Melatonin Promotes SGT1-Involved Signals to Ameliorate Drought Stress Adaption in Rice
Abstract
:1. Introduction
2. Results
2.1. Determination of Optimal Concentration of Melatonin on Drought Stress
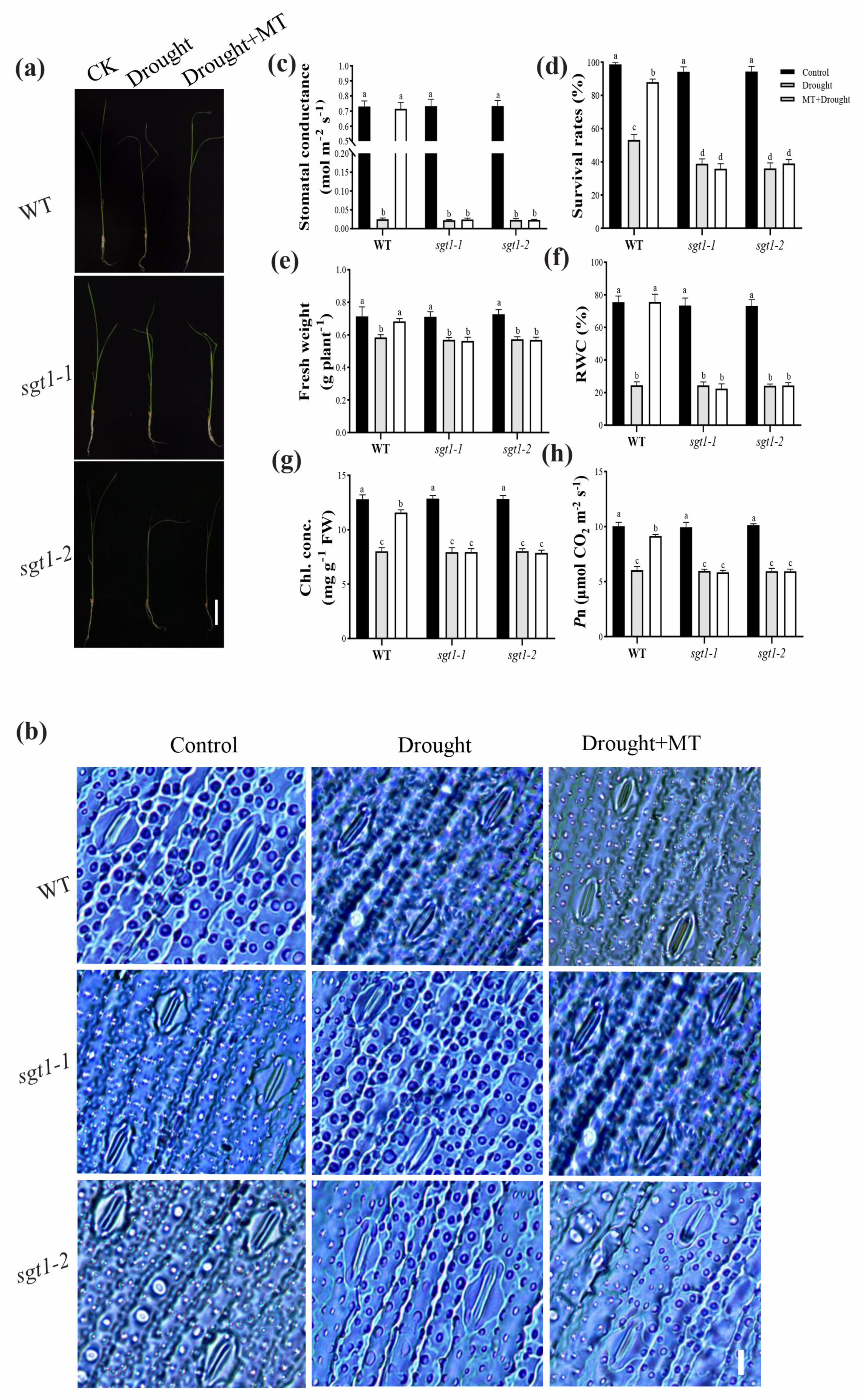
2.2. Effects of Melatonin on the Seedling Performance in sgt1 Mutants under Drought Stress
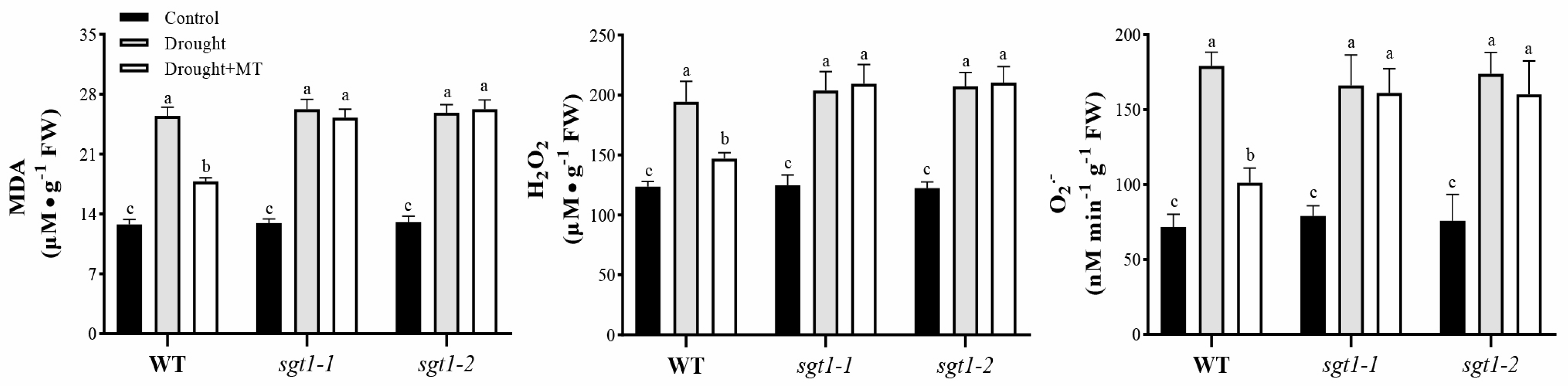
2.3. Effects of Melatonin on the Oxidative Damages in sgt1 Mutants under Drought Stress
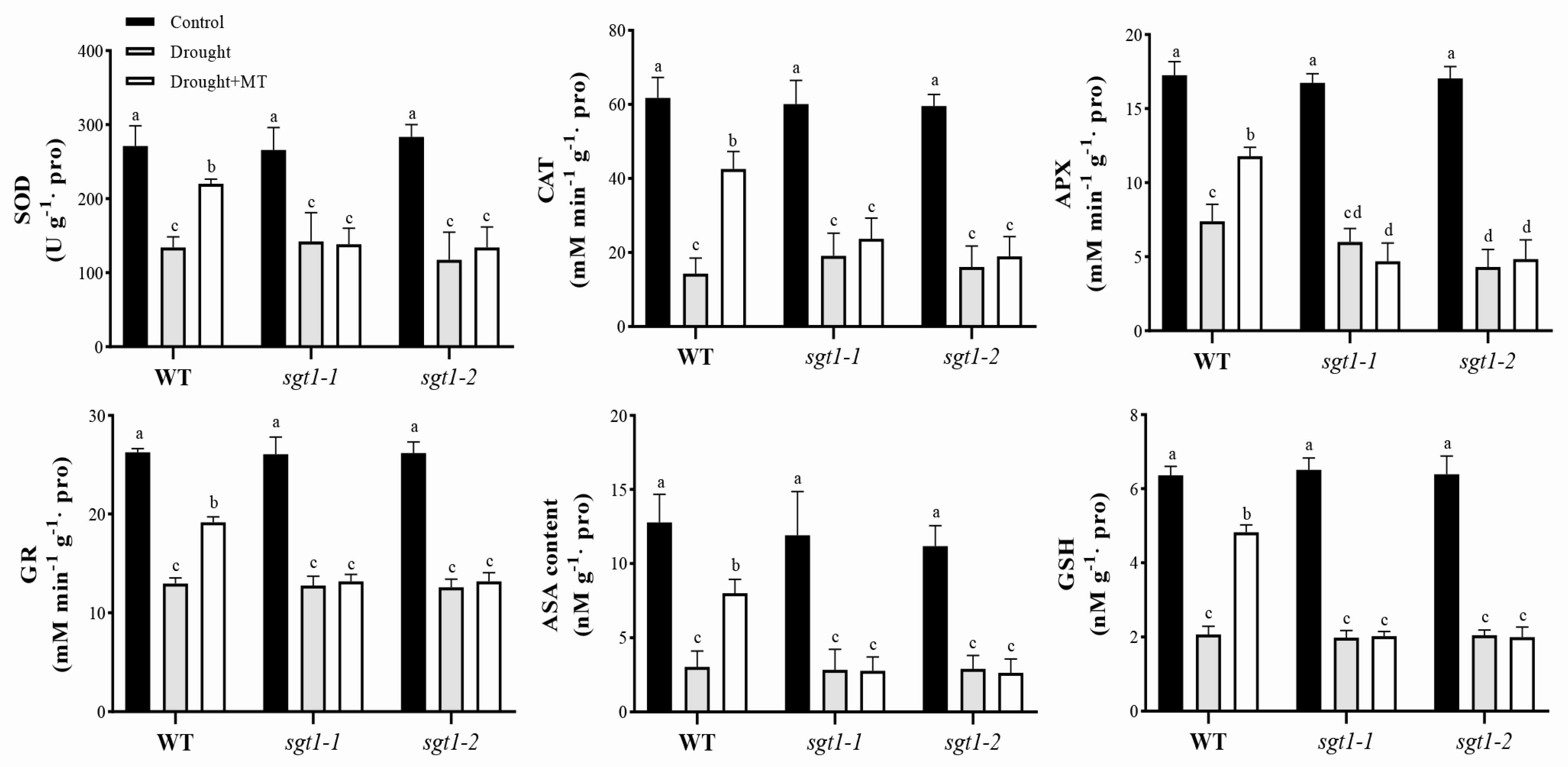
2.4. Effects of Melatonin on the Antioxidant Activity under Drought Stress
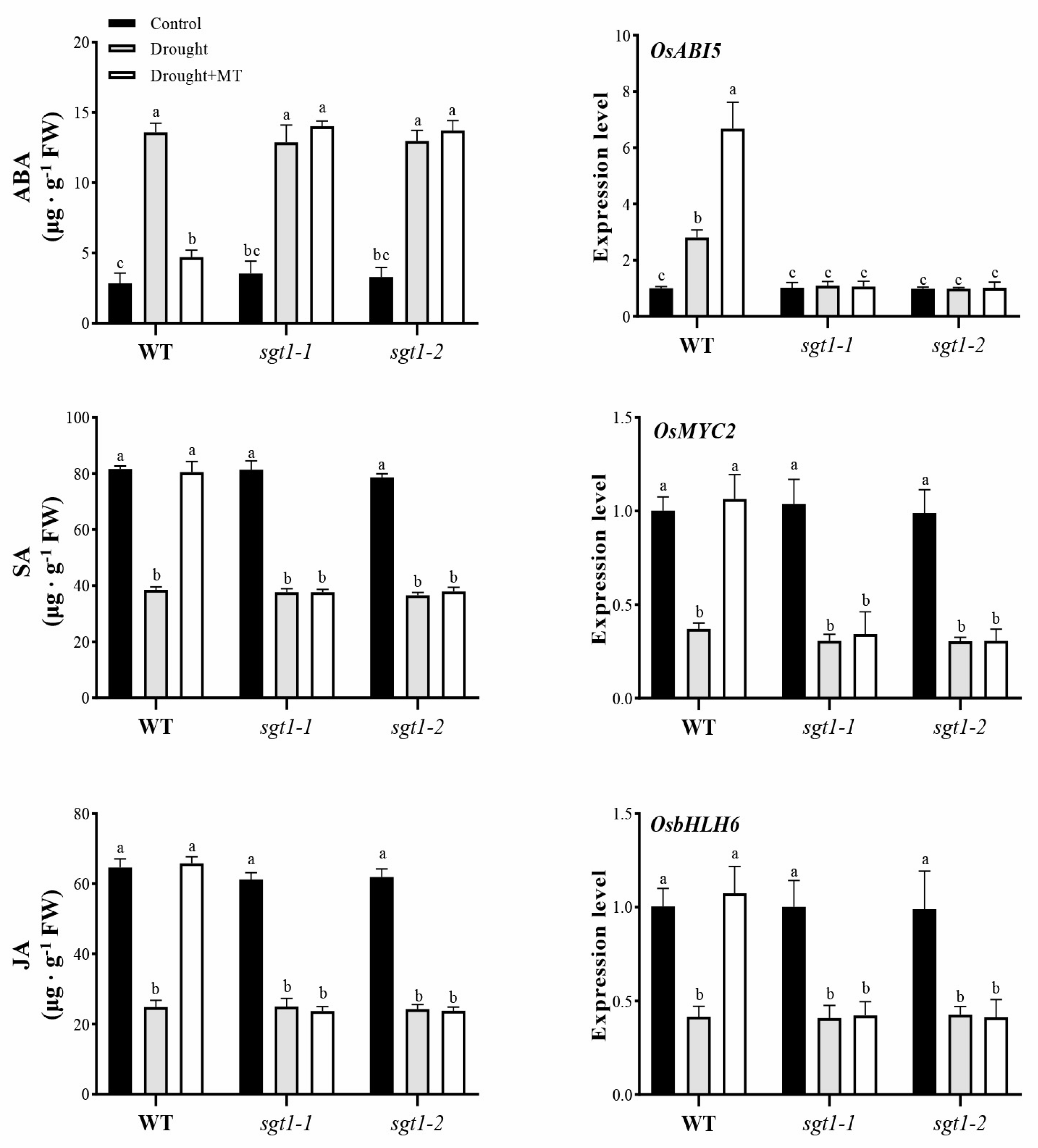
2.5. Effects of Melatonin on the Synthesis of Endogenous ABA, SA and JA under Drought Stress
2.6. Effects of Dual Mutation of OsABI5 and OsSGT1 on the Melatonin-Alleviated Drought Stress
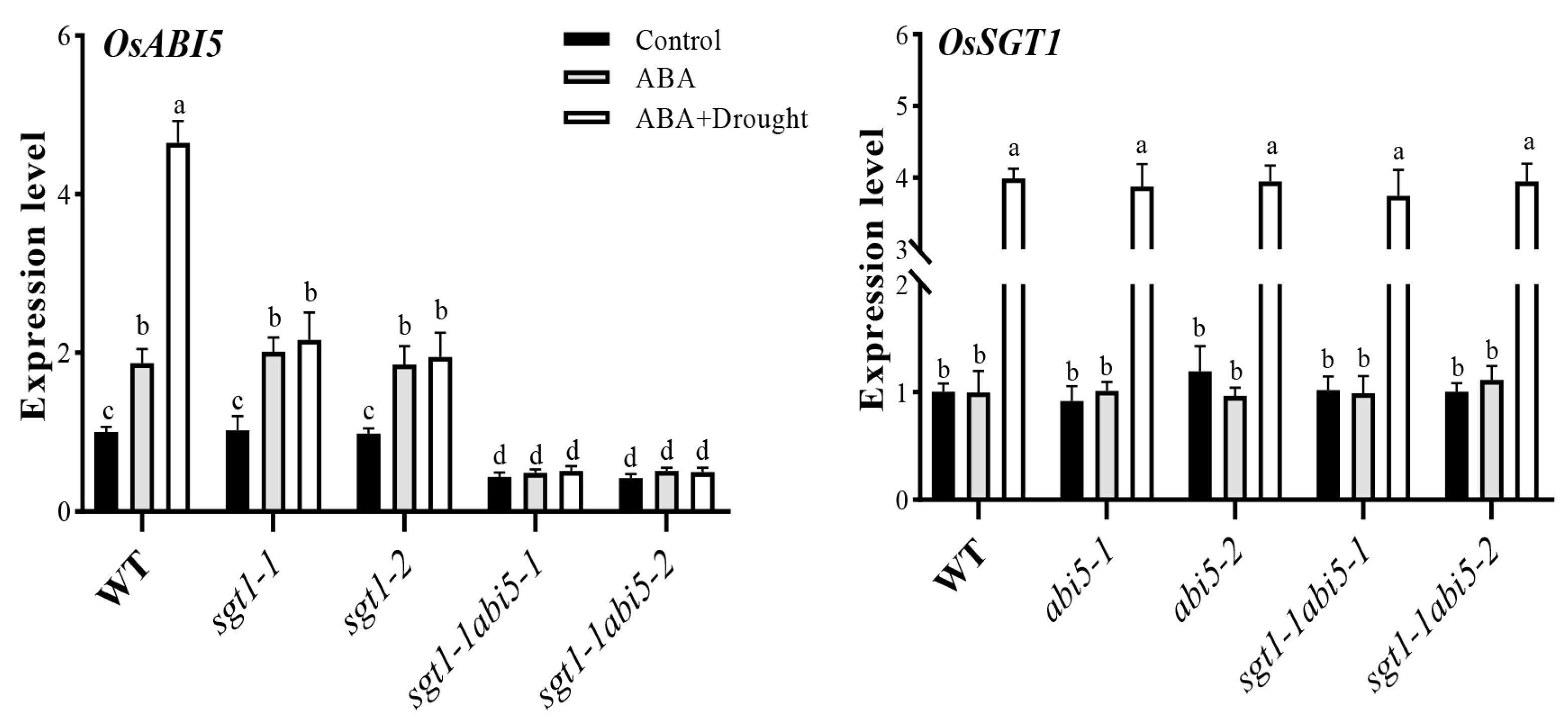
2.7. Effects of Exogenous ABA Feeding on the Expression Levels of OsABI5 and OsSGT1 under Drought Stress
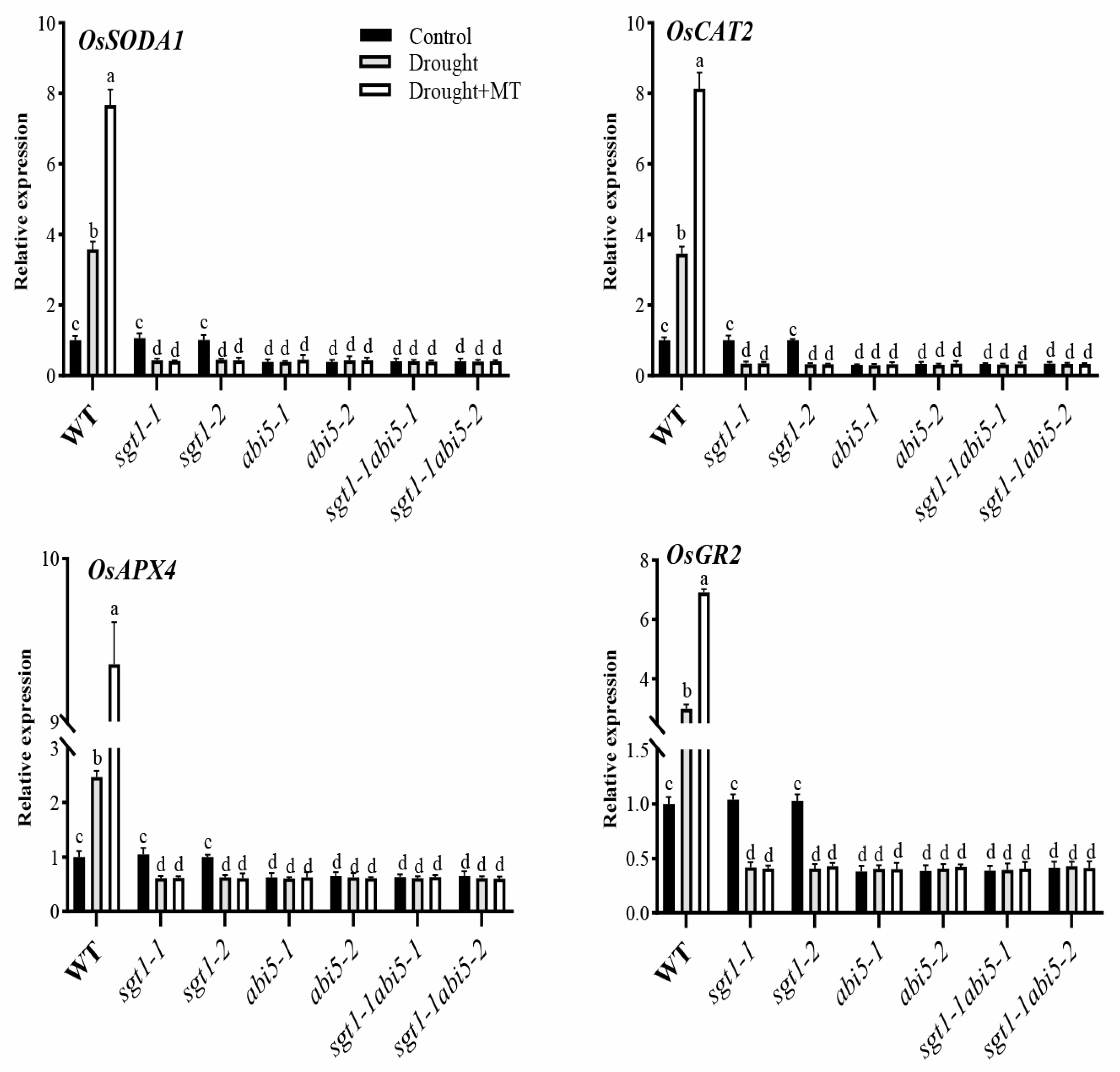
2.8. Effects of Dual Mutation of OsABI5 and OsSGT1 on the Melatonin-Induced Expression of Antioxidant Genes under Drought Stress
3. Discussion
3.1. Melatonin Alleviates Drought Stress to Promote Seedling Growth
3.2. SGT1 Is Crucial for the Melatonin Alleviated Effects on Drought Stress
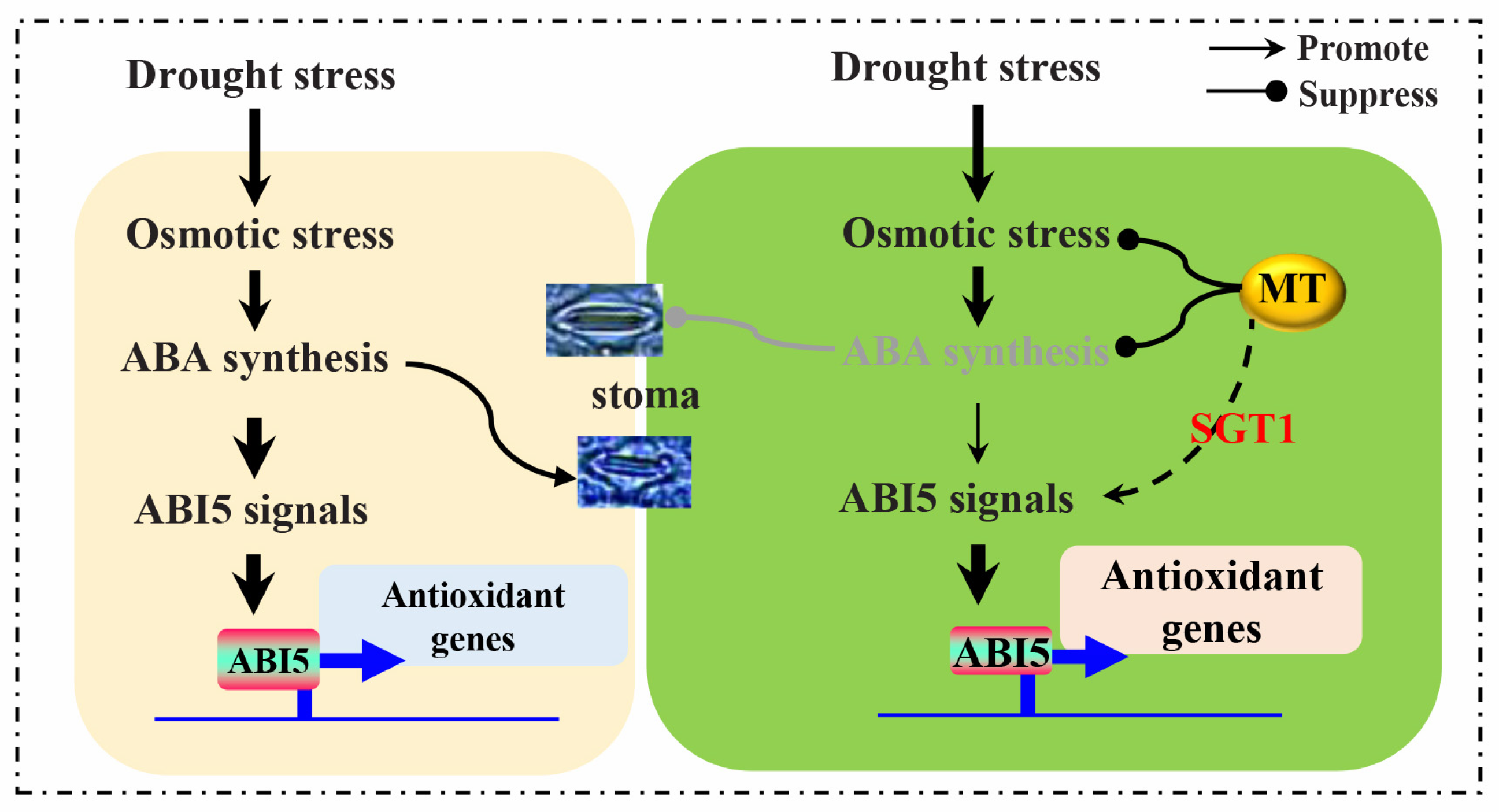
3.3. The Ameliorated Effects of Melatonin on Drought Adaption Is Tightly Associated with SGT1-Involved Signals
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Determination of Physiological Parameters
4.3. Measurement of Oxidant Products
4.4. Activity Assays of Antioxidant System
4.5. Measurement of ABA, SA and JA Contents
4.6. Gene Expression Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Demidchik, V. Mechanisms of oxidative stress in plants: From classical chemistry to cell biology. Environ. Exp. Bot. 2015, 109, 212–228. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Y.; Zhu, J.-K. Thriving under Stress: How plants balance growth and the stress response. Dev. Cell 2020, 55, 529–543. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Dubey, R.S. Drought induces oxidative stress and enhances the activities of antioxidant enzymes in growing rice seedlings. Plant Growth Regul. 2005, 46, 209–221. [Google Scholar] [CrossRef]
- Lafitte, H.; Li, Z.; Vijayakumar, C.; Gao, Y.; Shi, Y.; Xu, J.; Fu, B.; Yu, S.; Ali, A.; Domingo, J.; et al. Improvement of rice drought tolerance through backcross breeding: Evaluation of donors and selection in drought nurseries. Field Crop. Res. 2006, 97, 77–86. [Google Scholar] [CrossRef]
- Kim, T.-W.; Jehanzaib, M. Drought risk analysis, forecasting and assessment under climate change. Water 2020, 12, 1862. [Google Scholar] [CrossRef]
- Tiwari, R.K.; Lal, M.K.; Kumar, R.; Chourasia, K.N.; Naga, K.C.; Kumar, D.; Das, S.K.; Zinta, G. Mechanistic insights on mel-atonin-mediated drought stress mitigation in plants. Physiol. Plant 2021, 172, 121–1226. [Google Scholar] [CrossRef]
- Leng, G.; Tang, Q.; Rayburg, S. Climate change impacts on meteorological, agricultural and hydrological droughts in China. Glob. Planet. Chang. 2015, 126, 23–34. [Google Scholar] [CrossRef]
- Boogar, A.R.; Salehi, H.; Jowkar, A. Exogenous nitric oxide alleviates oxidative damage in turfgrasses under drought stress. S. Afr. J. Bot. 2014, 92, 78–82. [Google Scholar] [CrossRef] [Green Version]
- Cui, G.; Zhao, X.; Liu, S.; Sun, F.; Zhang, C.; Xi, Y. Beneficial effects of melatonin in overcoming drought stress in wheat seedlings. Plant Physiol. Biochem. 2017, 118, 138–149. [Google Scholar] [CrossRef]
- Kapoor, D.; Bhardwaj, S.; Landi, M.; Sharma, A.; Ramakrishnan, M.; Sharma, A. The impact of drought in plant metabolism: How to exploit tolerance mechanisms to increase crop production. Appl. Sci. 2020, 10, 5692. [Google Scholar] [CrossRef]
- Soares, C.; Carvalho, M.E.A.; Azevedo, R.A.; Fidalgo, F. Plants facing oxidative challenges—A little help from the antioxidant networks. Environ. Exp. Bot. 2019, 161, 4–25. [Google Scholar] [CrossRef]
- Munemasa, S.; Hauser, F.; Park, J.; Waadt, R.; Brandt, B.; Schroeder, J.I. Mechanisms of abscisic acid-mediated control of sto-matal aperture. Curr. Opin. Plant Biol. 2015, 28, 154–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, T.; Noman, M.; Manzoor, N.; Shahid, M.; Abdullah, M.; Ali, L.; Wang, G.; Hashem, A.; Al-Arjani, A.-B.F.; Alqarawi, A.A.; et al. Nanoparticle-based amelioration of drought stress and cadmium toxicity in rice via triggering the stress responsive genetic mechanisms and nutrient acquisition. Ecotoxicol. Environ. Saf. 2021, 209, 111829. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Wang, L.; Yang, Y.; Wang, P.; Guo, P.; Guo, T.; Kang, G. Abscisic acid enhances tolerance of wheat seedlings to drought and regulates transcript levels of genes encoding ascorbate-glutathione biosynthesis. Front. Plant Sci. 2015, 6, 458. [Google Scholar] [CrossRef]
- Sohag, A.A.M.; Tahjib-Ul-Arif, M.; Polash, M.A.S.; Chowdhury, M.B.; Afrin, S.; Burritt, D.J.; Murata, Y.; Hossain, M.A.; Hossain, M.A. Exogenous glutathione-mediated drought stress tolerance in rice (Oryza sativa L.) is associated with lower oxidative damage and favorable ionic homeostasis. Iran. J. Sci. Technol. Trans. A Sci. 2020, 44, 955–971. [Google Scholar] [CrossRef]
- Hossain, S.; Li, J.; Sikdar, A.; Hasanuzzaman, M.; Uzizerimana, F.; Muhammad, I.; Yuan, Y.; Zhang, C.; Wang, C.; Feng, B. Exogenous melatonin modulates the physiological and biochemical mechanisms of drought tolerance in Tartary Buckwheat (Fagopyrum tataricum (L.) Gaertn). Molecules 2020, 25, 2828. [Google Scholar] [CrossRef]
- Hanif, S.; Saleem, M.F.; Sarwar, M.; Irshad, M.; Shakoor, A.; Wahid, M.A.; Khan, H.Z. Biochemically triggered heat and drought stress tolerance in rice by proline application. J. Plant Growth Regul. 2021, 40, 305–312. [Google Scholar] [CrossRef]
- Zou, J.N.; Jin, X.J.; Zhang, Y.X.; Ren, C.Y.; Zhang, M.C.; Wang, M.X. Effects of melatonin on photosynthesis and soybean seed growth during grain filling under drought stress. Photosynthetica 2019, 57, 512–520. [Google Scholar] [CrossRef] [Green Version]
- Imran, M.; Shazad, R.; Bilal, S.; Imran, Q.M.; Khan, M.; Kang, S.M.; Khan, A.L.; Yun, B.W.; Lee, I.J. Exogenous melatonin me-diates the regulation of endogenous nitric oxide in Glycine max L. to reduce effects of drought stress. Environ. Exp. Bot. 2021, 188, 104511. [Google Scholar] [CrossRef]
- Alharby, H.F.; Fahad, S. Melatonin application enhances biochar efficiency for drought tolerance in maize varieties: Modifications in physio-biochemical machinery. Agron. J. 2020, 112, 2826–2847. [Google Scholar] [CrossRef]
- Khan, M.N.; Khan, Z.; Luo, T.; Liu, J.; Rizwan, M.; Zhang, J.; Xu, Z.; Wu, H.; Hu, L. Seed priming with gibberellic acid and melatonin in rapeseed: Consequences for improving yield and seed quality under drought and non-stress conditions. Ind. Crop. Prod. 2020, 156, 112850. [Google Scholar] [CrossRef]
- Arnao, M.B.; Ruiz, J.H. Melatonin: A new plant hormone and/or a plant master regulator? Trends Plant Sci. 2019, 24, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. Is phytomelatonin a new plant hormone? Agronomy 2020, 10, 95. [Google Scholar] [CrossRef] [Green Version]
- Moustafa-Farag, M.; Mahmoud, A.; Arnao, M.; Sheteiwy, M.; Dafea, M.; Soltan, M.; Elkelish, A.; Hasanuzzaman, M.; Ai, S. Melatonin-induced water stress tolerance in plants: Recent advances. Antioxidants 2020, 9, 809. [Google Scholar] [CrossRef]
- Aimar, D.; Calafat, M.; Andrade, A.; Carassay, L.; Bouteau, F.; Abdala, G.; Molas, M. Drought effects on the early development stages of Panicum virgatum L.: Cultivar differences. Biomass Bioenergy 2014, 66, 49–59. [Google Scholar] [CrossRef]
- Li, C.; Tan, D.-X.; Liang, D.; Chang, C.; Jia, D.; Ma, F. Melatonin mediates the regulation of ABA metabolism, free-radical scavenging, and stomatal behaviour in two Malus species under drought stress. J. Exp. Bot. 2015, 66, 669–680. [Google Scholar] [CrossRef] [Green Version]
- Prakash, V.; Singh, V.P.; Tripathi, D.K.; Sharma, S.; Corpas, F.J. May crosstalk between nitric oxide (NO) and abscisic acid (ABA) signalling molecules in higher plants. Environ. Exp. Bot. 2019, 161, 419. [Google Scholar] [CrossRef]
- Degenkolbe, T.; Do, P.T.; Zuther, E.; Repsilber, D.; Walther, D.; Hincha, D.K.; Köhl, K.I. Expression profiling of rice cultivars differing in their tolerance to long-term drought stress. Plant Mol. Biol. 2009, 69, 133–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mckersie, B.D.; Lesham, Y.Y. Stress and Stress Coping in Cultivated Plants; Springer: Dordrecht, The Netherlands, 1994; pp. 148–180. [Google Scholar]
- Sandhu, N.; Yadav, S.; Kumar, A. Chapter 8: Recent efforts in developing high-yield, drought-tolerant rice varieties. In Ad-vancement in Crop Improvement Techniques; Tuteja, N., Tuteja, R., Passricha, N., Saifi, S.K., Eds.; Woodhead Publishing Sawston: Cambridge, UK, 2020; pp. 111–128. [Google Scholar]
- Azevedo, C.; Betsuyaku, S.; Peart, J.; Takahashi, A.; Noël, L.; Sadanandom, A.; Casais, C.; Parker, J.; Shirasu, K. Role of SGT1 in resistance protein accumulation in plant immunity. EMBO J. 2006, 25, 2007–2016. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.C.; Millet, Y.; Cheng, Z.; Bush, J.; Ausubel, F.M. Jasmonate signalling in Arabidopsis involves SGT1b–HSP70–HSP90 chaperone complexes. Nat. Plant. 2015, 1, 15049. [Google Scholar] [CrossRef] [Green Version]
- Cazalé, A.C.; Clément, M.; Chiarenza, S.; Roncato, M.A.; Pochon, N.; Creff, A.; Marin, E.; Leonhardt, N.; Noël, L.D. Altered expression of cytosolic/nuclear HSC70-1 molecular chaperone affects development and abiotic stress tolerance in Arabidopsis thaliana. J. Exp. Bot. 2009, 60, 2653–2664. [Google Scholar] [CrossRef] [Green Version]
- Shanmugam, A.; Thamilarasan, S.K.; Park, J.I.; Jung, M.Y.; Nou, I.S. Characterization and abiotic stress-responsive expression analysis of SGT1 genes in Brassica oleracea. Genome 2016, 59, 243–251. [Google Scholar] [CrossRef] [Green Version]
- Fu, D.; Ghabrial, S.; Kachroo, A. GmRAR1 and GmSGT1 are required for basal, R gene-mediated and systemic acquired re-sistance in soybean. Mol. Plant-Microbe Interact. 2009, 22, 86–95. [Google Scholar] [CrossRef] [Green Version]
- Noël, L.D.; Cagna, G.; Stuttmann, J.; Wirthmüller, L.; Betsuyaku, S.; Witte, C.P.; Bhat, R.; Pochon, N.; Colby, T.; Parker, J.E.; et al. Interaction between SGT1 and cyto-solic/nuclear HSC70 chaperones regulates Arabidopsis immune responses. Plant Cell 2007, 19, 4061–4076. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, P.; Bhardwaj, R.; Tuteja, N. Plant Signaling Under Abiotic Stress Environment. In Environmental Adaptations and Stress Tolerance of Plants in the Era of Climate Change; Ahmad, P., Prasad, M., Eds.; Springer: New York, NY, USA, 2012; pp. 297–323. [Google Scholar]
- Hosseini, M.S.; Samsampour, D.; Zahedi, S.M.; Zamanian, K.; Rahman, M.; Mostofa, M.G.; Tran, L.P. Melatonin alleviates drought impact on growth and essential oil yield of lemon verbena by enhancing antioxidant responses, mineral balance, and abscisic acid content. Physiol. Plant. 2021, 172, 1363–1375. [Google Scholar] [CrossRef]
- Skubacz, A.; Daszkowska-Golec, A.; Szarejko, I. The role and regulation of ABI5 (ABA-Insensitive 5) in plant development, abiotic stress responses and phytohormone crosstalk. Front. Plant Sci. 2016, 7, 1884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, W.; Cao, Y.; Loka, D.A.; Harris-Shultz, K.R.; Reiter, R.J.; Ali, S.; Liu, Y.; Zhou, Z. Exogenous melatonin improves cotton (Gossypium hirsutum L.) pollen fertility under drought by regulating carbohydrate metabolism in male tissues. Plant Physiol. Biochem. 2020, 151, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, C.; Chatzimichail, G.; Xenofontos, R.; Pavlou, J.J.; Panagiotou, E.; Christou, A.; Fotopoulos, V. Melatonin system-ically ameliorates drought stress-induced damage in Medicago sativa plants by modulating nitro-oxidative homeostasis and proline metabolism. J. Pineal Res. 2017, 62, e12401. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zhao, B.; Zhang, H.-J.; Weeda, S.; Yang, C.; Yang, Z.-C.; Ren, S.; Guo, Y.-D. Melatonin promotes water-stress tolerance, lateral root formation, and seed germination in cucumber (Cucumis sativus L.). J. Pineal Res. 2012, 54, 15–23. [Google Scholar] [CrossRef]
- Liu, L.; Li, D.; Ma, Y.; Shen, H.; Zhao, S.; Wang, Y. Combined application of arbuscular mycorrhizal fungi and exogenous melatonin alleviates drought stress and improves plant growth in tobacco seedlings. J. Plant Growth Regul. 2021, 40, 1074–1087. [Google Scholar] [CrossRef]
- Lei, K.; Sun, S.; Zhong, K.; Li, S.; Hu, H.; Sun, C.; Zheng, Q.; Tian, Z.; Dai, T.; Sun, J. Seed soaking with melatonin promotes seed germination under chromium stress via enhancing reserve mobilization and antioxidant metabolism in wheat. Ecotoxicol. Environ. Saf. 2021, 220, 112241. [Google Scholar] [CrossRef] [PubMed]
- Handa, N.; Kohli, S.K.; Thukral, A.; Bhardwaj, R.; Alyemeni, M.N.; Wijaya, L.; Ahmad, P. Protective role of selenium against chromium stress involving metabolites and essential elements in Brassica juncea L. seedlings. 3 Biotech 2018, 8, 66. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yu, B.; Gao, Y.; Dai, A.; Bai, J.G. Cinnamic acid pretreatment mitigates chilling stress of cucumber leaves through al-tering antioxidant enzyme activity. J. Plant Physiol. 2011, 168, 927–934. [Google Scholar] [CrossRef]
- Guo, W.; Yang, H.; Guo, Y.; Chen, B.; Mu, J.; Wang, Y.; Li, X.; Zhou, J. Improved powdery mildew resistance of transgenic tobacco overexpressing the Cucurbita moschata CmSGT1 gene. Front. Plant Sci. 2019, 10, 955. [Google Scholar] [CrossRef]
- Hasan, M.K.; Ahammed, G.J.; Sun, S.; Li, M.; Yin, H.; Zhou, J. Melatonin inhibits cadmium translocation and enhances plant tolerance by regulating sulfur uptake and assimilation in Solanum lycopersicum L. J. Agric. Food Chem. 2019, 67, 10563–10576. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Jiang, M.; Song, Y.; Zhang, H. Melatonin alleviates low-temperature stress via ABI5-mediated signals during seed germination in rice (Oryza sativa L.). Front. Plant Sci. 2021, 12, 727596. [Google Scholar] [CrossRef]
- Li, H.; Guo, Y.; Lan, Z.; Zhang, Z.; Ahammed, G.J.; Chang, J.; Zhang, Y.; Wei, C.; Zhang, X. Melatonin antagonizes ABA action to promote seed germination by regulating Ca2+ efflux and H2O2 accumulation. Plant Sci. 2021, 303, 110761. [Google Scholar] [CrossRef]
- Møller, I.M.; Jensen, P.E.; Hansson, A. Oxidative modifications to cellular components in plants. Annu. Rev. Plant Biol. 2007, 58, 459–481. [Google Scholar] [CrossRef] [Green Version]
- Arnao, M.B.; Ruiz, J.H. Melatonin and its relationship to plant hormones. Ann. Bot. 2018, 121, 195–207. [Google Scholar] [CrossRef] [Green Version]
- Ishibashi, Y.; Aoki, N.; Kasa, S.; Sakamoto, M.; Kai, K.; Tomokiyo, R.; Watabe, G.; Yuasa, T.; Iwaya-Inoue, M. The inter rela-tionship between abscisic acid and reactive oxygen species plays a key role in barley seed dormancy and germination. Front. Plant Sci. 2017, 8, 275. [Google Scholar] [CrossRef] [Green Version]
- Bi, C.; Ma, Y.; Wu, Z.; Yu, Y.-T.; Liang, S.; Lu, K.; Wang, X.-F. Arabidopsis ABI5 plays a role in regulating ROS homeostasis by activating CATALASE 1 transcription in seed germination. Plant Mol. Biol. 2017, 94, 197–213. [Google Scholar] [CrossRef] [Green Version]
- Lei, Y.; Lu, L.; Liu, H.-Y.; Li, S.; Xing, F.; Chen, L.-L. CRISPR-P: A web tool for synthetic single-guide RNA design of CRISPR-System in Plants. Mol. Plant 2014, 7, 1494–1496. [Google Scholar] [CrossRef] [Green Version]
- Nachabe, M.H. Refining the definition of field capacity in the literature. J. Irrig. Drain. Eng. 1998, 124, 230–232. [Google Scholar] [CrossRef]
- Weatherley, P.E. Studies in the water relations of the cotton plant. I. the field measurement of water deficits in leaves. New Phytol. 1950, 49, 81–97. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef] [Green Version]
- Fairbairn, N.J. A modified anthrone reagent. Chem. Ind. Lond. 1953, 4, 86. [Google Scholar]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Song, Y.; Jiang, M.; Zhang, H.; Li, R. Zinc oxide nanoparticles alleviate chilling stress in rice (Oryza Sativa L.) by regulating antioxidative system and chilling response transcription factors. Molecules 2021, 26, 2196. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Pro-tective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Elstner, E.F.; Heupel, A. Inhibition of nitrite formation from hydroxylammoniumchloride: A simple assay for superoxide dismutase. Anal. Biochem. 1976, 70, 616–620. [Google Scholar] [CrossRef]
- Su, X.; Fan, X.; Shao, R.; Guo, J.; Wang, Y.; Yang, J.; Yang, Q.; Guo, L. Physiological and iTRAQ-based proteomic analyses reveal that melatonin alleviates oxidative damage in maize leaves exposed to drought stress. Plant Physiol. Biochem. 2019, 142, 263–274. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, J.; Li, X.; Xia, X.-J.; Zhou, Y.-H.; Shi, K.; Chen, Z.; Yu, J.-Q. H2O2 mediates the crosstalk of brassinosteroid and abscisic acid in tomato responses to heat and oxidative stresses. J. Exp. Bot. 2014, 65, 4371–4383. [Google Scholar] [CrossRef]
- Qi, Q.; Rose, P.A.; Abrams, G.D.; Taylor, D.C.; Abrams, S.R.; Cutler, A.J. (+)-Abscisic acid metabolism, 3-ketoacyl-coenzyme a synthase gene expression, and very-long-chain monounsaturated fatty acid biosynthesis in Brassica napus embryos 1. Plant Physiol. 1998, 117, 979–987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seskar, M.; Shulaev, V.; Raskin, I. Endogenous Methyl Salicylate in Pathogen-Inoculated Tobacco Plants1. Plant Physiol. 1998, 116, 387–392. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Li, R.-Q.; Jiang, M.; Liu, Y.-H.; Zheng, Y.-C.; Huang, J.-Z.; Wu, J.-M.; Shu, Q.-Y. The xantha marker trait is associated with altered tetrapyrrole biosynthesis and deregulated transcription of PhANGs in rice. Front. Plant Sci. 2017, 8, 901. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, R.; Yang, R.; Zheng, W.; Wu, L.; Zhang, C.; Zhang, H. Melatonin Promotes SGT1-Involved Signals to Ameliorate Drought Stress Adaption in Rice. Int. J. Mol. Sci. 2022, 23, 599. https://doi.org/10.3390/ijms23020599
Li R, Yang R, Zheng W, Wu L, Zhang C, Zhang H. Melatonin Promotes SGT1-Involved Signals to Ameliorate Drought Stress Adaption in Rice. International Journal of Molecular Sciences. 2022; 23(2):599. https://doi.org/10.3390/ijms23020599
Chicago/Turabian StyleLi, Ruiqing, Ruifang Yang, Wenyin Zheng, Liquan Wu, Can Zhang, and Huali Zhang. 2022. "Melatonin Promotes SGT1-Involved Signals to Ameliorate Drought Stress Adaption in Rice" International Journal of Molecular Sciences 23, no. 2: 599. https://doi.org/10.3390/ijms23020599
APA StyleLi, R., Yang, R., Zheng, W., Wu, L., Zhang, C., & Zhang, H. (2022). Melatonin Promotes SGT1-Involved Signals to Ameliorate Drought Stress Adaption in Rice. International Journal of Molecular Sciences, 23(2), 599. https://doi.org/10.3390/ijms23020599




