Staphylococcus aureus Keratitis in Taiwan: Genotyping, Antibiotic Susceptibility, and Clinical Features
Abstract
1. Introduction
2. Results
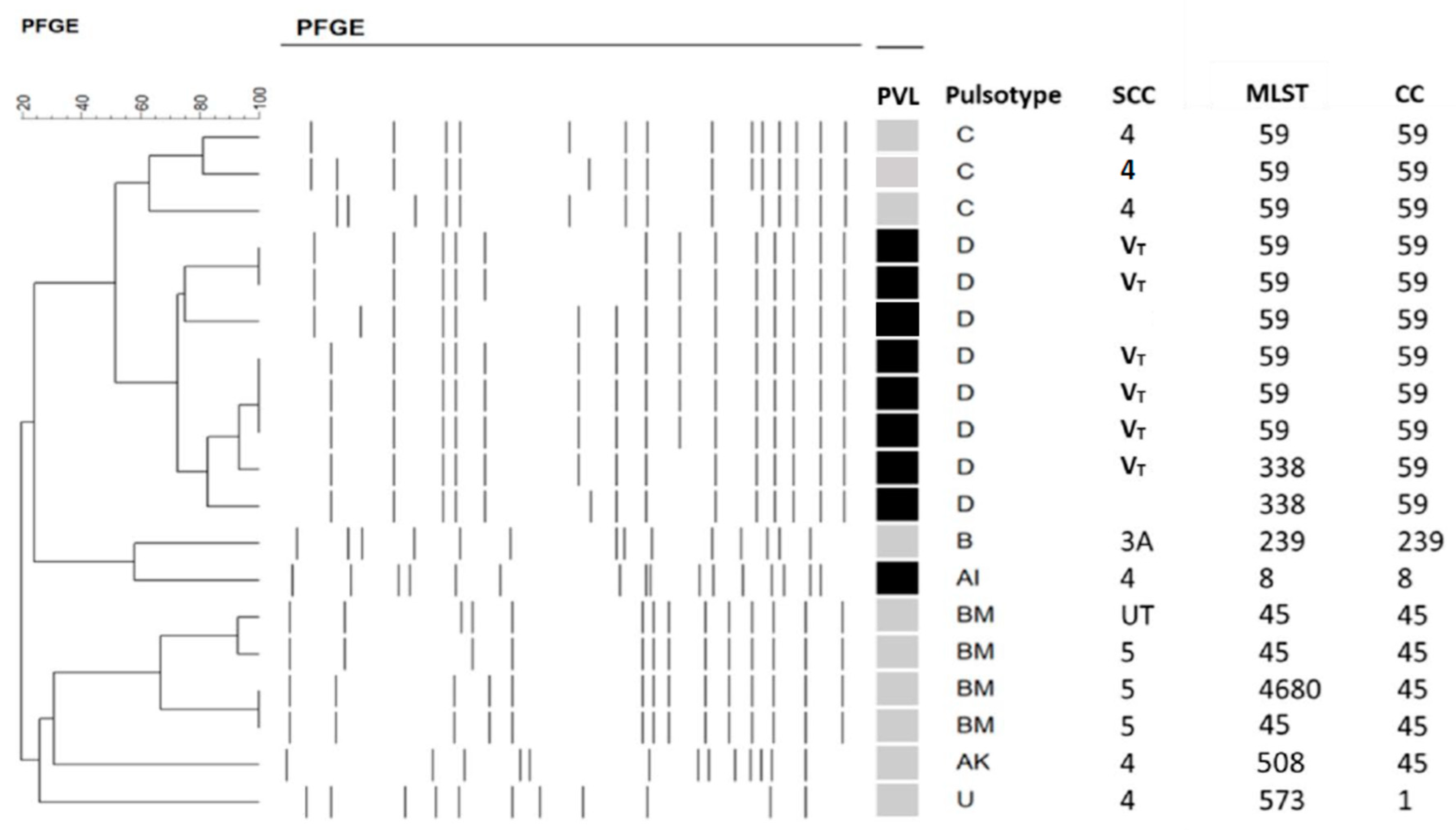
2.1. Molecular Typing of MRSA Isolates and PVL Presence
2.2. Antibiotics Susceptibility
2.3. Demographics and Predisposing Factors
2.4. Clinical Findings
2.5. Treatment and Outcome
3. Discussion
4. Material and Methods
4.1. Study Population and Data Collection
4.2. Drug Susceptibility Tests
4.3. Molecular Typing and Detection of PVL Gene
4.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Herold, B.C.; Immergluck, L.C.; Maranan, M.C.; Lauderdale, D.S.; Gaskin, R.E.; Boyle-Vavra, S.; Leitch, C.D.; Daum, R.S. Community-acquired methicillin-resistant Staphylococcus aureus in children with no identified predisposing risk. JAMA 1998, 279, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.T. Community-associated methicillin-resistant Staphylococcus aureus. Clin. Infect. Dis. 2005, 41 (Suppl. S4), S269–S272. [Google Scholar] [CrossRef] [PubMed]
- Petrillo, F.; Folliero, V.; Santella, B.; Franci, G.; Foglia, F.; Trotta, M.C.; Della Rocca, M.T.; Avitabile, T.; Gagliano, C.; Galdiero, M. Prevalence and Antibiotic Resistance Patterns of Ocular Bacterial Strains Isolated from Pediatric Patients in University Hospital of Campania “Luigi Vanvitelli,” Naples, Italy. Int. J. Microbiol. 2020, 2020, 8847812. [Google Scholar] [CrossRef]
- Hsiao, C.-H.; Chuang, C.-C.; Tan, H.-Y.; Ma, D.H.; Lin, K.-K.; Chang, C.-J.; Huang, Y.-C. Methicillin-resistant Staphylococcus aureus ocular infection: A 10-year hospital-based study. Ophthalmology 2012, 119, 522–527. [Google Scholar] [CrossRef]
- Chuang, C.-C.; Hsiao, C.-H.; Tan, H.-Y.; Ma, D.H.-K.; Lin, K.-K.; Chang, C.-J.; Huang, Y.-C. Staphylococcus aureus ocular infection: Methicillin-resistance, clinical features, and antibiotic susceptibilities. PLoS ONE 2012, 8, e42437. [Google Scholar] [CrossRef]
- Ong, S.J.; Huang, Y.-C.; Tan, H.-Y.; Ma, D.H.K.; Lin, H.-C.; Yeh, L.-K.; Chen, P.Y.F.; Chen, H.-C.; Chuang, C.-C.; Chang, C.-J.; et al. Staphylococcus aureus keratitis: A review of hospital cases. PLoS ONE 2013, 8, e80119. [Google Scholar] [CrossRef]
- Hsiao, C.H.; Ong, S.J.; Chuang, C.C.; Ma, D.H.; Huang, Y.C. A Comparison of Clinical Features between Community-Associated and Healthcare-Associated Methicillin-Resistant Staphylococcus aureus Keratitis. J. Ophthalmol. 2015, 2015, 923941. [Google Scholar] [CrossRef]
- Archer, G.L. Staphylococcus aureus: A well-armed pathogen. Clin. Infect. Dis. 1998, 26, 1179–1181. [Google Scholar] [CrossRef]
- O’Callaghan, R.J. The Pathogenesis of Staphylococcus aureus Eye Infections. Pathogens 2018, 7, 9. [Google Scholar] [CrossRef]
- Astley, R.; Miller, F.C.; Mursalin, M.H.; Coburn, P.S.; Callegan, M.C. An Eye on Staphylococcus aureus Toxins: Roles in Ocular Damage and Inflammation. Toxins 2019, 11, 356. [Google Scholar] [CrossRef]
- Lina, G.; Piémont, Y.; Godail-Gamot, F.; Bes, M.; Peter, M.-O.; Gauduchon, V.; Vandenesch, F.; Etienne, J. Involvement of Panton-Valentine leukocidin-producing Staphylococcus aureus in primary skin infections and pneumonia. Clin. Infect. Dis. 1999, 29, 1128–1132. [Google Scholar] [CrossRef] [PubMed]
- Diep, B.A.; Otto, M. The role of virulence determinants in community-associated MRSA pathogenesis. Trends Microbiol. 2008, 16, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Lo, W.T.; Wang, C.C. Panton-Valentine leukocidin in the pathogenesis of community-associated methicillin-resistant Staphylococcus aureus infection. Pediatr. Neonatol. 2011, 52, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Shallcross, L.J.; Fragaszy, E.; Johnson, A.M.; Hayward, A.C. The role of the Panton-Valentine leucocidin toxin in staphylococcal disease: A systematic review and meta-analysis. Lancet Infect. Dis. 2013, 13, 43–54. [Google Scholar] [CrossRef]
- Tromp, A.T.; van Strijp, J.A.G. Studying Staphylococcal Leukocidins: A Challenging Endeavor. Front. Microbiol. 2020, 11, 611. [Google Scholar] [CrossRef]
- Kaneko, J.; Kamio, Y. Bacterial two-component and hetero-heptameric pore-forming cytolytic toxins: Structures, pore-forming mechanism, and organization of the genes. Biosci. Biotechnol. Biochem. 2004, 68, 981–1003. [Google Scholar] [CrossRef]
- Boyle-Vavra, S.; Daum, R.S. Community-acquired methicillin-resistant Staphylococcus aureus: The role of Panton-Valentine leukocidin. Lab. Investig. 2007, 87, 3–9. [Google Scholar] [CrossRef]
- Saeed, K.; Gould, I.; Esposito, S.; Ahmad-Saeed, N.; Ahmed, S.S.; Alp, E.; Bal, A.M.; Bassetti, M.; Bonnet, E.; Chan, M.; et al. Panton-Valentine leukocidin-positive Staphylococcus aureus: A position statement from the International Society of Chemotherapy. Int. J. Antimicrob. Agents 2018, 51, 16–25. [Google Scholar] [CrossRef]
- Rutar, T.; Chambers, H.F.; Crawford, J.B.; Perdreau-Remington, F.; Zwick, O.M.; Karr, M.; Diehn, J.J.; Cockerham, K.P. Ophthalmic manifestations of infections caused by the USA300 clone of community-associated methicillin-resistant Staphylococcus aureus. Ophthalmology 2006, 113, 1455–1462. [Google Scholar] [CrossRef]
- Sueke, H.; Shankar, J.; Neal, T.; Winstanley, C.; Tuft, S.; Coates, R.; Horsburgh, M.J.; Kaye, S. lukSF-PV in Staphylococcus aureus keratitis isolates and association with clinical outcome. Investig. Ophthalmol. Vis. Sci. 2013, 54, 3410–3416. [Google Scholar] [CrossRef]
- Chen, Y.-L.; Kang, E.Y.-C.; Yeh, L.-K.; Ma, D.H.K.; Tan, H.-Y.; Chen, H.-C.; Hung, K.-H.; Huang, Y.-C.; Hsiao, C.-H. Clinical Features and Molecular Characteristics of Methicillin-Susceptible Staphylococcus aureus Ocular Infection in Taiwan. Antibiotics 2021, 10, 1445. [Google Scholar] [CrossRef] [PubMed]
- Seybold, U.; Kourbatova, E.V.; Johnson, J.G.; Halvosa, S.J.; Wang, Y.F.; King, M.D.; Ray, S.M.; Blumberg, H.M. Emergence of community-associated methicillin-resistant Staphylococcus aureus USA300 genotype as a major cause of health care-associated blood stream infections. Clin. Infect. Dis. 2006, 42, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.C.; Chen, C.J. Community-associated meticillin-resistant Staphylococcus aureus in children in Taiwan, 2000s. Int. J. Antimicrob. Agents 2011, 38, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Nimmo, G.R.; Coombs, G.; Pearson, J.C.; O’brien, F.G.; Christiansen, K.J.; Turnidge, J.D.; Gosbell, I.; Collignon, P.; McLaws, M.-L. Methicillin-resistant Staphylococcus aureus in the Australian community: An evolving epidemic. Med. J. Aust. 2006, 184, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Chini, V.; Petinaki, E.; Foka, A.; Paratiras, S.; Dimitracopoulos, G.; Spiliopoulou, I. Spread of Staphylococcus aureus clinical isolates carrying Panton-Valentine leukocidin genes during a 3-year period in Greece. Clin. Microbiol. Infect. 2006, 12, 29–34. [Google Scholar] [CrossRef]
- Dar, J.A.; Thoker, M.A.; Khan, J.A.; Ali, A.; Khan, M.A.; Rizwan, M.; Bhat, K.H.; Dar, M.J.; Ahmed, N.; Ahmad, S. Molecular epidemiology of clinical and carrier strains of methicillin resistant Staphylococcus aureus (MRSA) in the hospital settings of north India. Ann. Clin. Microbiol. Antimicrob. 2006, 5, 22. [Google Scholar] [CrossRef]
- Huang, Y.C.; Ho, C.F.; Chen, C.J.; Su, L.H.; Lin, T.Y. Comparative molecular analysis of community-associated and healthcare-associated methicillin-resistant Staphylococcus aureus isolates from children in northern Taiwan. Clin. Microbiol. Infect. 2008, 14, 1167–1172. [Google Scholar] [CrossRef]
- Huang, Y.C.; Chen, C.J.; Lauderdale, T.Y. Detection, spread and phylogeny of meticillin-resistant Staphylococcus aureus sequence type 45 in Taiwan. Microb Genom. 2021, 7, 000555. [Google Scholar] [CrossRef]
- Huang, Y.C.; Chen, C.J.; Kuo, C.C.; Lu, M.C. Emergence, transmission and phylogeny of meticillin-resistant Staphylococcus aureus sequence type 8 (USA300) in Taiwan. J. Hosp. Infect. 2018, 100, 355–358. [Google Scholar] [CrossRef]
- Alexandrakis, G.; Alfonso, E.C.; Miller, D. Shifting trends in bacterial keratitis in south Florida and emerging resistance to fluoroquinolones. Ophthalmology 2000, 107, 1497–1502. [Google Scholar] [CrossRef]
- Goldstein, M.H.; Kowalski, R.P.; Gordon, Y.J. Emerging fluoroquinolone resistance in bacterial keratitis: A 5-year review. Ophthalmology 1999, 106, 1313–1318. [Google Scholar] [CrossRef]
- Marangon, F.B.; Miller, D.; Muallem, M.S.; Romano, A.C.; Alfonso, E.C. Ciprofloxacin and levofloxacin resistance among methicillin-sensitive Staphylococcus aureus isolates from keratitis and conjunctivitis. Am. J. Ophthalmol. 2004, 137, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Asbell, P.A.; Colby, K.A.; Deng, S.; McDonnell, P.; Meisler, D.M.; Raizman, M.B.; Sheppard, J.D.; Sahm, D.F. Ocular TRUST: Nationwide antimicrobial susceptibility patterns in ocular isolates. Am. J. Ophthalmol. 2008, 145, 951–958. [Google Scholar] [CrossRef]
- Haas, W.; Pillar, C.M.; Torres, M.; Morris, T.W.; Sahm, D.F. Monitoring antibiotic resistance in ocular microorganisms: Results from the Antibiotic Resistance Monitoring in Ocular micRorganisms (ARMOR) 2009 surveillance study. Am. J. Ophthalmol. 2011, 152, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.K.; Melton, R.; Asbell, P.A. Antibiotic resistance among ocular pathogens: Current trends from the ARMOR surveillance study (2009–2016). Clin. Optom. 2019, 11, 15–26. [Google Scholar] [CrossRef]
- Zaidi, T.; Zaidi, T.; Yoong, P.; Pier, G.B. Staphylococcus aureus corneal infections: Effect of the Panton-Valentine leukocidin (PVL) and antibody to PVL on virulence and pathology. Invest. Ophthalmol. Vis. Sci. 2013, 54, 4430–4438. [Google Scholar] [CrossRef] [PubMed]
- Day, N.P. Panton-Valentine leucocidin and staphylococcal disease. Lancet Infect. Dis. 2013, 13, 5–6. [Google Scholar] [CrossRef]
- Huang, Y.-C.; Su, L.-H.; Chen, C.-J.; Lin, T.-Y. Nasal carriage of methicillin-resistant Staphylococcus aureus in school children without identifiable risk factors in northern taiwan. Pediatr. Infect. Dis. J. 2005, 24, 276–278. [Google Scholar] [CrossRef]
- Huang, Y.-C.; Su, L.-H.; Wu, T.-L.; Lin, T.-Y. Changing molecular epidemiology of methicillin-resistant Staphylococcus aureus bloodstream isolates from a teaching hospital in Northern Taiwan. J. Clin. Microbiol. 2006, 44, 2268–2270. [Google Scholar] [CrossRef][Green Version]
- Chen, F.-J.; Lauderdale, T.-L.; Huang, I.-W.; Lo, H.-J.; Lai, J.-F.; Wang, H.-Y.; Shiau, Y.-R.; Chen, P.-C.; Ito, T.; Hiramatsu, K. Methicillin-resistant Staphylococcus aureus in Taiwan. Emerg. Infect. Dis. 2005, 11, 1760–1763. [Google Scholar] [CrossRef]
- Chen, C.J.; Unger, C.; Hoffmann, W.; Lindsay, J.A.; Huang, Y.C.; Gotz, F. Characterization and comparison of 2 distinct epidemic community-associated methicillin-resistant Staphylococcus aureus clones of ST59 lineage. PLoS ONE 2013, 8, e63210. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Guo, Y.; Chu, X. In Vitro generation of Panton-Valentine leukocidin (PVL) in clinical Methicillin-Resistant Staphylococcus aureus (MRSA) and its correlation with PVL variant, clonal complex, infection type. Sci. Rep. 2018, 8, 7696. [Google Scholar] [CrossRef] [PubMed]
- Minnesota Department of Health. Community-Associated Methicillin-Resistant Staphylococcus aureus in Minnesota. Dis. Control. Newsl. 2004, 32, 61–72. [Google Scholar]
- Scott, I.U.; Schein, O.D.; West, S.; Bandeen-Roche, K.; Enger, C.; Folstein, M.F. Functional status and quality of life measurement among ophthalmic patients. Arch. Ophthalmol. 1994, 112, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.C.; Su, L.H.; Wu, T.L.; Liu, C.E.; Young, T.G.; Chen, P.Y.; Hseuh, P.R.; Lin, T.Y. Molecular epidemiology of clinical isolates of methicillin-resistant Staphylococcus aureus in Taiwan. J. Clin. Microbiol. 2004, 42, 307–310. [Google Scholar] [CrossRef]
- Tenover, F.C.; Arbeit, R.D.; Goering, R.V.; Mickelsen, P.A.; Murray, B.E.; Persing, D.H.; Swaminathan, B. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: Criteria for bacterial strain typing. J. Clin. Microbiol. 1995, 33, 2233–2239. [Google Scholar] [CrossRef]
- Enright, M.C.; Day, N.P.; Davies, C.E.; Peacock, S.J.; Spratt, B.G. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J. Clin. Microbiol. 2000, 38, 1008–1015. [Google Scholar] [CrossRef]
- Oliveira, D.C.; de Lencastre, H. Multiplex PCR strategy for rapid identification of structural types and variants of the mec element in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2002, 46, 2155–2161. [Google Scholar] [CrossRef]
| Antibiotics | MRSA (n = 17) No.a (%) | PVL (+) MRSA (n = 7) No. (%) | PVL (−) MRSA (n = 10) No. (%) | MSSA (n = 32) No. (%) | PVL (+) MSSA (n = 2) No. (%) | PVL (−) MSSA (n = 30) No. (%) |
|---|---|---|---|---|---|---|
| Clindamycin | 7 (41.2) | 1 (14.3) | 6 (60.0) | 32 (100) | 2 (100) | 30 (100) |
| Erythromycin | 5 (29.4) | 0 (0) | 5 (50.0) | 32 (100) | 2 (100) | 30 (100) |
| TMP-SMX | 16 (94.1) | 7 (100) | 9 (90.0) | 32 (100) | 2 (100) | 30 (100) |
| Teicoplanin | 17 (100) | 7 (100) | 10 (100) | 32 (100) | 2 (100) | 30 (100) |
| Tigecycline | 17 (100) | 7 (100) | 10 (100) | 32 (100) | 2 (100) | 30 (100) |
| Vancomycin | 17 (100) | 7 (100) | 10 (100) | 32 (100) | 2 (100) | 30 (100) |
| Ciprofloxacin | 10 (58.8) | 6 (85.7) | 4 (40.0) | 32 (100) | 2 (100) | 30 (100) |
| Levofloxacin | 11 (64.7) | 6 (85.7) | 5 (50.0) | 32 (100) | 2 (100) | 30 (100) |
| Gatifloxacin | 11 (64.7) | 6 (85.7) | 5 (50.0) | 32 (100) | 2 (100) | 30 (100) |
| Moxifloxacin | 11 (64.7) | 6 (85.7) | 5 (50.0) | 32 (100) | 2 (100) | 30 (100) |
| Characteristics | MRSA | PVL (+) MRSA | PVL (−) MRSA | MSSA | PVL (+) MSSA | PVL (−) MSSA |
|---|---|---|---|---|---|---|
| (n = 17) | (n = 7) | (n = 10) | (n = 32) | (n = 2) | (n = 30) | |
| Age: median (range) | 49 (2–93) | 46 (8–93) | 53 (2–88) | 52 (6–88) | 43.5 (18–69) | 52 (6–88) |
| Sex: M/F | 9/8 | 4/3 | 5/5 | 17/15 | 0/2 | 17/13 |
| Community associated/ | 9 (52.9)/ | 4 (57.1)/ | 5 (50)/ | 25 (78.1)/ | 1 (50)/ | 24 (80)/ |
| Healthcare-associated: n. (%) | 8 (47.1) | 3 (42.9) | 5 (50) | 7 (21.9) | 1 (50) | 6 (20) |
| Local factors: n (%) a | ||||||
| Contact lens wear | 4 (23.5) | 1 (14.3) | 3 (30) | 10 (31.3) | 1 (50) | 9 (30) |
| Trauma | 1 (5.9) | 0 (0) | 1 (10) | 3 (9.4) | 0 (0) | 3 (10) |
| Ocular surface disease | 11 (64.7) | 4 (57.1) | 7 (70) | 18 (56.3) | 1 (50) | 17 (56.7) |
| Previous ocular surgery | 11 (64.7) | 4 (57.1) | 7 (70) | 16 (50) | 1 (50) | 15 (50) |
| Usage of topical antibiotics/immunosuppressants | 2 (11.8) | 0 (0) | 2 (20) | 3 (9.4) | 0 (0) | 3 (10) |
| Systemic factors: n (%) | ||||||
| Systemic comorbidities | 8 (47.1) | 3 (42.9) | 5 (50) | 11 (34.4) | 1 (50) | 10 (33.3) |
| Immunosuppressant | 3 (17.6) | 1 (14.3) | 2 (20) | 2 (6.3) | 1 (50) | 1 (3.3) |
| Systemic antibiotics | 3 (17.6) | 1 (14.3) | 2 (20) | 0 (0) | 0 (0) | 0 (0) |
| Clinical Findings | MRSA | PVL (+) MRSA | PVL (−) MRSA | MSSA | PVL (+) MSSA | PVL (−) MSSA |
|---|---|---|---|---|---|---|
| (n = 17) | (n = 7) | (n = 10) | (n = 32) | (n = 2) | (n = 30) | |
| Location: a No. (%) | ||||||
| Central | 9 (52.9) | 4 (57.1) | 5 (50) | 13 (40.6) | 1 (50) | 12 (40) |
| Paracentral | 7 (41.2) | 2 (28.6) | 5 (50) | 10 (31.3) | 1 (50) | 9 (30) |
| Peripheral | 1 (5.9) | 1 (14.3) | 0 (0) | 9 (28.1) | 0 (0) | 9 (30) |
| Infiltration size (mm): No. (%) | ||||||
| Small (<2) | 7 (41.2) | 3 (42.9) | 4 (40) | 19 (59.4) | 0 (0) | 19 (63.3) |
| Medium (2~6) | 8 (47.1) | 4 (57.1) | 4 (40) | 11 (34.4) | 2 (100) | 9 (30) |
| Large (>6) | 2 (11.8) | 0 (0) | 2 (20) | 2 (6.3) | 0 (0) | 2 (6.7) |
| Hypopyon | 2 (11.8) | 1 (14.3) | 1 (10) | 8 (25) | 1 (50) | 7 (23.3) |
| MRSA | PVL (+) MRSA | PVL (−) MRSA | MSSA | PVL (+) MSSA | PVL (−) MSSA | |
|---|---|---|---|---|---|---|
| (n = 17) | (n = 7) | (n = 10) | (n = 32) | (n = 2) | (n = 30) | |
| Modification of antibiotics: n (%) | 5 (29.4) | 3 (42.9) | 2 (20) | 10 (31.3) | 1 (50) | 9 (30) |
| Surgical intervention: n (%) | 3 (17.6) | 2 (28.6) | 1 (10) | 4 (12.5) | 0 (0) | 4 (13.3) |
| Admission: n (%) | 6 (35.3) | 4 (57.1) | 2 (20) | 15 (46.9) | 2 (100) | 13 (43.3) |
| Healing time a (days) | 21.3 ± 33.0 | 17.0 ± 15.3 | 24.4 ± 42.6 | 15.1 ± 11.7 | 12.5 ± 0.7 | 16.4 ± 12.2 |
| mean + standard deviation | ||||||
| Healing time (days)/ulcer area median (range) | 2.11 (0.12–30) | 3.6 (1.5–4) | 2 (0.12–30) | 1.82 (0.4–20) | 6.29 (0.58–12) | 2.19 (0.4–20) |
| VA (LogMAR): median (range) | ||||||
| At presentation | 2.3 (0.2–3.2) | 1.4 (0.2–3.2) | 2.3 (0.2–3.2) | 2.3 (0–3.2) | 2.3 (2.3–2.3) | 1.4 (0–3.2) |
| After treatment | 2.3 (0.2–3.2) | 1.5 (0.2–3.2) | 2.3 (0.2–3.2) | 1.5 (0–3.2) | 1.4 (0.5–2.3) | 1.0 (0–3.2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsiao, C.-H.; Kang, E.Y.-C.; Yeh, L.-K.; Ma, D.H.K.; Chen, H.-C.; Hung, K.-H.; Huang, Y.-C. Staphylococcus aureus Keratitis in Taiwan: Genotyping, Antibiotic Susceptibility, and Clinical Features. Int. J. Mol. Sci. 2022, 23, 11703. https://doi.org/10.3390/ijms231911703
Hsiao C-H, Kang EY-C, Yeh L-K, Ma DHK, Chen H-C, Hung K-H, Huang Y-C. Staphylococcus aureus Keratitis in Taiwan: Genotyping, Antibiotic Susceptibility, and Clinical Features. International Journal of Molecular Sciences. 2022; 23(19):11703. https://doi.org/10.3390/ijms231911703
Chicago/Turabian StyleHsiao, Ching-Hsi, Eugene Yu-Chuan Kang, Lung-Kun Yeh, David H. K. Ma, Hung-Chi Chen, Kuo-Hsuan Hung, and Yhu-Chering Huang. 2022. "Staphylococcus aureus Keratitis in Taiwan: Genotyping, Antibiotic Susceptibility, and Clinical Features" International Journal of Molecular Sciences 23, no. 19: 11703. https://doi.org/10.3390/ijms231911703
APA StyleHsiao, C.-H., Kang, E. Y.-C., Yeh, L.-K., Ma, D. H. K., Chen, H.-C., Hung, K.-H., & Huang, Y.-C. (2022). Staphylococcus aureus Keratitis in Taiwan: Genotyping, Antibiotic Susceptibility, and Clinical Features. International Journal of Molecular Sciences, 23(19), 11703. https://doi.org/10.3390/ijms231911703








