Calcium-Bound S100P Protein Is a Promiscuous Binding Partner of the Four-Helical Cytokines
Abstract
1. Introduction
2. Results and Discussion
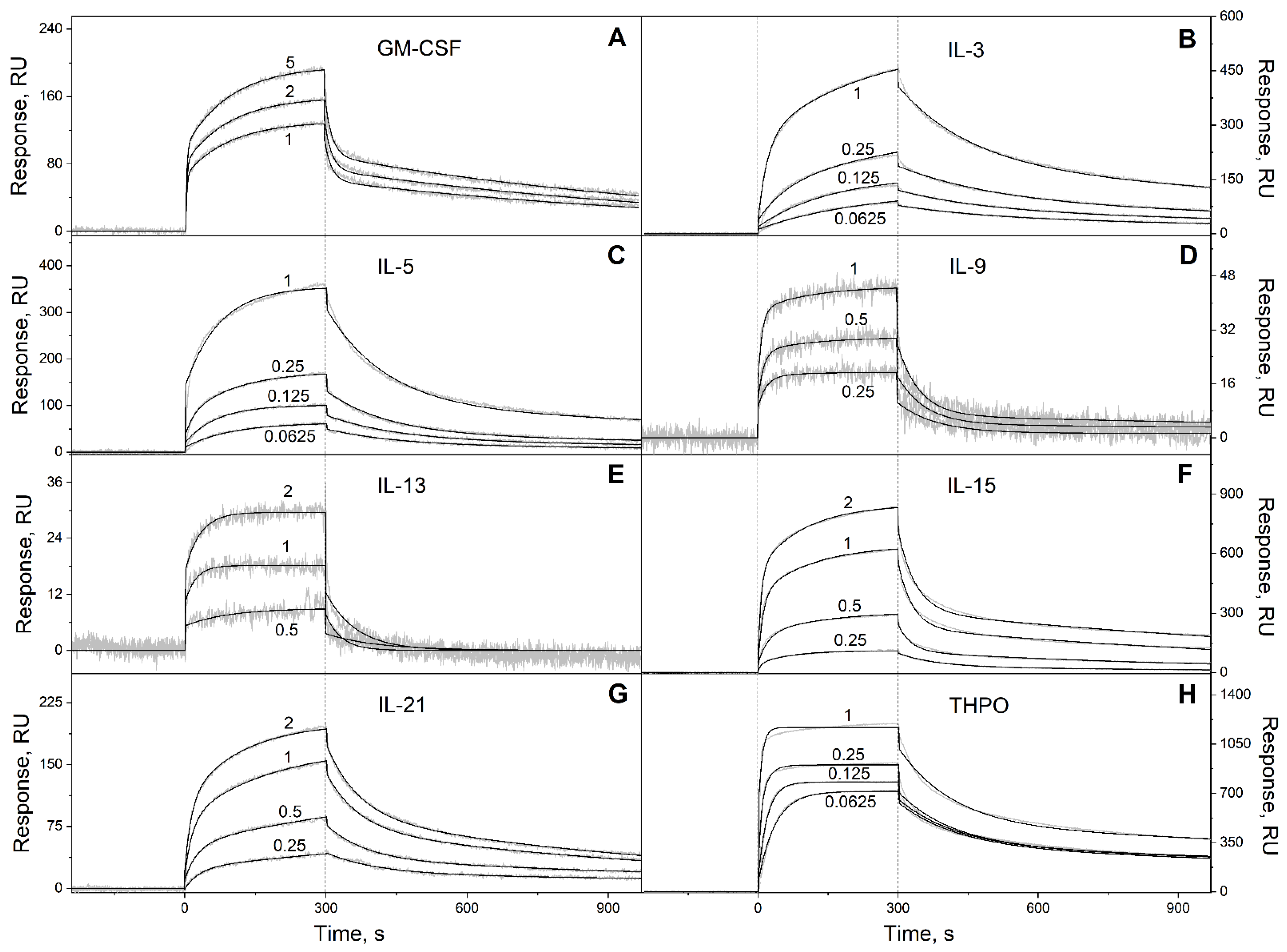
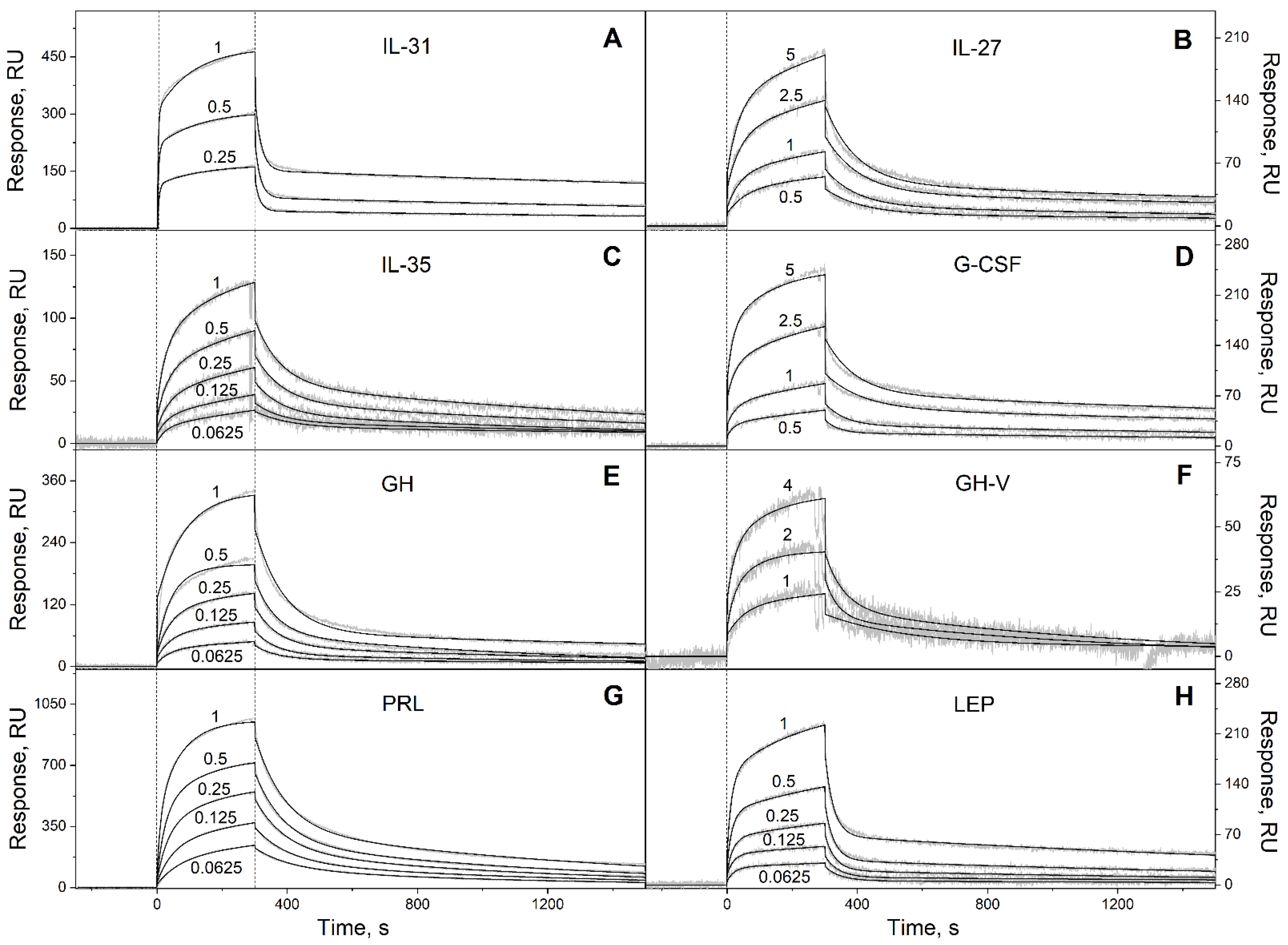
2.1. S100P Interaction with Specific Four-Helical Cytokines
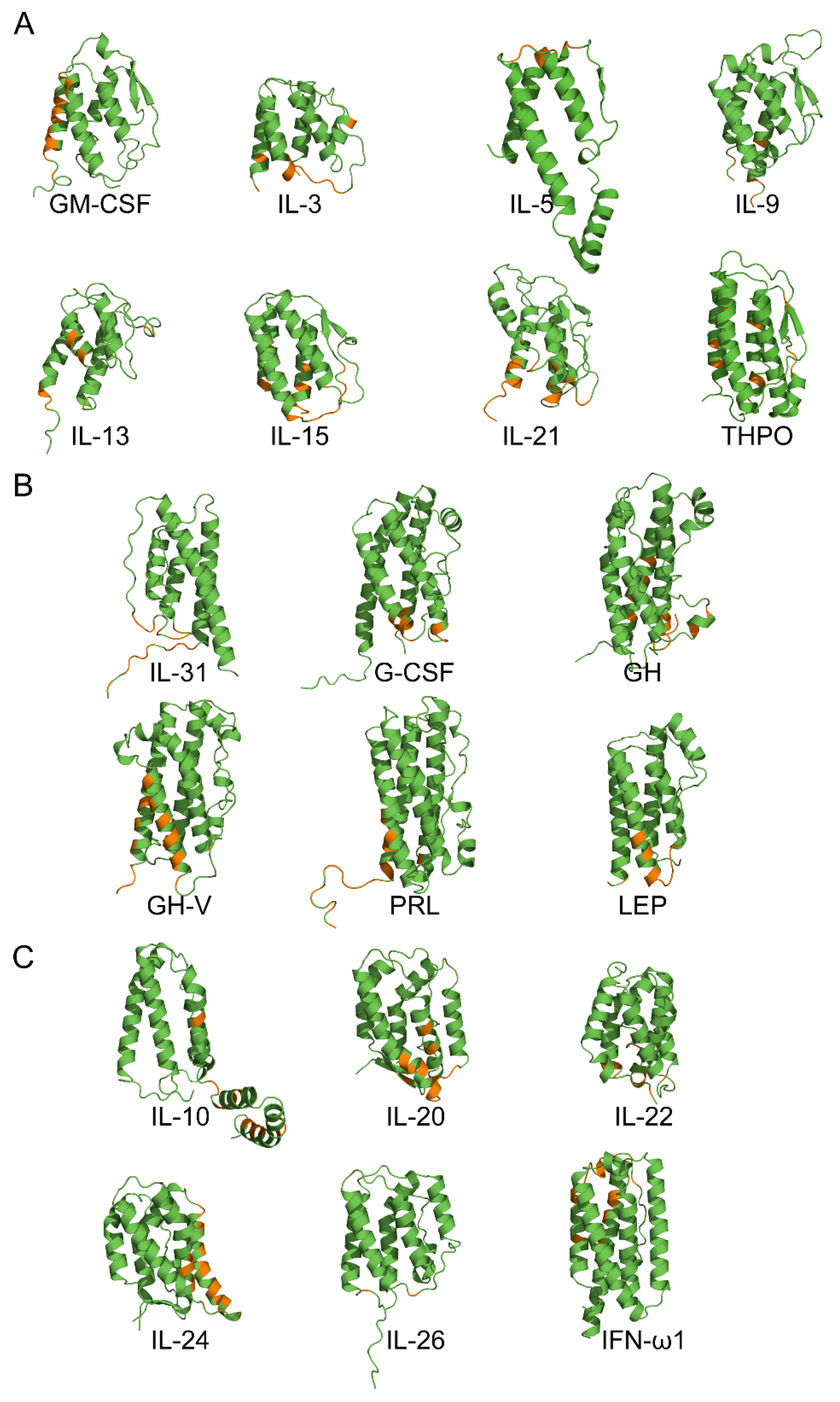
2.2. Modeling of the S100P–Cytokine Complexes
3. Materials and Methods
3.1. Materials
3.2. Surface Plasmon Resonance Studies
| ka1 | ka2 | |||||
| L1 + A | → ← | L1A | L2 + A | → ← | L2A | (1) |
| kd1 Kd1 | kd2 Kd2 |
3.3. Structural Classification of Cytokines
3.4. Modeling of the S100P–Cytokine Complexes
3.5. Comparison of Structural Properties of WT S100P and Its Mutants
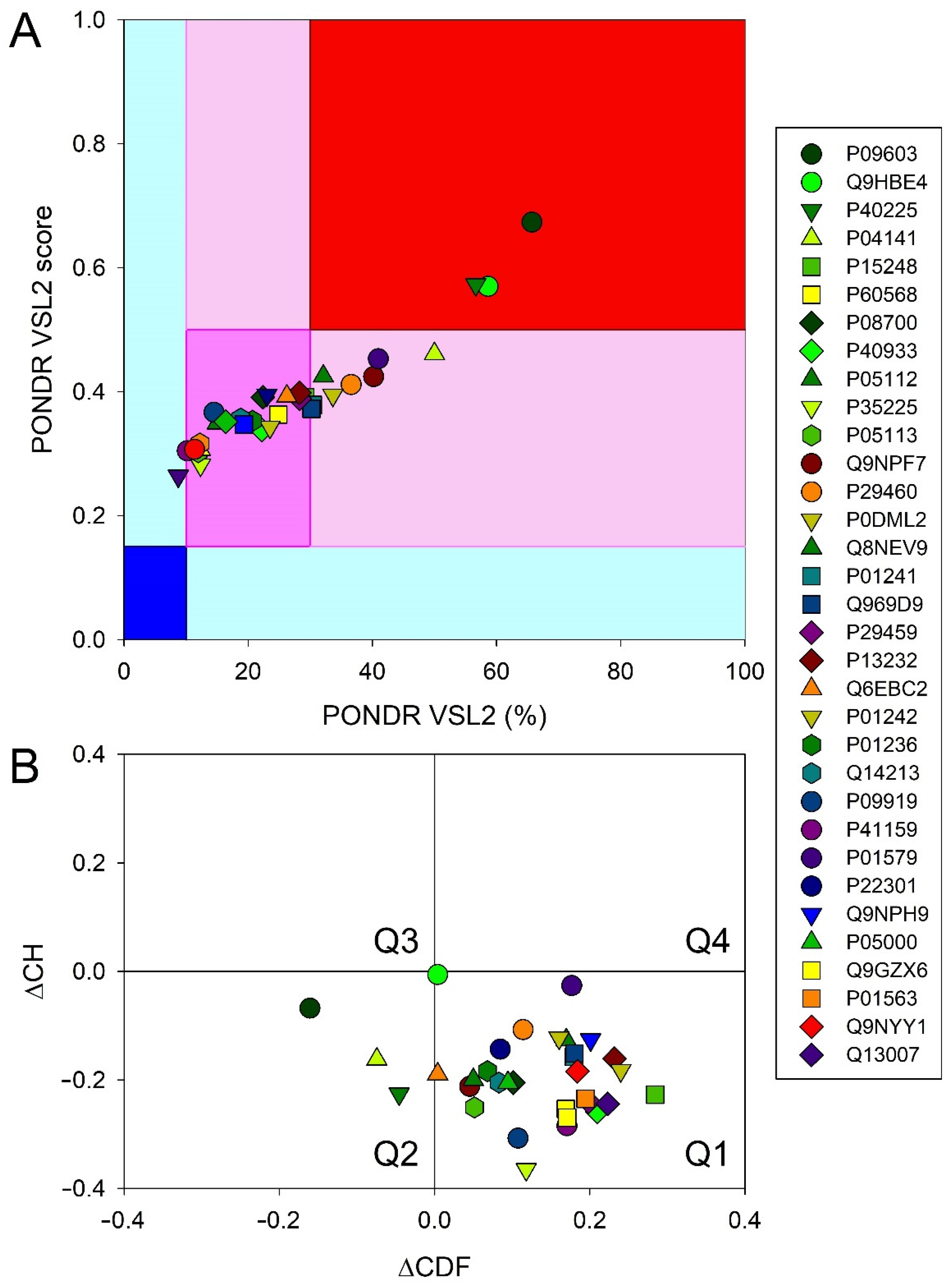
3.6. Intrinsic Disorder Analysis of Human Four-Helical Cytokines
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CDF | cumulative distribution function |
| CH | charge-hydropathy |
| CHO | Chinese hamster ovary cells |
| EDTA | ethylenediaminetetraacetic acid |
| EPO | erythropoietin |
| FGF | fibroblast growth factor |
| HEK293 | human embryonic kidney 293 cells |
| HEPES | 4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid |
| G-CSF | granulocyte colony-stimulating factor |
| GH | growth hormone/somatotropin |
| GH-V | growth hormone variant |
| GM-CSF | granulocyte-macrophage colony-stimulating factor |
| IFN | interferon |
| IL | interleukin |
| LEP | leptin |
| M-CSF | macrophage colony-stimulating factor 1 |
| NMR | nuclear magnetic resonance |
| PDB | protein data bank |
| PL | chorionic somatomammotropin hormone 1 |
| PRL | prolactin |
| RAGE | receptor for advanced glycation end products |
| SCOP | structural classification of proteins |
| SDS–PAGE | sodium dodecyl sulfate–polyacrylamide gel electrophoresis |
| SPR | surface plasmon resonance |
| RU | resonance unit |
| THPO | thrombopoietin |
| TSLP | thymic stromal lymphopoietin |
| Δ42–4 | human S100P mutant lacking PGFLQS sequence in the ‘hinge’ region |
| WT | wild-type protein |
References
- Donato, R.; Cannon, B.R.; Sorci, G.; Riuzzi, F.; Hsu, K.; Weber, D.J.; Geczy, C.L. Functions of S100 Proteins. Curr. Mol. Med. 2013, 13, 24–57. [Google Scholar] [CrossRef] [PubMed]
- Sreejit, G.; Flynn, M.C.; Patil, M.; Krishnamurthy, P.; Murphy, A.J.; Nagareddy, P.R. S100 family proteins in inflammation and beyond. In Advances of Clinical Chemistry; Makowski, G.S., Ed.; Academic Press: Cambridge, MA, USA, 2020; Volume 98, pp. 173–231. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Ali, S.A. Multifunctional Role of S100 Protein Family in the Immune System: An Update. Cells 2022, 11, 2274. [Google Scholar] [CrossRef] [PubMed]
- Nockolds, C.E.; Kretsinger, R.H.; Coffee, C.J.; Bradshaw, R.A. Structure of a Calcium-Binding Carp Myogen. Proc. Natl. Acad. Sci. USA 1972, 69, 581–584. [Google Scholar] [CrossRef]
- Sigrist, C.J.A.; de Castro, E.; Cerutti, L.; Cuche, B.A.; Hulo, N.; Bridge, A.; Bougueleret, L.; Xenarios, I. New and continuing developments at PROSITE. Nucleic Acids Res. 2012, 41, D344–D347. [Google Scholar] [CrossRef]
- Zimmer, D.B.; Eubanks, J.O.; Ramakrishnan, D.; Criscitiello, M.F. Evolution of the S100 family of calcium sensor proteins. Cell Calcium 2013, 53, 170–179. [Google Scholar] [CrossRef]
- Heizmann, C.W. 3D Structures of the Calcium and Zinc Binding S100 Proteins. In Handbook of Metalloproteins; Messerschmidt, A., Hubert, R., Poulos, T., Wieghardt, K., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar] [CrossRef]
- Streicher, W.W.; Lopez, M.M.; Makhatadze, G.I. Modulation of quaternary structure of S100 proteins by calcium ions. Biophys. Chem. 2010, 151, 181–186. [Google Scholar] [CrossRef]
- Gilston, B.A.; Skaar, E.P.; Chazin, W.J. Binding of transition metals to S100 proteins. Sci. China Life Sci. 2016, 59, 792–801. [Google Scholar] [CrossRef]
- Kazakov, A.S.; Mayorov, S.A.; Deryusheva, E.; Avkhacheva, N.V.; Denessiouk, K.A.; Denesyuk, A.I.; Rastrygina, V.A.; Permyakov, E.A.; Permyakov, S.E. Highly specific interaction of monomeric S100P protein with interferon beta. Int. J. Biol. Macromol. 2019, 143, 633–639. [Google Scholar] [CrossRef]
- Bresnick, A.R.; Weber, D.J.; Zimmer, D.B. S100 proteins in cancer. Nat. Cancer 2015, 15, 96–109. [Google Scholar] [CrossRef] [PubMed]
- Cristóvão, J.S.; Gomes, C.M. S100 Proteins in Alzheimer’s Disease. Front. Neurosci. 2019, 13, 463. [Google Scholar] [CrossRef] [PubMed]
- Holzinger, D.; Tenbrock, K.; Roth, J. Alarmins of the S100-Family in Juvenile Autoimmune and Auto-Inflammatory Diseases. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Sattar, Z.; Lora, A.; Jundi, B.; Railwah, C.; Geraghty, P. The S100 Protein Family as Players and Therapeutic Targets in Pulmonary Diseases. Pulm. Med. 2021, 2021, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, L.L.; Garrie, K.; Turner, M.D. Role of S100 proteins in health and disease. Biochim. Biophys. Acta 2020, 1867, 118677. [Google Scholar] [CrossRef]
- Allgöwer, C.; Kretz, A.-L.; Von Karstedt, S.; Wittau, M.; Henne-Bruns, D.; Lemke, J. Friend or Foe: S100 Proteins in Cancer. Cancers 2020, 12, 2037. [Google Scholar] [CrossRef]
- Bresnick, A.R. S100 proteins as therapeutic targets. Biophys. Rev. 2018, 10, 1617–1629. [Google Scholar] [CrossRef]
- Rumpret, M.; von Richthofen, H.J.; van der Linden, M.; Westerlaken, G.H.A.; Ormeño, C.T.; Low, T.Y.; Ovaa, H.; Meyaard, L. Recognition of S100 proteins by Signal Inhibitory Receptor on Leukocytes-1 negatively regulates human neutrophils. Eur. J. Immunol. 2021, 51, 2210–2217. [Google Scholar] [CrossRef]
- Kazakov, A.; Sofin, A.; Avkhacheva, N.; Denesyuk, A.; Deryusheva, E.; Rastrygina, V.; Sokolov, A.; Permyakova, M.; Litus, E.; Uversky, V.; et al. Interferon Beta Activity Is Modulated via Binding of Specific S100 Proteins. Int. J. Mol. Sci. 2020, 21, 9473. [Google Scholar] [CrossRef]
- Kazakov, A.S.; Sofin, A.D.; Avkhacheva, N.V.; Deryusheva, E.I.; Rastrygina, V.A.; Permyakova, M.E.; Uversky, V.N.; Permyakov, E.A.; Permyakov, S.E. Interferon-β Activity Is Affected by S100B Protein. Int. J. Mol. Sci. 2022, 23, 1997. [Google Scholar] [CrossRef]
- Kazakov, A.S.; Sokolov, A.S.; Permyakova, M.E.; Litus, E.A.; Uversky, V.N.; Permyakov, E.A.; Permyakov, S.E. Specific cytokines of interleukin-6 family interact with S100 proteins. Cell Calcium 2021, 101, 102520. [Google Scholar] [CrossRef] [PubMed]
- Kazakov, A.S.; Deryusheva, E.I.; Sokolov, A.S.; Permyakova, M.E.; Litus, E.A.; Rastrygina, V.A.; Uversky, V.N.; Permyakov, E.A.; Permyakov, S.E. Erythropoietin Interacts with Specific S100 Proteins. Biomolecules 2022, 12, 120. [Google Scholar] [CrossRef] [PubMed]
- Klingelhöfer, J.; Møller, H.D.; Sumer, E.U.; Berg, C.H.; Poulsen, M.; Kiryushko, D.; Soroka, V.; Ambartsumian, N.; Grigorian, M.; Lukanidin, E.M. Epidermal growth factor receptor ligands as new extracellular targets for the metastasis-promoting S100A4 protein. FEBS J. 2009, 276, 5936–5948. [Google Scholar] [CrossRef]
- Mohan, S.K.; Yu, C. The IL1α-S100A13 Heterotetrameric Complex Structure. J. Biol. Chem. 2011, 286, 14608–14617. [Google Scholar] [CrossRef] [PubMed]
- Carreira, C.M.; LaVallee, T.M.; Tarantini, F.; Jackson, A.; Lathrop, J.T.; Hampton, B.; Burgess, W.H.; Maciag, T. S100A13 Is Involved in the Regulation of Fibroblast Growth Factor-1 and p40 Synaptotagmin-1 Release in Vitro. J. Biol. Chem. 1998, 273, 22224–22231. [Google Scholar] [CrossRef]
- Gupta, A.A.; Chou, R.-H.; Li, H.; Yang, L.-W.; Yu, C. Structural insights into the interaction of human S100B and basic fibroblast growth factor (FGF2): Effects on FGFR1 receptor signaling. Biochim. Biophys. Acta BBA Proteins Proteom. 2013, 1834, 2606–2619. [Google Scholar] [CrossRef]
- Andreeva, A.; Kulesha, E.; Gough, J.; Murzin, A.G. The SCOP database in 2020: Expanded classification of representative family and superfamily domains of known protein structures. Nucleic Acids Res. 2019, 48, D376–D382. [Google Scholar] [CrossRef]
- Kazakov, A.S.; Sokolov, A.S.; Rastrygina, V.A.; Solovyev, V.V.; Ismailov, R.G.; Mikhailov, R.V.; Ulitin, A.B.; Yakovenko, A.R.; Mirzabekov, T.A.; Permyakov, E.A.; et al. High-affinity interaction between interleukin-11 and S100P protein. Biochem. Biophys. Res. Commun. 2015, 468, 733–738. [Google Scholar] [CrossRef]
- Kazakov, A.S.; Sokolov, A.S.; Vologzhannikova, A.A.; Permyakova, M.E.; Khorn, P.A.; Ismailov, R.G.; Denessiouk, K.A.; Denesyuk, A.I.; Rastrygina, V.A.; Baksheeva, V.E.; et al. Interleukin-11 binds specific EF-hand proteins via their conserved structural motifs. J. Biomol. Struct. Dyn. 2016, 35, 78–91. [Google Scholar] [CrossRef]
- Penumutchu, S.R.; Chou, R.-H.; Yu, C. Structural Insights into Calcium-Bound S100P and the V Domain of the RAGE Complex. PLoS ONE 2014, 9, e103947. [Google Scholar] [CrossRef]
- Orchard, S.; Ammari, M.; Aranda, B.; Breuza, L.; Briganti, L.; Broackes-Carter, F.; Campbell, N.H.; Chavali, G.; Chen, C.; Del-Toro, N.; et al. The MIntAct project—IntAct as a common curation platform for 11 molecular interaction databases. Nucleic Acids Res. 2013, 42, D358–D363. [Google Scholar] [CrossRef]
- Oughtred, R.; Rust, J.; Chang, C.; Breitkreutz, B.; Stark, C.; Willems, A.; Boucher, L.; Leung, G.; Kolas, N.; Zhang, F.; et al. TheBioGRIDdatabase: A comprehensive biomedical resource of curated protein, genetic, and chemical interactions. Protein Sci. 2020, 30, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Madeira, F.; Park, Y.M.; Lee, J.; Buso, N.; Gur, T.; Madhusoodanan, N.; Basutkar, P.; Tivey, A.R.N.; Potter, S.C.; Finn, R.D.; et al. The EMBL-EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Res. 2019, 47, W636–W641. [Google Scholar] [CrossRef]
- Lee, Y.-C.; Volk, D.E.; Thiviyanathan, V.; Kleerekoper, Q.; Gribenko, A.V.; Zhang, S.; Gorenstein, D.G.; Makhatadze, G.I.; Luxon, B.A. Letter to the Editor: NMR Structure of the Apo-S100P Protein. J. Biomol. NMR 2004, 29, 399–402. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, G.; Ding, Y.; Wang, Z.; Barraclough, R.; Rudland, P.S.; Fernig, D.; Rao, Z. The Crystal Structure at 2Å Resolution of the Ca2+-binding Protein S100P. J. Mol. Biol. 2002, 325, 785–794. [Google Scholar] [CrossRef]
- Wu, Z.; Boonmars, T.; Nagano, I.; Boonjaraspinyo, S.; Srinontong, P.; Ratasuwan, P.; Narong, K.; Nielsen, P.S.; Maekawa, Y. Significance of S100P as a biomarker in diagnosis, prognosis and therapy of opisthorchiasis-associated cholangiocarcinoma. Int. J. Cancer 2015, 138, 396–408. [Google Scholar] [CrossRef] [PubMed]
- Permyakov, S.E.; Denesyuk, A.I.; Denessiouk, K.A.; Permyakova, M.E.; Kazakov, A.S.; Ismailov, R.G.; Rastrygina, V.A.; Sokolov, A.S.; Permyakov, E.A. Monomeric state of S100P protein: Experimental and molecular dynamics study. Cell Calcium 2019, 80, 152–159. [Google Scholar] [CrossRef]
- Spadaro, A.; Rinaldi, T.; Riccieri, V.; Valesini, G.; Taccari, E. Interleukin 13 in synovial fluid and serum of patients with psoriatic arthritis. Ann. Rheum. Dis. 2002, 61, 174–176. [Google Scholar] [CrossRef]
- Rousseau, F.; Gauchat, J.-F.; McLeod, J.G.; Chevalier, S.; Guillet, C.; Guilhot, F.; Cognet, I.; Froger, J.; Hahn, A.F.; Knappskog, P.M.; et al. Inactivation of cardiotrophin-like cytokine, a second ligand for ciliary neurotrophic factor receptor, leads to cold-induced sweating syndrome in a patient. Proc. Natl. Acad. Sci. USA 2006, 103, 10068–10073. [Google Scholar] [CrossRef]
- Desta, I.T.; Porter, K.A.; Xia, B.; Kozakov, D.; Vajda, S. Performance and Its Limits in Rigid Body Protein-Protein Docking. Structure 2020, 28, 1071–1081. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Permyakov, S.E.; Ismailov, R.G.; Xue, B.; Denesyuk, A.I.; Uversky, V.N.; Permyakov, E.A. Intrinsic disorder in S100 proteins. Mol. BioSyst. 2011, 7, 2164–2180. [Google Scholar] [CrossRef] [PubMed]
- Leonard, W.J.; Lin, J.-X.; O’Shea, J.J. The γc Family of Cytokines: Basic Biology to Therapeutic Ramifications. Immunity 2019, 50, 832–850. [Google Scholar] [CrossRef] [PubMed]
- Quaglia, F.; Mészáros, B.; Salladini, E.; Hatos, A.; Pancsa, R.; Chemes, L.B.; Pajkos, M.; Lazar, T.; Peña-Díaz, S.; Santos, J.; et al. DisProt in 2022: Improved quality and accessibility of protein intrinsic disorder annotation. Nucleic Acids Res. 2021, 50, D480–D487. [Google Scholar] [CrossRef]
- Pace, C.N.; Vajdos, F.; Fee, L.; Grimsley, G.; Gray, T. How to measure and predict the molar absorption coefficient of a protein. Protein Sci. 1995, 4, 2411–2423. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- Blum, H.E.; Lehky, P.; Kohler, L.; Stein, E.A.; Fischer, E.H. Comparative properties of vertebrate parvalbumins. J. Biol. Chem. 1977, 252, 2834–2838. [Google Scholar] [CrossRef]
- Burstein, E.A.; Emelyanenko, V.I. Log-Normal Description of Fluorescence Spectra of Organic Fluorophores. Photochem. Photobiol. 1996, 64, 316–320. [Google Scholar] [CrossRef]
- Schägger, H.; von Jagow, G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 1987, 166, 368–379. [Google Scholar] [CrossRef]
- Peng, K.; Vucetic, S.; Radivojac, P.; Brown, C.J.; Dunker, A.K.; Obradovic, Z. Optimizing Long Intrinsic Disorder Predictors with Protein Evolutionary Information. J. Bioinform. Comput. Biol. 2005, 3, 35–60. [Google Scholar] [CrossRef]
- Peng, Z.L.; Kurgan, L. Comprehensive Comparative Assessment of In-Silico Predictors of Disordered Regions. Curr. Protein Pept. Sci. 2012, 13, 6–18. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Kurgan, L. Accurate prediction of disorder in protein chains with a comprehensive and empirically designed consensus. J. Biomol. Struct. Dyn. 2013, 32, 448–464. [Google Scholar] [CrossRef] [PubMed]
- Necci, M.; Piovesan, D.; CAID Predictors; DisProt Curators; Tosatto, S.C.E. Critical assessment of protein intrinsic disorder prediction. Nat. Methods 2021, 18, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Uversky, V.N.; Gillespie, J.R.; Fink, A.L. Why are “natively unfolded” proteins unstructured under physiologic conditions? Proteins 2000, 41, 415–427. [Google Scholar] [CrossRef]
- Oldfield, C.J.; Cheng, Y.; Cortese, M.; Brown, C.J.; Uversky, V.N.; Dunker, A.K. Comparing and Combining Predictors of Mostly Disordered Proteins. Biochemistry 2005, 44, 1989–2000. [Google Scholar] [CrossRef] [PubMed]
- Xue, B.; Oldfield, C.J.; Dunker, A.K.; Uversky, V.N. CDF it all: Consensus prediction of intrinsically disordered proteins based on various cumulative distribution functions. FEBS Lett. 2009, 583, 1469–1474. [Google Scholar] [CrossRef]
- Huang, F.; Oldfield, C.J.; Xue, B.; Hsu, W.-L.; Meng, J.; Liu, X.; Shen, L.; Romero, P.; Uversky, V.N.; Dunker, A.K. Improving protein order-disorder classification using charge-hydropathy plots. BMC Bioinform. 2014, 15, S4. [Google Scholar] [CrossRef]
- Mohan, A.; Sulliivan, W.J., Jr.; Radivojac, P.; Dunker, A.K.; Uversky, V.N. Intrinsic disorder in pathogenic and non-pathogenic microbes: Discovering and analyzing the unfoldomes of early-branching eukaryotes. Mol. BioSyst. 2008, 4, 328–340. [Google Scholar] [CrossRef]
- Huang, F.; Oldfield, C.; Meng, J.; Hsu, W.-L.; Xue, B.; Uversky, V.N.; Romero, P.; Dunker, A.K. Subclassifying Disordered Proteins by the Ch-Cdf Plot Method. Biocomputing 2012 2011, 128–139. [Google Scholar] [CrossRef]
- Simon, M.A.; Ecsédi, P.; Kovács, G.M.; Póti, L.; Reményi, A.; Kardos, J.; Gógl, G.; Nyitray, L. High-throughput competitive fluorescence polarization assay reveals functional redundancy in the S100 protein family. FEBS J. 2020, 287, 2834–2846. [Google Scholar] [CrossRef]
- Simon, M.A.; Bartus, É; Mag, B.; Boros, E.; Roszjár, L.; Gógl, G.; Travé, G.; Martinek, T.A.; Nyitray, L. Promiscuity mapping of the S100 protein family using a high-throughput holdup assay. Sci. Rep. 2022, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]



| Cytokine | Kd1, M | Kd2, M | Reference |
|---|---|---|---|
| Short-chain cytokines | |||
| EPO | (5.4 ± 1.2) × 10−7 | (1.8 ± 0.5) × 10−6 | [22] |
| Long-chain cytokines | |||
| IL-11 | (3.2 ± 0.3) × 10−8 | (2.88 ± 0.01) × 10−7 | [28] |
| Cardiotrophin-like cytokine factor 1 | (8.1 ± 2.6) × 10−8 | (1.4 ± 0.8) × 10−7 | [21] |
| Ciliary neurotrophic factor | (1.0 ± 0.6) × 10−7 | (1.1 ± 0.8) × 10−7 | [21] |
| Cardiotrophin-1 | (1.9 ± 0.5) × 10−8 | (9.8 ± 2.7) × 10−7 | [21] |
| Oncostatin-M | (7.0 ± 4.2) × 10−7 | (2.0 ± 0.9) × 10−6 | [21] |
| Interferons/IL-10 | |||
| IFN-β | (5.34 ± 0.10) × 10−8 | (6.1 ± 2.3) × 10−7 | [10] |
| Full Name | Abbreviation | UniProt ID | Manufacturer | Cat. Number | Source |
|---|---|---|---|---|---|
| Short-chain cytokines | |||||
| Macrophage colony-stimulating factor 1 | M-CSF | P09603 | PeproTech | 300-25 | E. coli |
| Granulocyte-macrophage colony-stimulating factor | GM-CSF | P04141 | PeproTech | 300-03 | E. coli |
| Interleukin-2 | IL-2 | P60568 | PeproTech | AF-200-02 | E. coli |
| Interleukin-3 | IL-3 | P08700 | SCI-Store (Russia) | PSG160-10 | CHO |
| Interleukin-4 | IL-4 | P05112 | PeproTech | AF-200-04 | E. coli |
| Interleukin-5 | IL-5 | P05113 | PeproTech | 200-05 | E. coli |
| Interleukin-9 | IL-9 | P15248 | PeproTech | 200-09 | E. coli |
| Interleukin-13 | IL-13 | P35225 | PeproTech | 200-13 | E. coli |
| Interleukin-15 | IL-15 | P40933 | PeproTech | 200-15 | E. coli |
| Interleukin-21 | IL-21 | Q9HBE4 | PeproTech | 200-21 | E. coli |
| Thrombopoietin | THPO | P40225 | SCI-Store (Russia) | PSG090-10 | CHO |
| Long-chain cytokines | |||||
| Interleukin-7 | IL-7 | P13232 | SCI-Store (Russia) | PSG240-10 | CHO |
| Interleukin-31 | IL-31 | Q6EBC2 | PeproTech | 200-31 | E. coli |
| Granulocyte colony-stimulating factor | G-CSF | P09919 | Pharmstandard (Russia) | n/a | E. coli |
| Somatotropin | GH | P01241 | PeproTech | AF-100-40 | E. coli |
| Growth hormone variant | GH-V | P01242 | R&D Systems | 7668-GH/CF | E. coli |
| Prolactin | PRL | P01236 | PeproTech | 100-07 | E. coli |
| Leptin | LEP | P41159 | PeproTech | AF-300-27 | E. coli |
| Thymic stromal lymphopoietin | TSLP | Q969D9 | PeproTech | 300-62 | E. coli |
| Chorionic somatomammotropin hormone 1 | PL | P0DML2 | R&D Systems | 5757-PL/CF | CHO |
| Interleukin-12 | IL-12 | P29459 * and P29460 | PeproTech | 200-12H | HEK293 |
| Interleukin-23 | IL-23 | Q9NPF7 * and P29460 | PeproTech | 200-23 | Hi-5 |
| Interleukin-27 | IL-27 | Q8NEV9 * and Q14213 | PeproTech | 200-38 | HEK293 |
| Interleukin-35 | IL-35 | P29459 * and Q14213 | PeproTech | 200-37 | HEK293 |
| Interferons/IL-10 | |||||
| Interleukin-10 | IL-10 | P22301 | PeproTech | AF-200-10 | E. coli |
| Interleukin-20 | IL-20 | Q9NYY1 | PeproTech | 200-20 | E. coli |
| Interleukin-22 | IL-22 | Q9GZX6 | PeproTech | 200-22 | E. coli |
| Interleukin-24 | IL-24 | Q13007 | PeproTech | 200-35 | CHO |
| Interleukin-26 | IL-26 | Q9NPH9 | R&D Systems | 1375-IL/CF | E. coli |
| Interferon α-2 | IFN-α2 | P01563 | Vector-Medica (Russia) | n/a | E. coli |
| Interferon γ | IFN-γ | P01579 | Pharmaclon (Russia) | n/a | E. coli |
| Interferon ω-1 | IFN-ω1 | P05000 | PeproTech | 300-02J | E. coli |
| Cytokine | kd1, s−1 | Kd1, M | kd2, s−1 | Kd2, M |
|---|---|---|---|---|
| Short-chain cytokines | ||||
| GM-CSF | (1.13 ± 0.23) × 10−3 | (2.45 ± 1.45) × 10−7 | (6.72 ± 2.13) × 10−2 | (4.50 ± 1.75) × 10−7 |
| IL-3 | (6.27 ± 0.38) × 10−4 | (2.32 ± 0.81) × 10−8 | (5.29 ± 0.86) × 10−3 | (5.79 ± 2.19) × 10−7 |
| IL-5 | (6.12 ± 1.12) × 10−4 | (3.66 ± 1.13) × 10−9 | (7.12 ± 0.77) × 10−3 | (8.33 ± 3.58) × 10−7 |
| IL-9 | (5.31 ± 1.69) × 10−4 | (3.47 ± 2.66) × 10−8 | (1.70 ± 0.48) × 10−2 | (1.36 ± 0.78) × 10−7 |
| IL-13 * | (1.83 ± 1.09) × 10−2 | (2.30 ± 0.44) × 10−6 | n/a | n/a |
| IL-15 | (1.09 ± 0.25) × 10−3 | (1.02 ± 0.72) × 10−7 | (2.72 ± 1.03) × 10−2 | (9.80 ± 5.84) × 10−7 |
| IL-21 | (9.28 ± 2.85) × 10−4 | (2.72 ± 0.43) × 10−7 | (1.26 ± 0.26) × 10−2 | (2.85 ± 1.73) × 10−7 |
| THPO | (4.09 ± 0.49) × 10−4 | (8.34 ± 2.79) × 10−10 | (8.29 ± 0.38) × 10−3 | (4.12 ± 2.44) × 10−8 |
| Long-chain cytokines | ||||
| IL-31 | (2.30 ± 0.52) × 10−4 | (2.52 ± 1.30) × 10−8 | (5.83 ± 0.79) × 10−2 | (2.02 ± 1.12) × 10−7 |
| IL-27 # | (9.94 ± 0.75) × 10−3 | (8.59 ± 2.67) × 10−7 | (3.57 ± 0.64) × 10−4 | (1.69 ± 0.59) × 10−6 |
| IL-35 # | (5.31 ± 1.31) × 10−4 | (9.62 ± 4.92) × 10−8 | (1.20 ± 0.13) × 10−2 | (2.55 ± 1.77) × 10−7 |
| G-CSF | (3.15 ± 0.46) × 10−4 | (2.13 ± 0.15) × 10−7 | (1.54 ± 0.72) × 10−2 | (8.54 ± 2.80) × 10−7 |
| GH | (6.69 ± 2.77) × 10−4 | (4.29 ± 0.93) × 10−8 | (1.42 ± 0.18) × 10−2 | (2.66 ± 1.54) × 10−7 |
| GH-V | (1.77 ± 1.05) × 10−3 | (1.04 ± 0.88) × 10−6 | (1.81 ± 0.71) × 10−2 | (2.73 ± 0.15) × 10−6 |
| PRL | (9.81 ± 0.84) × 10−4 | (5.46 ± 2.35) × 10−8 | (1.03 ± 0.10) × 10−2 | (9.06 ± 0.17) × 10−7 |
| LEP | (4.52 ± 0.77) × 10−4 | (1.43 ± 0.32) × 10−7 | (3.07 ± 0.75) × 10−2 | (4.04 ± 2.35) × 10−7 |
| Interferons/IL-10 | ||||
| IL-10 | (1.06 ± 0.17) × 10−2 | (2.55 ± 0.54) × 10−6 | (5.19 ± 0.43) × 10−4 | (2.66 ± 1.68) × 10−6 |
| IL-20 | (4.42 ± 0.97) × 10−4 | (4.03 ± 1.29) × 10−7 | (2.46 ± 0.51) × 10−2 | (5.37 ± 0.90) × 10−7 |
| IL-22 | (7.64 ± 5.76) × 10−3 | (4.82 ± 0.96) × 10−7 | (2.76 ± 1.93) × 10−3 | (7.80 ± 0.77) × 10−7 |
| IL-24 | (4.83 ± 0.21) × 10−4 | (1.58 ± 0.20) × 10−7 | (7.94 ± 0.34) × 10−3 | (9.18 ± 1.24) × 10−7 |
| IL-26 | (1.44 ± 0.78) × 10−4 | (9.02 ± 4.24) × 10−9 | (5.09 ± 1.38) × 10−3 | (1.32 ± 0.24) × 10−6 |
| IFN-ω1 | (5.71 ± 0.09) × 10−4 | (1.74 ± 0.19) × 10−7 | (8.39 ± 0.56) × 10−3 | (5.78 ± 1.18) × 10−7 |
| Cytokine\S100P | Wild-Type | F89A | Δ42–47 | ||
|---|---|---|---|---|---|
| Kd1, M | Kd2, M | Kd1, M | Kd1, M | Kd, M | |
| Short-chain cytokines | |||||
| GM-CSF | (2.45 ± 1.45) × 10−7 | (4.50 ± 1.75) × 10−7 | n.d. | n.d. | |
| IL-3 | (2.32 ± 0.81) × 10−8 | (5.79 ± 2.19) × 10−7 | n.d. | n.d. | |
| IL-5 | (3.66 ± 1.13) × 10−9 | (8.33 ± 3.58) × 10−7 | n.d. | n.d. | |
| IL-9 | (3.47 ± 2.66) × 10−8 | (1.36 ± 0.78) × 10−7 | n.d. | n.d. | |
| IL-13 * | (2.30 ± 0.44) × 10−6 | n/a | >10−4 | >10−4 | |
| IL-15 | (1.02 ± 0.72) × 10−7 | (9.80 ± 5.84) × 10−7 | (1.57 ± 0.57) × 10−7 | (2.83 ± 0.34) × 10−7 | >10−4 |
| IL-21 | (2.72 ± 0.43) × 10−7 | (2.85 ± 1.73) × 10−7 | (4.01 ± 1.64) × 10−7 | (9.50 ± 4.52) × 10−7 | >10−4 |
| THPO | (8.34 ± 2.79) × 10−10 | (4.12 ± 2.44) × 10−8 | n.d. | n.d. | |
| Long-chain cytokines | |||||
| IL-31 | (2.52 ± 1.30) × 10−8 | (2.02 ± 1.12) × 10−7 | (2.02 ± 0.59) × 10−7 | (1.18 ± 0.53) × 10−6 | >10−4 |
| IL-27 # | (8.59 ± 2.67) × 10−7 | (1.69 ± 0.59) × 10−6 | n.d. | n.d. | |
| IL-35 # | (9.62 ± 4.92) × 10−8 | (2.55 ± 1.77) × 10−7 | >10−4 | >10−5 | |
| G-CSF | (2.13 ± 0.15) × 10−7 | (8.54 ± 2.80) × 10−7 | n.d. | n.d. | |
| GH | (4.29 ± 0.93) × 10−8 | (2.66 ± 1.54) × 10−7 | (2.92 ± 2.66) × 10−5 | (1.38 ± 0.52) × 10−4 | >10−4 |
| GH-V | (1.04 ± 0.88) × 10−6 | (2.73 ± 0.15) × 10−6 | >10−4 | >10−4 | |
| PRL | (5.46 ± 2.35) × 10−8 | (9.06 ± 0.17) × 10−7 | (6.89 ± 3.20) × 10−7 | (3.12 ± 0.50) × 10−6 | >10−4 |
| LEP | (1.43 ± 0.32) × 10−7 | (4.04 ± 2.35) × 10−7 | (4.37 ± 1.71) × 10−6 | (3.57 ± 1.77) × 10−5 | >10−4 |
| Interferons/IL-10 | |||||
| IL-10 | (2.55 ± 0.54) × 10−6 | (2.66 ± 1.68) × 10−6 | (4.74 ± 3.41) × 10−6 | (6.05 ± 1.47) × 10−6 | >10−4 |
| IL-20 | (4.03 ± 1.29) × 10−7 | (5.37 ± 0.90) × 10−7 | n.d. | n.d. | |
| IL-22 | (4.82 ± 0.96) × 10−7 | (7.80 ± 0.77) × 10−7 | n.d. | n.d. | |
| IL-24 | (1.58 ± 0.20) × 10−7 | (9.18 ± 1.24) × 10−7 | n.d. | n.d. | |
| IL-26 | (9.02 ± 4.24) × 10−9 | (1.32 ± 0.24) × 10−6 | >10−4 | >10−4 | |
| IFN-ω1 | (1.74 ± 0.19) × 10−7 | (5.78 ± 1.18) × 10−7 | n.d. | n.d. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kazakov, A.S.; Deryusheva, E.I.; Permyakova, M.E.; Sokolov, A.S.; Rastrygina, V.A.; Uversky, V.N.; Permyakov, E.A.; Permyakov, S.E. Calcium-Bound S100P Protein Is a Promiscuous Binding Partner of the Four-Helical Cytokines. Int. J. Mol. Sci. 2022, 23, 12000. https://doi.org/10.3390/ijms231912000
Kazakov AS, Deryusheva EI, Permyakova ME, Sokolov AS, Rastrygina VA, Uversky VN, Permyakov EA, Permyakov SE. Calcium-Bound S100P Protein Is a Promiscuous Binding Partner of the Four-Helical Cytokines. International Journal of Molecular Sciences. 2022; 23(19):12000. https://doi.org/10.3390/ijms231912000
Chicago/Turabian StyleKazakov, Alexey S., Evgenia I. Deryusheva, Maria E. Permyakova, Andrey S. Sokolov, Victoria A. Rastrygina, Vladimir N. Uversky, Eugene A. Permyakov, and Sergei E. Permyakov. 2022. "Calcium-Bound S100P Protein Is a Promiscuous Binding Partner of the Four-Helical Cytokines" International Journal of Molecular Sciences 23, no. 19: 12000. https://doi.org/10.3390/ijms231912000
APA StyleKazakov, A. S., Deryusheva, E. I., Permyakova, M. E., Sokolov, A. S., Rastrygina, V. A., Uversky, V. N., Permyakov, E. A., & Permyakov, S. E. (2022). Calcium-Bound S100P Protein Is a Promiscuous Binding Partner of the Four-Helical Cytokines. International Journal of Molecular Sciences, 23(19), 12000. https://doi.org/10.3390/ijms231912000









