Molecular Research of Lipid Peroxidation and Antioxidant Enzyme Activity of Comamonas testosteroni Bacterial Cells under the Hexachlorobenzene Impact
Abstract
:1. Introduction
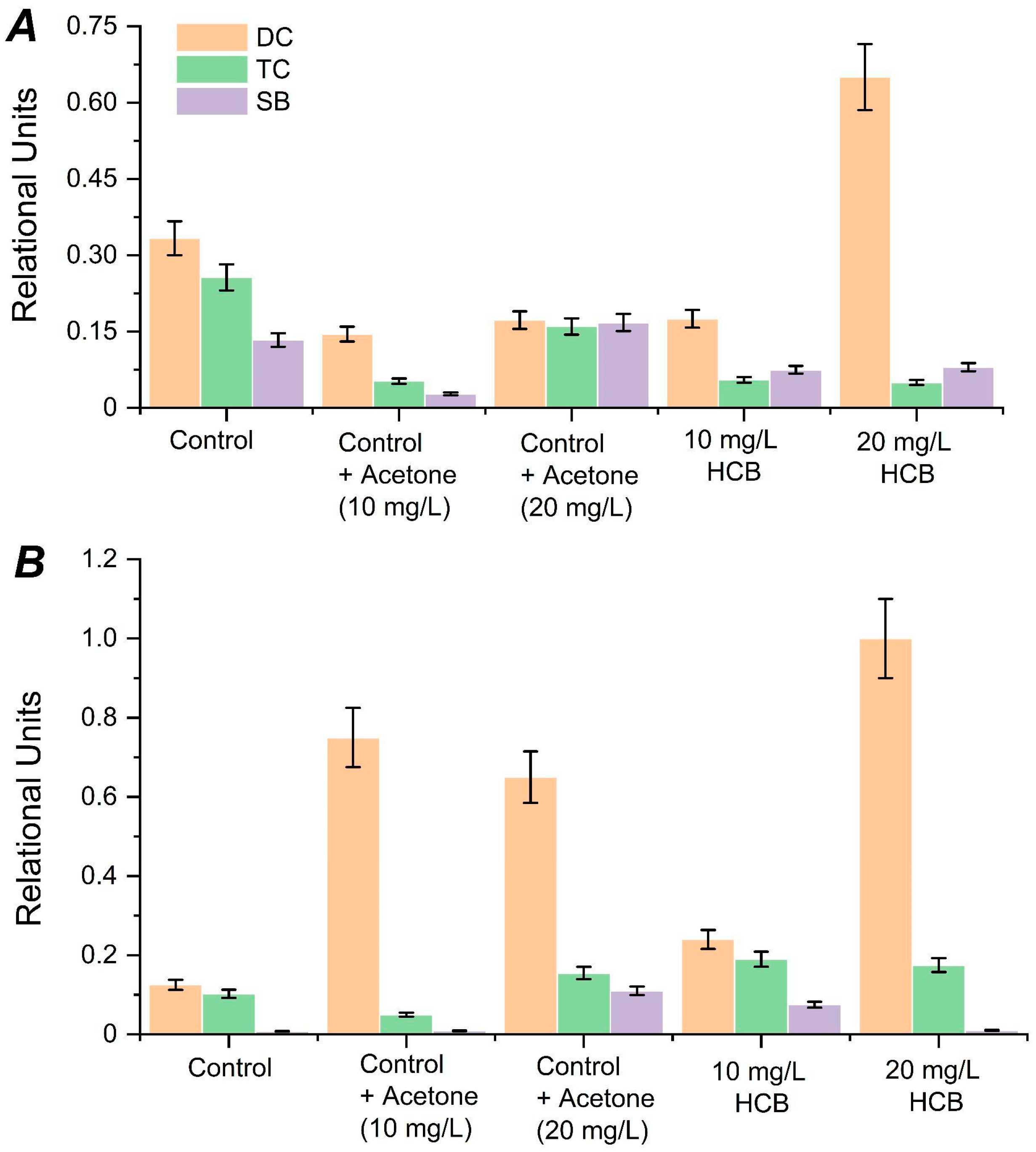
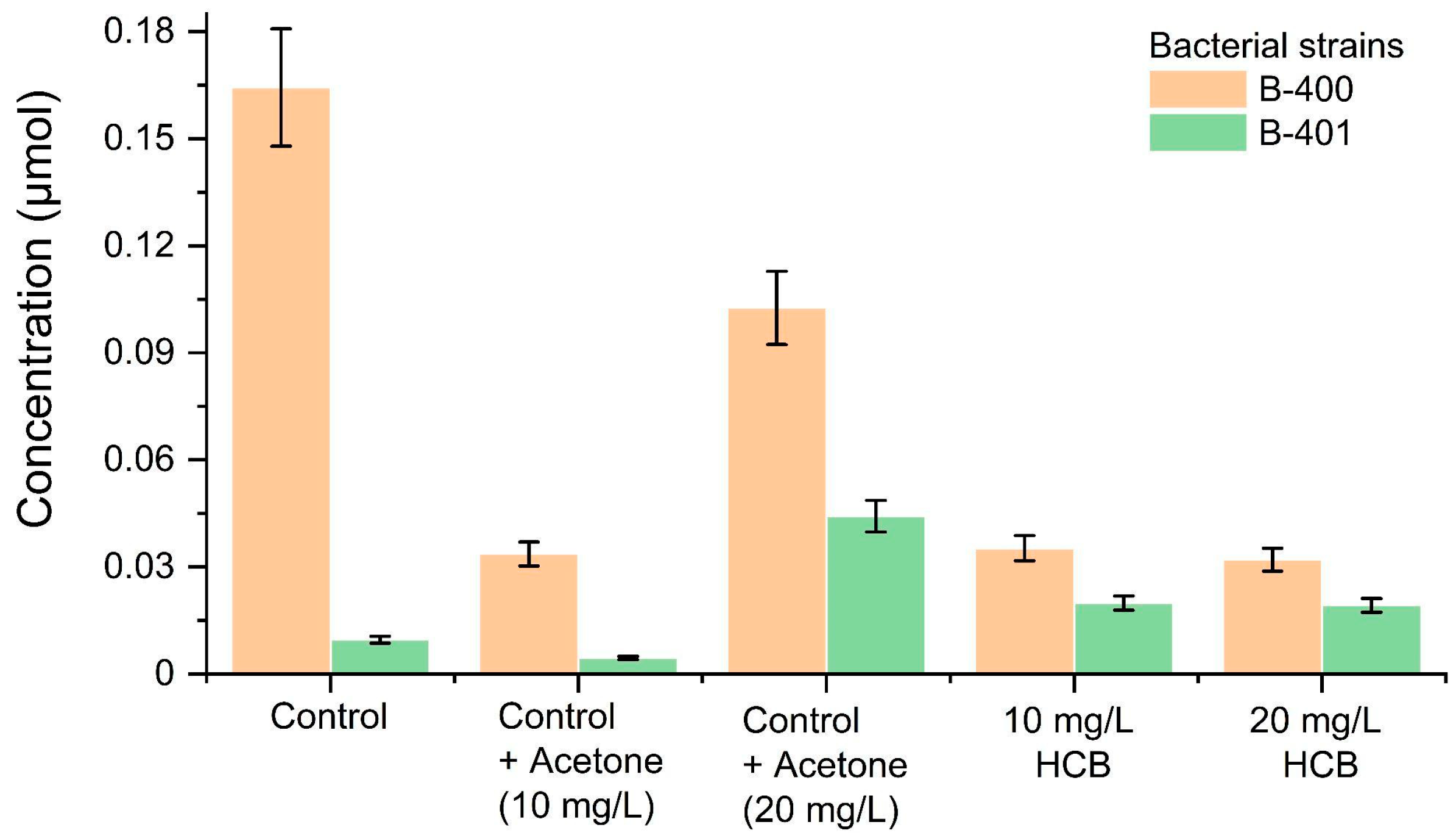
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HCB | hexachlorobenzene |
| MDA | malondialdehyde |
| LP | lipid peroxidation |
| DC | diene conjugates |
| TC | trine conjugates |
| SB | Schiff bases |
| LB | Luria-Bertrani medium |
| ABTS | 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic) acid |
References
- Lipuma, J.J.; Currie, B.J.; Peacock, S.J.; Vandamme, P.A.R. Burkholderia, Stenotrophomonas, Ralstonia, Cupriavidus, Pandoraea, Brevundimonas, Comamonas, Delftia and Acidovorax. In Manual of Clinical Microbiology; Jorgensen, J.H., Carroll, K.C., Funke, G., Pfaller, M.A., Landry, M.L., Richter, S.S., Warnock, D.W., Eds.; ASM Press: Washington, DC, USA, 2015; pp. 791–812. ISBN 978-1-68367-280-7. [Google Scholar]
- Tamaoka, J.; Ha, D.-M.; Komagata, K. Reclassification of Pseudomonas Acidovorans Den Dooren de Jong 1926 and Pseudomonas Testosteroni Marcus and Talalay 1956 as Comamonas Acidovorans Comb. Nov. and Comamonas Testosteroni Comb. Nov., with an Emended Description of the Genus Comamonas. Int. J. Syst. Bacteriol. 1987, 37, 52–59. [Google Scholar] [CrossRef]
- Lu, Q.; Sun, X.; Jiang, Z.; Cui, Y.; Li, X.; Cui, J. Effects of Comamonas Testosteroni on Dissipation of Polycyclic Aromatic Hydrocarbons and the Response of Endogenous Bacteria for Soil Bioremediation. Environ. Sci. Pollut. Res. Int. 2022. [Google Scholar] [CrossRef] [PubMed]
- Vítková, M.; Dercová, K.; Molnárová, J.; Tóthová, L.; Polek, B.; Godočíková, J. The Effect of Lignite and Comamonas Testosteroni on Pentachlorophenol Biodegradation and Soil Ecotoxicity. Water Air Soil Pollut. 2011, 218, 145–155. [Google Scholar] [CrossRef]
- Nguyen, O.T.; Ha, D.D. Degradation of Chlorotoluenes and Chlorobenzenes by the Dual-Species Biofilm of Comamonas Testosteroni Strain KT5 and Bacillus Subtilis Strain DKT. Ann. Microbiol. 2019, 69, 267–277. [Google Scholar] [CrossRef]
- Ni, B.; Huang, Z.; Fan, Z.; Jiang, C.-Y.; Liu, S.-J. Comamonas Testosteroni Uses a Chemoreceptor for Tricarboxylic Acid Cycle Intermediates to Trigger Chemotactic Responses towards Aromatic Compounds: New Mechanism for Chemotaxis towards Aromatic Compounds. Mol. Microbiol. 2013, 90, 813–823. [Google Scholar] [CrossRef]
- Liu, L.; Jiang, C.-Y.; Liu, X.-Y.; Wu, J.-F.; Han, J.-G.; Liu, S.-J. Plant-Microbe Association for Rhizoremediation of Chloronitroaromatic Pollutants with Comamonas Sp. Strain CNB-1. Environ. Microbiol. 2007, 9, 465–473. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, B.; Dai, X.; Li, S.; Lu, G.; Zhou, Y. Structure and Function of the Bacterial Communities during Rhizoremediation of Hexachlorobenzene in Constructed Wetlands. Environ. Sci. Pollut. Res. 2017, 24, 11483–11492. [Google Scholar] [CrossRef]
- Tang, Q.; Lu, T.; Liu, S.-J. Developing a Synthetic Biology Toolkit for Comamonas Testosteroni, an Emerging Cellular Chassis for Bioremediation. ACS Synth. Biol. 2018, 7, 1753–1762. [Google Scholar] [CrossRef]
- Sun, X.; Li, X.; Cui, Y.; Jiang, Z.; Wang, Q.; Lu, Q.; Cui, J. Interaction with Endogenous Microorganisms, Comamonas Testosteroni Enhanced the Degradation of Polycyclic Aromatic Hydrocarbon in Soil. Res. Sq. 2021, 1–22. [Google Scholar] [CrossRef]
- Stockholm Convention on Persistent Organic Pollutants (POPs); Secretariat of the Stockholm Convention (SSC): Stockholm, Sweden, 2002; Volume 2020, Available online: https://www.state.gov/key-topics-office-of-environmental-quality-and-transboundary-issues/stockholm-convention-on-persistent-organic-pollutants (accessed on 25 September 2022).
- Ashraf, M.A. Persistent Organic Pollutants (POPs): A Global Issue, a Global Challenge. Environ. Sci. Pollut. Res. 2017, 24, 4223–4227. [Google Scholar] [CrossRef]
- Cheng, Z.; Chen, M.; Xie, L.; Peng, L.; Yang, M.; Li, M. Bioaugmentation of a Sequencing Batch Biofilm Reactor with Comamonas Testosteroni and Bacillus Cereus and Their Impact on Reactor Bacterial Communities. Biotechnol. Lett. 2015, 37, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Gabelish, C.L. Isolation and Investigation of an Aerobic Hexachlorobenzene-Degrading Bacterium; UNSW: Sydney, Australia, 2002. [Google Scholar]
- Gęgotek, A.; Skrzydlewska, E. Biological Effect of Protein Modifications by Lipid Peroxidation Products. Chem. Phys. Lipids 2019, 221, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Altomare, A.; Baron, G.; Gianazza, E.; Banfi, C.; Carini, M.; Aldini, G. Lipid Peroxidation Derived Reactive Carbonyl Species in Free and Conjugated Forms as an Index of Lipid Peroxidation: Limits and Perspectives. Redox Biol. 2021, 42, 101899. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxidative Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef] [PubMed]
- Prione, L.P.; Olchanheski, L.R.; Tullio, L.D.; Santo, B.C.E.; Reche, P.M.; Martins, P.F.; Carvalho, G.; Demiate, I.M.; Pileggi, S.A.V.; Dourado, M.N.; et al. GST Activity and Membrane Lipid Saturation Prevents Mesotrione-Induced Cellular Damage in Pantoea Ananatis. AMB Express 2016, 6, 70. [Google Scholar] [CrossRef] [PubMed]
- Ogliari, J.; Freitas, S.P.; Ramos, A.C.; Bressan Smith, R.E.; Façanha, A.R. Sistemas Primários de Transporte de Prótons Integram Os Mecanismos de Desintoxicação Do Mesotrione Em Plantas de Milho. Planta Daninha 2009, 27, 799–807. [Google Scholar] [CrossRef]
- Rodríguez-Vargas, S.; Sánchez-García, A.; Martínez-Rivas, J.M.; Prieto, J.A.; Randez-Gil, F. Fluidization of Membrane Lipids Enhances the Tolerance of Saccharomyces Cerevisiae to Freezing and Salt Stress. Appl. Environ. Microbiol. 2007, 73, 110–116. [Google Scholar] [CrossRef]
- Duc, H.D. Degradation of Chlorotoluenes by Comamonas Testosterone KT5. Appl. Biol. Chem. 2017, 60, 457–465. [Google Scholar] [CrossRef]
- Dimova, M.; Dankevych, L.; Yamborko, N.; Iutynska, G. Polyphasic Taxonomy Analyse of Comamonas Testosteroni Resistant to Hexachlorobenzene. J. Microbiol. Biotechnol. Food Sci. 2022, 11, e4711. [Google Scholar] [CrossRef]
- Dimova, M.; Iutynska, G. Fatty Acid Composition of Comamonas Testosteroni under Hexachlorobenzene Loading Conditions. Mikrobiol. Z. 2022, 2022, 3–14. [Google Scholar]
- Cabiscol, E.; Tamarit, J.; Ros, J. Oxidative Stress in Bacteria and Protein Damage by Reactive Oxygen Species. Int. Microbiol. 2000, 3, 3–8. [Google Scholar] [PubMed]
- Olchanheski, L.R.; Dourado, M.N.; Beltrame, F.L.; Zielinski, A.A.F.; Demiate, I.M.; Pileggi, S.A.V.; Azevedo, R.A.; Sadowsky, M.J.; Pileggi, M. Mechanisms of Tolerance and High Degradation Capacity of the Herbicide Mesotrione by Escherichia Coli Strain DH5-α. PLoS ONE 2014, 9, e99960. [Google Scholar] [CrossRef] [PubMed]
- Kibinza, S.; Bazin, J.; Bailly, C.; Farrant, J.M.; Corbineau, F.; El-Maarouf-Bouteau, H. Catalase Is a Key Enzyme in Seed Recovery from Ageing during Priming. Plant Sci. 2011, 181, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Kabel, A.M. Free Radicals and Antioxidants: Role of Enzymes and Nutrition. World J. Nutr. Health 2014, 2, 35–38. [Google Scholar] [CrossRef]
- Murínová, S.; Dercová, K. Response Mechanisms of Bacterial Degraders to Environmental Contaminants on the Level of Cell Walls and Cytoplasmic Membrane. Int. J. Microbiol. 2014, 2014, 873081. [Google Scholar] [CrossRef]
- Balague, C.; Sturtz, N.; Duffard, R.; Evangelista de Duffard, A.M. Effect of 2,4-Dichlorophenoxyacetic Acid Herbicide OnEscherichia Coli Growth, Chemical Composition, and Cellular Envelope. Environ. Toxicol. 2001, 16, 43–53. [Google Scholar] [CrossRef]
- Sánchez de Medina, F.; Romero-Calvo, I.; Mascaraque, C.; Martínez-Augustin, O. Intestinal Inflammation and Mucosal Barrier Function: Inflamm. Bowel Dis. 2014, 20, 2394–2404. [Google Scholar] [CrossRef]
- Halliwell, B. Free Radicals and Antioxidants: Updating a Personal View. Nutr. Rev. 2012, 70, 257–265. [Google Scholar] [CrossRef]
- Lü, Z.; Sang, L.; Li, Z.; Min, H. Catalase and Superoxide Dismutase Activities in a Stenotrophomonas Maltophilia WZ2 Resistant to Herbicide Pollution. Ecotoxicol. Environ. Saf. 2009, 72, 136–143. [Google Scholar] [CrossRef]
- de Oliveira, E.P.; da Silva Rovida, A.F.; Martins, J.G.; Pileggi, S.A.V.; Schemczssen-Graeff, Z.; Pileggi, M. Tolerance of Pseudomonas Strain to the 2,4-D Herbicide through a Peroxidase System. PLoS ONE 2021, 16, e0257263. [Google Scholar] [CrossRef]
- Recknagel, R.O.; Glende, E.A. Spectrophotometric Detection of Lipid Conjugated Dienes. Methods Enzym. 1984, 105, 331–337. [Google Scholar] [CrossRef]
- Ramishvili, L.; Zibzibadze, M.; Alibegashvili, M.; Chigogidze, T.; Gordeziani, M.; Gabunia, N.; Khazaradze, A.; Kotrikadze, N. Some Criteria for the Evaluation of Endogenous Intoxication in Men with Prostate Tumours. JBPC 2016, 16, 167–171. [Google Scholar] [CrossRef]
- Barriuso, B.; Astiasarán, I.; Ansorena, D. A Review of Analytical Methods Measuring Lipid Oxidation Status in Foods: A Challenging Task. Eur. Food Res. Technol. 2013, 236, 1–15. [Google Scholar] [CrossRef]
- Hadwan, M.H.; Abed, H.N. Data Supporting the Spectrophotometric Method for the Estimation of Catalase Activity. Data Brief 2016, 6, 194–199. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Thiyagarajan, A. Optimization of Extracellular Peroxidsae Production from Coprinus sp. IJST 2008, 1, 1–5. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dimova, M.; Tugai, A.; Tugai, T.; Iutynska, G.; Dordevic, D.; Kushkevych, I. Molecular Research of Lipid Peroxidation and Antioxidant Enzyme Activity of Comamonas testosteroni Bacterial Cells under the Hexachlorobenzene Impact. Int. J. Mol. Sci. 2022, 23, 11415. https://doi.org/10.3390/ijms231911415
Dimova M, Tugai A, Tugai T, Iutynska G, Dordevic D, Kushkevych I. Molecular Research of Lipid Peroxidation and Antioxidant Enzyme Activity of Comamonas testosteroni Bacterial Cells under the Hexachlorobenzene Impact. International Journal of Molecular Sciences. 2022; 23(19):11415. https://doi.org/10.3390/ijms231911415
Chicago/Turabian StyleDimova, Mariia, Andrii Tugai, Tetiana Tugai, Galyna Iutynska, Dani Dordevic, and Ivan Kushkevych. 2022. "Molecular Research of Lipid Peroxidation and Antioxidant Enzyme Activity of Comamonas testosteroni Bacterial Cells under the Hexachlorobenzene Impact" International Journal of Molecular Sciences 23, no. 19: 11415. https://doi.org/10.3390/ijms231911415
APA StyleDimova, M., Tugai, A., Tugai, T., Iutynska, G., Dordevic, D., & Kushkevych, I. (2022). Molecular Research of Lipid Peroxidation and Antioxidant Enzyme Activity of Comamonas testosteroni Bacterial Cells under the Hexachlorobenzene Impact. International Journal of Molecular Sciences, 23(19), 11415. https://doi.org/10.3390/ijms231911415







