Portable Plasmonic Paper-Based Biosensor for Simple and Rapid Indirect Detection of CEACAM5 Biomarker via Metal-Enhanced Fluorescence
Abstract
1. Introduction
2. Results and Discussion
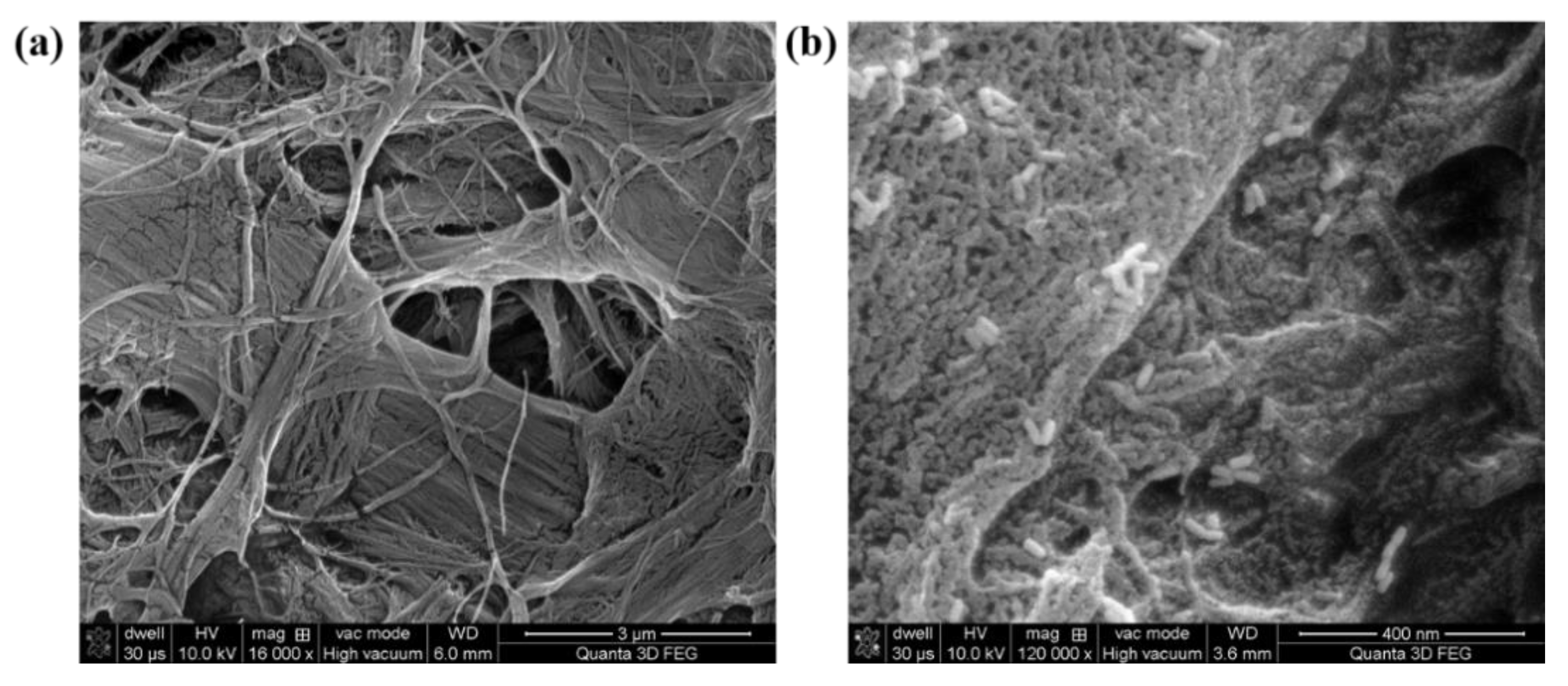
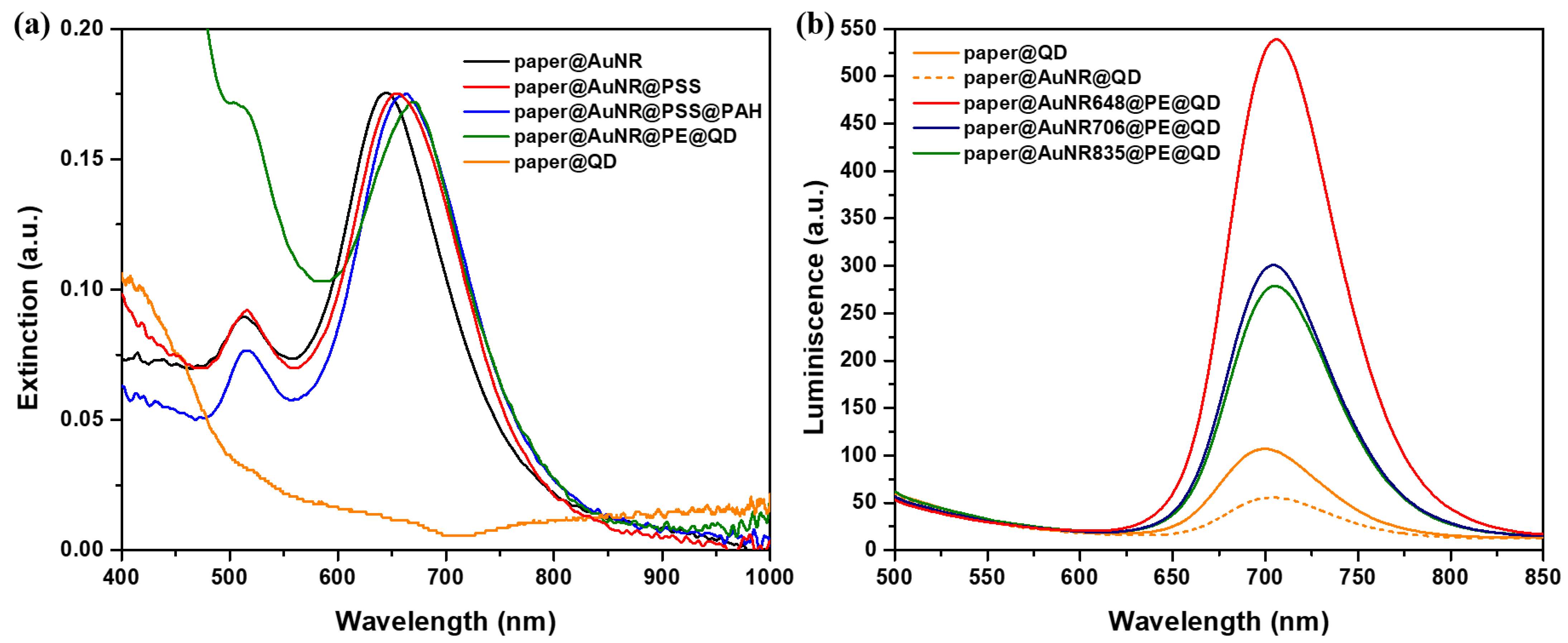
2.1. Optical and Morphological Characterization of the Designed Paper-Based Nanoplatforms
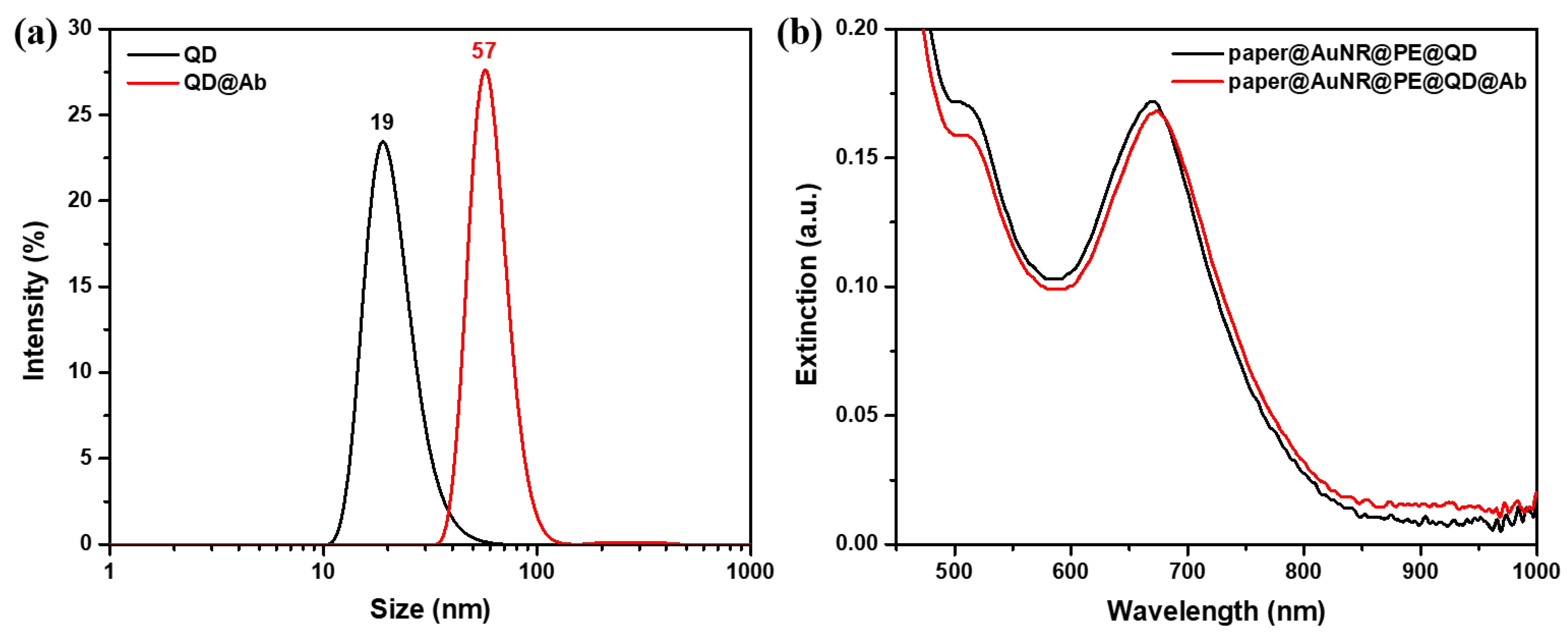
2.2. Characterization of the Active QD@Ab Complex
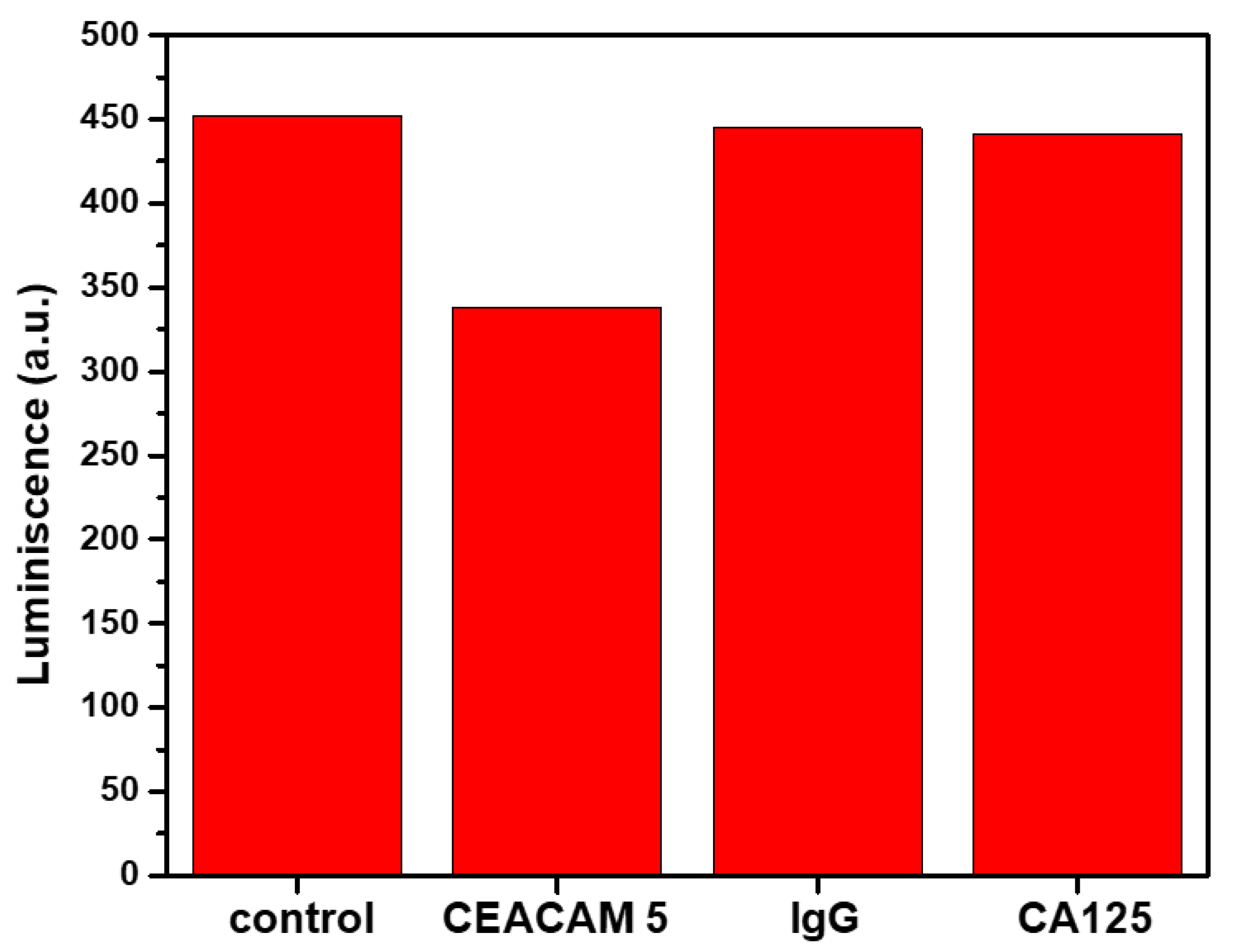
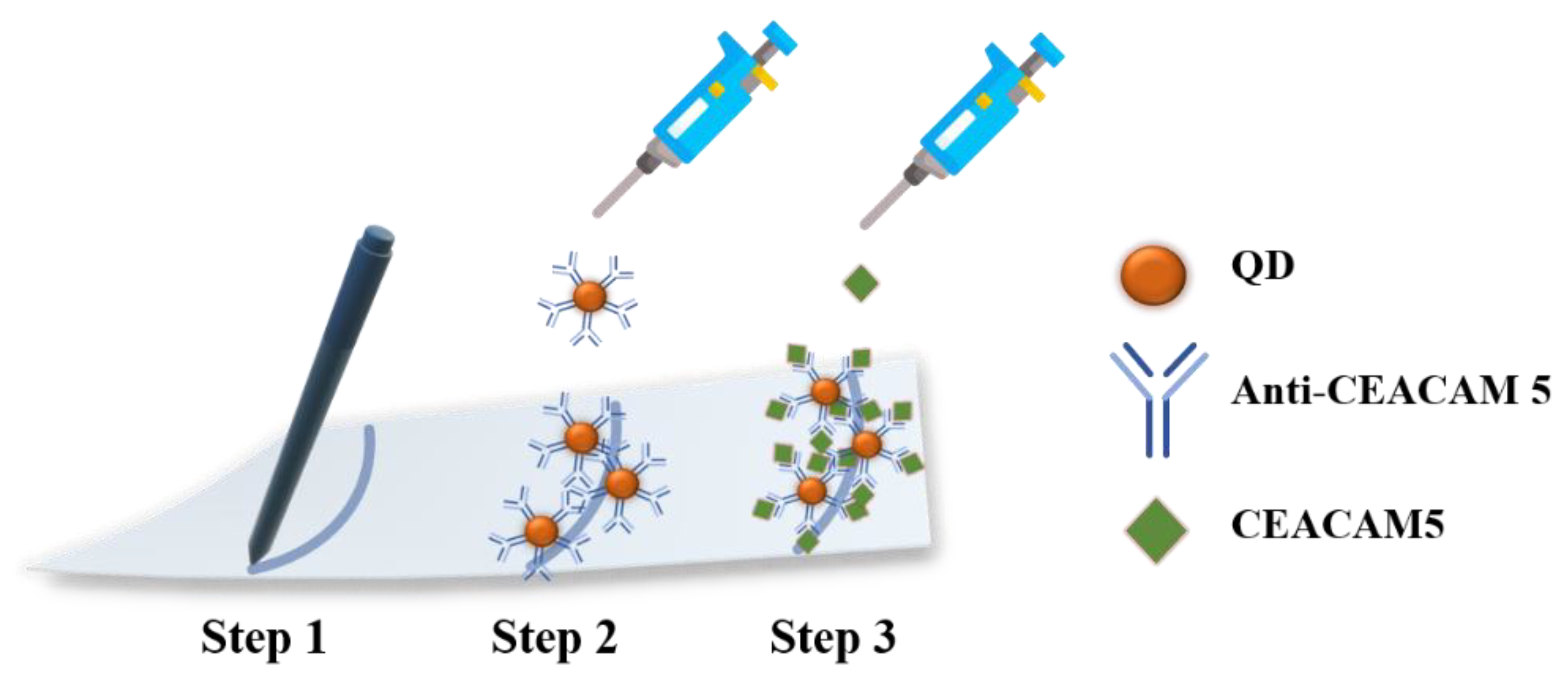
2.3. Quantitative Detection of CEACAM5 Biomarker via Indirect MEF Sensing
3. Materials and Methods
3.1. Materials
3.2. Colloidal Gold Nanorods Synthesis
3.3. Preparation of the Plasmonic Nanoplatforms
3.4. Biosensing Protocol
3.5. Characterization Techniques
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Crosby, D.; Bhatia, S.; Brindle, K.M.; Coussens, L.M.; Dive, C.; Emberton, M.; Esener, S.; Fitzgerald, R.C.; Gambhir, S.S.; Kuhn, P.; et al. Early Detection of Cancer. Science 2022, 375, eaay9040. [Google Scholar] [CrossRef] [PubMed]
- Pashayan, N.; Pharoah, P.D.P. The Challenge of Early Detection in Cancer. Science 2020, 368, 589–590. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, M.C.C.G.; Rodrigues, L.R.; Moreira, F.T.C.; Sales, M.G.F. Colorimetric Paper-Based Sensors against Cancer Biomarkers. Sensors 2022, 22, 3221. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, T.; de la Guardia, M.; Baradaran, B. Lateral Flow Assays towards Point-of-Care Cancer Detection: A Review of Current Progress and Future Trends. TrAC Trends Anal. Chem. 2020, 125, 115842. [Google Scholar] [CrossRef]
- Gubala, V.; Harris, L.F.; Ricco, A.J.; Tan, M.X.; Williams, D.E. Point of Care Diagnostics: Status and Future. Anal. Chem. 2012, 84, 487–515. [Google Scholar] [CrossRef]
- Jin, C.; Wu, Z.; Molinski, J.H.; Zhou, J.; Ren, Y.; Zhang, J.X.J. Plasmonic Nanosensors for Point-of-Care Biomarker Detection. Mater. Today Bio 2022, 14, 100263. [Google Scholar] [CrossRef]
- Wu, L.; Qu, X. Cancer Biomarker Detection: Recent Achievements and Challenges. Chem. Soc. Rev. 2015, 44, 2963–2997. [Google Scholar] [CrossRef]
- Kuswandi, B.; Ensafi, A.A. Perspective—Paper-Based Biosensors: Trending Topic in Clinical Diagnostics Developments and Commercialization. J. Electrochem. Soc. 2019, 167, 037509. [Google Scholar] [CrossRef]
- Nie, J.; Zhang, Y.; Lin, L.; Zhou, C.; Li, S.; Zhang, L.; Li, J. Low-Cost Fabrication of Paper-Based Microfluidic Devices by One-Step Plotting. Anal. Chem. 2012, 84, 6331–6335. [Google Scholar] [CrossRef]
- Yaraki, M.T.; Tan, Y.N. Metal Nanoparticles-Enhanced Biosensors: Synthesis, Design and Applications in Fluorescence Enhancement and Surface-enhanced Raman Scattering. Chem. Asian J. 2020, 15, 3180–3208. [Google Scholar] [CrossRef]
- Becker, J.; Trügler, A.; Jakab, A.; Hohenester, U.; Sönnichsen, C. The Optimal Aspect Ratio of Gold Nanorods for Plasmonic Bio-Sensing. Plasmonics 2010, 5, 161–167. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, J.; Li, J. Construction of Plasmonic Nano-Biosensor-Based Devices for Point-of-Care Testing. Small Methods 2017, 1, 1700197. [Google Scholar] [CrossRef]
- Gong, X.; Cai, J.; Zhang, B.; Zhao, Q.; Piao, J.; Peng, W.; Gao, W.; Zhou, D.; Zhao, M.; Chang, J. A Review of Fluorescent Signal-Based Lateral Flow Immunochromatographic Strips. J. Mater. Chem. B 2017, 5, 5079–5091. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Luan, J.; Wang, Z.; Jiang, Q.; Gupta, R.; Cao, S.; Liu, K.-K.; Morrissey, J.J.; Kharasch, E.D.; Naik, R.R.; et al. Gold Nanorod Size-Dependent Fluorescence Enhancement for Ultrasensitive Fluoroimmunoassays. ACS Appl. Mater. Interfaces 2021, 13, 11414–11423. [Google Scholar] [CrossRef] [PubMed]
- You, P.-Y.; Li, F.-C.; Liu, M.-H.; Chan, Y.-H. Colorimetric and Fluorescent Dual-Mode Immunoassay Based on Plasmon-Enhanced Fluorescence of Polymer Dots for Detection of PSA in Whole Blood. ACS Appl. Mater. Interfaces 2019, 11, 9841–9849. [Google Scholar] [CrossRef]
- Zhong, W. Nanomaterials in Fluorescence-Based Biosensing. Anal. Bioanal. Chem. 2009, 394, 47–59. [Google Scholar] [CrossRef]
- Camacho, S.A.; Sobral-Filho, R.G.; Aoki, P.H.B.; Constantino, C.J.L.; Brolo, A.G. Immunoassay Quantification Using Surface-Enhanced Fluorescence (SEF) Tags. Analyst 2017, 142, 2717–2724. [Google Scholar] [CrossRef]
- Cui, Q.; He, F.; Li, L.; Möhwald, H. Controllable Metal-Enhanced Fluorescence in Organized Films and Colloidal System. Adv. Colloid Interface Sci. 2014, 207, 164–177. [Google Scholar] [CrossRef]
- Campu, A.; Lerouge, F.; Chateau, D.; Chaput, F.; Baldeck, P.; Parola, S.; Maniu, D.; Craciun, A.M.; Vulpoi, A.; Astilean, S.; et al. Gold NanoBipyramids Performing as Highly Sensitive Dual-Modal Optical Immunosensors. Anal. Chem. 2018, 90, 8567–8575. [Google Scholar] [CrossRef]
- Kochuveedu, S.T.; Son, T.; Lee, Y.; Lee, M.; Kim, D.; Kim, D.H. Revolutionizing the FRET-Based Light Emission in Core-Shell Nanostructures via Comprehensive Activity of Surface Plasmons. Sci. Rep. 2014, 4, 4735. [Google Scholar] [CrossRef]
- Petryayeva, E.; Algar, W.R. Proteolytic Assays on Quantum-Dot-Modified Paper Substrates Using Simple Optical Readout Platforms. Anal. Chem. 2013, 85, 8817–8825. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, R.; Naderi-Manesh, H.; Farzin, L.; Vaezi, Z.; Ayarri, N.; Samandari, L.; Shamsipur, M. Fluorescence Sensing and Imaging with Carbon-Based Quantum Dots for Early Diagnosis of Cancer: A Review. J. Pharm. Biomed. Anal. 2022, 212, 114628. [Google Scholar] [CrossRef] [PubMed]
- Sultangaziyev, A.; Akhmetova, A.; Kunushpayeva, Z.; Rapikov, A.; Filchakova, O.; Bukasov, R. Aluminum Foil as a Substrate for Metal Enhanced Fluorescence of Bacteria Labelled with Quantum Dots, Shows Very Large Enhancement and High Contrast. Sens. Bio-Sens. Res. 2020, 28, 100332. [Google Scholar] [CrossRef]
- Song, H.Y.; Wong, T.I.; Guo, S.; Deng, J.; Tan, C.; Gorelik, S.; Zhou, X. Nanoimprinted Thrombin Aptasensor with Picomolar Sensitivity Based on Plasmon Excited Quantum Dots. Sens. Actuators B Chem. 2015, 221, 207–216. [Google Scholar] [CrossRef]
- Sun, L.L.; Leo, Y.S.; Zhou, X.; Ng, W.; Wong, T.I.; Deng, J. Localized Surface Plasmon Resonance Based Point-of-Care System for Sepsis Diagnosis. Mater. Sci. Energy Technol. 2020, 3, 274–281. [Google Scholar] [CrossRef]
- Khunkitchai, N.; Nuchtavorn, N.; Rypar, T.; Vlcnovska, M.; Nejdl, L.; Vaculovicova, M.; Macka, M. UV-Light-Actuated in-Situ Preparation of Paper@ZnCd Quantum Dots for Paper-Based Enzymatic Nanoreactors. Chem. Eng. J. 2022, 428, 132508. [Google Scholar] [CrossRef]
- Durán, G.M.; Benavidez, T.E.; Ríos, Á.; García, C.D. Quantum Dot-Modified Paper-Based Assay for Glucose Screening. Microchim. Acta 2016, 183, 611–616. [Google Scholar] [CrossRef]
- De França, C.C.L.; Meneses, D.; Silva, A.C.A.; Dantas, N.O.; de Abreu, F.C.; Petroni, J.M.; Lucca, B.G. Development of Novel Paper-Based Electrochemical Device Modified with CdSe/CdS Magic-Sized Quantum Dots and Application for the Sensing of Dopamine. Electrochim. Acta 2021, 367, 137486. [Google Scholar] [CrossRef]
- Xiao, A.; Huang, Y.; Zheng, J.; Chen, P.; Guan, B.-O. An Optical Microfiber Biosensor for CEACAM5 Detection in Serum: Sensitization by a Nanosphere Interface. ACS Appl. Mater. Interfaces 2020, 12, 1799–1805. [Google Scholar] [CrossRef]
- Chen, H.; Shao, L.; Li, Q.; Wang, J. Gold Nanorods and Their Plasmonic Properties. Chem. Soc. Rev. 2013, 42, 2679–2724. [Google Scholar] [CrossRef]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose Nanocrystals: Chemistry, Self-Assembly, and Applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef] [PubMed]
- Kirk, K.A.; Othman, A.; Andreescu, S. Nanomaterial-Functionalized Cellulose: Design, Characterization and Analytical Applications. Anal. Sci. 2018, 34, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Bardhan, R.; Grady, N.K.; Cole, J.R.; Joshi, A.; Halas, N.J. Fluorescence Enhancement by Au Nanostructures: Nanoshells and Nanorods. ACS Nano 2009, 3, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Yun, B.J.; Kwon, J.E.; Lee, K.; Koh, W.-G. Highly Sensitive Metal-Enhanced Fluorescence Biosensor Prepared on Electrospun Fibers Decorated with Silica-Coated Silver Nanoparticles. Sens. Actuators B Chem. 2019, 284, 140–147. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, X.; Qian, W.; Yang, Q.; Qi, Y.; Chen, Y.; Wang, A. Quantum Dots-based Fluorescence Immunoassay for Detection of Tiamulin in Pork. J. Food Saf. 2021, 41, e12930. [Google Scholar] [CrossRef]
- Yao, J.; Xing, G.; Han, J.; Sun, Y.; Wang, F.; Deng, R.; Hu, X.; Zhang, G. Novel Fluoroimmunoassays for Detecting Ochratoxin A Using CdTe Quantum Dots. J. Biophotonics 2017, 10, 657–663. [Google Scholar] [CrossRef]
- Qdot Nanocrystals—Section 6.6–RO. Available online: https://www.thermofisher.com/tr/en/home/references/molecular-probes-the-handbook/ultrasensitive-detection-technology/qdot-nanocrystal-technology.html (accessed on 1 August 2022).
- Qdot® ITKTM Carboxyl Quantum Dots; Version 11; Thermo Fisher Scientific: Waltham, MA, USA, 2007.
- Blumenthal, R.D.; Leon, E.; Hansen, H.J.; Goldenberg, D.M. Expression Patterns of CEACAM5 and CEACAM6 in Primary and Metastatic Cancers. BMC Cancer 2007, 7, 2. [Google Scholar] [CrossRef]
- Liu, J.-N.; Wang, H.-B.; Zhou, C.-C.; Hu, S.-Y. CEACAM5 Has Different Expression Patterns in Gastric Non-Neoplastic and Neoplastic Lesions and Cytoplasmic Staining Is a Marker for Evaluation of Tumor Progression in Gastric Adenocarcinoma. Pathol. Res. Pract. 2014, 210, 686–693. [Google Scholar] [CrossRef]
- Topdagi, O.; Timuroglu, A. Evaluation of the Relationship between Carcinoembryonic Antigen and TNM Stage in Colorectal Cancer. Eurasian J. Med. 2018, 50, 96–98. [Google Scholar] [CrossRef]
- Bae, O.S. CEA Expressions in Colorectal Tumor. J. Korean Soc. Coloproctol. 2004, 20, 39–45. [Google Scholar]
- Semeniak, D.; Cruz, D.F.; Chilkoti, A.; Mikkelsen, M.H. Plasmonic Fluorescence Enhancement in Diagnostics for Clinical Tests at Point-of-Care: A Review of Recent Technologies. Adv. Mater. 2022, 2022, 2107986. [Google Scholar] [CrossRef]
- Jawad, Z.A.R.; Theodorou, I.G.; Jiao, L.R.; Xie, F. Highly Sensitive Plasmonic Detection of the Pancreatic Cancer Biomarker CA 19-9. Sci. Rep. 2017, 7, 14309. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Wang, L.; Zhang, R.; Sun, X.; Huang, L.; Su, H.; Wei, X.; Chen, C.-C.; Lou, J.; Dai, H.; et al. Diagnosis and Prognosis of Myocardial Infarction on a Plasmonic Chip. Nat. Commun. 2020, 11, 1654. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, W.; Yang, Q.; Gong, X.; Guo, W.; Dong, C.; Liu, J.; Xuan, L.; Chang, J. Rapid and Quantitative Detection of Prostate Specific Antigen with a Quantum Dot Nanobeads-Based Immunochromatography Test Strip. ACS Appl. Mater. Interfaces 2014, 6, 6406–6414. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, Y.; Wang, J.; Tang, Z.; Pounds, J.G.; Lin, Y. Rapid and Sensitive Detection of Protein Biomarker Using a Portable Fluorescence Biosensor Based on Quantum Dots and a Lateral Flow Test Strip. Anal. Chem. 2010, 82, 7008–7014. [Google Scholar] [CrossRef]
- Wang, C.; Hou, F.; Ma, Y. Simultaneous Quantitative Detection of Multiple Tumor Markers with a Rapid and Sensitive Multicolor Quantum Dots Based Immunochromatographic Test Strip. Biosens. Bioelectron. 2015, 68, 156–162. [Google Scholar] [CrossRef]
- Ferro, M.; De Cobelli, O.; Lucarelli, G.; Porreca, A.; Busetto, G.M.; Cantiello, F.; Damiano, R.; Autorino, R.; Musi, G.; Vartolomei, M.D.; et al. Beyond PSA: The Role of Prostate Health Index (Phi). Int. J. Mol. Sci. 2020, 21, 1184. [Google Scholar] [CrossRef]
- Rong, Z.; Bai, Z.; Li, J.; Tang, H.; Shen, T.; Wang, Q.; Wang, C.; Xiao, R.; Wang, S. Dual-Color Magnetic-Quantum Dot Nanobeads as Versatile Fluorescent Probes in Test Strip for Simultaneous Point-of-Care Detection of Free and Complexed Prostate-Specific Antigen. Biosens. Bioelectron. 2019, 145, 111719. [Google Scholar] [CrossRef]
- Li, J.; Lv, Y.; Li, N.; Wu, R.; Li, J.; You, J.; Shen, H.; Chen, X.; Li, L.S. Dual Protecting Encapsulation Synthesis of Ultrastable Quantum-Dot Nanobeads for Sensitive and Accurate Detection of Cardiac Biomarkers. Sens. Actuators B Chem. 2021, 344, 130275. [Google Scholar] [CrossRef]
- Nikoobakht, B.; El-Sayed, M.A. Preparation and Growth Mechanism of Gold Nanorods (NRs) Using Seed-Mediated Growth Method. Chem. Mater. 2003, 15, 1957–1962. [Google Scholar] [CrossRef]
- Dvorakova, V.; Cadkova, M.; Datinska, V.; Kleparnik, K.; Foret, F.; Bilkova, Z.; Korecka, L. An Advanced Conjugation Strategy for the Preparation of Quantum Dot-Antibody Immunoprobes. Anal. Methods 2017, 9, 1991–1997. [Google Scholar] [CrossRef]
- Shrivastava, A.; Gupta, V. Methods for the Determination of Limit of Detection and Limit of Quantitation of the Analytical Methods. Chron. Young Sci. 2011, 2, 21. [Google Scholar] [CrossRef]



| Excitation (nm) | Sample | τ1 (ns) | τ2 (ns) | ⟨τ⟩ (ns) | χ2 |
|---|---|---|---|---|---|
| 405 | QD | 15 ± 0.29 | 1.15 ± 0.01 | 14 ± 0.28 | 1.06 |
| QD@Ab | 16 ± 0.24 | 0.99 ± 0.01 | 15 ± 0.23 | 1.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Susu, L.; Vulpoi, A.; Astilean, S.; Focsan, M. Portable Plasmonic Paper-Based Biosensor for Simple and Rapid Indirect Detection of CEACAM5 Biomarker via Metal-Enhanced Fluorescence. Int. J. Mol. Sci. 2022, 23, 11982. https://doi.org/10.3390/ijms231911982
Susu L, Vulpoi A, Astilean S, Focsan M. Portable Plasmonic Paper-Based Biosensor for Simple and Rapid Indirect Detection of CEACAM5 Biomarker via Metal-Enhanced Fluorescence. International Journal of Molecular Sciences. 2022; 23(19):11982. https://doi.org/10.3390/ijms231911982
Chicago/Turabian StyleSusu, Laurentiu, Adriana Vulpoi, Simion Astilean, and Monica Focsan. 2022. "Portable Plasmonic Paper-Based Biosensor for Simple and Rapid Indirect Detection of CEACAM5 Biomarker via Metal-Enhanced Fluorescence" International Journal of Molecular Sciences 23, no. 19: 11982. https://doi.org/10.3390/ijms231911982
APA StyleSusu, L., Vulpoi, A., Astilean, S., & Focsan, M. (2022). Portable Plasmonic Paper-Based Biosensor for Simple and Rapid Indirect Detection of CEACAM5 Biomarker via Metal-Enhanced Fluorescence. International Journal of Molecular Sciences, 23(19), 11982. https://doi.org/10.3390/ijms231911982








