A Single Oral Vitamin D3 Bolus Reduces Inflammatory Markers in Healthy Saudi Males
Abstract
1. Introduction
2. Results
2.1. Characteristics of the Participants
2.2. Biochemical Parameters
2.2.1. Ca2+, PO4− and PTH
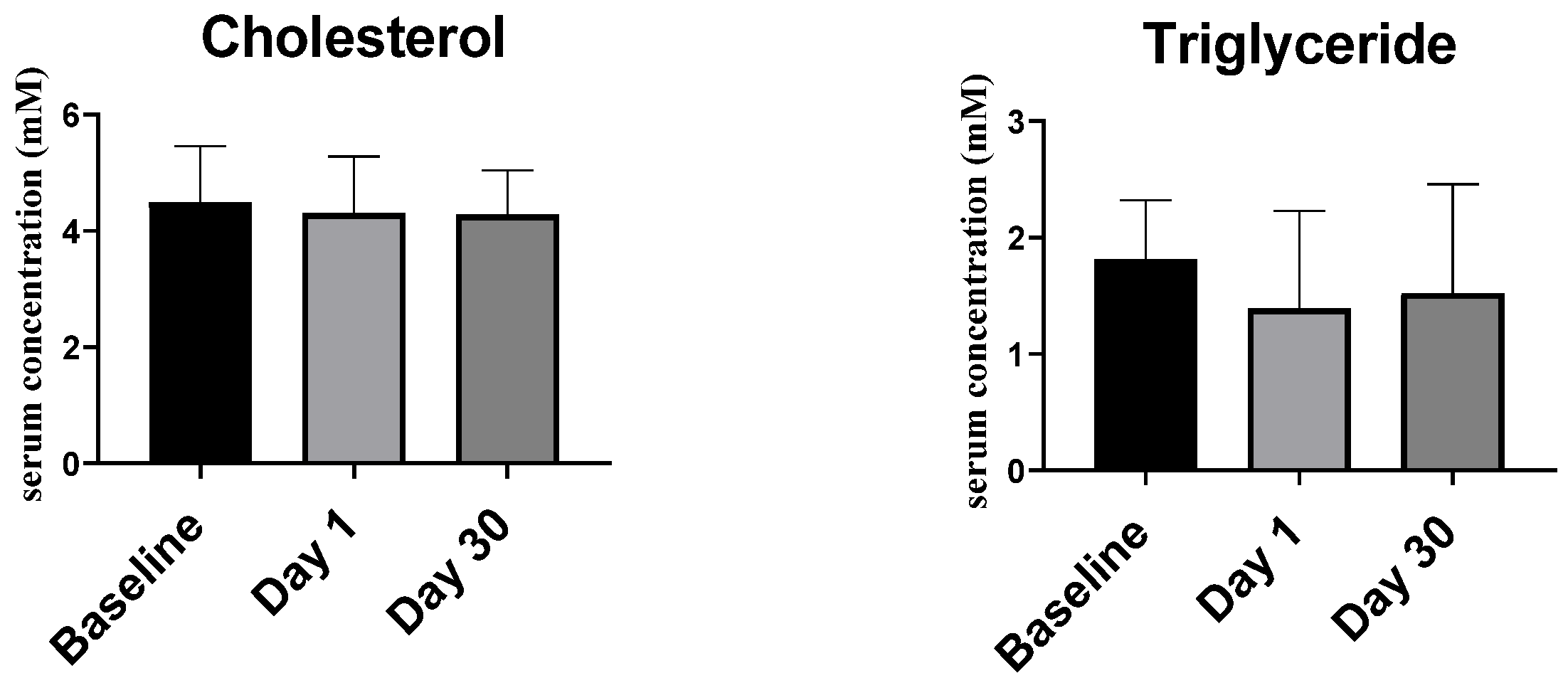
2.2.2. Lipid Profile
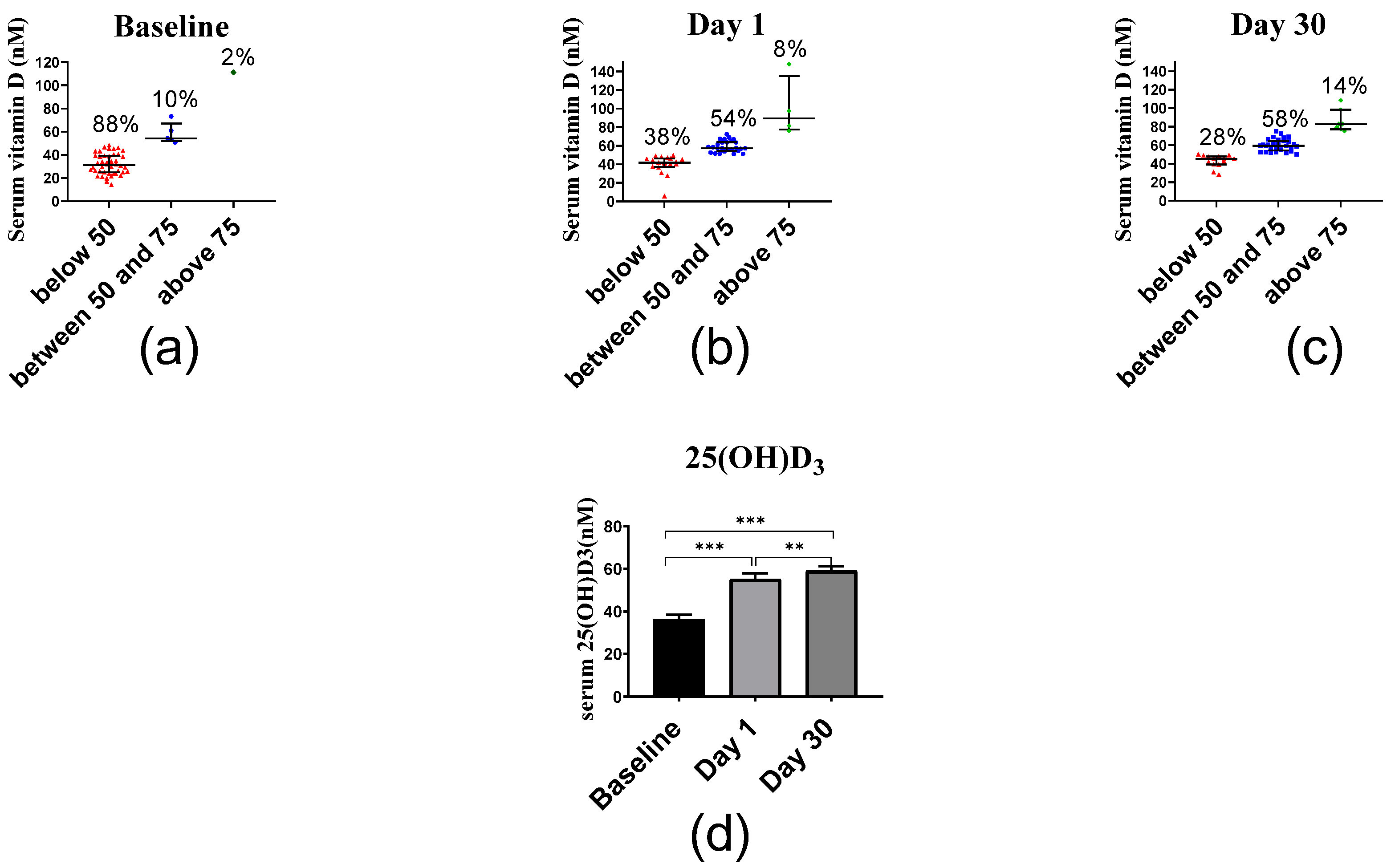
2.2.3. Serum Vitamin D
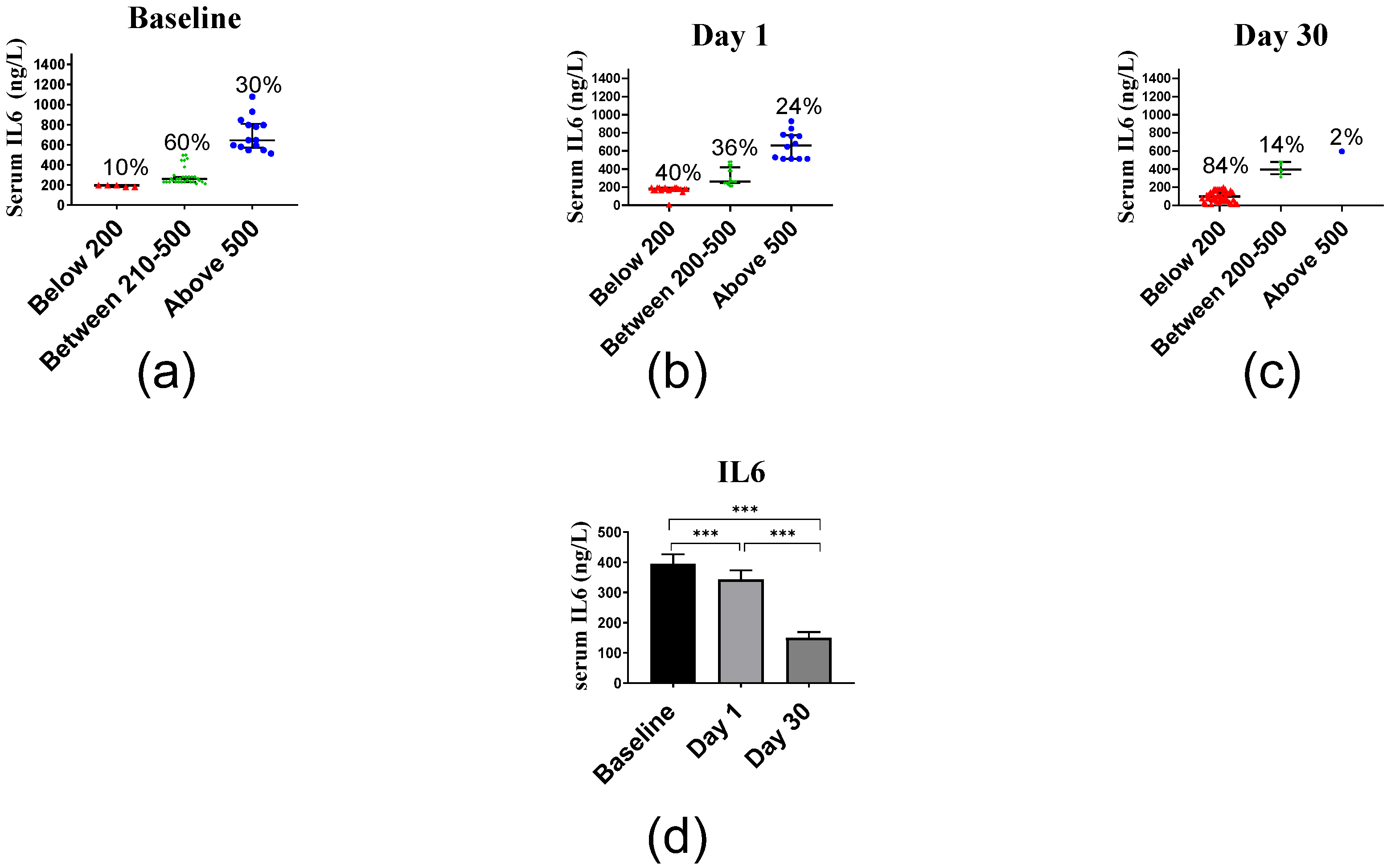
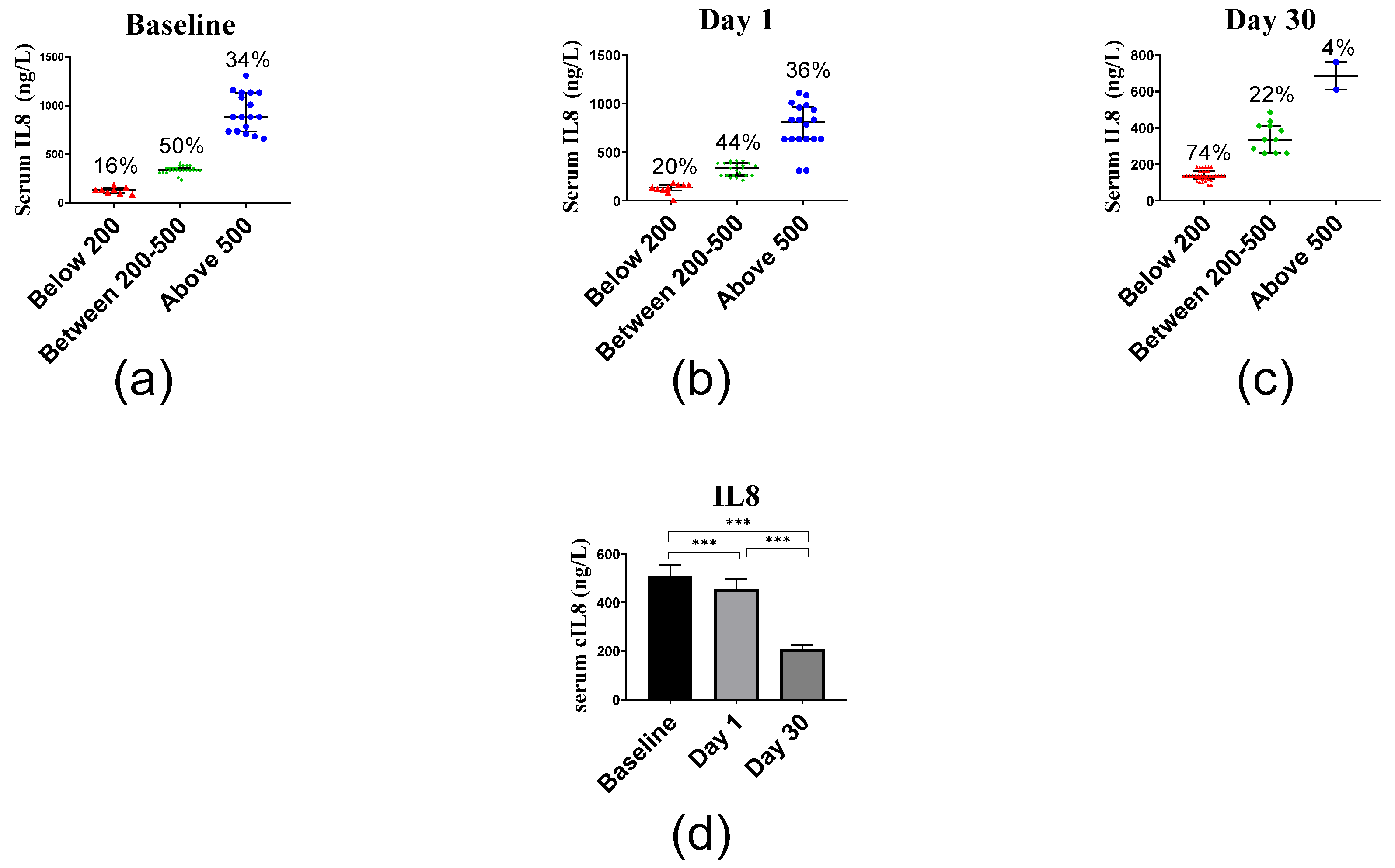
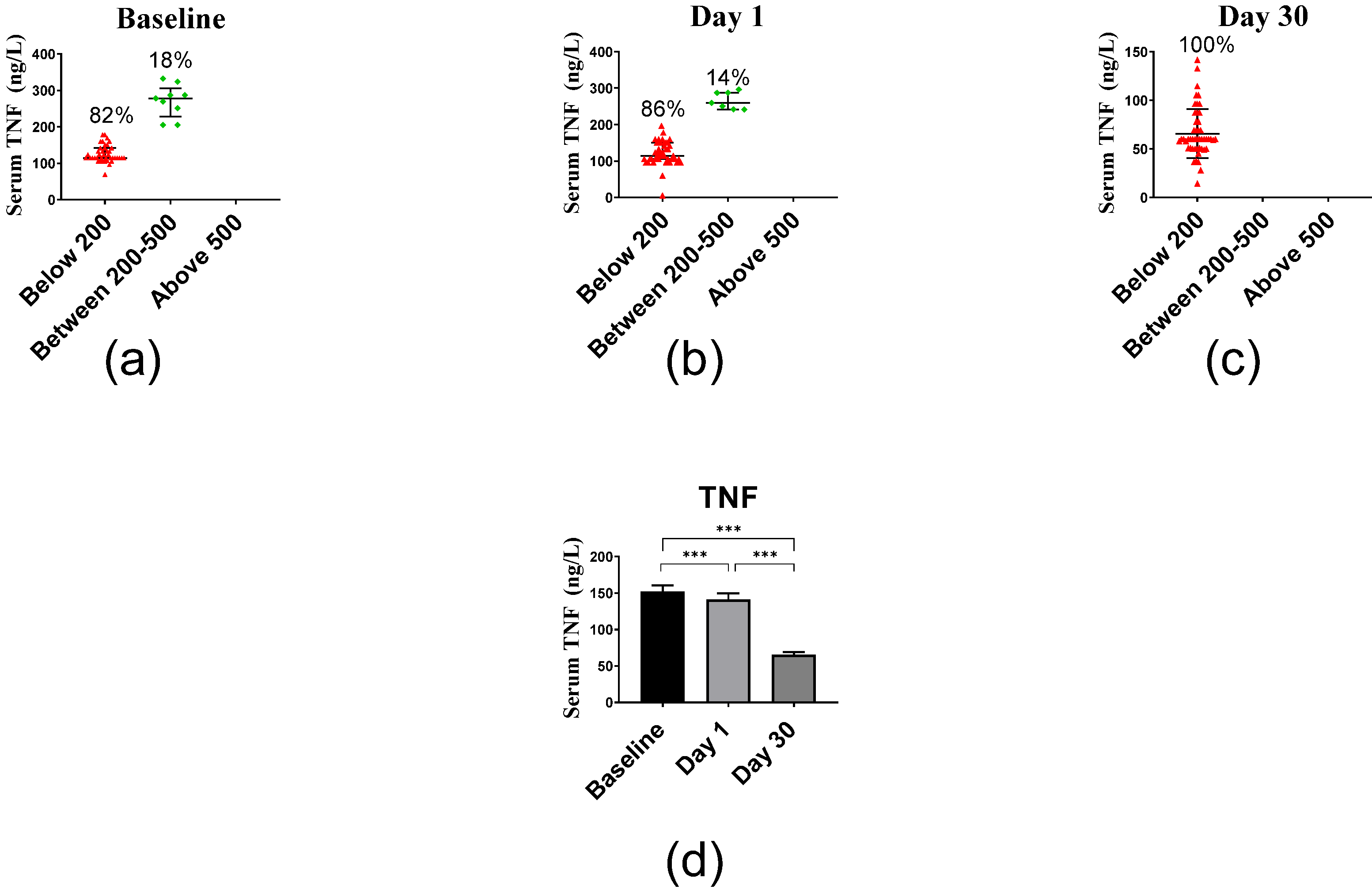
2.2.4. Inflammatory Markers
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Questionnaire and Anthropometric Measurements
4.3. Serum Specimen
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef]
- Pahwa, R.; Goyal, A.; Jialal, I. Chronic Inflammation; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Curley, S.; Gall, J.; Byrne, R.; Yvan-Charvet, L.; McGillicuddy, F.C. Metabolic inflammation in obesity-at the crossroads between fatty acid and cholesterol metabolism. Mol. Nutr. Food Res. 2021, 65, e1900482. [Google Scholar] [CrossRef]
- Krainer, J.; Siebenhandl, S.; Weinhäusel, A. Systemic autoinflammatory diseases. J. Autoimmun. 2020, 109, 102421. [Google Scholar] [CrossRef]
- Joshipura, K.J.; Hu, F.B.; Manson, J.E.; Stampfer, M.J.; Rimm, E.B.; Speizer, F.E.; Colditz, G.; Ascherio, A.; Rosner, B.; Spiegelman, D.; et al. The effect of fruit and vegetable intake on risk for coronary heart disease. Ann. Intern. Med. 2001, 134, 1106–1114. [Google Scholar] [CrossRef]
- Gandini, S.; Merzenich, H.; Robertson, C.; Boyle, P. Meta-analysis of studies on breast cancer risk and diet: The role of fruit and vegetable consumption and the intake of associated micronutrients. Eur. J. Cancer 2000, 36, 636–646. [Google Scholar] [CrossRef]
- Yoon, J.H.; Baek, S.J. Molecular targets of dietary polyphenols with anti-inflammatory properties. Yonsei Med. J. 2005, 46, 585–596. [Google Scholar] [CrossRef]
- Maroon, J.C.; Bost, J.W.; Maroon, A. Natural anti-inflammatory agents for pain relief. Surg. Neurol. Int. 2010, 1, 80. [Google Scholar] [CrossRef] [PubMed]
- Dogan-Sander, E.; Mergl, R.; Willenberg, A.; Baber, R.; Wirkner, K.; Riedel-Heller, S.G.; Röhr, S.; Schmidt, F.M.; Schomerus, G.; Sander, C. Inflammation and the association of vitamin D and depressive symptomatology. Nutrients 2021, 13, 1972. [Google Scholar] [CrossRef]
- Pittas, A.G.; Harris, S.S.; Stark, P.C.; Dawson-Hughes, B. The effects of calcium and vitamin D supplementation on blood glucose and markers of inflammation in nondiabetic adults. Diabetes Care 2007, 30, 980–986. [Google Scholar] [CrossRef]
- Bui, L.; Zhu, Z.; Hawkins, S.; Cortez-Resendiz, A.; Bellon, A. Vitamin D regulation of the immune system and its implications for COVID-19: A mini review. SAGE Open Med. 2021, 9, 20503121211014073. [Google Scholar] [CrossRef] [PubMed]
- Tiosano, D.; Wildbaum, G.; Gepstein, V.; Verbitsky, O.; Weisman, Y.; Karin, N.; Eztioni, A. The role of vitamin D receptor in innate and adaptive immunity: A study in hereditary vitamin D-resistant rickets patients. J. Clin. Endocrinol. Metab. 2013, 98, 1685–1693. [Google Scholar] [CrossRef] [PubMed]
- Gruber-Bzura, B.M. Vitamin D and influenza—Prevention or therapy? Int. J. Mol. Sci. 2018, 19, 2419. [Google Scholar] [CrossRef]
- Rondanelli, M.; Miccono, A.; Lamburghini, S.; Avanzato, I.; Riva, A.; Allegrini, P.; Faliva, M.A.; Peroni, G.; Nichetti, M.; Perna, S. Self-care for common colds: The pivotal role of vitamin D, vitamin C, zinc, and Echinacea in three main immune interactive clusters (physical barriers, innate and adaptive immunity) involved during an episode of common colds—Practical advice on dosages and on the time to take these nutrients/botanicals in order to prevent or treat common catolds. Evid. Based Complement. Alternat. Med. 2018, 2018, 5813095. [Google Scholar] [CrossRef]
- Gombart, A.F.; Pierre, A.; Maggini, S. A review of micronutrients and the immune system–working in harmony to reduce the risk of infection. Nutrients 2020, 12, 236. [Google Scholar] [CrossRef]
- Roffe-Vazquez, D.N.; Huerta-Delgado, A.S.; Castillo, E.C.; Villarreal-Calderón, J.R.; Gonzalez-Gil, A.M.; Enriquez, C.; Garcia-Rivas, G.; Elizondo-Montemayor, L. Correlation of vitamin D with inflammatory cytokines, atherosclerotic parameters, and lifestyle factors in the setting of heart failure: A 12-month follow-up study. Int. J. Mol. Sci. 2019, 20, 5811. [Google Scholar] [CrossRef] [PubMed]
- Maigoro, A.Y.; An, D.; Lee, S. Exploring the link between vitamin D deficiency and cytokine storms in COVID-19 patients: An in silico analysis. J. Med. Food 2022, 25, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Ali, N. Role of vitamin D in preventing of COVID-19 infection, progression and severity. J. Infect. Public Health 2020, 13, 1373–1380. [Google Scholar] [CrossRef]
- Giustina, A.; Adler, R.A.; Binkley, N.; Bollerslev, J.; Bouillon, R.; Dawson-Hughes, B.; Ebeling, P.R.; Feldman, D.; Formenti, A.M.; Lazaretti-Castro, M.; et al. Consensus Statement from 2nd International Conference on Controversies in Vitamin D. Rev. Endocr. Metab. Disord. 2020, 21, 89–116. [Google Scholar] [CrossRef]
- Ish-Shalom, S.; Segal, E.; Salganik, T.; Raz, B.; Bromberg, I.L.; Vieth, R. Comparison of daily, weekly, and monthly vitamin D3 in ethanol dosing protocols for two months in elderly hip fracture patients. J. Clin. Endocrinol. Metab. 2008, 93, 3430–3435. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.C.; Manson, J.E.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: What clinicians need to know. J. Clin. Endocrinol. Metab. 2011, 96, 53–88. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Endocrine society evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Shirvani, A.; Kalajian, T.A.; Song, A.; Holick, M.F. Disassociation of vitamin D’s calcemic activity and non-calcemic genomic activity and individual responsiveness: A randomized controlled double-blind clinical trial. Sci. Rep. 2019, 9, 17685. [Google Scholar] [CrossRef] [PubMed]
- Bacon, C.J.; Gamble, G.D.; Horne, A.M.; Scott, M.A.; Reid, I.R. High-dose oral vitamin D3 supplementation in the elderly. Osteoporos. Int. 2009, 20, 1407–1415. [Google Scholar] [CrossRef]
- Neme, A.; Seuter, S.; Malinen, M.; Nurmi, T.; Tuomainen, T.-P.; Virtanen, J.K.; Carlberg, C. In vivo transcriptome changes of human white blood cells in response to vitamin D. J. Steroid Biochem. Mol. Biol. 2019, 188, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Vukić, M.; Neme, A.; Seuter, S.; Saksa, N.; de Mello, V.D.F.; Nurmi, T.; Uusitupa, M.; Tuomainen, T.-P.; Virtanen, J.K.; Carlberg, C. Relevance of vitamin D receptor target genes for monitoring the vitamin D responsiveness of primary human cells. PLoS ONE 2015, 10, e0124339. [Google Scholar] [CrossRef]
- Seuter, S.; Virtanen, J.K.; Nurmi, T.; Pihlajamäki, J.; Mursu, J.; Voutilainen, S.; Tuomainen, T.-P.; Neme, A.; Carlberg, C. Molecular evaluation of vitamin D responsiveness of healthy young adults. J. Steroid Biochem. Mol. Biol. 2017, 174, 314–321. [Google Scholar] [CrossRef]
- Carlberg, C.; Seuter, S.; Nurmi, T.; Tuomainen, T.-P.; Virtanen, J.K.; Neme, A. In vivo response of the human epigenome to vitamin D: A proof-of-principle study. J. Steroid Biochem. Mol. Biol. 2018, 180, 142–148. [Google Scholar] [CrossRef]
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef] [PubMed]
- DeNicola, E.; Aburizaiza, O.S.; Siddique, A.; Khwaja, H.; Carpenter, D.O. Obesity and public health in the kingdom of Saudi Arabia. Rev. Environ. Health 2015, 30, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Guh, D.P.; Zhang, W.; Bansback, N.; Amarsi, Z.; Birmingham, C.L.; Anis, A.H. The incidence of co-morbidities related to obesity and overweight: A systematic review and meta-analysis. BMC Public Health 2009, 9, 88. [Google Scholar] [CrossRef]
- Ding, C.; Gao, D.; Wilding, J.; Trayhurn, P.; Bing, C. Vitamin D signalling in adipose tissue. Br. J. Nutr. 2012, 108, 1915–1923. [Google Scholar] [CrossRef] [PubMed]
- Matute Wilander, A.; Kåredal, M.; Axmon, A.; Nordander, C. Inflammatory biomarkers in serum in subjects with and without work related neck/shoulder complaints. BMC Musculoskelet. Disord. 2014, 15, 103. [Google Scholar] [CrossRef] [PubMed]
- Kershaw, E.E.; Flier, J.S. Adipose tissue as an endocrine organ. J. Clin. Endocrinol. Metabol. 2004, 89, 2548–2556. [Google Scholar] [CrossRef]
- Zorena, K.; Jachimowicz-Duda, O.; Ślęzak, D.; Robakowska, M.; Mrugacz, M. Adipokines and obesity. Potential link to metabolic disorders and chronic complications. Int. J. Mol. Sci. 2020, 21, 3570. [Google Scholar] [CrossRef]
- Vgontzas, A.N.; Papanicolaou, D.A.; Bixler, E.O.; Hopper, K.; Lotsikas, A.; Lin, H.M.; Kales, A.; Chrousos, G.P. Sleep apnea and daytime sleepiness and fatigue: Relation to visceral obesity, insulin resistance, and hypercytokinemia. J. Clin. Endocrinol. Metab. 2000, 85, 1151–1158. [Google Scholar] [CrossRef]
- Kern, L.; Mittenbühler, M.J.; Vesting, A.J.; Ostermann, A.L.; Wunderlich, C.M.; Wunderlich, F.T. Obesity-induced TNFα and IL-6 signaling: The missing link between obesity and inflammation-driven liver and colorectal cancers. Cancers 2018, 11, 24. [Google Scholar] [CrossRef]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Li, W.; Xie, J.; Hou, Y.; You, C. Cytokine storm induced by SARS-CoV-2. Clin. Chim. Acta 2020, 509, 280–287. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Ardawi, M.S.M.; Sibiany, A.M.; Bakhsh, T.M.; Qari, M.H.; Maimani, A.A. High prevalence of vitamin D deficiency among healthy Saudi Arabian men: Relationship to bone mineral density, parathyroid hormone, bone turnover markers, and lifestyle factors. Osteoporos. Int. 2012, 23, 675–686. [Google Scholar] [CrossRef]
- Elsammak, M.Y.; Al-Wossaibi, A.A.; Al-Howeish, A.; Alsaeed, J. High prevalence of vitamin D deficiency in the sunny eastern region of Saudi Arabia: A hospital-based study. East. Mediterr. Heal. J. 2011, 17, 317–322. [Google Scholar] [CrossRef]
- Alsuwadia, A.O.; Farag, Y.M.; Al Sayyari, A.A.; Mousa, D.H.; Alhejaili, F.F.; Al-Harbi, A.S.; Housawi, A.A.; Mittal, B.V.; Singh, A.K. Prevalence of vitamin D deficiency in Saudi Adults. Saudi Med. J. 2013, 34, 814–818. [Google Scholar]
- Farhat, K.H.; Arafa, M.A.; Rabah, D.M.; Amin, H.S.; Ibrahim, N.K. Vitamin D status and its correlates in Saudi male population. BMC Public Health 2019, 19, 211. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.N.; Alkhenizan, A.H.; El Shaker, M.; Raef, H.; Gabr, A. Increasing trends and significance of hypovitaminosis D: A population-based study in the kingdom of Saudi Arabia. Arch. Osteoporos. 2014, 9, 190. [Google Scholar] [CrossRef] [PubMed]
- Carlberg, C. Molecular approaches for optimizing vitamin D supplementation. Vitam. Horm. 2016, 100, 255–271. [Google Scholar] [CrossRef]
- Carlberg, C.; Raunio, H. From pharmacogenomics to integrated personal omics profiling: A gap in implementation into healthcare. Per. Med. 2014, 11, 625–629. [Google Scholar] [CrossRef]
- Levine, B.S.; Rodríguez, M.; Felsenfeld, A.J. Serum calcium and bone: Effect of PTH, phosphate, vitamin D and uremia. Nefrologia 2014, 34, 658–669. [Google Scholar] [CrossRef]
- Kearns, M.D.; Binongo, J.N.G.; Watson, D.; Alvarez, J.A.; Lodin, D.; Ziegler, T.R.; Tangpricha, V. The effect of a single, large bolus of vitamin D in healthy adults over the winter and following year: A randomized, double-blind, placebo-controlled trial. Eur. J. Clin. Nutr. 2015, 69, 193–197. [Google Scholar] [CrossRef][Green Version]
- Laird, E.; McNulty, H.; Ward, M.; Hoey, L.; McSorley, E.; Wallace, J.M.W.; Carson, E.; Molloy, A.M.; Healy, M.; Casey, M.C.; et al. Vitamin D deficiency is associated with inflammation in older Irish adults. J. Clin. Endocrinol. Metab. 2014, 99, 1807–1815. [Google Scholar] [CrossRef]
- Hashemi, R.; Morshedi, M.; Jafarabadi, M.A.; Altafi, D.; Hosseini-Asl, S.S.; Rafie-Arefhosseini, S. Anti-Inflammatory effects of dietary vitamin D3 in patients with multiple sclerosis. Neurol. Genet. 2018, 4, e278. [Google Scholar] [CrossRef]
- Erta, M.; Quintana, A.; Hidalgo, J. Interleukin-6, a major cytokine in the central nervous system. Int. J. Biol. Sci. 2012, 8, 1254–1266. [Google Scholar] [CrossRef]
- Giulietti, A.; van Etten, E.; Overbergh, L.; Stoffels, K.; Bouillon, R.; Mathieu, C. Monocytes from type 2 diabetic patients have a pro-inflammatory profile. 1,25-dihydroxyvitamin D3 works as anti-inflammatory. Diabetes Res. Clin. Pract. 2007, 77, 47–57. [Google Scholar] [CrossRef]
- Grossmann, R.E.; Zughaier, S.M.; Liu, S.; Lyles, R.H.; Tangpricha, V. Impact of vitamin D supplementation on markers of inflammation in adults with cystic fibrosis hospitalized for a pulmonary exacerbation. Eur. J. Clin. Nutr. 2012, 66, 1072–1074. [Google Scholar] [CrossRef]
- Dadaei, T.; Safapoor, M.H.; Asadzadeh Aghdaei, H.; Balaii, H.; Pourhoseingholi, M.A.; Naderi, N.; Zojaji, H.; Azimzadeh, P.; Mohammadi, P.; Zali, M.R. Effect of vitamin D3 supplementation on TNF-α serum level and disease activity index in Iranian IBD patients. Gastroenterol. Hepatol. Bed Bench 2015, 8, 49–55. [Google Scholar]
- Borazan, A.; Üstün, H.; Cefle, A.; Sekitmez, N.; Yilmaz, A. Comparative efficacy of oral and intravenous calcitriol treatment in haemodialysis patients: Effects on serum biochemistry and cytokine levels. J. Int. Med. Res. 2003, 31, 489–496. [Google Scholar] [CrossRef]
- Dauletbaev, N.; Herscovitch, K.; Das, M.; Chen, H.; Bernier, J.; Matouk, E.; Bérubé, J.; Rousseau, S.; Lands, L.C. Down-regulation of IL-8 by high-dose vitamin D is specific to hyperinflammatory macrophages and involves mechanisms beyond up-regulation of DUSP1. Br. J. Pharmacol. 2015, 172, 4757–4771. [Google Scholar] [CrossRef]
- Zhang, Y.; Fang, F.; Tang, J.; Jia, L.; Feng, Y.; Xu, P.; Faramand, A. Association between vitamin D supplementation and mortality: Systematic review and meta-analysis. BMJ 2019, 366, 14673. [Google Scholar] [CrossRef]
- Hopkins, M.H.; Owen, J.; Ahearn, T.; Fedirko, V.; Flanders, W.D.; Jones, D.P.; Bostick, R.M. Effects of supplemental vitamin D and calcium on biomarkers of inflammation in colorectal adenoma patients: A randomized, controlled clinical trial. Cancer Prev. Res. 2011, 4, 1645–1654. [Google Scholar] [CrossRef]
- Jakobsen, J.; Andersen, E.A.W.; Christensen, T.; Andersen, R.; Bügel, S. Vitamin D vitamers affect vitamin D status differently in young healthy males. Nutrients 2017, 10, 12. [Google Scholar] [CrossRef]
- Azizieh, F.; Alyahya, K.; Raghupathy, R. Association between levels of vitamin D and inflammatory markers in healthy women. J. Inflamm. Res. 2016, 9, 51–57. [Google Scholar] [CrossRef]

| Mean | SD | Range | |
| Age (years) | 30.3 | ±6.2 | 18–53 |
| Age N (%) | All participants (n = 50) | ||
| 20 to less than 40 years | 47 (94) | ||
| 40 to less than 60 years | 3 (6) | ||
| 60 years | 0 (0) | ||
| Mean | SD | Range | |
| Height (cm) | 174.3 | ±6.2 | 160–188 |
| Body weight (kg) | 83.8 | ±15.2 | 53–121 |
| BMI (kg/m2) | 27.6 | ±4.7 | 20.1–39.1 |
| BMI classification | All participants (n = 50) | ||
| Underweight | 0 (0) | ||
| Normal (18.5–24.99) | 17 (34%) | ||
| Overweight (25–29.99) | 16 (32%) | ||
| Obese (>30) | 17 (34%) | ||
| Mean | SD | Range | |
| Waist circumference (cm) | 94.4 | ±14.8 | 59–126 |
| Hip circumference (cm) | 104.9 | ±15.7 | 39–128 |
| WHR | 0.91 | ±0.13 | 0.67–1.51 |
| Health status N (%) | All participants (n = 50) | ||
| Liver disease | 0 (0%) | ||
| Kidney diseases | 0 (0%) | ||
| Healthy | 50 (100%) | ||
| Taking any medicines N (%) | All participants (n = 50) | ||
| Yes | 0 (0) | ||
| No | 50 (100%) | ||
| Broken any bones N (%) | |||
| Yes | 35 (70%) | ||
| No | 15 (30%) | ||
| Taking any supplements N (%) | |||
| Minerals | 2 (4%) | ||
| Vitamin D | 0 (0%) | ||
| Ca2+ | 0 (0)% | ||
| Nothing | 48 (96%) | ||
| Awareness to vitamin D (if taking test of vitamin D concentration) N (%) | |||
| Yes | 15 (30%) | ||
| No | 35 (70%) | ||
| Sun exposure N (%) | |||
| Less than 5 min/day | 1 (2%) | ||
| 5–15 min/day | 7 (14%) | ||
| 15–30 min/day | 12 (24%) | ||
| More than 30 min/day | 30 (60%) | ||
| Exercise rate N (%) | |||
| Always | 15 (30%) | ||
| Usually | 30 (60%) | ||
| Rarely | 5 (10%) | ||
| Smoke status N (%) | |||
| Smoker | 19 (38%) | ||
| Non-smoker | 31 (62%) | ||
| Inflammatory Markers | Baseline, IL6 n = 50 | Day 1, IL6 n = 50 | Day 30, IL6 n = 50 | |||
| r | p | r | p | r | p | |
| IL8 | 0.50 ** | <0.01 | 0.51 ** | <0.01 | 0.54 ** | <0.01 |
| TNF | 0.64 ** | <0.01 | 0.56 ** | <0.01 | 0.03 | 0.82 |
| Inflammatory Markers | Baseline, IL8 n = 50 | Day 1, IL8 n = 50 | Day 30, IL8 n = 50 | |||
| r | p | r | p | r | p | |
| TNF | 0.50 ** | <0.01 | 0.43 ** | <0.01 | 0.05 | 0.74 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
AlGhamdi, S.A.; Enaibsi, N.N.; Alsufiani, H.M.; Alshaibi, H.F.; Khoja, S.O.; Carlberg, C. A Single Oral Vitamin D3 Bolus Reduces Inflammatory Markers in Healthy Saudi Males. Int. J. Mol. Sci. 2022, 23, 11992. https://doi.org/10.3390/ijms231911992
AlGhamdi SA, Enaibsi NN, Alsufiani HM, Alshaibi HF, Khoja SO, Carlberg C. A Single Oral Vitamin D3 Bolus Reduces Inflammatory Markers in Healthy Saudi Males. International Journal of Molecular Sciences. 2022; 23(19):11992. https://doi.org/10.3390/ijms231911992
Chicago/Turabian StyleAlGhamdi, Shareefa A., Nusaibah N. Enaibsi, Hadeil M. Alsufiani, Huda F. Alshaibi, Sawsan O. Khoja, and Carsten Carlberg. 2022. "A Single Oral Vitamin D3 Bolus Reduces Inflammatory Markers in Healthy Saudi Males" International Journal of Molecular Sciences 23, no. 19: 11992. https://doi.org/10.3390/ijms231911992
APA StyleAlGhamdi, S. A., Enaibsi, N. N., Alsufiani, H. M., Alshaibi, H. F., Khoja, S. O., & Carlberg, C. (2022). A Single Oral Vitamin D3 Bolus Reduces Inflammatory Markers in Healthy Saudi Males. International Journal of Molecular Sciences, 23(19), 11992. https://doi.org/10.3390/ijms231911992







