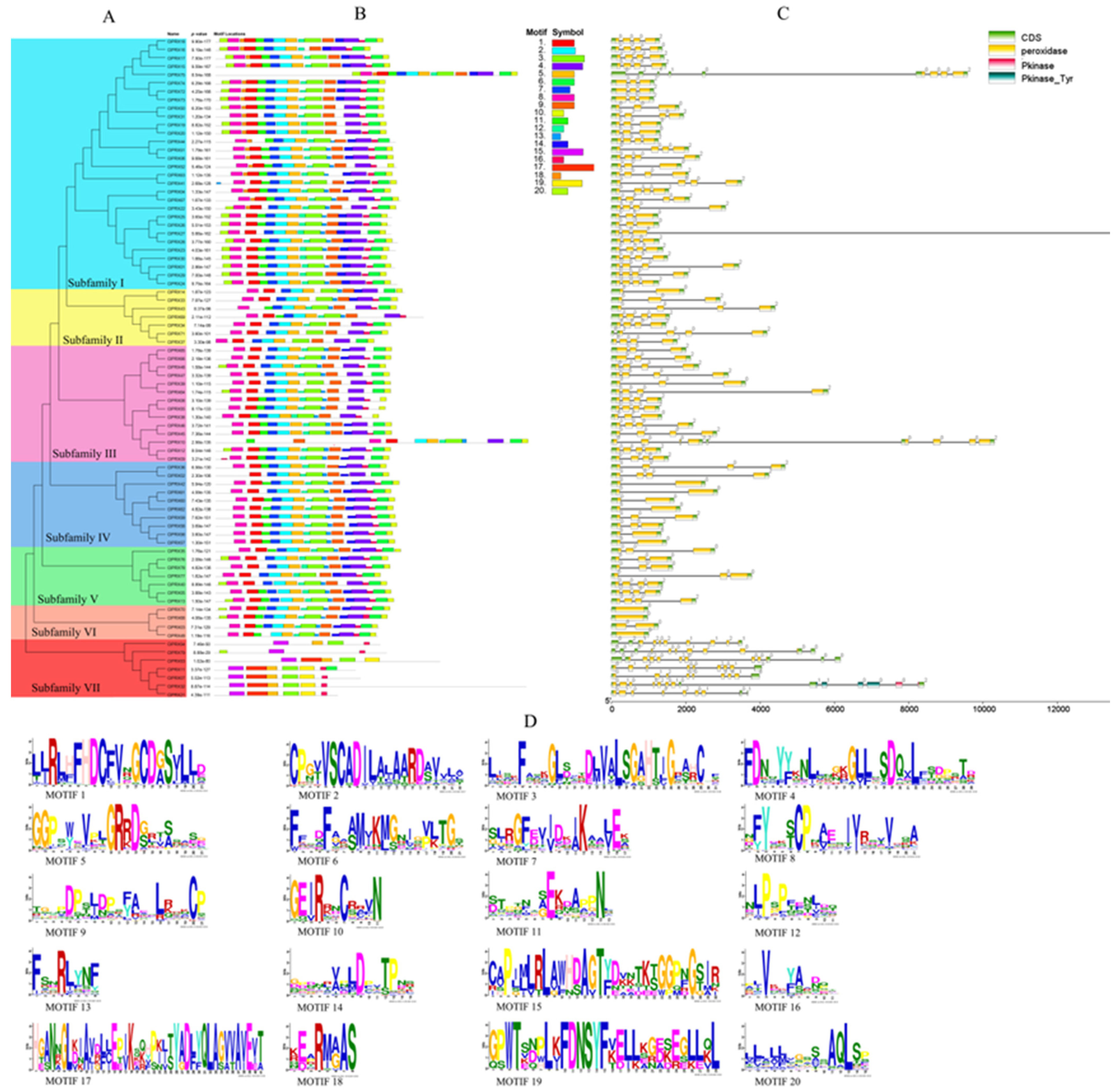
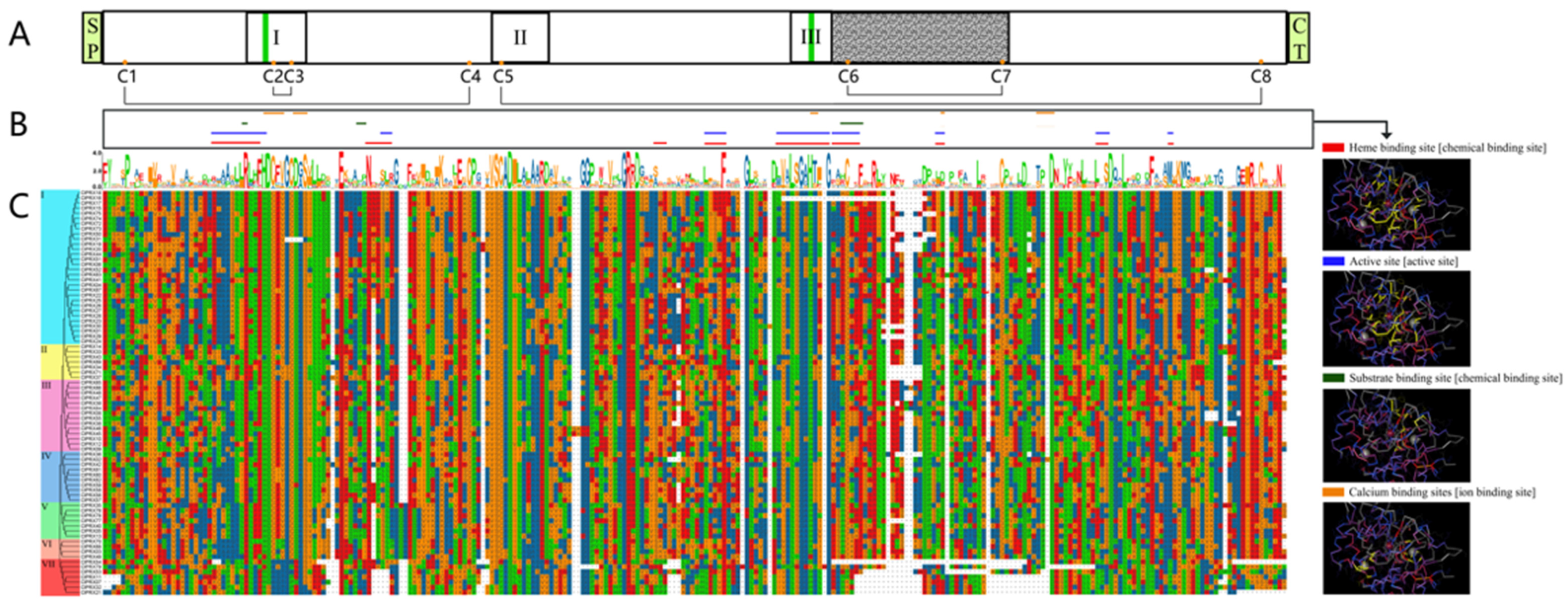
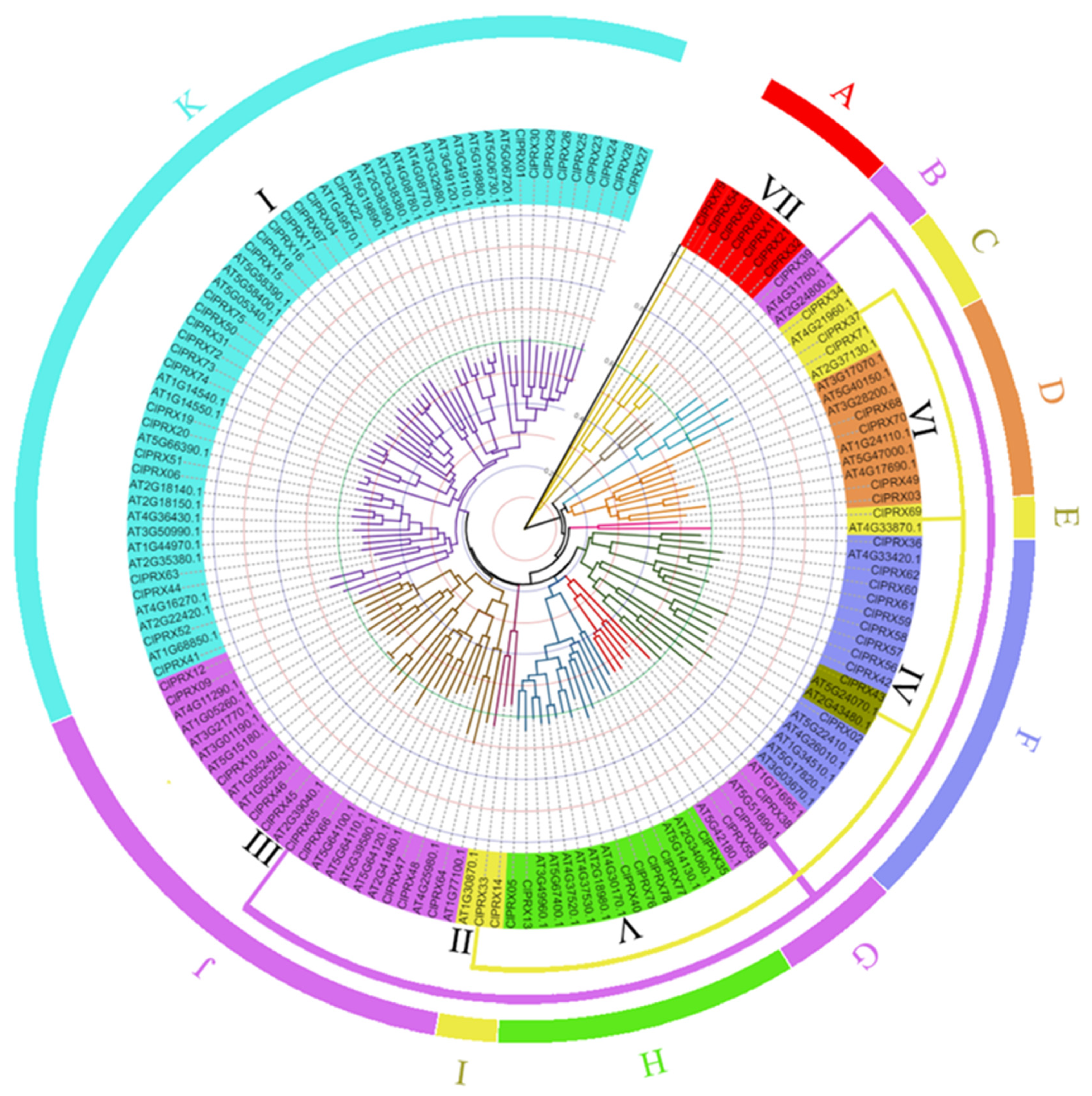
2.4. ClPRXs’ Evolutionary Tree, Gene Structure, Protein Sequence Comparison, and Conserved Structure
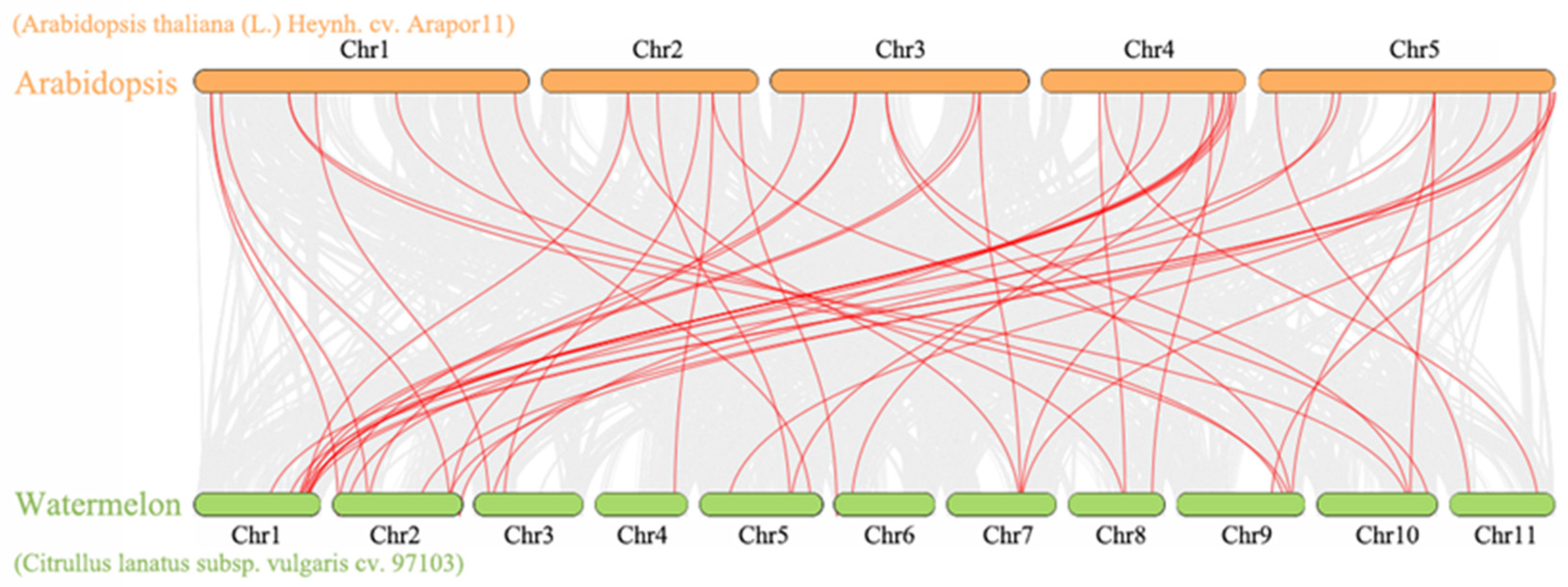
2.5. Comparative Analysis of PRXs between Watermelon and Arabidopsis
2.6. Prediction of Subcellular Localization of ClPRX Proteins
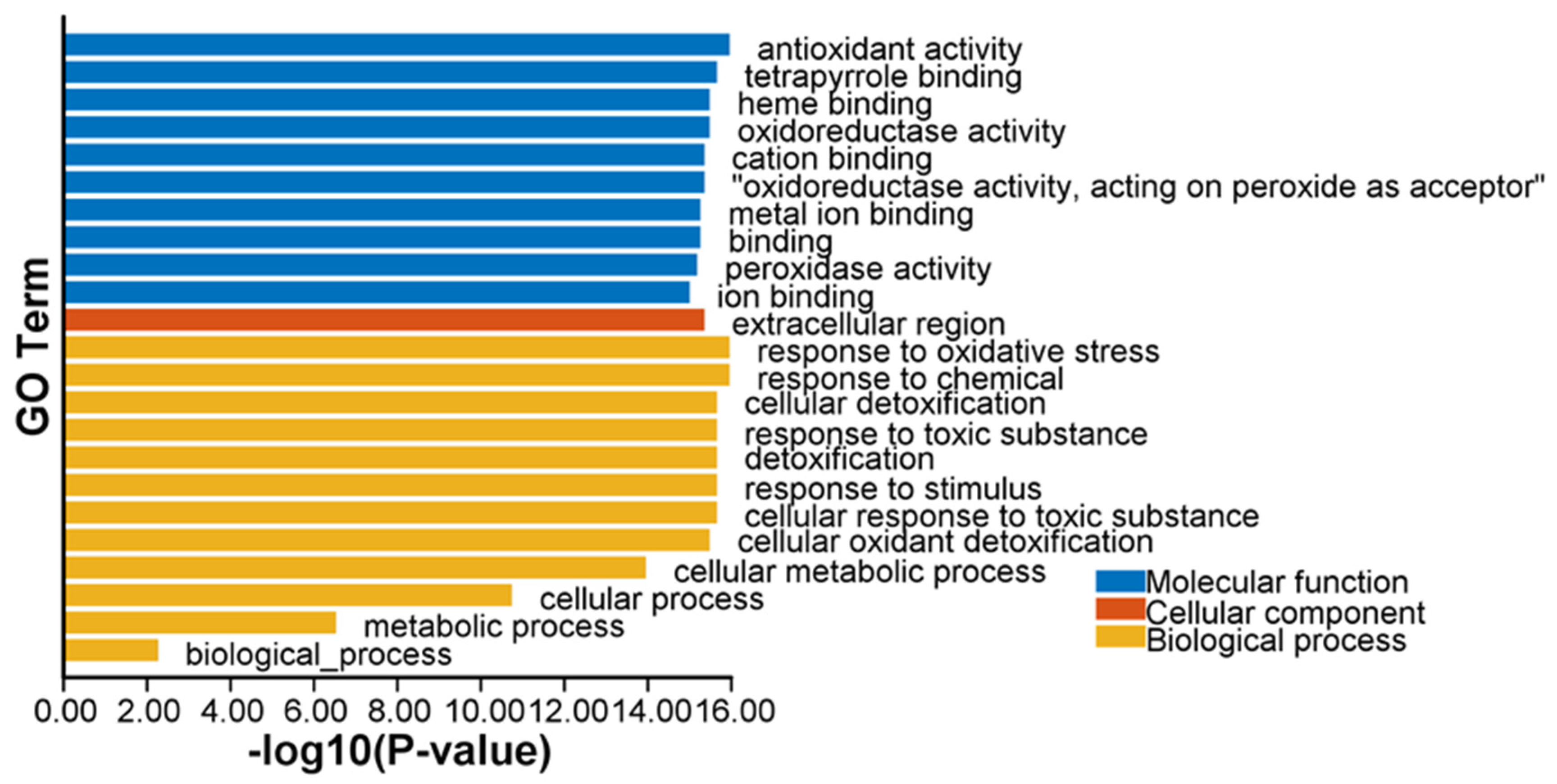
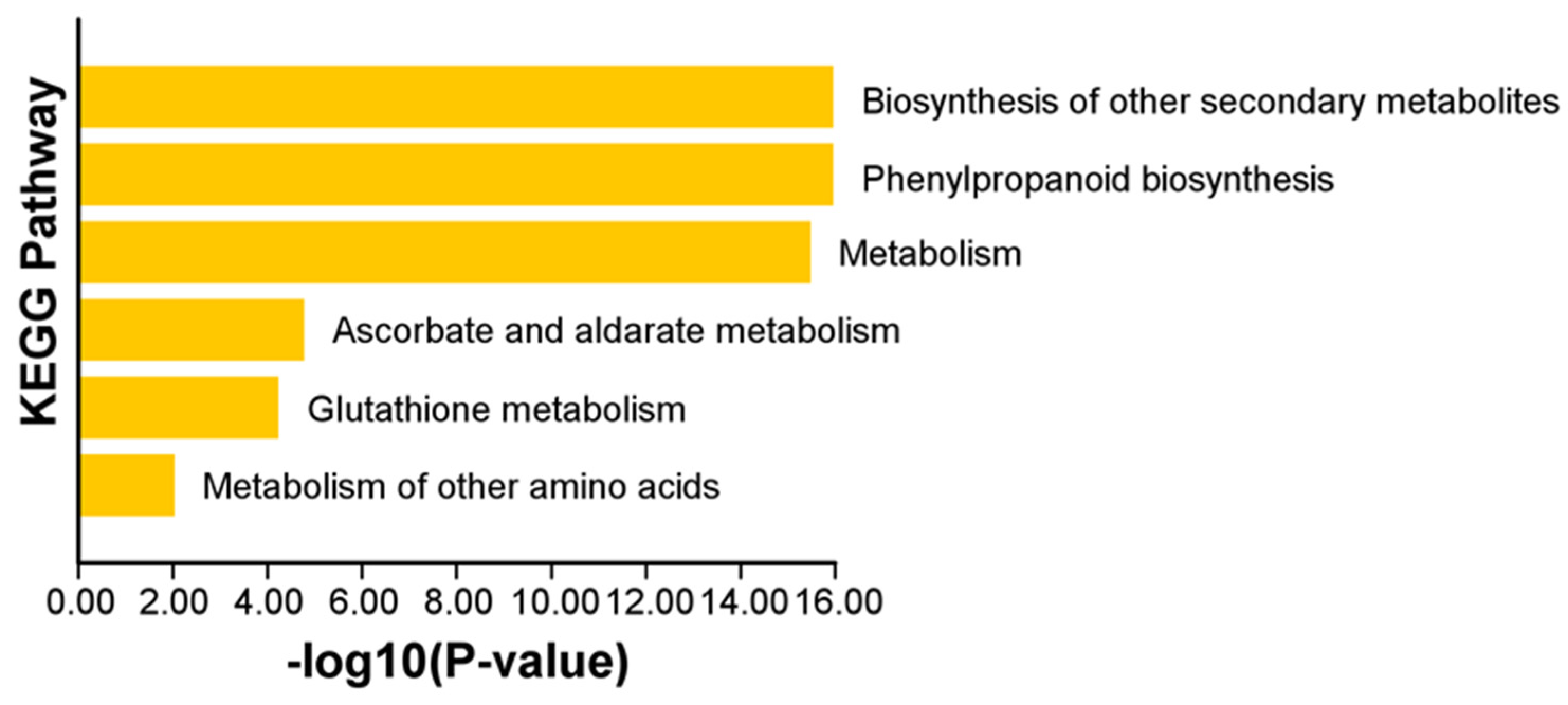
2.7. Functional Gene Ontology and Kyoto Encyclopedia of Genes and Genomes Enrichment of ClPRXs
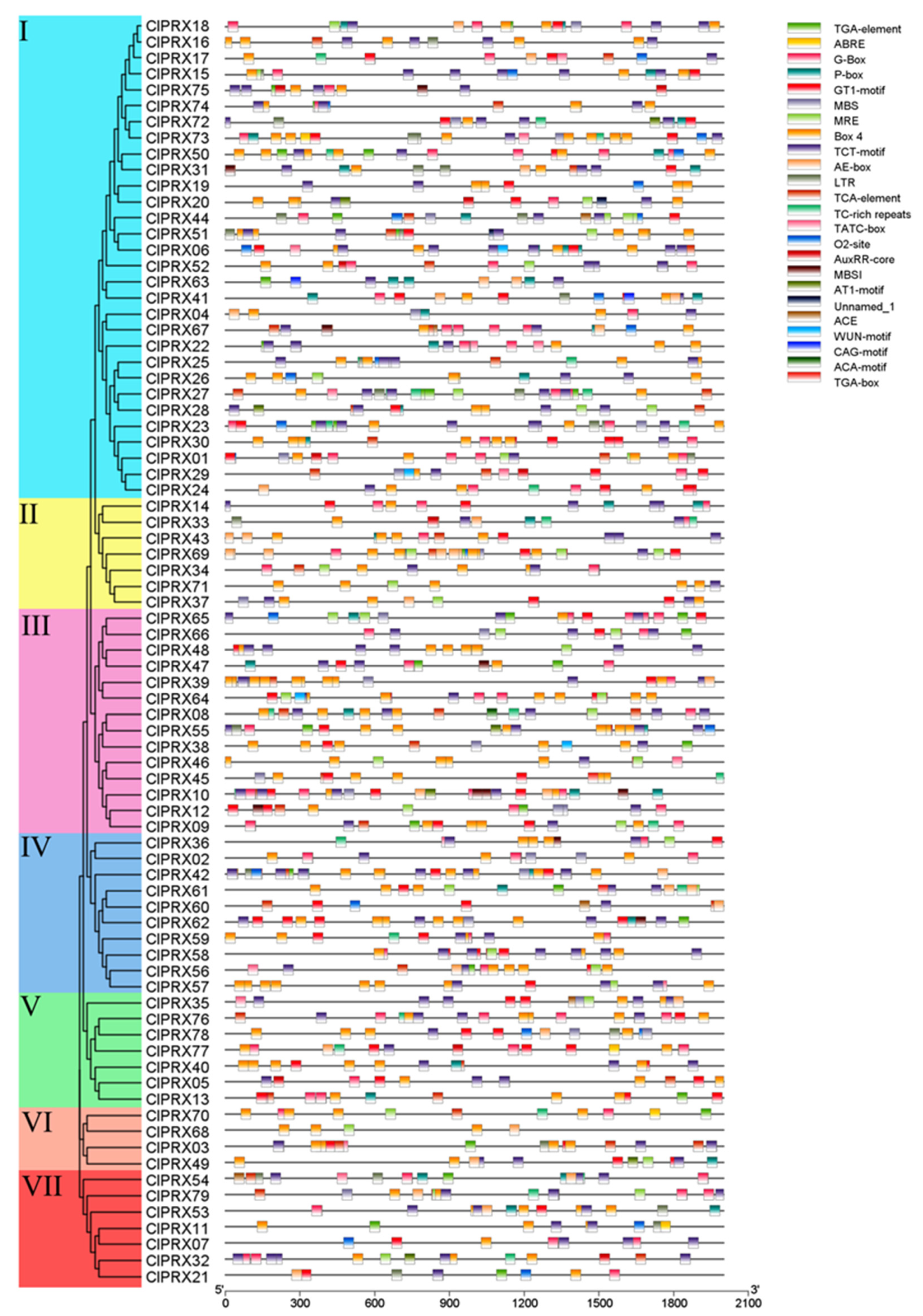
2.8. Promoter Elements of ClPRXs
2.9. Expression Analysis of ClPRXs in Watermelon Fruits
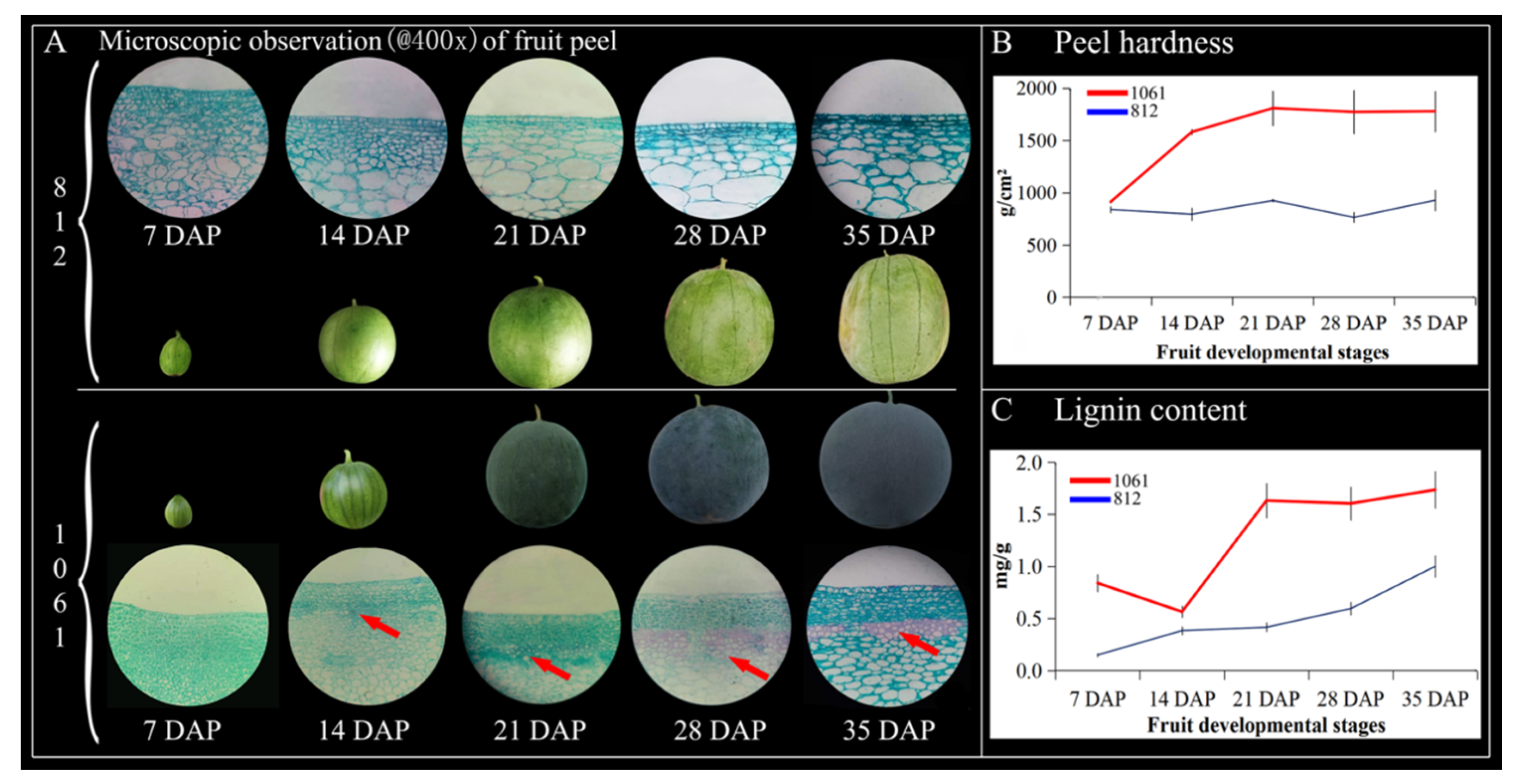
2.10. Observation of the Microstructure, Hardness, and Lignin Content of Watermelon Peel at Different Periods
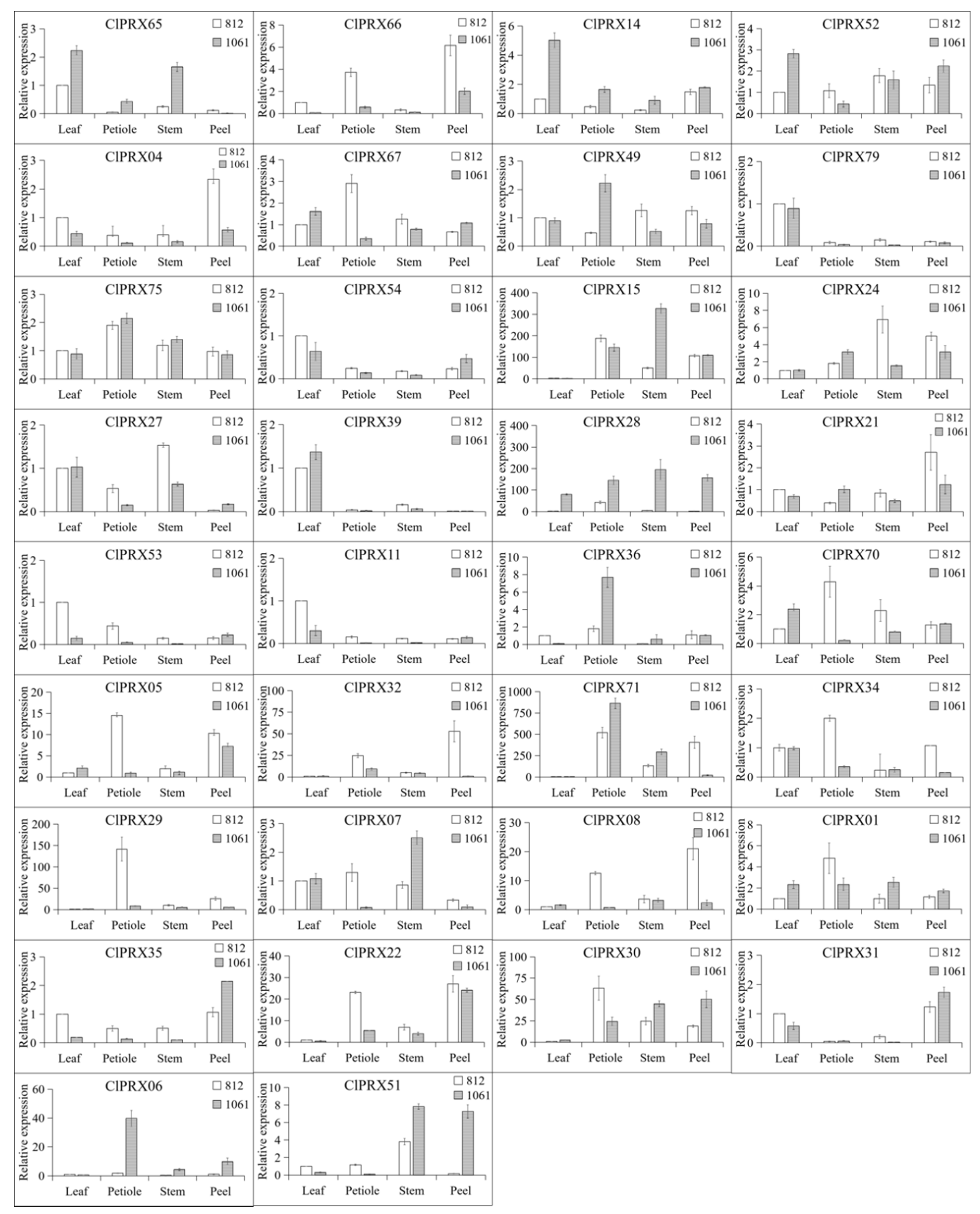
2.11. Expression Patterns of ClPRXs in Different Tissues
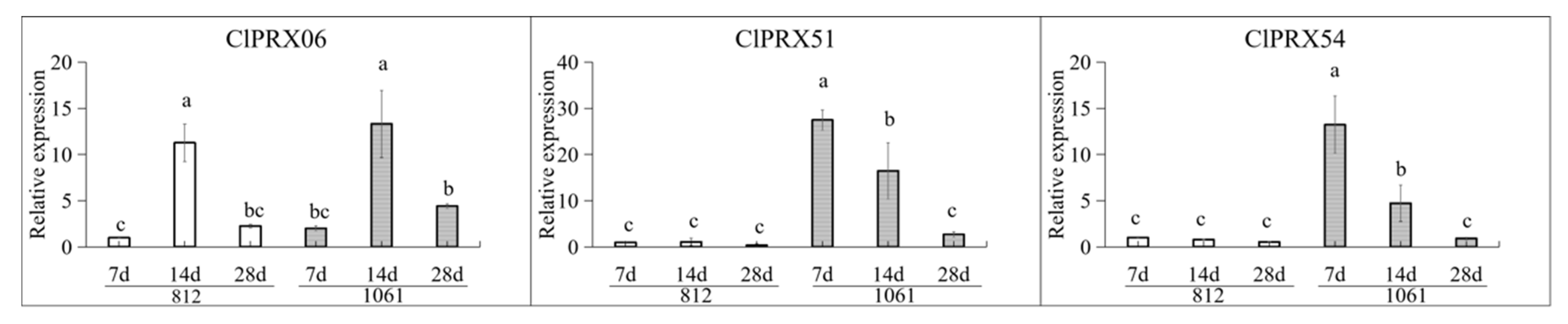
2.12. Expression Patterns of Candidate ClPRXs at Different Developmental Stages of Peel
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Sampling
4.2. Preliminary Search and Identification of ClPRXs in the Watermelon Genome
4.3. Chromosome Localization, Gene Replication Relationship, and Interspecific and Intraspecific Collinearity Analysis
4.4. ClPRXs’ Structure and Protein Domain Analysis
4.5. Construction of the Phylogenetic Tree for Watermelon and Arabidopsis
4.6. Functional Enrichment Analysis of ClPRXs
4.7. Transcriptome Analysis of ClPRXs at Different Periods of Peel and Flesh
4.8. RNA Extraction, cDNA Synthesis, and Gene Expression Analysis
4.9. Determination of Watermelon Peel Hardness and Paraffin Sectioning
4.10. Endogenous Quantification of Lignin Contents in Watermelon Peel
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, X.; Yuan, J.; Luo, W.; Qin, M.; Yang, J.; Wu, W.; Xie, X. Genome-Wide Identification and Expression Analysis of the Class III Peroxidase Gene Family in Potato (Solanum tuberosum L.). Front. Genet. 2020, 11, 3577. [Google Scholar] [CrossRef] [PubMed]
- Gabaldón, T.; Snel, B.; van Zimmeren, F.; Hemrika, W.; Tabak, H.; Huynen, M. Origin and evolution of the peroxisomal proteome. Biol. Direct 2006, 1, 8. [Google Scholar] [CrossRef]
- Bindschedler, L.V.; Dewdney, J.; Blee, K.A.; Stone, J.M.; Asai, T.; Plotnikov, J.; Denoux, C.; Hayes, T.; Gerrish, C.; Davies, D.R.; et al. Peroxidase-dependent apoplastic oxidative burst in Arabidopsis required for pathogen resistance. Plant J. 2006, 47, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Almagro, L.; Gómez, R.L.V.; Belchi-Navarro, S.; Bru, R.; Ros, B.A.; Pedreño, M.A. Class III perozidases in plant defence reaction. J. Exp. Bot. 2009, 2, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Valério, L.; De Meyer, M.; Penel, C.; Dunand, C. Expression analysis of the Arabidopsis peroxidase multigenic family. Phytochemistry 2004, 65, 1331–1342. [Google Scholar] [CrossRef] [PubMed]
- Passardi, F.; Longet, D.; Penel, C.; Dunand, C. The class III peroxidase multigenic family in rice and its evolution in land plants. Phytochemistry 2004, 65, 1879–1893. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Q.; Zhao, Y.; Han, G.; Zhu, S. Systematic analysis of maize class III peroxidase gene family reveals a conserved subfamily involved in abiotic stress response. Gene 2015, 566, 95–108. [Google Scholar] [CrossRef]
- Cao, Y.; Han, Y.; Meng, D.; Li, D.; Jin, Q.; Lin, Y.; Cai, Y. Structural, Evolutionary, and Functional Analysis of the Class III Peroxidase Gene Family in Chinese Pear (Pyrus bretschneideri). Front. Plant Sci. 2016, 7, 1874. [Google Scholar] [CrossRef]
- Wu, C.; Ding, X.; Ding, Z.; Tie, W.; Yan, Y.; Wang, Y.; Yang, H.; Hu, W. The Class III Peroxidase (POD) Gene Family in Cassava: Identification, Phylogeny, Duplication, and Expression. Int. J. Mol. Sci. 2019, 20, 2730. [Google Scholar] [CrossRef]
- Cai, K.W.; Liu, H.; Chen, S.; Zhao, X.Y.; Chen, S. Genome-wide identification and analysis of Class III peroxidases in Betula pendula. BMC Genom. 2021, 22, 314. [Google Scholar] [CrossRef]
- Hiraga, S.; Sasaki, K.; Ito, H.; Ohashi, Y.; Matsui, H. A Large Family of Class III Plant Peroxidases. Plant Cell Physiol. 2001, 42, 462–468. [Google Scholar] [CrossRef]
- Passardi, F.; Tognolli, M.; De Meyer, M.; Penel, C.; Dunand, C. Two cell wall associated peroxidases from Arabidopsis influence root elongation. Planta 2005, 223, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Francoz, E.; Ranocha, P.; Nguyen-Kim, H.; Jamet, E.; Burlat, V.; Dunand, C. Roles of cell wall peroxidases in plant development. Phytochemistry 2015, 112, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Kärkönen, A.; Warinowski, T.; Teeri, T.H.; Simola, L.K.; Fry, S.C. On the mechanism of apoplastic H2O2 production during lignin formation and elicitation in cultured spruce cells—peroxidases after elicitation. Planta 2009, 230, 553–567. [Google Scholar] [CrossRef]
- Fagerstedt, K.V.; Kukkola, E.M.; Koistinen, V.V.T.; Takahashi, J.; Marjamaa, K. Cell wall lignin is polymerized by Class Ⅲ secretable plant peroxidases in norway spruce. J. Integr. Plant Biol. 2010, 52, 186–194. [Google Scholar] [CrossRef]
- Herrero, J.; Fernández-Pérez, F.; Yebra, T.; Novo-Uzal, E.; Pomar, F.; Pedreño, M.; Cuello, J.; Guéra, A.; Esteban-Carrasco, A.; Zapata, J.M. Bioinformatic and functional characterization of the basic peroxidase 72 from Arabidopsis thaliana involved in lignin biosynthesis. Planta 2013, 237, 1599–1612. [Google Scholar] [CrossRef]
- Jun, S.; Yuko, K.; Koki, F.; Ryuichiro, K.; Yuj, T. Putative cationic cell-wall-bound peroxidase homologues in Arabidopsis, AtPrx2, AtPrx25, and AtPrx71, are involved in lignification. J. Agric. Food Chem. 2013, 61, 3781–3788. [Google Scholar] [CrossRef]
- Ipekçi, Z.; Ogras, T.; Altlnkut, A.; Bajrovic, K.; Kazan, K.; Gözükirmizi, N.; Boydak, M.; Tank, T.; Akalp, T.; Özden, Ö.; et al. Reduced leaf peroxidase activity is associated with reduced lignin content in transgenic poplar. Plant Biotechnol. 1999, 16, 381–387. [Google Scholar] [CrossRef]
- Mei, W.; Qin, Y.; Song, W.; Li, J.; Zhu, Y. Cotton GhPOX1 encoding plant class III peroxidase may be responsible for the high level of reactive oxygen species production that is related to cotton fiber elongation. J. Genet. Genom. 2009, 36, 141–150. [Google Scholar] [CrossRef]
- Zhu, X.; Jiang, L.; Cai, Y.; Cao, Y. Functional analysis of four Class III peroxidases from Chinese pear fruit: A critical role in lignin polymerization. Physiol. Mol. Biol. Plants 2021, 27, 515–522. [Google Scholar] [CrossRef]
- Yang, T.; Amanullah, S.; Pan, J.; Chen, G.; Liu, S.; Ma, S.; Wang, J.; Gao, P.; Wang, X. Identification of putative genetic regions for watermelon rind hardness and related traits by BSA-seq and QTL mapping. Euphytica 2021, 217, 1–18. [Google Scholar] [CrossRef]
- Zhao, D.; Xu, C.; Luan, Y.; Shi, W.; Tang, Y.; Tao, J. Silicon enhances stem strength by promoting lignin accumulation in herbaceous peony (Paeonia lactiflora Pall.). Int. J. Biol. Macromol. 2021, 190, 769–779. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Pérez, F.; Pomar, F.; Pedreño, M.A.; Novo-Uzal, E. Suppression of Arabidopsis peroxidase 72 alters cell wall and phenylpropanoid metabolism. Plant Sci. 2015, 239, 192–199. [Google Scholar] [CrossRef]
- Passardi, F.; Cosio, C.; Penel, C.; Dunand, C. Peroxidases have more functions than a Swiss army knife. Plant Cell Rep. 2005, 24, 255–265. [Google Scholar] [CrossRef]
- Moore, C.; Purugganan, R.; Michael, D. The early stages of duplicate gene evolution. Proc. Natl. Acad. Sci. USA 2003, 100, 15682–15687. [Google Scholar] [CrossRef]
- Han, Y.; Ding, T.; Su, B.; Jiang, H. Genome-Wide Identification, Characterization and Expression Analysis of the Chalcone Synthase Family in Maize. Int. J. Mol. Sci. 2016, 17, 161. [Google Scholar] [CrossRef]
- Martinez, C.; Geiger, J.P.; Bresson, E.; Daniel, J.F.; Dai, G.H.; Andray, A.; Nicole, M. Plant peroxidases: Biochemistry and physiology. Plant Growth Regul. 1996, 12, 303–312. [Google Scholar]
- Buffard, D.; Breda, C.; van Huystee, R.B.; Asemota, O.; Pierre, M.; Ha, D.B.; Esnault, R. Molecular cloning of complementary DNAs encoding two cationic peroxidases from cultivated peanut cells. Proc. Natl. Acad. Sci. USA. 1990, 87, 8874–8878. [Google Scholar] [CrossRef]
- Welinder, K.G.; Justesen, A.F.; Jaersgard, I.V.K.; Jensen, R.B.; Rasmussen, S.K.; Jespersen, H.M.; Duroux, L. Structural diversity and transcription of Class-IIl peroxidases from Arabidopsis thaliana. Eur. J. Biochem. 2002, 269, 6063–6081. [Google Scholar] [CrossRef] [PubMed]
- Rogozin, I.; Wolf, Y.; Sorokin, A.V.; Mirkin, B.; Koonin, E.V. Remarkable Interkingdom Conservation of Intron Positions and Massive, Lineage-Specific Intron Loss and Gain in Eukaryotic Evolution. Curr. Biol. 2003, 13, 1512–1517. [Google Scholar] [CrossRef]
- Yang, L.; Takuno, S.; Waters, E.R.; Gaut, B.S. Lowly Expressed Genes in Arabidopsis thaliana Bear the Signature of Possible Pseudogenization by Promoter Degradation. Mol. Biol. Evol. 2010, 28, 1193–1203. [Google Scholar] [CrossRef][Green Version]
- Li, Z.Q.; Li, J.T.; Bing, J.; Zhang, G.F. The role analysis of APX gene family in the growth and developmental processes and in response to abiotic stresses in Arabidopsis thaliana. Yi Chuan Hered. 2019, 41, 534–547. [Google Scholar]
- Wang, X.; Yang, T.; Liu, Z.; Sun, L.; Zhu, Z.; Gao, P.; Liu, S.; Luan, F. Analysis on hardness related characters of watermelon rind. J. Northeast Agric. Univ. 2020, 51, 35–44. [Google Scholar]
- Wang, R.; Xue, Y.; Fan, J.; Yao, J.-L.; Qin, M.; Lin, T.; Lian, Q.; Zhang, M.; Li, X.; Li, J.; et al. A systems genetics approach reveals PbrNSC as a regulator of lignin and cellulose biosynthesis in stone cells of pear fruit. Genome Biol. 2021, 22, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Liu, S.; Gao, P.; Liu, H.; Wang, X.; Luan, F.; Zhang, Q.; Dai, Z. Expression of ClPAP and ClPSY1 in watermelon correlates with chromoplast differentiation, carotenoid accumulation, and flesh color formation. Sci. Hortic. 2020, 270, 109437. [Google Scholar] [CrossRef]
- Finn, R.; Jody, C.; William, A.; Miller, B.; Wheeler, T.; Fabian, S.; Alex, B.; Eddy, S. HMMER web server: 2015 update. Nucleic Acids Res. 2015, 43, 30–38. [Google Scholar] [CrossRef]
- Tognolli, M.; Penel, C.; Greppin, H.; Simon, P. Analysis and expression of the class III peroxidase large gene family in Arabidopsis thaliana. Gene 2002, 288, 129–138. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Gasteiger, E.; Hoogland, C.; Gattiker, A.; Wilkins, M.R.; Appel, R.D.; Bairoch, A. Chapter 52: Protein identification and analysis tools on the ExPASy Server. In The Proteomics Protocols Handbook; Walker, J.M., Ed.; Humana Press: Moscow, Russia, 2005; Volume 71, pp. 571–607. [Google Scholar]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Guo, S.; Sun, H.; Zhang, H.; Liu, J.; Ren, Y.; Gong, G.; Jiao, C.; Zheng, Y.; Yang, W.; Fei, Z.; et al. Comparative Transcriptome Analysis of Cultivated and Wild Watermelon during Fruit Development. PLoS ONE 2015, 10, e0130267. [Google Scholar] [CrossRef]
- Singh, V.K.; Mangalam, A.; Dwivedi, S.; Naik, S. Primer Premier: Program for Design of Degenerate Primers from a Protein Sequence. BioTechniques 1998, 24, 318–319. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]

| Gene Symbol | Gene ID | Chromosome | Starting Point | End Position | Length of Coding Region | Length of CDS | Number of Exons | Coding Protein Characteristics | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amino Acid (aa) Length | Molecular Weight (MW)/ 1000-Dalton (kDa) | Isoelectric Point (PI) | Secondary Structure Characteristics | ||||||||||
| α-Helix (%) | β-Sheet (%) | Random Coil (%) | |||||||||||
| ClPRX01 | Cla97C01G005010.1 | 1 | 4723552 | 4726977 | 3426 | 1011 | 4 | 336 | 35,957.3 | 4.42 | 25.30 | 14.58 | 60.12 |
| ClPRX02 | Cla97C01G011510.1 | 1 | 20830864 | 20835096 | 4233 | 975 | 4 | 324 | 35,565.6 | 5.58 | 29.32 | 14.51 | 56.17 |
| ClPRX03 | Cla97C01G013060.1 | 1 | 26885053 | 26886313 | 1261 | 1005 | 2 | 334 | 37,268.8 | 6.17 | 23.95 | 19.16 | 56.89 |
| ClPRX04 | Cla97C01G015760.1 | 1 | 29495599 | 29497136 | 1538 | 984 | 4 | 327 | 36,838.1 | 6.88 | 33.03 | 17.43 | 49.54 |
| ClPRX05 | Cla97C01G016570.1 | 1 | 30277513 | 30278818 | 1306 | 987 | 4 | 328 | 35,737.8 | 9.14 | 30.79 | 19.21 | 50.00 |
| ClPRX06 | Cla97C01G019360.1 | 1 | 32325769 | 32328138 | 2370 | 1002 | 4 | 333 | 36,365.2 | 9.05 | 27.33 | 16.82 | 55.86 |
| ClPRX07 | Cla97C01G019890.1 | 1 | 32769843 | 32773816 | 3974 | 891 | 9 | 296 | 32,914.6 | 8.30 | 38.18 | 12.50 | 49.32 |
| ClPRX08 | Cla97C01G020000.1 | 1 | 32847704 | 32849046 | 1343 | 960 | 4 | 319 | 34,676.6 | 9.14 | 33.86 | 8.78 | 57.37 |
| ClPRX09 | Cla97C02G028190.1 | 2 | 1675720 | 1677245 | 1526 | 978 | 4 | 325 | 35,449.7 | 8.62 | 31.38 | 17.85 | 50.77 |
| ClPRX10 | Cla97C02G028200.1 | 2 | 1681804 | 1692106 | 10303 | 1755 | 9 | 584 | 64,150.5 | 8.95 | 26.54 | 20.38 | 53.08 |
| ClPRX11 | Cla97C02G030990.1 | 2 | 3941579 | 3945614 | 4036 | 861 | 9 | 286 | 31,559.9 | 6.67 | 40.21 | 10.14 | 49.65 |
| ClPRX12 | Cla97C02G035070.1 | 2 | 10386004 | 10387325 | 1322 | 987 | 4 | 328 | 35,847.9 | 8.09 | 39.02 | 13.41 | 47.56 |
| ClPRX13 | Cla97C02G037820.1 | 2 | 24964862 | 24967129 | 2268 | 1005 | 4 | 334 | 36,089.3 | 8.86 | 27.84 | 20.66 | 51.50 |
| ClPRX14 | Cla97C02G044750.1 | 2 | 32912405 | 32914360 | 1956 | 1065 | 2 | 354 | 39,631.8 | 9.21 | 32.49 | 12.71 | 54.80 |
| ClPRX15 | Cla97C02G045070.1 | 2 | 33199676 | 33201140 | 1465 | 981 | 4 | 326 | 35,326.4 | 9.38 | 22.39 | 19.02 | 58.59 |
| ClPRX16 | Cla97C02G045080.1 | 2 | 33206687 | 33208047 | 1361 | 870 | 4 | 289 | 31,399.8 | 8.72 | 24.22 | 19.38 | 56.40 |
| ClPRX17 | Cla97C02G045090.1 | 2 | 33211116 | 33212531 | 1416 | 975 | 4 | 324 | 35,542.8 | 9.41 | 32.41 | 15.43 | 52.16 |
| ClPRX18 | Cla97C02G045100.1 | 2 | 33215577 | 33216865 | 1289 | 942 | 4 | 313 | 34,070.4 | 5.47 | 23.96 | 17.25 | 58.79 |
| ClPRX19 | Cla97C02G045120.1 | 2 | 33230817 | 33232144 | 1328 | 960 | 3 | 319 | 34,792.8 | 9.27 | 24.76 | 21.63 | 53.61 |
| ClPRX20 | Cla97C02G045130.1 | 2 | 33237622 | 33238928 | 1307 | 960 | 3 | 319 | 34,574.3 | 8.53 | 21.94 | 19.75 | 58.31 |
| ClPRX21 | Cla97C02G046770.1 | 2 | 34511814 | 34515485 | 3672 | 753 | 9 | 250 | 27,274.9 | 5.60 | 43.60 | 8.40 | 48.00 |
| ClPRX22 | Cla97C02G049870.1 | 2 | 37212086 | 37215158 | 3073 | 1020 | 4 | 339 | 36,812.2 | 7.53 | 26.84 | 21.83 | 51.33 |
| ClPRX23 | Cla97C02G049880.1 | 2 | 37219380 | 37220760 | 1381 | 1014 | 4 | 337 | 36,152.5 | 4.73 | 22.26 | 18.99 | 58.75 |
| ClPRX24 | Cla97C02G049890.1 | 2 | 37226317 | 37227586 | 1270 | 1023 | 4 | 340 | 36,696.6 | 8.60 | 26.47 | 16.18 | 57.35 |
| ClPRX25 | Cla97C02G049900.1 | 2 | 37234294 | 37235546 | 1253 | 993 | 3 | 330 | 35,335.4 | 5.71 | 33.64 | 15.45 | 50.91 |
| ClPRX26 | Cla97C02G049910.1 | 2 | 37238447 | 37239716 | 1270 | 990 | 3 | 329 | 35,869.1 | 9.07 | 32.83 | 12.46 | 54.71 |
| ClPRX27 | Cla97C02G049920.1 | 2 | 37245856 | 37260161 | 14306 | 990 | 4 | 329 | 36,370.9 | 5.30 | 27.96 | 14.29 | 57.75 |
| ClPRX28 | Cla97C02G049930.1 | 2 | 37271693 | 37272975 | 1283 | 1026 | 4 | 341 | 36,810.4 | 6.53 | 31.38 | 13.78 | 54.84 |
| ClPRX29 | Cla97C02G049940.1 | 2 | 37280732 | 37282786 | 2055 | 960 | 4 | 319 | 34,277.4 | 4.60 | 24.76 | 18.81 | 56.43 |
| ClPRX30 | Cla97C02G049950.1 | 2 | 37290261 | 37291772 | 1512 | 963 | 4 | 320 | 34,345.3 | 4.70 | 23.12 | 23.75 | 53.12 |
| ClPRX31 | Cla97C03G051030.1 | 3 | 255043 | 256982 | 1940 | 945 | 4 | 314 | 33,950.3 | 5.75 | 29.94 | 16.24 | 53.82 |
| ClPRX32 | Cla97C03G053010.1 | 3 | 2050061 | 2058477 | 8417 | 1899 | 14 | 632 | 71,578.4 | 5.41 | 40.19 | 12.97 | 46.84 |
| ClPRX33 | Cla97C03G055260.1 | 3 | 4235106 | 4238033 | 2928 | 1029 | 3 | 342 | 37,922.2 | 5.75 | 29.82 | 16.08 | 54.09 |
| ClPRX34 | Cla97C03G055890.1 | 3 | 4781189 | 4782652 | 1464 | 996 | 4 | 331 | 37,689.3 | 8.66 | 40.48 | 11.78 | 47.73 |
| ClPRX35 | Cla97C03G059200.1 | 3 | 8602556 | 8605339 | 2784 | 1044 | 3 | 347 | 37,903.6 | 9.27 | 37.75 | 14.41 | 47.84 |
| ClPRX36 | Cla97C04G070210.1 | 4 | 10427630 | 10432305 | 4676 | 960 | 4 | 319 | 35,032.0 | 8.10 | 31.35 | 18.18 | 50.47 |
| ClPRX37 | Cla97C04G075580.1 | 4 | 23076560 | 23078327 | 1768 | 894 | 4 | 297 | 33,467.3 | 5.70 | 37.71 | 12.46 | 49.83 |
| ClPRX38 | Cla97C05G089640.1 | 5 | 7906134 | 7907486 | 1353 | 918 | 3 | 305 | 33,412.1 | 8.04 | 32.79 | 14.10 | 53.11 |
| ClPRX39 | Cla97C05G097050.1 | 5 | 26403410 | 26407012 | 3603 | 986 | 3 | 328 | 34,981.4 | 5.21 | 17.90 | 27.10 | 54.80 |
| ClPRX40 | Cla97C05G098800.1 | 5 | 27994171 | 27995541 | 1371 | 963 | 4 | 320 | 35,772.8 | 9.05 | 26.80 | 18.10 | 55.00 |
| ClPRX41 | Cla97C05G105280.1 | 5 | 32937084 | 32940587 | 3504 | 1020 | 4 | 339 | 37,654.1 | 5.28 | 32.10 | 15.60 | 52.20 |
| ClPRX42 | Cla97C05G107830.1 | 5 | 34625160 | 34627681 | 2522 | 1032 | 2 | 343 | 37,943.1 | 5.24 | 32.60 | 16.90 | 50.40 |
| ClPRX43 | Cla97C06G110270.1 | 6 | 920898 | 925297 | 4400 | 1017 | 4 | 338 | 37,923.6 | 9.08 | 35.80 | 14.20 | 50.00 |
| ClPRX44 | Cla97C06G113900.1 | 6 | 4822850 | 4824110 | 1261 | 1014 | 4 | 337 | 36,341.1 | 4.93 | 33.20 | 12.70 | 54.00 |
| ClPRX45 | Cla97C06G115010.1 | 6 | 5937272 | 5940105 | 2834 | 995 | 4 | 329 | 36,275.5 | 9.47 | 34.30 | 13.30 | 52.20 |
| ClPRX46 | Cla97C06G115020.1 | 6 | 5956825 | 5959012 | 2188 | 990 | 4 | 329 | 36,069.2 | 8.37 | 28.80 | 16.40 | 54.70 |
| ClPRX47 | Cla97C06G120340.1 | 6 | 22349470 | 22352615 | 3146 | 978 | 4 | 325 | 35,998.0 | 6.95 | 33.50 | 11.00 | 55.30 |
| ClPRX48 | Cla97C06G120350.1 | 6 | 22368798 | 22371140 | 2343 | 963 | 4 | 320 | 35,447.3 | 5.62 | 28.70 | 18.10 | 53.10 |
| ClPRX49 | Cla97C06G126320.1 | 6 | 28110200 | 28111195 | 996 | 996 | 1 | 331 | 36,316.6 | 6.95 | 37.70 | 12.60 | 49.50 |
| ClPRX50 | Cla97C07G131030.1 | 7 | 2626605 | 2628423 | 1819 | 799 | 3 | 315 | 34,150.5 | 8.79 | 29.50 | 14.60 | 55.80 |
| ClPRX51 | Cla97C07G135790.1 | 7 | 21625198 | 21627270 | 2073 | 1004 | 4 | 333 | 36,548.5 | 9.13 | 36.60 | 12.30 | 51.00 |
| ClPRX52 | Cla97C07G144420.1 | 7 | 31806093 | 31807972 | 1880 | 1024 | 3 | 339 | 38,007.4 | 6.01 | 25.60 | 16.80 | 57.50 |
| ClPRX53 | Cla97C08G148570.1 | 8 | 16719601 | 16725763 | 6163 | 1710 | 12 | 457 | 49,685.2 | 7.63 | 31.90 | 12.20 | 55.80 |
| ClPRX54 | Cla97C08G156740.1 | 8 | 24456600 | 24460113 | 3514 | 1011 | 10 | 336 | 36,670.9 | 6.97 | 33.60 | 13.90 | 52.30 |
| ClPRX55 | Cla97C08G157670.1 | 8 | 25164283 | 25165558 | 1276 | 954 | 4 | 317 | 34,657.7 | 9.31 | 34.70 | 11.00 | 54.20 |
| ClPRX56 | Cla97C09G167060.1 | 9 | 3951866 | 3953193 | 1328 | 999 | 2 | 331 | 36,014.3 | 9.57 | 36.50 | 15.70 | 47.70 |
| ClPRX57 | Cla97C09G167070.1 | 9 | 3957503 | 3958980 | 1478 | 1008 | 2 | 335 | 36,338.0 | 6.39 | 34.90 | 15.20 | 49.80 |
| ClPRX58 | Cla97C09G167080.1 | 9 | 3961741 | 3963120 | 1380 | 1020 | 3 | 339 | 36,849.0 | 9.00 | 27.10 | 21.50 | 51.30 |
| ClPRX59 | Cla97C09G167090.1 | 9 | 3968829 | 3971128 | 2300 | 1011 | 3 | 336 | 36,458.7 | 9.21 | 31.80 | 18.70 | 49.40 |
| ClPRX60 | Cla97C09G167100.1 | 9 | 3973203 | 3974880 | 1678 | 1008 | 2 | 335 | 36,590.7 | 5.84 | 27.40 | 16.40 | 56.10 |
| ClPRX61 | Cla97C09G167110.1 | 9 | 3978060 | 3980910 | 2851 | 987 | 2 | 328 | 36,322.7 | 8.37 | 34.70 | 11.80 | 53.30 |
| ClPRX62 | Cla97C09G167120.1 | 9 | 3983474 | 3985318 | 1845 | 999 | 2 | 332 | 35,972.0 | 8.36 | 27.10 | 18.00 | 54.80 |
| ClPRX63 | Cla97C09G175150.1 | 9 | 13236273 | 13238337 | 2065 | 998 | 4 | 331 | 37,419.7 | 5.94 | 23.20 | 23.80 | 52.80 |
| ClPRX64 | Cla97C09G177060.1 | 9 | 26136385 | 26142213 | 5829 | 990 | 4 | 329 | 35,419.4 | 5.64 | 33.70 | 13.30 | 52.80 |
| ClPRX65 | Cla97C09G177290.1 | 9 | 27493392 | 27495394 | 2003 | 989 | 3 | 329 | 35,713.6 | 8.39 | 19.40 | 24.00 | 56.50 |
| ClPRX66 | Cla97C09G177300.1 | 9 | 27526270 | 27528397 | 2128 | 987 | 4 | 328 | 36,178.3 | 8.11 | 21.00 | 24.70 | 54.20 |
| ClPRX67 | Cla97C09G178920.1 | 9 | 32289531 | 32291626 | 2096 | 1044 | 4 | 347 | 38,431.5 | 4.93 | 30.20 | 14.40 | 55.30 |
| ClPRX68 | Cla97C09G180150.1 | 9 | 33769566 | 33770531 | 966 | 966 | 1 | 321 | 35,223.1 | 8.06 | 32.70 | 13.40 | 53.80 |
| ClPRX69 | Cla97C09G184240.1 | 9 | 37364363 | 37365922 | 1560 | 1170 | 4 | 389 | 43,464.0 | 5.18 | 32.10 | 14.90 | 52.90 |
| ClPRX70 | Cla97C10G197400.1 | 10 | 27216069 | 27217049 | 981 | 983 | 1 | 326 | 35,890.8 | 8.33 | 28.20 | 12.80 | 58.90 |
| ClPRX71 | Cla97C10G203590.1 | 10 | 33215489 | 33219675 | 4187 | 969 | 5 | 322 | 36,313.8 | 7.58 | 30.10 | 13.30 | 56.50 |
| ClPRX72 | Cla97C11G206940.1 | 11 | 765876 | 767014 | 1139 | 951 | 3 | 316 | 34,727.6 | 9.36 | 25.90 | 19.30 | 54.70 |
| ClPRX73 | Cla97C11G207220.1 | 11 | 1081415 | 1082545 | 1131 | 945 | 3 | 314 | 33,974.5 | 8.07 | 24.80 | 20.00 | 55.10 |
| ClPRX74 | Cla97C11G207230.1 | 11 | 1086466 | 1087611 | 1146 | 954 | 3 | 317 | 34,413.2 | 8.36 | 26.80 | 20.80 | 52.30 |
| ClPRX75 | Cla97C11G212730.1 | 11 | 6071711 | 6081296 | 9586 | 7195 | 9 | 564 | 61,162.5 | 9.10 | 25.80 | 10.40 | 63.60 |
| ClPRX76 | Cla97C11G214520.1 | 11 | 7856001 | 7857609 | 1609 | 972 | 3 | 323 | 34,727.5 | 5.35 | 35.60 | 16.40 | 47.90 |
| ClPRX77 | Cla97C11G214530.1 | 11 | 7867036 | 7870803 | 3768 | 927 | 2 | 308 | 33,039.3 | 6.90 | 25.00 | 19.40 | 55.50 |
| ClPRX78 | Cla97C11G214540.1 | 11 | 7899468 | 7901107 | 1640 | 984 | 3 | 327 | 35,316.9 | 4.90 | 34.80 | 15.20 | 49.80 |
| ClPRX79 | Cla97C11G220240.1 | 11 | 26276590 | 26282064 | 5475 | 1050 | 11 | 349 | 38,057.1 | 6.06 | 36.30 | 8.60 | 55.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, T.; Zhang, P.; Pan, J.; Amanullah, S.; Luan, F.; Han, W.; Liu, H.; Wang, X. Genome-Wide Analysis of the Peroxidase Gene Family and Verification of Lignin Synthesis-Related Genes in Watermelon. Int. J. Mol. Sci. 2022, 23, 642. https://doi.org/10.3390/ijms23020642
Yang T, Zhang P, Pan J, Amanullah S, Luan F, Han W, Liu H, Wang X. Genome-Wide Analysis of the Peroxidase Gene Family and Verification of Lignin Synthesis-Related Genes in Watermelon. International Journal of Molecular Sciences. 2022; 23(2):642. https://doi.org/10.3390/ijms23020642
Chicago/Turabian StyleYang, Tiantian, Pengyu Zhang, Jiahui Pan, Sikandar Amanullah, Feishi Luan, Wenhao Han, Hongyu Liu, and Xuezheng Wang. 2022. "Genome-Wide Analysis of the Peroxidase Gene Family and Verification of Lignin Synthesis-Related Genes in Watermelon" International Journal of Molecular Sciences 23, no. 2: 642. https://doi.org/10.3390/ijms23020642
APA StyleYang, T., Zhang, P., Pan, J., Amanullah, S., Luan, F., Han, W., Liu, H., & Wang, X. (2022). Genome-Wide Analysis of the Peroxidase Gene Family and Verification of Lignin Synthesis-Related Genes in Watermelon. International Journal of Molecular Sciences, 23(2), 642. https://doi.org/10.3390/ijms23020642







