Abstract
Polycystic ovary syndrome (PCOS) is an endocrine-gynecology disorder affecting many women of childbearing age. Although a part of the involved mechanism in PCOS occurrence is discovered, the exact etiology and pathophysiology are not comprehensively understood yet. We searched PubMed for PCOS pathogenesis and management in this article and ClinicalTrials.gov for information on repurposed medications. All responsible factors behind PCOS were thoroughly evaluated. Furthermore, the complete information on PCOS commonly prescribed and repurposed medications is summarized through tables. Epigenetics, environmental toxicants, stress, diet as external factors, insulin resistance, hyperandrogenism, inflammation, oxidative stress, and obesity as internal factors were investigated. Lifestyle modifications and complementary and alternative medicines are preferred first-line therapy in many cases. Medications, including 3-hydroxy-3-methyl-3-glutaryl-coenzyme A (HMG-CoA) reductase inhibitors, thiazolidinediones, sodium-glucose cotransporter-2 inhibitors, dipeptidyl peptidase-4 inhibitors, glucose-like peptide-1 receptor agonists, mucolytic agents, and some supplements have supporting data for being repurposed in PCOS. Since there are few completed clinical trials with a low population and mostly without results on PCOS repurposed medications, it would be helpful to do further research and run well-designed clinical trials on this subject. Moreover, understanding more about PCOS would be beneficial to find new medications implying the effect via the novel discovered routes.
1. Introduction
Polycystic ovary syndrome (PCOS) is a heterogeneous endocrine disorder that impacts many women of the reproductive age worldwide [1]. This syndrome is often associated with enlarged and dysfunctional ovaries, excess androgen levels, resistance to insulin, etc. [2]. It is estimated that approximately every 1 in 10 women face PCOS before menopause and struggle with its complications [3].
Although the high ratio of luteinizing hormone (LH) to follicle-stimulating hormone (FSH) and increased frequency of gonadotropin-releasing hormone (GnRH) is known as the underlying causes of PCOS [4], the exact etiology and pathology have not been comprehensively well-known [4,5]. Evidence suggests the role of different external and internal factors, including insulin resistance (IR), hyperandrogenism (HA), environmental factors, genetic, and epigenetics. In addition, it is worth mentioning that PCOS increases the risk of further complications like cardiovascular diseases [5,6], type 2 diabetes mellitus [5,6], metabolic syndrome [6], depression, and anxiety [7].
To manage this condition, the most crucial step is to lose at least 5% of the weight; therefore, having a regular exercise plan and fat and sugar-free diets are also recommended to every woman with PCOS. Furthermore, in some cases, taking complementary and alternative medicine strategies with or without other treatments is preferable due to their prior beliefs, lower costs, etc.
Physicians tend to use (combined) oral contraceptives, antiandrogen agents, insulin sensitizers, and ovulation inducers [4]. Up until today, there is no United States Food and Drug Administration (USFDA) approved medication specifically for PCOS, and all mentioned medications are used off-label [8]. Apart from the essential need for improvement in the research and development of new drug molecules and new drug discovery, novel medications could be found with drug repurposing methods [9]. On this very spot, there are plenty of medications, previously approved by USFDA for indications rather than PCOS; and, today, there is a desire to implement them as the therapeutic options in the management of PCOS.
These agents vary from anti-diabetic medications such as pioglitazone, empagliflozin, sitagliptin, liraglutide to 3-hydroxy-3-methyl-3-glutaryl-coenzyme A (HMG-CoA) reductase inhibitors like simvastatin and atorvastatin, as well as mucolytic drugs like N-acetyl cysteine.
Given that PCOS is a growing issue that is unfortunately followed by many unwanted complications and that available methods and medications are not 100% effective, it is essential to investigate its pathogenesis and find out new pharmacological targets carefully. This could be done through repositioning approaches, saving time and cost.
This review discusses PCOS’s definition, diagnosis, and etiology, focusing on the pathogenesis and management of this syndrome. Internal and external factors contributing to PCOS have been comprehensively studied, and several commonly prescribed medications with their complete drug information are provided. Subsequently, a couple of repurposed medications are mentioned thoroughly, reviewing the related clinical trials over the past five years.
2. Methods
PubMed, Google Scholar, ScienceDirect, TRIP database, and UpToDate were comprehensively searched for publications including PCOS relevant keywords in different areas, focusing on the new ones (since 2016) and excluding those with a language rather than English or animal studies. In addition, Clinicaltrials.gov was searched to find data about completed or running trials of repurposed drugs in PCOS over the past five years.
3. Diagnosis
PCOS is among the conditions that cannot be diagnosed with basic diagnostic tests, including blood tests, culture, and biopsy; thus, there is no certain test for PCOS diagnosis. Differential diagnosis is called excluding the relevant disorders according to the symptoms and narrowing the choices. In order to establish a differential diagnosis for PCOS, hyperprolactinemia, thyroid disease, Cushing’s syndrome, and hyperplasia of adrenal should be excluded based on the associated investigations [10,11]. Although considering past medical history, weight changes, and symptoms of insulin resistance might be helpful, pelvic examination, a transvaginal ultrasound, and measuring the level of hormones are among the most frequently recommended investigations [12]. According to the National Health Service (NHS), irregular or infrequent periods, high levels of androgenic hormones or symptoms, and scans showing polycystic ovaries are the specified criteria for PCOS [13]. In addition, Rotterdam PCOS diagnostic criteria in adults are the most commonly used method. In an ultrasound, the presence of two clinical or biochemical hyperandrogenism, ovulatory dysfunction, or polycystic ovaries would finalize a PCOS diagnosis [14].
4. Etiology and Risk Factors
4.1. External Factors
4.1.1. Epigenetic Mechanism
Epigenetic refers to inheritable alterations in genome and gene expression without any changes in DNA sequence [15,16]. These changes involve adding or omitting chemical components on DNA or histone [17]. Increased LH activity is a seen phenomenon in PCOS women. It may relate to the problems in follicle development and HA, which are common among PCOS patients [18]. LH/choriogonadotropin receptor (LHCGR) is responsible for the steroidogenesis process in theca cells [19]. This receptor hypomethylation leads to higher gene expression and sensitivity to LH [18,20].
A study on PCOS patients approved that hypomethylated sites are related to overexpression of LHCGR [15,19] on theca cells surface [19]. In addition, epoxide hydrolase 1 (EPHX1) is an active enzyme in degrading aromatic compounds [15,19,21]. Its gene promoter hypomethylation [15,19] increases enzyme expression [15]. Overproduction of EPHX1 reduces the transformation of testosterone to estradiol, which can contribute to PCOS [15]. Furthermore, peroxisome proliferator-activated receptor gamma (PPAR-γ) plays a role in ovaries’ function [15,18,19,22]. Hypermethylation of PPARγ, hypomethylation of nuclear co-repressor 1 [19,22], and alteration in acetylation of histone deacetylase 3, for which both are PPARγ co-repressors [15], are observed in PCOS patients showing HA [15,19,22]. These alterations were noticed in PCOS women’s granulosa cells [18,23].
4.1.2. Environmental Toxicants
The United States Environmental Protection Agency (USEPA) defines endocrine-disrupting chemical (EDC) as “an exogenous agent that interferes with the synthesis, secretion, transport, binding, action, or elimination of natural hormones in the body that are responsible for the maintenance of homeostasis, reproduction, development and/or behavior” [24].
EDCs may act as hormones’ agonists or antagonists in binding to their receptors [25]. EDCs are almost parts of everything we use in our daily life [21]. Their structures consist of phenols or halogens like chlorine and bromine, so they imitate steroid hormones’ actions [21]. Studies have approved the higher serum concentration of EDCs in PCOS suffering women [21,26]. Prolonged and continuous exposure to EDCs from prenatal to adolescence can cause susceptibility to PCOS [21,27].
As an example, bisphenol A (BPA) BPA is a synthetic compound used in polycarbonate plastics, epoxy resins [25,28], dental filling, food and drink packages [25], baby bottles, and polyvinyl chloride (PVC) [28], which affects metabolism through different pathways. BPA directly affects oogenesis [29] by interacting with estrogen receptor (ER) α and β, non-classical membrane ER, and G-protein coupled receptor 30 (GPCR30) [21,28,29]. It also triggers androgen secretion and restrains testosterone catabolism in theca cells [21,29].
Another effect of BPA on interstitial theca cells is the overproduction of androgens by dysregulation of 17β-hydroxylase (P450c17) [28,30], cholesterol side-chain cleavage enzyme (P450scc), and steroidogenic acute regulatory protein [30]. BPA’s influence on granulosa cells refers to reducing the expression of aromatase enzyme and production of estrogen [21,29]. Lastly, it disturbs the intrafollicular environment and damages the oocyte development and maturation [21,29]. BPA’s indirect effect on HA involves downregulation of testosterone 2a-hydroxylase and testosterone 6b-hydroxylase enzymes in liver level, and thus a higher concentration of testosterone [30,31].
In addition, BPA is a potent ligand for sex hormone-binding globulin (SHBG) and replaces testosterone; thereby, free testosterone concentration increases. Androgen and BPA have a two-way relation; high androgen inactivates the uridine diphosphate-glucuronosyl transferase enzyme and reduces BPA clearance in the liver. This process causes a high concentration of free BPA in blood and worsens its negative effects on the ovaries [21,29,30,31].
Additionally, it is believed that BPA may act as an obesogen [28,30]. Its obesogenic influence includes upregulation of adipogenesis-related genes [30], stimulation of adipocytes differentiation [28,30], potentiation of the accumulation of lipid in cells incorporated in medical syndrome, and triggering the conversion of target cells to adipocytes via phosphatidylinositol 3-kinase pathway [30].
Adipogenesis due to BPA happens because of the activation of the glucocorticoid receptor. Activation of the receptor upregulates the enzyme involved in the conversion of cortisone to cortisol, thus inducing adipogenesis [28]. Moreover, BPA prompts the release of interleukin-6 (IL-6) and tumor necrosis factor α (TNF-α) [30,31], both involving adiposity and IR [30]. In addition, it restrains the release of adiponectin [28,29,30,31] and the beneficial compound in protecting against IR [28,30,31].
It can also change glucose homeostasis [28,29,31] by directly influencing the pancreatic cells [29]. BPA causes a chronic increase in insulin and further IR in long exposure [30] by affecting the mitochondrial activity and metabolic pathways of β-pancreatic cells [28]. BPA reduces glucagon secretion by inhibiting the intracellular calcium ion fluctuating pattern with a lack of glucose condition [30].
Advanced glycation end products (AGEs), also called glycotoxins, are another chemical group affecting body health. AGEs are pro-inflammatory molecules [21,23,29,32] that interact with their surface receptor called RAGE (receptor for AGE) [21,23,29] and stimulate pro-inflammatory pathways and oxidative stress [21,23,29,32]. AGEs can be absorbed into the body as exogenous compounds or derived from nonenzymatic glycation and oxidation of proteins and lipids [21]. Increased concentration of AGEs in serum has been detected in PCOS patients [21]. AGEs interrupt pre-ovulatory follicles growth via ERK1/MAPK pathway and damage follicles by oxidative stress caused by interaction with RAGEs [21]. This interaction increases intracellular inflammatory molecules [21].
In vitro studies on 3T3-L1 cell lines showed that glycotoxins are likely to trigger adipogenesis [21]. On the other hand, a higher body mass index corresponds to a lower extent of soluble RAGEs, which is responsible for glycotoxin clearance and deposition of AGEs in the reproductive system, especially in ovaries [21,29]. This bilateral relation worsens inflammatory processes and metabolic syndrome in PCOS [21]. AGEs also play a role in IR [21,29]. These compounds disrupted glucose transport in the human granulosa KGN cell line [21] and reduced glucose uptake by adipocytes in previous research [21,29]. They also involve IR by causing oxidative stress, inflammation, and glycation of proteins, which considerably diminishes insulin sensitivity [21]. Moreover, increased concentration of AGEs changes the insulin signaling pathway and interferes with glucose transporter 4 (GLUT-4) translocation [23].
4.1.3. Physical and Emotional Stress
Although there is minimal information on the role of stress in PCOS, it is known that PCOS possesses adverse effects on self-esteem and mental health. Chronic stress results in hypertrophy and hyperplasia of adipocytes. This phenomenon happens as a result of glucocorticoids’ effect on pre-adipocytes maturation. Chronic stress is also associated with adipokine secretion, attraction, and activation of stromal fat immune cells [33].
In addition, it is responsible for making an inflammatory condition by leading to high levels of inflammatory cytokines like IL-6 and TNF-α, along with disrupting oxidant-antioxidant balance [33]. In addition, chronic stress plays a vital role in IR.
Stress triggers the hypothalamic-pituitary-adrenal (HPA) axis to release cortisol [34,35]. Cortisol leads to IR by stimulating visceral fat accumulation, gluconeogenesis, and lipolysis [35]. Moreover, cortisol arouses glucose production in the liver [35]. Stress is also involved in enhancing insulin levels [34]. Other stress influences on PCOS may refer to inference with anti-mullerian hormone (AMH) and changing sex hormone levels [34,35].
4.1.4. Diet
Although nutrition contributions to PCOS is unclear, studies showed a relationship between some nutrient levels and PCOS indices.
Saturated fatty acids (SFAs) intake plays a role in PCOS by producing an inflammatory status [36] and reducing insulin sensitivity [37]. Taking SFAs induces inflammation by triggering an increase in TNF-α level in circulation and expressing a specific cytokine suppressor [36].
Vitamin D deficiency may exacerbate PCOS [37,38] or the comorbidities induced by PCOS [38]. Calcitriol upregulates insulin receptors at mRNA and protein levels. It also increases insulin sensitivity directly and indirectly. The direct effect occurs by activating PPAR-δ, the involved receptor in fatty acids metabolism in adipose tissue and skeletal muscle. The indirect impact is the regulation of intracellular calcium, which is vital for insulin-mediated signaling in fat and muscle [38]. On the other hand, vitamin D deficiency may result in insulin resistance by causing an inflammatory response [37,39]. Furthermore, vitamin D downregulates the AMH promoter [39].
4.2. Internal Factors
4.2.1. Insulin Resistance
IR means an insufficient cells response to insulin [40]. IR is independent of patients’ adiposity, body fat topography, and androgen levels [18,41]; i.e., it has been reported in lean patients as well [18,42]. It should be mentioned that IR is tissue-selective in PCOS women [18,43], although skeletal muscles [18,43,44], adipose tissue, and liver lose their sensitivity to insulin, adrenal glands [18,43], and ovaries remain sensitive [18,28,43,45].
Insulin directly triggers androgens production in ovarian theca cells [32,44,46,47,48] and grow [48]. Insulin effectively stimulates ovarian follicle growth and hormone secretion by stimulating its receptors in the follicle membrane cells [49]. It also triggers ovarian P450c17 [18,23,50] and P450scc enzyme activity to promote ovarian steroidogenesis [18,51] and increases them with the synergistic effect of chorionic gonadotropin [52]. This hormone, as well as insulin-like growth factor 1 (IGF-1) [18], synergizes with luteinizing hormone [18,45]. Hyperinsulinemia increases LH-binding sites and androgen-producing response to LH [44]. LH and insulin interaction enhance steroidogenic acute regulatory enzyme and CYP450c17 mRNA expression [52,53]. CYP450c17 is involved in androgen production [23,44]. Likewise, IR independently enhances CYP17A1 activity, the productive enzyme in androstenedione and testosterone production [52].
On the other hand, hyperinsulinemia reduces hepatic SHBG [18,32,40,49,52,54,55,56], increasing free testosterone levels in blood [18,32,52,54,56]. In addition, hyperinsulinemia inhibits IGF-1 binding protein production in the liver. IGF-1 is responsible for triggering the production of androgens in thecal cells. Inhibition of the production of IGF-1 binding proteins leads to a higher concentration of this substance in blood circulation and then higher production of androgens in thecal cells [18,46]. Moreover, IGF-1 upregulation decreases a specific miRNA and thus accelerates granulosa cells apoptosis and inhibits folliculogenesis [52]. HA [46] and hyperinsulinemia [45,46,57] both play a role in stopping follicles growth [45,46]. This stoppage is attributed to menstrual irregularity, anovulatory sub-fertility, and amassing of immature follicles [46].
Furthermore, hyperinsulinemia contributes to PCOS by affecting the pituitary gland. Excessive insulin stimulates its receptors in the pituitary gland to release LH [49]. Accumulation of insulin stimulates GnRH and LH pulse secretion via influencing both amplitude and frequency [23]. Insulin’s indirect effect on PCOS is augmented by pituitary gonadotropin sensitivity to GnRH [18], and hyperinsulinemia increases GnRH neuron activity [58].
The insulin’s influence on adipose tissue and inflammation is another essential PCOS pathogenesis topic. Insulin stimulates adipogenesis and lipogenesis and inhibits lipolysis [42], resulting in fat accumulation [44]. IR leads to enhanced plasma levels of free fatty acids (FFAs), affecting the liver and adipose tissue [32]. Moreover, IR causes a reduction in omentin level independent of the patient’s body mass index (BMI). In addition, hyperglycemia can lead to inflammation by producing TNF-α from mononuclear cells (MNCs) [50].
4.2.2. Hyperandrogenism
Generally, hyperandrogenism (HA) reduces the SHBG level, leading to a higher concentration of free testosterone [18,59]. It was observed that PCOS women have higher concentrations of testosterone in plasma which can convert to estrone in adipose tissue. Increased alteration of estrone to estradiol affect follicle growth and increases the LH to FSH ratio causing ovulatory dysfunction [23].
HA can result in AMH upregulation, which inhibits ovulation and the development of follicles by a different mechanism. Furthermore, the IGF-II level is negatively related to androgen levels, and HA reduces IGF-II in follicular fluid. IGF-II positively relates to follicle diameters and estradiol concentration in follicular fluid [23]. In addition, HA increases LH indirectly [58,60]. Estradiol and progesterone are responsible for GnRH and LH secretion via negative feedback [58,61,62]. HA disrupts the negative feedback on secretion [18,23,61,62] resulting in increased LH levels [18,62]. Interaction of androgen and its receptor interferes with progesterone receptor transcription. Moreover, this receptor is involved in converting high levels of androgens to compounds that modulate the gamma-aminobutyric acid A (GABAA). Modulation of the GABAA receptor triggers GnRH neurons and weakens the response to negative progesterone feedback [58]. In addition, it is assumed that androgens might decrease hepatic nuclear factor-4α (HNF-4α) levels by inhibiting lipid synthesis. HNF-4α stimulates SHBG expression by binding to its promoter [63].
HA contributes to other influential factors of PCOS, including IR, inflammation, and oxidative stress.
HA aggravates IR via different routes; it reduces the insulin sensitivity, expression of GLUT-4 and inhibits insulin degradation in the liver [23,32]. Moreover, HA increases a type of skeletal muscle fibers that have low insulin sensitivity [32]. On the other hand, HA worsens central adiposity, which is involved in IR [23,32]. Additionally, it was observed that testosterone increases inflammatory chemicals such as lipopolysaccharide-induced IL-6 in 3T3-L1 adipocytes by activating some signaling pathways [64]. One way androgen results in oxidative stress is by increasing MNC sensitivity to glucose and aggravating glucose-stimulated oxidative stress [65]. It is worth mentioning that dehydroepiandrosterone as an androgen decreases interferon-γ (IFN-γ), an essential regulator in normal ovarian physiology and cell function [64].
In addition, it should be mentioned that studies on PCOS women approved the resemblance of their fatty tissue to men, and hence the effect of HA on adipose tissue dysfunction [8]. In addition, HA is a cause of adipocyte hypertrophy and consequential damages to adipokine secretion [55].
4.2.3. Inflammation
Appropriate inflammation is a vital cause of oocyte growth and ovulation [66]. However, high levels of white blood cell [46,66], C-reactive protein (CRP) [4,46,50,66,67], and other inflammatory biomarkers in peripheral blood are associated with PCOS [4,46,66,67,68]. Inflammation is a cause of HA [44,69]. TNF-α is a pro-inflammatory chemical that can worsen IR. Contribution to IR happens due to interference of pro-inflammatory molecules with insulin signaling pathways [32,67] and reduction of GLUT-4 expression [23]. Some studies showed that the insulin receptor substrate (IRS) serine residue phosphorylation inhibits insulin receptor signaling [32,70]. This phenomenon results in the prevention of GLUT-4 translocation and glucose reuptake [70]. In addition, TNF-α showed the ability to prompt theca cells proliferation in vitro [71]. Furthermore, IL-1 hinders the FSH and LH receptors. Inhibition of these receptors leads to inhibition of follicular development and ovulation [66]. Both TNF-α and IL-1β inhibit activation of HNF-4α by different mechanisms [23]. In addition, NLRP3 inflammasomes induce follicular pyroptosis, ovarian fibrosis, and disturbance of follicular formation [66]. An increase in CRP level is another cause of IR in insulin-sensitive tissues. IR occurs because of increased pro-inflammatory factors secreted by the liver and monocytes. CRP stimulates this increase in secretion [72]. Moreover, another study approved the higher-than-normal level of IL-6 mRNA in granulosa cells [66].
4.2.4. Oxidative Stress
Oxidative stress (OS) is an imbalance between pro-oxidants and antioxidants [71,72,73]. Oxidative molecules include different chemicals such as reactive oxygen species (ROS) [73,74,75] (e.g., O2−, H2O2, and OH−) [76] and reactive nitrogen species (RNS) [74,75]. ROS plays a role in different mechanisms like signaling pathways [71,73,76], cell growth [71,73], and differentiation, as well as RNS [73]. RONS also acts on ovaries functions like steroidogenesis [67,77] and affects neurons responsible for feeding behavior to induce hunger [71]. Overproductions of oxidative chemicals cause various damage to vital molecules such as lipids, proteins, and DNA [73,74,75,77].
Increased OS has been seen in PCOS patients in different studies [74,78,79]. Increased levels of OS activate the nuclear factor-kappa B (NF-κB) [72,75]. NF-κB is involved in inflammatory pathways [75] and affects the production of pro-inflammatory cytokines like TNF-α and IL-6 [72,80]; the effect in IR and PCOS was explained above. A high level of OS also increases the release of TNF-α [77]. On the other hand, increased OS actuates some protein kinases that trigger serine/threonine phosphorylation instead of normal tyrosine phosphorylation of IRS. Thus, the insulin signaling pathway is inhibited, and OS leads to IR [67]. OS also plays a role in obesity. It increases mature adipocyte size and consequently stimulates pre-adipocyte proliferation and adipocyte differentiation. OS also imposes a major effect on obesity [71].
4.2.5. Obesity
Obesity is a key in low-grade chronic inflammation [72]. Accumulation of adipocytes in visceral fat leads to hypoxia and consequent necrosis, which causes inflammatory cytokines production [66]. Adipocyte death due to hypertrophy causes an inflammatory state [44,69]. The mononuclear cells of adipose tissue produce pro-inflammatory cytokines [6,44,81]. Excess abdominal fat is also responsible for the inflammatory condition [6,44,81].
Obesity also plays a role in hyperinsulinemia, IR, and HA occurrence. Visceral obesity arouses an increase in non-esterified fatty acids (NEFAs) levels in the blood. Skeletal muscles uptake NEFAs as the energy source instead of glucose. This hyperglycemia leads to a pancreas rapid reaction and hyperinsulinemia [55]. In addition, the lipolytic response of visceral fat to catecholamines causes lipotoxicity [44] and impairment of insulin clearance and activity [81].
FFA stimulates IRS-1 serine/threonine phosphorylation and reduces tyrosine phosphorylation. Increased FFAs reduce insulin and glucose uptake sensitivity in intramyocellular lipids [52]. Notably, that visceral fat is weightier in IR than abdominal [44] and subcutaneous fat [81] as the visceral fat lipolytic response to catecholamines is more severe [44,81]. The reason is the increased function of the β3 and higher expression of β1 and β2 receptors [81]. Moreover, the type 1 isoenzyme of 11β-hydroxysteroid dehydrogenase (11β-HSD) is involved in converting cortisone to active cortisol, which is highly expressed in adipose tissue, especially in adipose tissue visceral ones. Glucocorticoids reduce glucose uptake and insulin signaling in omental adipocytes [81]. In addition, visceral fat’s adiponectin secretion is less than subcutaneous fats, and this phenomenon leads to decreased adiponectin secretion in obesity [46].
In addition to all adipose tissue’s functions mentioned above, this tissue has endocrine function and secretes chemicals called adipokines or adipocytokines. Adipocytes produce leptin, a high concentration of which inhibits the expression of aromatase mRNA in granulosa cells—thus interrupting androgens to estrogen conversion [52]. In addition, it is suggested that increased leptin levels are related to the absence of folliculogenesis [81]. Moreover, adiponectin, secreted by adipocytes [52], has insulin-sensitizing, anti-diabetic, and anti-inflammatory effects [46]. The adiponectin insulin-sensitizing effect causes a reduction in FFA uptake and gluconeogenesis. It also plays a role in progesterone and estrogen production, ovulation, and decreased GnRH secretion [52]. Furthermore, adiponectin reduces LH secretion from the pituitary, triggers estradiol secretion in granulosa, and is associated with androgen production in ovaries [81]. Omentin-1, another adipose tissue secreted chemical, improves IGF-1-induced progesterone and estradiol secretion in different ways, including increasing the steroidogenic acute regulatory protein and CYP450 aromatase expression and enhancing IGF-1 receptor signaling [82].
Adipose tissue also has several enzymes responsible for converting androstenedione to testosterone and testosterone to dihydrotestosterone [45]. 17β-HSD converts androstenedione to testosterone [44,81] and estrone to estradiol [81]. This enzyme is expressed in adipose tissue [44,81]. As a result of this process, excess adiposity exacerbates HA [45].
Furthermore, the accumulation of lipid in non-adipose tissues, called lipotoxicity, causes oxidative/endoplasmic reticulum stress linked with inflammation and IR. Excess fatty acids in muscles and liver induce IR via serine phosphorylation of insulin receptor by diacylglycerol [83]. In addition, lipid accumulation in the liver diminishes HNF-4α levels leading to reduced SHBG production [63].
A summary of the most representative molecular mechanisms of PCOS pathogenesis is presented in Figure 1.
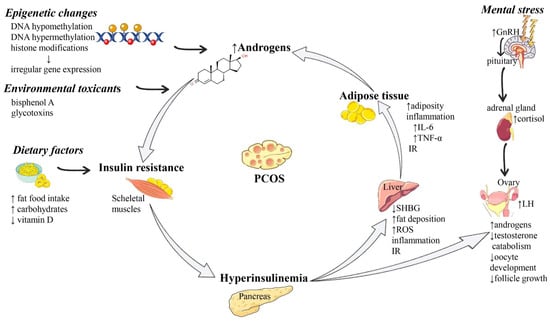
Figure 1.
Summarized scheme regarding the pathophysiology of PCOS. Abbreviations and symbols: ↑ (increased), ↓ (decreased), DNA (deoxyribonucleic acid), GnRH (Gonadotropin-releasing hormone), IL-6 (interleukin 6), IR (insulin resistance), LH (luteinizing hormone), PCOS (polycystic ovary syndrome), SHBG (sex hormone-binding globulin), TNF-α (tumor necrosis alpha).
5. Management
The management approach and selection of the best therapy option depend on the target patient and her priorities [4]. The complications may vary from seeking fertility, regulation of menstrual disturbances to weight reduction or relief from hyperandrogenic symptoms, including acne, hirsutism, or androgenic alopecia [84]. Indeed, the approach should be individualized for each person to meet the optimal result [8]. There is no one ideal treatment for all women diagnosed with PCOS, which leaves physicians no choice but symptomatic therapy [85].
5.1. Lifestyle Modification and Non-Pharmacological Approaches
5.1.1. Weight Loss
Elevated androgenic hormone levels lead to weight gain in women with PCOS, mainly in the abdominal area. As a result, many PCOS women have an apple shape body instead of a pear shape [37]. The first step for women diagnosed with PCOS would be weight reduction and calorie intake restriction [86]. Many studies demonstrate that even a 5% to 10% reduction in weight can restore the regular menstruation cycle [87]. For obese women, it would be best if they could reach their normal range of body mass index (BMI). Along with weight loss, the level of free testosterone decreases, and the incidence of metabolic syndrome reduces [84].
5.1.2. Diet
As mentioned above, to achieve specific goals for each woman, the best diet or nutrient regimen would be the tailored one [87]. However, some suggestions may help choose what to eat more or less. An ideal diet would be rich in fibers and low in saturated fats and carbohydrates. There is a carbohydrate classification considering the blood glucose response they cause within 2 h: low and high glycemic index carbohydrates. Low glycemic index carbohydrates are at the top of our agenda; they include foods and vegetables like broccoli, raw carrot, lentils, soy, bran breakfast cereals, whole-grain bread, etc. Patients should also be aware that foods with a high glycemic index for prevention, white rice, cakes and cookies, fries or chips, and some fruits such as pineapple or watermelon are actual examples [37].
5.1.3. Exercise
Exercise and physical activity play a key role in weight reduction. They may be beneficial to improve insulin sensitivity [88]. Different studies suggest various times for exercise during the week, but the American Heart Association (AHA) recommends approximately 150 min of moderate or 75 min of vigorous and intense exercise per week [84]. Several studies show that exercise, with or without being on a diet, can resume ovulation in women with PCOS. Exercise probably can affect ovulation through modulation of the hypothalamic-pituitary-gonadal (HPG) axis. In overweight and obese women, exercise leads to lower insulin and free androgen levels, inducing the restoration of HPA regulation of ovulation [88].
5.2. Complementary and Alternative Medicine (CAM)
Current management and accessible medications are only moderately effective in PCOS, and there are still some cases left untreated despite non-pharmacological and pharmacological treatments. Some literature claims that pharmacologically based therapies are only effective in 60% of patients [64]. Recent studies have demonstrated that using complementary and alternative medicine (CAM) as adjunctive therapy may benefit the management [89]. Today, CAM is a well-known approach that has been used at least at one point in more than 70% of PCOS patients during their diseases [90]. Several manners can categorize it; according to the latest edition of the National Center for Complementary and Integrative Health (NCCIH), complementary approaches can be classified by their primary therapeutic input into three classes of nutritional, psychological, physical, or all of them in combination [68]. One of the significant merits of CAM is that people often tend to accept these methods due to their beliefs and cultures; this leads to their improved adherence or tolerance to the therapy. Taking a look at prior studies, various methods of CAM including traditional Chinese medicine (TCM), immunotherapy, diet therapy (herbal and medicinal foods, probiotics, and vitamin or supplementation therapy), psychotherapy, spa, yoga, Tai Chi, and oxygen therapy have been considered as effective strategies in reducing the severity of PCOS and its complications [89,91,92,93,94,95]. Two critical subgroups of CAM effective in PCOS management are discussed in the following sections.
5.2.1. Acupuncture
Acupuncture, a fundamental part of CAM, has been used in China for more than 3000 years [89]. It is a kind of sensory stimulation in which thin needles are placed into the skin and muscles. Acupuncture improves clinical manifestations of PCOS by activating somatic afferent nerves of the skin and muscles, modulating somatic and autonomic nervous system activity and endocrine/metabolic functions [91]. Within acupuncture, β-endorphin production increases, affecting the secretion of gonadotropin-releasing hormone, ovulation, and menstrual cycle. This means that acupuncture may induce ovulation and restore the menstrual cycle [64].
5.2.2. Supplementations
Apart from medications with USFDA approval, plenty of supplementation products has been shown to be effective in some women with PCOS. These products include vitamin D supplements, resveratrol, α-lipoic acid, omega-3, berberine, folic acid, myoinositol (MI), and d-chiro-inositol (DCI).
Vitamin D is effective in several studies, especially in cold seasons of the year. The deficiency of this vitamin is thought to be important in the pathogenesis of PCOS, so just the compensatory amount would be suggested [96].
Resveratrol is among the most recommended supplements for the treatment of PCOS. It is assumed to possess chemopreventive, anti-inflammatory and antioxidant, cardioprotective, and neuroprotective effects [97]. Resveratrol may play a beneficial role in PCOS by inhibiting HMG-CoA reductase expression and activity, just like statins [98]. Clinical use of this product has been shown to reduce IR and the risk of type 2 diabetes development [99].
Alpha-lipoic acid and omega-3 are the two supplements found to improve women’s lipid profile and insulin sensitivity through their anti-inflammatory and antioxidant properties [4].
Berberine is a nutraceutical compound with possible, desirable effects against IR and obesity, particularly against visceral adipose tissue (VAT) [100]. Folic acid is usually an agent given to PCOS women seeking fertility [24].
Last but not least, MI and DCI are other essential and well-studied supplements for PCOS treatment. MI has been demonstrated to improve the activity of insulin receptors and can potentially restore the ovulatory function in most women with PCOS [85]. Inositol influences intracellular metabolic processes; it activates key enzymes controlling glucose’s oxidative and nonoxidative metabolism. Studies conducted on PCOS women taken MI alone, DCI alone, and these combinations of the two showed that they cause increased frequency of ovulation, decreased need for FSH therapy for triggering the ovulation, and a significant improvement in the pregnancy rate [97].
5.3. Pharmacological Treatments
Before heading to pharmacological approaches, healthy lifestyle advice must be given to all women diagnosed with PCOS regardless of their weight, complaint, or anything else. This is because, in most cases, and especially in mild to moderate forms, women can solely benefit from diet and exercise [101]. However, the treatment would rely mainly on the patient’s choices and condition in others. If the patient does not want to get pregnant and complains mostly about her menstruation irregularity, combined oral contraceptives (COCs) or progestins are the drugs of choice. The physician can choose the best oral contraceptive with a look on other symptoms rather than menstruation irregularity; for example, Yasmin®, Yaz®, or some other agents can show antiandrogenic effects and can, on the other hand, result in the reduction of androgen production. As a result, they might be helpful in those with hirsutism and/or acne complications.
Metformin, from the biguanides category, is usually prescribed along with the first-choice drugs (COCs) to restore the ovulation cycle in PCOS women because of its insulin sensitivity-increasing properties. Metformin has an antihyperandrogenic effect in the short term too.
In other patients who just want relief from dermatological manifestations due to hyperandrogenism, agents such as aldosterone receptor antagonists (e.g., spironolactone) and 5-alpha reductases (e.g., finasteride) would be more beneficial. Therapy options change for those with infertility who should take agents for ovulation induction like clomiphene citrate and/or aromatase inhibitors [84].
Of course, there are lots of limitations and precautions, and not everyone can benefit from the agents mentioned above owing to their adverse effects or contraindications. Many COC agents cause nausea and vomiting as they try to stimulate the pregnancy situation for the body. In addition, depression, headaches, and migraine are commonly seen in those taking them. Metformin also causes nausea and vomiting in the first days of consumption which may not be tolerated in all patients and leads to abandonment of the therapy. Spironolactone, a widely used and prescribed agent for androgen-related complications, can cause hyperkalemia. Therefore, it is suggested to look up the adverse reactions or contraindications in reliable drug literature or ask the patient’s history of any possible reaction before the prescription.
The complete list of the routine medications used to treat PCOS and the step-by-step treatment pathway considering the patient’s complaints are provided here in Table 1.

Table 1.
Commonly prescribed medications in PCOS.
5.4. Drug Repurposing in PCOS
Drug repurposing, or in other terms drug repositioning or drug re-tasking, actually means finding new indications in other diseases or conditions for a medication that has previously been in the market and has USFDA approval for a specific therapeutic goal [9]. Using this method has shortened the duration of the research and development process, given the thought that the medicines have passed pre-clinical and clinical, safety, and immunological tests. As mentioned before in this review, PCOS still does not have a single ideal pharmacological treatment, and doctors typically tend to cure patients’ symptoms with other agents. Taking a look at other drugs—mostly diabetes agents—may be helpful to recognize some new medications for women with PCOS-related complications.
Table 2 and Table 3, respectively, present general information and clinical trials of drugs primarily approved for other indications (e.g., diabetes type II, hyperlipidemia, weight reduction, etc.), which are now being examined to see their potential effect in PCOS.

Table 2.
Repurposed medications for the treatment of PCOS.

Table 3.
Clinical trials of the repurposed medications for PCOS since 2016.
6. Conclusions
Although the pathogenesis of PCOS is not fully understood, it is believed that different factors from epigenetic alterations to obesity, inflammation, and inactivity may aggravate this syndrome. Since there is still no certain medication or definite cure for this condition, the routine approach after advising on some lifestyle modification and supplementary tips is symptomatic therapy with plenty of agents, including contraceptives, oral antidiabetics, or antiandrogens. In terms of the repurposing, there is a good chance that other approved agents could exert beneficial effects on PCOS. Since the complete profiles of these agents are available, and their efficacy and safety have already been comprehensively studied, the pathway for finding novel treatments becomes a little more straightforward. However, there is still very much to discover and examine for a better understanding of the pathogenesis, and, as a result, targeting the mechanism by proper medication.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas—that is, revising or critically reviewing the article; giving final approval of the version to be published; agreeing on the journal to which the article has been submitted; and confirming to be accountable for all aspects of the work. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
References
- Deans, R. Polycystic ovary syndrome in adolescence. Med. Sci. 2019, 7, 101. [Google Scholar] [CrossRef] [Green Version]
- Witchel, S.F.; E Oberfield, S.; Peña, A.S. Polycystic Ovary Syndrome: Pathophysiology, Presentation, and Treatment With Emphasis on Adolescent Girls. J. Endocr. Soc. 2019, 3, 1545–1573. [Google Scholar] [CrossRef] [PubMed]
- Polycystic Ovary Syndrome. Available online: https://www.womenshealth.gov/a-z-topics/polycystic-ovary-syndrome (accessed on 22 September 2021).
- Bednarska, S.; Siejka, A. The pathogenesis and treatment of polycystic ovary syndrome: What’s new? Adv. Clin. Exp. Med. 2017, 26, 359–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganie, M.A.; Vasudevan, V.; Wani, I.A.; Baba, M.S.; Arif, T.; Rashid, A. Epidemiology, pathogenesis, genetics & management of polycystic ovary syndrome in India. Indian J. Med Res. 2019, 150, 333–344. [Google Scholar] [CrossRef]
- Glueck, C.J.; Goldenberg, N. Characteristics of obesity in polycystic ovary syndrome: Etiology, treatment, and genetics. Metab. 2019, 92, 108–120. [Google Scholar] [CrossRef]
- Damone, A.L.; Joham, A.E.; Loxton, D.; Earnest, A.; Teede, H.J.; Moran, L.J. Depression, anxiety and perceived stress in women with and without PCOS: A community-based study. Psychol. Med. 2019, 49, 1510–1520. [Google Scholar] [CrossRef]
- Escobar-Morreale, H.F. Polycystic ovary syndrome: Definition, aetiology, diagnosis and treatment. Nat. Rev. Endocrinol. 2018, 14, 270–284. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, H.M.; Adeli, I.; Mousavi, T.; Daniali, M.; Nikfar, S.; Abdollahi, M. Drug Repurposing for the Management of Depression: Where Do We Stand Currently? Life 2021, 11, 774. [Google Scholar] [CrossRef]
- Differential Diagnosis of PCOS. Available online: https://www.verywellhealth.com/what-is-the-differential-diagnosis-of-pcos-2616642 (accessed on 6 December 2021).
- Witchel, S.F.; Burghard, A.C.; Tao, R.H.; Oberfield, S.E. The diagnosis and treatment of PCOS in adolescents. Curr. Opin. Pediatr. 2019, 31, 562–569. [Google Scholar] [CrossRef]
- Polycystic Ovary Syndrome (PCOS). Available online: https://www.mayoclinic.org/diseases-conditions/pcos/diagnosis-treatment/drc-20353443 (accessed on 6 December 2021).
- Diagnosis of Polycystic Ovary Syndrome. Available online: https://www.nhs.uk/conditions/polycystic-ovary-syndrome-pcos/diagnosis/ (accessed on 22 September 2021).
- European Society of Human Reproduction and Embryology. International Evidence-Based Guideline for the Assessment and Management of Polycystic Ovary Syndrome. 2018. Available online: https://www.eshre.eu/Guidelines-and-Legal/Guidelines/Polycystic-Ovary-Syndrome (accessed on 22 September 2021).
- Ilie, I.R.; Georgescu, C.E. Polycystic Ovary Syndrome-Epigenetic Mechanisms and Aberrant MicroRNA. Adv. Virus Res. 2015, 71, 25–45. [Google Scholar] [CrossRef]
- Casadesús, J.; Noyer-Weidner, M. Epigenetics. In Brenner’s Encyclopedia of Genetics, 2nd ed.; Maloy, S., Hughes, K., Eds.; Academic Press: San Diego, CA, USA, 2013; pp. 500–503. [Google Scholar]
- Mukherjee, S.; Sagvekar, P.; Azarnezhad, R.; Patil, K. Pathomechanisms of polycystic ovary syndrome Multidimensional approaches. Front. Biosci. 2018, 10, 384–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibanez, L.; Oberfield, S.E.; Witchel, S.F.; Auchus, R.J.; Chang, R.J.; Codner, E.; Dabadghao, P.; Darendeliler, F.; Elbarbary, N.; Gambineri, A.; et al. An International Consortium Update: Pathophysiology, Diagnosis, and Treatment of Polycystic Ovarian Syndrome in Adolescence. Horm. Res. Paediatr. 2017, 88, 371–395. [Google Scholar] [CrossRef]
- Fenichel, P.; Rougier, C.; Hieronimus, S.; Chevalier, N. Which origin for polycystic ovaries syndrome: Genetic, environmental or both? Ann. d’Endocrinol. 2017, 78, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Abbott, D.H.; A Dumesic, D.; E Levine, J. Hyperandrogenic origins of polycystic ovary syndrome – implications for pathophysiology and therapy. Expert Rev. Endocrinol. Metab. 2019, 14, 131–143. [Google Scholar] [CrossRef]
- Rutkowska, A.; Diamanti-Kandarakis, E. Polycystic ovary syndrome and environmental toxins. Fertil. Steril. 2016, 106, 948–958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qu, F.; Wang, F.-F.; Yin, R.; Ding, G.-L.; El-Prince, M.; Gao, Q.; Shi, B.-W.; Pan, H.-H.; Huang, Y.-T.; Jin, M.; et al. A molecular mechanism underlying ovarian dysfunction of polycystic ovary syndrome: Hyperandrogenism induces epigenetic alterations in the granulosa cells. J. Mol. Med. 2012, 90, 911–923. [Google Scholar] [CrossRef]
- Li, Y.; Chen, C.; Ma, Y.; Xiao, J.; Luo, G.; Li, Y.; Wu, D. Multi-system reproductive metabolic disorder: Significance for the pathogenesis and therapy of polycystic ovary syndrome (PCOS). Life Sci. 2019, 228, 167–175. [Google Scholar] [CrossRef]
- Rocha, A.L.; Oliveira, F.R.; Azevedo, R.C.; Silva, V.A.; Peres, T.M.; Candido, A.L.; Gomes, K.B.; Reis, F.M. Recent advances in the understanding and management of polycystic ovary syndrome. F1000Research 2019, 8, 565. [Google Scholar] [CrossRef] [Green Version]
- Jones, L.; Regan, F. Endocrine Disrupting Chemicals. In Encyclopedia of Analytical Science, 3rd ed.; Worsfold, P., Poole, C., Townshend, A., Miró, M., Eds.; Academic Press: Oxford, UK, 2019; pp. 31–38. [Google Scholar]
- Merkin, S.S.; Phy, J.L.; Sites, C.K.; Yang, D. Environmental determinants of polycystic ovary syndrome. Fertil. Steril. 2016, 106, 16–24. [Google Scholar] [CrossRef] [Green Version]
- Calina, D.; Docea, A.O.; Golokhvast, K.S.; Sifakis, S.; Tsatsakis, A.; Makrigiannakis, A. Management of Endocrinopathies in Pregnancy: A Review of Current Evidence. Int. J. Environ. Res. Public Health 2019, 16, 781. [Google Scholar] [CrossRef] [Green Version]
- Sobolewski, M.; Barrett, E.S. Polycystic Ovary Syndrome: Do Endocrine-Disrupting Chemicals Play a Role? Semin. Reprod. Med. 2014, 32, 166–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soave, I.; Occhiali, T.; Assorgi, C.; Marci, R.; Caserta, D. Environmental toxin exposure in polycystic ovary syndrome women and possible ovarian neoplastic repercussion. Curr. Med Res. Opin. 2020, 36, 693–703. [Google Scholar] [CrossRef]
- Palioura, E.; Diamanti-Kandarakis, E. Polycystic ovary syndrome (PCOS) and endocrine disrupting chemicals (EDCs). Rev. Endocr. Metab. Disord. 2015, 16, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Palioura, E.; Diamanti-Kandarakis, E. Industrial endocrine disruptors and polycystic ovary syndrome. J. Endocrinol. Investig. 2013, 36, 1105–1111. [Google Scholar] [CrossRef]
- Wang, J.; Wu, D.; Guo, H.; Li, M. Hyperandrogenemia and insulin resistance: The chief culprit of polycystic ovary syndrome. Life Sci. 2019, 236, 116940. [Google Scholar] [CrossRef] [PubMed]
- Stefanaki, C.; Pervanidou, P.; Boschiero, D.; Chrousos, G.P. Chronic stress and body composition disorders: Implications for health and disease. Hormones 2018, 17, 33–43. [Google Scholar] [CrossRef] [Green Version]
- Steegers-Theunissen, R.; Wiegel, R.; Jansen, P.; Laven, J.; Sinclair, K. Polycystic Ovary Syndrome: A Brain Disorder Characterized by Eating Problems Originating during Puberty and Adolescence. Int. J. Mol. Sci. 2020, 21, 8211. [Google Scholar] [CrossRef]
- Yang, S.; Yang, C.; Pei, R.; Li, C.; Li, X.; Huang, X.; Wu, S.; Liu, D. Investigation on the association of occupational stress with risk of polycystic ovary syndrome and mediating effects of HOMA-IR. Gynecol. Endocrinol. 2018, 34, 961–964. [Google Scholar] [CrossRef]
- Szczuko, M.; Kikut, J.; Szczuko, U.; Szydłowska, I.; Nawrocka-Rutkowska, J.; Ziętek, M.; Verbanac, D.; Saso, L. Nutrition Strategy and Life Style in Polycystic Ovary Syndrome—Narrative Review. Nutrients 2021, 13, 2452. [Google Scholar] [CrossRef]
- Faghfoori, Z.; Fazelian, S.; Shadnoush, M.; Goodarzi, R. Nutritional management in women with polycystic ovary syndrome: A review study. Diabetes Metab. Syndr. Clin. Res. Rev. 2017, 11, S429–S432. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Altieri, B.; de Angelis, C.; Palomba, S.; Pivonello, R.; Colao, A.; Orio, F. Shedding new light on female fertility: The role of vitamin D. Rev. Endocr. Metab. Disord. 2017, 18, 273–283. [Google Scholar] [CrossRef]
- Ciebiera, M.; Esfandyari, S.; Siblini, H.; Prince, L.; Elkafas, H.; Wojtyła, C.; Al-Hendy, A.; Ali, M. Nutrition in Gynecological Diseases: Current Perspectives. Nutrients 2021, 13, 1178. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, E.A.; Huddleston, H.G. Insulin resistance in polycystic ovary syndrome: Concept versus cutoff. Fertil. Steril. 2019, 112, 827–828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrakis, D.; Vassilopoulou, L.; Mamoulakis, C.; Psycharakis, C.; Anifantaki, A.; Sifakis, S.; Docea, A.O.; Tsiaoussis, J.; Makrigiannakis, A.; Tsatsakis, A.M. Endocrine Disruptors Leading to Obesity and Related Diseases. Int. J. Environ. Res. Public Health 2017, 14, 1282. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Zhou, H.; Hu, M.; Feng, H. Effect of Diet on Insulin Resistance in Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2020, 105. [Google Scholar] [CrossRef] [PubMed]
- Dabadghao, P. Polycystic ovary syndrome in adolescents. Best Pr. Res. Clin. Endocrinol. Metab. 2019, 33, 101272. [Google Scholar] [CrossRef]
- Rosenfield, R.L.; Ehrmann, D.A. The Pathogenesis of Polycystic Ovary Syndrome (PCOS): The Hypothesis of PCOS as Functional Ovarian Hyperandrogenism Revisited. Endocr. Rev. 2016, 37, 467–520. [Google Scholar] [CrossRef]
- Rothenberg, S.S.; Beverley, R.; Barnard, E.; Baradaran-Shoraka, M.; Sanfilippo, J.S. Polycystic ovary syndrome in adolescents. Best Pr. Res. Clin. Obstet. Gynaecol. 2018, 48, 103–114. [Google Scholar] [CrossRef]
- Jeanes, Y.; Reeves, S. Metabolic consequences of obesity and insulin resistance in polycystic ovary syndrome: Diagnostic and methodological challenges. Nutr. Res. Rev. 2017, 30, 97–105. [Google Scholar] [CrossRef]
- Polak, K.; Czyzyk, A.; Simoncini, T.; Meczekalski, B. New markers of insulin resistance in polycystic ovary syndrome. J. Endocrinol. Investig. 2017, 40, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Hu, J.; Wang, W.; Sun, Y.; Sun, K. HMGB1-induced aberrant autophagy contributes to insulin resistance in granulosa cells in PCOS. FASEB J. 2020, 34, 9563–9574. [Google Scholar] [CrossRef]
- He, F.-F.; Li, Y.-M. Role of gut microbiota in the development of insulin resistance and the mechanism underlying polycystic ovary syndrome: A review. J. Ovarian Res. 2020, 13, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bannigida, D.M.; Nayak, B.S.; Vijayaraghavan, R. Insulin resistance and oxidative marker in women with PCOS. Arch. Physiol. Biochem. 2018, 126, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Avery, P.J.; Jorgensen, A.; Hamberg, A.K.; Wadelius, M.; Pirmohamed, M.; Kamali, F. A Proposal for an Individualized Pharmacogenetics-Based Warfarin Initiation Dose Regimen for Patients Commencing Anticoagulation Therapy. Clin. Pharmacol. Ther. 2011, 90, 701–706. [Google Scholar] [CrossRef]
- Zeng, X.; Xie, Y.-J.; Liu, Y.-T.; Long, S.-L.; Mo, Z.-C. Polycystic ovarian syndrome: Correlation between hyperandrogenism, insulin resistance and obesity. Clin. Chim. Acta 2020, 502, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Docea, A.O.; Vassilopoulou, L.; Fragou, D.; Arsene, A.L.; Fenga, C.; Kovatsi, L.; Petrakis, D.; Rakitskii, V.N.; Nosyrev, A.E.; Izotov, B.N.; et al. CYP polymorphisms and pathological conditions related to chronic exposure to organochlorine pesticides. Toxicol. Rep. 2017, 4, 335–341. [Google Scholar] [CrossRef]
- Cassar, S.; Misso, M.L.; Hopkins, W.G.; Shaw, C.S.; Teede, H.; Stepto, N.K. Insulin resistance in polycystic ovary syndrome: A systematic review and meta-analysis of euglycaemic–hyperinsulinaemic clamp studies. Hum. Reprod. 2016, 31, 2619–2631. [Google Scholar] [CrossRef] [Green Version]
- Condorelli, R.A.; Calogero, A.E.; Di Mauro, M.; La Vignera, S. PCOS and diabetes mellitus: From insulin resistance to altered beta pancreatic function, a link in evolution. Gynecol. Endocrinol. 2017, 33, 665–667. [Google Scholar] [CrossRef] [Green Version]
- Lizneva, D.V.; Gavrilova-Jordan, L.; Walker, W.; Azziz, R. Androgen excess: Investigations and management. Best Pr. Res. Clin. Obstet. Gynaecol. 2016, 37, 98–118. [Google Scholar] [CrossRef]
- Macut, D.; Bjekić-Macut, J.; Rahelić, D.; Doknić, M. Insulin and the polycystic ovary syndrome. Diabetes Res. Clin. Pr. 2017, 130, 163–170. [Google Scholar] [CrossRef]
- Baskind, N.E.; Balen, A.H. Hypothalamic–pituitary, ovarian and adrenal contributions to polycystic ovary syndrome. Best Pr. Res. Clin. Obstet. Gynaecol. 2016, 37, 80–97. [Google Scholar] [CrossRef] [PubMed]
- Ianoşi, S.; Ianoşi, G.; Neagoe, D.; Ionescu, O.; Zlatian, O.; Docea, A.O.; Badiu, C.; Sifaki, M.; Tsoukalas, D.; Tsatsakis, A.; et al. Age-dependent endocrine disorders involved in the pathogenesis of refractory acne in women. Mol. Med. Rep. 2016, 14, 5501–5506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, A.M.; Campbell, R.E. Polycystic ovary syndrome: Understanding the role of the brain. Front. Neuroendocr. 2017, 46, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Coyle, C.; Campbell, R.E. Pathological pulses in PCOS. Mol. Cell. Endocrinol. 2019, 498, 110561. [Google Scholar] [CrossRef]
- Ruddenklau, A.; E Campbell, R. Neuroendocrine Impairments of Polycystic Ovary Syndrome. Endocrinology 2019, 160, 2230–2242. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, Z.; Feng, W.-J.; Long, S.-L.; Mo, Z.-C. Sex hormone-binding globulin and polycystic ovary syndrome. Clin. Chim. Acta 2019, 499, 142–148. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, Q.; Sun, D.; Cui, X.; Chen, S.; Bulbul, A.; Liu, S.; Yan, Q. Dehydroepiandrosterone stimulates inflammation and impairs ovarian functions of polycystic ovary syndrome. J. Cell. Physiol. 2018, 234, 7435–7447. [Google Scholar] [CrossRef]
- Sanchez-Garrido, M.A.; Tena-Sempere, M. Metabolic dysfunction in polycystic ovary syndrome: Pathogenic role of androgen excess and potential therapeutic strategies. Mol. Metab. 2020, 35, 100937. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, H.; Li, Z.; Fan, H.; Yan, X.; Liu, X.; Xuan, J.; Feng, D.; Wei, X. The Release of Peripheral Immune Inflammatory Cytokines Promote an Inflammatory Cascade in PCOS Patients via Altering the Follicular Microenvironment. Front. Immunol. 2021, 12, 685724. [Google Scholar] [CrossRef]
- Zuo, T.; Zhu, M.; Xu, W. Roles of Oxidative Stress in Polycystic Ovary Syndrome and Cancers. Oxidative Med. Cell. Longev. 2016, 2016, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Rudnicka, E.; Suchta, K.; Grymowicz, M.; Calik-Ksepka, A.; Smolarczyk, K.; Duszewska, A.; Smolarczyk, R.; Meczekalski, B. Chronic Low Grade Inflammation in Pathogenesis of PCOS. Int. J. Mol. Sci. 2021, 22, 3789. [Google Scholar] [CrossRef]
- Shorakae, S.; Ranasinha, S.; Abell, S.; Lambert, G.; Lambert, E.; De Courten, B.; Teede, H. Inter-related effects of insulin resistance, hyperandrogenism, sympathetic dysfunction and chronic inflammation in PCOS. Clin. Endocrinol. 2018, 89, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Stepto, N.K.; Moreno-Asso, A.; McIlvenna, L.; A Walters, K.; Rodgers, R.J. Molecular Mechanisms of Insulin Resistance in Polycystic Ovary Syndrome: Unraveling the Conundrum in Skeletal Muscle? J. Clin. Endocrinol. Metab. 2019, 104, 5372–5381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mancini, A.; Bruno, C.; Vergani, E.; D′abate, C.; Giacchi, E.; Silvestrini, A. Oxidative Stress and Low-Grade Inflammation in Polycystic Ovary Syndrome: Controversies and New Insights. Int. J. Mol. Sci. 2021, 22, 1667. [Google Scholar] [CrossRef] [PubMed]
- Mizgier, M.; Jarząbek-Bielecka, G.; Wendland, N.; Jodłowska-Siewert, E.; Nowicki, M.; Brożek, A.; Kędzia, W.; Formanowicz, D.; Opydo-Szymaczek, J. Relation between Inflammation, Oxidative Stress, and Macronutrient Intakes in Normal and Excessive Body Weight Adolescent Girls with Clinical Features of Polycystic Ovary Syndrome. Nutrients 2021, 13, 896. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Liu, H.; Bai, H.; Zhang, Y.; Liu, Q.; Guan, L.; Fan, P. Oxidative stress status in Chinese women with different clinical phenotypes of polycystic ovary syndrome. Clin. Endocrinol. 2017, 86, 88–96. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, Z.; Zhao, S.; Cheng, L.; Man, Y.; Gao, X.; Zhao, H. Oxidative stress markers in the follicular fluid of patients with polycystic ovary syndrome correlate with a decrease in embryo quality. J. Assist. Reprod. Genet. 2021, 38, 471–477. [Google Scholar] [CrossRef]
- Di Segni, C.; Silvestrini, A.; Fato, R.; Bergamini, C.; Guidi, F.; Raimondo, S.; Meucci, E.; Romualdi, D.; Apa, R.; Lanzone, A.; et al. Plasmatic and Intracellular Markers of Oxidative Stress in Normal Weight and Obese Patients with Polycystic Ovary Syndrome. Exp. Clin. Endocrinol. Diabetes 2017, 125, 506–513. [Google Scholar] [CrossRef] [Green Version]
- Lai, Q.; Xiang, W.; Li, Q.; Zhang, H.; Li, Y.; Zhu, G.; Xiong, C.; Jin, L. Oxidative stress in granulosa cells contributes to poor oocyte quality and IVF-ET outcomes in women with polycystic ovary syndrome. Front. Med. 2018, 12, 518–524. [Google Scholar] [CrossRef]
- Lu, J.; Wang, Z.; Cao, J.; Chen, Y.; Dong, Y. A novel and compact review on the role of oxidative stress in female reproduction. Reprod. Biol. Endocrinol. 2018, 16, 1–18. [Google Scholar] [CrossRef]
- Uyanikoglu, H.; Sabuncu, T.; Dursun, H.; Sezen, H.; Aksoy, N. Circulating levels of apoptotic markers and oxidative stress parameters in women with polycystic ovary syndrome: A case-controlled descriptive study. Biomarkers 2017, 46, 1–5. [Google Scholar] [CrossRef]
- Özer, A.; Bakacak, M.; Kiran, H.; Ercan, O.; Kostu, B.; Pektas, M.K.; Kilinç, M.; Aslan, F. Increased oxidative stress is associated with insulin resistance and infertility in polycystic ovary syndrome. Ginekol. Pol. 2016, 87, 733–738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guzmán Hernández, E.A.; Díaz Portillo, S.A.; Villafuerte Anaya, Ó.C.; González Valle, M.D.R.; Benítez Flores, J.D.C.; Chávez, R.S.M.; Galindo, G.C.; Mondragón, L.D.V.; Cobos, D.S.; Guerrero, G.A.M.; et al. Renoprotective and Hepatoprotective Effects Of Hippocratea Excelsa On Metabolic Syndrome In Fructose-Fed Rats. Farmacia 2020, 68, 1106–1119. [Google Scholar] [CrossRef]
- Delitala, A.; Capobianco, G.; Delitala, G.; Cherchi, P.L.; Dessole, S. Polycystic ovary syndrome, adipose tissue and metabolic syndrome. Arch. Gynecol. Obstet. 2017, 296, 405–419. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Watanabe-Kominato, K.; Takahashi, Y.; Kojima, M.; Watanabe, R. Adipose Tissue-Derived Omentin-1 Function and Regulation. Compr. Pshysiol. 2017, 7, 765–781. [Google Scholar] [CrossRef]
- Dumesic, D.A.; Abbott, D.H.; Sanchita, S.; Chazenbalk, G.D. Endocrine–metabolic dysfunction in polycystic ovary syndrome: An evolutionary perspective. Curr. Opin. Endocr. Metab. Res. 2020, 12, 41–48. [Google Scholar] [CrossRef]
- Zeind, C.S.; Carvalho, M.G. Applied Therapeutics: The Clinical Use of Drugs; Wolters Kluwer Health: Philadelphia, PA, USA, 2017. [Google Scholar]
- Liu, H.-Y.; Liu, J.-Q.; Mai, Z.-X.; Zeng, Y.-T. A Subpathway-Based Method of Drug Reposition for Polycystic Ovary Syndrome. Reprod. Sci. 2014, 22, 423–430. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zheng, Y.; Guo, Y.; Lai, Z. The Effect of Low Carbohydrate Diet on Polycystic Ovary Syndrome: A Meta-Analysis of Randomized Controlled Trials. Int. J. Endocrinol. 2019, 2019, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Brennan, L.; Teede, H.; Skouteris, H.; Linardon, J.; Hill, B.; Moran, L. Lifestyle and Behavioral Management of Polycystic Ovary Syndrome. J. Women’s Health 2017, 26, 836–848. [Google Scholar] [CrossRef]
- Hakimi, O.; Cameron, L.-C. Effect of Exercise on Ovulation: A Systematic Review. Sports Med. 2016, 47, 1555–1567. [Google Scholar] [CrossRef]
- Jia, L.-Y.; Feng, J.-X.; Li, J.-L.; Liu, F.-Y.; Xie, L.-Z.; Luo, S.-J.; Han, F.-J. The Complementary and Alternative Medicine for Polycystic Ovary Syndrome: A Review of Clinical Application and Mechanism. Evid.-Based Complement. Altern. Med. 2021, 2021, 1–12. [Google Scholar] [CrossRef]
- Shen, W.; Jin, B.; Pan, Y.; Han, Y.; You, T.; Zhang, Z.; Qu, Y.; Liu, S.; Zhang, Y. The Effects of Traditional Chinese Medicine-Associated Complementary and Alternative Medicine on Women with Polycystic Ovary Syndrome. Evid.-Based Complement. Altern. Med. 2021, 2021, 1–26. [Google Scholar] [CrossRef]
- Raja-Khan, N.; Stener-Victorin, E.; Wu, X.; Legro, R.S. The physiological basis of complementary and alternative medicines for polycystic ovary syndrome. Am. J. Physiol. Metab. 2011, 301, E1–E10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Guo, X.; Ma, S.; Ma, H.; Li, H.; Wang, Y.; Qin, Z.; Wu, X.; Han, Y.; Han, Y. The Treatment with Complementary and Alternative Traditional Chinese Medicine for Menstrual Disorders with Polycystic Ovary Syndrome. Evid.-Based Complement. Altern. Med. 2021, 2021, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Shirvani-Rad, S.; Tabatabaei-Malazy, O.; Mohseni, S.; Hasani-Ranjbar, S.; Soroush, A.-R.; Hoseini-Tavassol, Z.; Ejtahed, H.-S.; Larijani, B. Probiotics as a Complementary Therapy for Management of Obesity: A Systematic Review. Evid.-Based Complement. Altern. Med. 2021, 2021, 1–11. [Google Scholar] [CrossRef]
- Li, Y.; Peng, C.; Cao, G.; Li, W.; Hou, L. Tai chi for overweight/obese adolescent and young women with polycystic ovary syndrome: Study protocol for a randomized controlled trial. Trials 2018, 19, 512. [Google Scholar] [CrossRef]
- Mohseni, M.; Eghbali, M.; Bahrami, H.; Dastaran, F.; Amini, L. Yoga Effects on Anthropometric Indices and Polycystic Ovary Syndrome Symptoms in Women Undergoing Infertility Treatment: A Randomized Controlled Clinical Trial. Evid.-Based Complement. Altern. Med. 2021, 2021, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Thomson, R.L.; Spedding, S.; Brinkworth, G.D.; Noakes, M.; Buckley, J.D. Seasonal effects on vitamin D status influence outcomes of lifestyle intervention in overweight and obese women with polycystic ovary syndrome. Fertil. Steril. 2013, 99, 1779–1785. [Google Scholar] [CrossRef]
- Legro, R.S.; Duguech, L.M.M. Pharmacologic Treatment of Polycystic Ovary Syndrome: Alternate and Future Paths. Semin. Reprod. Med. 2017, 35, 326–343. [Google Scholar] [CrossRef]
- Ortega, I.; A Villanueva, J.; Wong, D.H.; Cress, A.B.; Sokalska, A.; Stanley, S.D.; Duleba, A.J. Resveratrol potentiates effects of simvastatin on inhibition of rat ovarian theca-interstitial cells steroidogenesis. J. Ovarian Res. 2014, 7, 21. [Google Scholar] [CrossRef] [Green Version]
- Crandall, J.P.; Oram, V.; Trandafirescu, G.; Reid, M.; Kishore, P.; Hawkins, M.; Cohen, H.W.; Barzilai, N. Pilot Study of Resveratrol in Older Adults With Impaired Glucose Tolerance. J. Gerontol. Ser. A Boil. Sci. Med Sci. 2012, 67, 1307–1312. [Google Scholar] [CrossRef] [Green Version]
- Rondanelli, M.; Infantino, V.; Riva, A.; Petrangolini, G.; Faliva, M.A.; Peroni, G.; Naso, M.; Nichetti, M.; Spadaccini, D.; Gasparri, C.; et al. Polycystic ovary syndrome management: A review of the possible amazing role of berberine. Arch. Gynecol. Obstet. 2020, 301, 53–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naka, K.K.; Kalantaridou, S.N.; Kravariti, M.; Bechlioulis, A.; Kazakos, N.; Calis, K.A.; Makrigiannakis, A.; Katsouras, C.S.; Chrousos, G.P.; Tsatsoulis, A.; et al. Effect of the insulin sensitizers metformin and pioglitazone on endothelial function in young women with polycystic ovary syndrome: A prospective randomized study. Fertil. Steril. 2011, 95, 203–209. [Google Scholar] [CrossRef]
- Ethinyl Estradiol and Levonorgestrel. Available online: https://www.drugs.com/mtm/ethinyl-estradiol-and-levonorgestrel.html (accessed on 8 October 2021).
- Mircette® Drug Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/020713s010lbl.pdf (accessed on 4 September 2021).
- Diane-35® Drug Information. Available online: https://www.bayer.com/sites/default/files/DIANE_35_EN_PI.pdf (accessed on 4 September 2021).
- Yasmin® Drug Infromation. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/021098s019lbl.pdf (accessed on 4 September 2021).
- Yaz® Drug Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/021676s012lbl.pdf (accessed on 4 September 2021).
- Natazia® Drug Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/022252s001lbl.pdf (accessed on 4 September 2021).
- Zahradnik, H. Belara®–A Reliable Oral Contraceptive with Additional Benefits for Health and Efficacy in Dysmenorrhoea. Eur. J. Contracept. Reprod. Health Care 2005, 10, 12–18. [Google Scholar] [CrossRef]
- Provera® Drug Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2007/011839s071lbl.pdf (accessed on 4 September 2021).
- Food and Drug Administration (FDA). GLUCOPHAGE®(Metformin Hydrochloride) Tablets. GLUCOPHAGE® XR (Metformin Hydrochloride) Extended-Release Tablets. Label. 2017. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/020357s037s039,021202s021s023lbl.pdf (accessed on 4 September 2021).
- Highlights of Prescribing Information-Aldactone® (Spironolactone) Tablets, for Oral Use. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/012151s075lbl.pdf (accessed on 10 April 2021).
- Propecia® Drug Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/020788s020s021s023lbl.pdf (accessed on 4 September 2021).
- Clomid® Drug Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/016131s026lbl.pdf (accessed on 4 September 2021).
- Femara® Drug Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/020726s027lbl.pdf (accessed on 4 September 2021).
- Zocor® Drug Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/019766s085lbl.pdf (accessed on 4 September 2021).
- Cassidy-Vu, L.; Joe, E.; Kirk, J.K. Role of Statin Drugs for Polycystic Ovary Syndrome. J. Fam. Reprod. Health 2016, 10, 165–175. [Google Scholar]
- Lipitor® Drug Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/020702s056lbl.pdf (accessed on 4 September 2021).
- Food and Drug Administration (FDA). ACTOS (Pioglitazone Hydrochloride) Tablets for Oral Use. Label. 2011. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/021073s043s044lbl.pdf (accessed on 4 September 2021).
- Xu, Y.; Wu, Y.; Huang, Q. Comparison of the effect between pioglitazone and metformin in treating patients with PCOS:a meta-analysis. Arch. Gynecol. Obstet. 2017, 296, 661–677. [Google Scholar] [CrossRef] [Green Version]
- Jardiance® Drug Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/204629s023lbl.pdf (accessed on 4 September 2021).
- Marinkovic-Radosevic, J.; Berkovic, M.C.; Kruezi, E.; Bilic-Curcic, I.; Mrzljak, A. Exploring new treatment options for polycystic ovary syndrome: Review of a novel antidiabetic agent SGLT2 inhibitor. World J. Diabetes 2021, 12, 932–938. [Google Scholar] [CrossRef]
- Farxiga® Drug Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/202293s020lbl.pdf (accessed on 4 September 2021).
- Invokana® Drug Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/204042s027lbl.pdf (accessed on 4 September 2021).
- Januvia® Drug Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/021995s019lbl.pdf (accessed on 4 September 2021).
- Abdalla, M.A.; Deshmukh, H.; Atkin, S.; Sathyapalan, T. The potential role of incretin-based therapies for polycystic ovary syndrome: A narrative review of the current evidence. Ther. Adv. Endocrinol. Metab. 2021, 12, 2042018821989238. [Google Scholar] [CrossRef] [PubMed]
- Victoza® Drug Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/022341s027lbl.pdf (accessed on 4 September 2021).
- Cena, H.; Chiovato, L.; E Nappi, R. Obesity, Polycystic Ovary Syndrome, and Infertility: A New Avenue for GLP-1 Receptor Agonists. J. Clin. Endocrinol. Metab. 2020, 105, e2695–e2709. [Google Scholar] [CrossRef]
- Ozempic® Drug Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/209637lbl.pdf (accessed on 4 September 2021).
- Byetta® Drug Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/021773s9s11s18s22s25lbl.pdf (accessed on 4 September 2021).
- Cetylev® Drug Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/207916s000lbl.pdf (accessed on 4 September 2021).
- Sandhu, J.K.; Waqar, A.; Jain, A.; Joseph, C.; Srivastava, K.; Ochuba, O.; Alkayyali, T.; Ruo, S.W.; Poudel, S. Oxidative Stress in Polycystic Ovarian Syndrome and the Effect of Antioxidant N-Acetylcysteine on Ovulation and Pregnancy Rate. Cureus 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Tehran University of Medical Sciences. The Effect of Astaxanthin on Oxidative Stress Indices in Patients With Polycystic Ovary Syndrome. Available online: https://ClinicalTrials.gov/show/NCT03991286 (accessed on 14 September 2021).
- Azienda di Servizi alla Persona di Pavia. Berberine and Polycystic Ovary Syndrome. Available online: https://ClinicalTrials.gov/show/NCT04932070 (accessed on 14 September 2021).
- Woman’s; AstraZeneca. EQW, DAPA, EQW/DAPA, DAPA/MET ER and PHEN/TPM ER in Obese Women With PolycysticOvary Syndrome (PCOS). Available online: https://ClinicalTrials.gov/show/NCT02635386 (accessed on 14 September 2021).
- Biosearch, S.A. Evaluation of the Mixture Myoinositol:D-Chiro-Inositol 3.6:1 in Women With Polycystic Ovary Syndrome. Available online: https://ClinicalTrials.gov/show/NCT03201601 (accessed on 14 September 2021).
- Hull University Teaching Hospitals NHS Trust. Empagliflozin vs. Metformin in PCOS. Available online: https://ClinicalTrials.gov/show/NCT03008551 (accessed on 14 September 2021).
- Javed, Z.; Papageorgiou, M.; Deshmukh, H.; Rigby, A.S.; Qamar, U.; Abbas, J.; Khan, A.Y.; Kilpatrick, E.S.; Atkin, S.L.; Sathyapalan, T. Effects of empagliflozin on metabolic parameters in polycystic ovary syndrome: A randomized controlled study. Clin. Endocrinol. 2019, 90, 805–813. [Google Scholar] [CrossRef] [PubMed]
- RenJi Hospital. Research of Exenatide for Overweight/Obese PCOS Patients With IGR. Available online: https://ClinicalTrials.gov/show/NCT03352869 (accessed on 14 September 2021).
- Tao, T.; Zhang, Y.; Zhu, Y.-C.; Fu, J.-R.; Wang, Y.-Y.; Cai, J.; Ma, J.-Y.; Xu, Y.; Gao, Y.-N.; Sun, Y.; et al. Exenatide, Metformin, or Both for Prediabetes in PCOS: A Randomized, Open-label, Parallel-group Controlled Study. J. Clin. Endocrinol. Metab. 2021, 106, e1420–e1432. [Google Scholar] [CrossRef] [PubMed]
- AGUNCO Obstetrics and Gynecology Centre; Hospital Juarez de Mexico. Myo-inositol, Alpha-Lactalbumin and Folic Acid Treatment in PCOS. Available online: https://ClinicalTrials.gov/show/NCT04645745 (accessed on 14 September 2021).
- Lo.Li.Pharma s.r.l. Improved Effects of MI Plus Alpha-LA in PCOS. Available online: https://ClinicalTrials.gov/show/NCT03422289 (accessed on 14 September 2021).
- Oliva, M.M.; Buonomo, G.; Calcagno, M.; Unfer, V. Effects of myo-inositol plus alpha-lactalbumin in myo-inositol-resistant PCOS women. J. Ovarian Res. 2018, 11, 38. [Google Scholar] [CrossRef]
- Pharmarte srl. Myoinositol Plus L-Tyrosine, Selenium and Chromium in PCOS. Available online: https://ClinicalTrials.gov/show/NCT03673995 (accessed on 14 September 2021).
- Medical University of Vienna. Micronutrient Supplementation in PCO-Syndrome. Available online: https://ClinicalTrials.gov/show/NCT03306745 (accessed on 14 September 2021).
- Hager, M.; Nouri, K.; Imhof, M.; Egarter, C.; Ott, J. The impact of a standardized micronutrient supplementation on PCOS-typical parameters: A randomized controlled trial. Arch. Gynecol. Obstet. 2019, 300, 455–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Universitaire Ziekenhuizen Leuven. The Gut Microbiome in Women With Polycystic Ovary Syndrome. Available online: https://ClinicalTrials.gov/show/NCT03642600 (accessed on 14 September 2021).
- Ain Shams University. L-Carnitine and Clomiphene Citrate for Induction of Ovulation in Women With Polycystic Ovary Syndrome. Available online: https://ClinicalTrials.gov/show/NCT03476356 (accessed on 14 September 2021).
- University, A. Adding L-Carnitine to Clomiphene Citrate for Induction of Ovulation in Women with Polycystic Ovary Syndrome. Available online: https://ClinicalTrials.gov/show/NCT03630341 (accessed on 14 September 2021).
- Woman’s; A/S, N.N. Liraglutide 3mg (Saxenda) on Weight, Body Composition, Hormonal and Metabolic Parameters in Obese Women with PCOS. Available online: https://ClinicalTrials.gov/show/NCT03480022 (accessed on 14 September 2021).
- University, A.S. NAC in CC Resistant PCOS After LOD. Available online: https://ClinicalTrials.gov/show/NCT02775734 (accessed on 14 September 2021).
- Shiraz University of Medical Sciences. Effects of Cyproterone Compound-spironolactone, Metformin and Pioglitazone on Inflammatory Markers in PCOS. Available online: https://ClinicalTrials.gov/show/NCT02689843 (accessed on 14 September 2021).
- Peshawar, K.M.U. Treatment with Metformin and Combination of Metformin and Pioglitazone in Polycystic Ovarian Syndrome. Available online: https://ClinicalTrials.gov/show/NCT03117517 (accessed on 14 September 2021).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).