Genome-Wide Identification and Expression Analysis of Cytokinin Response Regulator (RR) Genes in the Woody Plant Jatropha curcas and Functional Analysis of JcRR12 in Arabidopsis
Abstract
1. Introduction
2. Results
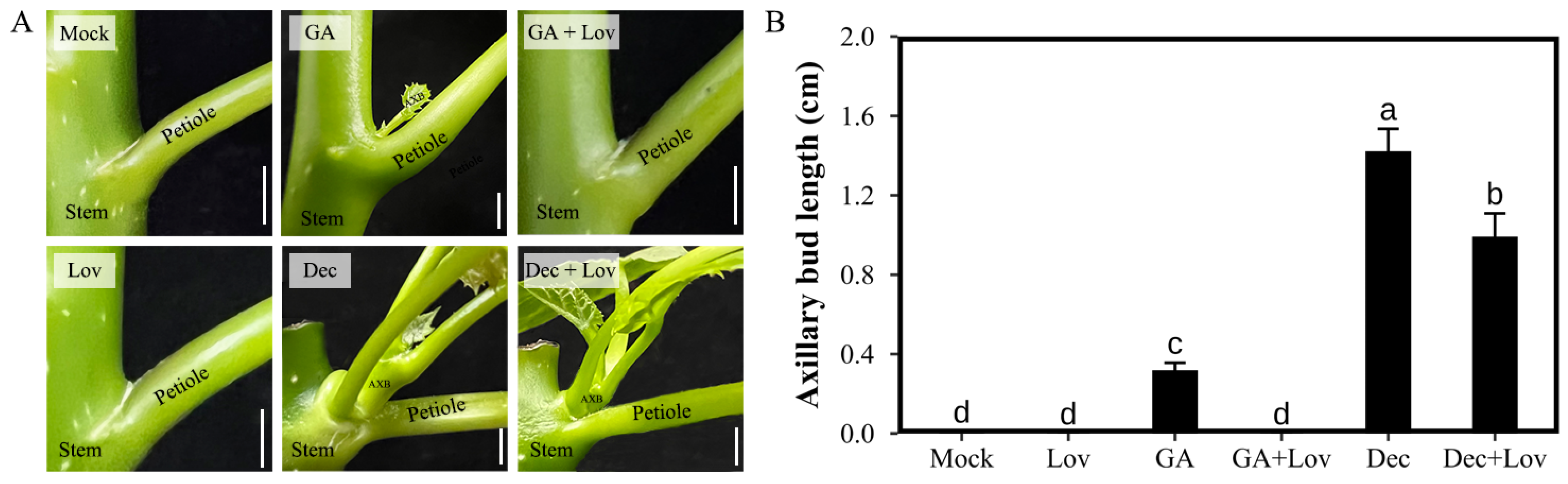
2.1. CK Is Required for GA- and Decapitation-Promoted Axillary Bud Outgrowth in J. curcas
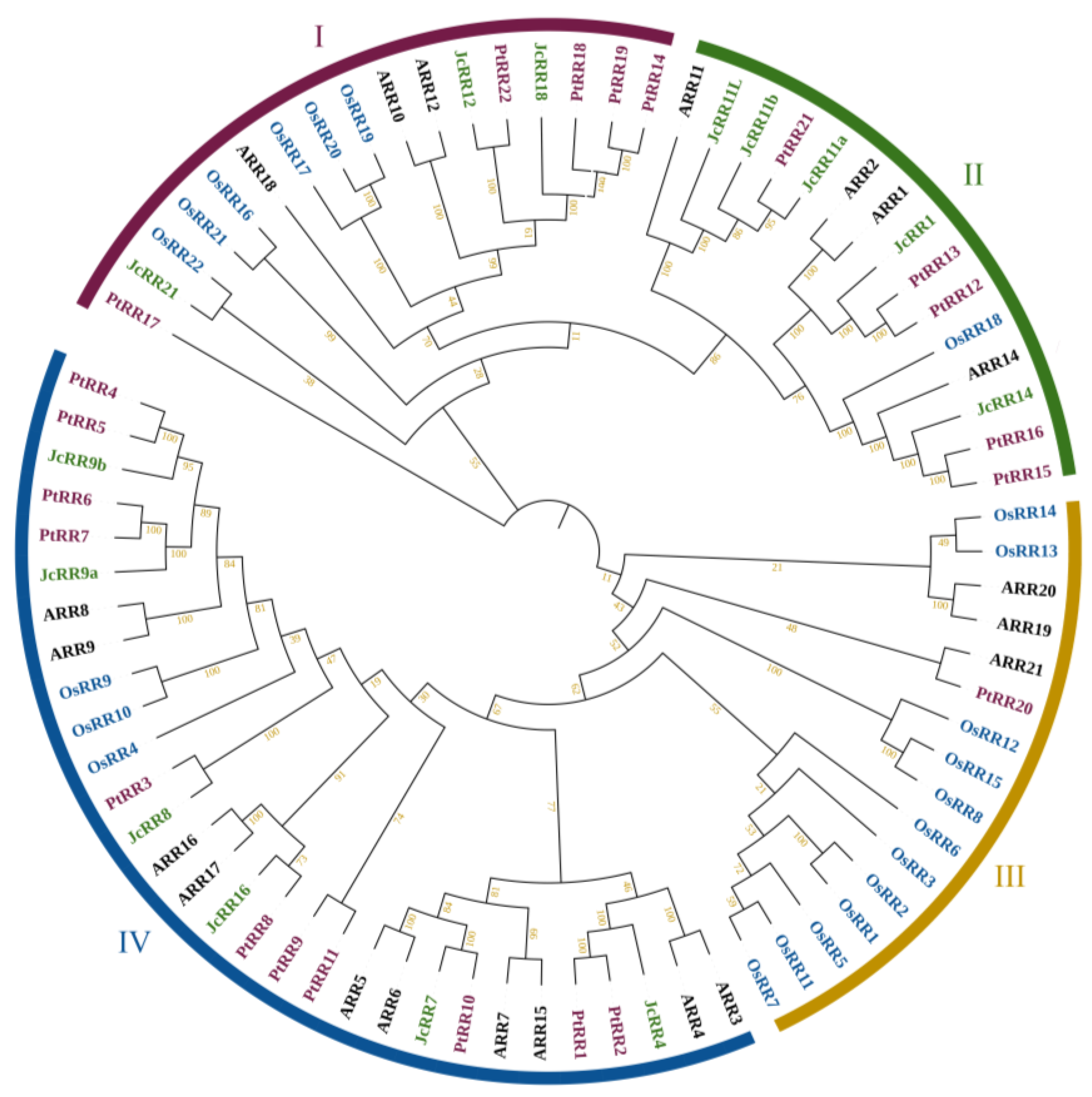
2.2. Identification of the RR Gene Family in J. curcas
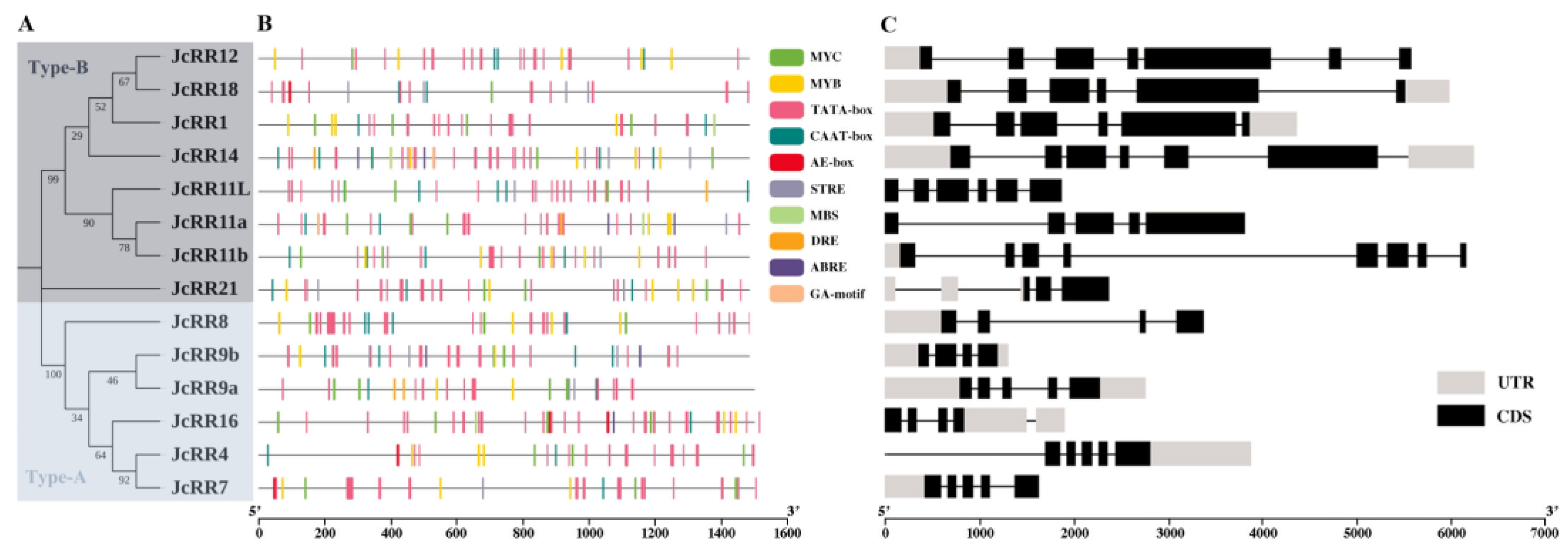
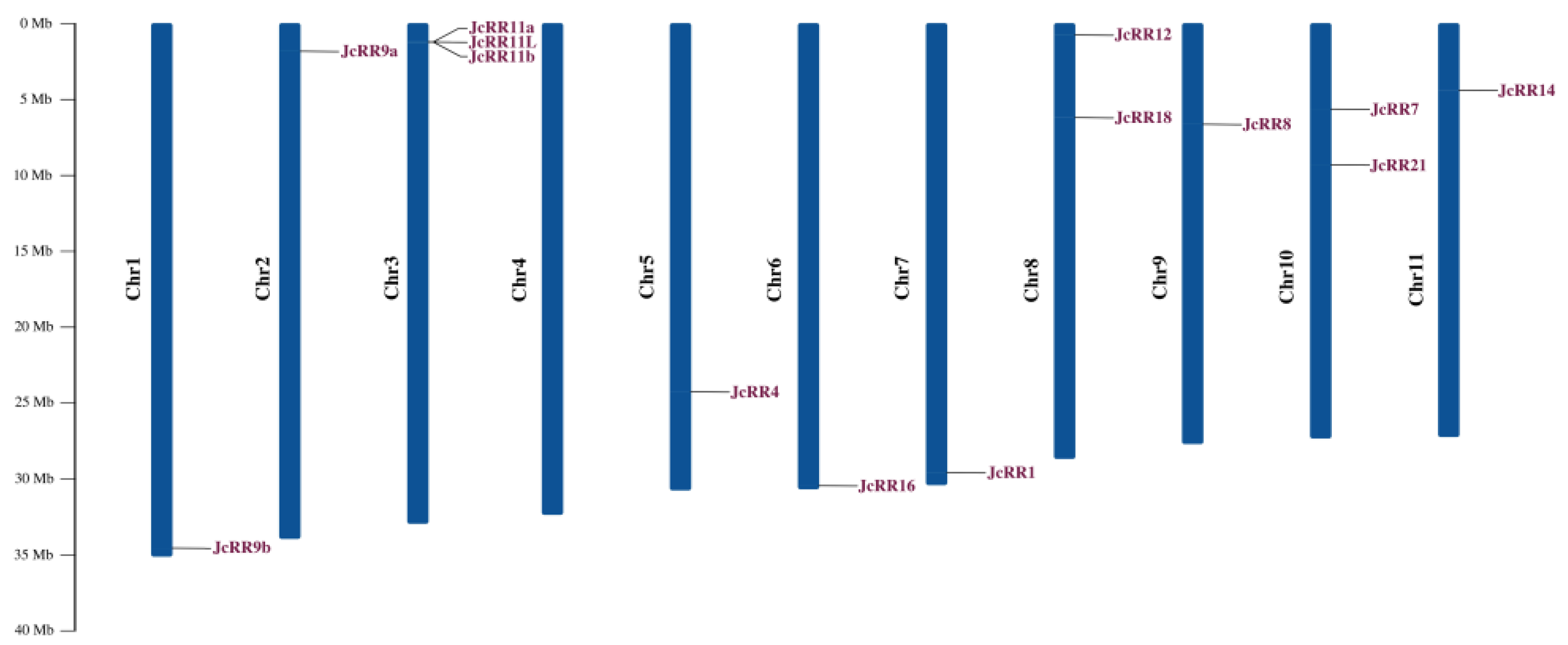
2.3. Gene Structure Analysis and Chromosome Locations
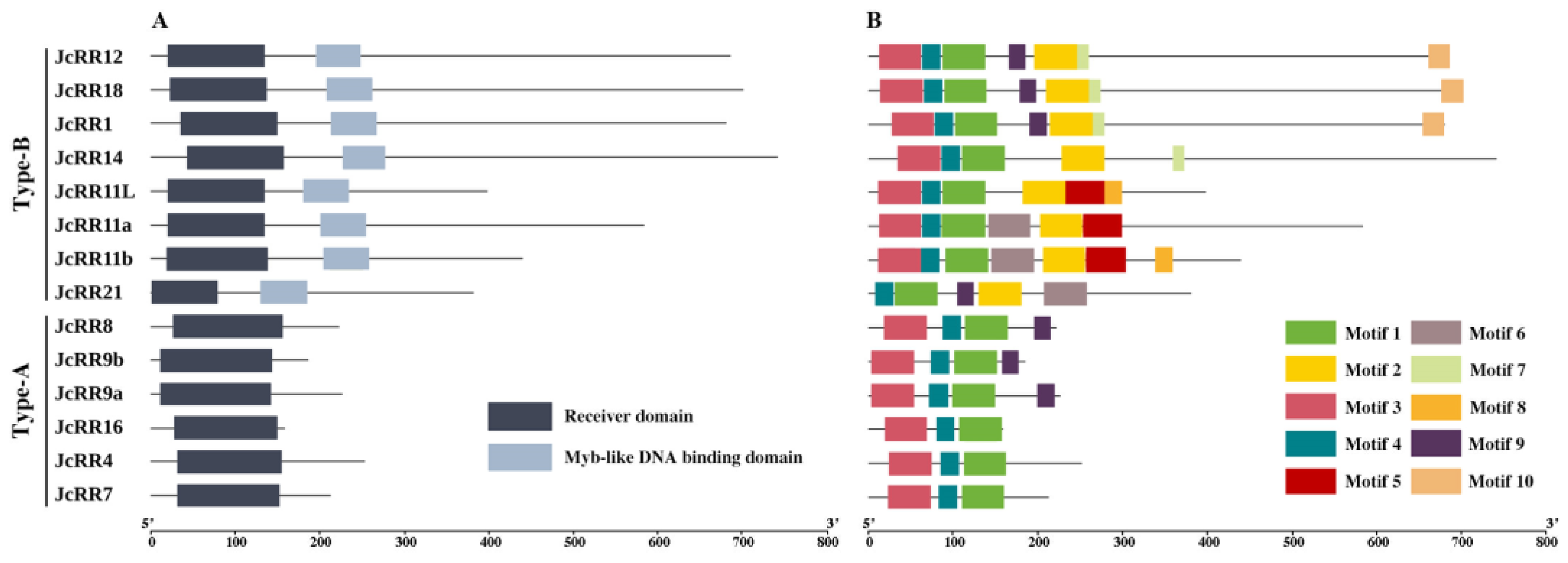
2.4. Conserved Domain and Motif Analysis
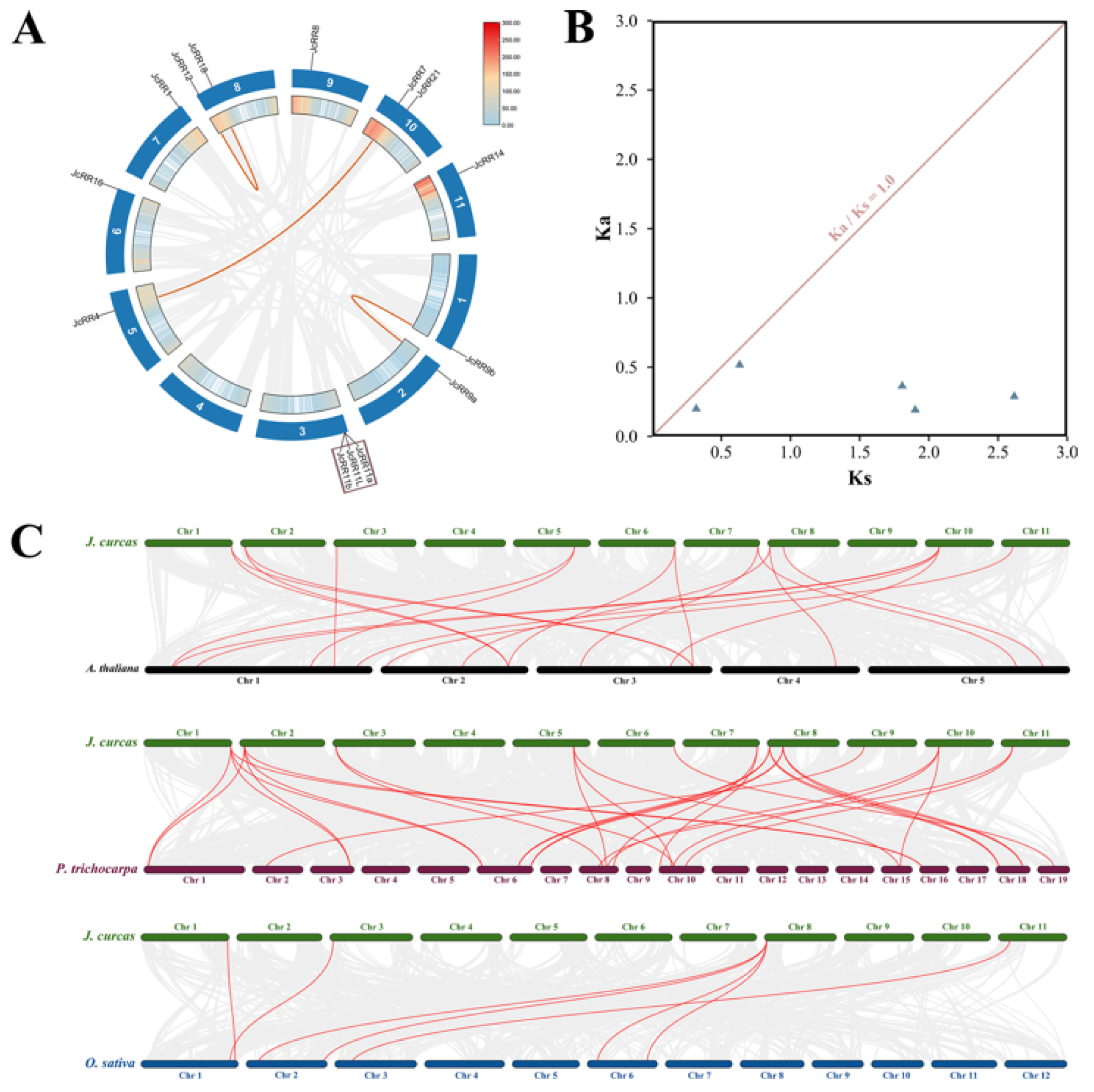
2.5. Chromosomal Distribution and Synteny Analysis
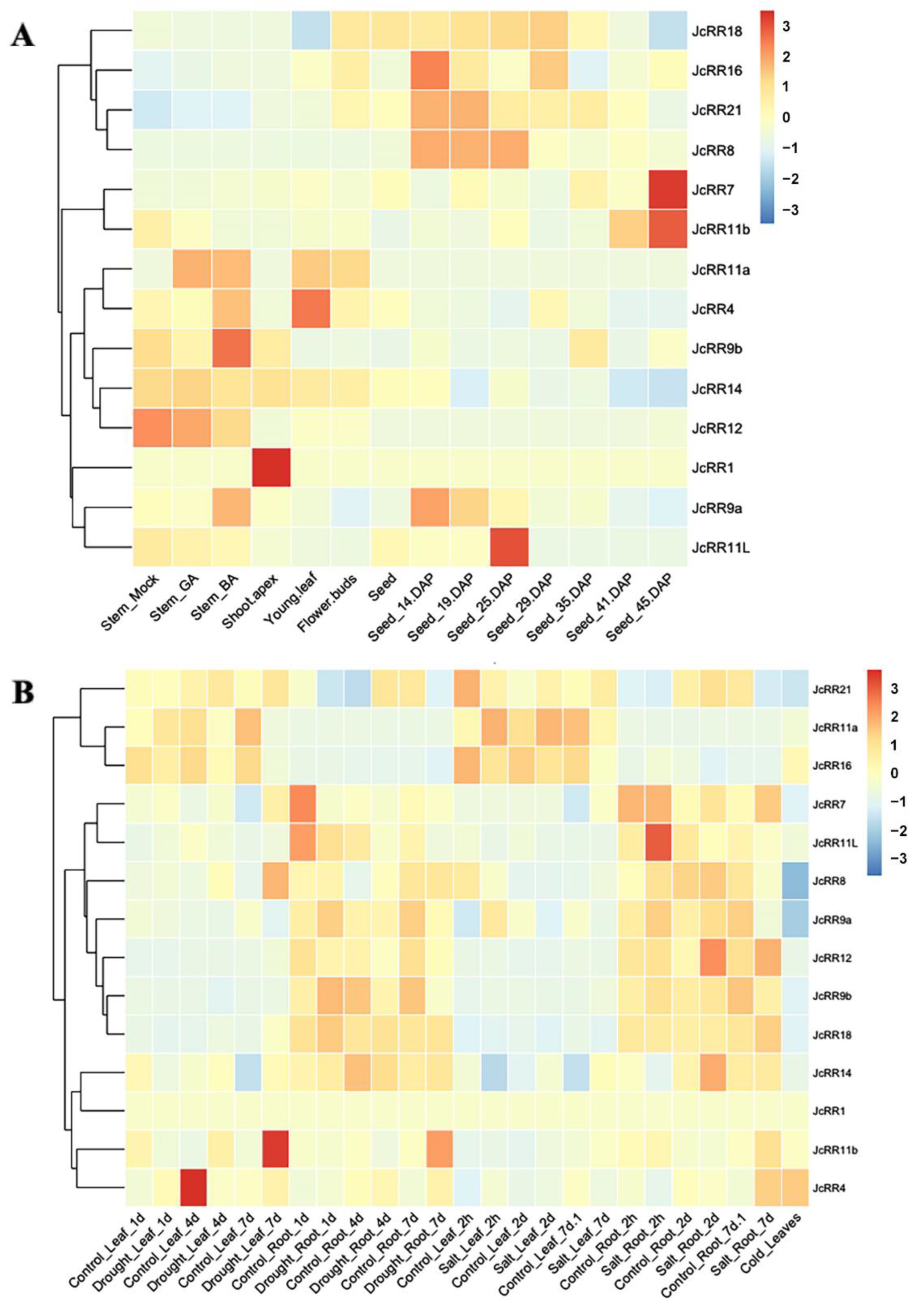
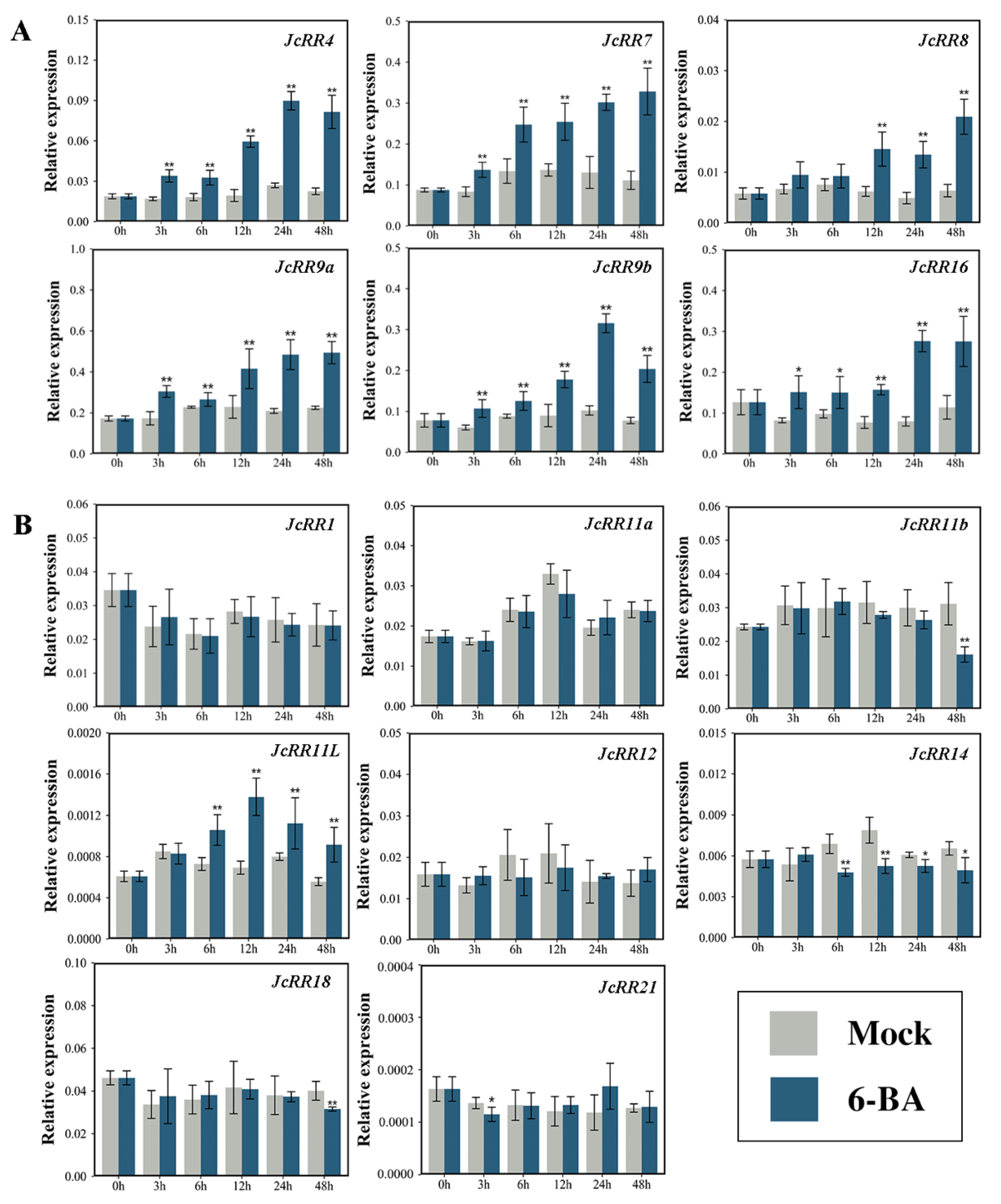
2.6. Expression Profiles of JcRRs
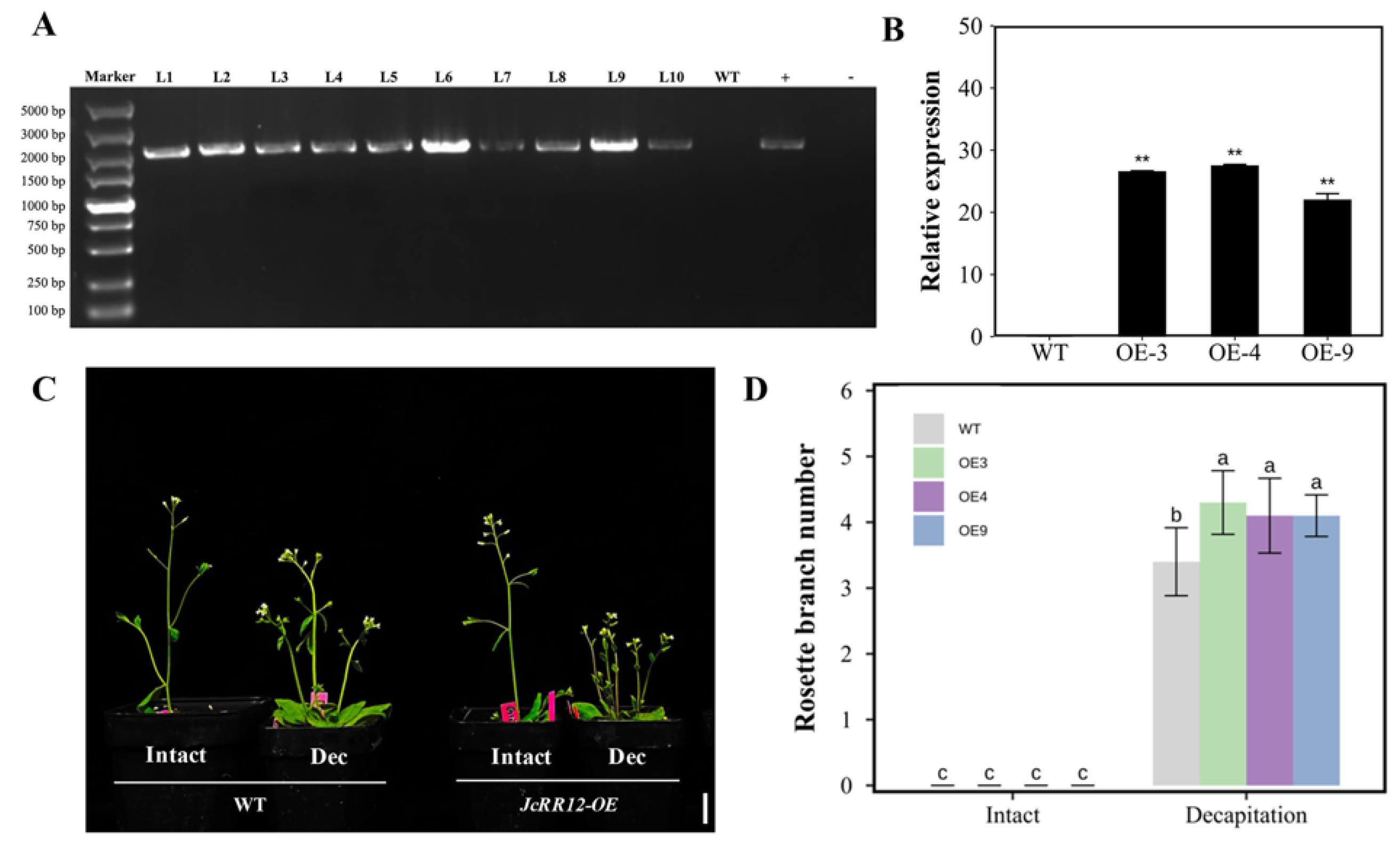
2.7. Overexpression of J. curcas JcRR12 in Arabidopsis Led to Slightly Increased Numbers of Rosette Branches after Decapitation Treatment
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Sequence Identification and Phylogenetic Tree Construction
4.3. Analysis of Protein Structure and Physicochemical Properties
4.4. Cis-Acting Elements in the Promoter Region and Gene Structure Analysis
4.5. Conserved Motif and Domain Prediction
4.6. Chromosomal Distribution and Synteny Analysis
4.7. Hormone, Stress Response, and Spatiotemporal Expression Analysis of JcRRs According to Transcriptome Data
4.8. Exogenous CK Treatment in J. curcas
4.9. mRNA Extraction and qRT–PCR
4.10. Construction of the JcRR12 Overexpression Vector and Arabidopsis Transformation
4.11. Decapitation Assays and Branch Counts in Arabidopsis
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lindsay, D.L.; Sawhney, V.K.; Bonham-Smith, P.C. Cytokinin-induced changes in CLAVATA1 and WUSCHEL expression temporally coincide with altered floral development in Arabidopsis. Plant Sci. 2006, 170, 1111–1117. [Google Scholar] [CrossRef]
- Werner, T.; Motyka, V.; Laucou, V.; Smets, R.; Van Onckelen, H.; Schmulling, T. Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity. Plant Cell 2003, 15, 2532–2550. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.P.; Chickarmane, V.S.; Ohno, C.; Meyerowitz, E.M. Multiple feedback loops through cytokinin signaling control stem cell number within the Arabidopsis shoot meristem. Proc. Natl. Acad. Sci. USA 2009, 106, 16529–16534. [Google Scholar] [CrossRef]
- Aloni, R. Role of cytokinin in differentiation of secondary xylem fibers. Plant Phyisol. 1982, 70, 1631–1633. [Google Scholar] [CrossRef]
- Lara, M.E.B.; Garcia, M.C.G.; Fatima, T.; Ehness, R.; Lee, T.K.; Proels, R.; Tanner, W.; Roitsch, T. Extracellular invertase is an essential component of cytokinin-mediated delay of senescence. Plant Cell 2004, 16, 1276–1287. [Google Scholar] [CrossRef] [PubMed]
- Bishopp, A.; Help, H.; El-Showk, S.; Weijers, D.; Scheres, B.; Friml, J.; Benkova, E.; Mahonen, A.P.; Helariutta, Y. A mutually inhibitory interaction between auxin and cytokinin specifies vascular pattern in roots. Curr. Biol. 2011, 21, 917–926. [Google Scholar] [CrossRef] [PubMed]
- Jameson, P.E.; Song, J. Cytokinin: A key driver of seed yield. J. Exp. Bot. 2016, 67, 593–606. [Google Scholar] [CrossRef] [PubMed]
- Peleg, Z.; Reguera, M.; Tumimbang, E.; Walia, H.; Blumwald, E. Cytokinin-mediated source/sink modifications improve drought tolerance and increase grain yield in rice under water-stress. Plant Biotechnol. J. 2011, 9, 747–758. [Google Scholar] [CrossRef] [PubMed]
- Ramireddy, E.; Chang, L.; Schmulling, T. Cytokinin as a mediator for regulating root system architecture in response to environmental cues. Plant Signal. Behav. 2014, 9, e27771. [Google Scholar] [CrossRef]
- Roman, H.; Girault, T.; Barbier, F.; Péron, T.; Brouard, N.; Pěnčík, A.; Novák, O.; Vian, A.; Sakr, S.; Lothier, J.; et al. Cytokinins are initial targets of light in the control of bud outgrowth. Plant Physiol. 2016, 172, 489–509. [Google Scholar] [CrossRef]
- Werner, T.; Schmülling, T. Cytokinin action in plant development. Curr. Opin. Plant Biol. 2009, 12, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Hutchison, C.E.; Kieber, J.J. Cytokinin signaling in Arabidopsis. Plant Cell 2002, 14, S47–S59. [Google Scholar] [CrossRef]
- Imamura, A.; Hanaki, N.; Nakamura, A.; Suzuki, T.; Taniguchi, M.; Kiba, T.; Ueguchi, C.; Sugiyama, T.; Mizuno, T. Compilation and characterization of Arabiopsis thaliana response regulators implicated in His-Asp phosphorelay signal transduction. Plant Cell Physiol. 1999, 40, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Zubo, Y.O.; Blakley, I.C.; Yamburenko, M.V.; Worthen, J.M.; Street, I.H.; Franco-Zorrilla, J.M.; Zhang, W.; Hill, K.; Raines, T.; Solano, R.; et al. Cytokinin induces genome-wide binding of the type-B response regulator ARR10 to regulate growth and development in Arabidopsis. Proc. Natl. Acad. Sci. USA 2017, 114, E5995–E6004. [Google Scholar] [CrossRef] [PubMed]
- To, J.P.C.; Deruere, J.; Maxwell, B.B.; Morris, V.F.; Hutchison, C.E.; Ferreira, F.J.; Schaller, G.E.; Kieber, J.J. Cytokinin regulates type-A Arabidopsis response regulator activity and protein stability via two-component phosphorelay. Plant Cell 2007, 19, 3901–3914. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Carvajal, G.A.; Morse, A.M.; Davis, J.M. Transcript profiles of the cytokinin response regulator gene family in Populus imply diverse roles in plant development. New Phytol. 2008, 177, 77–89. [Google Scholar] [CrossRef]
- Zeng, J.; Zhu, X.; Haider, M.S.; Wang, X.; Zhang, C.; Wang, C. Genome-wide identification and analysis of the type-B authentic response regulator gene family in peach (Prunus persica). Cytogenet. Genome Res. 2017, 151, 41–49. [Google Scholar] [CrossRef]
- Li, H.; Chen, R.; Chen, Z.; Lin, J.; Jin, X.; Ren, C.; Chen, Q.; Chen, F.; Yu, G.; Zhang, Y. The molecular characteristics of soybean ARR-B transcription factors. Biocell 2022, 46, 1575–1592. [Google Scholar] [CrossRef]
- Chen, C.; Liu, A.; Ren, H.; Yu, Y.; Duanmu, H.; Duan, X.; Sun, X.; Liu, B.; Zhu, Y. Genome-wide analysis of Glycine soja response regulator GsRR genes under alkali and salt stresses. Front. Plant Sci. 2018, 9, 1306. [Google Scholar] [CrossRef]
- D’Agostino, I.B.; Deruere, J.; Kieber, J.J. Characterization of the response of the Arabidopsis response regulator gene family to cytokinin. Plant Physiol. 2000, 124, 1706–1717. [Google Scholar] [CrossRef]
- Liu, Z.; Dai, X.; Li, J.; Liu, N.; Liu, X.; Li, S.; Xiang, F. The type-B cytokinin response regulator ARR1 inhibits shoot regeneration in an ARR12-dependent manner in Arabidopsis. Plant Cell 2020, 32, 2271–2291. [Google Scholar] [CrossRef]
- Waldie, T.; Leyser, O. Cytokinin targets auxin transport to promote shoot branching. Plant Phyisol. 2018, 177, 803–818. [Google Scholar] [CrossRef]
- Xia, X.; Dong, H.; Yin, Y.; Song, X.; Gu, X.; Sang, K.; Zhou, J.; Shi, K.; Zhou, Y.; Foyer, C.H.; et al. Brassinosteroid signaling integrates multiple pathways to release apical dominance in tomato. Proc. Natl. Acad. Sci. USA 2021, 118, e2004384118. [Google Scholar] [CrossRef]
- Yamburenko, M.V.; Worthen, J.M.; Zeenat, A.; Azhar, B.J.; Swain, S.; Couitt, A.R.; Shakeel, S.N.; Kieber, J.J.; Schaller, G.E. Functional analysis of the rice type-B response regulator RR22. Front. Plant Sci. 2020, 11, 577676. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.; Wang, M.; An, Y. Overexpression of a B-type cytokinin response regulator (OsORR2) reduces plant height in rice. Plant Signal. Behav. 2020, 15, 1780405. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Carvajal, G.A.; Morse, A.M.; Dervinis, C.; Davis, J.M. The cytokinin type-B response regulator PtRR13 Is a negative regulator of adventitious root development in Populus. Plant Physiol. 2009, 150, 759–771. [Google Scholar] [CrossRef] [PubMed]
- Müller, B.; Sheen, J. Cytokinin and auxin interaction in root stem-cell specification during early embryogenesis. Nature 2008, 453, 1094–1097. [Google Scholar] [CrossRef] [PubMed]
- Ishida, K.; Yamashino, T.; Yokoyama, A.; Mizuno, T. Three type-B response regulators, ARR1, ARR10 and ARR12, play essential but redundant roles in cytokinin signal transduction throughout the life cycle of Arabidopsis thaliana. Plant Cell Physiol. 2008, 49, 47–57. [Google Scholar] [CrossRef]
- Nguyen, K.H.; Van Ha, C.; Nishiyama, R.; Watanabe, Y.; Leyva-González, M.A.; Fujita, Y.; Tran, U.T.; Li, W.; Tanaka, M.; Seki, M.; et al. Arabidopsis type B cytokinin response regulators ARR1, ARR10, and ARR12 negatively regulate plant responses to drought. Proc. Natl. Acad. Sci. USA 2016, 113, 3090–3095. [Google Scholar] [CrossRef]
- Zeng, R.; Li, Z.; Shi, Y.; Fu, D.; Yin, P.; Cheng, J.; Jiang, C.; Yang, S. Natural variation in a type-A response regulator confers maize chilling tolerance. Nat.Commun. 2021, 12, 4713. [Google Scholar] [CrossRef] [PubMed]
- Pandey, V.C.; Singh, K.; Singh, J.S.; Kumar, A.; Singh, B.; Singh, R.P. Jatropha curcas: A potential biofuel plant for sustainable environmental development. Renew. Sustain. Energy Rev. 2012, 16, 2870–2883. [Google Scholar] [CrossRef]
- Makkar, H.P.S.; Becker, K. Jatropha curcas, a promising crop for the generation of biodiesel and value-added coproducts. Eur. J. Lipid. Sci. Technol. 2009, 111, 773–787. [Google Scholar] [CrossRef]
- Singh, D.; Sharma, D.; Soni, S.L.; Inda, C.S.; Sharma, S.; Sharma, P.K.; Jhalani, A. A comprehensive review of physicochemical properties, production process, performance and emissions characteristics of 2nd generation biodiesel feedstock: Jatropha curcas. Fuel 2021, 285, 119110. [Google Scholar] [CrossRef]
- Divakara, B.N.; Upadhyaya, H.D.; Wani, S.P.; Gowda, C.L.L. Biology and genetic improvement of Jatropha curcas L.: A review. Appl. Energy 2010, 87, 732–742. [Google Scholar] [CrossRef]
- Mazumdar, P.; Singh, P.; Babu, S.; Siva, R.; Harikrishna, J.A. An update on biological advancement of Jatropha curcas L.: New insight and challenges. Renew. Sustain. Energy Rev. 2018, 91, 903–917. [Google Scholar] [CrossRef]
- Ewunie, G.A.; Morken, J.; Lekang, O.I.; Yigezu, Z.D. Factors affecting the potential of Jatropha curcas for sustainable biodiesel production: A critical review. Renew. Sustain. Energy Rev. 2021, 137, 110500. [Google Scholar] [CrossRef]
- Lama, A.D.; Klemola, T.; Saloniemi, I.; Niemelä, P.; Vuorisalo, T. Factors affecting genetic and seed yield variability of Jatropha curcas (L.) across the globe: A review. Energy Sustain. Dev. 2018, 42, 170–182. [Google Scholar] [CrossRef]
- Ming, X.; Tao, Y.-B.; Fu, Q.; Tang, M.; He, H.; Chen, M.-S.; Pan, B.-Z.; Xu, Z.-F. Flower-specific overproduction of cytokinins altered flower development and sex expression in the perennial woody plant Jatropha curcas L. Int. J. Mol. Sci. 2020, 21, 640. [Google Scholar] [CrossRef]
- Ni, J.; Zhao, M.-L.; Chen, M.-S.; Pan, B.-Z.; Tao, Y.-B.; Xu, Z.-F. Comparative transcriptome analysis of axillary buds in response to the shoot branching regulators gibberellin A3 and 6-benzyladenine in Jatropha curcas. Sci. Rep. 2017, 7, 11417. [Google Scholar] [CrossRef]
- Chen, M.-S.; Zhao, M.-L.; Wang, G.-J.; He, H.-Y.; Bai, X.; Pan, B.-Z.; Fu, Q.-T.; Tao, Y.-B.; Tang, M.-Y.; Martínez-Herrera, J.; et al. Transcriptome analysis of two inflorescence branching mutants reveals cytokinin is an important regulator in controlling inflorescence architecture in the woody plant Jatropha curcas. BMC Plant Biol. 2019, 19, 468. [Google Scholar] [CrossRef]
- Chen, M.-S.; Niu, L.; Zhao, M.-L.; Xu, C.; Pan, B.-Z.; Fu, Q.; Tao, Y.-B.; He, H.; Hou, C.; Xu, Z.-F. De novo genome assembly and Hi-C analysis reveal an association between chromatin architecture alterations and sex differentiation in the woody plant Jatropha curcas. GigaScience 2020, 9, giaa009. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Pan, B.-Z.; Chen, M.; Chen, W.; Li, J.; Xu, Z.-F.; Liu, C. JCDB: A comprehensive knowledge base for Jatropha curcas, an emerging model for woody energy plants. BMC Genom. 2019, 20, 958. [Google Scholar] [CrossRef]
- Niu, L.; Tao, Y.-B.; Chen, M.-S.; Fu, Q.; Dong, Y.; He, H.; Xu, Z.-F. Identification and characterization of tetraploid and octoploid Jatropha curcas induced by colchicine. Caryologia 2016, 69, 58–66. [Google Scholar] [CrossRef]
- Xu, G.; Huang, J.; Yang, Y.; Yao, Y.-A. Transcriptome analysis of flower sex differentiation in Jatropha curcas L. using RNA sequencing. PLoS ONE 2016, 11, e0145613. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Wu, P.; Zhang, S.; Song, C.; Chen, Y.; Li, M.; Jia, Y.; Fang, X.; Chen, F.; Wu, G. Global analysis of gene expression profiles in developing physic nut (Jatropha curcas L.) seeds. PLoS ONE 2012, 7, e36522. [Google Scholar] [CrossRef]
- Juntawong, P.; Sirikhachornkit, A.; Pimjan, R.; Sonthirod, C.; Sangsrakru, D.; Yoocha, T.; Tangphatsornruang, S.; Srinives, P. Elucidation of the molecular responses to waterlogging in Jatropha roots by transcriptome profiling. Front. Plant Sci. 2014, 5, 658. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, C.; Wu, P.; Chen, Y.; Li, M.; Jiang, H.; Wu, G. Global analysis of gene expression profiles in physic nut (Jatropha curcas L.) seedlings exposed to salt stress. PLoS ONE 2014, 9, e97878. [Google Scholar]
- Wang, H.; Zou, Z.; Wang, S.; Gong, M. Global analysis of transcriptome responses and gene expression profiles to cold stress of Jatropha curcas L. PLoS ONE 2013, 8, e82817. [Google Scholar] [CrossRef]
- Ni, J.; Gao, C.; Chen, M.-S.; Pan, B.-Z.; Ye, K.; Xu, Z.-F. Gibberellin promotes shoot branching in the perennial woody plant Jatropha curcas. Plant Cell Physiol. 2015, 56, 1655–1666. [Google Scholar] [CrossRef]
- Hwang, I.; Sheen, J.; Müller, B. Cytokinin signaling networks. Annu. Rev. 2012, 63, 353–380. [Google Scholar] [CrossRef]
- Du, L.; Jiao, F.; Chu, J.; Jin, G.; Chen, M.; Wu, P. The two-component signal system in rice (Oryza sativa L.): A genome-wide study of cytokinin signal perception and transduction. Genomics 2007, 89, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Manevski, A.; Bertoni, G.; Bardet, C.; Tremousaygue, D.; Lescure, B. In synergy with various cis-acting elements, plant insterstitial telomere motifs regulate gene expression in Arabidopsis root meristems. FEBS Lett. 2000, 483, 43–46. [Google Scholar] [CrossRef]
- Yamaguchi-Shinozaki, K.; Shinozaki, K. Organization of cis-acting regulatory elements in osmotic- and cold-stress-responsive promoters. Trends Plant Sci. 2005, 10, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Narusaka, Y.; Nakashima, K.; Shinwari, Z.K.; Sakuma, Y.; Furihata, T.; Abe, H.; Narusaka, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Interaction between two cis-acting elements, ABRE and DRE, in ABA-dependent expression of Arabidopsis rd29A gene in response to dehydration and high-salinity stresses. Plant J. 2003, 34, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Sutoh, K.; Yamauchi, D. Two cis-acting elements necessary and sufficient for gibberellin-upregulated proteinase expression in rice seeds. Plant J. 2003, 34, 635–645. [Google Scholar] [CrossRef]
- Sleckman, B.; Gorman, J.; Alt, F. Accessibility control of antigen-receptor variable-region gene assembly: Role of cis-acting elements. Annu. Rev. Immunol. 1996, 14, 459–481. [Google Scholar] [CrossRef] [PubMed]
- Marin-de la Rosa, N.; Pfeiffer, A.; Hill, K.; Locascio, A.; Bhalerao, R.P.; Miskolczi, P.; Gronlund, A.L.; Wanchoo-Kohli, A.; Thomas, S.G.; Bennett, M.J.; et al. Genome wide binding site analysis reveals transcriptional coactivation of cytokinin-responsive genes by DELLA proteins. PLoS Genet. 2015, 11, e1005337. [Google Scholar] [CrossRef] [PubMed]
- Cannon, S.B.; Mitra, A.; Baumgarten, A.; Young, N.D.; May, G. The roles of segmental and tandem gene duplication in the evolution of large gene families in Arabidopsis thaliana. BMC Plant Biol. 2004, 4, 10. [Google Scholar] [CrossRef]
- Moubayidin, L.; Perilli, S.; Dello Ioio, R.; Di Mambro, R.; Costantino, P.; Sabatini, S. The rate of cell differentiation controls the Arabidopsis root meristem growth phase. Curr. Biol. 2010, 20, 1138–1143. [Google Scholar] [CrossRef]
- Fleishon, S.; Shani, E.; Ori, N.; Weiss, D. Negative reciprocal interactions between gibberellin and cytokinin in tomato. New Phytol. 2011, 190, 609–617. [Google Scholar] [CrossRef]
- Weiss, D.; Ori, N. Mechanisms of cross talk between gibberellin and other hormones. Plant Physiol. 2007, 144, 1240–1246. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Yu, H.; Yuan, K.; Liao, Z.; Meng, X.; Jing, Y.; Liu, G.; Chu, J.; Li, J. Strigolactone promotes cytokinin degradation through transcriptional activation of CYTOKININ OXIDASE/DEHYDROGENASE 9 in rice. Proc. Natl. Acad. Sci. USA 2019, 116, 14319–14324. [Google Scholar] [CrossRef]
- Huang, X.; Hou, L.; Meng, J.; You, H.; Li, Z.; Gong, Z.; Yang, S.; Shi, Y. The antagonistic action of abscisic acid and cytokinin signaling mediates drought stress response in Arabidopsis. Mol. Plant 2018, 11, 970–982. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; Tyagi, A.K.; Khurana, J.P. Molecular characterization and differential expression of cytokinin-responsive type-A response regulators in rice (Oryza sativa). BMC Plant Biol. 2006, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Müller, D.; Leyser, O. Auxin, cytokinin and the control of shoot branching. Ann. Bot. 2011, 107, 1203–1212. [Google Scholar] [CrossRef]
- Miyawaki, K.; Matsumoto-Kitano, M.; Kakimoto, T. Expression of cytokinin biosynthetic isopentenyltransferase genes in Arabidopsis: Tissue specificity and regulation by auxin, cytokinin, and nitrate. Plant J. 2004, 37, 128–138. [Google Scholar] [CrossRef]
- Jameson, P.E. Cytokinins. In Encyclopedia of Applied Plant Sciences, 2nd ed.; Thomas, B., Murray, B.G., Murphy, D.J., Eds.; Academic Press: Oxford, UK, 2017; pp. 391–402. [Google Scholar]
- Worthen, J.M.; Yamburenko, M.V.; Lim, J.; Nimchuk, Z.L.; Kieber, J.J.; Schaller, G.E. Type-B response regulators of rice play key roles in growth, development and cytokinin signaling. Development 2019, 146, dev174870. [Google Scholar] [CrossRef]
- Zhang, F.; May, A.; Irish, V.F. Type-B ARABIDOPSIS resonse regulators directly activate WUSCHEL. Trends Plant Sci. 2017, 22, 815–817. [Google Scholar] [CrossRef]
- Gasteiger, E.; Hoogland, C.; Gattiker, A.; Duvaud, S.; Wilkins, M.R.; Appel, R.D.; Bairoch, A. Protein identification and analysis tools on the ExPASy server. In The Proteomics Protocols Handbook; Springer: Berlin/Heidelberg, Germany, 2005; pp. 571–607. [Google Scholar]
- Chou, K.-C.; Shen, H.-B. Plant-mPLoc: A top-down strategy to augment the power for predicting plant protein subcellular localization. PLoS ONE 2010, 5, e11335. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Zhang, L.; He, L.-L.; Fu, Q.-T.; Xu, Z.-F. Selection of reliable reference genes for gene expression studies in the biofuel plant Jatropha curcas using real-time quantitative PCR. Int. J. Mol. Sci. 2013, 14, 24338–24354. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Chen, Z.X. Potentiation of developmentally regulated plant defense response by AtWRKY18, a pathogen-induced Arabidopsis transcription factor. Plant Phyisol. 2002, 129, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef] [PubMed]

| Name | Gene ID | Type | Protein Length (aa) | MW (kDa) | pI | Subcellular Localization | Instability Index | GRAVY |
|---|---|---|---|---|---|---|---|---|
| JcRR4 | 105649825 | A | 252 | 27.79 | 8.18 | Nucleus | 87.84 | −0.392 |
| JcRR7 | 105640448 | A | 212 | 23.60 | 8.28 | Nucleus | 66.37 | −0.248 |
| JcRR8 | 105638952 | A | 222 | 25.06 | 4.86 | Nucleus | 69.70 | −0.817 |
| JcRR9a | 105634414 | A | 226 | 25.52 | 5.80 | Nucleus | 74.07 | −0.733 |
| JcRR9b | 110009224 | A | 185 | 20.90 | 5.19 | Nucleus | 53.56 | −0.523 |
| JcRR16 | 105649171 | A | 158 | 17.77 | 7.58 | Nucleus | 31.31 | −0.313 |
| JcRR1 | 105643496 | B | 680 | 74.15 | 5.64 | Nucleus | 44.46 | −0.459 |
| JcRR11a | 105644735 | B | 583 | 65.66 | 5.65 | Nucleus | 45.23 | −0.618 |
| JcRR11b | 105644733 | B | 439 | 49.37 | 5.76 | Nucleus | 48.08 | −0.608 |
| JcRR11L | 105644734 | B | 398 | 44.86 | 6.52 | Nucleus | 36.01 | −0.387 |
| JcRR12 | 105637574 | B | 685 | 75.29 | 5.79 | Nucleus | 32.63 | −0.488 |
| JcRR14 | 105634128 | B | 741 | 80.28 | 6.27 | Nucleus | 44.31 | −0.272 |
| JcRR18 | 105633481 | B | 701 | 76.85 | 5.47 | Nucleus | 43.80 | −0.566 |
| JcRR21 | 110009834 | B | 381 | 43.11 | 5.55 | Nucleus | 45.77 | −0.671 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geng, X.; Zhang, C.; Wei, L.; Lin, K.; Xu, Z.-F. Genome-Wide Identification and Expression Analysis of Cytokinin Response Regulator (RR) Genes in the Woody Plant Jatropha curcas and Functional Analysis of JcRR12 in Arabidopsis. Int. J. Mol. Sci. 2022, 23, 11388. https://doi.org/10.3390/ijms231911388
Geng X, Zhang C, Wei L, Lin K, Xu Z-F. Genome-Wide Identification and Expression Analysis of Cytokinin Response Regulator (RR) Genes in the Woody Plant Jatropha curcas and Functional Analysis of JcRR12 in Arabidopsis. International Journal of Molecular Sciences. 2022; 23(19):11388. https://doi.org/10.3390/ijms231911388
Chicago/Turabian StyleGeng, Xianchen, Chun Zhang, Lida Wei, Kai Lin, and Zeng-Fu Xu. 2022. "Genome-Wide Identification and Expression Analysis of Cytokinin Response Regulator (RR) Genes in the Woody Plant Jatropha curcas and Functional Analysis of JcRR12 in Arabidopsis" International Journal of Molecular Sciences 23, no. 19: 11388. https://doi.org/10.3390/ijms231911388
APA StyleGeng, X., Zhang, C., Wei, L., Lin, K., & Xu, Z.-F. (2022). Genome-Wide Identification and Expression Analysis of Cytokinin Response Regulator (RR) Genes in the Woody Plant Jatropha curcas and Functional Analysis of JcRR12 in Arabidopsis. International Journal of Molecular Sciences, 23(19), 11388. https://doi.org/10.3390/ijms231911388







