Microsporum gypseum Isolated from Ailuropoda melanoleuca Provokes Inflammation and Triggers Th17 Adaptive Immunity Response
Abstract
1. Introduction
2. Results
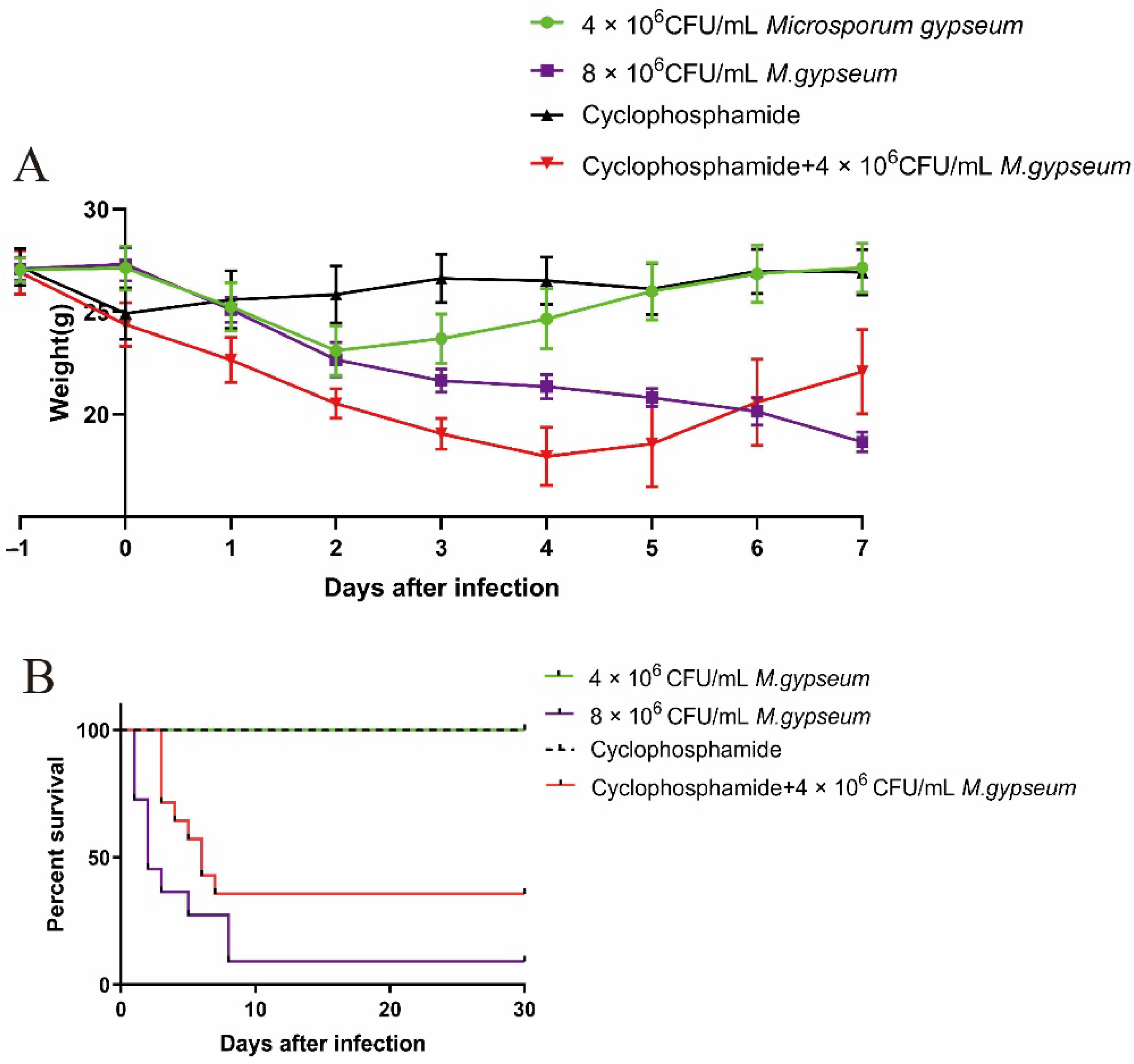
2.1. Murine Weight and Mortality
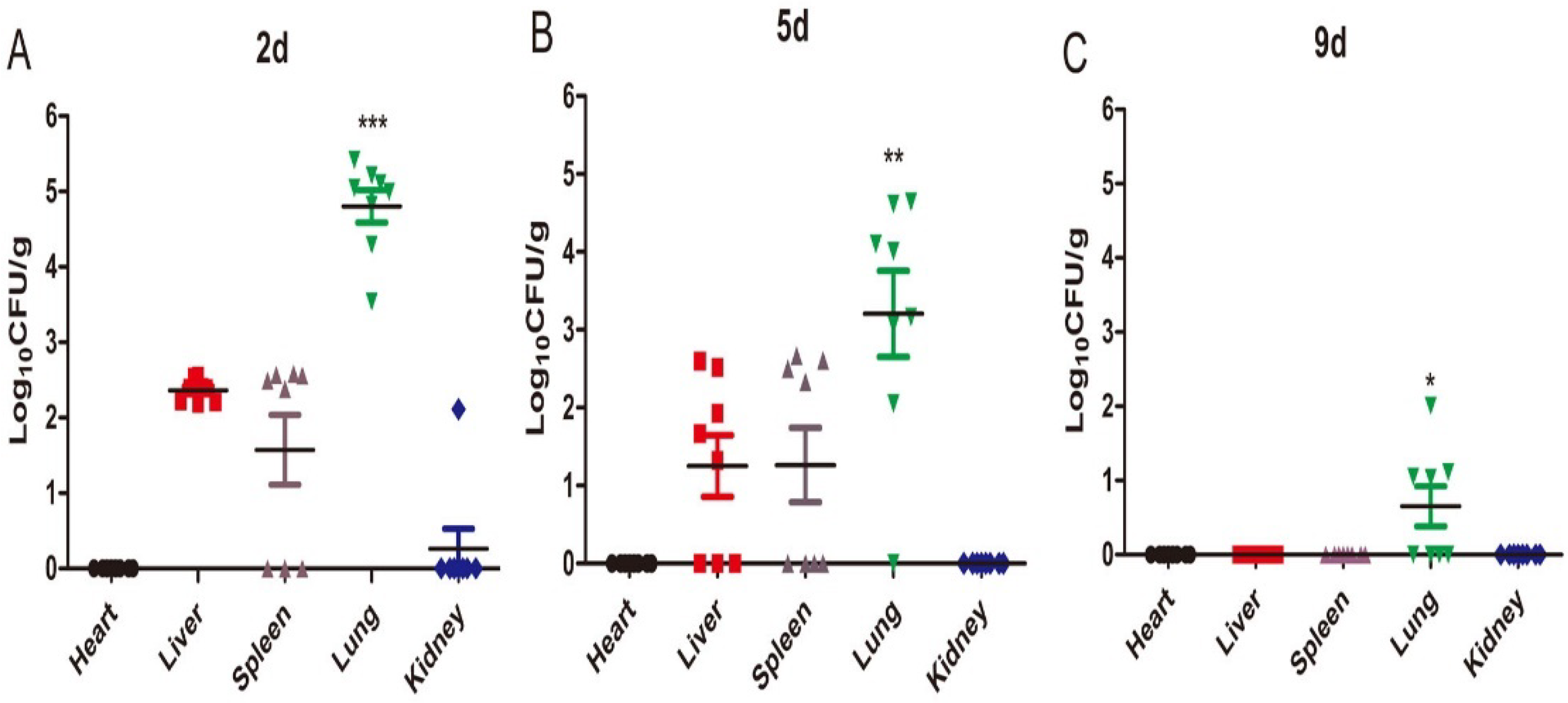
2.2. Tissue Fungal Burden
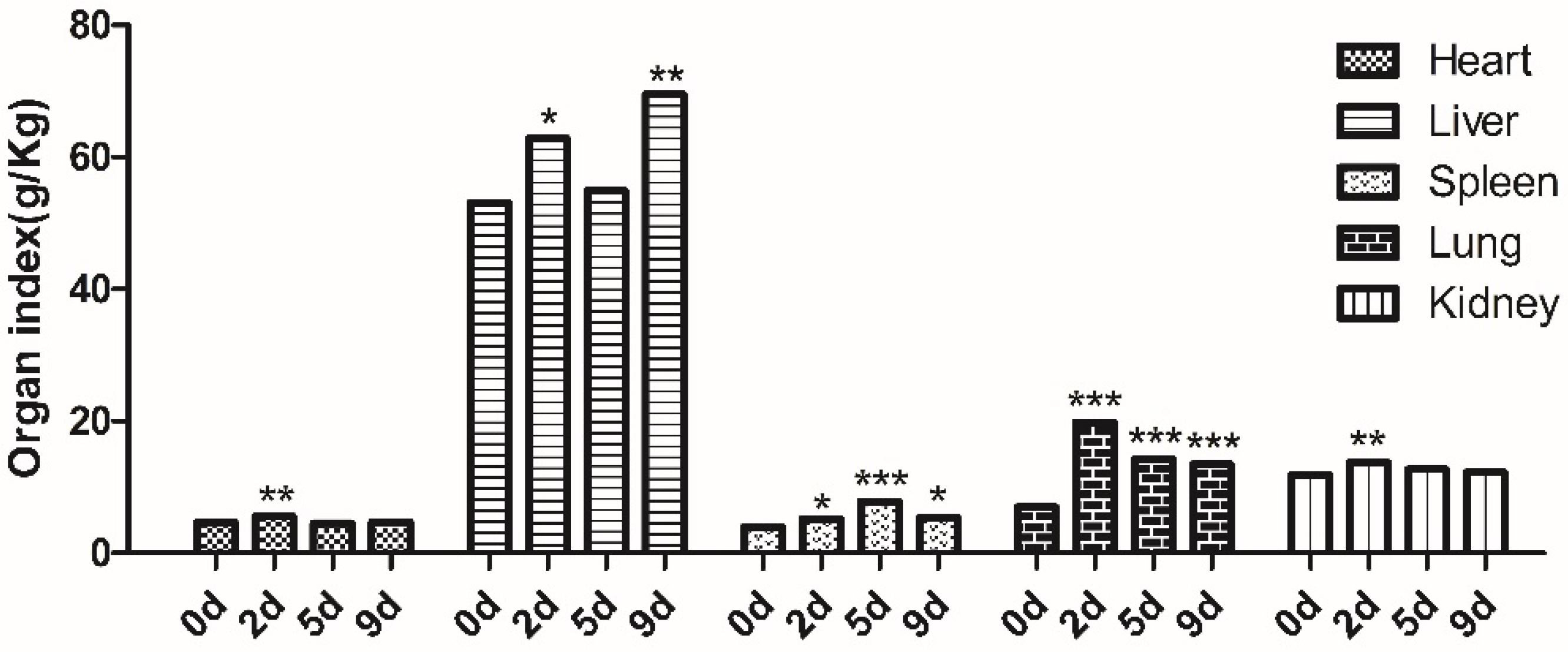
2.3. Tissue Weight and Organ Index
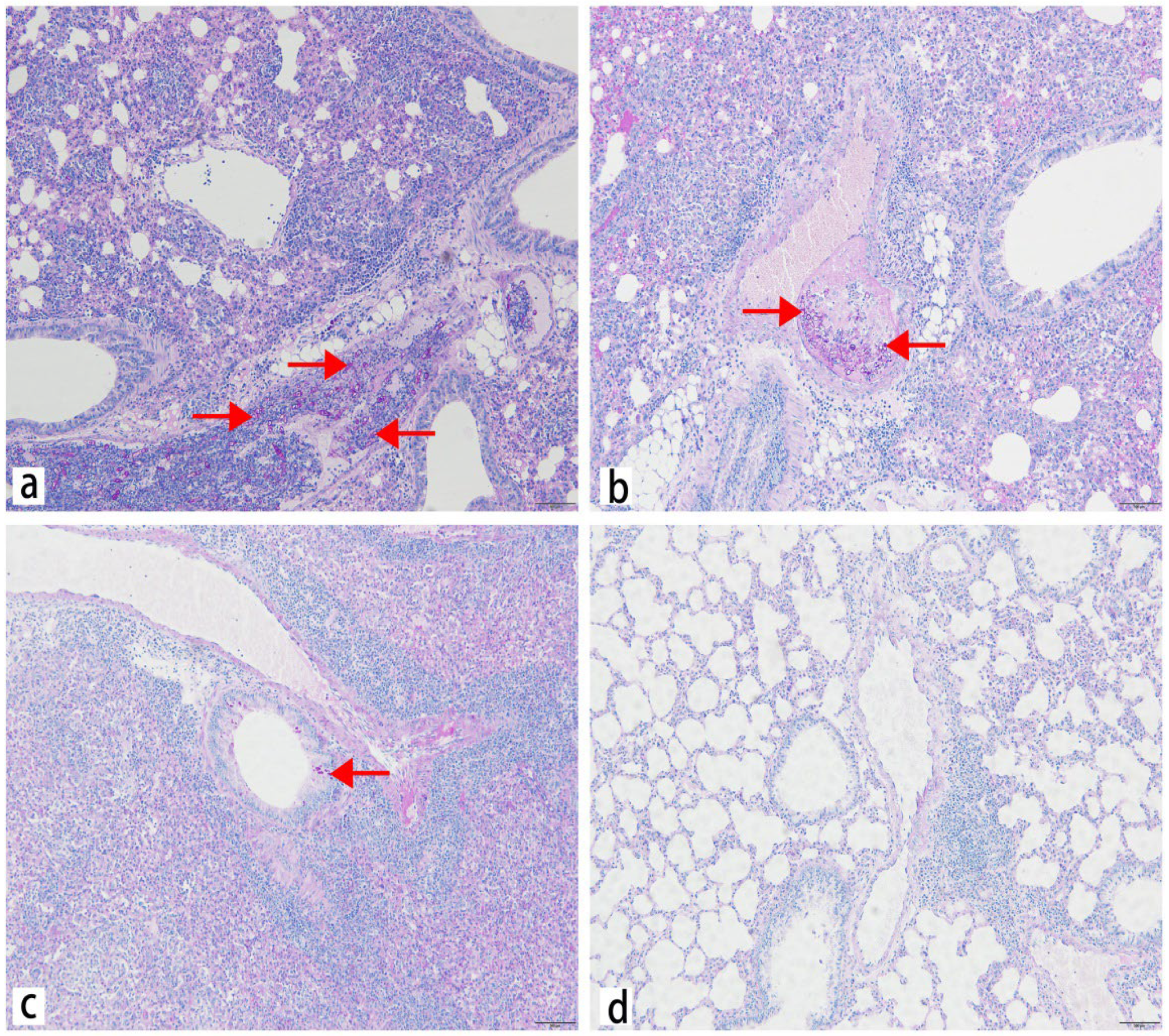
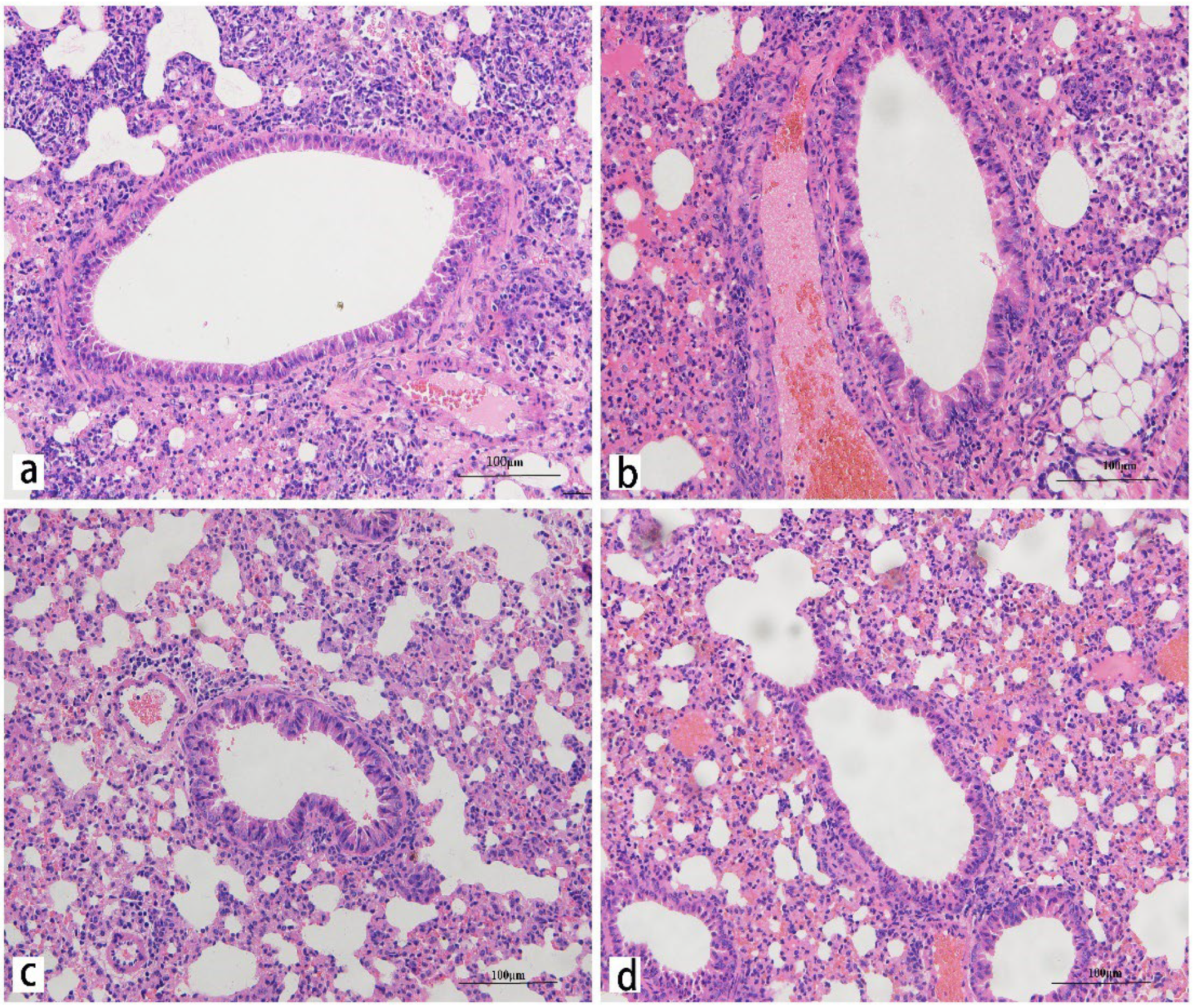
2.4. Histology
2.5. Quantification of Receptor mRNA Levels in the Lung after M. gypseum Infection
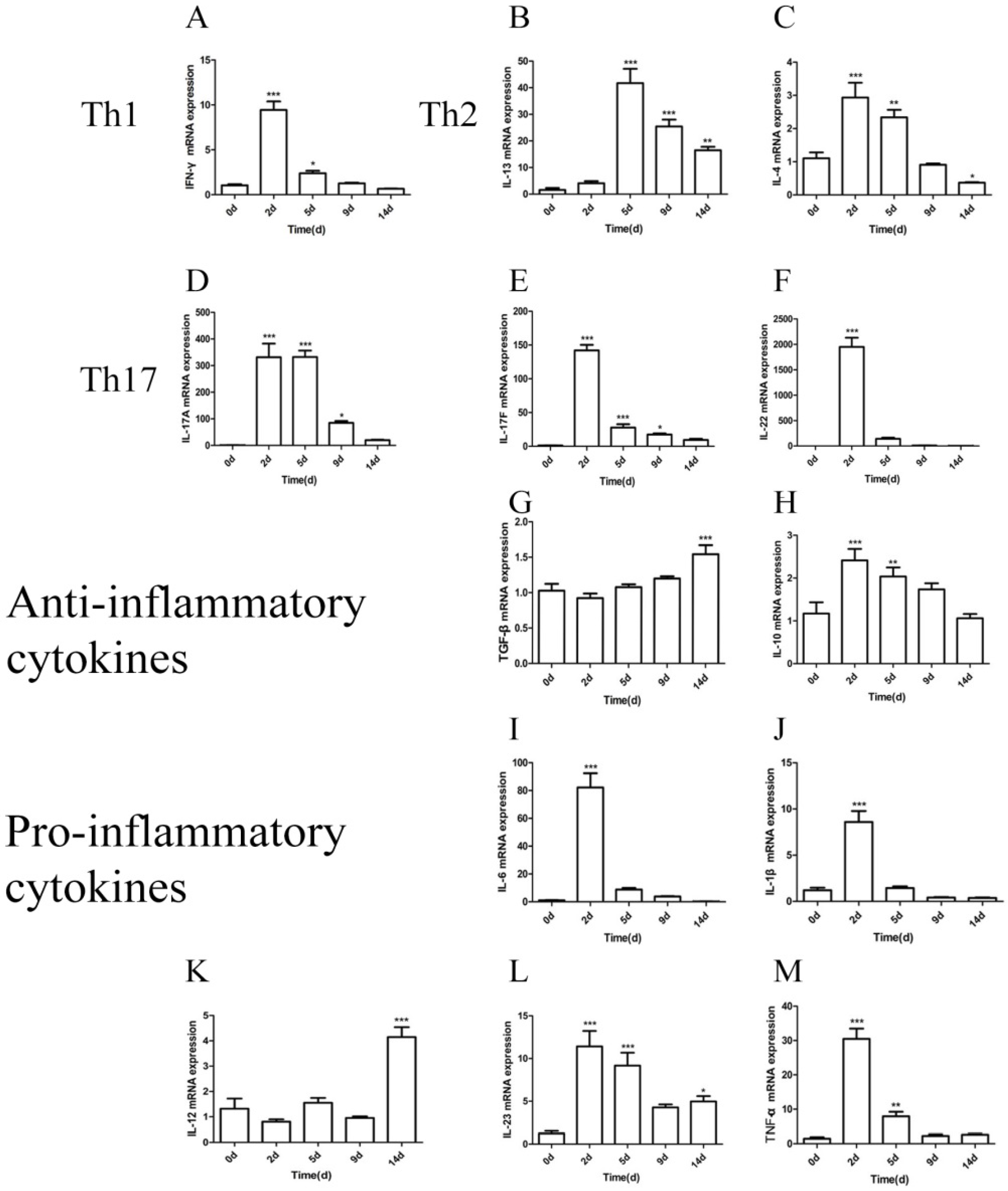
2.6. Quantification of Cytokine mRNA Levels in the Lung after M. gypseum Infection
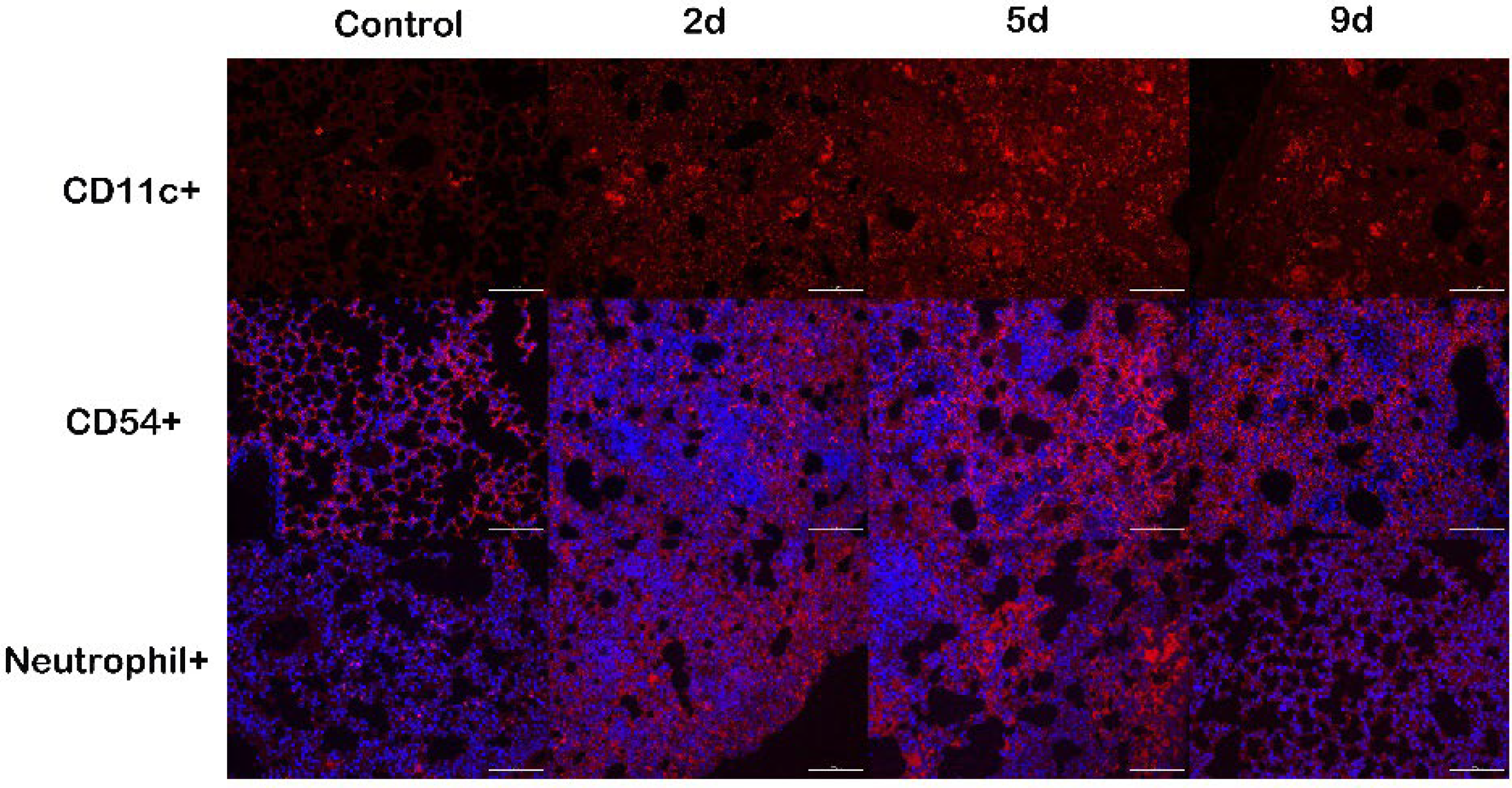
2.7. Immune Cells Recruitment following M. gypseum Infection
2.8. Th Cells Detection after M. gypseum Infection
3. Discussion
3.1. Selection of the M. gypseum Infection Model
3.2. Selection of Spore Infection Concentration of M. gypseum
3.3. Combined Analysis of Cytokine mRNA Expression and Flow Cytometry Results after M. gypseum Infection
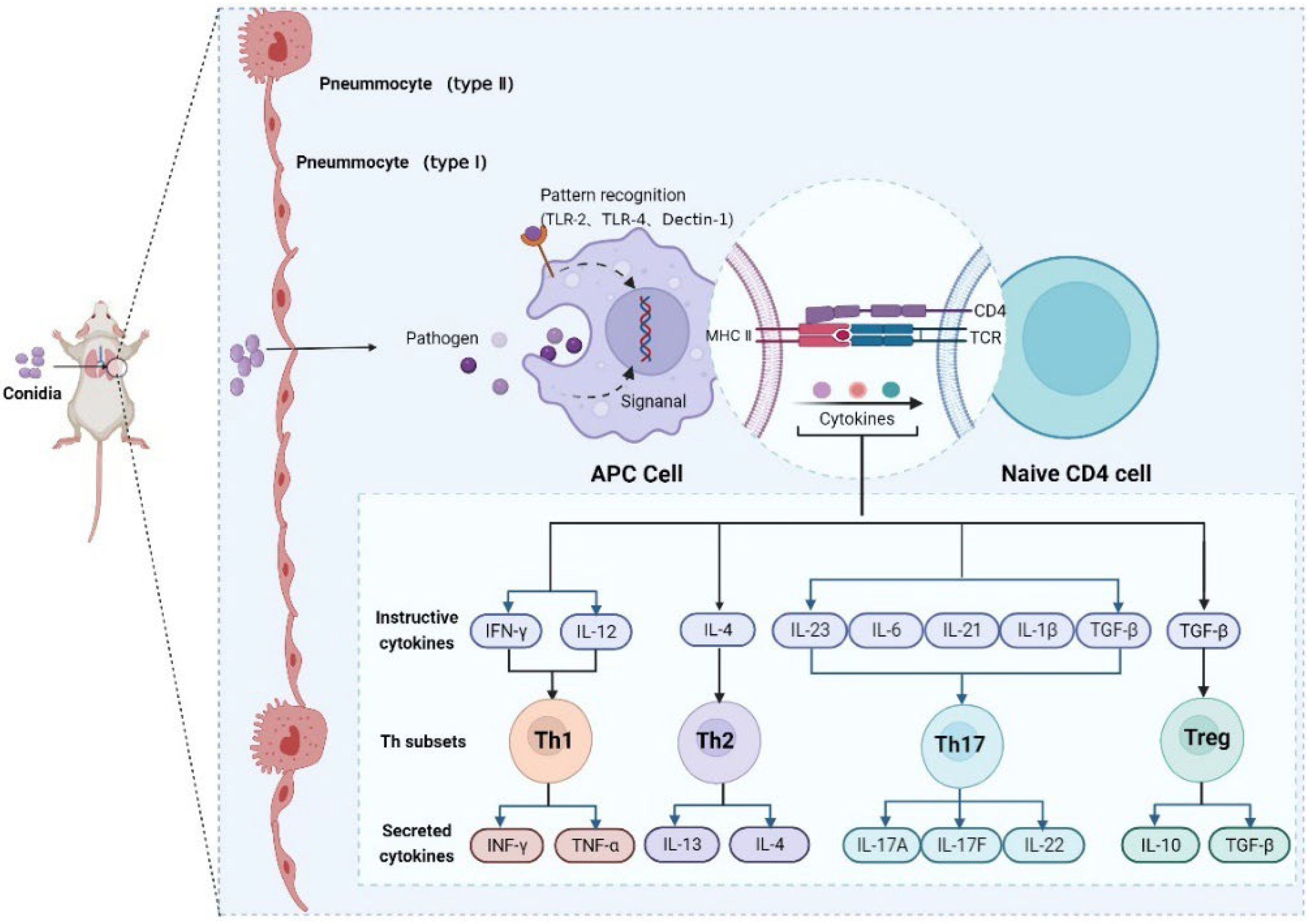
3.4. In-Depth Analysis of Th1, Th2, Th17, and Treg Signaling Pathways after M. gypseum Infection
3.5. Immune Response Patterns of M. gypseum Infection In Vivo
4. Materials and Methods
4.1. Fungal Strain and Conidia Preparation
4.2. Animals
4.3. Determination of Weight and Mortality
4.4. Determination of Organ Index
4.5. Tissue Fungal Burden Analysis
4.6. Histology
4.7. Quantitative Real Time-Polymerase Chain Reaction (qRT-PCR)
4.8. Immunofluorescence Staining
4.9. Flow Cytometry
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garcia-Agudo, L.; Espinosa-Ruiz, J.J. Tinea capitis by Microsporum gypseum, an infrequent species. Arch. Argent. Pediatr. 2018, 116, e296–e299. [Google Scholar]
- Boever, W.J.; Rush, D.M. Microsporum gypseum infection in a dromedary camel. Vet. Med. Small Anim. Clin. 1975, 70, 1190–1192. [Google Scholar] [PubMed]
- Fischman, O.; Siqueira, P.A.; Baptista, G. Microsporum gypseum infection in a gray wolf (Canis lupus) and a camel (Camelus bactrianus) in a zoological garden. Mykosen 1987, 30, 295–297. [Google Scholar] [CrossRef] [PubMed]
- Hackworth, C.E.; Eshar, D.; Nau, M.; Bagladi-Swanson, M.; Andrews, G.A.; Carpenter, J.W. Diagnosis and Successful Treatment of a Potentially Zoonotic Dermatophytosis Caused by Microsporum gypseum in a Zoo-Housed North American Porcupine (Erethizon dorsatum). J. Zoo Wildl. Med. 2017, 48, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Nardoni, S.; Mugnaini, L.; Papini, R.; Fiaschi, M.; Mancianti, F. Canine and feline dermatophytosis due to Microsporum gypseum: A retrospective study of clinical data and therapy outcome with griseofulvin. J. Mycol. Med. 2013, 23, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Pascoe, R.R.; Connole, M.D. Dermatomycosis due to Microsporum gypseum in horses. Aust. Vet. J. 1974, 50, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Xavier, G.A.; da Silva, L.B.; da Silva, D.R.; de Moraes Peixoto, R.; Lino, G.C.; Mota, R.A. Dermatophytosis caused by Microsporum canis and Microsporum gypseum in free-living Bradypus variegatus (Schiz, 1825) in the state of Pernambuco, Brazil. Braz. J. Microbiol. 2008, 39, 508–510. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, X.; Gu, Y.; Yuan, S.; Li, D.; Huang, X.; Cao, S.; Huang, X.; Ling, S.; Yu, S.; et al. Identification and pathogenicity of Microsporum gypseum isolated from Ailuropoda melanoleuca. Vet. Sci. China 2015, 45, 551–559. [Google Scholar] [CrossRef]
- Araviysky, A.N.; Araviysky, R.A.; Eschkov, G.A. Deep generalized trichophytosis. (Endothrix in tissues of different origin). Mycopathologia 1975, 56, 47–65. [Google Scholar] [CrossRef]
- Belda Junior, W.; Criado, P.R. Atypical clinical presentation of an Arthroderma gypseum infection in a renal transplant recipient. Rev. Inst. Med. Trop. Sao Paulo 2020, 62, e42. [Google Scholar] [CrossRef]
- Heinen, M.P.; Cambier, L.; Fievez, L.; Mignon, B. Are Th17 Cells Playing a Role in Immunity to Dermatophytosis? Mycopathologia 2017, 182, 251–261. [Google Scholar] [CrossRef]
- Netea, M.G.; Gow, N.A.; Munro, C.A.; Bates, S.; Collins, C.; Ferwerda, G.; Hobson, R.P.; Bertram, G.; Hughes, H.B.; Jansen, T.; et al. Immune sensing of Candida albicans requires cooperative recognition of mannans and glucans by lectin and Toll-like receptors. J. Clin. Investig. 2006, 116, 1642–1650. [Google Scholar] [CrossRef] [PubMed]
- Reid, D.M.; Gow, N.A.; Brown, G.D. Pattern recognition: Recent insights from Dectin-1. Curr. Opin. Immunol. 2009, 21, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Geijtenbeek, T.B.; Gringhuis, S.I. C-type lectin receptors in the control of T helper cell differentiation. Nat. Rev. Immunol. 2016, 16, 433–448. [Google Scholar] [CrossRef] [PubMed]
- Jouault, T.; Ibata-Ombetta, S.; Takeuchi, O.; Trinel, P.A.; Sacchetti, P.; Lefebvre, P.; Akira, S.; Poulain, D. Candida albicans phospholipomannan is sensed through toll-like receptors. J. Infect. Dis. 2003, 188, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Meier, A.; Kirschning, C.J.; Nikolaus, T.; Wagner, H.; Heesemann, J.; Ebel, F. Toll-like receptor (TLR) 2 and TLR4 are essential for Aspergillus-induced activation of murine macrophages. Cell. Microbiol. 2003, 5, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Cambier, L.; Mathy, A.; Baldo, A.; Bagut, E.T.; Tabart, J.; Antoine, N.; Mignon, B. Feline polymorphonuclear neutrophils produce pro-inflammatory cytokines following exposure to Microsporum canis. Vet. Microbiol. 2013, 162, 800–805. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.M.; Klein, B.S. Dendritic cells in antifungal immunity and vaccine design. Cell Host Microbe 2012, 11, 436–446. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, F.S.; Ferreira, L.G.; de Almeida, F.G.; de Almeida, S.R. An In Vitro Model for the Study of the Macrophage Response Upon Trichophyton rubrum Challenge. Mycopathologia 2017, 182, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Dennehy, K.M.; Willment, J.A.; Williams, D.L.; Brown, G.D. Reciprocal regulation of IL-23 and IL-12 following co-activation of Dectin-1 and TLR signaling pathways. Eur. J. Immunol. 2009, 39, 1379–1386. [Google Scholar] [CrossRef] [PubMed]
- Gerosa, F.; Baldani-Guerra, B.; Lyakh, L.A.; Batoni, G.; Esin, S.; Winkler-Pickett, R.T.; Consolaro, M.R.; De Marchi, M.; Giachino, D.; Robbiano, A.; et al. Differential regulation of interleukin 12 and interleukin 23 production in human dendritic cells. J. Exp. Med. 2008, 205, 1447–1461. [Google Scholar] [CrossRef] [PubMed]
- Patin, E.C.; Thompson, A.; Orr, S.J. Pattern recognition receptors in fungal immunity. In Seminars in Cell & Developmental Biology; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef] [PubMed]
- Cambier, L.C.; Heinen, M.P.; Bagut, E.T.; Antoine, N.A.; Mignon, B.R. Overexpression of TLR-2 and TLR-4 mRNA in feline polymorphonuclear neutrophils exposed to Microsporum canis. Vet. Dermatol. 2016, 27, 78–81e22. [Google Scholar] [CrossRef]
- Burstein, V.L.; Guasconi, L.; Beccacece, I.; Theumer, M.G.; Mena, C.; Prinz, I.; Cervi, L.; Herrero, M.; Masih, D.T.; Chiapello, L.S. IL-17-Mediated Immunity Controls Skin Infection and T Helper 1 Response during Experimental Microsporum canis Dermatophytosis. J. Investig. Dermatol. 2018, 138, 1744–1753. [Google Scholar] [CrossRef] [PubMed]
- Luckheeram, R.V.; Zhou, R.; Verma, A.D.; Xia, B. CD4(+)T cells: Differentiation and functions. Clin. Dev. Immunol. 2012, 2012, 925135. [Google Scholar] [CrossRef] [PubMed]
- Baltazar Lde, M.; Santos, P.C.; Paula, T.P.; Rachid, M.A.; Cisalpino, P.S.; Souza, D.G.; Santos, D.A. IFN-gamma impairs Trichophyton rubrum proliferation in a murine model of dermatophytosis through the production of IL-1beta and reactive oxygen species. Med. Mycol. 2014, 52, 293–302. [Google Scholar] [CrossRef]
- Yoshikawa, F.S.; Yabe, R.; Iwakura, Y.; de Almeida, S.R.; Saijo, S. Dectin-1 and Dectin-2 promote control of the fungal pathogen Trichophyton rubrum independently of IL-17 and adaptive immunity in experimental deep dermatophytosis. Innate Immun. 2016, 22, 316–324. [Google Scholar] [CrossRef]
- Cambier, L.; Weatherspoon, A.; Defaweux, V.; Bagut, E.T.; Heinen, M.P.; Antoine, N.; Mignon, B. Assessment of the cutaneous immune response during Arthroderma benhamiae and A. vanbreuseghemii infection using an experimental mouse model. Br. J. Dermatol. 2014, 170, 625–633. [Google Scholar] [CrossRef]
- Conti, H.R.; Shen, F.; Nayyar, N.; Stocum, E.; Sun, J.N.; Lindemann, M.J.; Ho, A.W.; Hai, J.H.; Yu, J.J.; Jung, J.W.; et al. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J. Exp. Med. 2009, 206, 299–311. [Google Scholar] [CrossRef]
- Zelante, T.; De Luca, A.; Bonifazi, P.; Montagnoli, C.; Bozza, S.; Moretti, S.; Belladonna, M.L.; Vacca, C.; Conte, C.; Mosci, P.; et al. IL-23 and the Th17 pathway promote inflammation and impair antifungal immune resistance. Eur. J. Immunol. 2007, 37, 2695–2706. [Google Scholar] [CrossRef]
- Lee, S.J.; Han, J.I.; Lee, G.S.; Park, M.J.; Choi, I.G.; Na, K.J.; Jeung, E.B. Antifungal effect of eugenol and nerolidol against Microsporum gypseum in a guinea pig model. Biol. Pharm. Bull. 2007, 30, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Cambier, L.; Heinen, M.P.; Mignon, B. Relevant Animal Models in Dermatophyte Research. Mycopathologia 2017, 182, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Hu, J.; Wang, C.; Gu, Y.; Cao, S.; Huang, X.; Wen, Y.; Zhao, Q.; Wu, R.; Zuo, Z.; et al. Innate and mild Th17 cutaneous immune responses elicited by subcutaneous infection of immunocompetent mice with Cladosporium cladosporioides. Microb. Pathog. 2022, 163, 105384. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Hu, J.; Yu, Y.; Wang, C.; Gu, Y.; Cao, S.; Huang, X.; Wen, Y.; Zhao, Q.; Wu, R.; et al. Assessment of the pulmonary adaptive immune response to Cladosporium cladosporioides infection using an experimental mouse model. Sci. Rep. 2021, 11, 909. [Google Scholar] [CrossRef]
- Hu, S.; Dai, J.; Chen, X. Vitamin D reduces autophagy by regulating NF-κB resistance to Aspergillus fumigatus infection. Gene 2020, 753, 144819. [Google Scholar] [CrossRef]
- Marine, M.; Bom, V.L.; de Castro, P.A.; Winkelstroter, L.K.; Ramalho, L.N.; Brown, N.A.; Goldman, G.H. The development of animal infection models and antifungal efficacy assays against clinical isolates of Trichosporon asahii, T. asteroides and T. inkin. Virulence 2015, 6, 476–486. [Google Scholar] [CrossRef]
- Esche, C.; Stellato, C.; Beck, L.A. Chemokines: Key players in innate and adaptive immunity. J. Investig. Dermatol. 2005, 125, 615–628. [Google Scholar] [CrossRef] [PubMed]
- Olivier, D.; Hélène, B.; Pascale, C. The bacterial pathogen Listeria monocytogenes and the interferon family: Type I, type II and type III interferons. Front. Cell. Infect. Microbiol. 2014, 4, 50. [Google Scholar]
- Wang, M.-Y.; Li, R.-Y. Immune responses to cutaneous fungal infections. Int. J. Dermatol. Venereol. 2006, 32, 90–92. [Google Scholar]
- Zhang, Z.; Myers, J.M.B.; Brandt, E.B.; Ryan, P.H.; Lindsey, M.; Mintz-Cole, R.A.; Reponen, T.; Vesper, S.J.; Forde, F.; Ruff, B.; et al. beta-Glucan exacerbates allergic asthma independent of fungal sensitization and promotes steroid-resistant TH2/TH17 responses. J. Allergy Clin. Immunol. 2017, 139, 54–65e8. [Google Scholar] [CrossRef]
- Eyerich, K.; Dimartino, V.; Cavani, A. IL-17 and IL-22 in immunity: Driving protection and pathology. Eur. J. Immunol. 2017, 47, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.R.; Roy, S.; Leal, S.M., Jr.; Sun, Y.; Howell, S.J.; Cobb, B.A.; Li, X.; Pearlman, E. Autocrine IL-17A–IL-17RC neutrophil activation in fungal infections is regulated by IL-6, IL-23, RORγt and Dectin-2. Nat. Immunol. 2014, 15, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Conti, H.R.; Gaffen, S.L. IL-17-Mediated Immunity to the Opportunistic Fungal Pathogen Candida albicans. J. Immunol. 2015, 195, 780–788. [Google Scholar] [CrossRef]
- Vautier, S.; Sousa Mda, G.; Brown, G.D. C-type lectins, fungi and Th17 responses. Cytokine Growth Factor Rev. 2010, 21, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Wolk, K.; Kunz, S.; Witte, E.; Friedrich, M.; Asadullah, K.; Sabat, R. IL-22 increases the innate immunity of tissues. Immunity 2004, 21, 241–254. [Google Scholar] [CrossRef]
- Imperiale, B.R.; García, A.; Minotti, A.; González Montaner, P.; Moracho, L.; Morcillo, N.S.; Palmero, D.J.; Sasiain, M.D.C.; de la Barrera, S. Th22 response induced by Mycobacterium tuberculosis strains is closely related to severity of pulmonary lesions and bacillary load in patients with multi-drug-resistant tuberculosis. Clin. Exp. Immunol. 2021, 203, 267–280. [Google Scholar] [CrossRef]
- Sonnenberg, G.F.; Fouser, L.A.; Artis, D. Functional biology of the IL-22-IL-22R pathway in regulating immunity and inflammation at barrier surfaces. Adv. Immunol. 2010, 107, 1–29. [Google Scholar] [PubMed]
- Neumann, C.; Scheffold, A.; Rutz, S. Functions and regulation of T cell-derived interleukin-10. Semin. Immunol. 2019, 44, 101344. [Google Scholar] [CrossRef]
- Bozza, S.; Gaziano, R.; Spreca, A.; Bacci, A.; Montagnoli, C.; di Francesco, P.; Romani, L. Dendritic cells transport conidia and hyphae of Aspergillus fumigatus from the airways to the draining lymph nodes and initiate disparate Th responses to the fungus. J. Immunol. 2002, 168, 1362–1371. [Google Scholar] [CrossRef]
- Rengarajan, J.; Mowen, K.A.; McBride, K.D.; Smith, E.D.; Singh, H.; Glimcher, L.H. Interferon regulatory factor 4 (IRF4) interacts with NFATc2 to modulate interleukin 4 gene expression. J. Exp. Med. 2002, 195, 1003–1012. [Google Scholar] [CrossRef]
- Coquet, J.M.; Schuijs, M.J.; Smyth, M.J.; Deswarte, K.; Beyaert, R.; Braun, H.; Boon, L.; Karlsson Hedestam, G.B.; Nutt, S.L.; Hammad, H.; et al. Interleukin-21-Producing CD4(+) T Cells Promote Type 2 Immunity to House Dust Mites. Immunity 2015, 43, 318–330. [Google Scholar] [CrossRef]
- Ballesteros-Tato, A.; Randall, T.D.; Lund, F.E.; Spolski, R.; Leonard, W.J.; León, B. T Follicular Helper Cell Plasticity Shapes Pathogenic T Helper 2 Cell-Mediated Immunity to Inhaled House Dust Mite. Immunity 2016, 44, 259–273. [Google Scholar] [CrossRef]
- Stadhouders, R.; Lubberts, E.; Hendriks, R.W. A cellular and molecular view of T helper 17 cell plasticity in autoimmunity. J. Autoimmun. 2018, 87, 1–15. [Google Scholar] [CrossRef]
- Szabo, S.J.; Sullivan, B.M.; Peng, S.L.; Glimcher, L.H. Molecular mechanisms regulating Th1 immune responses. Annu. Rev. Immunol. 2003, 21, 713. [Google Scholar] [CrossRef]
- Gorelik, L.; Fields, P.E.; Flavell, R.A. Cutting edge: TGF-beta inhibits Th type 2 development through inhibition of GATA-3 expression. J. Immunol. 2000, 165, 4773–4777. [Google Scholar] [CrossRef]
- Zhu, J.; Davidson, T.S.; Wei, G.; Jankovic, D.; Cui, K.; Schones, D.E.; Guo, L.; Zhao, K.; Shevach, E.M.; Paul, W.E. Down-regulation of Gfi-1 expression by TGF-beta is important for differentiation of Th17 and CD103+ inducible regulatory T cells. J. Exp. Med. 2009, 206, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.F.S.; Rogério, D.A.S. The Role of Phagocytes and NETs in Dermatophytosis. Mycopathologia 2017, 182, 263–272. [Google Scholar]
- Tittel, A.P.; Heuser, C.; Ohliger, C.; Llanto, C.; Yona, S.; Hämmerling, G.J.; Engel, D.R.; Garbi, N.; Kurts, C. Functionally relevant neutrophilia in CD11c diphtheria toxin receptor transgenic mice. Nat. Methods 2012, 9, 385–390. [Google Scholar] [CrossRef]
- Lambrecht, B.N. Alveolar macrophage in the driver’s seat. Immunity 2006, 24, 366–368. [Google Scholar] [CrossRef]
- Staudt, V.; Bothur, E.; Klein, M.; Lingnau, K.; Reuter, S.; Grebe, N.; Gerlitzki, B.; Hoffmann, M.; Ulges, A.; Taube, C.; et al. Interferon-regulatory factor 4 is essential for the developmental program of T helper 9 cells. Immunity 2010, 33, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Knight, V.; Lourensz, D.; Tchongue, J.; Correia, J.; Tipping, P.; Sievert, W. Cytoplasmic domain of tissue factor promotes liver fibrosis in mice. World J. Gastroenterol. 2017, 23, 5692–5699. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Martin, R.J.; Rino, J.G.; Breed, R.; Torres, R.M.; Chu, H.W. IL-23-dependent IL-17 production is essential in neutrophil recruitment and activity in mouse lung defense against respiratory Mycoplasma pneumoniae infection. Microbes Infect. 2007, 9, 78–86. [Google Scholar] [CrossRef] [PubMed]


| Target Gene | Primer Sequence (5′ > 3′) | PCR Product Size (bp) | |
|---|---|---|---|
| Forward | Reverse | ||
| 12s rRNA | GGAAGGCATAGCTGCTGGAGGT | CGATGACATCCTTGGCCTGA | 164 |
| β-Actin | TTCCAGCGTTCCTTCTTGGGT | GTTGGCATAGAGGTGTTTACG | 90 |
| IL-6 | GTTCTCTGGGAAATCGTGGA | TGTACTCCAGGTAGCTATGG | 208 |
| IL-1β | TTGACGGACCCCAAAAGATG | AGAAGGTGCTCATGTCCTCA | 204 |
| TNF-α | TCTCATCAGTTCTATGGCCC | GGGAGTAGACAAGGTACAAC | 164 |
| IL-22 | GGCCAGCCTTGCAGATAACA | GCTGATGTGACAGGAGCTGA | 220 |
| IL-10 | AGCCGGGAAGACAATAACTG | CATTTCCGATAAGGCTTGG | 189 |
| TGF-β | TGCCCTCTACAACCAACACA | GTTGGACAACTGCTCCACCT | 277 |
| IL-23 | TGTGCCCCGTATCCAGTGT | CGGATCCTTTGCAAGCAGAA | 81 |
| IL-17F | CTGAGGCCCAGTGCAGACA | GCTGAATGGCGACGGAGTT | 189 |
| IL-17A | GCTCCAGAAGGCCCTCAGA | AGCTTTCCCTCCGCATTGA | 187 |
| IFN-γ | AAAGACAATCAGGCCATCAG | TGGGTTGTTGACCTCAAACT | 129 |
| IL-4 | CATCGGCATTTTGAACGAG | TTGGAAGCCCTACAGACGAG | 120 |
| IL-9 | CTGATGATTGTACCACACCGTGC | GCCTTTGCATCTCTGTCTTCTGG | 237 |
| IL-13 | AGACTCCCCTGTGCAACGGCA | GGAGACCGTAGTGGGGGCCTT | 167 |
| TLR-2 | CGACATCCATCACCTGACTCTTC | GCCTCGGAATGCCAGCTTCTTC | 182 |
| TLR-4 | ACAAGGCATGGCATGGCTTACAC | TGTCTCCACAGCCACCAGATTCTC | 125 |
| Dectin-1 | CCCTCCAAGGCATCCCAAAC | CCTAGCTGGGAGCAGTGTCT | 140 |
| Primary Antibody | Target | Secondary Antibody | Source |
|---|---|---|---|
| Rat antimouse CD54 (ICAM-I) biotin | Macrophages | Goat Anti-rat CY3 | eBioscience (San Diego, CA, U.S.A.) |
| Hamster antimouse CD11c biotin | DCs | Goat Anti-rat CY3 | eBioscience |
| Rat antimouse neutrophil | PMNs | Goat Anti-rat CY3 | Abcam (Cambridge, U.K.) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.; Liu, Z.; Yu, Y.; Jiang, Y.; Wang, C.; Zuo, Z.; Ling, S.; He, M.; Cao, S.; Wen, Y.; et al. Microsporum gypseum Isolated from Ailuropoda melanoleuca Provokes Inflammation and Triggers Th17 Adaptive Immunity Response. Int. J. Mol. Sci. 2022, 23, 12037. https://doi.org/10.3390/ijms231912037
Ma X, Liu Z, Yu Y, Jiang Y, Wang C, Zuo Z, Ling S, He M, Cao S, Wen Y, et al. Microsporum gypseum Isolated from Ailuropoda melanoleuca Provokes Inflammation and Triggers Th17 Adaptive Immunity Response. International Journal of Molecular Sciences. 2022; 23(19):12037. https://doi.org/10.3390/ijms231912037
Chicago/Turabian StyleMa, Xiaoping, Zhen Liu, Yan Yu, Yaozhang Jiang, Chengdong Wang, Zhicai Zuo, Shanshan Ling, Ming He, Sanjie Cao, Yiping Wen, and et al. 2022. "Microsporum gypseum Isolated from Ailuropoda melanoleuca Provokes Inflammation and Triggers Th17 Adaptive Immunity Response" International Journal of Molecular Sciences 23, no. 19: 12037. https://doi.org/10.3390/ijms231912037
APA StyleMa, X., Liu, Z., Yu, Y., Jiang, Y., Wang, C., Zuo, Z., Ling, S., He, M., Cao, S., Wen, Y., Zhao, Q., Wu, R., Huang, X., Zhong, Z., Peng, G., & Gu, Y. (2022). Microsporum gypseum Isolated from Ailuropoda melanoleuca Provokes Inflammation and Triggers Th17 Adaptive Immunity Response. International Journal of Molecular Sciences, 23(19), 12037. https://doi.org/10.3390/ijms231912037








